Abstract
Mumps is a vaccine-preventable disease; however, outbreaks have been reported in a number of countries with childhood immunization programs, particularly among young adults at the tertiary stage of education. We have retrospectively investigated the epidemiological, virological, and serological factors associated with mumps cases identified in Ireland from 2004 to 2009. Genetic analysis of mumps virus strain variability demonstrated that a single genotype, genotype G, was circulating, and it was also detected in cerebrospinal fluid samples obtained from patients with meningitis. We observed that younger individuals were disproportionately affected with neurological sequelae following mumps virus infection, and the average age of patients with mumps virus RNA detected in cerebrospinal fluid was 19.25 years (median, 19 years; range, 14 to 24 years). Our analysis showed a 4-fold rise in mumps cases in 2008-2009 and an increased incidence in infection in those ≥30 years of age. Over a 6-year period (2004 to 2009), a total of 7,805 serum samples were investigated; of this number, 1,813 (23%) were positive for mumps virus-specific IgM. We observed a strong bias for acute mumps virus infection in males compared to females (P < 10−32) that was independent of vaccination status.
Mumps virus (MuV) is the etiological agent responsible for an acute viral infection which presents clinically with parotitis (usually unilateral in nature), a low-grade fever, headache, malaise, anorexia, rash, and abdominal pain (5). Symptoms are normally benign and not life threatening; however, complications necessitating hospitalization may occur following MuV infection in approximately 10% of cases (12). Complications include pancreatitis, orchitis, oophoritis, mastitis, and neurological involvement (meningoencephalitis), which can result in deafness and other severe neurological sequelae (13, 21). Invasion of the central nervous system (CNS) by MuV appears in >50% of patients with clinical mumps as evidenced by cerebrospinal fluid (CSF) pleocytosis; however, symptomatic CNS infection (aseptic meningitis) is much less frequent, occurring in <10% of cases, and encephalitis occurs in <1% of mumps cases (5). Interestingly, there is a gender bias for neurological manifestations, with males disproportionately affected (ca. 3:1 male/female ratio) (17, 18, 20).
MuV is an enveloped, nonsegmented, negative-sense, single-stranded RNA virus and is classified as a member of the family Paramyxoviridae, subfamily Paramyxovirinae and genus Rubulavirus. The MuV genome is a 15.3-kb RNA with seven contiguous transcription units, encoding the nucleocapsid-associated protein (NP), V protein (V)/phosphoprotein (P), matrix (M) protein, fusion (F) protein, small hydrophobic (SH) protein, hemagglutinin-neuraminidase (HN) protein, and large (L) protein. The SH gene encodes a small protein of 57 amino acids which is thought to inhibit tumor necrosis factor alpha (TNF-α)-mediated apoptosis in virally infected cells (28). The SH gene is the most variable region of the MuV genome and is employed in molecular epidemiology to assign genotypes to determine transmission patterns in populations (16), and 12 MuV genotypes (A to L) have been described to date (1, 16).
Routine childhood vaccination with measles-mumps-rubella (MMR) vaccine was introduced in the Republic of Ireland in 1988 for children between 12 to 15 months of age. In 1992, a second dose of the MMR vaccine (MMR2) was introduced for children 10 to 14 years of age; however, the age for MMR2 was subsequently reduced in 1999 to 4 to 5 years of age, following primary-school outbreaks (12). This resulted in a potentially partially protected cohort of individuals who received only one dose of the MMR vaccine (12, 27), and during recent outbreaks in the United States, mumps cases have occurred in individuals who had received two doses of MMR vaccine (10, 24). Data from the European Sero-Epidemiology Network (ESEN2) project in 2004 suggested that 80 to 85% of 15- to 24-year-olds in Ireland were immune to mumps, through either natural immunity or immunization (11). MMR immunization uptake is currently 92%; however, this is still below the World Health Organization-recommended target of 95% (11).
The aim of this study was to investigate the recent resurgence of mumps outbreaks in Ireland. Phylogenetic analysis revealed that only one genotype, genotype G (predominantly the G5 subgenotype), was circulating, and it was also detected in CSF samples obtained from patients with meningitis. This study showed a 4-fold increase in mumps cases in 2008-2009, with a significant male gender bias in addition to an increased incidence in the older age group (≥30 years old). Importantly, we observed a strong bias for acute mumps virus infection in males compared to females that was independent of vaccination status.
MATERIALS AND METHODS
Specimen collection.
During the study period (January 2004 to June 2009), specimens were collected from individuals with clinical presentations suggestive of mumps virus infection. Totals of 1,602 oral fluid samples and 7,805 serum samples were submitted to the National Virus Reference Laboratory (NVRL), University College Dublin (UCD) for laboratory confirmation of mumps virus infection. Ethical approval for the study was obtained from the Human Research Ethics Committee of University College Dublin.
Detection of MuV-specific IgG and IgM antibodies.
Oral fluid specimens were collected using OraCol devices (Malvern Medical Developments, United Kingdom), eluted into 1.5 ml of phosphate-buffered saline containing 0.5% Tween 20, and stored at −20°C. MuV-specific IgM was detected in both oral fluid and serum samples by using an IgM class capture enzyme immunoassay (Microimmune Ltd., United Kingdom). MuV-specific IgG was detected only in sera by using a commercial enzyme immunoassay (Captia, Trinity Biotech, Ireland). Assays were performed in accordance with the manufacturer's instructions.
Nucleic acid extraction, MuV real-time RT-PCR, and MuV genotyping.
Total nucleic acids were extracted from 140 μl of specimen with a QIAamp viral RNA minikit by following the manufacturer's instructions (Qiagen, Crawley, United Kingdom). The real-time reverse transcription-PCR (RT-PCR) was carried out using the MuV SH gene-targeting primers described by Boddicker and colleagues (4), employing the SuperScript One-Step RT-PCR system with Platinum Taq DNA polymerase (Invitrogen, Paisley, United Kingdom) with concentrations of 300 nM forward primer and 900 nM reverse primer and 100 nM concentrations of a 5′ Cy5-labeled mumps virus-specific TaqMan probe and 3′ black hole quencher (Metabion, Jena, Germany). rtPCR was performed on a TaqMan 7500SDS (Applied Biosystems, Warrington, United Kingdom) with the following cycling parameters: 50°C for 15 min, 95°C for 2 min, and then 40 cycles of 95°C for 15 min and 60°C for 1 min, with data acquisition in the annealing/extension phase. MuV genotyping was carried out by amplification of the SH gene from RT-PCR-positive RNA extracts as previously described (14).
Phylogenetic analysis.
MuV SH gene sequences were aligned with published reference sequences (16), and multiple sequence alignments were assembled in Clustal W (http://www.ebi.ac.uk/Tools/clustalw2/index.html). A model of evolution for maximum likelihood was selected in Modeltest (http://darwin.uvigo.es/software/modeltest.html). Maximum likelihood tree generation and bootstrap analysis were carried out using Paup* 4.0 beta (http://paup.csit.fsu.edu/downl.html) and the Clustal tool of the Megalign program in DNAStar.
Statistical analysis.
Chi-square analysis was performed to compare the numbers of samples in male and female cohorts positive for mumps virus-specific IgG and IgM.
Nucleotide sequence accession numbers.
The GenBank accession numbers assigned for this study were GU937425 (strain MuVs/Louth/IRL/36.06), GU937426 (strain MuVs/Dublin/IRL/41.06), GU937427 (strain MuVs/Cork/IRL/48.08), and GU937428 (strain MuVcsf/Sligo/IRL/16.09).
RESULTS
Serological analysis.
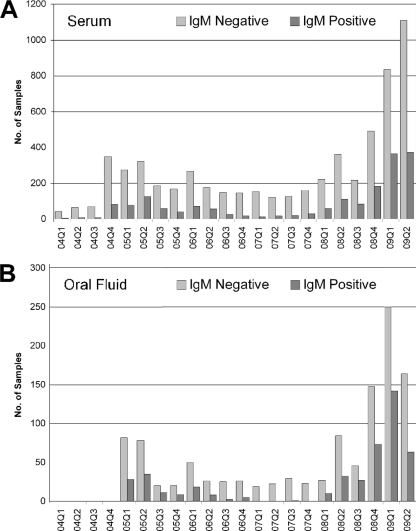
During the study period (January 2004 to June 2009), 1,602 oral fluid samples and 7,805 serum samples received were tested and analyzed. Quarterly analysis showed the occurrence of outbreaks of differing severities spanning from the end of 2004 to the middle of 2006, and the last outbreak was from 2008 to the middle of 2009, according to clinical diagnosis. The total numbers of serum samples received per quarter and the numbers positive for mumps virus-specific IgM are shown in Fig. 1 A. A total of 7,805 serum samples were tested; of this number, 1,813 (23%) were positive for mumps virus-specific IgM. The total numbers of samples tested and the actual numbers of IgM-positive cases were approximately 4-fold higher in the recent outbreak. However, the percentages of positive samples were similar in the two outbreaks (range from 2004 to 2006, 18.7 to 27.9%; range in 2008-2009, 21.1 to 30.4%).
FIG. 1.
Quarterly analysis of numbers of samples demonstrating mumps virus-specific-IgM-negative and -positive results. Panel A shows data for serum samples, and panel B shows data for oral fluid samples received for laboratory testing. Q1 through Q4 indicate the first through fourth quarters of the years 2004 to 2009.
Of the 1,602 oral fluid samples received, 464 (29%) samples were positive for mumps virus-specific IgM. The quarterly breakdown is shown in Fig. 1B, and although the numbers of positive samples are greater in the more-recent outbreak, the rates of positivity were similar (range from 2004 to 2006, 23.5 to 35.5%; range in 2008-2009, 27 to 37%) to the results of serum samples tested.
Quarterly analysis over the study period (2004 to 2009) of a total of 1,414 sera (904 from males and 510 from females) which were positive for mumps virus-specific IgM revealed the presence of mumps virus-specific IgG as well (range, 94.1 to 100%). In the 2008-2009 outbreak, 847 serum samples showed 97.2% positivity for both mumps virus-specific IgG and IgM antibodies.
Impact of gender on the number of laboratory-confirmed cases positive for mumps virus-specific IgM.
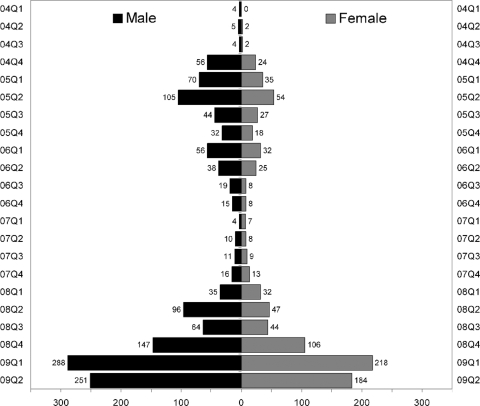
The total numbers of samples received from males (4,559) and females (4,649) were similar. Gender was not included on 199 sample forms, and these cases were excluded from this analysis. Chi-square analysis performed on the 2,273 mumps virus-specific-IgM-positive samples from males (1,370 positives) and females (903 positives) showed a significantly predominant positivity among males throughout the study period (P = 10−32) (Fig. 2).
FIG. 2.
Observed gender-specific bias in mumps virus-specific IgM serology for males. Quarterly data and numbers positive are shown for each study period analyzed.
Age profile of mumps virus-specific-IgM-positive individuals.
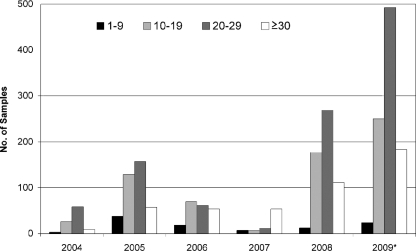
In each year, the incidence of mumps was predominantly restricted to three age groups: 15 to 19, 20 to 24, and 25 to 29 years. Figure 3 shows the age profile of patients with laboratory-confirmed mumps by calendar year. Even though the figures for 2009 include only the first half of the year, there is a higher incidence of mumps in individuals ≥30 years of age in the most recent outbreak (66/485 [13.6%] individuals for 2004-2005, compared to 294/1,118 [26.3%] individuals for 2008-2009).
FIG. 3.
Patient age profile of laboratory-confirmed mumps virus-specific-IgM-positive samples analyzed on a yearly basis. An increase in the ≥30-year-old age group is evident in this population. *, it should be noted that the figures for 2009 are for quarters 1 and 2 only.
Molecular epidemiology of MuV in Ireland 2006 to 2009.
In order to genotype the strains of MuV circulating in Ireland from 2006 to 2009, we initially tested by RT-PCR mumps virus isolated in fetal rhesus kidney (FRK) cell monolayers (n = 15) from oral fluid specimens positive for mumps virus-specific IgM. The median threshold cycle (CT) value of these isolates was 23.71 (mean, 22.81; range, 17.03 to 28.16). Subsequent screening of mumps virus-specific-IgM-positive oral fluid specimens by RT-PCR found that approximately one-third, 13 of 37 (35%), were positive for mumps virus RNA. The median CT value of these 13 positives was 32.50 (mean, 32.75; range, 25.17 to 38.45).
The mumps virus RT-PCR assay also detected mumps virus RNA in 12 of 27 CSF specimens (44% positivity). The median CT was 35.98 (mean, 35.89; range, 31.6 to 39.6), and no strong gender bias was observed corresponding to CSF specimens positive for MuV RNA (seven males and five females). The average age of patients with MuV RNA in CSF was 19.25 years (median, 19 years; range, 14 to 24 years). No herpesvirus DNA (herpes simplex virus type 1 [HSV-1], HSV-2, varicella-zoster virus [VZV], or human herpesvirus 6 [HHV-6]) or enterovirus RNA was found in the CSF specimens collected from these patients who presented with neurological symptoms.
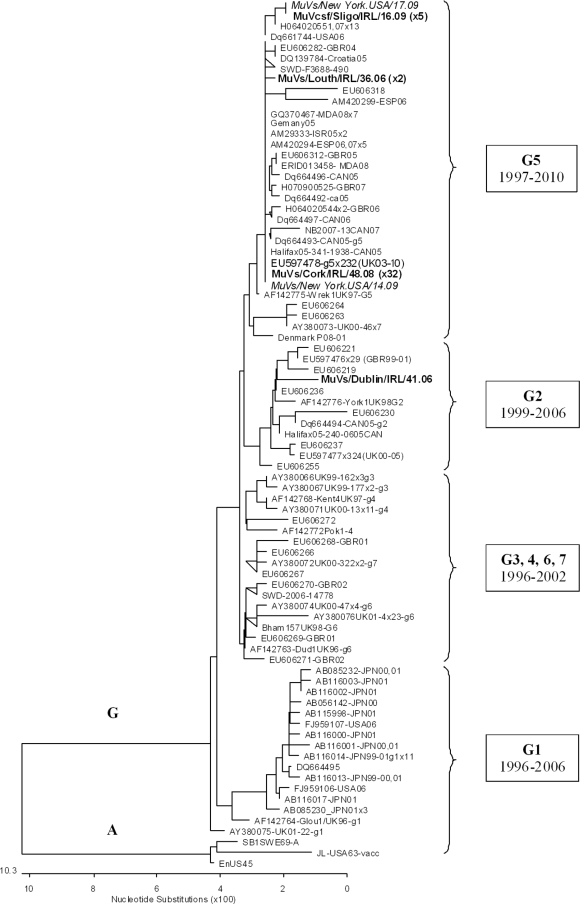
A phylogenetic analysis of circulating MuV strains in Ireland (n = 40) from 2006 to 2009, comprising MuV-positive culture, oral fluid, and CSF specimens, is shown in Fig. 4. All cases segregated with MuV genotype G and specifically genotype G5 in all cases except for a single 2006 specimen (MuVs/Dublin/IRL/41.06), which was identified as genotype G2 per the nomenclature in reference 15. During the study period, mumps virus infection was identified in two U.S. adolescents in New York who had a history of recent travel to Ireland. Figure 4 shows that these two cases (MuVs/New York.USA/14.09 and MuVs/New York.USA/17.09) were 100% identical in the MuV SH gene to the MuV genotype G5 strains circulating in Ireland before importation to the United States. This MuV genotype G5 strain (exemplified by GenBank accession number EU597478) was also frequently detected in the United Kingdom since 2003 (8) and some other countries, such as Canada, Germany, Spain, Israel, Moldova, and Australia. Strains MuVs/Louth/IRL/36.06 and MuVs/Louth/IRL/40.06 were 100% identical over the SH gene, with a single point mutation compared to all publicly available MuV SH genes and the predominant MuV G5 strain (EU597478) which interestingly leads to a N-terminal mutation in the SH protein (Y9D).
FIG. 4.
Phylogenetic analysis of MuV in Ireland from 2006 to 2009. MuV SH genes were either amplified directly from clinical specimens (oral fluids/saliva [s] or cerebrospinal fluid [csf]) or first propagated in FRK monolayer isolates (i). GenBank and Irish MuV SH gene sequences are named by virus and sample type/place/country/week.year. The tree depicts the relationship of Irish MuV SH gene genotype G (316 nucleotides [nt]) sequences to MuV sequences identified internationally. The scale bar indicates nucleotide substitutions per site, and MuV genotype A sequences were employed as outgroups. The transition of MuV genotype G variants over time is indicated. The 40 sequences analyzed in this study are represented on the tree by four unique MuV sequences, namely, MuVs/Louth/IRL/36.06 (2 strains), MuVs/Dublin/IRL/41.06, MuVs/Cork/IRL/48.08 (2006 to 2009; 32 strains), and MuVcsf/Sligo/IRL/16.09 (2007 to 2009; 5 strains). The two New York cases (MuVs/New York.USA/14.09 and MuVs/New York.USA/17.09) involving a history of recent travel to Ireland are shown in italics. The number of identical sequences is shown in parentheses beside each of the unique sequences.
DISCUSSION
The 2008-2009 mumps outbreak in Ireland has been the largest since the introduction of the MMR childhood vaccination schedule in 1988. Prior to the introduction of MMR vaccination in Ireland, mumps occurred mainly in children between the ages of 5 and 15 years. The epidemiological picture of mumps from 2004 to 2009, however, now shows that the majority of mumps cases occur in young adults, with those in the 18- to 24-year-old age category most affected in the recent mumps outbreaks. This is consistent with the findings in other studies showing an upsurge of cases in tertiary colleges (27).
The age profiles of the predominantly affected group in our study were young adults (18 to 24 years) in university or college. This contrasts with the case in the United States, where a shift in peak age-specific attack rates from 5 to 9 years to 18 to 24 years and then back has been observed (3). We also noted a dramatic increase in the ≥30-year-old age group presenting with mumps virus infection. The reasons for this finding remain unclear and may include incomplete vaccination, as mumps virus-specific IgG was detectable, increase in overseas travel, and attendance at mass gatherings similar to the crowded conditions existing at colleges.
Analysis of the ratio of males to females with mumps cases in Ireland from 2004 to 2009 revealed a clear gender bias of MuV-specific-IgM-positive results in males compared to females. Similar numbers of samples from males and females were submitted over this period of time for IgM testing, and males and females were drawn from the same population, so this bias was unexpected. Both the numbers of samples that were mumps virus-specific IgG positive and the age distributions were similar between males and females, and there is no reason to suggest that the uptake of vaccination would be greater in females than in males to account for an increased susceptibility of males. A recent study of mumps epidemiology in the midwest of Ireland suggested that the authors' observation of a 1.4:1 male-to-female ratio for infection could reflect patterns of attendance at tertiary colleges where the majority of outbreak cases have been identified (27). However, the Central Statistics Office estimates that 61% of 19-year-olds in tertiary education in Ireland are female, suggesting that it would be more likely for females rather than males to test positive for mumps virus-specific IgM in a year. The male gender bias observed in the current study is not likely to be solely due to behavioral patterns, as observed in a recent study in Jerusalem, where mainly male adolescents were affected because the mumps outbreaks occurred in religious boarding schools which were attended by males only (25). In our study, there was no strong gender bias observed related to the 12 CSF specimens positive for MuV RNA, of which 7 came from males. In vitro studies of human lymphoid cells have shown gender differences in immune function, and also gender-based differences in humoral immunity have been noted with several vaccines, including those for influenza, hepatitis A, and measles, and this may be extrapolated to natural infection (6, 7).
The dramatic rise in the numbers of mumps cases in Ireland and elsewhere has resulted in increasing demands to confirm mumps virus infection in a timely manner, particularly in CSF specimens from patients with neurological symptoms, and this necessitates the implementation of molecular testing. Traditional MuV detection methods by culture are insensitive, costly, and time-consuming. Early serological diagnosis often relies on detection of anti-mumps virus IgM, which may be absent in the first 7 to 10 days of illness and has a low positive predictive value for CSF specimens. The relatively low percentage of oral fluid positive for IgM which was detected by the RT-PCR (∼35%) was not unexpected, as the presence of viral nucleic acid is largely dependent on the time of sampling and/or storage of the specimen. Indeed, the results of this study are in agreement with a previous study from this laboratory which showed that 6 of 17 IgM-positive oral fluid specimens, including both weak (6%) and strong (29%) positives, had detectable MuV RNA (∼35%) (22). MuV RNA was detected in ∼44% of CSF specimens tested, and no herpesvirus DNA (HSV-1, HSV-2, VZV, or HHV-6) or enterovirus RNA was found in these specimens from patients with neurological symptoms. A large study from the United Kingdom (19) analyzed 88 CSF specimens and detected five cases of meningitis (5.7%) by using the real-time method of Uchida and coworkers (26) which targets the highly conserved F gene in the MuV genome. The assay employed in the present study targets the SH gene (4), and the MuV CSF positivity of 44% compares well to the findings described above. It should also be noted that the study by Krause et al. (19) reported CT values in the range of 35.8 to 43.7, which is marginally higher than that from the SH gene assay in our study (CT range, 31.6 to 39.6); however, direct comparison is problematic in the absence of a side-by-side evaluation of the available real-time methodologies for MuV detection.
The Jeryl Lynn vaccine strain consists of two distinct viral isolates (JL-2 and JL-5) belonging to genotype A, while the mumps virus strain responsible for the outbreaks in Ireland over the period 2006 to 2009 belongs to genotype G. Previous phylogenetic analysis of circulating mumps virus strains in 2004 and 2005 revealed circulation of mixed genotypes of types G and J (22). It appears that genotype G is now endemic in Ireland, with sustained indigenous transmission occurring, as genotype G was identified in the 42 saliva, culture fluid, and CSF samples, including the two U.S. mumps cases which were associated with a history of recent travel to Ireland. No difference in genotype was noted between the CSF samples submitted from patients with aseptic meningitis and the saliva and culture fluid samples, indicating that the meningitis cases seen in the 2009 outbreak were not attributable to a different mumps virus genotype.
In response to the recent resurgence in mumps outbreaks in Ireland, a national outbreak control team was convened in early 2009. They have recommended reinforcing the current control measures and adopting additional active interventions, both regionally and nationally, in an attempt to control the future transmission of mumps at secondary schools and third-level colleges. An additional dose of MMR vaccine was offered to schoolgoing students 16 to 19 years old in the spring, and this has also been implemented in the United States (9). Uptake rates of 97% were achieved, and notification of mumps cases declined from July 2009 onwards, most probably due to a combination of factors, including the end of the academic year in addition to the booster vaccine.
The mumps virus strains responsible for the outbreaks in Ireland, the United Kingdom, Canada, and the United States belong to genotypes phylogenetically distinct from the vaccine strains employed in these countries. Despite the fact that the Jeryl Lynn vaccine (genotype A) has been shown to be effective in outbreaks caused by MuV belonging to genotype G, more-intensive assessments of mumps outbreaks should potentially include IgG avidity testing as well as a comprehensive assessment of the functional antibody response in order to ascertain the ability of serum antibody to neutralize both the vaccine virus and the endemic challenge virus strain (2, 10).
The first genotype G strain identified in 1996 (Glou1/UK96, GenBank accession number AF142764), later named G1, was not detected in this study; however, numerous genotype G variants have been detected since 2000 (8, 23), and the subgenotype genetic diversity can be up to 7%. Most of the G1 variants were detected in Japan during 2000-2001 and again in the United States in 2006; the only G2 strain found in Ireland, MuVs/Dublin/IRL/41.06, was genetically distinguishable from strain EU597477, which was detected in over 300 cases in the United Kingdom from 2000 to 2005. Some of the G5 variants, e.g., EU606282, detected in the United Kingdom in 2004 were detected in Croatia in 2005 (DQ139784); EU606312 was detected in the United Kingdom in 2005 and subsequently detected in Moldova in 2008 with 100% identity in the SH gene, although without any confirmed transmission link. Interestingly, strain EU597478, the predominantly circulating strain in the United Kingdom since 2003, has been found in many other countries, including Ireland, and is mostly linked to the mumps endemic in the United Kingdom. The viral fitness and replication capacity of these strains need to be ascertained. In the meantime, it is important that surveillance of genotype distribution is continued in Ireland in order to fully understand the epidemiology of mumps virus infection.
Acknowledgments
We are grateful to Joanne Moran (NVRL, UCD) for mumps surveillance data, Paul O'Reilly (NVRL, UCD) for isolation of mumps virus in FRK monolayers, and Paul Rota, Centers for Disease Control and Prevention, Atlanta, GA, for providing information on the two New York mumps cases.
Footnotes
Published ahead of print on 21 July 2010.
REFERENCES
- 1.Afzal, M. A., J. Buchanan, A. B. Heath, and P. D. Minor. 1997. Clustering of mumps virus isolates by SH gene sequence only partially reflects geographical origin. Arch. Virol. 142:227-238. [DOI] [PubMed] [Google Scholar]
- 2.Atrasheuskaya, A. V., E. M. Blatun, M. V. Kulak, A. Atrasheuskaya, I. A. Karpov, S. Rubin, and G. M. Ignatyev. 2007. Investigation of mumps vaccine failures in Minsk, Belarus, 2001-2003. Vaccine 25:4651-4658. [DOI] [PubMed] [Google Scholar]
- 3.Barskey, A. E., J. W. Glasser, and C. W. LeBaron. 2009. Mumps resurgences in the United States: a historical perspective on unexpected elements. Vaccine 27:6186-6195. [DOI] [PubMed] [Google Scholar]
- 4.Boddicker, J. D., P. A. Rota, T. Kreman, A. Wangeman, L. Lowe, K. B. Hummel, R. Thompson, W. J. Bellini, M. Pentella, and L. E. Desjardin. 2007. Real-time reverse transcription-PCR assay for detection of mumps virus RNA in clinical specimens. J. Clin. Microbiol. 45:2902-2908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carbone, K. M., and S. Rubin. 2007. Mumps virus, p. 1527-1550. In D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology, 5th ed. Lippincott-Raven, Philadelphia, PA.
- 6.Casimir, G. J., S. Mulier, L. Hanssens, K. Zylberberg, and J. Duchateau. 2010. Gender differences in inflammatory markers in children. Shock 33:258-262. [DOI] [PubMed] [Google Scholar]
- 7.Cook, I. F. 2008. Sexual dimorphism of humoral immunity with human vaccines. Vaccine 26:3551-3555. [DOI] [PubMed] [Google Scholar]
- 8.Cui, A., R. Myers, W. Xu, and L. Jin. 2009. Analysis of the genetic variability of the mumps SH gene in viruses circulating in the UK between 1996 and 2005. Infect. Genet. Evol. 9:71-80. [DOI] [PubMed] [Google Scholar]
- 9.Date, A. A., M. H. Kyaw, A. M. Rue, J. Klahn, L. Obrecht, T. Krohn, J. Rowland, S. Rubin, T. J. Safranek, W. J. Bellini, and G. H. Dayan. 2008. Long-term persistence of mumps antibody after receipt of 2 measles-mumps-rubella (MMR) vaccinations and antibody response after a third MMR vaccination among a university population. J. Infect. Dis. 197:1662-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dayan, G. H., M. P. Quinlisk, A. A. Parker, A. E. Barskey, M. L. Harris, J. M. Schwartz, K. Hunt, C. G. Finley, D. P. Leschinsky, A. L. O'Keefe, J. Clayton, L. K. Kightlinger, E. G. Dietle, J. Berg, C. L. Kenyon, S. T. Goldstein, S. K. Stokley, S. B. Redd, P. A. Rota, J. Rota, D. Bi, S. W. Roush, C. B. Bridges, T. A. Santibanez, U. Parashar, W. J. Bellini, and J. F. Seward. 2008. Recent resurgence of mumps in the United States. N. Engl. J. Med. 358:1580-1589. [DOI] [PubMed] [Google Scholar]
- 11.Di Renzi, M., S. Jackson, S. Gee, and S. Cotter. 2004. Increase in mumps in Ireland in late 2004. Euro Surveill. 8:pii=2608. [Google Scholar]
- 12.Gee, S., D. O'Flanagan, M. Fitzgerald, and S. Cotter. 2008. Mumps in Ireland, 2004-2008. Euro Surveill. 13:pii=18857. [DOI] [PubMed] [Google Scholar]
- 13.Hall, R., and H. Richards. 1987. Hearing loss due to mumps. Arch. Dis. Child. 62:189-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin, L., S. Beard, and D. W. Brown. 1999. Genetic heterogeneity of mumps virus in the United Kingdom: identification of two new genotypes. J. Infect. Dis. 180:829-833. [DOI] [PubMed] [Google Scholar]
- 15.Jin, L., D. W. Brown, P. A. Litton, and J. M. White. 2004. Genetic diversity of mumps virus in oral fluid specimens: application to mumps epidemiological study. J. Infect. Dis. 189:1001-1008. [DOI] [PubMed] [Google Scholar]
- 16.Jin, L., B. Rima, D. Brown, C. Orvell, T. Tecle, M. Afzal, K. Uchida, T. Nakayama, S. W. Song, C. Kang, P. A. Rota, W. Xu, and D. Featherstone. 2005. Proposal for genetic characterisation of wild-type mumps strains: preliminary standardisation of the nomenclature. Arch. Virol. 150:1903-1909. [DOI] [PubMed] [Google Scholar]
- 17.Kanra, G., P. Isik, A. Kara, A. B. Cengiz, G. Seçmeer, and M. Ceyhan. 2004. Complementary findings in clinical and epidemiologic features of mumps and mumps meningoencephalitis in children without mumps vaccination. Pediatr. Int. 46:663-668. [DOI] [PubMed] [Google Scholar]
- 18.Koskiniemi, M., M. Donner, and O. Pettay. 1983. Clinical appearance and outcome in mumps encephalitis in children. Acta Paediatr. Scand. 72:603-609. [DOI] [PubMed] [Google Scholar]
- 19.Krause, C. H., K. Eastick, and M. M. Ogilvie. 2006. Real-time PCR for mumps diagnosis on clinical specimens—comparison with results of conventional methods of virus detection and nested PCR. J. Clin. Virol. 37:184-189. [DOI] [PubMed] [Google Scholar]
- 20.Leboreiro-Fernandez, A., M. V. Moura-Ribeiro, I. E. Leboreiro, F. M. Sawan, A. R. Ventura, and K. C. Barbosa. 1997. Mumps meningoencephalitis. An epidemiological approach. Arq. Neuropsiquiatr. 55:12-15. [DOI] [PubMed] [Google Scholar]
- 21.Nussinovitch, M., B. Volovitz, and I. Varsano. 1995. Complications of mumps requiring hospitalization in children. Eur. J. Pediatr. 154:732-734. [DOI] [PubMed] [Google Scholar]
- 22.Reid, F., J. Hassan, F. Irwin, A. Waters, W. Hall, and J. Connell. 2008. Epidemiologic and diagnostic evaluation of a recent mumps outbreak using oral fluid samples. J. Clin. Virol. 41:134-137. [DOI] [PubMed] [Google Scholar]
- 23.Rota, J. S., J. C. Turner, M. K. Yost-Daljev, M. Freeman, D. M. Toney, E. Meisel, N. Williams, S. B. Sowers, L. Lowe, P. A. Rota, L. A. Nicolai, L. Peake, and W. J. Bellini. 2009. Investigation of a mumps outbreak among university students with two measles-mumps-rubella (MMR) vaccinations, Virginia, September-December. J. Med. Virol. 81:1819-1825. [DOI] [PubMed] [Google Scholar]
- 24.Savage, E., M. Ramsay, J. White, S. Beard, H. Lawson, R. Hunjan, and D. Brown. 2005. Mumps outbreaks across England and Wales in 2004: observational study. BMJ 330:1119-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stein-Zamir, C., H. Shoob, N. Abramson, E. Tallen-Gozani, I. Sokolov, and G. Zentner. 2009. Mumps outbreak in Jerusalem affecting mainly male adolescents. Euro Surveill. 14:pii=19440. [PubMed] [Google Scholar]
- 26.Uchida, K., M. Shinohara, S. Shimada, Y. Segawa, R. Doi, A. Gotoh, and R. Hondo. 2005. Rapid and sensitive detection of mumps virus RNA directly from clinical samples by real-time PCR. J. Med. Virol. 75:470-474. [DOI] [PubMed] [Google Scholar]
- 27.Whyte, D., F. O'Dea, C. McDonnell, N. H. O'Connell, S. Callinan, E. Brosnan, J. Powell, R. Monahan, R. FitzGerald, M. Mannix, T. Greally, A. Dee, and P. O'Sullivan. 2009. Mumps epidemiology in the mid-west of Ireland 2004-2008: increasing disease burden in the university/college setting. Euro Surveill. 14:pii=19182. [PubMed] [Google Scholar]
- 28.Wilson, R. L., S. M. Fuentes, P. Wang, E. C. Taddeo, A. Klatt, A. J. Henderson, and B. He. 2006. Function of small hydrophobic proteins of paramyxovirus. J. Virol. 80:1700-1709. [DOI] [PMC free article] [PubMed] [Google Scholar]