Abstract
The specific identification of broad tapeworms (genus Diphyllobothrium) infecting humans is very difficult to perform by morphological observation. Molecular analysis by PCR and sequencing represents the only reliable tool to date to identify these parasites to the species level. Due to the recent spread of human diphyllobothriosis in several countries, a correct diagnosis has become crucial to better understand the distribution and the life cycle of human-infecting species as well as to prevent the introduction of parasites to disease-free water systems. Nevertheless, PCR and sequencing, although highly precise, are too complicated, long, and expensive to be employed in medical laboratories for routine diagnostics. In the present study we optimized a cheap and rapid molecular test for the differential identification of the most common Diphyllobothrium species infecting humans (D. latum, D. dendriticum, D. nihonkaiense, and D. pacificum), based on a multiplex PCR with the cytochrome c oxidase subunit 1 gene of mitochondrial DNA.
Human diphyllobothriosis is a widespread fish-borne zoonosis caused by tapeworms of the genus Diphyllobothrium Cobbold, 1858 (Cestoda: Diphyllobothriidea). Their life cycles are complex and involve two intermediate hosts (a copepod and a fish) and a definitive host (humans or other piscivorous mammals and aquatic birds). Infection takes place through the consumption of raw or undercooked fish harboring plerocercoid larvae and often remains unnoticed until the excretion of segments of the adult parasite (proglottids) in stools. Symptoms include various minor digestive problems occurring a few weeks after infection (mostly nausea, diarrhea, and abdominal pain); less commonly, prolonged or heavy infections can result in a pernicious anemia (7). About 14 species of Diphyllobothrium have been reported to infect humans. From a medical perspective, Diphyllobothrium latum, Diphyllobothrium nihonkaiense, Diphyllobothrium dendriticum, and Diphyllobothrium pacificum are the most important, because humans represent their preferred definitive hosts (17).
The identification of Diphyllobothrium tapeworms by physicians and medical laboratories is generally based on the morphological observation of operculated eggs and segments of adult worms (7). Morphoanatomical criteria allow identification to the genus level but are not reliable to assess species identity, because the different taxa are extremely similar one another, and species differentiation relies on characteristics of the scolex or the genital apparatus observed on mature proglottids, which are often unavailable during human infections (20). To this end, molecular methods have been recommended (17). In the past, biochemical techniques (isoenzymatic assay or immunoelectrophoresis) have been used as alternatives to traditional tools for species identification (5, 6). Matsuura et al. (13) previously discriminated between D. latum and D. nihonkaiense by using restriction fragment length polymorphism (RFLP) of ribosomal DNA (rDNA), as the profiles generated with three different restriction enzymes provided valuable species-specific markers. Since the late 1990s, the development of molecular biology based on the sequencing of nuclear and mitochondrial DNA (mtDNA) targets resulted in a better knowledge of the genus Diphyllobothrium. Notable results for the phylogenetic relationships among some taxa have been obtained by the sequencing of the 18S rRNA, cytochrome c oxidase subunit 1 (cox1 or COI), and NADH dehydrogenase subunit 3 (NADH3) genes and internal transcribed spacer 1 (ITS1) and ITS2 (19, 27, 29, 31).
However, molecular methods are still rarely employed in routine laboratories due to economical (need of reagents and equipment such as a thermal cycler and sequencer) and technical (procedures are complicated and time-consuming and require trained personnel) reasons. Moreover, all human-infecting Diphyllobothrium species cause similar symptoms, and infections are successfully treated with praziquantel, which can lead one to think that a further, specific identification of the causative agent is useless for the practice.
In contrast, the specific diagnostic identification of Diphyllobothrium parasites isolated from patients is of great importance from an epidemiological point of view. Human diphyllobothriosis has been estimated to affect 20 million people worldwide (4, 14), and recent studies indicate a recrudescence in some well-developed countries (26). Thanks to the use of molecular tools, locally acquired infections with allochtonous species are being reported more and more frequently. For instance, D. nihonkaiense, usually present in Japan and South Korea (8), has recently been detected in Switzerland (18, 23), France (15, 30), Finland (26), and North America (25). D. dendriticum, common in northern Europe, was found in a Swiss patient (24). A human case due to D. latum was reported in Taiwan (11). In Sao Paulo, Brazil, an outbreak with a significant economic and public health impact linked to the ingestion of raw salmon was proven to be caused by D. latum (16). In most of these cases the infective source consisted of imported fish; on the other hand, the origin of the intermediate host even brought into question the known geographical repartition of some species, i.e., D. nihonkaiense, harbored by Pacific salmons in North America (a hypothesis which is currently under verification). The molecular identification of broad tapeworms infecting humans can therefore contribute to map the present species distribution, to understand their life cycles (for example, the intermediate hosts of D. pacificum are still unknown), and to clarify which fish represent the most important sources of human infections.
To date, the sequencing of some genomic DNA targets allowed the identification of many Diphyllobothrium species (17), and the use of RFLP enabled the differentiation between D. latum and D. nihonkaiense (13). Nevertheless, only a PCR-based method for routine discrimination between D. latum and D. nihonkaiense has been developed for use in diagnostic laboratories (9). In this study, we adapted an easy and rapid molecular technique (one that does not require the sequencing of PCR products) to be routinely used for the differential diagnosis of all principal Diphyllobothrium species infecting humans. The method is based on a multiplex PCR, which was successfully employed for cestode identification in previous studies (9, 28).
MATERIALS AND METHODS
Target gene.
The cytochrome c oxidase subunit 1 (cox1) gene of mitochondrial DNA (mtDNA) represents a suitable gene for the inter- and intraspecific identification of various Diphyllobothrium species (17, 27, 29, 31). It was therefore used as a target for multiplex PCR. Due to its variability, the primers for the multiplex PCR were designed on the basis of several representative sequences for each species, i.e., samples from different geographical origins and developmental stages. The alignment was built by using (i) sequences deposited in public databases (DDJB/EMBL/GenBank accession numbers AM747494 [D. pacificum]; AB268585, NC_009463, AM412559, AM412560, EF420138, and AB015755 [D. nihonkaiense]; NC_008945, AB269325, DQ985706, FM209180, and FM209181 [D. latum]; and AM412738 and AB374223 [D. dendriticum]) and (ii) sequences obtained from other samples (Table 1); among the latter, two plerocercoid larvae isolated from Lorna drum (Sciaena deliciosa; Perciformes: Sciaenidae) in Peru had been identified as D. pacificum according to morphological characteristics only (2). The other specimens had been previously identified by genetic analysis (18S rRNA gene and ITS1-ITS2 regions) (26).
TABLE 1.
Diphyllobothrium samples sequenced for the complete cox1 gene
| Species | Stage | Host | Originb |
|---|---|---|---|
| D. latum | Adult | Human | St. Gallen, SWI |
| D. latum | Plerocercoid | Perca fluviatilis | POL |
| D. dendriticum | Plerocercoid | Freshwater fish | EST |
| D. dendriticum | Plerocercoid | Freshwater fish | EST |
| D. dendriticum | Plerocercoid | Coregonus lavaretus | Loch Lomond, GBR |
| D. dendriticum | Plerocercoid | Coregonus lavaretus | Loch Lomond, GBR |
| D. dendriticum | Plerocercoid | Coregonus lavaretus | Loch Lomond, GBR |
| D. dendriticum | Plerocercoid | Coregonus autumnalis | Olkhon Island, RUS |
| D. pacificum | Adult | Human | Lima, PER |
| D. pacificum | Adult | Human | Lima, PER |
| D. pacificum | Adult | Human | PER |
| D. pacificum | Adult | Human | Lima, PER |
| D. pacificuma | Plerocercoid | Sciaena deliciosa | Lima, PER |
| D. pacificuma | Plerocercoid | Sciaena deliciosa | Lima, PER |
Identified by morphological observation.
SWI, Switzerland; POL, Poland; EST, Estonia; GBR, Great Britain; RUS, Russia; PER, Peru.
Cox1 sequencing and analysis.
DNA from parasite samples was extracted from 25 mg tissue (about two proglottids) or the whole plerocercoid larva with the DNeasy blood and tissue kit according to the manufacturer's protocol for the purification of total DNA from animal tissues (Qiagen, Hilden, Germany). The complete cox1 gene was amplified by PCR in a T3000 thermocycler (Biometra, Goettingen, Germany) by using primers Cox1Forward (5′-TATCAAATTAAGTTAAGTAGACTA-3′) and Cox1Reverse (5′-CCAAATAGCATGATGCAAAAG-3′) in 50-μl reaction mixtures containing 2.5 U of Taq DNA polymerase, 1.5 mM MgCl2, 200 μM each deoxynucleoside triphosphate (dNTP) (Top Bio, Prague, Czech Republic), and 0.3 μM each primer. The thermal cycle consisted of 1 cycle at 95°C for 5 min; 40 cycles of 95°C for 30 s, 50°C for 1 min, and 72°C for 1 min; and a final cycle at 72°C for 10 min. The PCR products were visualized on a 0.8% agarose gel and purified by using the QIAquick PCR purification kit according to the manufacturer's instructions for the direct purification of DNA fragments with a microcentrifuge (Qiagen, Hilden, Germany). Sequencing was performed with an ABI Prism 3130xl automatic sequencer by using a Big Dye Terminator v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA). The products of the sequencing reaction were purified by ethanol precipitation with sodium acetate. The sequences obtained were analyzed and corrected with the software MEGA v. 4.0 (21).
Primer design.
Five primers for the multiplex PCR were designed on the basis of the alignment of cox1 sequences by using the software MEGA v. 4.0 (21). One reverse primer was common for all Diphyllobothrium species (MulRevCom, located at positions 1492 to 1512). Four forward primers were specific: MulLat3 (positions 1055 to 1077) for D. latum, MulDen4 (positions 1174 to 1200) for D. dendriticum, MulPac2 (positions 765 to 786) for D. pacificum, and MulNih5 (positions 260 to 279) for D. nihonkaiense (Table 2). The expected product sizes were 437 bp for D. latum, 318 bp for D. dendriticum, 727 bp for D. pacificum, and 1,232 bp for D. nihonkaiense.
TABLE 2.
Primers designed for multiplex PCR
| Primer | Specificity | Strand | Sequence (5′→3′) |
|---|---|---|---|
| MulRevCom | Common | Reverse | ATGATAAGGGAYAGGRGCYCA |
| MulLat3 | D. latum | Forward | GGGGTGTTACGGGTATTATACTC |
| MulDen4 | D. dendriticum | Forward | GTGTTTTTCATTTGATGATGACCAGTC |
| MulPac2 | D. pacificum | Forward | ACATGTGTGTAGTAACCTTGGC |
| MulNih5 | D. nihonkaiense | Forward | CTTTGTTGTCTGGCCTTCCT |
Multiplex PCR differential identification.
The multiplex PCR was tested on 24 samples of D. latum, 17 samples of D. dendriticum, 17 samples of D. pacificum, and 21 samples of D. nihonkaiense (Table 3). To estimate the specificity of the primers, three other Diphyllobothrium species were also tested: Diphyllobothrium ursi (a species infecting bears in the Pacific coast of North America, which occasionally infects humans), Diphyllobothrium ditremum (present in northern Europe and North America, infecting mainly fish-eating birds), and Diphyllobothrium hottai (present along the northern coast of Japan; the definitive hosts are unknown).
TABLE 3.
Diphyllobothrium samples tested by multiplex PCR
| Species | Stage | Hostb | Originc | Yr of isolation | Lanea |
|---|---|---|---|---|---|
| D. latum | Plerocercoid | Esox lucius | RUS | 2005 | 1 |
| Plerocercoid | Perca fluviatilis | POL | 2007 | 2 | |
| Adult | Human | Kotka, FIN | 2004 | 3 | |
| Adult | Human | Grenoble, FRA | 2005 | 4 | |
| Plerocercoid | Fish (NI) | Lake Peipsi, EST | 2004 | 5 | |
| Plerocercoid | Fish (NI) | Lake Peipsi, EST | 2006 | 6 | |
| Plerocercoid | Perca fluviatilis | Como, ITA | 2006 | 7 | |
| Plerocercoid | Perca fluviatilis | Lake Maggiore, SWI | 2006 | 8 | |
| Plerocercoid | Perca fluviatilis | Lake Geneva, SWI | 2007 | 9 | |
| Plerocercoid | Perca fluviatilis | Lake Geneva, SWI | 2007 | 10 | |
| Plerocercoid | Perca fluviatilis | Lake Maggiore, SWI | 2007 | 11 | |
| Eggs | Human | Geneva, SWI | 2005 | 12 | |
| Eggs | Human | Geneva, SWI | 2005 | 13 | |
| Adult | Human | Le Lignon, SWI | 2005 | 14 | |
| Adult | Human | Vaud, SWI | 2005 | 15 | |
| Adult | Human | Geneva, SWI | 2006 | 16 | |
| Adult | Human | St Gallen, SWI | 2006 | 17 | |
| Adult | Human | Vésenaz, SWI | 2006 | 18 | |
| Adult | Human | Baveno, ITA | 2006 | 19 | |
| Eggs | Human | Bubendorf, SWI | 2006 | 20 | |
| Eggs | Human | Montreux, SWI | 2006 | 21 | |
| Eggs | Human | Chiasso, SWI | 2006 | 22 | |
| Adult | Dog | Bern, SWI | 2002 | 23 | |
| Adult | Vulpes vulpes | Grisons, SWI | 2002 | 24 | |
| D. dendriticum | Plerocercoid | Coregonus lavaretus | Lake Peipsi, EST | 2004 | 25 |
| Plerocercoid | Coregonus lavaretus | Lake Peipsi, EST | 2004 | 26 | |
| Plerocercoid | Coregonus lavaretus | Lake Peipsi, EST | 2004 | 27 | |
| Plerocercoid | Coregonus lavaretus | Lake Peipsi, EST | 2004 | 28 | |
| Plerocercoid | Coregonus lavaretus | Lake Peipsi, EST | 2004 | 29 | |
| Plerocercoid | Coregonus lavaretus | Loch Lomond, GBR | 2001 | 30 | |
| Plerocercoid | Coregonus lavaretus | Loch Lomond, GBR | 2002 | 31 | |
| Plerocercoid | Salmo trutta | Loch Doyne, GBR | 2002 | 32 | |
| Plerocercoid | Coregonus lavaretus | Loch Lomond, GBR | 2004 | 33 | |
| Plerocercoid | Coregonus lavaretus | Loch Lomond, GBR | 2004 | 34 | |
| Plerocercoid | Coregonus lavaretus | Loch Lomond, GBR | 2004 | 35 | |
| Plerocercoid | Coregonus lavaretus | Loch Lomond, GBR | 2004 | 36 | |
| Plerocercoid | Coregonus lavaretus | Loch Lomond, GBR | 2004 | 37 | |
| Plerocercoid | Coregonus lavaretus | Loch Lomond, GBR | 2004 | 38 | |
| Plerocercoid | Coregonus lavaretus | Loch Lomond, GBR | 2004 | 39 | |
| Plerocercoid | Coregonus autumnalis | Olkhon Island, RUS | 2005 | 40 | |
| Adult | Human | Bern, SWI | 2006 | 41 | |
| D. pacificum | Adult | Human | Piura, PER | 2004 | 42 |
| Adult | Human | Lima, PER | 2005 | 43 | |
| Adult | Human | Tumbes, PER | 1994 | 44 | |
| Adult | Human | Tumbes, PER | 2003 | 45 | |
| Adult | Human | Lima, PER | 2006 | 46 | |
| Adult | Human | Lima, PER | 2006 | 47 | |
| Adult | Human | Tumbes, PER | 2006 | 48 | |
| Adult | Human | Tumbes, PER | 2006 | 49 | |
| Adult | Human | La Libertad, PER | 2006 | 50 | |
| Adult | Human | Tumbes, PER | 2007 | 51 | |
| Adult | Human | Tumbes, PER | 2007 | 52 | |
| Adult | Human | Tumbes, PER | 2007 | 53 | |
| Adult | Human | Lima, PER | 2007 | 54 | |
| Adult | Human | La Libertad, PER | 2007 | 55 | |
| Adult | Human | La Libertad, PER | 2007 | 56 | |
| Adult | Human | La Libertad, PER | 2007 | 57 | |
| Adult | Human | La Libertad, PER | 2008 | 58 | |
| D. nihonkaiense | Plerocercoid | Oncorhynchus keta | Hokkaido, JPN | 2006 | 59 |
| Plerocercoid | Oncorhynchus keta | Hokkaido, JPN | 2006 | 60 | |
| Plerocercoid | Oncorhynchus keta | Hokkaido, JPN | 2006 | 61 | |
| Plerocercoid | Oncorhynchus keta | Hokkaido, JPN | 2006 | 62 | |
| Plerocercoid | Oncorhynchus keta | Hokkaido, JPN | 2006 | 63 | |
| Plerocercoid | Oncorhynchus keta | Hokkaido, JPN | 2006 | 64 | |
| Plerocercoid | Oncorhynchus keta | Hokkaido, JPN | 2006 | 65 | |
| Plerocercoid | Oncorhynchus keta | Hokkaido, JPN | 2006 | 66 | |
| Plerocercoid | Oncorhynchus keta | Hokkaido, JPN | 2006 | 67 | |
| Plerocercoid | Oncorhynchus keta | Hokkaido, JPN | 2006 | 68 | |
| Plerocercoid | Oncorhynchus keta | Hokkaido, JPN | 2006 | 69 | |
| Plerocercoid | Oncorhynchus keta | Hokkaido, JPN | 2006 | 70 | |
| Plerocercoid | Oncorhynchus masou | Hokkaido, JPN | 2006 | 71 | |
| Adult | Human | JPN | 2006 | 72 | |
| Adult | Human | JPN | 2007 | 73 | |
| Adult | Human | JPN | 2007 | 74 | |
| Adult | Human | JPN | 2006 | 75 | |
| Adult | Human | JPN | 2007 | 76 | |
| Adult | Human | JPN | 2007 | 77 | |
| Adult | Human | JPN | 2006 | 78 | |
| Adult | Human | JPN | 2007 | 79 |
The lane numbers correspond to those reported in Fig. 1.
NI, not identified.
RUS, Russia; POL, Poland; ITA, Italy; SWI, Switzerland; EST, Estonia; FRA, France; GBR, Great Britain; PER, Peru; JPN, Japan.
DNA extraction and the multiplex PCR procedure were performed as described above for the cox1 gene analysis. Eggs were isolated from fecal samples by formalin-ether sediment concentration. One thousand to 4,000 eggs per sample were resuspended in 30 μl of Tris-EDTA (TE) solution and subsequently used for DNA extraction. DNA was amplified by multiplex PCR using primers MulRevCom, MulLat3, MulDen4, MulPac2, and MulNih5 in a single reaction at the same concentration (0.3 μM each). Different annealing temperatures (ranging from 50°C to 60°C) were tested to avoid unspecific amplification. The thermal cycle was optimized as follows: 94°C for 15 min; 25 cycles of 94°C for 30 s, 60°C for 1 min 30 s, 72°C for 1 min 30 s; 72°C for 10 min; storage at 4°C. A negative control (5 μl sterile water) was included for each species. The presence of the amplicon was verified by loading 5 μl of PCR product into a 1.5% agarose gel, which was electrophoresed for 50 min at 100 V in 1% Tris-acetate-EDTA (TAE) buffer and stained in ethidium bromide. For each species, four of the PCR products obtained by multiplex PCR were purified and directly sequenced as described above.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in the present study have been deposited in the DDJB/EMBL/GenBank databases under the accession numbers GU997612, GU997613, GU997614, and GU997615 (D. latum); AB548647, AB548648, AB548649, and AB548650 (D. nihonkaiense); GU997616, GU997617, GU997618, and GU997619 (D. dendriticum); and AB548651, AB548652, AB548653, and AB548654 (D. pacificum).
RESULTS
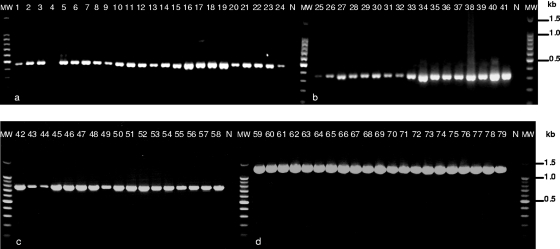
A total of 23 samples of D. latum, 17 samples of D. dendriticum, 17 samples of D. pacificum, and 21 samples of D. nihonkaiense were successfully amplified by multiplex PCR, yielding specific products of the expected sizes (Fig. 1). One sample of D. dendriticum (no. 25) yielded a faint band, and one sample of D. latum (no. 4) did not yield any amplicons. There were no amplification products from three other Diphyllobothrium species (data not shown) as well as from the negative control. The sequenced PCR products were confirmed as the target site of each species.
FIG. 1.
Differential identification by multiplex PCR of human-infecting species of Diphyllobothrium. (a) D. latum (lanes 1 to 24). (b) D. dendriticum (lanes 25 to 41). (c) D. pacificum (lanes 42 to 58). (d) D. nihonkaiense (lanes 59 to 79). N, negative control; MW, molecular weight (100-bp ladder; Promega, Madison, WI).
The amplification of diagnostic products by multiplex PCR was successfully obtained with an equal ratio of each reverse and forward primer. The optimal annealing temperature was set at 60°C, because the amplification at lower temperatures was unspecific and in some cases yielded multiple bands.
The analysis of cox1 gene sequences obtained from two plerocercoid larvae isolated from S. deliciosa allowed us to identify them as D. pacificum, the main species infecting humans in South America (17). As an additional tool for identification, their DNA was also tested by PCR with primers for the amplification of ITS1 and ITS2 according to a method described previously by Luton et al. (12); the analysis of the two obtained partial sequences (1,247-bp fragment) by a BLAST search (1) supported their identity (99.8% similarity with the reference sequence of D. pacificum under DDJB/EMBL/GenBank accession number FM204788).
DISCUSSION
In light of the recent appearance of both local and imported diphyllobothriosis in several regions, a reliable technique for the routine identification of broad tapeworms infecting humans is necessary for the surveillance, control, and better understanding of the epidemiology of this zoonosis. In particular, D. latum, D. dendriticum, D. nihonkaiense, and D. pacificum are of great medical importance and represent an emerging public health problem, especially in countries where the demand for fish is increasing and new culinary habits involving raw or uncooked preparations (sushi, sashimi, ceviche, carpaccio, etc.) are gaining ground. General practitioners and health workers usually identify Diphyllobothrium species on the basis of their morphological characteristics, often according to previously reported cases in the geographical area where their patients live. It has been widely proven that such methods may easily lead to misidentification, because entire worms with scolex and mature proglottids are almost never available, and that specific identification can be achieved only by using molecular data.
Our results indicate that the multiplex PCR assay is a promising method for the routinely identification of the principal species of Diphyllobothrium infecting humans. It represents a suitable alternative for medical laboratories and physicians, replacing the more expensive and time-consuming PCR and sequencing, and can be employed by nonspecialized personnel. Compared to other techniques described in the literature (5, 6, 13), the multiplex PCR is easier, faster, cheaper, and highly reliable, due to the specificity of cox1 gene sequences. On the contrary, immunoelectrophoretic methods require manual expertise, are rather work intensive, and require large amounts of polyclonal antibodies. Other molecular methods based on the random restriction of DNA, such as RFLP, can be used even when no previous data on DNA sequences are available for a particular organism. However, they are not necessarily species specific and are often difficult to interpret, as profiles generated from a whole DNA sample can include several fragments; such methods are better employed to discriminate relatively few DNA sequences.
The use of the multiplex PCR allows the differential identification of proglottids and eggs shed by patients, which in turn can help trace the origins of the infective plerocercoids present in fish (through the analysis of anamnestic data). Moreover, it has been demonstrated that allochtonous Diphyllobothrium species can be introduced into new habitats that satisfy their ecological requirements (i.e., the presence of adequate hosts and suitable biotic and abiotic factors), as was the case for D. latum and D. dendriticum in South America (22). Given that a single person harboring a broad tapeworm produces up to 1 million eggs per day and is able to infect a lake, a correct diagnosis may help prevent the introduction of exotic parasites in disease-free water systems. Specific identification by molecular methods is also of key importance to clarify the geographical range of the zoonosis and to prevent its spread. It should be applied especially in cases of atypical specimens and/or in cases of patients who have been abroad.
The differential PCR test can be applied to any developmental stage of Diphyllobothrium parasites provided that the DNA is extracted from frozen or ethanol-preserved samples (17). Other fixatives such as formalin or denatured alcohol irreversibly affect the quality of DNA and the chemical reactions for its amplification. Although some authors reported successful PCRs of short DNA fragments from formalin-fixed materials, the results were highly dependent on the formaldehyde concentration and time of sample fixation (3, 10). In our study, the lack of an amplicon for sample no. 4 is most probably due to the fact that it was stored in formalin at a high concentration (10%). The fact that sample no. 25 yielded a faint signal might be due to a handling problem, because a higher specific signal was produced in previous multiplex PCRs carried out to test different annealing temperatures.
Besides medical purposes, multiplex PCR can be useful in other fields, for example, in the study of Diphyllobothrium life cycles, through the identification of larval stages (sharing, like adults and eggs, similar morphologies among the different taxa). In the present study, we had the opportunity to identify plerocercoids of D. pacificum isolated from the fish S. deliciosa, providing the first evidence of this parasite in this intermediate (or paratenic) host.
A limitation of the present study concerned the evaluation of the cross-reaction with other Diphyllobothrium species (both human-infecting and non-human-infecting strains), an aspect that should be considered especially in the analysis of samples collected in the field. Also, the occurrence of broad tapeworms belonging to species other than D. latum, D. dendriticum, D. nihonkaiense, and D. pacificum in humans is quite rare but not impossible (17). Due to the lack of samples, we were able to evaluate the primers' specificity using only D. ursi, D. ditremum, and D. hottai. Moreover, molecular data are currently available for only a few species in the genus. Thus, even though cox1 sequences of other Diphyllobothrium and other human-infecting cestodes (such as taeniids and Diplogonoporus grandis) are theoretically different enough at the primer binding site, further investigation is required to evaluate the possibilities of cross-reactions.
In conclusion, further research is needed to optimize a broader, cheap, and rapid molecular diagnostic test for the identification of all Diphyllobothrium parasites to be used, for example, for the identification of larvae isolated from copepods and fish. Such a tool would noticeably facilitate the comprehension of parasitic life cycles and be employed in ecological and epidemiological studies.
Acknowledgments
We gratefully acknowledge the following people, who provided part of the samples for this study: H. H. García and J. Cárdenas (D. pacificum), J. Štefka, M. Nolvak, and A. P. Shinn (D. dendriticum); and F. de Marval, A. Gustinelli, and J. Dupouy-Camet (D. latum). We give special thanks to all staff of the laboratories of helminthology and molecular taxonomy (Institute of Parasitology, ASCR, České Budějovice, Czech Republic) for the valuable collaboration and to two anonymous reviewers who provided helpful remarks and suggestions.
This study was supported by the Swiss National Science Foundation (grant IZK0Z3-125824/1), the Institute of Parasitology of the ASCR (project no. Z60220518 and LC522), the International Collaboration Research Fund from the Japan Society for the Promotion of Science (grant 21256003) to A.I., and the Fogarty International Center, NIH (grant TW001140).
Footnotes
Published ahead of print on 30 June 2010.
REFERENCES
- 1.Altschul, S. F., W. Gish, W. Miller, E. W. Myers, and D. J. Lipman. 1990. Basic Local Alignment Search Tool. J. Mol. Biol. 215:403-410. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, K., and D. I. Gibson. 1989. A key to three species of larval Diphyllobothrium Cobbold, 1858 (Cestoda: Pseudophyllidea) occurring in European and North American freshwater fishes. Syst. Parasitol. 13:3-9. [Google Scholar]
- 3.Bhadury, P., M. C. Austen, D. T. Bilton, P. J. D. Lambshead, A. D. Rogers, and G. R. Smerdon. 2007. Exploitation of archived marine nematodes—a hot lysis DNA extraction protocol for molecular studies. Zool. Scr. 36:93-98. [Google Scholar]
- 4.Chai, J. Y., K. D. Murrell, and A. J. Lymbery. 2005. Fish-borne parasitic zoonoses: status and issues. Int. J. Parasitol. 35:1233-1254. [DOI] [PubMed] [Google Scholar]
- 5.de Vos, T., and T. A. Dick. 1989. Differentiation between Diphyllobothrium dendriticum and D. latum using isozymes, restriction profiles and ribosomal gene probes. Syst. Parasitol. 13:161-166. [Google Scholar]
- 6.Fukumoto, S., S. Yazaki, H. Kamo, Y. Yamane, and M. Tsuji. 1988. Distinction between Diphyllobothrium nihonkaiense and Diphyllobothrium latum by immunoelectrophoresis. Jpn. J. Parasitol. 37:91-95. [Google Scholar]
- 7.Garcia, L. S., and D. A. Bruckner. 1993. Diagnostic medical parasitology. American Society for Microbiology, Washington, DC.
- 8.Jeon, H.-K., K.-H. Kim, S. Huh, J.-Y. Chai, D.-Y. Min, H.-J. Rim, and K. S. Eom. 2009. Morphologic and genetic identification of Diphyllobothrium nihonkaiense in Korea. Kor. J. Parasitol. 47:369-375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, K., H. Jeon, S. Kang, T. Sultana, G. J. Kim, K. S. Eom, and J. K. Park. 2007. Characterization of the complete mitochondrial genome of Diphyllobothrium nihonkaiense (Diphyllobothriidae: Cestoda), and development of molecular markers for differentiating fish tapeworms. Mol. Cells 23:379-390. [PubMed] [Google Scholar]
- 10.Li, J., X. Liao, and H. Yang. 2000. Molecular characterization of a parasitic tapeworm (Ligula) based on DNA sequences from formalin-fixed specimens. Biochem. Genet. 38:309-322. [DOI] [PubMed] [Google Scholar]
- 11.Lou, H. Y., P. C. Tsai, C. C. Chang, Y. H. Lin, C. W. Liao, T. C. Kao, H. C. Lin, W. C. Lee, and C. K. Fan. 2007. A case of human diphyllobothriasis in northern Taiwan after eating raw fish fillets. J. Microbiol. Immunol. Infect. 40:452-456. [PubMed] [Google Scholar]
- 12.Luton, K., D. Walker, and D. Blair. 1992. Comparisons of ribosomal internal transcribed spacers from two congeneric species of flukes (Platyhelminthes: Trematoda: Digenea). Mol. Biochem. Parasitol. 56:323-327. [DOI] [PubMed] [Google Scholar]
- 13.Matsuura, T., G. Bylund, and K. Sugane. 1992. Comparison of restriction fragment length polymorphisms of ribosomal DNA between Diphyllobothrium nihonkaiense and D. latum. J. Helminthol. 66:261-266. [DOI] [PubMed] [Google Scholar]
- 14.Muller, R. 2002. Worms and human disease, 2nd ed. CABI Publishing, Wallingford, United Kingdom.
- 15.Paugam, A., H. Yera, P. Poirier, A. Lebuisson, and J. Dupouy-Camet. 2008. Bothriocéphalose à Diphyllobothrium nihonkaiense: un nouveau risque lié à la consommation de saumon. Presse Méd. 38:675-677. [DOI] [PubMed] [Google Scholar]
- 16.Sampaio, J. L., V. P. de Andrade, M. C. Lucas, L. Fung, S. M. Gagliardi, S. R. Santos, C. M. Mendes, M. B. Eduardo, and T. Dick. 2005. Diphyllobothriasis, Brazil. Emerg. Infect. Dis. 11:1598-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scholz, T., H. H. García, R. Kuchta, and B. Wicht. 2009. Update on the human broad tapeworm (genus Diphyllobothrium), including clinical relevance. Clin. Microbiol. Rev. 22:146-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimizu, H., H. Kawakatsu, T. Shimizu, M. Yamada, T. Tegoshi, R. Uchikawa, and N. Arizono. 2008. Diphyllobothriasis nihonkaiense: possibly acquired in Switzerland from imported Pacific salmon. Int. Med. 47:1359-1362. [DOI] [PubMed] [Google Scholar]
- 19.Škeříková, A., J. Brabec, R. Kuchta, J. A. Jiménez, H. H. García, and T. Scholz. 2006. Is the human-infecting Diphyllobothrium pacificum a valid species or just a South American population of the Holarctic fish broad tapeworm, D. latum? Am. J. Trop. Med. Hyg. 75:307-310. [PubMed] [Google Scholar]
- 20.Stunkard, H. W. 1965. Variation and criteria for generic and specific determination of diphyllobothriid cestodes. J. Helminthol. 39:281-296. [DOI] [PubMed] [Google Scholar]
- 21.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 22.Torres, P., C. Cuevas, M. Tang, M. Barra, R. Franjola, N. Navarrete, A. Montefusco, L. Otth, G. Wilson, and S. Puga. 2004. Introduced and native fishes as infection foci of Diphyllobothrium spp. in humans and dogs from two localities at Lake Panguipulli in Southern Chile. Comp. Parasitol. 71:111-117. [Google Scholar]
- 23.Wicht, B., F. de Marval, and R. Peduzzi. 2007. Diphyllobothrium nihonkaiense (Yamane et al., 1986) in Switzerland: first molecular evidence and case reports. Parasitol. Int. 56:195-199. [DOI] [PubMed] [Google Scholar]
- 24.Wicht, B., F. de Marval, B. Gottstein, and R. Peduzzi. 2008. Imported diphyllobothriasis in Switzerland: molecular evidence of Diphyllobothrium dendriticum (Nitzsch, 1824). Parasitol. Res. 102:201-204. [DOI] [PubMed] [Google Scholar]
- 25.Wicht, B., T. Scholz, R. Peduzzi, and R. Kuchta. 2008. First record of human infection with the tapeworm Diphyllobothrium nihonkaiense in North America. Am. J. Trop. Med. Hyg. 78:235-238. [PubMed] [Google Scholar]
- 26.Wicht, B. 2009. Ecology, epidemiology and molecular identification of the genus Diphyllobothrium Cobbold, 1858 in the sub-alpine lakes region. Ph.D. thesis no. 4046. University of Geneva, Geneva, Switzerland.
- 27.Wicht, B., N. Ruggeri-Bernardi, T. Yanagida, M. Nakao, R. Peduzzi, and A. Ito. 2010. Inter- and intra-specific characterization of tapeworms of the genus Diphyllobothrium (Cestoda: Diphyllobothriidea) from Switzerland, using nuclear and mitochondrial DNA targets. Parasitol. Int. 59:35-39. [DOI] [PubMed] [Google Scholar]
- 28.Yamasaki, H., J. C. Allan, M. O. Sato, M. Nakao, Y. Sako, K. Nakaya, D. Qiu, W. Mamuti, P. S. Craig, and A. Ito. 2004. DNA differential diagnosis of taeniasis and cysticercosis by multiplex PCR. J. Clin. Microbiol. 42:548-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanagida, T., H. Matsuoka, T. Kanai, M. Nakao, and A. Ito. 2010. Anomalous segmentation of Diphyllobothrium nihonkaiense. Parasitol. Int. 59:268-270. [DOI] [PubMed] [Google Scholar]
- 30.Yera, H., C. Estran, P. Delaunay, M. Gari-Toussaint, J. Dupouy-Camet, and P. Marty. 2006. Putative Diphyllobothrium nihonkaiense acquired from a Pacific salmon (Oncorhynchus keta) eaten in France: genomic identification and case report. Parasitol. Int. 55:45-49. [DOI] [PubMed] [Google Scholar]
- 31.Yera, H., J. Nicoulaud, and J. Dupouy-Camet. 2008. Use of nuclear and mitochondrial DNA PCR and sequencing for molecular identification of Diphyllobothrium isolates potentially infective for humans. Parasite (Paris) 15:402-407. [DOI] [PubMed] [Google Scholar]