Abstract
Although screening for maternal toxoplasmic seroconversion during pregnancy is based on immunodiagnostic assays, the diagnosis of clinically relevant toxoplasmosis greatly relies upon molecular methods. A problem is that this molecular diagnosis is subject to variation of performances, mainly due to a large diversity of PCR methods and primers and the lack of standardization. The present multicentric prospective study, involving eight laboratories proficient in the molecular prenatal diagnosis of toxoplasmosis, was a first step toward the harmonization of this diagnosis among university hospitals in France. Its aim was to compare the analytical performances of different PCR protocols used for Toxoplasma detection. Each center extracted the same concentrated Toxoplasma gondii suspension and tested serial dilutions of the DNA using its own assays. Differences in analytical sensitivities were observed between assays, particularly at low parasite concentrations (≤2 T. gondii genomes per reaction tube), with “performance scores” differing by a 20-fold factor among laboratories. Our data stress the fact that differences do exist in the performances of molecular assays in spite of expertise in the matter; we propose that laboratories work toward a detection threshold defined for a best sensitivity of this diagnosis. Moreover, on the one hand, intralaboratory comparisons confirmed previous studies showing that rep529 is a more adequate DNA target for this diagnosis than the widely used B1 gene. But, on the other hand, interlaboratory comparisons showed differences that appear independent of the target, primers, or technology and that hence rely essentially on proficiency and care in the optimization of PCR conditions.
Toxoplasmosis is a worldwide endemic protozoan disease, acquired mainly through infected meat. The consequences of fetal infections range from severe neurological abnormalities and chorioretinitis to subclinical infection at birth which, however, still poses a risk of late onset of ocular lesions (37). A rapid and accurate diagnosis is required in order to start the antiparasitic treatment. Prenatal diagnosis of congenital toxoplasmosis has considerably improved the prognosis and outcome for infected children wherever it has been implemented and has been a national policy in France since 1978 (35). The detection of the parasite DNA by PCR-based molecular diagnostic tests using amniotic fluid (AF) has largely superseded more classical methods (1). However, the sensitivity of this molecular prenatal diagnosis remains a problem because parasitic loads are generally low (11, 30) and, even in proficient laboratories, the diagnostic sensitivity generally remains below 80% (4, 31, 36; reviewed in references 1 and 34). In addition, most Toxoplasma-PCR assays used for this application are “in-house” or “laboratory-developed” methods, set up independently in each laboratory, which leads to important variations in the PCR protocols between laboratories (regarding DNA extraction, DNA target, PCR primers, amplification conditions, and amplicon detection) (32). This situation has well-known drawbacks, particularly a lack of standardization and variations in efficiency (12, 17, 21, 25). In addition to this diversity, external quality assessments or interlaboratory comparative studies for the molecular detection of Toxoplasma gondii are scarce: five of these have been carried out in the last 10 years, all in Europe and all demonstrating wide divergences in the performances of PCR methods (2, 12, 17, 21, 25). In France, a network has been set up for the improvement and standardization of this molecular diagnosis within the framework of the recently created National Reference Centre for Toxoplasmosis (http://www.chu-reims.fr/professionnels/cnr-toxoplasmose-1/). This network is made up of eight university hospital laboratories, all experienced in the use of PCR for detection of T. gondii in clinical specimens, all participating in external quality assessments for this diagnosis, and all considered expert laboratories for performing this diagnosis in their geographical region. Within this network, a multicentric prospective study was launched to compare the performances of the different laboratories in the molecular detection of T. gondii using methods routinely used for hospital diagnosis in each center. The specific aims of the study were (i) to compare the two DNA targets most commonly used for this diagnosis and (ii) to be able to propose to diagnostic laboratories a PCR sensitivity threshold as a minimal objective for an optimal molecular diagnosis of toxoplasmosis. Our work was voluntarily conducted with low concentrations of parasites (i) because it has been established that AF from a large proportion of infected patients contains Toxoplasma loads of <10 tachyzoites per ml (11, 30) and (ii) because diagnostic methods for pathogens are particularly fallible with low concentrations of pathogens in the biological sample (10, 21-23, 25). In spite of the expertise of the participants, our study revealed relevant differences in the performances of the molecular assays compared. Our data also emphasize the fact that the use of the repetitive noncoding rep529 DNA target (20) should generally be preferred for the prenatal diagnosis of congenital toxoplasmosis; but, still, they stress the importance of PCR “optimization” and proficiency in obtaining a high-quality in-house molecular diagnosis.
MATERIALS AND METHODS
Study scheme.
T. gondii suspensions were prepared in one center (Toulouse, France) and distributed to eight participant laboratories in France on two occasions, in October 2006 and October 2007. The study scheme was established after a concerted decision between all participants in 2006 and reproduced unchanged in 2007. All the participating laboratories were proficient in detecting T. gondii in clinical specimens and were authorized by the Ministry of Health to practice prenatal diagnosis of toxoplasmosis.
Preparation and distribution of panels.
Parasites were collected from ascitic fluid containing tachyzoites drawn from a Swiss Webster female mouse infected with the RH strain of T. gondii. Harvested peritoneal parasites were washed twice and then resuspended in sterile 0.9% NaCl. Counting of tachyzoites was done in triplicate, and the mean number was calculated. The concentration was adjusted to get a concentration of 106 tachyzoites/ml; 200 μl of this stock suspension (2 × 105 tachyzoites) were then dispensed into two microtubes and immediately sent to every participant by courier. Each of the eight participants thus received identical 200-μl samples drawn at the same time from the same pool (here termed T. gondii stock suspension). The homogeneity of the stock suspensions was verified by performing a quantitative Toxoplasma PCR in triplicate using the first and last sample tubes that were prepared during tachyzoite distribution in laboratory C and two distinct samples in laboratory A: the variation in crossing-point (CP) values observed between the means of both PCR results was close to zero (0.07; intra-PCR standard deviations, 0.13 and 0.15). The proficiency panel arrived at its destination within 24 h. Apart from one exception (laboratory F, where the panel was kept for 24 h at +4°C), all samples were processed on the day of arrival.
DNA extraction.
DNA extraction was performed using the whole volume received for each sample (200 μl). The DNA extraction methods varied among laboratories (Table 1): basically, either the Tween-Nonidet-NaOH (TNN; 0.5% Tween 20, 0.5% Nonidet P40, 10 mM NaOH) lysis buffer method (19) or commercial kits were used, the latter according to the manufacturer's specifications (High Pure PCR template [Roche Molecular Biochemicals, Meylan, France] and QIAamp DNA kits using the blood or body fluids protocol [mini kit no. 51304 or blood mini kit no. 51104; Qiagen, Courtaboeuf, France]). The extracted DNA was resuspended in either elution buffer, sterile distilled water, or TNN buffer (depending on the method used in the respective laboratories) for a Toxoplasma DNA serial dilution assay.
TABLE 1.
Overview of the methods and primers used in the study scheme for molecular detection of T. gondii
| Center | DNA extraction method (reference) | DNA target | Primer(s) (reference[s]) | PCR technologya | Amplicon detectionb | Inhibition controlc |
|---|---|---|---|---|---|---|
| A | Tween-Noninet-NaOH method (19) | B1 gene | (6) | cnPCR | Gel electrophoresis + ethidium bromide staining | T. gondii DNA internal control |
| rep529 | H1-H2 (unpublished) | cnPCR | Gel electrophoresis + ethidium bromide staining | T. gondii DNA internal control | ||
| B | Qiagen QIAamp DNA mini kit | B1 gene | (29) | cnPCR | ELISA | Plasmidic internal control |
| rep529 | (33, 38) | rtPCR | TaqMan MGB and LNAe | Plasmidic internal control | ||
| C | Roche HighPure PCR template kit | B1 gene | (13) | rtPCR | FRET | β-Globin gene |
| rep529 | (8) | rtPCR | FRET | β-Globin gene | ||
| D | Qiagen QIAamp DNA mini kit | B1 gene | Primers 1-4 (7) | qrtPCR | Sybr Green | Plasmidic internal control |
| rep529 | (20) | rtPCR | Sybr Green | Plasmidic internal controld | ||
| E | Qiagen QIAamp DNA mini kit | B1 gene | (26) | rtPCR | FRET | Plasmidic competitive internal control (5) |
| F | Qiagen QIAamp DNA blood mini kit | B1 gene | (15) | rtPCR | TaqMan | T. gondii DNA internal control |
| rep529 | (15) | rtPCR | TaqMan | T. gondii DNA internal control | ||
| G | Qiagen QIAamp DNA mini kit | B1 gene | (13) | rtPCR | FRET | β-Globin gene |
| rep529 | (27) | rtPCR | FRET | β-Globin gene | ||
| H | Qiagen QIAamp DNA blood mini kit | rep529 | (27) | cnPCR | Gel electrophoresis + ethidium bromide staining | T. gondii DNA internal control |
| rep529 | (16) | rtPCR | FRET | T. gondii DNA internal control |
cnPCR, conventional PCR; rtPCR: real-time PCR.
Gel electrophoresis + ethidium bromide staining, direct visualization of DNA by ethidium bromide staining after agarose gel electrophoresis; ELISA, PCR-ELISA based upon the PCR-ELISA DIG labeling and PCR-ELISA DIG detection kits (Roche Diagnostics); TaqMan MGB and LNA, hydrolysis DNA probes (TaqMan technology; Applied Biosystems, Courtaboeuf, France); FRET, FRET hybridization DNA probes; Sybr green, Sybr green-based real-time PCR.
The absence of reaction inhibition was verified by amplifying a positive internal control concurrently and in the same reaction tube as the test DNA after the addition of a control sequence of target DNA; this control DNA may be either highly diluted T. gondii genomic DNA (equivalent to 1 or 0.5 tachyzoite genome), an artificial plasmidic DNA construct containing the primer sequences (amplified by the test primers), or a defined sequence of DNA amplified by a second primer pair, e.g., β-globin or albumin amplified under stringent conditions (to increase the PCR sensitivity to the presence of inhibitors in the sample).
Laboratory D also systematically performed one PCR with the matrix DNA diluted.
The two types of probes gave similar results.
Toxoplasma DNA serial dilution assay.
The serial dilution assay consisted of the performance of serial dilution steps of the primary DNA in elution buffer, distilled water, or TNN (depending on the first solution used as described above for resuspension). In all subsequent tests (as well as throughout this report), the concentrations of the DNA solutions are expressed as the number of T. gondii genomes (Tgg) per PCR tube. Since the laboratories used different volumes for DNA elution and for the PCR, this choice allowed a straightforward comparison between laboratories. As a previous study in our laboratory had suggested that the protocol of dilution used may influence the PCR outcome (unpublished data), we proposed a common dilution protocol to all participants. The equivalents of 50, 10, 5, 2, 1, 0.5, 0.2, and 0.1 Tgg per PCR tube were tested. These concentrations were chosen because they define a large range of parasite loads that likely represent the range found in clinical specimens. The reproducibility of the serial dilution was attested by the realization of the analysis in duplicate in one center. No difference was seen in the percentage of positives among multiple tests; and the ΔCP was very low, ranging from 0.08 to 1.87 for the highest to the lowest dilution, respectively, (data not shown).
PCR amplification.
Two DNA targets were used for PCR: (i) the B1 gene (7) (GenBank accession no. AF179871) and (ii) the repetitive noncoding element described by Homan et al. (20) and Reischl et al. (27) and termed rep529 (GenBank accession no. AF146527 and AF487550, respectively). Each laboratory used its own “laboratory-developed” PCR assay, whether a conventional PCR (cnPCR), enzyme-linked immunosorbent assay (ELISA), or real-time PCR (rtPCR) (5-8, 13, 15, 16, 20, 26-29, 33, 38). The PCR protocol employed by each laboratory differed in a number of ways (Table 1): DNA extraction, DNA target, primers, PCR technology, amplicon detection, DNA extraction control, and PCR inhibition control.
All laboratories used uracyl-DNA glycosylase (UNG; Roche Diagnostics, Meylan, France) as a measure for preventing contamination as well as all recommended physical separation measures. Contamination-negative controls and PCR inhibition-positive controls were used in all laboratories. For the latter, each laboratory amplified the same control as the one used in its routine practice (Table 1). Briefly, laboratories A, F, and H used highly diluted T. gondii DNA (down to 1 genome equivalent per reaction in laboratories A and F and 0.5 genome equivalent in laboratory H) that was amplified together with the patient's sample DNA in a separate tube using the same PCR conditions and primers as the sample reaction tube; given that the reaction is inconsistently positive at this concentration, laboratories A and F amplified the positive control in duplicate and inferred that the reaction was inhibited only if both reactions were negative. Laboratories C and G amplified the human beta-globin gene in a different reaction tube. Laboratories B and D used a commercially available internal plasmidic control that was coamplified with the sample DNA using specific primers. Laboratory E used a laboratory-developed plasmidic competitive internal control (5) that was coamplified with the sample DNA in a single tube using the same primers and that is distinguished from the Toxoplasma amplicon by a specific set of hybridization probes (fluorescence resonance energy transfer [FRET]) labeled with LC-Red 705.
Data analysis.
At and around the sensitivity threshold of a given PCR method, only a proportion of the reaction tubes appear positive, which implies that for very low concentrations of the pathogen, several PCRs have to be carried out for each experiment (thus increasing the probability of amplifying the pathogen DNA) (9, 10, 23, 38). As a consequence, here, in each laboratory, the concentrations of 50 and 10 Tgg per reaction tube were tested in duplicate, the concentrations of 5, 2, and 1 Tgg per reaction tube were tested in quadruplicate, the concentrations of 0.5 and 0.2 Tgg per reaction tube were tested eight times, and the concentration of 0.1 Tgg per reaction tube was tested 16 times. To facilitate interlaboratory comparisons, a scoring system was then applied; this system consisted of calculating a ratio of the number of positive reactions to the total number of reactions performed at low concentrations: the so-called “0.2” score included results at the 5, 2, 1, 0.5, and 0.2 Tgg per reaction tube concentrations (28 reactions in total), and the more selective “0.1” score included the results at the 0.1 Tgg per reaction tube concentration in addition to the previous five (44 reactions in total). Results were expressed as raw numbers and percentages. Greater explanation and a detailed example are given in Table S1 in the supplemental material. Chi-square tests were used for comparative statistical analysis of the 0.1 scores between the two years of study and between targets for each laboratory. When necessary (for calculated frequencies of <5, i.e., for laboratory G), the Yates correction was applied.
RESULTS
General design of the study.
In order to avoid any bias due to the preanalytic step of sample preparation and to minimize the possible effect of the host DNA upon the performances of these methods, the test material sent to every laboratory consisted of a 200-μl stock suspension of live T. gondii tachyzoites (200 μl being the volume generally employed for the subsequent DNA extraction in commercial kits). The analytical sensitivity of each molecular diagnostic method was then assessed using serial dilutions of the DNA solution extracted independently by each participant from this stock tachyzoite suspension. It should be noted that we decided not to send already extracted DNA, as a previous study using the same T. gondii DNA extracted by a Roche system and then sent to every participant revealed variations in the results that did not reflect the exact efficiency of the different PCR methods: the performances of all PCR methods but the one which used the Roche extraction system were reduced. In our sense, this could be explained only by a lack of conjoint optimization between the DNA extraction method of the sender and the PCR methods used by the participants (unpublished data). In contrast, our protocol allowed us to test the whole process (extraction, amplification, and detection) of each molecular diagnosis set up independently by each participant and to reliably compare the processes of all participants.
Each of the eight participating laboratories used its own laboratory-developed molecular diagnosis method(s), differing in the DNA extraction, DNA target, primers, PCR technology, and amplicon detection methods (Table 1). Eleven different primer pairs, targeting two DNA sequences, were used: the 35-fold repetitive B1 gene (7) and the 200- to 300-fold repetitive 529-bp DNA fragment (20), here termed rep529. Six laboratories used both DNA targets and returned two or three data sets. In total, 16 methods were tested and, for both years, 31 data sets were compared.
Overall results.
The raw data of the comparative study are shown in Table S1 in the supplemental material for each method and year. All methods reliably detected 50 and 10 Tgg per reaction tube. But, below 10 Tgg per reaction tube, certain methods gave partially but reproducibly positive results; i.e., only a portion of the reactions were positive. This generally indicates that the method is close to its detection limit (9, 10, 22, 23).
At 5 and 2 Tgg per reaction tube, only three and four methods, respectively, did not obtain 100% of the positive reactions out of the four reactions performed. As expected, this number increased as the DNA concentration decreased. At 0.2 Tgg per reaction tube, all methods but three detected the parasite, but only four of the 31 data sets showed eight positive reactions out of the eight test reactions performed (see Table S1 in the supplemental material). In order to estimate the sensitivity of each method, we used a scoring system based on the proportion of positive reactions to the total number of reactions performed for concentrations between 5 Tgg and 0.2 (or 0.1) Tgg per reaction tube (0.2 and 0.1 scores, respectively; see Materials and Methods). Large score differences among the methods were apparent, as shown by 0.2 scores ranging from 7% to 100% and by 0.1 scores ranging from 4.5% to 95.4% (see Table S1 and details below).
The specificity of all assays was 100%, as shown by a total of 186 negative controls that proved negative within the framework of this study.
Comparison of performances according to the DNA targets and primers.
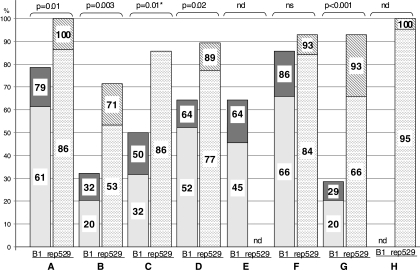
The 0.2 and the 0.1 scores of each participant for each method in 2007 are shown in Fig. 1 and summarized in Table S2 in the supplemental material. The difference between the two DNA targets was evident in all laboratories and with all methods. Whatever the DNA primers and type of PCR method used, both scores were better with the rep529 element than with the B1 gene in all laboratories that tested both targets; the difference was statistically significant in five out of six (Fig. 1).
FIG. 1.
Performances of the different PCR methods for each DNA target in 2007. A to H, participating centers. The two DNA targets, the B1 gene (left bar) and rep529 (right bar), are compared for each center. The numbers within the bars represent the 0.2 (top) and 0.1 (bottom) scores obtained by the different participants in 2007 (score decimal numbers were rounded to the nearest whole number). Chi-square tests showed that rep529 yielded significantly better performances than the B1 gene in five out of six laboratories that tested both targets (P values shown at the top of the figure). *, an 0.1 score was not available for laboratory C and, consequently, the chi-square test was performed using the 0.2 score; nd, not done; ns, not statistically significant at 95% confidence (P = 0.08). Summarized details of the scores, PCR technology, and primers for each participant can be found in Table S2 in the supplemental material.
In contrast, with regard to primers, no clear correlation could be made. Indeed, PCR assays using exactly the same primers displayed large variations in sensitivity among different laboratories, both for the B1 gene (see Table S1 in the supplemental material, participants C and G) and for rep 529 (Table S1, participants G and H). Similarly, participants D and H both used the primers used by Homan et al. (20) and showed 0.2 scores of 53.6 and 100 and 0.1 scores of 34.1 and 63.6, respectively, in 2006 (Table S1).
Influence of the type of PCR technology.
If the 0.2 and 0.1 scores of the participants are analyzed according to the type of PCR technology used, several conclusions can be drawn: (i) the use of rtPCR does not ensure a better sensitivity than that of cnPCR (and vice versa); (ii) for rtPCR, the use of Sybr green (only one method) or hybridization probes does not appear to clearly affect the performances of the methods; and (iii) PCR-ELISA (only one method) does not bring any obvious improvement to the sensitivity of the method.
DISCUSSION
This multicentric comparative external assessment was performed twice according to a strict prospective and standardized protocol and involved only laboratories proficient in the molecular diagnosis of toxoplasmosis. Still, it allowed the demonstration of large differences in the performances of the different assays used by the participants.
The bias of the preanalytic step interfering with the results was avoided by sending each participant a small volume of 200 μl of the stock solution. In contrast, it is noteworthy that the rest of the method was considered as a whole without the ability to distinguish between what, in the results, is precisely linked with the efficiency of extraction or of amplification.
The study was based upon the extraction of a high concentration of parasites in suspension (106 Toxoplasma tachyzoites per ml), followed by serial dilution of the DNA in buffer. The rationale for the choice of this material has been described above. One may consider that this is an artificial situation far from routine diagnosis; however, (i) in the experience of the French national External Quality Assessment for Toxoplasma-PCR (2), the use of live tachyzoites diluted in a volume of biological sample fluid used in routine testing was associated with problems (likely due to cell death and DNA degradation) rendering data interpretation difficult; (ii) we could not send very low concentrations of T. gondii because of the well-known inefficient DNA recovery from low parasite concentrations in a noncellular fluid; (iii) the distribution of tachyzoites at low concentrations in sample tubes would be less rigorous than the protocol we used, as it is even more subject to the Poisson law of large numbers than the distribution of extracted DNA in solution. Therefore, we believe that our results can be extrapolated to certain clinical samples such as fluids with low cellularity. Indeed, host DNA (a factor able to negatively influence the reaction) and PCR inhibitors are generally considered to be at very low levels in AF, aqueous humor, or cerebrospinal fluid. Moreover, three centers verified by two different extraction methods that extraction efficiencies were similar in saline and AF: two by comparing the PCR efficiencies in the two systems and one by comparing both the limits of detection and the PCR efficiencies (data not shown). A confirmation of this extrapolation might be achieved by repeating the same study using (ideally lyophilized) artificial samples made from spiked negative AF or by diluting pooled positive AF samples in pooled negative AF. Finally, to further investigate whether our study also applies to other types of samples, more multicentric studies using blood, buffy coat, or placenta samples will be needed.
The use of 0.1 and 0.2 scores allows the enhancement of differences between the methods compared. The more selective 0.1 score magnifies the differences observed with the 0.2 score. The pitfall of the Poisson law (which is applicable at very low pathogen concentrations) was avoided by the use of dilutions of extracted DNA, combined with the repetition of the PCR DNA targets. This has been validated by the good reproducibility of the results between the 2 years of the study. It is important to note that these scores are merely useful tools that highlight differences in sensitivity and that the sensitivity is not proportional to the given figures; i.e., a score at 45% does not exactly mean that the method is half as sensitive as a method with a score at 90%.
The interlaboratory comparison showed large differences in the performances of the methods used. The DNA target appears to be a major source of variability (see below) and must be taken into account for these comparisons. As regards the B1 gene target, methods in laboratories A and F clearly performed better, whereas laboratory B and G methods showed a markedly lower sensitivity. When the rep529 DNA target was used, the range was narrower, but still with laboratories A, F, and H being highly performing (the latter with the method using the primers from Reischl et al. [27]) and laboratories B and C being at the lower end. The main interest of such ranking is to be able to propose standards that could ideally be attained by laboratories involved in this molecular diagnosis. One way of determining such standards is to consider that the PCR sensitivity threshold is reached when approximately 50% of the reactions are positive (9, 10, 22, 23). Here, this threshold varies by a 50-fold factor, i.e., from 0.1 to 5 Tgg per reaction tube, according to the methods used. We would recommend that, for a best sensitivity of the molecular diagnosis of T. gondii, laboratories work toward a threshold of 0.5 Tgg per reaction tube (which would then correspond to 0.75 to 2.5 tachyzoites/ml of AF depending upon the different preanalytic protocols used in routine practice in the participating centers). Obviously, the specificity of the PCR assay remains an essential parameter to take into account, but this can be more easily established and assessed than sensitivity. In our study, the specificity appeared to be 100% for all assays. Moreover, none of the participating centers had experienced any false positives in the annual national external quality controls over the previous 8 years (2; unpublished data).
We show here that the PCR assays based upon the rep529 DNA target generally perform better than those based upon the B1 gene, independently of the DNA primers and PCR technology used. This was true each time the two targets were tested within the same laboratory, when rep529-based assays systematically proved more efficient than B1-based ones, regardless of the primers used. This multicentric evaluation thus confirms previous findings by individual groups using a variety of primers (8, 14, 18, 24, 27). In contrast, interlaboratory comparisons show that B1-based assays in certain laboratories may be more efficient than rep529-based methods in others (Fig. 1; see Table S2 in the supplemental material). Similarly, in this study, as reported by others (reviewed in reference 3), we show that for the same DNA target, cnPCR methods can perform better than rtPCR methods. This does not detract from the fact that rtPCR shows undeniable advantages over cnPCR (particularly reliability, speed, and reduction of contamination) (3). In any case, we infer that the main factors that may explain the differences observed here among similar assays in different centers are the proficiency and care in optimization of the PCR conditions as a whole (including DNA extraction). But, within the same laboratory (which will ideally optimize both targets equally well), rep529 should perform better. We therefore recommend the use of the DNA target rep529 over the more classical B1 gene for the molecular diagnosis of toxoplasmosis.
With regard to primers, the diversity of primers used here for the two targets prevents us from indicating one primer pair as superior to another. We can only note that one intralaboratory comparison in our study (participant H [see Table S1]), as well as unpublished data by F. Dalle et al., suggests that the primers of Homan et al. (20) may be less efficient than those of Reischl et al. (27). In contrast, as underlined in a previous report (3), interlaboratory comparisons show that the use of identical primer pairs can yield variable results depending on the laboratory. Similarly, we did not observe that the use of TaqMan probes (laboratories B and F) was less efficient than that of fluorescence resonance energy transfer (FRET) probes on the rep529 target, perhaps because there were no intralaboratory comparisons addressing this point. Again, interlaboratory comparisons cannot be taken into account here.
In conclusion, although multiple factors can influence the results of a PCR assay even when using the “right” DNA target and the “right” primers (reviewed in reference 3), the whole of our data underlines the crucial importance of four independent and nonexclusive general parameters for the quality of molecular diagnosis in infectious diseases: (i) the proficiency of the laboratory performing the diagnosis, relying in particular upon (ii) the mastering of the optimization of PCR conditions, and taking into account (iii) the fine-tuning of the combination “DNA extraction-PCR conditions” (our unpublished data), and, finally, (iv) the need for test surveys or external quality assessments of each molecular diagnostic method. We suggest that these should go through the definition of standard sensitivity thresholds, complementing classical quality assessments.
Supplementary Material
Acknowledgments
We thank Agnès Pacot (Dijon), Catherine Barois (Grenoble), Michèle Wauquier and Filoména Naji (Lille), Céline Fraissinet and Guillaume Bresson (Montpellier), Christelle Lehuen (Paris-Cochin), Nathalie Chartrel (Paris-Pitié-Salpétrière), Rachel Huber and Sylvie Matern (Strasbourg), and S. Chalmeton, Elodie Duthu, Séverine Gisquet, and C. Paris (Toulouse), for their technical assistance.
This work was supported by the National Reference Centre (CNR) for Toxoplasmosis and the Institut de Veille Sanitaire.
Footnotes
Published ahead of print on 7 July 2010.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Bastien, P. 2002. Molecular diagnosis of toxoplasmosis. Trans. R. Soc. Trop. Med. Hyg. 96(Suppl. 1):S205-S215. [DOI] [PubMed] [Google Scholar]
- 2.Bastien, P., E. Jumas-Bilak, E. Varlet-Marie, and P. Marty. 2007. Three years of multi-laboratory external quality control for the molecular detection of Toxoplasma gondii in amniotic fluid in France. Clin. Microbiol. Infect. 13:430-433. [DOI] [PubMed] [Google Scholar]
- 3.Bastien, P., G. W. Procop, and U. Reischl. 2008. Quantitative real-time PCR is not more sensitive than “conventional” PCR. J. Clin. Microbiol. 46:1897-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bessieres, M. H., A. Berrebi, S. Cassaing, J. Fillaux, J. P. Cambus, A. Berry, C. Assouline, J. M. Ayoubi, and J. F. Magnaval. 2009. Diagnosis of congenital toxoplasmosis: prenatal and neonatal evaluation of methods used in Toulouse University Hospital and incidence of congenital toxoplasmosis. Mem. Inst. Oswaldo Cruz 104:389-392. [DOI] [PubMed] [Google Scholar]
- 5.Brenier-Pinchart, M. P., V. Morand-Bui, H. Fricker-Hidalgo, V. Equy, R. Marlu, and H. Pelloux. 2007. Adapting a conventional PCR assay for Toxoplasma gondii detection to real-time quantitative PCR including a competitive internal control. Parasite 14:149-154. [DOI] [PubMed] [Google Scholar]
- 6.Bretagne, S., J. M. Costa, M. Vidaud, J. Tran, V. Nhieu, and J. Fleury-Feith. 1993. Detection of Toxoplasma gondii by competitive DNA amplification of bronchoalveolar lavage samples. J. Infect. Dis. 168:1585-1588. [DOI] [PubMed] [Google Scholar]
- 7.Burg, J. L., C. M. Grover, P. Pouletty, and J. C. Boothroyd. 1989. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J. Clin. Microbiol. 27:1787-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassaing, S., M. H. Bessieres, A. Berry, A. Berrebi, R. Fabre, and J. F. Magnaval. 2006. Comparison between two amplification sets for molecular diagnosis of toxoplasmosis by real-time PCR. J. Clin. Microbiol. 44:720-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chabbert, E., L. Lachaud, L. Crobu, and P. Bastien. 2004. Comparison of two widely used PCR primer systems for detection of toxoplasma in amniotic fluid, blood, and tissues. J. Clin. Microbiol. 42:1719-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chernesky, M., M. Smieja, J. Schachter, J. Summersgill, L. Schindler, N. Solomon, K. Campbell, L. Campbell, A. Cappuccio, C. Gaydos, S. Chong, J. Moncada, J. Phillips, D. Jang, B. Wood, A. Petrich, M. Hammerschlag, M. Cerney, and J. Mahony. 2002. Comparison of an industry-derived LCx Chlamydia pneumoniae PCR research kit to in-house assays performed in five laboratories. J. Clin. Microbiol. 40:2357-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costa, J. M., P. Ernault, E. Gautier, and S. Bretagne. 2001. Prenatal diagnosis of congenital toxoplasmosis by duplex real-time PCR using fluorescence resonance energy transfer hybridization probes. Prenat. Diagn. 21:85-88. [DOI] [PubMed] [Google Scholar]
- 12.Costa, J. M., C. Munoz, D. Kruger, R. Martino, T. K. Held, M. L. Darde, C. Cordonnier, and S. Bretagne. 2001. Quality control for the diagnosis of Toxoplasma gondii reactivation in SCT patients using PCR assays. Bone Marrow Transplant. 28:527-528. [DOI] [PubMed] [Google Scholar]
- 13.Costa, J. M., C. Pautas, P. Ernault, F. Foulet, C. Cordonnier, and S. Bretagne. 2000. Real-time PCR for diagnosis and follow-up of Toxoplasma reactivation after allogeneic stem cell transplantation using fluorescence resonance energy transfer hybridization probes. J. Clin. Microbiol. 38:2929-2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Edvinsson, B., M. Lappalainen, and B. Evengård. 2006. Real-time PCR targeting a 529-bp repeat element for diagnosis of toxoplasmosis. Clin. Microbiol. Infect. 12:131-136. [DOI] [PubMed] [Google Scholar]
- 15.Fekkar, A., B. Bodaghi, F. Touafek, P. Le Hoang, D. Mazier, and L. Paris. 2008. Comparison of immunoblotting, calculation of the Goldmann-Witmer coefficient, and real-time PCR using aqueous humor samples for diagnosis of ocular toxoplasmosis. J. Clin. Microbiol. 46:1965-1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Filisetti, D., M. Gorcii, E. Pernot-Marino, O. Villard, and E. Candolfi. 2003. Diagnosis of congenital toxoplasmosis: comparison of targets for detection of Toxoplasma gondii by PCR. J. Clin. Microbiol. 41:4826-4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guy, E. C., H. Pelloux, M. Lappalainen, H. Aspock, A. Hassl, K. K. Melby, M. Holberg-Pettersen, E. Petersen, J. Simon, and P. Ambroise-Thomas. 1996. Interlaboratory comparison of polymerase chain reaction for the detection of Toxoplasma gondii DNA added to samples of amniotic fluid. Eur. J. Clin. Microbiol. Infect. Dis. 15:836-839. [DOI] [PubMed] [Google Scholar]
- 18.Hierl, T., U. Reischl, P. Lang, H. Hebart, M. Stark, P. Kyme, and I. Autenrieth. 2004. Preliminary evaluation of one conventional nested and two real-time PCR assays for the detection of Toxoplasma gondii in immunocompromised patients. J. Med. Microbiol. 53:629-632. [DOI] [PubMed] [Google Scholar]
- 19.Hohlfeld, P., F. Daffos, J. M. Costa, P. Thulliez, F. Forestier, and M. Vidaud. 1994. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. N. Engl. J. Med. 331:695-699. [DOI] [PubMed] [Google Scholar]
- 20.Homan, W. L., M. Vercammen, J. De Braekeleer, and H. Verschueren. 2000. Identification of a 200- to 300-fold repetitive 529 bp DNA fragment in Toxoplasma gondii, and its use for diagnostic and quantitative PCR. Int. J. Parasitol. 30:69-75. [DOI] [PubMed] [Google Scholar]
- 21.Kaiser, K., A. M. Van Loon, H. Pelloux, J. Ferrandiz, S. Picot, P. Wallace, and F. Peyron. 2007. Multicenter proficiency study for detection of Toxoplasma gondii in amniotic fluid by nucleic acid amplification methods. Clin. Chim Acta 375:99-103. [DOI] [PubMed] [Google Scholar]
- 22.Lachaud, L., E. Chabbert, P. Dubessay, J. Reynes, J. Lamothe, and P. Bastien. 2001. Comparison of various sample preparation methods for PCR diagnosis of visceral leishmaniasis using peripheral blood. J. Clin. Microbiol. 39:613-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lachaud, L., S. Marchergui-Hammami, E. Chabbert, J. Dereure, J. Dedet, and P. Bastien. 2002. Comparison of six PCR methods using peripheral blood for detection of canine visceral leishmaniasis. J. Clin. Microbiol. 40:210-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menotti, J., Y. J. Garin, P. Thulliez, M. C. Serugue, J. Stanislawiak, P. Ribaud, N. de Castro, S. Houze, and F. Derouin. 2010. Evaluation of a new 5′-nuclease real-time PCR assay targeting the Toxoplasma gondii AF146527 genomic repeat. Clin. Microbiol. Infect. 16:363-368. [DOI] [PubMed] [Google Scholar]
- 25.Pelloux, H., E. Guy, M. C. Angelici, H. Aspock, M. H. Bessieres, R. Blatz, M. Del Pezzo, V. Girault, R. Gratzl, M. Holberg-Petersen, J. Johnson, D. Kruger, M. Lappalainen, A. Naessens, and M. Olsson. 1998. A second European collaborative study on polymerase chain reaction for Toxoplasma gondii, involving 15 teams. FEMS Microbiol. Lett. 165:231-237. [DOI] [PubMed] [Google Scholar]
- 26.Pelloux, H., J. Weiss, J. Simon, F. Muet, H. Fricker-Hidalgo, A. Goullier-Fleuret, and P. Ambroise-Thomas. 1996. A new set of primers for the detection of Toxoplasma gondii in amniotic fluid using polymerase chain reaction. FEMS Microbiol. Lett. 138:11-15. [DOI] [PubMed] [Google Scholar]
- 27.Reischl, U., S. Bretagne, D. Kruger, P. Ernault, and J. M. Costa. 2003. Comparison of two DNA targets for the diagnosis of toxoplasmosis by real-time PCR using fluorescence resonance energy transfer hybridization probes. BMC Infect. Dis. 3:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robert, F., T. Ouatas, P. Blanche, C. Tourte-Schaefer, D. Sicard, and J. Dupouy-Camet. 1996. Retrospective evaluation of the detection of Toxoplasma gondii by polymerase chain reaction in AIDS patients. Presse Med. 25:541-545. [In French.] [PubMed] [Google Scholar]
- 29.Robert-Gangneux, F., M. F. Gavinet, T. Ancelle, J. Raymond, C. Tourte-Schaefer, and J. Dupouy-Camet. 1999. Value of prenatal diagnosis and early postnatal diagnosis of congenital toxoplasmosis: retrospective study of 110 cases. J. Clin. Microbiol. 37:2893-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romand, S., M. Chosson, J. Franck, M. Wallon, F. Kieffer, K. Kaiser, H. Dumon, F. Peyron, P. Thulliez, and S. Picot. 2004. Usefulness of quantitative polymerase chain reaction in amniotic fluid as early prognostic marker of fetal infection with Toxoplasma gondii. Am. J. Obstet Gynecol. 190:797-802. [DOI] [PubMed] [Google Scholar]
- 31.Romand, S., M. Wallon, J. Franck, P. Thulliez, F. Peyron, and H. Dumon. 2001. Prenatal diagnosis using polymerase chain reaction on amniotic fluid for congenital toxoplasmosis. Obstet. Gynecol. 97:296-300. [DOI] [PubMed] [Google Scholar]
- 32.Sterkers, Y., E. Varlet-Marie, P. Marty, and P. Bastien et al. 2 November 2009, posting date. Diversity and evolution of methods and practices for the molecular diagnosis of congenital toxoplasmosis in France: a four years survey. Clin. Microbiol. Infect. [Epub ahead of print.] [DOI] [PubMed]
- 33.Talabani, H., M. Asseraf, H. Yera, E. Delair, T. Ancelle, P. Thulliez, A. P. Brezin, and J. Dupouy-Camet. 2009. Contributions of immunoblotting, real-time PCR, and the Goldmann-Witmer coefficient to diagnosis of atypical toxoplasmic retinochoroiditis. J. Clin. Microbiol. 47:2131-2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thalib, L., L. Gras, S. Romand, A. Prusa, M. H. Bessieres, E. Petersen, and R. E. Gilbert. 2005. Prediction of congenital toxoplasmosis by polymerase chain reaction analysis of amniotic fluid. BJOG 112:567-574. [DOI] [PubMed] [Google Scholar]
- 35.Thulliez, P. 1992. Screening programme for congenital toxoplasmosis in France. Scand. J. Infect. Dis. Suppl. 84:43-45. [PubMed] [Google Scholar]
- 36.Vidigal, P. V., D. V. Santos, F. C. Castro, J. C. Couto, R. W. Vitor, and G. Brasileiro Filho. 2002. Prenatal toxoplasmosis diagnosis from amniotic fluid by PCR. Rev. Soc. Bras. Med. Trop. 35:1-6. [DOI] [PubMed] [Google Scholar]
- 37.Wallon, M., L. Kodjikian, C. Binquet, J. Garweg, J. Fleury, C. Quantin, and F. Peyron. 2004. Long-term ocular prognosis in 327 children with congenital toxoplasmosis. Pediatrics 113:1567-1572. [DOI] [PubMed] [Google Scholar]
- 38.Yera, H., D. Filisetti, P. Bastien, T. Ancelle, P. Thulliez, and L. Delhaes. 2009. Multicentre comparative evaluation of five commercial methods for Toxoplasma DNA extraction from amniotic fluid. J. Clin. Microbiol. 47:3881-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.