Abstract
Canine vector-borne diseases (CVBDs) pose a diagnostic challenge, particularly when a dog is coinfected with more than one pathogen. The purpose of this study was to generate information about the diagnosis of CVBDs in young dogs following their first exposure to flea, tick, sand fly, louse, and mosquito vectors. From March 2008 to May 2009, 10 purpose-bred young naive beagle dogs and a cohort of 48 mixed-breed dogs living in an area to which CVBD is endemic in southern Italy were monitored using different diagnostic tests (cytology, serology, and PCR). Overall, PCR detected the highest number of dogs infected with Anaplasma platys, Babesia vogeli, and Ehrlichia canis, whereas seroconversion was a more sensitive indicator of exposure to Leishmania infantum. For A. platys infection, combining blood and buffy coat cytology in parallel enhanced the relative sensitivity (SErel) (87.3%). For B. vogeli, the best diagnostic combination was buffy coat cytology and serology used in parallel (SErel, 67.5%), whereas serology and PCR used in parallel (SErel, 100%) was the best combination for L. infantum. Overall, 12 (20.7%) dogs were coinfected; however, the percentage of new coinfections decreased from baseline (50%) to the first (33.3%) and second (16.6%) follow-up time points. Numbers of coinfections with A. platys and B. vogeli were significantly higher (P < 0.05) than coinfections with other pathogen combinations. The data generated in this study provide insights on the incidence of certain pathogens infecting young dogs in southern Italy, highlight important diagnostic testing limitations, and support the use of multiple diagnostic modalities when attempting to confirm a tick-borne infection in an individual dog or in a canine population.
Canine vector-borne diseases (CVBDs) comprise a group of infectious diseases caused by diverse pathogens (e.g., bacteria, protozoa, and helminths), which are transmitted by arthropod vectors, including ticks, fleas, lice, mosquitoes, and phlebotomine sand flies (27). Because some CVBD-causing pathogens (e.g., Anaplasma phagocytophilum, Bartonella spp., Ehrlichia canis, Leishmania infantum, Rickettsia spp.) are of major zoonotic concern, these diseases constitute an emerging worldwide public health threat for pet dogs and their owners (4, 6, 27).
In Italy, CVBDs, such as anaplasmosis, babesiosis, dirofilariosis, ehrlichiosis, and leishmaniosis, are highly prevalent, with some evidence supporting the spread of vectors and pathogens into new geographic locations (26). For instance, new foci of canine leishmaniosis have recently been detected in the northern regions of Italy (29). The reasons for the spread of various CVBDs in Europe are not fully understood but are probably linked to changes in vector ecology caused by alterations in climate and habitat, the introduction or reintroduction of competent vectors into previously disease-free areas, and the inadvertent transport of CVBD-causing pathogens during travel and relocation of occult, infected animals (pets, strays, and wildlife) from areas of endemicity to areas in which they are not endemic (26). However, the occurrence and comparative medical importance of the aforementioned CVBDs in pet dogs, as well as infection with other little-known vector-borne pathogens in Italy, might still be underestimated. For example, DNA of Bartonella vinsonii subsp. berkhoffii, a zoonotic emerging pathogen, was recently sequenced from ticks and dogs from southern Italy (10).
CVBDs represent a substantial diagnostic challenge for veterinarians because clinical signs induced by various vector-borne pathogens may be similar and because coinfections with two or more agents may lead to overlapping or atypical clinical signs (for examples, see references 22 and 35). Diagnostic confirmation of CVBDs should include historical exposure to arthropod vectors, compatible clinical signs and physical examination findings, and laboratory confirmation, supported by cytological, serological, and molecular test results. During the past decade, molecular techniques (e.g., PCR-based methods) have proven to be useful for the diagnostic confirmation of many CVBDs, whereas serology or cytology has been used historically in epidemiological surveys or to confirm a clinical diagnosis, respectively (28).
Few longitudinal studies have been carried out in order to assess the utility of various diagnostic modalities for confirming CVBDs in dogs living in areas of endemicity. In the Mediterranean Basin, most of these studies have been focused on L. infantum (e.g., 12, 14, 24, 30) and a few other pathogens, such as E. canis (1, 22). However, there is little information regarding the diagnosis of a diverse spectrum of CVBDs in young dogs in areas of endemicity following natural exposure to fleas, ticks, and sand fly vectors. Therefore, the objective of this study was to evaluate the comparative diagnostic utility of cytology, serology, and PCR alone or in combination for the diagnosis of single or multiple infections with Anaplasma platys, Babesia vogeli, Bartonella spp., E. canis, Hepatozoon canis, and L. infantum in young, naive, autochthonous (i.e., indigenous animals whelped in the study area), and sentinel dogs following their first natural exposure to arthropod vectors.
MATERIALS AND METHODS
Animals and sampling procedures.
From March 2008 to May 2009, samples were collected from 58 dogs living in a private animal shelter in Putignano (province of Bari, Apulia region, Italy), a region in which the targeted CVBDs are endemic. In particular, 48 mixed-breed autochthonous puppies that were whelped during the winter and ranged in age from 90 to 145 days (in March and April 2008) were included in the study. In May 2008, 10 purpose-bred beagles (120 days old), purchased from Green Hill SRL (Montichiari, Brescia, Italy), were introduced into the animal shelter to be included as naive controls in the study. All dogs were kept in wire mesh cages (10 m by 20 m) surrounded by free-ranging dogs, which were not directly enrolled in the study. Dogs were kept under their usual housing conditions and were purposely not treated for ectoparasites. At the time of initial testing (March and April 2008), all blood and skin samples were obtained from all dogs entered into the trial at four time points, i.e., at their enrolment (baseline), in July and October 2008 (first and second follow-up times, respectively), and in April 2009 (third follow-up). A bone marrow aspiration biopsy was performed at baseline and at the final follow-up time.
Blood samples were collected from the brachial or jugular veins, allowed to clot at room temperature, and centrifuged at 1,678 × g for 10 min, after which the serum was separated and stored at −20°C until tested. Skin samples were collected from the right shoulder region using disposable scalpels after shaving the hair over an area of about 0.5 by 0.5 by 0.6 cm. Bone marrow samples were aspirated from the iliac crest using Rosenthal needles (16 or 18 gauge). The dogs were restrained in the lateral recumbent position, and local anesthesia (lidocaine, 2%; Azienda Terapeutica Italiana, Ozzano Emilia, Bologna, Italy) was applied prior to bone marrow puncture. Bone marrow samples were placed in individual Eppendorf tubes containing 1 ml of sterile phosphate-buffered saline (PBS) (pH 7.2) and stored at −20°C until DNA extraction. All procedures were approved by the Animal Ethics Committee from the Faculty of Veterinary Medicine, University of Bari, Bari, Italy. Dogs in this study were examined clinically in conjunction with routine blood tests, which were done at the baseline and at each follow-up time point.
Diagnostic categorization.
Unless specified, a “positive dog” is defined here as a dog that was cytology, serology, or PCR positive for one or more than one CVBD-causing pathogen in one or more than one diagnostic test.
Serology.
An in-house immunofluorescent antibody test (IFAT) was used to detect anti-Leishmania IgG antibodies. Promastigotes of L. infantum zymodeme MON-1 were used as an antigen, and all procedures were performed as described elsewhere (29). Samples were scored as positive when they produced a clear cytoplasmatic or membrane fluorescence with promastigotes using a cutoff dilution of 1:80. Positive sera were titrated until negative.
A commercial IFAT, MegaScreen Fluobabesia canis (MegaCor Diagnostic, Hörbranz, Austria), was used to detect anti-Babesia canis IgG antibodies, using canine erythrocytes infected with B. canis as an antigen. Positive and negative controls were supplied by the company. Slide wells were exposed to sera diluted (1:32) in PBS in a moist chamber and then to fluoresceinated rabbit anti-dog IgG serum diluted 1:40 (Rabbit anti-dog IgG; Sigma-Aldrich, Milan, Italy), both at 37°C for 30 min. Samples were scored as positive when fluorescence was noticed within the cytoplasm or outside infected erythrocytes.
Anti-E. canis antibodies were detected by IFAT using slides containing fixed E. canis in DH82 cells (Canine Ehrlichiosis FA substrate slide; VMRD, Pullmann, WA). The infected cells were exposed to sera diluted (1:50) in PBS in a moist chamber and, after washing, to fluoresceinated rabbit anti-dog IgG diluted 1:60; both incubations were done at 37°C for 30 min. Samples that screened positive for cytoplasmic inclusion body fluorescence at a dilution of 1:50 were further titrated until the reaction became negative.
Dirofilaria immitis antigens were assessed at the third follow-up using a commercial canine heartworm test kit (Idex Laboratories, Milan, Italy) according to the manufacturer's instructions.
Cytology.
Blood, buffy coat, and bone marrow smears were prepared and stained using MGG quick stain (Bio Optica, Milan, Italy). Stained smears were examined by light microscopy for the presence of intracellular inclusions (or free forms) of common CVBD-causing pathogens. In particular, A. platys, E. canis, H. canis, and Babesia species inclusions were searched for in blood and/or buffy coat smears. In addition, intracellular and free amastigote forms of L. infantum were searched for in bone marrow smears. Each smear was examined for 10 min under an oil immersion objective at magnification ×100.
PCR.
For the detection of L. infantum, DNA was extracted from 100 μl of bone marrow using the QIAamp DNA microkit (Qiagen, Milan, Italy) and from about 50 mg of skin samples using a genomic DNA purification kit (Gentra Systems, Minneapolis, MN), following the producers' recommendations. A fragment (∼447 bp) of the L. infantum minicircle kinetoplast DNA (kDNA) was amplified using the primers MC1 and MC2 (5). The sample DNA (4 μl) was added to the PCR mix (46 μl) containing 2.5 mM MgCl2, 10 mM Tris-HCl, pH 8.3, and 50 mM KCl, 250 μM (each) deoxynucleoside triphosphates (dNTPs), 50 pmol of each primer, and 1.25 U of AmpliTaq Gold polymerase (Applied Biosystems, Milan, Italy). PCR conditions were standardized as follows: initial denaturation at 94°C for 12 min, 30 cycles consisting of denaturation at 94°C for 30 s, annealing at 60°C for 20 s, extension at 72°C for 30 s, and a final extension at 72°C for 5 min. Positive (L. infantum DNA) and negative (no DNA) controls were included in all the assays. Amplification products were visualized by 2% agarose gel electrophoresis under UV exposure.
For the detection of Ehrlichia and Anaplasma species, DNA was automatically extracted from EDTA-blood samples using the MagAttract DNA blood kit (Qiagen, Valencia, CA). Genomic DNA samples were initially screened by amplification of a fragment of the heat shock protein (groEL) gene of all known Ehrlichia and Anaplasma spp. as described previously (2). Samples positive at the genus level were subsequently tested at the species level by individual PCR assays detecting DNA from A. platys (3), A. phagocytophilum (36), and E. canis. For E. canis DNA detection, the following oligonucleotide primers were manually designed to amplify an approximately 410-bp fragment of the groEL gene: gro-E.canis163s (5′-AAA TGT AGT TGT AAC GGG TGA ACA G-3′) and gro-E.canis573as (5′-AGA TAA TAC CTC ACG CTT CAT AGA CA-3′). Amplifications were performed using conventional one-tube PCR in a 25-μl final volume reaction mixture containing 1× PCR mix (Premix Ex Taq; Takara Bio Inc., Japan), 12.5 pmol of each primer, and 5.0 μl of DNA template. After a single hot-start cycle at 95°C for 30 s, PCR cycled 55 times with the following parameters: denaturation for 10 s at 94°C, annealing for 15 s at 62°C, and elongation for 15 s at 72°C (Mastercycler EP; Eppendorf, Hamburg, Germany). Amplification was completed with a final cycle at 72°C for 1 min, and PCR products were analyzed by 1.5% agarose gel electrophoresis under UV exposure. To generate positive controls, PCR amplicons from a dog naturally infected with E. canis (similar to this sequence under GenBank accession number CP000107) were cloned into plasmid vectors (pGem-T easy vector; Promega, Madison, WI), and Escherichia coli was transformed according to the protocol of the manufacturer (E. coli DH-5α; Invitrogen, Carlsbad, CA). Recombinant clones were selected by blue-white screening of bacterial colonies, and DNA insertions were sequenced bidirectionally (Eton Bioscience, Research Triangle Park, NC). A clone with a partial sequence of groEL of E. canis was quantified by spectrophotometry (average of 5 measurements) and was diluted individually 10-fold, ranging from 1 × 109 to 0.1 plasmid(s)/μl. The limit of detection observed in PCR amplifications was 10 copies of target gene per reaction. Specificity of these primers to E. canis DNA was confirmed by testing them against DNA of A. platys, A. phagocytophilum, Bartonella henselae, Ehrlichia chaffeensis, and Ehrlichia ewingii.
Anaplasma platys DNA was detected by a conventional single-tube PCR assay targeting the groEL gene and performed as described previously (3). For A. phagocytophilum, a real-time PCR assay targeting the msp2 (p44) gene with a dually labeled fluorescent probe was used as described previously (36). Samples positive at the Ehrlichia/Anaplasma genus level by groEL PCR assay but negative in species-level PCR assays were purified and sequenced to identify amplified organisms. Babesia species DNA was amplified by conventional PCR, using primers described previously (21). Babesia vogeli DNA (similar to the sequence under GenBank accession number AY371196) was used as a positive control. Bartonella species DNA was amplified by conventional PCR targeting a fragment of the RNA polymerase b subunit (rpoB) gene as described previously (11). DNA from B. henselae (similar to this sequence under GenBank accession number BX897699) was used as a positive control for rpoB PCR. Spotted fever group Rickettsia DNA was amplified by real-time PCR with Sybr green as a detection method targeting a fragment of the outer membrane protein A (ompA) gene as described previously (20). Rickettsia conorii DNA (similar to the sequence under GenBank accession number DQ518245) was used as a positive control for ompA PCR. Dog genomic DNA (gDNA) from a healthy subject was used as a PCR-negative control, and negative controls for the DNA extraction were also tested.
In addition, in order to characterize the species of Babesia, the genomic DNA was extracted from 100 μl of whole-blood samples using the DNeasy tissue and blood Kit (Qiagen, Milan, Italy). DNA was subjected to a PCR assay to amplify a 410-bp portion of the small-subunit ribosomal DNA (ssu-rDNA) of Babesia spp. by using the primers PIRO-A and PIRO-B (25). The genomic DNA (4 μl) was added to the PCR mix (46 μl) containing 2.5 mM MgCl2, 10 mM Tris-HCl, pH 8.3, and 50 mM KCl, 250 mM [each] dNTPs, 50 pmol of each primer, and 1.25 U of AmpliTaq Gold polymerase (Applied Biosystems, Milan, Italy). Optimal conditions for PCR amplification were standardized as follows: initial denaturation at 94°C for 12 min, 40 cycles (denaturation at 95°C for 30 s, annealing at 62°C for 25 s, and extension at 72°C for 30 s), and a final extension at 72°C for 7 min. A positive control containing genomic Babesia species DNA and a negative control without DNA were included in all the assays. Amplification products (410 bp) were visualized by 2% agarose gel electrophoresis under UV exposure.
Amplification products of Bartonella species and Babesia species PCRs were purified using Ultrafree-DA columns (Amicon; Millipore, Milan, Italy) and sequenced directly in an automated DNA sequencer (Abi-Prism 377; Applied Biosystems, Milan, Italy) using the Taq DyeDeoxyTerminator cycle sequencing kit (Applied Biosystems, Milan, Italy). Sequences were determined in both directions (using the same primers individually as for the PCR), and the electropherograms were verified by eye. Sequences were compared with sequences of Bartonella spp. and Babesia spp. available in GenBank.
Data analysis.
Relative sensitivity (SErel) and specificity (SPrel) of each test were calculated at each follow-up, as described elsewhere (39), considering PCR the gold standard. The SErel and SPrel values of each test were also calculated in parallel and in series (multiple test evaluation in WIN Epi). The chi-square test (or Fisher's exact test) was used to assess whether the prevalence rates obtained with different tests differed significantly. The percentages of coinfections associated with different pathogens were compared using the chi-square test (or Fisher's exact test). Analyses were done using the Win Episcope 2.0 software program (9) and SPSS for Windows, version 15.0 (SPSS Inc., Chicago, Illinois). Data on Bartonella spp. and H. canis infections were excluded from statistical analysis due to the low prevalence of these organisms observed in this study.
RESULTS
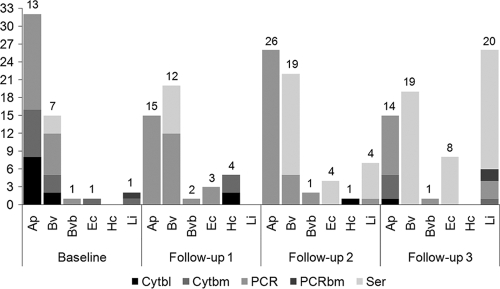
Prevalence rates for infection by different pathogens calculated using different tests and at each follow-up time are reported in Tables 1 to 4 and Fig. 1. Irrespective of pathogens involved or tests used, the overall number of dogs positive at baseline remained stable or decreased during the subsequent follow-up times, whereas among dogs that were initially negative by all testing modalities, there was an increase in positivity at subsequent follow-up times. Throughout the study, PCR was more sensitive than cytology or serology for documenting infection with A. platys, Babesia spp., and E. canis, whereas more dogs were seroreactive than positive for L. infantum in PCR or cytology (Fig. 1). Rickettsia conorii DNA was not amplified from any dog during the study. Dirofilaria immitis antigen also was not detected in any dog at the conclusion of the study.
TABLE 1.
Number and percentage of dogs cytologically positive for Anaplasma platys on blood or buffy coat smears or by PCR at each follow-up time intervala
| Sampling time | Method | Result for group (nb) |
|||||
|---|---|---|---|---|---|---|---|
| Autoc− (35) |
Autoc+ (13) |
Beagles (10) |
|||||
| No. (%) Pos | No. tested | No. (%) Pos | No. tested | No. (%) Pos | No. tested | ||
| Baseline | Cytblo | 26 | 8 (66.7) | 12 | 10 | ||
| Cytbcoa | 35 | 8 (61.5) | 13 | 10 | |||
| PCR | 33 | 13 (100) | 13 | 10 | |||
| Follow-up 1 | Cytblo | 26 | 12 | 10 | |||
| Cytbcoa | 29 | 12 | 10 | ||||
| PCR | 10 (34.5) | 29 | 5 (41.7) | 12 | 10 | ||
| Follow-up 2 | Cytblo | 26 | 12 | 10 | |||
| Cytbcoa | 2 (7.7) | 26 | 12 | 5 | |||
| PCR | 15 (57.7) | 26 | 6 (50) | 12 | 5 (50) | 10 | |
| Follow-up 3 | Cytblo | 1 (4.8) | 21 | 11 | 10 | ||
| Cytbcoa | 22 | 3 (27.3) | 11 | 1 (10) | 10 | ||
| PCR | 7 (30.4) | 23 | 11 | 3 (30) | 10 | ||
Dogs were categorized as autochthonous negative (Autoc−) or positive (Autoc+) at baseline or as sentinel beagles (all negative at baseline). Cytblo, cytology of blood smears; Cytbcoa, cytology of buffy coat smears; PCR, PCR analysis after extraction of DNA from blood; Pos, positive.
n, no. of animals in group.
TABLE 4.
Number and percentage of dogs cytologically positive for Ehrlichia canison blood smears or buffy coat smears, PCR positive following extraction of DNA from blood, or positive by serology at each follow-up timea
| Sampling time | Method | Results for group (nb) |
|||||
|---|---|---|---|---|---|---|---|
| Autoc− (47) |
Autoc+ (1) |
Beagles (10) |
|||||
| No. (%) Pos | No. tested | No. (%) Pos | No. tested | No. (%) Pos | No. tested | ||
| Baseline | Cytblo | 38 | 10 | ||||
| Cytbcoa | 47 | 1 (100) | 1 | 10 | |||
| PCR | 45 | 1 | 10 | ||||
| Serology | 47 | 1 | 10 | ||||
| Follow-up 1 | Cytblo | 38 | 10 | ||||
| Cytbcoa | 40 | 1 | 10 | ||||
| PCR | 2 (5) | 40 | 1 | 1 (10) | 10 | ||
| Serology | 40 | 1 | 10 | ||||
| Follow-up 2 | Cytblo | 38 | 10 | ||||
| Cytbcoa | 38 | 5 | |||||
| PCR | 38 | 10 | |||||
| Serology | 4 (10.5) | 38 | 10 | ||||
| Follow-up 3 | Cytblo | 32 | 10 | ||||
| Cytbcoa | 33 | 10 | |||||
| PCR | 34 | 10 | |||||
| Serology | 6 (17.6) | 34 | 2 (20) | 10 | |||
Dogs were categorized as autochthonous negative (Autoc−) or positive (Autoc+) at baseline or as sentinel beagles (all negative at baseline). Cytblo, cytology of blood smears; Cytbcoa, cytology of buffy coat smears; PCR, PCR analysis after extraction of DNA from blood; Pos, positive.
n, no. of animals in group.
FIG. 1.
Number of dogs positive (in one or more diagnostic tests) for Anaplasma platys (Ap), Babesia vogeli (Bv), Bartonella vinsonii subsp. berkhoffii genotype III (Bvb), Ehrlichia canis (Ec), Hepatozoon canis (Hc), or Leishmania infantum (Li) from baseline to the third follow-up. The overall number of dogs positive for each pathogen (in one or more than one tests) at each follow-up is reported above the bars. Abbreviations: Cytbl, cytology on blood or buffy coat; Cytbm, cytology on bone marrow; PCR, PCR on blood and skin; PCRbm, PCR on bone marrow; Ser, serology.
Among autochthonous dogs that were PCR negative for A. platys at baseline (Table 1), PCR positivity increased to 34.5% at the first follow-up and 57.7% at the second follow-up and remained high (30.4%) during the third follow-up time point. At the time of baseline testing, a high percentage of the A. platys PCR-positive dogs were also cytologically positive in blood (66.7%) and buffy coat (61.5%), with samples from many dogs being positive in two or three tests (Table 5).
TABLE 5.
Number and percentage of dogs positive for Anaplasma platys, Babesia vogeli, and Leishmania infantum in one or more diagnostic tests at different time pointsa
| Sampling time | No. of testsb | No. (%) of dogs positive for: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Anaplasma platys |
Babesiavogeli |
Leishmania infantum |
|||||||||||
| Autoc− | Autoc+ | Beagles | Tc | Autoc− | Autoc+ | Beagles | Tc | Autoc− | Autoc+ | Beagles | Tc | ||
| Baseline | 1 | 4 (100) | 4 | 2 (100) | 2 | ||||||||
| 2 | 2 (100) | 2 | 6 (100) | 6 | |||||||||
| 3 | 7 (100) | 7 | 1 (100) | 1 | |||||||||
| Follow-up 1 | 1 | 10 (66.7) | 5 (33.3) | 15 | 6 (66.7) | 2 (22.2) | 1 (11.1) | 9 | |||||
| 2 | 1 (33.3) | 2 (66.7) | 3 | ||||||||||
| Follow-up 2 | 1 | 13 (54.2) | 6 (25) | 5 (20.8) | 24 | 9 (56.3) | 4 (25) | 3 (18.8) | 16 | 7 (100) | 7 | ||
| 2 | 2 (100) | 2 | 3 (100) | 3 | |||||||||
| Follow-up 3 | 1 | 6 (46.2) | 3 (23.1) | 4 (30.8) | 13 | 10 (52.6) | 4 (21.1) | 5 (26.3) | 19 | 16 (94.1) | 1 (5.9) | 17 | |
| 2 | 1 (100) | 1 | 1 (100) | 1 | |||||||||
| 3 | 1 (100) | 1 | |||||||||||
| 4 | 1 (100) | 1 | |||||||||||
Dogs were categorized as autochthonous negative (Autoc−) or positive (Autoc+) at baseline or as sentinel beagles (all negative at baseline).
Tests include cytology, serology, and PCR assays.
Total number of dogs falling into each category.
By PCR testing, 7 of 48 dogs were positive for Babesia spp. at baseline, of which 33% and 42.9% were also positive as tested by cytology of blood and buffy coat smears, respectively (Table 2). At the second and third follow-up times, no organisms were visualized on blood or buffy coat smears, yet there were 5 Babesia PCR-positive dogs and 36 Babesia IFAT-positive dogs. The pairwise comparison of the sequences obtained found 100% similarity to the B. vogeli partial 18S rRNA gene sequence available in GenBank (accession number AM183216.1). Based upon DNA sequencing, B. vinsonii subsp. berkhoffii genotype III was the only species found in the blood of the two positive dogs.
TABLE 2.
Number and percentage of dogs cytologically positive for Babesia vogelion blood smears or buffy coat smears, by PCR, or by serology at each follow-up timea
| Sampling time | Method | Result for group (nb) |
|||||
|---|---|---|---|---|---|---|---|
| Autoc− (41) |
Autoc+ (7) |
Beagles (10) |
|||||
| No. (%) Pos | No. tested | No. (%) Pos | No. tested | No. (%) Pos | No. Tested | ||
| Baseline | Cytblo | 32 | 2 (33.3) | 6 | 10 | ||
| Cytbcoa | 41 | 3 (42.9) | 7 | 10 | |||
| PCR | 39 | 7 (100) | 7 | 10 | |||
| Serology | 41 | 3 (42.9) | 7 | 10 | |||
| Follow-up 1 | Cytblo | 32 | 6 | 10 | |||
| Cytbcoa | 35 | 6 | 10 | ||||
| PCR | 4 (11.4) | 35 | 3 (50) | 6 | 10 | ||
| Serology | 4 (11.4) | 35 | 3 (50) | 6 | 1 (10) | 10 | |
| Follow-up 2 | Cytblo | 32 | 6 | 10 | |||
| Cytbcoa | 32 | 6 | 5 | ||||
| PCR | 4 (12.5) | 32 | 6 | 1 (10) | 10 | ||
| Serology | 11 (34.4) | 32 | 4 (66.7) | 6 | 2 (20) | 10 | |
| Follow-up 3 | Cytblo | 28 | 4 | 10 | |||
| Cytbcoa | 29 | 4 | 10 | ||||
| PCR | 29 | 5 | 10 | ||||
| Serology | 10 (34.5) | 29 | 4 (80) | 5 | 5 (50) | 10 | |
Dogs were categorized as autochthonous negative (Autoc−) or positive (Autoc+) at baseline or as sentinel beagles (all negative at baseline). Cytblo, cytology of blood smears; Cytbcoa, cytology of buffy coat smears; PCR, PCR analysis after extraction of DNA from blood; Pos, positive.
n, no. of animals in group.
Of the autochthonous dogs that were Babesia negative at baseline, 8 were subsequently PCR positive whereas 11 seroconverted to Babesia. Of the sentinel beagles, one was Babesia positive by PCR, whereas five seroconverted. At the third follow-up testing time, serology supported prior exposure to B. vogeli, whereas tests to confirm infection were all negative (Table 5). After seroconversion, most dogs remaining seroreactive until the last sampling time (Table 2).
At baseline (March 2008), only two young dogs were L. infantum positive by both bone marrow cytology and PCR. The March testing date preceded the phlebotomine sand fly season in this region of Italy, and therefore, the majority of new Leishmania infections were documented by serology at the second follow-up time (October 2008) (Table 3). At the last follow-up, 56.4% of the dogs (18/32) were Leishmania seroreactive but only two dogs were positive by PCR analysis of skin, one of which was also positive by bone marrow analysis. At the beginning of the sand fly season (July 2008), no new Leishmania infections were diagnosed, whereas at the second follow-up time point (October 2008; after the sand fly season), evidence of L. infantum infection could be implicated by two, three, or even four tests (Table 5); however, most dogs were only seroreactive at this time point.
TABLE 3.
Number and percentage of dogs cytologically positive for Leishmania infantumin bone marrow smears, PCR positive following extraction of DNA from skin or bone marrow, or positive by serologya
| Sampling time | Method | Result for group (nb) |
|||||
|---|---|---|---|---|---|---|---|
| Autoc− (46) |
Autoc+ (2) |
Beagles (10) |
|||||
| No. (%) Pos | No. tested | No. (%) Pos | No. tested | No. (%) Pos | No. tested | ||
| Baseline | Cytbm | 46 | 1 (50) | 2 | 10 | ||
| Serology | 46 | 2 | 10 | ||||
| PCR skin | 46 | 2 | 10 | ||||
| PCRbm | 46 | 1 (50) | 2 | 10 | |||
| Follow-up 1 | Serology | 39 | 2 | 10 | |||
| PCR skin | 39 | 2 | 10 | ||||
| Follow-up 2 | Serology | 6 (16.7) | 36 | 2 | 10 | ||
| PCR skin | 1 (2.8) | 36 | 2 | 10 | |||
| Follow-up 3 | Cytbm | 29 | 2 | 1 (10) | 10 | ||
| Serology | 18 (56.4) | 32 | 2 | 2 (20) | 10 | ||
| PCR skin | 2 (6.3) | 32 | 2 | 1 (10) | 10 | ||
| PCRbm | 1 (2.9) | 34 | 2 | 1 (10) | 10 | ||
Dogs were categorized as autochthonous negative (Autoc−) or positive (Autoc+) at baseline or as sentinel beagles (all negative at baseline). Cytbm, cytology of bone marrow smears; PCR skin and PCRbm, PCR analysis after extraction of DNA from skin and bone marrow, respectively; Pos, positive.
n, no. of animals in group.
At baseline, one dog was infected with E. canis based upon visualization of morulae on the buffy coat smear, a positive PCR, and seroreactivity to E. canis antigens. New E. canis infections were detected in two autochthonous dogs and one beagle at the first follow-up by PCR, whereas only serology was positive during the second and third follow-up times (Table 4).
Overall, only one diagnostic test or less frequently two tests were supportive of infection or exposure to each pathogen examined in this study (Table 5). By comparing the positivity in each test at each follow-up time point, acute or recent infection with A. platys, B. vogeli, and L. infantum was more readily detected by PCR and cytology then by serology (excluding A. platys, for which serology was not performed; Fig. 1), most likely reflecting the time required for seroconversion.
SErel and SPrel values for cytology and serology (and PCR analysis of bone marrow for L. infantum) calculated at each of the follow-up times are reported in Table 6. Cytology for A. platys showed high SPrel values throughout the observation period, whereas the SErel value for cytology was moderate at baseline and then decreased. Of 10 A. platys PCR-positive dogs at the last follow-up time, only one was cytologically positive by blood smear analysis, and no organisms were visualized on buffy coat smear. Compared to PCR at baseline, both cytology and serology showed high SPrel and low SErel values for B. vogeli. At the end of the study, 19 dogs (33%) were B. vogeli seroreactive, but all were PCR negative. Bone marrow cytology and PCR for L. infantum were very specific and less sensitive (SErel, 33% and 67%, respectively, at the last sampling) than serology when the latter test was 100% sensitive and less specific (58.5%) (only 3 out of 20 seroreactive dogs were confirmed by PCR analysis of skin).
TABLE 6.
Relative sensitivity and specificity of cytology of blood, buffy coat, and bone marrow, serology, and PCR analysis of bone marrow for Anaplasma platys, Babesia vogeli, and Leishmania infantum at each follow-upa
| Pathogen and method | Baseline value |
Value at follow-up: |
||||||
|---|---|---|---|---|---|---|---|---|
| 2 |
3 |
4 |
||||||
| SErel | SPrel | SErel | SPrel | SErel | SPrel | SErel | SPrel | |
| Anaplasma platys | ||||||||
| Cytblo | 67 | 100b | 11 | 100 | ||||
| Cytbcoa | 61.5 | 100b | 9 | 100 | 88 | |||
| Babesia vogeli | ||||||||
| Cytblo | 33 | 100b | ||||||
| Cytbcoa | 43 | 100b | ||||||
| Serology | 43 | 100b | 43 | 89 | 60 | 67 | ||
| Leishmania infantum | ||||||||
| Cytbm | 33 | 100 | ||||||
| PCRbm | 67 | 100 | ||||||
| Serology | 0 | 87 | 100 | 58.5b | ||||
In many cases, both SErel and SPrel could not be calculated because of the absence of positive dogs. Cytblo, cytology of blood; Cytbcoa, cytology of buffy coat; Cytbm, cytology of bone marrow; PCRbm, PCR analysis of bone marrow.
Statistically significant difference (P < 0.05).
Table 7 reports the predicted variation of SErel and SPrel when two tests are used in parallel (i.e., both tests are performed on the same sample) or in series (i.e., a second test is used to confirm a positive result from the first test). For diagnosis of A. platys infection, combining cytologic examination of both blood and buffy coat smears in parallel enhanced the SErel from 61.5 and 67% for each smear, respectively, to 87.3% if both smears are examined (Table 7). For B. vogeli, the best test combination was cytological examination of a buffy coat smear and serology in parallel, which increased the SErel from 43 to 67.5%. When the results of L. infantum serology and PCR on bone marrow were combined, the SErel (in parallel) and SPrel (in series) were 100%. In the latter case, since the SPrel values of all tests used in the study were very high, the use of an individual testing modality in series had no additional positive effect.
TABLE 7.
Relative sensitivity and specificity of cytology of blood, buffy coat, and bone marrow and serology and PCR analysis of bone marrow for Anaplasma platys, Babesia vogeli, and Leishmania infantum when two tests were used in parallel or in seriesa
| Pathogen and method | Baseline value |
Value at follow-up 4 |
||||||
|---|---|---|---|---|---|---|---|---|
| Parallel |
Series |
Parallel |
Series |
|||||
| SErel | SPrel | SErel | SPrel | SErel | SPrel | SErel | SPrel | |
| Anaplasma platys | ||||||||
| Cytblo + Cytbcoa | 87.3 | 100 | 41.2 | 100 | 11.9 | 100 | 0.1 | 100 |
| Babesia vogeli | ||||||||
| Cytblo + Cytbcoa | 61.8 | 100 | 14.2 | 100 | ||||
| Cytblo + serology | 61.8 | 100 | 14.2 | 100 | ||||
| Cytbcoa + serology | 67.5 | 100 | 18.5 | 100 | ||||
| Leishmania infantum | ||||||||
| Cytbm + PCRbm | 77.9 | 100 | 22.1 | 100 | ||||
| Cytbm + serology | 100 | 58.5 | 33 | 100 | ||||
| PCRbm + serology | 100 | 58.5 | 67 | 100 | ||||
Cytblo, cytology of blood; Cytbcoa, cytology of buffy coat; Cytbm, cytology of bone marrow; PCRbm, PCR analysis of bone marrow.
When the entire observation period is considered, 12 (20.7%) dogs were found to be coinfected with more than one CVBD-causing pathogen. The percentage of coinfected dogs identified by PCR and/or cytology decreased from baseline testing (50%) to the first (33.3%) and the second (16.6%) follow-up time points, and no new coinfections were detected at the final follow-up time (data not shown). The highest number of coinfections, based upon PCR or cytology, was represented by A. platys plus B. vogeli (11/18), followed by A. platys plus H. canis (3/18), A. platys plus L. infantum, and A. platys plus B. vinsonii subsp. berkhoffii (2/18). Coinfection with A. platys and B. vogeli was significantly higher than any other pathogen combinations (P < 0.05). No clinical signs were recorded for animals included in the trial, and thus, no treatments were administrated.
DISCUSSION
In areas of endemicity, the diagnosis of single and multiple infections with CVBD-causing pathogens depends on a wide range of factors, including animal exposure to arthropod vectors (e.g., vector density and seasonality) and the immunopathogenesis of infection among individual animals, which can be influenced by a variety of other host-related factors (e.g., age, sex, breed, and nutritional status). This longitudinal study was designed to assess potential changes in diagnostic patterns in dogs naturally exposed to multiple CVBD-causing pathogens. Therefore, this study was conducted using immunologically naive sentinel beagles, as well as a group of puppies that were born during winter in an area of high CVBD endemicity. Dogs were sequentially tested from the time of first pathogen exposure and then through one season of vector activity. This longitudinal study allowed the comparative analysis of at least three diagnostic assays for each pathogen with the exception of B. vinsonii subsp. berkhoffii, H. canis, D. immitis, and R. conori, for which only two or one test was employed. In addition, the sensitivities and specificities of individual tests at different points in time following vector exposure were compared.
Throughout the observation period, cytology and PCR were used to diagnose new infections with A. platys, B. vogeli, B. vinsonii subsp. berkhoffii, E. canis, H. canis, and L. infantum. For dogs that were negative by all testing modalities at baseline, the diagnostic pattern following initial exposure could be characterized during subsequent follow-up testing. In this study, the molecular and cytological prevalence of tick-borne pathogens in naive dogs increased from the baseline to the first (for A. platys and B. canis) and second (A. platys) follow-up time points, which occurred during July to October, when the highest density of tick infestations was recorded (unpublished data). At baseline, 13 of 48 dogs were PCR positive for A. platys, for which the organism was visualized cytologically in 66.7% of blood smears and in 61.5% of buffy coat smears. These results suggest that during the early phase of A. platys infection in naive young dogs, careful cytological examination of blood or buffy coat smears will allow the detection of the pathogen in approximately two-thirds of the infected dogs. At the July and October follow-up times, most of the dogs previously infected by A. platys remained infected, whereas newly acquired infections were detected by PCR and cytological examination of blood or buffy coat smears.
Also at baseline, there were a number of B. vogeli-infected dogs, based on PCR and blood and buffy coat cytology. However, at subsequent time points, there was an increase in seroreactivity to Babesia antigens among the study population but minimal diagnostic evidence (PCR and cytology) to support chronic infection. Most B. vogeli seroconverters remained seroreactive until the last follow-up time, indicating a persistence of humoral immunity throughout the observation period. However, it is uncertain whether these dogs cleared the infection (by B. vogeli) through their immune response or whether they were persistently infected but with a low parasite load, even below the limit of detection of the PCR. In this study, the higher SE of buffy coat examination than of blood smear examination for the cytological detection of B. vogeli could be due to the higher concentration of parasitized red cells at the buffy coat interface than in the red blood cell component. A possible explanation is that parasitized erythrocytes would approximate the buffy coat (top) and be collected during the separation step whereas uninfected erythrocytes are at the bottom.
Following PCR amplification, B. vogeli was confirmed in this study as the infecting species by DNA sequencing, which is consistent with a previous report describing the occurrence of this species in southern Italy (37). The PCR prevalence of B. vogeli in naive dogs in this study ranged from 11.4 to 14.5% and was similar to the previously reported prevalence (16.3%) in dogs from central and southern Italy (37).
New infections by E. canis were detected by PCR in two autochthonous dogs and one sentinel beagle at the first follow-up time point; however, by the end of the study, six autochthonous dogs and two beagles had seroconverted. This finding supports previous observations that E. canis bacteremia can be transient and that immunocompetent dogs may eliminate the parasite from blood (13, 18, 19). Immunological clearance of the organisms in the blood is further supported by finding no E. canis PCR- or cytology-positive dogs at the last two follow-up time points. In addition, the infections within deeper tissues may not be detected by the assays used in the present study. As with B. vogeli, it is also possible that E. canis organisms were sequestered in tissues such as the bone marrow or spleen or were present in systemic circulation below the level of PCR detection.
In this study, following initial vector exposure, PCR and cytology detected the highest number of infected dogs. For A. platys-infected dogs, PCR results remained positive even when the rickettsemia was very low, i.e., undetectable on blood or buffy coat smears. In the context of host factors that can influence interpretation of diagnostic test results, two young dogs were infected with L. infantum at the time of baseline testing. One dog was positive by bone marrow cytology, whereas the other dog was positive only by bone marrow PCR. Since neither of these dogs seroconverted and with the absence of phlebotomine sand fly vectors immediately prior to initiation of the study in March 2008, it is possible that these two puppies were infected by transplacental transmission and immunologically cleared the L. infantum infection (8, 34). Transient bone marrow PCR positivity has been previously found in a population of naturally exposed dogs in an area where leishmaniosis is endemic (24). Conversely, positivity of PCR on skin followed by seroconversion to L. infantum antigens at the second (October 2008) and third (March 2009) follow-up time points but not at the beginning of phlebotomine sand fly activity (July 2008) indicates that these dogs were exposed to sand flies during the summer season. Indeed, recent entomological surveys carried out in the northern (15), central (33), and southern regions (32) indicate that the activity of Phlebotomus perniciosus (the most important vector of L. infantum in the Mediterranean area) is restricted to the months from late May to early October.
The diagnosis of CVBDs is potentially more challenging when the clinician is confronted with a dog that is coinfected with two or more CVBD-causing pathogens (21), and therefore, a combination of different testing modalities is often required to clarify the exposure and infection status of individual dogs or kennel populations. Cytological examination of buffy coat, bone marrow, and lymph node smears may be useful, particularly for clinical diagnosis of multiple vector-borne infections in dogs (23). However, direct visualization of A. platys, B. canis, and E. canis in blood smears of acutely infected dogs might be time-consuming, technically challenging, and diagnostically insensitive due to low-level parasitemia, which occurs more frequently in the chronic phase of these infections (7, 41). Based upon PCR testing, coinfections were detected throughout the observation period and at all follow-up time points. The percentage of newly coinfected animals decreased from baseline (50%) to the first (33.3%) and second (16.6%) follow-up times, whereas no new coinfections were detected at the final follow-up time point. Although rarely studied in experimental infection models, the complex interactions occurring between the host immune system and single pathogens can be modified by simultaneous or sequential infections with two or more pathogens, which may influence the serological and parasitological diagnosis (16).
Although several CVBDs are endemic in Italy, there is scant information on their epidemiology (26). In addition to the evaluation of the diagnostic methods used in this study, the data provides some insights as to the incidence rates for certain pathogens infecting a population of young dogs in a defined geographic region. The E. canis seroprevalence (i.e., 17.6%) recorded at the third follow-up time period was similar to the seroprevalence reported in another study of kenneled dogs from southern Italy (i.e., 14.9%) (31). However, the PCR prevalence of A. platys infection (from 30.4 to 57.7%) in naive dogs was higher than the prevalence previously reported in kenneled dogs from central (23%) (38) or southern (11.3%) (31) Italy. The higher prevalence in this study was potentially due to the incorporation of three different diagnostic testing results for each dog at each time point.
In the Mediterranean region, there have been many reports of coinfections with L. infantum and E. canis (40). However, in this study, coinfections with A. platys and B. vogeli were more frequently detected. Finding a high prevalence of A. platys and B. vogeli coinfections further supports the suspicion that Rhipicephalus sanguineus ticks are likely vectors for both pathogens. Additional studies are needed to define the pathogenic role of A. platys in single infections and coinfections and the extent to which infection with this organism influences the clinical presentation, disease manifestations, or hematological alterations in dogs infected with another vector-borne pathogen. A recent experimental study involving A. platys and E. canis identified alterations in the anticipated serological response in dogs that were coinfected or sequentially infected compared to that of those infected with a single organism (16). In addition, obtaining a better understanding of the immunological and pathological interactions that are induced by different combinations of vector-borne pathogens would provide potentially useful diagnostic, prognostic, and therapeutic insights. For instance, hypothetically, A. platys could also impair the host immune response and thereby potentiate the transmission of another pathogen or increase the severity of infection induced by the newly introduced pathogen. Other authors have suggested that downregulation of major histocompatibility complex (MHC) class II receptors in dogs infected with E. canis might potentiate the clinical progression of canine leishmaniosis (17).
The combination of blood and buffy coat cytology in parallel enhanced the SErel of visualization from 61.5 and 67% to 87.3% for A. platys. Similarly, an increase in the SErel was obtained by combining buffy cytology and serology for B. vogeli or serology and PCR for L. infantum.
Overall, the results of this study support the combined use of cytology, PCR, and serology for the diagnosis of CVBDs in the field. For the tick-borne diseases investigated in this study, cytology had a high specificity, but it was less sensitive than PCR, particularly after the initial phase of the infection. Because diagnostic samples will be obtained at different time points in naturally infected dogs and because the cellular pathogenesis induced by each pathogen may vary among dogs of different ages, breeds, and sexes, the sensitivity differences among the testing modalities used in this study are an expected finding. Numerous vector, pathogen, and host factors might influence the specificity and sensitivity of diagnostic tests, and therefore, clinicians must appreciate the limitations of each test in detecting different pathogens. For tick-borne pathogens, cytology and PCR are more useful than serology during the acute phase of the infection. Subsequently, however, dogs will seroconvert, and antibodies may persist for months to years, even in dogs that have immunologically or therapeutically cleared their infection. Alternatively, dogs can become chronically infected with organisms such as B. vogeli or E. canis but the organism load is below the threshold for PCR amplification or cytological identification.
In conclusion, in the present study, cytology and PCR were the most reliable (highest specificity and sensitivity, respectively) tests for the diagnostic detection of CVBD-causing pathogens, especially if the infection is recent. However, over the time course of this study, most dogs became cytologically and PCR negative. Conversely, many dogs seroconverted, and antibody titers persisted for several months. Finally, these results should assist veterinary practitioners during their daily routine when choosing laboratory tests for confirming the clinical diagnosis of vector-borne diseases.
Acknowledgments
This work was supported by Bayer Animal Health (Germany).
For the field work, we are grateful to Viviana Domenica Tarallo and Vincenzo Lorusso (Faculty of Veterinary Medicine, University of Bari), to Sabrina Gallo and Angelo Carucci (practitioners at the Centro Veterinario S. Francesco, Putignano, Bari), and to Alexandru Bejan (Cluj Napoca, Romania). Luciana A. Figueredo (Faculty of Veterinary Medicine, University of Bari), Paola Mesto, Roldano Sottili, and Paola Ghergo (Laboratorio di Analisi Cliniche Veterinarie, Bari) deserve thanks for some lab work (cytology).
Footnotes
Published ahead of print on 21 July 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Baneth, G., S. Harrus, F. S. Ohnona, and Y. Schlesinger. 2009. Longitudinal quantification of Ehrlichia canis in experimental infection with comparison to natural infection. Vet. Microbiol. 136:321-325. [DOI] [PubMed] [Google Scholar]
- 2.Barber, R. M., Q. Li, P. P. V. P. Diniz, B. F. Porter, E. B. Breitschwerdt, M. K. Claiborne, A. J. Birkenheuer, J. M. Levine, G. J. Levine, K. Chandler, P. Kenny, P. Nghiem, S. Wei, C. E. Greene, M. Kent, S. R. Platt, K. Greer, and S. J. Schatzberg. 2010. Evaluation of brain tissue or cerebrospinal fluid with broadly reactive polymerase chain reaction for Ehrlichia, Anaplasma, spotted fever group Rickettsia, Bartonella, and Borrelia species in canine neurological diseases (109 Cases). J. Vet. Intern. Med. 24:372-378. [DOI] [PubMed] [Google Scholar]
- 3.Beall, M. J., R. Chandrashekar, M. D. Eberts, K. E. Cyr, P. P. V. P. Diniz, C. Mainville, B. C. Hegarty, J. M. Crawford, and E. B. Breitschwerdt. 2008. Serological and molecular prevalence of Borrelia burgdorferi, Anaplasma phagocytophilum, and Ehrlichia species in dogs from Minnesota. Vector Borne Zoonotic Dis. 8:455-464. [DOI] [PubMed] [Google Scholar]
- 4.Beugnet, F., and J. L. Marié. 2009. Emerging arthropod-borne diseases of companion animals in Europe. Vet. Parasitol. 163:298-305. [DOI] [PubMed] [Google Scholar]
- 5.Cortes, S., N. Rolão, J. Ramada, and L. Campino. 2004. PCR as a rapid and sensitive tool in the diagnosis of human and canine leishmaniasis using Leishmania donovani s.l.-specific kinetoplastid primers. Trans. R. Soc. Trop. Med. Hyg. 98:12-17. [DOI] [PubMed] [Google Scholar]
- 6.Dantas-Torres, F. 2007. The role of dogs as reservoirs of Leishmania parasites, with emphasis on Leishmania (Leishmania) infantum and Leishmania (Viannia) braziliensis. Vet. Parasitol. 149:139-146. [DOI] [PubMed] [Google Scholar]
- 7.Dantas-Torres, F., and L. A. Figueredo. 2006. Canine babesiosis: a Brazilian perspective. Vet. Parasitol. 141:197-203. [DOI] [PubMed] [Google Scholar]
- 8.da Silva, L. A., C. D. de Sousa, G. C. da Graça, R. Porrozzi, and E. Cupolillo. 2010. Sequence analysis and PCR-RFLP profiling of the hsp70 gene as a valuable tool for identifying Leishmania species associated with human leishmaniasis in Brazil. Infect. Genet. Evol. 10:77-83. [DOI] [PubMed] [Google Scholar]
- 9.de Blas, N., C. Ortega, K. Frankena, J. Noordhuizen, and M. V. Thrusfield. 2000. —Win Episcope 2.0. http://www.clive.ed.ac.uk/cliveCatalogueItem.asp?id=B6BC9009-C10F-4393-A22D-48F436516AC4. [DOI] [PubMed]
- 10.Diniz, P. P. V. P., S. A. Billeter, D. Otranto, D. de Caprariis, T. Petanides, M. E. Mylonakis, A. F. Koutinas, and E. B. Breitschwerdt. 2009. Molecular documentation of Bartonella spp. infection in dogs from Greece and Italy. J. Clin. Microbiol. 47:1565-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diniz, P. P., R. G. Maggi, D. S. Schwartz, M. B. Cadenas, J. M. Bradley, B. Hegarty, and E. B. Breitschwerdt. 2007. Canine bartonellosis: serological and molecular prevalence in Brazil and evidence of co-infection with Bartonella henselae and Bartonella vinsonii subsp. berkhoffii. Vet. Res. 38:697-710. [DOI] [PubMed] [Google Scholar]
- 12.Diouani, M. F., N. Ben Alaya Bouafif, J. Bettaib, H. Louzir, S. Jedidi, A. Ftaiti, A. Zaâtour, I. Jomaâ, K. Dellagi, R. Ben Ismail, and A. Ben Salah. 2008. Dogs L. infantum infection from an endemic region of the north of Tunisia: a prospective study. Arch. Inst. Pasteur Tunis. 85:55-61. [PubMed] [Google Scholar]
- 13.Eddlestone, S. M., P. P. Diniz, T. M. Neer, S. D. Gaunt, R. Corstvet, D. Cho, G. Hosgood, B. Hegarty, and E. B. Breitschwerdt. 2007. Doxycycline clearance of experimentally induced chronic Ehrlichia canis infection in dogs. J. Vet. Intern. Med. 21:1237-1242. [DOI] [PubMed] [Google Scholar]
- 14.Falqueto, A., A. L. Ferreira, C. B. dos Santos, R. Porrozzi, M. V. da Costa, A. Teva, E. Cupolillo, A. Campos-Neto, and G. Grimaldi, Jr. 2009. Cross-sectional and longitudinal epidemiologic surveys of human and canine Leishmania infantum visceral infections in an endemic rural area of southeast Brazil (Pancas, Espirito Santo). Am. J. Trop. Med. Hyg. 80:559-565. [PubMed] [Google Scholar]
- 15.Ferroglio, E., M. Maroli, S. Gastaldo, W. Mignone, and L. Rossi. 2005. Canine leishmaniasis, Italy. Emerg. Infect. Dis. 11:1618-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaunt, S., M. Beall, B. Stillman, L. Lorentzen, P. Diniz, R. Chandrashekar, and E. Breitschwerdt. 2010. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasit. Vectors 3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrus, S., T. Waner, D. Friedmann-Morvinski, Z. Fishman, H. Bark, and A. Harmelin. 2003. Down-regulation of MHC class II receptors of DH82 cells, following infection with Ehrlichia canis. Vet. Immunol. Immunopathol. 96:239-243. [DOI] [PubMed] [Google Scholar]
- 18.Hegarty, B. C., P. P. de Paiva Diniz, J. M. Bradley, L. Lorentzen, and E. Breitschwerdt. 2009. Clinical relevance of annual screening using a commercial enzyme-linked immunosorbent assay (SNAP 3Dx) for canine ehrlichiosis. J. Am. Anim. Hosp. Assoc. 45:118-124. [DOI] [PubMed] [Google Scholar]
- 19.Hibler, S. C., and C. E. Greene. 1986. Rickettsial infections in dogs, part II. Ehrlichiosis and infectious cyclic thrombocytopenia. Compend. Contin. Educ. Pract. Vet. 8:106-114. [Google Scholar]
- 20.Kidd, L., R. Maggi, P. P. Diniz, B. Hegarty, M. Tucker, and E. Breitschwerdt. 2008. Evaluation of conventional and real-time PCR assays for detection and differentiation of spotted fever group Rickettsia in dog blood. Vet. Microbiol. 129:294-303. [DOI] [PubMed] [Google Scholar]
- 21.Kordick, S. K., E. B. Breitschwerdt, B. C. Hegarty, K. L. Southwick, C. M. Colitz, S. I. Hancock, J. M. Bradley, R. Rumbough, J. T. Mcpherson, and J. N. MacCormack. 1999. Coinfection with multiple tick-borne pathogens in a Walker Hound kennel in North Carolina. J. Clin. Microbiol. 37:2631-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mekuzas, Y., L. Gradoni, G. Oliva, V. Foglia Manzillo, and G. Baneth. 2009. Ehrlichia canis and Leishmania infantum co-infection: a 3-year longitudinal study in naturally exposed dogs. Clin. Microbiol. Infect. 15:30-31. [DOI] [PubMed] [Google Scholar]
- 23.Mylonakis, M. E., A. F. Koutinas, G. Baneth, Z. Polizopoulou, and A. Fytianou. 2004. Mixed Ehrlichia canis, Hepatozoon canis, and presumptive Anaplasma phagocytophilum infection in a dog. Vet. Clin. Pathol. 33:249-251. [DOI] [PubMed] [Google Scholar]
- 24.Oliva, G., A. Scalone, V. Foglia Manzillo, M. Gramiccia, A. Pagano, T. Di Muccio, and L. Gradoni. 2006. Incidence and time course of Leishmania infantum infections examined by parasitological, serologic, and nested-PCR techniques in a cohort of naive dogs exposed to three consecutive transmission seasons. J. Clin. Microbiol. 44:1318-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Olmeda, A. S., P. M. Armstrong, B. M. Rosenthal, B. Valladares, A. del Castillo, F. de Armas, M. Miguelez, A. Gonzalez, J. A. Rodriguez Rodriguez, A. Spielman, and S. R. Telford III. 1997. A subtropical case of human babesiosis. Acta Trop. 67:229-234. [DOI] [PubMed] [Google Scholar]
- 26.Otranto, D., and F. Dantas-Torres. 2010. Canine and feline vector-borne diseases in Italy: current situation and perspectives. Parasit. Vectors 3:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otranto, D., F. Dantas-Torres, and E. B. Breitschwerdt. 2009. Managing canine vector-borne diseases of zoonotic concern: part one. Trends Parasitol. 25:157-163. [DOI] [PubMed] [Google Scholar]
- 28.Otranto, D., F. Dantas-Torres, and E. B. Breitschwerdt. 2009. Managing canine vector-borne diseases of zoonotic concern: part two. Trends Parasitol. 25:228-235. [DOI] [PubMed] [Google Scholar]
- 29.Otranto, D., G. Capelli, and C. Genchi. 2009. Changing distribution patterns of canine vector borne diseases in Italy: leishmaniosis vs. dirofilariosis. Parasit. Vectors 26(Suppl. 1):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otranto, D., P. Paradies, D. de Caprariis, D. Stanneck, G. Testini, F. Grimm, P. Deplazes, and G. Capelli. 2009. Toward diagnosing Leishmania infantum infection in asymptomatic dogs in an area where leishmaniasis is endemic. Clin. Vaccine Immunol. 16:337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otranto, D., P. Paradies, G. Testini, M. S. Latrofa, S. Weigl, C. Cantacessi, N. Mencke, D. de Caprariis, A. Parisi, G. Capelli, and D. Stanneck. 2008. Application of 10% imidacloprid/50% permethrin to prevent Ehrlichia canis exposure in dogs under natural conditions. Vet. Parasitol. 153:320-328. [DOI] [PubMed] [Google Scholar]
- 32.Otranto, D., P. Paradies, R. P. Lia, M. S. Latrofa, G. Testini, C. Cantacessi, N. Mencke, G. Galli, G. Capelli, and D. Stanneck. 2007. Efficacy of a combination of 10% imidacloprid/50% permethrin for the prevention of leishmaniasis in kennelled dogs in an endemic area. Vet. Parasitol. 144:270-278. [DOI] [PubMed] [Google Scholar]
- 33.Rossi, E., G. Bongiorno, E. Ciolli, T. Di Muccio, A. Scalone, M. Gramiccia, L. Gradoni, and M. Maroli. 2008. Seasonal phenology, host-blood feeding preferences and natural Leishmania infection of Phlebotomus perniciosus (Diptera, Psychodidae) in a high-endemic focus of canine leishmaniasis in Rome province, Italy. Acta Trop. 105:158-165. [DOI] [PubMed] [Google Scholar]
- 34.Rosypal, A. C., G. C. Troy, A. M. Zajac, G. Frank, and D. S. Lindsay. 2005. Transplacental transmission of a North American isolate of Leishmania infantum in an experimentally infected beagle. J. Parasitol. 91:970-972. [DOI] [PubMed] [Google Scholar]
- 35.Sasanelli, M., P. Paradies, G. Lubas, D. Otranto, and D. de Caprariis. 2009. Atypical clinical presentation of coinfection with Ehrlichia, Babesia and Hepatozoon species in a dog. Vet. Rec. 164:22-23. [DOI] [PubMed] [Google Scholar]
- 36.Scorpio, D. G., M. Akkoyunlu, E. Fikrig, and J. S. Dumler. 2004. CXCR2 blockade influences Anaplasma phagocytophilum propagation but not histopathology in the mouse model of human granulocytic anaplasmosis. Clin. Diagn. Lab. Immunol. 11:963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solano-Gallego, L., M. Trotta, E. Carli, B. Carcy, M. Caldin, and T. Furlanello. 2008. Babesia canis canis and Babesia canis vogeli clinicopathological findings and DNA detection by means of PCR-RFLP in blood from Italian dogs suspected of tick-borne disease. Vet. Parasitol. 157:211-221. [DOI] [PubMed] [Google Scholar]
- 38.Sparagano, O. A., A. P. de Vos, B. Paoletti, C. Cammà, P. de Santis, D. Otranto, and A. Giangaspero. 2003. Molecular detection of Anaplasma platys in dogs using polymerase chain reaction and reverse line blot hybridization. J. Vet. Diagn. Invest. 15:527-534. [DOI] [PubMed] [Google Scholar]
- 39.Thrusfield, M. 2005. Veterinary epidemiology, 3rd ed. Blackwell Science, Oxford, United Kingdom.
- 40.Trotz-Williams, L. A., and A. J. Trees. 2003. Systematic review of the distribution of the major vector-borne parasitic infections in dogs and cats in Europe. Vet. Rec. 152:97-105. [DOI] [PubMed] [Google Scholar]
- 41.Tuttle, A. D., A. J. Birkenheuer, T. Juopperi, M. G. Levy, and E. B. Breitschwerdt. 2003. Concurrent bartonellosis and babesiosis in a dog with persistent thrombocytopenia. J. Am. Vet. Med. Assoc. 223:1280-1281, 1306-1310. [DOI] [PubMed] [Google Scholar]