Abstract
During assembly and budding of retroviruses, host cell proteins are incorporated into viral particles. Identification of virion-associated proteins may help pinpoint key cellular components required for virus production and function. The cellular protein annexin 2 (Anx2) is incorporated into HIV-1 particles, and knockdown of Anx2 has been reported to cause defects in Gag processing and infectivity of HIV-1 particles in macrophages. Here, we tested whether Anx2 was required for HIV-1 production in other cell types capable of producing HIV-1 virions. Endogenous Anx2 levels were knocked down by ∼98% using lentivirus encoding short hairpin RNAs (shRNAs) or small interfering RNAs (siRNAs) targeting Anx2. Under these conditions, there was no reduction in HIV-1 virus-like particle (VLP) production in either COS-1, 293T, or Jurkat T cells or primary human monocyte-derived macrophages (MDMs). Murine embryonic fibroblasts derived from Anx2−/− mice produced the same levels of VLPs as matched cells from wild-type mice. The calcium-mediated spike in VLP production still occurred in Anx2-depleted COS-1 cells, and there was no apparent alteration in the intracellular Gag localization. Overexpression of Anx2 in trans had no effect on Gag processing or VLP production. Neither Anx2 depletion nor Anx2 overexpression altered the infectivity of HIV-1 particles produced by COS-1 or 293T cells. However, supernatants containing virus from Anx2 siRNA-treated primary human MDMs exhibited decreased infectivity. These data indicate that Anx2 is not required for HIV-1 assembly or Gag processing but rather plays a cell type-dependent role in regulating production of infectious HIV-1 by macrophages.
The Gag polyprotein generates the key structural proteins for all retroviruses. Gag is necessary and sufficient for the formation of virus-like particles (VLPs), which are morphologically similar to immature virions. Following its synthesis in the cytoplasm, HIV-1 Gag is trafficked to sites of particle production on membranes. Viral particle production depends on Gag-membrane interactions mediated by the myristoylated MA domain of Gag (18, 22, 31) and Gag-Gag interactions mediated by the CA and NC domains. Budding and release of the new virion are mediated by the Gag p6 domain. For successful particle production to occur, HIV-1 Gag must also interact with numerous host cell proteins and protein complexes. Identification of these interactions provides a crucial window into determining Gag trafficking intermediates as well as clues to the mechanism of virion production.
The host cell protein annexin 2 (Anx2) has recently attracted attention for its potential to regulate key processes in both cells and viruses (9, 14, 17, 24). Anx2 belongs to a family of conserved calcium-regulated proteins and interacts with actin, membranes, and negatively charged phospholipids. The major protein binding partner for Anx2 is p11, also known as S100A10. Two populations of Anx2 have been identified: a heterotetrameric complex with two molecules of Anx2 and two molecules of p11 (found predominantly at the plasma membrane) and a monomeric form found mainly in the cytoplasm. Anx2 performs multiple functions in the cell, including regulation of actin-based dynamics, fibrinolysis, calcium-mediated exocytosis, and transport of intermediates from early to late endosomes (10, 14-16) Anx2 also enhances binding and fusion of cytomegalovirus with phospholipid membranes (21). In addition, Anx2 can be detected within influenza virus particles (28), where it has been shown to aid in virus replication (9).
Several lines of evidence suggest that Anx2 may play a role in HIV-1 biogenesis. Both Anx2 and its binding partner p11 are incorporated in HIV-1 particles produced by macrophages (2). Anx2 interacts with Gag in macrophages, and annexin 2 knockdown has been reported to cause defective Gag processing and reduced infectivity of the released particles (24). Blockade of Anx2 function, with either anti-Anx2 antibody or small interfering RNA (siRNA)-mediated knockdown, results in suppression of HIV-1 infection in macrophages (11). Anx2 also binds to Gag in 293T cells, and expression of Anx2 in trans in these cells has been reported to lead to increased Gag processing and HIV-1 production (7). Taken together, these findings suggest that Anx2 might play a universal role in Gag trafficking and particle production. To test this hypothesis, we exploited methods to efficiently knock down Anx2 expression and determined the effect of Anx2 knockdown in a variety of cell lines capable of producing HIV-1 virions. Here we show that, in the absence of Anx2 expression, HIV-1 Gag is expressed, trafficked, and capable of mediating viral particle formation in a manner similar to that of control cells expressing Anx2. However, a cell type-dependent effect of Anx2 depletion on HIV-1 infectivity was detected in primary human monocyte-derived macrophages (MDMs). These findings suggest that Anx2 might be a macrophage-specific host cell factor that regulates HIV-1 infectivity.
MATERIALS AND METHODS
Plasmids and cells.
A plasmid encoding a Gag-green fluorescent protein (GFP) fusion, pCMV5Gag, and pHXB2ΔBalD25S have been described previously (12, 20). Plasmids encoding Anx2 and p11 were gifts from Kathryn Hajjar (Weill Cornell Medical College, New York, NY) (8). Plasmids encoding short hairpin RNA (shRNA) sequences for Anx2 cloned into the pLKO.1 vector were obtained from Open Biosystems (Huntsville, AL). The pHRD8.2 and pCMV-VSV-G plasmids were gifts from Filippo Giancotti (MSKCC, New York, NY). The COS-1, COS-7, HEK293, HEK293T, U937, and Jurkat cell lines were obtained from ATCC. Primary annexin 2-null mouse embryonic fibroblasts (MEFs) (C57BL/6) were obtained from Kathryn Hajjar. Matching wild-type control primary MEFs (C57BL/6) were obtained from ATCC.
Antibodies and reagents.
Anti-Anx2 (catalog no. 610069), anti-p11 (catalog no. 610070), anti-CD63 (catalog no. 556019), anti-EEA1 (catalog no. 610456), and anti-actin (catalog no. 612656) antibodies were obtained from BD Biosciences (San Jose, CA). Anti-Gag antibodies were obtained from the NIH AIDS Research and Reference Reagent program (catalog no. 6458) or generated in the lab and described previously (12). Anti-epidermal growth factor receptor (EGFR) antibody (catalog no. sc-03) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Alexa fluor 594-conjugated goat anti-mouse antibody (catalog no. A-11005), DAPI (4′,6-diamidino-2-phenylindole; catalog no. H3569), and Prolong Gold (catalog no. P36930) were obtained from Invitrogen (Carlsbad, CA). EGF (catalog no. 324831) was purchased from Calbiochem (San Diego, CA) and reconstituted according to the manufacturer's protocol. Ionomycin (catalog no. 13909) and cycloheximide (catalog no. 01810) were obtained from Sigma-Aldrich (St. Louis, MO).
Knockdown of Anx2.
Endogenous Anx2 was knocked down using shRNA delivered to cells via a lentiviral system. The targeting sequences for Anx2 shRNA and the control scrambled shRNA are CGGGATGCTTTGAACATTGAA and CCTAAGGTTAAGTCGCCCTCG, respectively. The shRNA-expressing lentiviruses were produced by cotransfecting confluent 293T cells in 15-cm plates with the pLKO.1 shRNA plasmid, HIV packaging vector pHRD8.2, and pcDNA3.1VSV-G. Viruses were harvested at 48 and 72 h. Transduction of cells with lentiviruses was carried out in the presence of 6 μg/ml Polybrene. Stable cells were produced by transducing target cells with either control scrambled or Anx2 shRNA-expressing lentiviruses, followed by selection in puromycin. Puromycin concentrations of 4 μg/ml and 1 μg/ml were used for COS-1 and Jurkat cells, respectively. HEK293T cells could not be stably transduced with Anx2 shRNA-expressing lentivirus; therefore, transient transduction experiments were carried out in these cells.
Immunofluorescence.
COS-1 cells stably transduced with lentiviruses expressing Anx2 or scrambled shRNA were transfected with a plasmid encoding Gag-GFP in 10-cm plates. The next day, the cells were seeded onto glass coverslips in six-well plates at a density of 2 × 105 cells/well. Transfected cells were fixed with 4% paraformaldyde and incubated with anti-EEA1 or anti-CD63 antibodies at a 1:250 dilution in 3% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) for 45 min and then washed. Cells were then incubated with Alexa fluor 594 goat anti-mouse antibody (1:500) and DAPI (1:10,000) for 30 min, washed, and mounted on slides using Prolong Gold. For cells stained for Anx2, the cells were fixed with methanol instead of paraformaldehyde; all other steps were the same.
VLP assays.
VLPs were harvested from COS-1, 293T, and MEFs as follows. Briefly, COS-1 cells were seeded in six-well plates at a density of 4 × 105 cells/well and were transfected with 2 to 3 μg of pHXB2ΔBalD25S cDNA, using Lipofectamine 2000. Media and cells were collected at 24 h posttransfection. The medium was passed through a 0.45-μm filter, layered on top of a 20% sucrose gradient, and then centrifuged at 34,500 rpm for 2 h. The VLP pellet was resuspended in 1× radioimmunoprecipitation assay (RIPA) buffer (150 mM NaCl, 1% NP-40, 0.5% Na deoxycholate, 1 mM EDTA, 50 mM Tris Cl, pH 7.4). Jurkat cells were transfected as follows. A total of 4 × 106 cells were electroporated (BTX Electro Cell Manipulator 600) with 10 μg of the pHXB2ΔBalD25S plasmid. Media and cells were harvested at 48 h posttransfection, and VLPs were harvested as described above. Relative VLP production = Gag in VLPs/(Gag in VLPs + Gag in cell lysates).
Calcium and ionomycin treatment.
Stimulation of cells with calcium and ionomycin was performed as described previously (20). Briefly, COS-1 cells stably transduced with lentiviruses expressing Anx2 or scrambled shRNA were plated in six-well plates at a density of 4 × 105 cells/well and were transfected with pHXB2ΔBalD25S. The next day, the culture medium was removed and cells were treated with or without medium containing 10 μM ionomycin and 3 mM CaCl2 for 5 min. Cells were washed with PBS, and medium was replaced. Cells and VLPs were harvested at 1 h following treatment, and levels of Gag were analyzed by Western blotting.
EGFR experiments.
COS-1 cells stably transduced with lentiviruses expressing Anx2 or scrambled shRNA were plated in six-well plates at a density of 5 × 105 cells/well. Cells were serum starved overnight. The following morning, cells were treated with medium containing either EGF (20 ng/ml) and cycloheximide (10 μM) or medium containing cycloheximide alone. Treated cells were harvested at 0, 1, and 4 h following treatment. Cells were lysed in RIPA buffer, and EGFR was analyzed by Western blotting.
Isolation and transfection of primary monocyte-derived macrophages.
Primary human monocyte-derived macrophages (MDMs) were isolated as follows. Peripheral blood mononuclear cells (PBMCs) were harvested from leukocytes (obtained from the New York Blood Center) using Ficoll gradient separation (Ficoll-Paque Plus; GE) according to the manufacturer's protocol. PBMCs were plated in six-well plates at a density of 30 × 106 cells/well. Nonadherent cells were washed off, and adherent monocytes were differentiated in culture for ∼14 days in the presence of Dulbecco's modified Eagle's medium (DMEM) containing 5% GCTCM (giant cell tumor conditioned medium; Irvine Scientific, CA) and 5% fetal bovine serum (FBS) as described previously (24). Differentiated macrophages were treated with 200 nM siRNA targeting Anx2 as described before (24) or β-galactosidase (β-Gal) using Lipofectamine 2000. One week after siRNA treatment, cells were transfected with 1.5 μg of pHXB2ΔBalD25S and 0.3 μg of Vpu with the jetPEI macrophage transfection reagent (Polyplus International) according to the manufacturer's protocol. Cells and supernatants were harvested 72 h posttransfection, and VLPs were isolated from the supernatants using sucrose density centrifugation. Cell lysates and VLPs were analyzed by Western blotting.
HIV-1 infectivity assays.
COS-1 cells stably expressing Anx2 or scrambled shRNA or 293T cells overexpressing Anx2 were seeded into six-well plates at a density of 4 × 105 cells/well. The next day, cells were transfected with a plasmid encoding the full-length infectious HIV-1 clone pNL4.3, using Lipofectamine 2000. The medium was changed the following day, and cell supernatants were harvested at 48 h posttransfection. The supernatants were clarified by centrifugation at 12,000 rpm for 5 min, followed by filtration through a 0.2-μm filter. The clarified supernatant was added in triplicate to TZM-bl cells in 96-well plates, which had been seeded the day before at 1 × 104 cells/well. Supernatants from the infected TZM-bl cells were harvested at 72 h postinfection and assayed for luciferase activity using the Bright-glo system. To determine p24 Gag levels, the supernatant was treated with detergent to inactivate infectious virus and a p24 enzyme-linked immunosorbent assay (ELISA) was performed. The luciferase readout for the infectivity was normalized against p24 Gag levels (ng). HIV-1 infection of primary MDMs was performed as follows. MDMs were treated with siRNAs on day 10 after differentiation. Seventy-two hours posttransfection, the MDMs were infected with HIV strain Bal at a multiplicity of infection (MOI) of 0.1 for 24 h. Cells were washed three times with PBS, and fresh medium was added. Supernatants and cells were harvested at days 6 and 9 postinfection. Supernatants were filtered through a 0.45-μm filter and stored at −80°C. Gag expression in cell lysates was analyzed by Western blotting. p24 levels in the medium were measured using an HIV-1 p24 ELISA kit (SAIC, Frederick, MD). TZM-bl cells were infected with equal amounts of p24-containing viral stocks for 72 h and assayed for infectivity as described above.
Infection of primary CD4+ cells.
Primary human CD4+ cells were separated by magnetic beads using a Miltenyi Biotech (Auburn, CA) CD4+ T-cell isolation kit. Phytohemagglutinin (PHA)-activated CD4+ lymphocytes were plated at 1 × 106 cells/well in a 24-well plate and were infected overnight with 20 ng of a p24 equivalent of viral stocks. After infection, cells were washed three times and maintained in complete RPMI 1640 medium with interleukin-2 (IL-2; 50 U/ml) over 5 to 8 days. Culture supernatants for p24 antigen quantification were collected every 4 days postinfection. p24 levels in the media were measured using an HIV-1 p24 ELISA kit (SAIC, Frederick, MD).
RESULTS
Anx2 and its binding partner p11 are expressed at different levels in multiple cell lines.
We first analyzed levels of endogenous Anx2 and p11 in several model cell lines typically used for HIV Gag studies. Anx2 was detected in all cell lines analyzed: the highest levels were observed in COS-1, COS-7, and HeLa cells, lower levels were observed in U937 differentiated monocyte-derived macrophages, HEK293, and U937 cells, and the lowest levels were observed in Jurkat and HEK293T cells (Fig. 1 A). Similarly, p11 levels were highest in COS-1, COS-7, and HeLa cells, with lower levels in HEK293, Jurkat, and U937 cells. p11 was at undetectable levels in HEK293T and U937 differentiated MDM cells (Fig. 1B).
FIG. 1.
Levels of endogenous Anx2 and its binding partner p11 in cell lines. Lysates from the indicated cell lines were resolved by SDS-PAGE and probed with anti-Anx2 and anti-actin antibodies (A) or anti-p11 and anti-actin antibodies (B) by Western blotting.
Disruption of endogenous Anx2 and its binding partner p11 results in increased VLP production.
We next examined the role of Anx2 in Gag-mediated VLP production by disrupting expression of endogenous Anx2 in COS-1 cells. COS-1 cells were transduced with lentiviruses, expressing either an shRNA sequence targeting Anx2 or a scrambled control sequence. Following transduction, COS-1 cells were placed under puromycin selection to screen for stably transduced cells with consistent Anx2 knockdown. We achieved knockdown efficiencies of ∼99% for Anx2 (Fig. 2 A) and ∼99% for p11 (Fig. 2B). It has been shown previously that Anx2 is required for p11 stability (8), which explains the decrease in levels of p11 observed in cells with stable Anx2 knockdown. COS-1 cells stably expressing scrambled control or Anx2 shRNA were transfected with cDNA encoding a noninfectious HIV-1 proviral construct (pHXB2ΔBalD25S). The kinetics of VLP production were monitored by harvesting VLPs at 1, 2, 4, and 8 h. Cells and VLPs were analyzed by Western blotting, probed with anti-Gag antibody, and quantified. Equivalent levels of Gag were expressed in both scrambled and Anx2 shRNA-transduced cells. Interestingly, increased production of VLPs was observed in Anx2-depleted cells compared to the scrambled controls (Fig. 2C). The difference in VLP production was ∼2-fold at the 1- and 2-h time points, with the difference becoming considerably less discernible at the 8-h time point. After 24 h, VLP production was ∼1.2-fold higher in the Anx2-depleted cells than that in the control cells (Fig. 2D and E).
FIG. 2.
Effects of Anx2 depletion on VLP production and infectivity in COS-1 cells. (A and B) COS-1 cells stably transduced with lentiviruses expressing shRNA sequences targeting Anx2 or control scrambled (Scr) sequences were generated. Cell lysates were analyzed by SDS-PAGE and Western blotting for Anx2 (A) and p11 (B) expression. Western blots were scanned, and the signal for the Scr control was set to 100. The inset in panel A shows a typical Western blot probed for Anx2 and actin. The inset in panel B shows a typical Western blot probed for p11 and actin. (C) COS-1 cells stably transduced as in panel A were transfected with pHXB2ΔBalD25S. The next day, the medium was changed, and VLPs were harvested at 1, 2, 4, and 8 h, purified by sucrose gradient centrifugation, resolved by SDS-PAGE, and probed with anti-Gag antibody by Western blotting. Gag expression levels were quantified using Image J software (NIH). The graph represents three independent experiments performed in duplicate. Relative VLP production was calculated as described in Materials and Methods. (D and E) Stably transduced COS-1 cells were transfected with pHXB2ΔBalD25S. VLPs and cells were harvested at the 24-h time point and analyzed as in panel C. (F) Stably transduced COS-1 cells were transfected with the full-length infectious HIV-1 clone pNL4.3. Virus was harvested at 48 h posttransfection, and p24 levels and infectivity were determined as described in Materials and Methods. Experiments were performed two times in triplicate, normalized to 100% for the Scr shRNA controls, and averaged.
We next examined whether Anx2 disruption affects the infectivity of HIV particles. COS-1 cells stably expressing control scrambled sequences and Anx2-depleted cells were transfected with a full-length infectious HIV clone (pNL4.3). Viruses harvested from these cells were used to infect TZM-bl reporter cells, and infectivity was determined and normalized against p24 levels. No significant differences in infectivity were observed in viruses obtained from the control scrambled or Anx2-depleted COS-1 cells (Fig. 2F).
The intracellular localization of Gag is not affected by annexin 2 disruption.
The intracellular localization pattern of Gag has been well characterized by us and others and provides a useful window into the trafficking and distribution of Gag within the cell. Since Anx2 is involved in transport of intermediates from the early to the late endosome, we analyzed the localization of EEA1 and CD63, markers of early and late endosomes, respectively. No obvious alterations in the distribution of Gag-GFP with relation to EEA1 (Fig. 3 A) or CD63 (Fig. 3B) were noted in COS-1 cells stably transduced with Anx2 shRNA versus the scrambled shRNA controls. Anx2-depleted cells and control cells were also fixed and stained with anti-Anx2 antibody, and the knockdown of Anx2 could be clearly visualized as well (Fig. 3C).
FIG. 3.
Effect of Anx2 depletion on the intracellular localization of Gag-GFP and endosomal markers. Stably transduced COS-1 cells (Scr and Anx2) were transfected with Gag-GFP and then seeded onto glass coverslips. Cells were fixed, permeabilized and stained with DAPI and anti-EEA1 antibody (A), anti-CD63 antibody (B), or anti-Anx2 antibody (C). The top panels in panels A and B are control cells, and the bottom panels are Anx2-depleted cells. In panel C, the panel on the left shows control cells and the panel on the right shows Anx2-depleted cells.
The calcium-mediated spike in VLP production is not significantly impaired by Anx2 disruption.
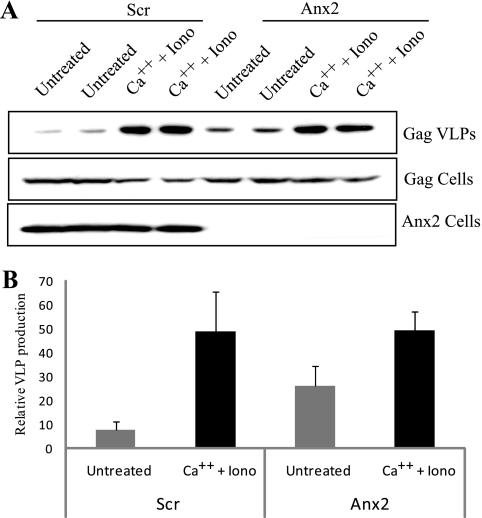
We have previously reported that a transient increase in intracellular calcium levels induced by the calcium ionophore ionomycin causes a spike in Gag-mediated VLP production (20). Since Anx2 is a calcium-regulated protein, we wanted to determine if Anx2 was involved in this process. Anx2-depleted cells or cells transduced with control scrambled shRNA were transfected with cDNA encoding a noninfectious HIV-1 proviral construct (pHXB2ΔBalD25S). Transfected cells were then treated for 5 min with or without ionomycin and calcium chloride. Cells were washed, and VLPs were harvested 1 h later. As observed previously (Fig. 2C), the basal level of VLP production was ∼2-fold higher in Anx2-depleted cells (Fig. 4 A and B). However, both Anx2-depleted and control shRNA-transduced cells generated a similar increase in VLP production in response to the calcium pulse (Fig. 4A and B).
FIG. 4.
Effects of Anx2 depletion on the calcium-induced spike in Gag VLP production. (A) Stably transduced COS-1 cells (Scr and Anx2) were transfected with pHXB2ΔBalD25S. The next day, transfected cells were treated with medium containing ionomycin (Iono) and CaCl2 (Ca++) or control medium for 5 min, and VLPs were harvested at 1 h following treatment. Cells and VLPs were resolved on SDS gels and probed with anti-Gag and anti-Anx2 antibodies by Western blotting. (B) Graphical representation of VLP production, representing three independent experiments performed in duplicate.
Anx2 depletion impairs downregulation of the EGFR.
To confirm that Anx2 depletion was affecting intrinsic trafficking functions in the cell, the effect of Anx2 disruption on the basal rate of EGFR degradation was monitored. COS-1 cells stably transduced with either control scrambled or Anx2 shRNA were serum starved overnight. The next day, cycloheximide was added to block new protein synthesis, and cells were incubated in cycloheximide-containing media with or without EGF. Cells were harvested at 0, 1, and 4 h following EGF treatment, and lysates were probed by Western blotting for EGFR. A striking difference in EGFR levels was observed between Anx2-depleted cells and control cells at 4 h post-EGF stimulation (Fig. 5 A). Cells expressing scrambled shRNA sequences had efficiently downregulated EGFR, with ∼20% of the EGFR signal remaining. By contrast, the Anx2-depleted cells exhibited considerably reduced downregulation of EGFR, with ∼85% of the EGFR signal still present after 4 h (Fig. 5B). These findings are consistent with the ability of Anx2 to regulate early to late endosome transport and indicate that Anx2 knockdown impaired endosomal trafficking functions in the cell.
FIG. 5.
Effects of Anx2 depletion on the basal rate of EGFR downregulation. (A) Stably transduced COS-1 cells (Scr and Anx2) were seeded in six-well plates and serum starved overnight. The next day, cells were treated with medium containing EGF and cylcoheximide or cycloheximide alone for 10 min. Cells were harvested immediately (0 h) and at 1 and 4 h following treatment. Cell lysates were analyzed by SDS-PAGE and Western blotting with anti-EGFR, anti-Anx2, and anti-actin antibodies. (B) Graphical representation of the experiments. EGFR levels in cells at 4 h following treatment were quantified using Image J software. EGFR levels in cells that received no EGF were normalized to 100. The graph represents two independent experiments.
Disruption of endogenous annexin 2 does not impair VLP production in multiple cell lines.
We next studied the effect of Anx2 depletion on Gag function in cells of the Jurkat T and 293T lines, which are widely used for HIV Gag studies. Jurkat T cells stably transduced with control scrambled or Anx2-specific shRNA were generated. Efficient knockdown of Anx2 was achieved, with depletion of ∼98% of the signal in Anx2 shRNA-expressing cells. (Fig. 6 B). When stable Jurkat T cells were transfected with pHXB2ΔBalD25S, there was no significant difference in VLP production between the cells transduced with control scrambled shRNA and those transduced with Anx2 shRNA (Fig. 6A and C). A similar experiment was performed by transducing 293T cells with lentiviruses expressing either control scrambled or Anx2 shRNA sequences. Again, efficient knockdown was achieved with a 99% reduction of Anx2 in Anx2-depleted cells (Fig. 6E). No difference in VLP production was detected between control and Anx2-depleted cells (Fig. 6D and F).
FIG. 6.
Effects of Anx2 disruption on Gag-mediated VLP production in multiple cell lines. (A to C) Jurkat cells stably transduced with lentiviruses expressing shRNA targeting Anx2 or control scrambled shRNA (Scr) were electroporated with pHXB2ΔBalD25S. Cells and VLPs were harvested at 48 h following electroporation, resolved by SDS-PAGE, and probed with anti-Gag (C) and anti-Anx2 antibodies by Western blotting. VLP production (A) was quantified from three independent experiments performed in duplicate and averaged. Levels of Anx2 were quantified (B); the inset shows a typical Western blot probed with anti-Anx2 and anti-actin antibodies. (D to F) 293T cells transiently transduced with lentiviruses expressing shRNA targeting Anx2 or control scrambled shRNA (Scr) were transfected with pHXB2ΔBalD25S. Cells and VLPs were harvested at 24 h following transfection, resolved by SDS-PAGE, and probed with anti-Gag (F) and anti-Anx2 antibodies by Western blotting. VLP production (D) was quantified from three independent experiments. Levels of Anx2 were quantified (E); the inset shows a typical Western blot probed with anti-Anx2 and anti-actin antibodies. (G to I) Wild-type (WT) MEFs (C57BL/6) and Anx2−/− MEFs were transfected with pCMVGag. VLPs and cells were harvested at 24 h posttransfection, resolved by SDS-PAGE, and probed with anti-Gag (I) and anti-Anx2 antibodies by Western blotting. VLP production (G) was quantified from three independent experiments performed in duplicate. (H) Levels of Anx2 were analyzed by Western blotting with anti-Anx2 antibody, with anti-actin antibody serving as a loading control.
Finally, we examined Gag function in primary mouse embroyonic fibroblasts (MEFs) derived from Anx2-null mice (C57BL/6). A codon-optimized, rev-independent vector encoding HIV-1 Gag was used to transfect the Anx2 null MEFs, and matched wild-type controls and VLPs were harvested (4). Anx2-null MEFs exhibited slightly higher VLP production (1.5-fold) than the matched wild-type cells (Fig. 6G to I). These findings establish that depletion of Anx2 in multiple cell types does not impair Gag function.
Expression of annexin 2 in trans has no effect on VLP production or infectivity.
A recent study reported that Anx2 overexpression increased VLP production in 293T cells (7). We repeated this experiment in both COS-1 and 293T cells. COS-1 cells were cotransfected with pHXB2ΔBalD25S and with either empty vector or vector encoding Anx2. No significant difference in VLP production was observed in control cells compared to cells with Anx2 overexpression (Fig. 7 A and B). However, as COS-1 cells have high endogenous levels of Anx2 (Fig. 1A), the expression of Anx2 in trans did not dramatically increase the levels of Anx2 in the cell (Fig. 7A, lowest panel). Since 293T cells have low levels of endogenous Anx2 (Fig. 1A), we tested the effect of overexpression of Anx2 in these cells to determine if Anx2 affected Gag function. 293T cells were transfected with either cDNA encoding pHXB2ΔBalD25S and either empty vector or vector encoding Anx2 or p11. Cells and VLPs were harvested and analyzed. No significant differences in Gag levels in the cells or VLP production were observed between cells expressing pHXB2ΔBalD25S and either empty vector or Anx2 or p11 (Fig. 7C and D). Anx2 overexpression was also reported to alter Gag processing in 293T cells (7). However, in our hands, no change in p24 generation was noted in 293T cells transfected with a full-length infectious clone of pNL4.3 (Fig. 7E). Moreover, no effect of Anx2 overexpression on the infectivity of particles produced by pNL4.3-transfected 293T cells was observed (Fig. 7F).
FIG. 7.
Effect of expression of Anx2 in trans on VLP production. (A and B) COS-1 cells were cotransfected with pHXB2ΔBalD25S and either empty vector (pCDNA3.1) or vector encoding Myc-His-tagged Anx2. Cells and VLPs were harvested at 24 h following transfection, resolved by SDS-PAGE, and probed with anti-Gag and anti-Anx2 antibodies. The upper band in the Anx2 blot represents the exogenous Myc-His-tagged Anx2, while the lower bands are endogenous Anx2 (A) Western blots of Gag and Anx2 expression. (B) VLP production was quantified; the graph represents three independent experiments. (C and D) 293T cells were cotransfected with pHXB2ΔBalD25S and either empty vector (pCDNA3.1) or vector encoding Myc-His-tagged Anx2 or Flag-tagged p11. Cells and VLPs were harvested at 24 h following transfection, resolved by SDS-PAGE and probed with anti-Gag, anti-Anx2, and anti-p11 antibodies. (C) Western blots of Gag, Anx2, and p11 expression. The upper band in the Anx2 blot represents the exogenous Myc-His-tagged Anx2, while the lower bands are endogenous Anx2. The upper band in the p11 blot represents exogenous Flag-tagged p11, and the lower band is endogenous p11 (D) VLP production was quantified; the graph represents three independent experiments. (E and F) 293T cells were transfected with pNL4.3 and either vector or Anx2. Cells and virus were harvested at 48 h posttransfection. Levels of Gag, actin, and Anx2 were determined by Western blotting (E), and p24 levels and infectivity (relative light units obtained in the luciferase assay per ng p24) (F) were determined as described in Materials and Methods. Experiments were performed two times in triplicate; results from a representative experiment are shown.
Depletion of Anx2 in human primary monocyte-derived macrophages does not affect VLP production.
We next examined the effect of Anx2 depletion on particle production in primary cells that are important for HIV-1, namely, human primary monocyte-derived macrophages (MDMs). A previous study by Ryzhova et al. reported that Anx2 depletion impairs Gag expression and processing and reduces the infectivity of particles produced in these cells (24). However, that study was performed by infecting primary MDMs with HIV-1 after Anx2 depletion. Since Anx2 is required for HIV-1 entry into macrophages (11, 29), it is difficult to determine if the observed defect in Anx2-depleted cells was due to an effect of Anx2 on entry or defects later in the viral life cycle. To address this issue, we first depleted Anx2 in primary MDMs using the transient siRNA transfection conditions developed by Ryzhova et al. (24) and then transfected the siRNA-treated cells with plasmids encoding HIV-1 cDNA. In this manner, we bypassed effects on entry and focused directly on examining a role for Anx2 in HIV-1 assembly.
Primary MDMs from three different donors were isolated, differentiated into macrophages, treated with siRNA directed against Anx2 or β-Gal for 7 days, and then transfected with pHXB2ΔBalD25S and Vpu for particle production (26). Results from these three independent experiments are shown in Fig. 8 A and B. A strong knockdown of endogenous Anx2 was achieved (80 to 98%) in each set of experiments. Slight differences in Gag expression levels were observed among the different batches of primary cells, and Anx2 depletion caused a small decrease in Gag expression in the cells (Fig. 8A). However, when normalized against cellular Gag levels, no defect in particle production was detected in Anx2-depleted cells. We conclude that Anx2 is not required for particle production in human primary MDMs.
FIG. 8.
Effect of Anx2 depletion on particle production in human primary MDMs. (A and B) MDMs from three different donors were treated with siRNA targeting Anx2 or β-Gal and then transfected with pHXB2ΔBalD25S and Vpu. Cells and VLPs were harvested and analyzed at 72 h posttransfection by Western blotting with anti-Gag antibodies (VLPs and cell lysates) and with antibodies to Anx2 and actin (cell lysates). The different batches of primary MDMs are denoted by 1, 2, and 3. The results from all three experiments carried out in triplicate are quantified in panel B. (C to H) Primary MDMs were transfected with siRNA directed against Anx2 or β-Gal. Cells were infected with HIV Bal virus at 72 h posttransfection. Supernatants were harvested at day 6 postinfection and analyzed for p24 levels and infectivity. Cells were harvested at day 9 postinfection, and lysates were analyzed for expression of Gag and Anx2. (C) Western blot and quantification of Anx2 expression in cell lysates. (D to F) The ratio of Pr55 to p24 in the cell lysates (D and E) and levels of p24 (ng) in supernatants (F) from HIV Bal-infected primary MDMs were quantified. (G and H) Infectivity was determined using supernatant containing an equal amount (16.5 pg) of p24 to infect TZM-bl cells (1 × 104 cells/well) (G) and primary CD4+ cells (1 × 106 cells/well) (H). The results from triplicate or quadruplicate experiments performed in triplicate or quadruplicate are quantified as relative light units (RLU) (G) or % of control p24 in supernatant (H).
We next determined the effects of Anx2 depletion in HIV-1-infected MDMs. Primary MDMs were transfected with siRNA directed against Anx2 or β-Gal. Cells were infected with HIV Bal virus at 72 h posttransfection, a time point where the cells still retain detectable levels of Anx2 (24) and thus should be susceptible to HIV-1 infection. Supernatants and cells were harvested at days 6 and 9 postinfection and analyzed for Anx2 and Gag expression and infectivity. Western blotting of cell lysates revealed that Anx2 levels were depleted to 47% of that of the β-Gal control by this protocol (Fig. 8C). Levels of Gag expression and the extent of Gag processing were equivalent in cells treated with either β-Gal or Anx2 siRNA (Fig. 8D and E). There was no detectable difference in the amount of p24 released into the supernatant of Anx2-depleted cells compared to β-Gal controls (Fig. 8F). However, when equal amounts of p24-containing viral stocks were assayed on TZM-bl reporter cells, supernatants containing virus from Anx2-depleted MDMs were 4.6-fold less infectious than the β-Gal controls (Fig. 8G). To ensure that these differences were not attributable to activation of the reporter plasmid by soluble Tat released into the supernatant of HIV-1-infected cells (30), infectivity assays were also performed on primary CD4+ cells. Supernatants containing virus from Anx2-depleted MDMs were 3.6-fold less infectious than the β-Gal controls (Fig. 8H). Taken together, these data imply that there is a cell type-dependent requirement for Anx2 in regulating HIV-1 infectivity in macrophages.
DISCUSSION
Anx2 and HIV-1 particle production.
In this study, we clearly demonstrate that Anx2 is not required for assembly of HIV-1. We used a variety of cell lines that express Anx2 at high, intermediate, or low levels and achieved knockdown of endogenous Anx2 expression levels of >99% compared to control cells. In addition, we examined human primary MDMs and also took advantage of the genetic null background of Anx2−/− MEFs to further solidify the conclusion that HIV-1 Gag-mediated viral particle production does not rely on the presence of Anx2 in the host cell. It should, however, be noted that these results pertain to cells in tissue culture, and we cannot exclude a potential role for Anx2 in HIV-1 pathogenesis in the live, human host.
Anx2 and HIV-1 infectivity.
Two recent studies reported that Anx2 is important for HIV-1 assembly and infectivity in macrophages and 293T cells (7, 24). Our results differ in several respects. First, we and others (3, 5, 13) detected endogenous Anx2 expression in both Jurkat T cells and 293T cells, whereas Harrist et al. claimed these cells do not express Anx2. Second, Harrist et al. reported that overexpression of Anx2 in 293T cells increases Gag processing, particle production, and infectivity (7). By contrast, we did not detect an effect of Anx2 overexpression on Gag processing, VLP production, or infectivity in 293T cells. Third, we disrupted Anx2 expression in several cell lines used for HIV-1 studies (293T, COS-1, and Jurkat T cells) as well as in primary MDMs and did not detect any significant differences in VLP production (Fig. 2, 6, and 8). We also tested if depletion or overexpression of Anx2 affected the infectivity of particles released from COS-1 and 293T cells and did not observe any significant differences in infectivity. Ryzhova et al. (24) reported that Anx2 is required for production of infectious particles in HIV-1-infected MDMs, and we have confirmed this finding (Fig. 8). However, in contrast to the findings of Ryzhova et al., we found no effect of Anx2 depletion on Gag expression or processing or on release of p24 from HIV-1-infected cells. It is possible that the differences in the two studies are related to use of different strains of HIV-1 virus, perhaps due to differential rates of Gag processing.
Host cell protein incorporation into virions and regulation of infectivity.
Retroviruses incorporate host cell proteins and lipids into the virion membrane during assembly and budding (2, 19, 25). Several of these host cell molecules have been shown to be important for HIV-1 function, including HLA class II, tetraspanins, integrins, and lipid raft components (1, 6, 19). By contrast, a recent study established that CD63, a tetraspanin protein that is incorporated into the HIV-1 viral membrane, is not required for assembly or infectivity of HIV-1 (23). In addition, there is a growing list of host cell proteins that actively participate in viral particle assembly and/or infectivity and are packaged within the HIV-1 virus particle (e.g., ESCRT subunits such as Tsg101, APOBEC3G, and cyclophilin). Here we present an example of a protein that functions as a cell type-dependent host cell factor for HIV-1 replication at the level of infectivity. Further studies will be needed to determine how Anx2 regulates infectivity and why this occurs in a macrophage-specific manner. It is possible that Anx2 regulates the extent of incorporation of viral proteins, viral RNA, or host cell factors into HIV-1 particles and/or secretion of cytokines or other factors that influence HIV-1 infectivity. Anx2 may function in macrophages at the level of endosomal trafficking (14-16) and/or by regulating association of viral components with cholesterol-rich lipid rafts (7). Moreover, it remains to be determined if cell types other than macrophages require the presence of Anx2 for production of infectious virus.
HIV-1, as well as other retroviruses, exploits the host cell machinery for replication, assembly, and budding. This had spurred several proteomic studies gearing toward identifying host cell proteins that are incorporated into the retrovirus virion. Over 250 proteins that associate with HIV-1 particles produced in macrophages and 30 proteins that are associated with Moloney murine leukemia virus were identified (2, 27). Proteins that actively participate in these processes are likely to be incorporated into the virion via a specific, active mechanism. By contrast, passive incorporation of host cell components into the budding virion can occur randomly, perhaps as a result of a bystander effect, and provides a fingerprint of the cell type that produced the viral particle. The presence of Anx2 in viral particles produced by MDMs (2) and its requirement for production of infectious HIV-1 by these cells suggest a mechanism for active incorporation of Anx2 specifically in macrophages. Further proteomic analysis will be required to determine if Anx2 is included or excluded from HIV-1 virions produced by other cell types and whether virion incorporation is necessarily an indicator of functionality.
Acknowledgments
We thank Kathryn Hajjar for Anx2 and p11 cDNAs and Anx2−/− MEFs; Nathaniel Martinez for performing the infectivity assays and for critical reading of the manuscript; John Moore for use of his biosafety level 2 (BSL-2) laboratory; the NIH AIDS Research and Reference Reagent Program for anti-p24 antibody; the New York Blood Center for supplying PBMCs; Raisa Louft-Nisenbaum for technical assistance; Goar Mosoyan for performing CD4 cell isolation, infection with HIV Bal, and p24 ELISAs; and the Molecular Cytology Core facility at MSKCC.
This research was supported by NIH grant CA72309.
Footnotes
Published ahead of print on 14 July 2010.
REFERENCES
- 1.Cantin, R., S. Methot, and M. J. Tremblay. 2005. Plunder and stowaways: incorporation of cellular proteins by enveloped viruses. J. Virol. 79:6577-6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chertova, E., O. Chertov, L. V. Coren, J. D. Roser, C. M. Trubey, J. W. Bess, Jr., R. C. Sowder II, E. Barsov, B. L. Hood, R. J. Fisher, K. Nagashima, T. P. Conrads, T. D. Veenstra, J. D. Lifson, and D. E. Ott. 2006. Proteomic and biochemical analysis of purified human immunodeficiency virus type 1 produced from infected monocyte-derived macrophages. J. Virol. 80:9039-9052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang, Y., A. Rizzino, Z. A. Sibenaller, M. S. Wold, and J. K. Vishwanatha. 1999. Specific down-regulation of annexin II expression in human cells interferes with cell proliferation. Mol. Cell. Biochem. 199:139-147. [DOI] [PubMed] [Google Scholar]
- 4.Diaz-Griffero, F., R. Taube, S. M. Muehlbauer, and J. Brojatsch. 2008. Efficient production of HIV-1 viral-like particles in mouse cells. Biochem. Biophys. Res. Commun. 368:463-469. [DOI] [PubMed] [Google Scholar]
- 5.Dubois, T., J. P. Oudinet, F. Russo-Marie, and B. Rothhut. 1995. In vivo and in vitro phosphorylation of annexin II in T cells: potential regulation by annexin V. Biochem. J. 310:243-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grigorov, B., V. Attuil-Audenis, F. Perugi, M. Nedelec, S. Watson, C. Pique, J. L. Darlix, H. Conjeaud, and D. Muriaux. 2009. A role for CD81 on the late steps of HIV-1 replication in a chronically infected T cell line. Retrovirology 6:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrist, A. V., E. V. Ryzhova, T. Harvey, and F. Gonzalez-Scarano. 2009. Anx2 interacts with HIV-1 Gag at phosphatidylinositol (4,5) bisphosphate-containing lipid rafts and increases viral production in 293T cells. PLoS One 4:e5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He, K. L., A. B. Deora, H. Xiong, Q. Ling, B. B. Weksler, R. Niesvizky, and K. A. Hajjar. 2008. Endothelial cell annexin A2 regulates polyubiquitination and degradation of its binding partner S100A10/p11. J. Biol. Chem. 283:19192-19200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.LeBouder, F., E. Morello, G. F. Rimmelzwaan, F. Bosse, C. Pechoux, B. Delmas, and B. Riteau. 2008. Annexin II incorporated into influenza virus particles supports virus replication by converting plasminogen into plasmin. J. Virol. 82:6820-6828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ling, Q., A. T. Jacovina, A. Deora, M. Febbraio, R. Simantov, R. L. Silverstein, B. Hempstead, W. H. Mark, and K. A. Hajjar. 2004. Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J. Clin. Invest. 113:38-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma, G., T. Greenwell-Wild, K. Lei, W. Jin, J. Swisher, N. Hardegen, C. T. Wild, and S. M. Wahl. 2004. Secretory leukocyte protease inhibitor binds to annexin II, a cofactor for macrophage HIV-1 infection. J. Exp. Med. 200:1337-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez, N. W., X. Xue, R. G. Berro, G. Kreitzer, and M. D. Resh. 2008. Kinesin KIF4 regulates intracellular trafficking and stability of the human immunodeficiency virus type 1 Gag polyprotein. J. Virol. 82:9937-9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsunaga, T., T. Inaba, H. Matsui, M. Okuya, A. Miyajima, T. Inukai, T. Funabiki, M. Endo, A. T. Look, and H. Kurosawa. 2004. Regulation of annexin II by cytokine-initiated signaling pathways and E2A-HLF oncoprotein. Blood 103:3185-3191. [DOI] [PubMed] [Google Scholar]
- 14.Mayran, N., R. G. Parton, and J. Gruenberg. 2003. Annexin II regulates multivesicular endosome biogenesis in the degradation pathway of animal cells. EMBO J. 22:3242-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morel, E., and J. Gruenberg. 2009. Annexin A2 binding to endosomes and functions in endosomal transport are regulated by tyrosine 23 phosphorylation. J. Biol. Chem. 284:1604-1611. [DOI] [PubMed] [Google Scholar]
- 16.Morel, E., and J. Gruenberg. 2007. The p11/S100A10 light chain of annexin A2 is dispensable for annexin A2 association to endosomes and functions in endosomal transport. PLoS One 2:e1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morel, E., R. G. Parton, and J. Gruenberg. 2009. Annexin A2-dependent polymerization of actin mediates endosome biogenesis. Dev. Cell 16:445-457. [DOI] [PubMed] [Google Scholar]
- 18.Ono, A., and E. O. Freed. 1999. Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J. Virol. 73:4136-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ott, D. E. 2008. Cellular proteins detected in HIV-1. Rev. Med. Virol. 18:159-175. [DOI] [PubMed] [Google Scholar]
- 20.Perlman, M., and M. D. Resh. 2006. Identification of an intracellular trafficking and assembly pathway for HIV-1 gag. Traffic 7:731-745. [DOI] [PubMed] [Google Scholar]
- 21.Raynor, C. M., J. F. Wright, D. M. Waisman, and E. L. Pryzdial. 1999. Annexin II enhances cytomegalovirus binding and fusion to phospholipid membranes. Biochemistry 38:5089-5095. [DOI] [PubMed] [Google Scholar]
- 22.Resh, M. D. 2004. A myristoyl switch regulates membrane binding of HIV-1 Gag. Proc. Natl. Acad. Sci. U. S. A. 101:417-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiz-Mateos, E., A. Pelchen-Matthews, M. Deneka, and M. Marsh. 2008. CD63 is not required for production of infectious human immunodeficiency virus type 1 in human macrophages. J. Virol. 82:4751-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryzhova, E. V., R. M. Vos, A. V. Albright, A. V. Harrist, T. Harvey, and F. Gonzalez-Scarano. 2006. Annexin 2: a novel human immunodeficiency virus type 1 Gag binding protein involved in replication in monocyte-derived macrophages. J. Virol. 80:2694-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saphire, A. C., P. A. Gallay, and S. J. Bark. 2006. Proteomic analysis of human immunodeficiency virus using liquid chromatography/tandem mass spectrometry effectively distinguishes specific incorporated host proteins. J. Proteome Res. 5:530-538. [DOI] [PubMed] [Google Scholar]
- 26.Schubert, U., K. A. Clouse, and K. Strebel. 1995. Augmentation of virus secretion by the human immunodeficiency virus type 1 Vpu protein is cell type independent and occurs in cultured human primary macrophages and lymphocytes. J. Virol. 69:7699-7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segura, M. M., A. Garnier, M. R. Di Falco, G. Whissell, A. Meneses-Acosta, N. Arcand, and A. Kamen. 2008. Identification of host proteins associated with retroviral vector particles by proteomic analysis of highly purified vector preparations. J. Virol. 82:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaw, M. L., K. L. Stone, C. M. Colangelo, E. E. Gulcicek, and P. Palese. 2008. Cellular proteins in influenza virus particles. PLoS Pathog. 4:e1000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wahl, S. M., T. Greenwell-Wild, and N. Vazquez. 2006. HIV accomplices and adversaries in macrophage infection. J. Leukoc. Biol. 80:973-983. [DOI] [PubMed] [Google Scholar]
- 30.Zauli, G., M. La Placa, M. Vignoli, M. C. Re, D. Gibellini, G. Furlini, D. Milani, M. Marchisio, M. Mazzoni, and S. Capitani. 1995. An autocrine loop of HIV type-1 Tat protein responsible for the improved survival/proliferation capacity of permanently Tat-transfected cells and required for optimal HIV-1 LTR transactivating activity. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10:306-316. [PubMed] [Google Scholar]
- 31.Zhou, W., L. J. Parent, J. W. Wills, and M. D. Resh. 1994. Identification of a membrane-binding domain within the amino-terminal region of human immunodeficiency virus type 1 Gag protein which interacts with acidic phospholipids. J. Virol. 68:2556-2569. [DOI] [PMC free article] [PubMed] [Google Scholar]