Abstract
HIV-1 gp140 envelope immunogens express conserved epitopes that are targeted by broadly cross-reactive neutralizing antibodies, but they fail to elicit similar antibodies upon immunization. The poor immunogenicity of conserved epitopes on gp140 could be linked to the high immunogenicity of variable Env regions on such constructs. Previous studies have shown that the first hypervariable region (V1 loop) is immunogenic on soluble gp140s but elicits type-specific antibodies. To address issues related to the high immunogenicity of the V1 loop, two conceptually opposite approaches were tested. In the first approach, we eliminated the V1 loop from our gp140 construct and examined how V1 deletion altered the immunogenic properties of other Env regions. In the second approach, we took advantage of the high immunogenicity of the V1 loop and engrafted four diverse V1 loops onto a common gp140 Env “scaffold.” These four scaffolds were used as a cocktail of immunogens to elicit diverse anti-V1 antibodies, under the hypothesis that eliciting diverse anti-V1 antibodies would expand the neutralizing breadth of immune sera. Our study indicates that three of four heterologous V1 loops were immunogenic on the common Env backbone “scaffold,” but heterologous anti-V1 neutralizing responses were observed in only one case. Both types of V1 modification dampened the immunogenicity of the V3 loop, differentially altered the immunogenicity of the transmembrane gp41 subunit, and altered the relative immunogenicities of unknown Env regions, including potentially the CD4-binding site (CD4-bs) and trimer-specific targets, which elicited cross-reactive neutralizing antibodies but of limited breadth.
An effective vaccine against human immunodeficiency virus type 1 (HIV-1) will need to incorporate an envelope-derived immunogen capable of eliciting potent and broadly cross-reactive neutralizing antibody responses against diverse primary HIV-1 isolates. The target of anti-HIV neutralizing antibodies (NAbs), the viral envelope (Env) glycoprotein, is expressed as a single transmembrane polypeptide precursor (gp160) that is glycosylated and cleaved into an extracellular subunit (gp120) and a transmembrane subunit (gp41) during intracellular processing (10, 20, 21, 54). The functional Env form on virion surfaces is a trimer composed of three noncovalently associated gp120-gp41 heterodimers. Soluble forms of the trimeric Env have been generated by introducing stop codons immediately upstream of the transmembrane domain of gp41. These constructs are commonly referred to as gp140 proteins and have been tested extensively as immunogens to elicit anti-HIV-1 NAbs. Soluble gp140s express epitopes that are targets of NAbs, including cross-reactive NAbs such as b12, 4E10, and 2G12 (5, 17, 34, 45, 47, 49, 50, 52, 57). Immunization with gp140 immunogens nonetheless does not result in a broadly cross-reactive neutralizing antibody response (2, 3, 17, 18, 26, 56, 58).
Epitope mapping analyses of the Abs elicited by soluble trimeric gp140 immunogens revealed that a large fraction of the gp140-induced neutralization response targets the first hypervariable region of gp120 (the V1 loop). In our hands, ∼40 to 70% of the neutralizing activity of sera from animals immunized with SF162 gp140 constructs is due to anti-V1 antibodies (17). In a study by Li et al. with YU2 gp140 (30) and a study by Wu et al. with HxB2/BaL gp145 (56), ∼10 to 80% of anti-YU2 neutralizing activity and 100% of anti-HxB2 neutralizing activity, respectively, were due to anti-V1 Abs. These anti-V1 Abs, however, are not cross-reactive. Previously, we also demonstrated that the diverse positionings of the V1 across heterologous strains limit access of broadly cross-reactive monoclonal antibodies (MAbs) to their targets (12).
Here, taking into consideration the V1 loop's high immunogenicity, we employed two opposing approaches aimed at the elicitation of cross-reactive neutralizing antibody responses to HIV-1. In the first approach, we deleted the V1 loop on our soluble trimeric gp140 construct (ΔV1SF162 gp140) and examined whether and how this modification altered the immunogenic properties of other Env regions. In the second approach, we substituted the V1 loop on our SF162 gp140 construct with the V1 loops from four heterologous HIV-1 viruses (89.6, YU2, JRFL, and HxB2) that differ in their amino acid compositions and in the number of potential N-linked glycosylation sites (PNGs). These four heterologous viruses display various neutralization phenotypes (7) and coreceptor utilization profiles (15, 35, 36, 48, 51). A total of four SF162 Env-based gp140 “scaffolds” expressing four different V1 loops were created and used as immunogens in a cocktail to test as a “proof of principle” the hypothesis that if diverse V1 loops are presented to the immune system simultaneously, the elicitation of anti-V1 NAbs with diverse specificities would broaden the overall neutralizing activity of immune sera. We also immunized animals with each of the four V1 chimeric scaffolds individually to ensure that all V1 loops were immunogenic when presented on the heterologous SF162 Env background.
All immunogens (including wild-type [WT] SF162 gp140 and ΔV1SF162 gp140) elicited homologous anti-SF162 NAbs. All immunogens except the scaffold construct expressing the YU2 V1 also elicited heterologous NAbs against the sensitive lab-adapted strain HxB2. The heterologous YU2, 89.6, and HxB2 V1 loops, but not the JRFL V1 loop, were immunogenic on the background of the SF162 Env scaffold. However, only anti-V1 neutralizing activity against the HxB2 virus was observed. Although neither approach resulted in the development of broad anti-HIV-1 cross-neutralizing antibody responses, cross-neutralizing antibody responses of narrow breadth were elicited. These responses were not due to antibodies that target to variable regions of gp120 but were due to antibodies that target either epitopes of the CD4-binding site (CD4-bs) or epitopes that are not present on monomeric gp120. These observations have implications for guiding rational Env-based immunogen design and for potentially eliciting broadly cross-reactive NAb responses.
MATERIALS AND METHODS
Cell Lines.
Human embryonic kidney 293-T cells and the U87 human astroglioma cell line, expressing human CD4 and CXCR4, were cultured as previously described (17). The HeLa-derived TZM-bl cell line (David Montefiori, Duke University, Durham, NC) stably expresses human CD4, CCR5, and CXCR4 and was cultured in complete Dulbecco's modified Eagle's medium (DMEM). TZM-bl cells contain integrated reporter cassettes for firefly luciferase (Luc) and β-galactosidase, both under the control of an HIV-1 long terminal repeat (LTR) (32, 38).
Antibodies.
Abs recognizing the CD4-binding site (CD4-bs) include IgG1-b12 and -b6 monoclonal antibodies (MAbs) (9), provided by Dennis Burton (Scripps Institute). IgG-CD4 was purchased from Progenics Pharmaceuticals Inc. (Tarrytown, NY). MAb 2G12 (41, 44), directed to a complex glycan epitope on gp120, and anti-gp41 MAbs 2F5 and 4E10 (60, 61) were obtained through Polymun Scientific (Vienna, Austria). The human anti-V3 MAb 447-52D was provided by Susan Zolla-Pazner and Mirek Gorny (New York University) (13, 59). The anti-V1 MAb P3C8 and anti-V3 MAb P3E1 were isolated from mice immunized with ΔV2SF162 gp140 and SF162 gp140, respectively (16).
Env-expressing plasmids.
Full-length SF162 Env was previously cloned into the pEMC* vector (11, 31). Full-length Envs for YU2, JRFL, 89.6, and HxB2 were provided by Joseph Sodroski (Dana Farber Cancer Institute, Boston, MA). YU2, JRFL, and 89.6 were cloned previously into the pSV7d vector and HxB2 into the E7 vector (55). The V1 amino acid sequence alignment for the wild-type (WT) form of each isolate is shown in Fig. 1 A.
FIG. 1.
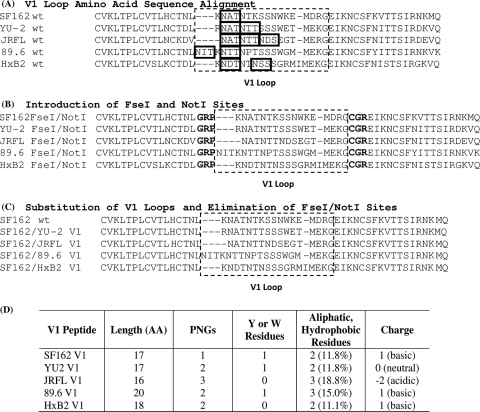
Generation of SF162 Envs expressing heterologous V1 loops. (A) Amino acid sequences of the SF162 V1 and of four heterologous V1s. Potential N-linked glycosylation sites (PNGs) within each V1 are boxed. (B) Introduction of FseI and NotI restriction sites at the 5′ and 3′ ends of the central regions of each V1 loop (Env F/N). The FseI site is represented by the flanking GRP sequence, and the NotI site is represented by the flanking CGR sequence. (C) Amino acid sequence for each SF162/Env V1 chimeric construct incorporating the V1 loop of either YU2, JRFL, 89.6, or HxB2 (SF162/Env V1); FseI and NotI sites have been eliminated. (D) Comparison of V1 loop amino acid length (AA), number of PNGs, number of tyrosine (Y) or tryptophan (W) residues, number of aliphatic hydrophobic residues (also the percentage of total residues that are aliphatic hydrophobic amino acids), and overall charge.
Replacing the V1 loop of SF162 with heterologous V1 loops.
First, FseI and NotI restriction sites at the 5′ and 3′ ends, respectively, of the V1 region were inserted into the gp160 env genes of SF162, YU2, JRFL, 89.6, and HxB2, as previously described (12) (Fig. 1B). These constructs will be referred to as “Env F/N”. A fragment between 400 and 500 bp (YU2 V1, 444 bp; JRFL V1, 447 bp; 89.6 V1, 462 bp; HxB2 V1, 453 bp) was PCR amplified (Platinum Pfx DNA polymerase; Invitrogen) from each heterologous “Env F/N” construct. Amplification was achieved using forward and reverse primers 216 (5′-AACCCACAAGAAATAGTATTG-3′) and 218 (5′-ATCATTACACTTTAGAATCGC-3′). All amplified V1 products were separated from the plasmid by agarose gel electrophoresis and purified using Qiagen's QIAquick gel extraction kit. The purified PCR product was then double digested with FseI and NotI restriction enzymes.
The SF162 Env construct containing the FseI/NotI sites (SF162 F/N) was simultaneously digested with FseI and NotI restriction enzymes to eliminate its V1 region. The previously digested and purified FseI/NotI-flanked heterologous V1 loop fragments were then ligated into the digested SF162 F/N vector plasmid using T4 DNA ligase (Promega, Madison, WI) (SF162/Env V1 F/N). The nucleotide sequences of all Env genes were verified by direct sequencing. Finally, the FseI and NotI restriction sites were eliminated by site-directed mutagenesis, as previously described but with a few modifications (12). Two rounds of mutagenesis were performed. The forward and reverse primers used to remove the FseI site on all chimeric Envs were as follows: SF162/YU2 V1 F/N, 5′-CATTGCACTAATTTGAGGAATGCTACTAATACC-3′ (forward) and 5′-GGTATTAGTAGCATTCCTCAAATTAGTGCAATG-3′ (reverse); SF162/JRFL V1 F/N, 5′-CTACATTGCACTAATTTGAATGCTACTAATACCACTAATG-3′ (forward) and 5′-CATTAGTGGTATTAGTAGCATTCAAATTAGTGCAATGTAG-3′ (reverse); SF162/89.6 V1 F/N, 5′-GCACTAATTTGAATATCACTAAGAATACTACTAATCCCAC-3′ (forward) and 5′-GTGGGATTAGTAGTATTCTTAGTGATATTCAAATTAGTGC-3′ (reverse); and SF162/HxB2 V1 F/N, 5′-CATTGCACTAATTTGAAGAATGATACTAATACC-3′ (forward) and 5′-GGTATTAGTATCATTCTTCAAATTAGTGCAATG-3′ (reverse). The forward and reverse primers used to remove the NotI site on all chimeric Envs were as follows: SF162/YU2 V1 F/N, 5′-ACGATGGAGAAAGGAGAAATAAAAAATTGC-3′ (forward) and 5′-GCAATTTTTTATTTCTCCTTTCTCCATCGT-3′ (reverse); SF162/JRFL V1 F/N, 5′-GGGAACGATGGAGAGAGGAGAAATAAAAAATTGC-3′ (forward) and 5′-GCAATTTTTTATTTCTCCTCTCTCCATCGTTCCC-3′ (reverse); SF162/89.6 V1 F/N, 5′-CTGGGGAATGATGGAGAAAGGAGAAATAAAAAATTGC-3′ (forward) and 5′-GCAATTTTTTATTTCTCCTTTCTCCATCATTCCCCAG-3′ (reverse); and SF162/HxB2 V1 F/N, 5′-ATGATAATGGAGAAAGGAGAAATAAAAAATTGC-3′ (forward) and 5′-GCAATTTTTTATTTCTCCTTTCTCCATTATCAT-3′ (reverse). The final chimeric SF162 constructs containing a heterologous V1 are designated “SF162/Env V1,” where “Env” designates the isolate from which the V1 loop sequence was derived (Fig. 1C). The entire Env genes for these constructs were sequence verified. Several properties of the incorporated heterologous V1 loops (i.e., length, number of potential N′-linked glycosylation sites, tryptophan and tyrosine residues, aliphatic hydrophobic residues, and charge) can be found in Fig. 1D.
Generation of single-round competent virions.
Single-replication, luciferase-reporter pseudovirions for each chimeric “SF162/Env V1” construct were generated as previously described (12, 17). Briefly, 2 × 105 293-T cells were cotransfected with vectors expressing the different gp160 envelopes using GeneJuice transfection reagent (Novagen, Gibbstown, NJ). The pNL4-3 luciferase-positive (luciferase+) Vpr− Env− construct was used as the backbone. The ratio of gp160-expressing to pNL4-3-expressing vectors used during transfection was 1:20. The p24 antigen concentration of each pseudovirus preparation was determined with the HIV-1 p24 antigen capture assay kit (AIDS Vaccine Program, NCI-Frederick Cancer Research and Development Center).
gp140 Env immunogens.
The immunogens tested were WT SF162 gp140, ΔV1SF162 gp140, SF162/YU2 V1 gp140, SF162/JRFL V1 gp140, SF162/89.6 V1 gp140, and SF162/HxB2 V1 gp140. gp140-encoding sequences were created by introducing two stop codons upstream of the transmembrane gp41 domain in the discussed gp160 genes, as previously described (1, 17). The primary and secondary gp120-gp41 cleavage sites were also eliminated by mutagenesis (1, 17). ΔV1SF162 gp140 lacks the central 17 amino acids (aa) of the SF162 V1 loop (from Lys at position 134 to Lys at position 150 [HxB2] numbering) (43, 53). The production and purification of trimeric gp140 proteins were previously described (42, 46).
Immunizations.
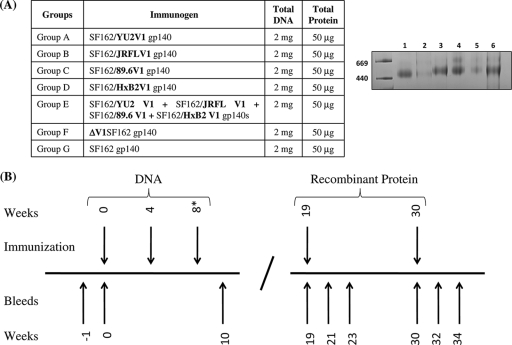
Guinea pigs (Pocono Rabbitry, Canadensis, PA) were immunized using the DNA-prime/protein-boost vaccination system (1, 8, 17). Seven immunization groups were tested (Fig. 2 A). Groups A to D (SF162/YU2 V1, SF162/JRFL V1, SF162/89.6 V1, and SF162/HxB2 V1) received one of the four chimeric SF162/Env V1 gp140 constructs, group E received a cocktail mixture of all four chimeric SF162/Env V1 gp140s, and group F and G animals were inoculated with the ΔV1SF162 gp140 and WT SF162 gp140 immunogens, respectively. As summarized in Fig. 2B, groups A to E received two immunizations with DNA vectors expressing the corresponding gp140 proteins, each administered 4 weeks apart. Following a brief period of rest, animals were boosted twice (11 weeks apart) with the corresponding purified trimeric gp140 protein emulsified in equal volume of Ribi adjuvant (Funakoshi Co., Tokyo, Japan). Group F and G animals were on a similar immunization schedule, but instead of receiving two DNA immunizations they received three DNA immunizations at 4-week intervals. In all cases, a total of 2 mg of DNA in 2 ml of endotoxin-free water was administered each time per animal intramuscularly at the quadriceps. Group E animals received a mixture of all four chimeric constructs (0.5 mg of each). A total of 50 μg of protein in a 0.2-ml total volume was administered intramuscularly to the deltoids of each animal. Animals in group E received 12.5 μg of each of the four gp140 proteins as a cocktail. Animals were bled at various times between each immunization phase and at several points following the protein boosts.
FIG. 2.
Immunogens and immunization schedule. (A) Left, seven immunization groups (A to G) with four guinea pigs each were used. The immunogen(s) in each group is indicated, as is the amount of DNA or recombinant protein used during immunization. Total DNA, total amount of DNA used per animal per immunization session. The total amount was split equally between the four constructs used to inoculate group E animals. Total protein, total amount of recombinant gp140 Env used per animal per immunization. The total amount was split equally between the four constructs used to inoculate group E animals. Protein was emulsified in an equal volume of Ribi adjuvant. Right, blue native PAGE of purified gp140 trimers (5 μg). Lanes: 1, WT SF162 trimer; 2, ΔV1SF162 trimer; 3, SF162/89.6 V1 trimer; 4, SF162/HxB2 V1 trimer; 5, SF162/JRFL V1 trimer; 6, SF162/YU2 V1 trimer. (B) Immunization schedule. Animals received two or three DNA immunizations with vectors expressing gp140 at 4-week intervals, followed by two immunizations with the corresponding recombinant gp140 proteins, spaced 11 weeks apart. Groups A to E received two DNA immunizations, whereas groups F and G received three DNA immunizations. Bleeds were collected throughout the immunization schedule, at the indicated times (weeks).
Recombinant proteins and peptides.
Linear peptides derived from the V1 variable region of SF162 (TNLKNATNTKSSNWKEMD), YU2 (RNATNTTSSSWETMEKG), JRFL (NATNTTNDSEGTMERG), 89.6 (NITKNTTNPTSSSWGMMEKG), and HxB2 (KNDTNTNSSSGRMIMEKG), as well as an SF162 N′-terminal V2 peptide (TTSIRNKMQKEYALF), an SF162 C′-terminal V2 peptide (YKLDVVPIDNDNTSY), and a scrambled SF162 V3 peptide (TRKSFYATPGRAITIG), were purchased from Sigma-Genosys (The Woodlands, TX). An SF162 V3 peptide (CTRKSITIGPGRAFYC) was provided by Genscript (Piscataway, NJ). The 2F5 (NEQELLELDKWASLWN) and 4E10 (NWFDITNWLWYIRKKK) peptides were also purchased from Genscript (Piscataway, NJ). The HxB2 gp41 ectodomain lacking the fusion peptide, membrane-spanning, and cytoplasmic domains was purchased from Viral Therapeutics (Ithaca, NY).
Anti-Env binding titers.
To determine the titer of Env-specific binding antibodies in immune plasma, two methodologies were implemented: enzyme-linked immunosorbent assay (ELISA) and Luminex.
(i) ELISA.
Fifty nanograms of peptide or purified trimeric gp140 protein was absorbed onto 96-well Immulon 2HB ELISA plates in 0.1 M sodium bicarbonate (pH 9.4) overnight at room temperature (RT). Plates were blocked for 2 h at RT in 100 μl of phosphate-buffered saline (PBS), 10% nonfat milk, and 0.3% Tween 20 and then washed three times in a plate washer. Serially diluted plasma (or MAb) was added in duplicate in 10% nonfat milk and 0.03% Tween 20 in PBS for 1 h at 37°C. Plates were washed, and bound guinea pig antibody was detected with 100 μl of a secondary IgG-horseradish peroxidase (HRP) antibody (Zymed, San Francisco, CA) diluted in PBS, 10% nonfat milk, and 0.03% Tween 20 for 1 h at 37°C. After a final set of washes, 50 μl of SureBlue Reserve TMB microwell peroxidase substrate (KPL, Gaithersburg, MD) and then 50 μl of stop solution (0.5 M H2SO4) were added, and the absorption at 450 nm was detected on a Versamaxx microplate reader (Molecular Devices, Sunnyvale, CA).
(ii) Luminex.
The same linear V1 or V3 loop-derived peptides used in ELISAs were coupled to carboxylated beads via free amines such as those present at the N′-terminal amino groups of all peptides, or on lysine residues, using a two-step carbodiimide coupling methodology provided through Luminex's xMAP technology. Multiplex analysis was carried out for each of the five V1 peptides (SF162, YU2, JRFL, 89.6, and HxB2 V1) and the SF162 V3 peptide, which were coupled to beads according to the manufacturer's guidelines (Luminex Corporation, Austin, TX). All V1 peptides could be coupled to the beads via the free amines at their N′-terminal ends. All but the JRFL V1 peptide also contain at least one lysine residue at their C′-terminal sides that can also be used to cross-link the peptides to the beads. Thus, the JRFL V1 peptide was engineered with a lysine residue at the C′ terminus. A total of 1,500 washed and blocked peptide-coupled beads were aliquoted and aspirated per well onto a multiwell filter plate (PALL Life Sciences, Ann Arbor, MI). To these beads, 100 μl of pooled heat-inactivated guinea pig plasma at a single dilution of 1:20 was added and incubated for 1 h at RT with gentle shaking at 600 rpm. Next, the plate was washed three times with 1× PBS and 0.05% Tween 20 (wash buffer) and then incubated with 100 μl/well of 1:1,000 rabbit anti-guinea pig-HRP (Zymed, San Francisco, CA) for 1 h with shaking (600 rpm). The beads were then washed three times with wash buffer and incubated for an additional hour with 100 μl/well of 1:500 goat-anti-rabbit-RPE 2o (Southern Biotechnology, Birmingham, AL). Before plate analysis, beads were run through a final sequence of three washes in 1× PBS-0.05% bovine serum albumin (BSA), followed by resuspension in 100 μl of dilution buffer. The net median fluorescence intensity (MFI) was acquired by reading plates on the Bioplex 200 system (Bio-Rad, Hercules, CA).
Plasma IgG purification.
Total IgG was isolated from guinea pig plasmas using a protein A-agarose antibody spin column kit according to the manufacturer's guidelines (Thermo Scientific, Rockford, IL). A total of 100 to 200 μl of heat-inactivated plasma from individual animals or from each of the pooled group plasmas were used (prebleed plasma and plasma collected 2 weeks following the final protein immunization). IgG was eluted in 500 μl of IgG elution buffer (Thermo Scientific, Rockford, IL) and then concentrated and buffer exchanged in PBS through a Microcon centrifugal 30-kDa-cutoff filter unit (Sigma-Aldrich, Saint Louis, MO). Samples were filter sterilized through ultrafree-MC 0.22-μm filters (Millipore, Billerica, MA). The final concentration of product was determined by UV absorbance at 280 nm.
Neutralization assays.
Neutralization assays were performed using pseudovirus preparations in the TZM-bl cell-based assay as previously described (12, 17, 32, 38). Briefly, serially diluted plasma, purified IgG, or monoclonal reagents were incubated for 90 min in the presence of single-round competent virions at 37°C. Ab dilutions were made in duplicate in complete DMEM in 96-well U-bottom tissue culture plates (Falcon). The virus-plasma mixture was added to TZM-bl cells plated 24 h prior at a density of 3 × 103 cells/well in a 96-well flat plate (Corning Inc., Lowell, MA) and left for 72 h at 37°C. Prior to inoculation, the cells were pretreated with Polybrene (2 μg/ml) in complete DMEM for 30 min at 37°C. Cell supernatants were removed, and 100 μl of Steady-Glo luciferase (Promega, Madison, WI) was added under gentle agitation for 3 min at RT to lyse cells. Seventy-five microliters of the cell lysate was transferred to 96-well white microtiter plates and read for luminescence to determine cell-associated luciferase activity. Percent neutralization at each plasma dilution was calculated using the following equation: [(RLUpreimmune − RLUimmune)/RLUpreimmune] × 100. Pseudovirions expressing the unrelated amphotropic murine leukemia virus (MLV) Env were implemented as a control for non-HIV-specific neutralizing activity.
Epitope mapping of vaccine-induced neutralizing antibodies.
To define the epitope specificities of the NAbs elicited during immunization, two complementary approaches were used: peptide competition and D368R gp120 competition.
(i) Peptide competitions.
Twenty microliters of 20-μg/ml peptide in DMEM was added to a 20-μl volume of serially diluted plasma (or MAb) and incubated at 37°C for 60 min. Pseudovirus (in 20 μl) was added to the plasma-peptide mixture and left for an additional 60 min at 37°C. The plasma-peptide-pseudovirus mixture was then incubated with Polybrene-treated TZM-bl cells plated at a density of 3 × 103 cells per well for 72 h at 37°C, after which cell-associated luciferase was determined. Percent neutralization was calculated at each plasma dilution in the presence and absence of peptide. For competitions done with polyclonal plasma, the percent reduction in plasma neutralizing activity in the presence of peptide was determined at the plasma dilution that resulted in 70% inhibition of infection in the absence of peptide. For competitions done with purified IgG, the percent reduction in plasma neutralizing activity was determined as a reduction in the “area under the curve” relative to neutralization in the absence of peptide. At the concentration used, the peptides did not interfere with viral entry.
(ii) D368R gp120 competitions.
Competition assays with a recombinant SF162 gp120 protein harboring a D368R (based on HxB2 Env numbering) mutation in the CD4-bs were performed. The D-to-R mutation at position 368 of the HIV Env abrogates the binding of most anti-CD4-bs Abs but maintains binding with most gp120 non-CD4-bs Abs such as MAbs 2G12 and 447-52D. The D368R gp120 mutant protein was previously used to define the presence of anti-CD4-bs NAbs in HIV+ sera (6, 19, 29, 33, 42). Here, we adopted the D368R gp120 protein for use in a standard peptide competition neutralization assay. A 20-μg/ml concentration of D368R gp120 protein in 20 μl was preincubated with 20 μl of serially diluted plasma (or MAb) for 1 h at 37°C. To the plasma-protein mixture, pseudovirus in 20 μl was added and incubated for an additional hour at 37°C. The final plasma-protein-pseudovirus mixture was then added to 3 × 103 TZM-bl cells and left for 72 h at 37°C, after which cells were lysed and cell-associated luciferase was determined. For plasma neutralization experiments, any reduction in neutralizing activity in the presence versus absence of D368R was determined at the plasma dilution that resulted in 70% neutralization in the absence of D368R. For purified IgG neutralization experiments, the percent reduction in neutralizing activity was determined from the “areas under the curve” in the presence and absence of D368R.
Statistical analysis.
InStat (GraphPad) was used for statistical analysis. Endpoint ELISA titer differences were analyzed nonparametrically using the two-tailed Mann-Whitney test. To examine whether 50% inhibition titers correlate with anti-gp140 or anti-V3 titers, the nonparametric Spearman correlation was calculated.
RESULTS
Neutralization phenotypes of single-round pseudoviruses expressing V1 modified Envs.
We previously reported that deletion of the V1 loop from the SF162 Env does not abrogate the ability of that Env to mediate virus-cell entry (43, 53). We also reported that the YU2, JRFL, 89.6, and HxB2 Envs remain functional when their respective V1 loops are replaced by that of SF162 (12). Here we first examined whether the SF162 Env remains functional when its V1 loop is replaced by that of YU2, JRFL, 89.6, or HxB2. Viruses expressing these four V1 chimeric Envs entered TZM-bl and U87-CD4-CCR5 cells as efficiently as the WT (data not shown).
We next examined how these substitutions affected the neutralization phenotype of the SF162 virus against known MAbs and IgG-CD4 (Table 1). V1 modification through either deletion or substitution did not significantly alter the neutralization susceptibility of SF162 to MAb b12 or IgG-CD4, but an ∼1-log10-unit increase in b6 resistance was observed for ΔV1SF162 and SF162/89.6 V1. Another Env region affected by V1 modification was the V3 loop. Several V1-modified viruses (i.e., SF162/JRFL V1, SF162/89.6 V1, and ΔV1SF162) were approximately 1 log10 unit more resistant than WT SF162 to neutralization by the anti-V3 MAbs P3E1 and 447-52D. In contrast, the neutralization susceptibilities of the chimeric viruses to MAbs 2G12, 2F5, and 4E10 (with the possible exception of the SF162/89.6 V1) were very similar to that of WT SF162. Overall, our neutralization data suggest that within the context of the virion-associated gp160 Env, properties within the V1 loop (i.e., length, number of PNGs, and amino acid composition [Fig. 1D]) greatly influence the exposure of gp120 epitopes within the V3 loop and the CD4-bs.
TABLE 1.
Effect of V1 modification on viral neutralization susceptibility to MAbs
| Virus | 50% Neutralization titer (IC50)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| b12 (anti-CD4) | IgG-CD4 (anti-CD4) | b6 (anti-CD4) | P3C8 (anti-V1) | P3E1 (anti-V3) | 447-52D (anti-V3) | 2G12 (antimannose) | 2F5 (anti-gp41) | 4E10 (anti-gp41) | |
| SF162 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.14 ± 0.03 | 0.16 ± 0.00 | 0.001 ± 0.00 | 0.06 ± 0.00 | 0.22 ± 0.00 | 0.21 ± 0.03 | 0.34 ± 0.03 |
| SF162/YU2 V1 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.17 ± 0.02 | — | 0.002 ± 0.00 | 0.28 ± 0.07 | 0.38 ± 0.01 | 0.24 ± 0.07 | 0.70 ± 0.08 |
| SF162/JRFL V1 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.14 ± 0.02 | — | 0.01 ± 0.01 | 0.15 ± 0.01 | 0.49 ± 0.19 | 0.23 ± 0.02 | 0.60 ± 0.03 |
| SF162/89.6 V1 | 0.05 ± 0.04 | 0.06 ± 0.01 | 1.55 ± 0.29 | — | 0.08 ± 0.01 | 2.41 ± 0.21 | 0.50 ± 0.19 | 0.41 ± 0.08 | 1.28 ± 0.27 |
| SF162/HxB2 V1 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.10 ± 0.03 | — | 0.0003 ± 0.00 | 0.06 ± 0.01 | 0.68 ± 0.02 | 0.22 ± 0.11 | 0.48 ± 0.02 |
| ΔV1SF162 | 0.01 ± 0.00 | 0.04 ± 0.01 | 1.79 ± 0.24 | — | 0.02 ± 0.00 | 0.56 ± 0.06 | 0.17 ± 0.02 | 0.14 ± 0.02 | 0.47 ± 0.07 |
The values represent the MAb concentration required to achieve 50% inhibition of viral infection. Results are averages and standard deviations from two independent experiments performed in duplicate. MAbs were tested at an initial dilution of 20 μg/ml. The Env region targeted is denoted in parentheses. —, MAb did not neutralize the isolate above 50% at the highest MAb concentration tested (20 μg/ml).
Antigenic profile of trimeric gp140 protein immunogens.
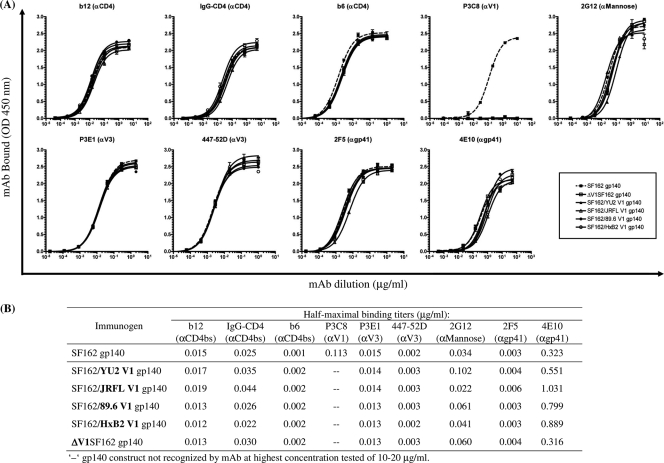
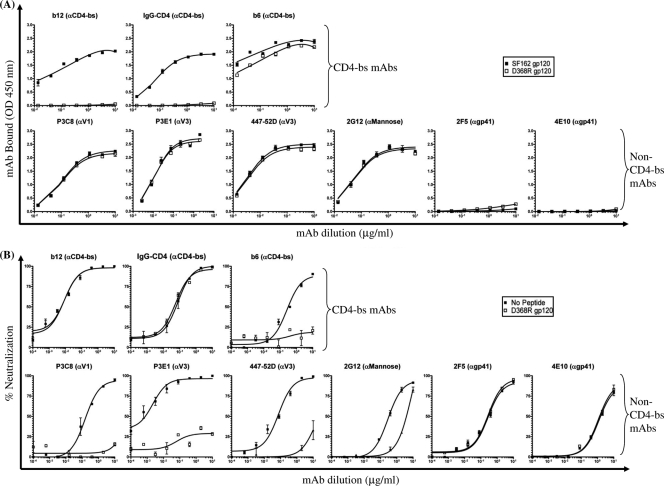
To examine whether diverse linear and conformational epitopes, particularly those within the V3 and CD4-bs, were also differentially exposed on the corresponding soluble trimeric gp140 proteins, we next evaluated the relative binding affinities of the same well-characterized MAbs to trimeric versions of our SF162 V1-deleted and SF162/Env V1-chimeric gp140 constructs by ELISA. Highly purified samples of each trimeric gp140 protein are shown by native PAGE (Fig. 2A); gp140 trimers have a predicted combined molecular mass of 480 kDa. Binding curves are shown in Fig. 3 A, and a summary of the half-maximum binding values is presented in Fig. 3B.
FIG. 3.
Antigenic characterization of purified trimeric SF162-derived gp140 constructs. (A) Binding curves of individual MAbs or IgG-CD4 to gp140 proteins. The epitope specificity of each MAb is shown in parentheses. MAbs and IgG-CD4 were tested at an initial concentration of 10 to 20 μg/ml against purified trimeric gp140 protein at 50 ng. Error bars indicate standard deviations. (B) The calculated half-maximal binding titers (μg/ml) are reported.
Recognition of trimeric SF162 gp140 by the two CD4-bs Abs (b12 and b6) and by IgG-CD4 was not altered upon deletion of the V1 loop or following its substitution with a heterologous V1 sequence. Thus, although several V1-modified viruses displayed various differences in their neutralization sensitivity to CD4-bs reagents such as MAb b6, these differences were not recorded when the soluble gp140 versions of those Envs were used in an ELISA format. As predicted, deletion of the central 17 amino acids from the V1 loop (ΔV1SF162) eliminated V1 recognition by the anti-V1 MAb P3C8. Also, P3C8 cross-reactivity was not observed with any of the SF162/Env V1 scaffolds harboring a heterologous V1 sequence. The anti-V3 MAbs P3E1 and 447-52D bound to all V1-modified gp140 constructs as well as SF162 gp140. These results also differ from the neutralization results presented above (Table 1), which depict several chimeric isolates as being more resistant than SF162 to P3E1 and/or 447-52D. Only small changes in binding were observed for the anti-gp41 MAbs 2F5 and 4E10 and for MAb 2G12. These results are in agreement with neutralization data, where no major shifts in neutralization sensitivity were observed with these three MAbs.
Immunogenic profiles of V1-modifed gp140 proteins.
Minor alterations to the antigenic phenotype of soluble SF162 gp140-derived trimeric proteins may have major and unpredictable effects on their immunogenic profiles. To assess and compare the immunogenic properties of SF162 gp140, ΔV1SF162 gp140, and the four V1 chimeric gp140s, we performed immunogenicity studies in guinea pigs, as described in Fig. 2.
(i) Anti-gp140 binding titers.
Relative endpoint titers for gp140-specific Abs were determined throughout the immunization protocol. In Table 2 we summarize the immunogenicity data with plasma collected at 2 weeks following the final recombinant gp140 protein immunization, since these are the samples we analyzed most extensively, both for epitope recognition and for neutralizing activity. Immune plasma from every animal in each of the seven immunization groups was evaluated against the immunogen with which the animals were immunized. Plasmas from group E animals, receiving a combination of all four V1 chimeric immunogens, were tested against a mixture of all four V1 chimeric gp140 proteins in equal parts.
TABLE 2.
Antibody titers and epitope specificities of immune plasma antibodies determined by ELISA
| Group | Animal | Antibody binding fora: |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| gp140b | N′ V2 (SF162) | C′ V2 (SF162) | V3 (SF162) | V1 (SF162) | V1 (YU2) | V1 (JRFL) | V1 (89.6) | V1 (HxB2) | gp41 (HxB2) | 2F5 | 4E10 | Scrambled V3 | ||
| A (SF162/YU2 V1) | 2071 | 69,894 | — | — | 5,879 | — | — | — | — | — | 21,778 | — | — | — |
| 2072 | 39,831 | — | — | 9,974 | — | — | — | — | — | 6,309 | — | — | — | |
| 2073 | 19,183 | — | — | 5,209 | — | — | — | — | — | 4,043 | — | — | — | |
| 2074 | 12,486 | — | — | 6,511 | — | — | — | — | — | 2,885 | — | — | — | |
| Avgc | 35,348 (P = 0.0286)d | — | — | 6,893 (P = 0.0286) | — | — | — | — | — | 8,754 (P = 0.200) | — | — | — | |
| B (SF162/JRFL V1) | 2075 | 52,690 | — | — | 9,844 | — | — | — | — | — | 24,854 | — | — | — |
| 2076 | 26,157 | — | — | 7,296 | — | — | — | — | — | 5,990 | — | — | — | |
| 2077 | 14,831 | — | — | 2,854 | — | — | — | — | — | 4,026 | — | — | — | |
| 2078 | 16,915 | — | — | 2,030 | — | — | — | — | — | 10,837 | — | — | — | |
| Avg | 27,648 (P = 0.0286) | — | — | 5,506 (P = 0.0286) | — | — | — | — | — | 11,427 (P = 0.3429) | — | — | — | |
| C (SF162/89.6 V1) | 2079 | 60,791 | — | — | 11,970 | — | — | — | 191 | — | 4,334 | — | — | — |
| 2080 | 307,200 | — | — | 95,925 | — | — | — | — | — | 20,263 | — | — | — | |
| 2081 | 49,491 | — | — | 17,765 | — | — | — | 398 | — | 1,287 | — | — | — | |
| 2082 | 86,042 | — | — | 24,402 | — | — | — | 479 | — | 7,069 | — | — | — | |
| Avg | 125,881 (P = 0.3429) | — | — | 37,515 (P = 0.3429) | — | — | — | 356 | — | 8,238 (P = 0.200) | — | — | — | |
| D (SF162/HxB2 V1) | 2083 | 14,429 | — | — | 1,266 | — | — | — | — | — | 2,265 | — | — | — |
| 2084 | 13,528 | — | — | 1,838 | — | — | — | — | — | 1,378 | — | — | — | |
| 2085 | 56,312 | — | — | 5,751 | — | — | — | — | — | 6,964 | — | — | — | |
| 2086 | 75,455 | — | — | 27,728 | — | — | — | — | — | 14,440 | — | — | — | |
| Avg | 39,931 (P = 0.0286) | — | — | 9,146 (P = 0.0571) | — | — | — | — | — | 6,261 (P = 0.200) | — | — | — | |
| E (V1 scaffold cocktail) | 2059 | 78,145 | — | — | 31,823 | — | — | — | — | — | 14,998 | — | — | — |
| 2060 | 57,647 | — | — | 24,543 | — | — | — | — | — | 12,049 | — | — | — | |
| 2061 | 32,430 | — | — | 8,399 | — | — | — | — | — | 7,157 | — | — | — | |
| 2062 | 20,450 | — | — | 6,971 | — | — | — | — | — | 3,484 | — | — | — | |
| Avg | 47,168 (P = 0.0286) | — | — | 17,934 (P = 0.1143) | — | — | — | — | — | 9,422 (P = 0.0286) | — | — | — | |
| F (ΔV1SF162) | 1918 | 93,142 | — | — | 13,872 | — | — | — | — | — | 29,758 | — | — | — |
| 1919 | 32,235 | — | — | 22,316 | — | — | — | — | — | 14,225 | — | — | — | |
| 1920 | 98,272 | — | — | 39,657 | — | — | — | — | — | 27,074 | — | — | — | |
| 1921 | 270,384 | — | — | 6,723 | — | — | — | — | — | 88,520 | — | — | — | |
| Avg | 123,508 (P = 0.0286) | — | — | 20,642 (P = 0.200) | — | — | — | — | — | 39,894 (P = 0.3429) | — | — | — | |
| G (SF162 gp140) | 1914 | 152,429 | — | — | 62,221 | 18,139 | — | — | — | — | 15,239 | — | — | — |
| 1915 | 210,929 | — | — | 97,148 | 354 | — | — | — | — | 23,081 | — | — | — | |
| 1916 | 146,451 | — | — | 22,316 | 43,652 | — | — | — | — | 19,150 | — | — | — | |
| 1917 | 151,417 | — | — | 32,703 | 2,919 | — | — | — | — | 15,607 | — | — | — | |
| Avg | 165,307 | — | — | 53,597 | 16,266 | — | — | — | — | 18,269 | — | — | — | |
Values indicate relative endpoint ELISA titers against the indicated recombinant proteins or linear peptides. Plasmas were collected at 2 weeks following the second gp140 protein immunization. Designations in parentheses indicate the viral isolate from which the peptide or protein is derived. —, reactivity not detected at the lowest plasma dilution tested (1:20).
SF162 and ΔV1SF162 immune plasmas were tested against SF162 and ΔV1SF162 gp140 trimeric proteins, respectively. Plasmas from groups A to D were tested against their matching SF162/EnvV1 gp140 protein. Group E animal plasma was tested against a mixture of each scaffold gp140 in equal parts.
Average group values include only animals with detectable antibody titers.
Statistical P values, comparing binding titers for each group with those of group G (SF162 gp140).
All animals, irrespective of the immunogen with which they were immunized, developed potent anti-Env binding titers. The binding Ab titers for groups A (SF162/YU2 V1, P = 0.0286), B (SF162/JRFL V1, P = 0.0286), D (SF162/HxB2 V1, P = 0.0286), and E (V1 scaffold cocktail, P = 0.0286) were significantly lower than those in group G (SF162 gp140). Within-group variability and small sample size (four animals per group) may account in part for the lack of a statistical difference with groups C (SF162/89.6 V1, P = 0.3429) and F (ΔV1SF162, P = 0.3429).
(ii) Immunogenicity of the V1, V2, and V3 loops on the V1-modified gp140 proteins.
To determine whether the decreased overall immunogenicity on the V1-modified gp140s was due to differences in the immunogenicities of the heterologous V1 loops or to changes in the immunogenicities of other variable regions, such as the V2 and V3 loops, we determined the anti-V1, anti-V2, and anti-V3 Ab titers (Table 2).
In agreement with previous observations (16, 17) we did not detect anti-V2 loop-directed antibodies in any of the immune sera. In contrast, the V3 loop was immunogenic across all constructs, although the chimeric constructs elicited lower anti-V3 loop antibody titers than SF162 gp140. However, only in groups A (SF162/YU2 V1, P = 0.0286) and B (SF162/JRFL V1, P = 0.0286) were these titers statistically significantly lower. These results are consistent with the observation that these same groups also elicited significantly lower anti-gp140 Ab titers. Taken together, these results suggest that V3 loop immunogenicity on SF162 was decreased by V1 deletion and by substitution with a heterologous V1 loop. Decreases in V3 immunogenicity may account in part for decreases in overall anti-gp140 titers among animals in these groups.
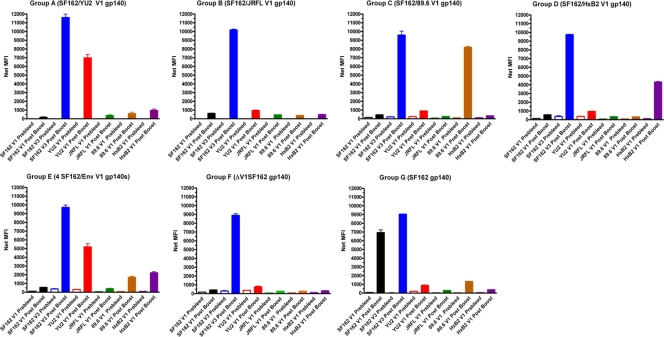
Consistent with previous immunogenicity studies in rabbits and in rhesus macaques (17, 28), SF162 gp140 elicited high titers of anti-SF162 V1 Abs (Table 2) which did not cross-react with any of the heterologous V1 peptides. In contrast, however, with the exception of the SF162/89.6 V1-immunized group, anti-V1 Abs were not detected in any of the other immune plasmas. The absence of anti-V1 antibodies in groups A, B, and D was unexpected. Thus, a different method (Luminex) was used to assess whether such antibodies were elicited but were for some reason undetectable by ELISA (Fig. 4). As a control we used the SF162 V3 peptide, which is common to all immunogens tested here. Consistent with our ELISA results, V3 Ab titers were detected across all immune groups. However, in contrast to our ELISA data, anti-V1 Abs were detectable in plasmas from all groups except B (SF162/JRFL V1). As expected, ΔV1SF162 plasma failed to react with any V1 peptide. Plasma Abs from group E animals, which received a combination of four different V1 scaffold immunogens, reacted with multiple V1 peptides (YU2, 89.6, and HxB2 but not JRFL V1). Compared to those in each of the monovalent groups that received only a single construct (groups A, C, and D), the anti-YU2, -89.6, and -HxB2 V1 titers were lower overall in group E plasma. These results suggest that three of four heterologous V1 loops were immunogenic in the context of the SF162 Env and that inclusion of multiple V1 scaffolds can expand the repertoire of anti-V1 Abs. It remains unclear why anti-V1 Abs were detectable by Luminex but not by ELISA. Potentially these differences could be due to the different ways that the peptides are oriented during the two assays.
FIG. 4.
Reactivity of immune plasmas to V1 or V3 peptides. Luminex technology was used to assess the presence of anti-V1 or anti-V3 antibodies in immune sera, as discussed in detail in Materials and Methods. In each case the signals recorded with plasma collected before the initiation of immunizations and sera collected at 2 weeks following the last recombinant Env immunization are shown. Pooled plasmas from each group were evaluated at a single dilution (1:20). The group code name and the immunogen used are shown at the top of each panel. The peptide used to screen immune antibodies is shown at the bottom of each panel. Error bars indicate standard deviations.
(iii) gp41 immunogenicity on soluble gp140s.
All immunogens elicited high titers of anti-gp41 binding Abs (Table 2), although the V1 modifications affected the immunogenicity of gp41. The V1 chimeric immunogens elicited lower overall anti-gp41 titers than SF162 gp140 (although statistically significantly lower titers were observed only in groups D [SF162/HxB2 V1, P = 0.0286] and E [V1 scaffold cocktail, P = 0.0286]). In contrast, deletion of the V1 loop (group F, ΔV1SF162 gp140) increased the immunogenicity of gp41. None of these gp41 antibodies target the 2F5 or 4E10 epitope, as defined by the lack of reactivity with the 2F5 and 4E10 peptides at the highest plasma dilution of 1:20.
Overall, our results suggest that all V1-modified immunogens elicited anti-V1 (with the exceptions of SF162/JRFL V1 gp140 and ΔV1SF162 gp140), anti-V3, and anti-gp41 binding Abs. While both types of V1 modification altered the relative immunogenicities of the V3 and gp41 regions, V2 loop immunogenicity remained unchanged, suggesting that V3 and gp41, but not V2 loop, immunogenicities are linked to the V1 loop (at least with the V1 modifications tested here). Differences in V1, V3, and gp41 immunogenicities may partially account for the observed differences in overall anti-gp140 binding titers for groups A to F compared to group G.
Virus neutralization.
To determine whether and how differences in epitope immunogenicity translate into differences in the neutralizing properties of the elicited Ab response, we tested for neutralizing activity against a small panel of six clade B viruses (depicting a range of neutralization sensitivities): SF162, YU2, JRFL, 89.6, HxB2, and SS1196. SF162 represents the homologous virus, and YU2, JRFL, 89.6, and HxB2 represent viruses whose V1 sequences were used to substitute the SF162 V1. Also tested were several “early” transmitted variants: 6535, QH0692, REJO, and BG1168 (data not shown).
(i) Homologous SF162 neutralization.
The V1 chimeric immunogens elicited lower anti-SF162 neutralizing antibody titers than the WT SF162 gp140 immunogen (Table 3), although only in groups A, B, and E were these titers statistically different from those in the WT SF162 gp140 group (group A, P = 0.0286; B, P = 0.0286; and E, P = 0.0286). Only three of four ΔV1SF162 gp140-immunized animals elicited anti-SF162 NAbs (Table 3). A positive linear correlation between the SF162 50% inhibitory concentration (IC50) neutralizing antibody titers of each animal and their anti-gp140 binding titers (r = 0.6581; P < 0.0003) was evident. Therefore, the lower neutralizing potencies of antibodies from groups A to F compared to group G (WT SF162 gp140) are in part explained by lower overall anti-gp140 titers in those groups. These results indicate that the potency of the homologous response was not improved upon V1 deletion or substitution.
TABLE 3.
Neutralization of homologous SF162 isolate
| Group | Animal | IC50a | % Reduction in neutralization |
||
|---|---|---|---|---|---|
| V1b | V3 | D368R | |||
| A (SF162/YU2 V1 gp140) | 2071 | 519 ± 182 | 0.0 ± 0.0 | 27.2 ± 2.9 | 55.0 ± 2.1 |
| 2072 | 4,025 ± 435 | 0.0 ± 0.0 | 62.9 ± 0.0 | 89.3 ± 2.1 | |
| 2073 | 1,071 ± 410 | 0.0 ± 0.0 | 49.3 ± 6.4 | 95.0 ± 0.7 | |
| 2074 | 2,218 ± 609 | 0.0 ± 0.0 | 77.9 ± 0.8 | 100.0 ± 0.0 | |
| Avgc | 1,958 (P = 0.0286)d | 0.0 | 54.3 | 84.8 | |
| B (SF162/JRFL V1 gp140) | 2075 | 1,262 ± 510 | 0.0 ± 0.0 | 40.7 ± 3.6 | 75.0 ± 16.4 |
| 2076 | 466 ± 71 | 0.0 ± 0.0 | 42.9 ± 0.0 | 56.2 ± 11.3 | |
| 2077 | 279 ± 0 | 0.0 ± 0.0 | 20.7 ± 3.6 | 9.5 ± 7.6 | |
| 2078 | 424 ± 26 | 0.0 ± 0.0 | 40.0 ± 0.0 | 53.8 ± 1.3 | |
| Avg | 608 (P = 0.0286) | 0.0 | 36.1 | 48.6 | |
| C (SF162/89.6 V1 gp140) | 2079 | 1,128 ± 214 | 0.0 ± 0.0 | 25.0 ± 0.7 | 38.6 ± 4.3 |
| 2080 | 46,306 ± 7,610 | 0.0 ± 0.0 | 67.9 ± 3.6 | 100.0 ± 0.0 | |
| 2081 | 9,328 ± 1,573 | 0.0 ± 0.0 | 57.9 ± 2.1 | 81.4 ± 10.1 | |
| 2082 | 2,035 ± 429 | 0.0 ± 0.0 | 50.7 ± 6.4 | 88.6 ± 5.7 | |
| Avg | 14,699 (P = 0.1143) | 0.0 | 50.4 | 77.1 | |
| D (SF162/HxB2 V1 gp140) | 2083 | — | — | — | — |
| 2084 | 815 ± 31 | 0.0 ± 0.0 | 26.4 ± 5.0 | 40.8 ± 10.8 | |
| 2085 | 506 ± 149 | 6.5 ± 2.2 | 0.0 ± 0.0 | 27.2 ± 2.9 | |
| 2086 | 903 ± 129 | 8.6 ± 2.9 | 49.3 ± 0.7 | 95.0 ± 5.0 | |
| Avg | 741 (P = 0.0571) | 5.0 | 25.2 | 54.3 | |
| E (V1 scaffold cocktail) | 2059 | 5,394 ± 575 | 0.0 ± 0.0 | 42.2 ± 2.1 | 87.2 ± 5.8 |
| 2060 | 2,278 ± 701 | 0.0 ± 0.0 | 27.9 ± 2.1 | 77.9 ± 6.5 | |
| 2061 | 1,303 ± 243 | 5.7 ± 0.0 | 32.9 ± 2.8 | 71.4 ± 4.3 | |
| 2062 | 1,291 ± 119 | 0.0 ± 0.0 | 30.7 ± 3.6 | 67.1 ± 0.0 | |
| Avg | 2,566 (P = 0.0286) | 1.4 | 33.4 | 75.9 | |
| F (ΔV1SF162 gp140) | 1918 | 1,773 ± 538 | 0.0 ± 0.0 | 73.6 ± 22.2 | 78.1 ± 10.6 |
| 1919 | — | — | — | — | |
| 1920 | 19,281 ± 6,180 | 0.0 ± 0.0 | 53.6 ± 2.2 | 95.0 ± 2.1 | |
| 1921 | 2,017 ± 170 | 0.0 ± 0.0 | 20.0 ± 11.2 | 96.4 ± 0.7 | |
| Avg | 7,690 (P = 0.1143) | 0.0 | 49.1 | 89.8 | |
| G (SF162 gp140) | 1914 | 56,407 ± 152 | 33.6 ± 5.0 | 2.2 ± 2.2 | 100.0 ± 0.0 |
| 1915 | 12,932 ± 6,972 | 0.0 ± 0.0 | 4.3 ± 4.3 | 100.0 ± 0.0 | |
| 1916 | 86,672 ± 6,435 | 67.9 ± 0.8 | 3.6 ± 3.6 | 100.0 ± 0.0 | |
| 1917 | 20,041 ± 926 | 12.2 ± 2.2 | 13.4 ± 0.7 | 97.2 ± 2.8 | |
| Avg | 44,013 | 28.4 | 5.9 | 99.3 | |
Plasma was collected at 2 weeks following the second gp140 immunization. The values represent the averages and standard deviations of results from two or three independent experiments performed in duplicate. —, plasma did not neutralize the isolate above 50% at lowest plasma dilution tested (1:20).
The SF162 V1 peptide was used for competitions with group F and G plasmas. The YU2, JRFL, 89.6, and HxB2 V1 peptides were used for group A to D plasma competitions, respectively. A combination of all four heterologous V1 peptides was used to compete out group E plasma.
Average group values include only animals that neutralized virus to 50% inhibition levels.
Statistical P values, comparing IC50 titers for each group with those for group G (SF162 gp140).
(ii) Heterologous HxB2 neutralization.
With the exception of the SF162/YU2 V1 gp140 immunogen, all other immunogens elicited NAbs against the lab-adapted strain HxB2 (Table 4). Because neutralization of HxB2 was significantly less potent and more inconsistent with plasma, neutralization experiments were performed using purified IgG. At a given IgG concentration (100 μg/ml), all immune groups neutralized HxB2, albeit with various potencies. The exception was group A (SF162/YU2 V1), where anti-HxB2 neutralizing activities were not recorded. Interestingly, only two of four WT SF162 gp140 animals neutralized HxB2, and of these two animals, only one (no. 1914) neutralized HxB2 above 70% (even at the highest IgG concentration tested). In contrast, three of four ΔV1SF162 and three of four group D animals neutralized HxB2, and all four animals in groups B, C, and E elicited HxB2 NAbs. Thus, anti-HxB2 neutralizing responses were more consistently elicited by the V1-modified constructs than by the WT SF162 immunogen. In total, eight animals did not neutralize HxB2. Of these, two (no. 1919 and 2083) also failed to neutralize SF162. IgG that neutralized SF162 most potently did not necessarily neutralize HxB2 most potently; thus, a strict correlation between SF162 and HxB2 neutralization potencies was not obvious.
TABLE 4.
Neutralization of heterologous HxB2 isolate
| Group | Animal | % Neutralization (100 μg/ml IgG)a | % Reduction in neutralization |
||
|---|---|---|---|---|---|
| V1b | V3 | D368R | |||
| A (SF162/YU2 V1 gp140) | 2071 | — | — | — | — |
| 2072 | — | — | — | — | |
| 2073 | — | — | — | — | |
| 2074 | — | — | — | — | |
| Avgc | — | — | — | — | |
| B (SF162/JRFL V1 gp140) | 2075 | 58.0 ± 2.2 | 2.7 ± 2.7 | 2.0 ± 0.0 | 11.0 ± 11.0 |
| 2076 | 54.5 ± 3.8 | 0.0 ± 0.0 | 2.1 ± 0.0 | 16.4 ± 1.8 | |
| 2077 | 66.6 ± 4.8 | 0.0 ± 0.0 | 0.0 ± 0.0 | 11.2 ± 0.8 | |
| 2078 | 56.8 ± 7.5 | 0.0 ± 0.0 | 0.0 ± 0.0 | 30.6 ± 6.8 | |
| Avg | 59.0 | 0.7 | 1.0 | 17.3 | |
| C (SF162/89.6 V1 gp140) | 2079 | 78.9 ± 4.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 7.2 ± 3.6 |
| 2080 | 79.1 ± 1.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 9.8 ± 2.7 | |
| 2081 | 57.2 ± 0.7 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | |
| 2082 | 76.6 ± 4.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 12.3 ± 7.3 | |
| Avg | 73.0 | 0.0 | 0.0 | 7.3 | |
| D (SF162/HxB2 V1 gp140) | 2083 | — | — | — | — |
| 2084 | 93.7 ± 2.7 | 27.0 ± 0.0 | 0.0 ± 0.0 | 8.3 ± 2.1 | |
| 2085 | 67.9 ± 6.7 | 8.3 ± 8.3 | 1.4 ± 0.0 | 23.0 ± 4.3 | |
| 2086 | 69.9 ± 1.6 | 35.7 ± 0.0 | 0.0 ± 0.0 | 34.3 ± 5.5 | |
| Avg | 77.2 | 23.6 | 0.5 | 21.8 | |
| E (V1 scaffold cocktail) | 2059 | 77.6 ± 2.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 31.5 ± 2.2 |
| 2060 | 86.7 ± 4.2 | 19.2 ± 0.0 | 0.1 ± 0.0 | 21.1 ± 1.3 | |
| 2061 | 84.2 ± 1.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 25.8 ± 4.1 | |
| 2062 | 58.7 ± 0.8 | 12.2 ± 0.0 | 0.0 ± 0.0 | 34.5 ± 0.0 | |
| Avg | 76.8 | 7.9 | 0.0 | 28.2 | |
| F (ΔV1SF162 gp140) | 1918 | 78.9 ± 3.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 42.9 ± 3.0 |
| 1919 | - | - | - | - | |
| 1920 | 81.5 ± 6.2 | 0.0 ± 0.0 | 1.1 ± 0.0 | 4.7 ± 2.9 | |
| 1921 | 81.5 ± 7.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 29.6 ± 4.2 | |
| Avg | 80.6 | 0.0 | 0.3 | 25.9 | |
| G (SF162 gp140) | 1914 | 95.5 ± 4.2 | 0.0 ± 0.0 | 0.0 ± 0.0 | 45.0 ± 6.5 |
| 1915 | 54.4 ± 0.3 | 0.0 ± 0.0 | 0.0 ± 0.0 | 42.6 ± 1.7 | |
| 1916 | — | — | — | — | |
| 1917 | — | — | — | — | |
| Avg | 75.0 | 0.0 | 0.0 | 43.8 | |
Total IgG was purified from plasma collected 2 weeks following the second gp140 immunization. The values represent percent neutralization at 100 μg/ml. The averages and standard deviations of results from two independent experiments performed in duplicate are presented. —, 50% neutralization was not achieved at the highest antibody concentration tested (100 μg/ml).
The SF162 V1 peptide was used for competitions with group F and G IgG. The YU2, JRFL, 89.6, and HxB2 V1 peptides were used for group A to D IgG competitions, respectively. A combination of all four heterologous V1 peptides was used to compete out group E IgG.
Average group values include only animals that neutralized virus to 50% inhibition levels.
Neutralization of all other viruses tested was not observed for any group at the highest IgG concentration tested (100 μg/ml) (data not shown). Therefore, neither deletion of the V1 loop nor V1 substitution enhanced the breadth of the neutralizing antibody response beyond the homologous SF162 and the heterologous HxB2 viruses. Also, despite the ability of several SF162/Env V1 gp140 immunogens to elicit heterologous V1 binding Abs (except SF162/JRFL V1 gp140), these V1 antibodies were unable to neutralize any of the heterologous isolates tested. Group E animals receiving a cocktail of all four SF162/Env V1 scaffolds likewise did not develop broader cross-reactive NAb responses despite eliciting multiple heterologous V1 binding Abs. Finally, despite the presence of anti-V3 and anti-gp41 antibodies across all groups (anti-gp41 titers were actually higher in the ΔV1 immune group compared to WT SF162 gp140), the lack of broad heterologous neutralization indicates that these antibodies are poorly cross-reactive.
Mapping the epitopes recognized by anti-SF162 and anti-HxB2 NAbs.
To define the epitope specificities of the autologous and heterologous NAb responses elicited by the gp140 immunogens evaluated here, we performed a series of peptide and D368R gp120 competition experiments. As discussed above, because neutralization of HxB2 was significantly less potent and more inconsistent with plasma, neutralization competition experiments were performed using purified IgG (100 μg/ml). We first analyzed the contributions of anti-V1, anti-V3, and anti-gp41 Abs to SF162 and HxB2 neutralization, since these regions were immunogenic on one or more of our immunogens (Tables 3 and 4).
(i) Anti-V1-matched Abs contribute partially to SF162 and HxB2 neutralization.
Peptide competition neutralization assays were conducted with V1 peptides that were matched to the V1 loop on the immunogen. The neutralization potency of group E plasma was competed using all four heterologous V1 peptides. Preincubation of SF162 gp140 immune plasma with V1 peptide reduced the plasma neutralizing activity against SF162 (mean group reduction of 28.4%) (Table 3), consistent with previous reports that SF162 gp140 elicits anti-V1 Abs that contribute to homologous SF162 neutralization (17). The extent to which anti-SF162 neutralizing activity was reduced appeared to be proportional to the anti-V1 binding antibody titers. As expected, the neutralizing potency of ΔV1 immune plasma was not competed out with the SF162 V1 peptide. Similarly, the anti-SF162 neutralizing activities of group A to E plasmas were not affected by any of the heterologous V1 peptides tested.
The anti-HxB2 neutralizing activities of plasma IgG from animals immunized with SF162/HxB2 V1 gp140 (groups D and E) were reduced in the presence of the HxB2 V1 peptide (by 23.6% and 7.9%, respectively) (Table 4). Thus, anti-V1 Abs in these animals only partially contributed to HxB2 neutralization. In contrast, the anti-HxB2 neutralizing activities of plasma IgG from animals immunized with the remaining three V1 scaffolds were not inhibited by the HxB2 V1 peptide.
(ii) Anti-V3 Abs contribute partially to homologous SF162 but not to heterologous neutralization.
Anti-V3 Abs in SF162 gp140 immune plasma contributed only weakly (5.9%) to SF162 neutralization (Table 3), consistent with previous reports that V3 Abs elicited by SF162 gp140 contribute less to homologous neutralization than V1 Abs (17). In contrast, an increased contribution (25.2 to 54.3%) of anti-V3 Abs to the overall anti-SF162 neutralizing activity was observed in the case of animals immunized with the ΔV1 or the V1 chimeric Envs. In general, V3 binding titers correlate positively with each animal's SF162 IC50 neutralizing antibody titers (r = 0.799; P < 0.0001). An exception is animal 1919 of the ΔV1SF162 group, which elicited high anti-V3 antibody titers (22,316) but still failed to neutralize SF162. Finally, V3 peptide competition did not deplete the neutralizing activities of pooled IgG against the HxB2 isolate (Table 4). The HxB2 V3 has a rare insertion of two amino acids in the V3 crown, a region highly targeted by Abs generated during infection and immunization. Anti-V3 Abs induced by the SF162 V3, which lacks this insertion, are not expected to recognize the HxB3 V3 loop.
In summary, both anti-V1 and anti-V3 Abs contribute partially to homologous SF162 neutralization. In contrast, neutralization of HxB2 was not due to anti-V3 antibodies, and only in the case of immunization with the SF162/HxB2 V1 gp140 immunogen was it due to anti-V1 antibodies. These results are in accordance with immunogenicity studies performed with YU2 gp140 and HxB2/BaL gp145 immunogens, which elicited HxB2 neutralizing antibody responses that could not be accounted for by anti-V3 Abs (30, 56).
(iii) Anti-gp41 Abs do not contribute to homologous or heterologous neutralization.
Anti-gp41 antibodies were detected in all immunized animals, irrespective of the gp140 immunogen used (Table 2). Thus, we evaluated the contribution of gp41-directed Abs to SF162 and HxB2 neutralization using a previously characterized HIV-2/1 MPER chimera (4, 23-25). This chimeric HIV-2/1 virus incorporates the complete MPER sequence of HIV-1 YU2 and is sensitive to MPER neutralizing Abs such as 2F5 and 4E10; the parental HIV-2 strain remains resistant. MPER-reactive NAbs were not detected, as defined by the inability of plasma Abs to neutralize the HIV-2/1 MPER chimeric virus (data not shown). Our study indicates that gp140-elicited gp41 antibodies (at least those directed to the MPER) are nonneutralizing. We acknowledge the possibility that anti-gp41 Abs outside the MPER, which were not accounted for in our HIV-2/1 MPER chimera assay, may have contributed to neutralization (14, 37).
D368R gp120 competitions.
Soluble Env-derived peptides employed during the neutralization competition experiments may not adopt conformations that are relevant to those found on the native Env. Thus, the contribution of Abs that recognize conformational epitopes on variable and conserved regions of gp120 may not be accounted for when soluble peptides are used. To overcome this problem, we developed a new competition assay that is based on a mutant form of monomeric gp120, termed D368R gp120. The D368R modification abrogates binding by the broadly neutralizing anti-CD4-bs MAb b12 and IgG-CD4 (Fig. 5 A). Interestingly, the D368R mutation reduces but does not abrogate the binding of the anti-CD4-bs MAb b6 to the SF162 gp120. b6 neutralizes only selected HIV-1 viruses (including SF162 and HxB2) (7). D368R gp120 proteins have been used to absorb anti-CD4-bs antibodies present in HIV-1+ sera (6, 19, 29, 33, 42). Here, we used the D368R gp120 protein as a competing reagent during our in vitro neutralization assays. D368R can be used in such assays because it does not bind to cellular CD4 molecules on the surface of target cells, and thus it does not interfere with viral entry (data not shown).
FIG. 5.
Properties of monomeric D368R gp120 protein. (A) Binding patterns of several anti-HIV Env MAbs, as well as IgG-CD4, with WT SF162 gp120 or SF162 D368R gp120. The epitope specificities of each MAb are shown. (B) Anti-SF162 neutralizing activities of the indicated MAbs and IgG-CD4 in the presence versus absence of D368R. Error bars indicate standard deviations.
We first validated the potential usage of D368R gp120 as a competing reagent with the use of known anti-HIV neutralizing MAbs (Fig. 5B). As expected, the neutralizing activity of MAb b12 (and F105 [data not shown]) or IgG-CD4 (but not that of MAb b6) against SF162 was unaffected by D368R. Similarly, the neutralizing activities of two anti-gp41 MAbs, 2F5 and 4E10, were unaffected by the presence of D368R, which lacks the gp41 region. In contrast, anti-SF162 V1 and V3 neutralizing activities were abrogated in the presence of D368R. MAb 2G12 neutralizing activity was equally abrogated but only at MAb concentrations below 5 μg/ml. Therefore, competition with D368R gp120 may be used to identify the contribution of CD4-bs Abs to neutralizing potency, with a few exceptions.
Next, we determined whether the presence of D368R gp120 had any effect on the overall neutralizing potential of immune plasma (or purified IgG) against the homologous SF162 virus and the heterologous HxB2 virus (Tables 3 and 4).
(i) Homologous SF162 neutralization in the presence of D368R.
Complete reductions in neutralizing potency against the SF162 virus were observed with group G plasma (WT SF162 gp140) in the presence of D368R gp120, indicating that this immunogen elicits autologous NAbs that target epitopes readily present on monomeric gp120, excluding the CD4-bs (Table 3). They also support the above-discussed results that anti-gp41-directed antibodies do not contribute to the homologous SF162 neutralizing activity of immune plasma. All V1-modified immunogens also elicited anti-SF162 NAbs directed to epitopes represented on D368R gp120. However, the contribution of these antibodies to the overall anti-SF162 neutralizing activities of immune sera was not as significant as that of sera from animals immunized with the WT SF162 gp140 immunogen. For example, in two cases (SF162/JRFL V1 and SF162/HxB2 V1), only half of the anti-SF162 neutralizing activities of plasmas targeted the D368R gp120 protein. The remaining anti-SF162 neutralizing activities could be due to anti-CD4-bs antibodies or antibodies that do not recognize monomeric gp120.
(ii) Heterologous HxB2 neutralization in the presence of D368R.
For groups B to F (group A did not neutralize HxB2, as discussed above), only 7 to 28% of the anti-HxB2 neutralizing activity targeted epitopes on D368R gp120. In contrast, approximately half of the anti-HxB2 cross-neutralizing activity of group G (WT SF162 gp140) IgG targeted the D368R protein. The remaining anti-HxB2 neutralizing activities could be due to anti-CD4-bs antibodies or antibodies that do not recognize monomeric gp120. Therefore, antibodies to elements not present on D368R are the major contributors to the anti-HxB2 cross-neutralizing activities of these plasmas. However, the fact that these plasmas do not neutralize other heterologous viruses suggests that these epitopes either are not present on other isolates or are not as exposed as they are on the HxB2 envelope. Overall, our results indicate that a considerable fraction of the heterologous HxB2 neutralizing potency of all plasmas is due to “non-D368R gp120-binding” Abs, with higher contributions in each of the V1-modified immune groups.
Summary of epitope mapping studies.
The results of our epitope mapping experiments are summarized in Table 5. Anti-gp41 Abs elicited by all of the immunogens do not contribute to neutralization of either the homologous SF162 isolate or the heterologous HxB2 isolate. Anti-V3 Abs do not contribute to heterologous HxB2 neutralization, but they do contribute to anti-SF162 neutralizing activity. While anti-V3 antibodies contribute only minimally to the neutralizing activity of WT SF162 immune plasma, up to approximately half of the anti-SF162 neutralizing activities in the ΔV1 and V1 chimeric groups were due to anti-V3 antibodies. Anti-V1 Abs, when matched in sequence to the target virus, also played a role in SF162 and HxB2 neutralization. Finally, neutralization of HxB2 by plasmas from all groups was attributed in large part to non-D368R gp120 binding Abs, which include but are not limited to Abs that overlap with the CD4-bs or target epitopes not present on monomeric gp120. Such Abs also partially explain the anti-SF162 neutralizing activities of plasmas from groups A to F but not group G.
TABLE 5.
Summary of epitope mapping
| Group | Immunogen | Contribution to neutralizationa |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| SF162 |
HxB2 |
||||||||
| gp41 | V3 | V1 | D368R | gp41 | V3 | V1 | D368R | ||
| Group A | SF162/YU2 V1 | − (0.0%) | +++ (54.3%) | − (0.0%) | +++++ (84.8%) | ND | ND | ND | ND |
| Group B | SF162/JRFL V1 | − (0.0%) | ++ (36.1%) | − (0.0%) | +++ (48.6%) | − (0.0%) | − (1.0%) | − (0.7%) | + (17.3%) |
| Group C | SF162/89.6 V1 | − (0.0%) | +++ (50.4%) | − (0.0%) | ++++ (77.1%) | − (0.0%) | − (0.0%) | − (0.0%) | + (7.3%) |
| Group D | SF162/HxB2 V1 | − (0.0%) | ++ (25.2%) | − (5.0%) | +++ (54.3%) | − (0.0%) | − (0.5%) | ++ (23.6%) | ++ (21.8%) |
| Group E | V1 scaffold cocktail | − (0.0%) | ++ (33.4%) | − (1.4%) | ++++ (75.9%) | − (0.0%) | − (0.0%) | + (7.9%) | ++ (28.2%) |
| Group F | ΔV1SF162 | − (0.0%) | +++ (49.1%) | − (0.0%) | +++++ (89.8%) | − (0.0%) | − (0.3%) | − (0.0%) | ++ (25.9%) |
| Group G | SF162 gp140 | − (0.0%) | + (5.9%) | ++ (28.4%) | +++++ (99.3%) | − (0.0%) | − (0.0%) | − (0.0%) | +++ (43.8%) |
Contribution of Abs directed to a given peptide or recombinant protein to neutralization: −, 0 to 5%; +, 5 to 20%; ++, 20 to 40%; +++, 40 to 60%; ++++, 60 to 80%; +++++, 80 to 100%. The group mean percent contribution to neutralization is given in parentheses. ND, 50% neutralization was not achieved at the highest antibody concentration tested (100 μg/ml) or the lowest plasma dilution tested of (1:20).
DISCUSSION
Our study indicates that the presence (or absence), as well as the nature, of the V1 loop greatly affects the immunogenicities of the V3 and gp41 domains of soluble gp140 protein immunogens. Both approaches tested here (immunization with soluble gp140s lacking the V1 loop or immunization with a mixture of gp140 scaffolds expressing heterologous V1 loops) resulted in decreases in V3 immunogenicity. In contrast, the first approach enhanced the immunogenicity of gp41, while the second approach reduced it. In the absence of structural information on Env that includes the V1 loop, we speculate that these V1 modifications altered V3 and gp41 positioning within the soluble trimeric gp140 Env in such a way that altered their accessibilities to B cells. Such changes could, for instance, be related to the repositioning of sugar molecules within or around the V1 (27, 31). The observed decrease in gp41 immunogenicity upon V1 substitution versus the increase in gp41 immunogenicity upon V1 deletion may be due to smaller changes in V1 loop positioning as opposed to more dramatic modifications through V1 deletion that may more profoundly affect the overall Env trimeric organization. Overall, neither modification enhanced or shifted the Ab response toward known conserved neutralization epitopes. Ultimately, the limited cross-neutralizing response achieved by the immunogens tested here may be due to the fact that these constructs imperfectly mimic the native Env trimer and thus elicit Abs incapable of efficiently recognizing functional, virion-associated Env. Thus, although the V1 loop provides one pathway for modulating Env immunogenicity, further modifications are required to improve the immunogenicities of conserved targets. In the absence, however, of structural information on the HIV Env trimeric spike, it is becoming very difficult to design Env-based immunogens that would elicit antibody responses to conserved elements of the HIV-1 Env.
Antibodies against linear epitopes in V1, V3, and gp41 that were elicited by the WT SF162 gp140 immunogen either did not contribute or contributed only minimally to neutralization of the homologous SF162 virus. The entire anti-SF162 neutralizing activity of WT SF162 gp140-elicted antibodies, however, appears to target epitopes that are present on D368R gp120. These observations suggest that homologous anti-SF162 neutralizing activity is most likely due to conformational epitopes present on D368R gp120. Our results also indicate that the nature of the V1 loop greatly influences the immunogenicities of epitopes recognized by such antibodies. For example, in the case of the SF162/YU2 V1, SF162/89.6 V1, and ΔV1SF162 gp140 immunogens, the contribution of anti-D368R gp120 antibodies was as significant as that of antibodies elicited by WT SF162 gp140. In the case of the SF162/JRFL V1 and SF162/HxB2 V1 immunogens, however, only half of the anti-SF162 neutralizing activity was due to anti-D368R gp120 antibodies. Most (targeting the HxB2 virus) of the modest cross-neutralizing activity of antibodies elicited by our immunogens did not target epitopes on D368R gp120. The epitopes recognized by these antibodies are not present (or are poorly exposed) on the other heterologous viruses tested here.
In this study we demonstrate that diverse V1 loops differing in loop length, in amino acid composition, and in the number of PNGs can be antigenically accessible and immunogenic in the context of a heterologous SF162 Env scaffold (Fig. 4). The reasons, however, for the poor immunogenicity of the JRFL V1 loop are currently unknown. Previous studies with a cleaved, disulfide-stabilized gp140 (SOSIP.R6) based on the JRFL isolate indicated that homologous JRFL neutralization could not be accounted for by V1 Abs (2). Therefore, it is possible that the JRFL V1 is inherently poorly immunogenic. Comparatively, the JRFL V1 has the shortest V1 sequence (16 aa versus 17 to 20 aa) but harbors the greatest number of PNGs (three versus one or two) (Fig. 1D). These attributes may make for a more occluded, less immunogenically accessible V1. Previous studies also reveal that B-cell epitopes trend toward having enriched numbers of tryptophan (W), tyrosine (Y), charged, and polar amino acids due to their capacity to form a multitude of interactions at protein-protein interfaces (40). They are also underrepresented by aliphatic hydrophobic residues (Gly, Leu, Val, Ile, and Ala). While most of the V1 loops evaluated here have a neutral or positive charge with at least one Y or W (except HxB2 V1), JRFL V1 lacks Y or W residues and is very acidic. Proportionally, JRFL V1 also contains the greatest percentage of aliphatic hydrophobic residues (18.8% versus 11.1 to 15%).
Previously, Li et al. reported that YU2 gp140 elicits anti-V1 Abs that neutralize YU2 (30). It is unclear, then, why the anti-V1 Abs elicited by SF162/YU2 V1 gp140, which recognize the matched V1 peptide, were unable to neutralize YU2. Possibly, the conformation and orientation of the YU2 V1 loop on the homologous YU2 Env backbone differ from those on the heterologous SF162 Env backbone. Such differences may result in the elicitation of anti-YU2 V1 antibodies that differ in their abilities to recognize the wild-type YU2 V1 conformation. Therefore, alternative strategies for stabilizing the V1 loop to more accurately mimic its presentation on the native virion should be explored. Potentially, follow-up studies will need to focus the B-cell response to semiconserved regions of the V1 (i.e., the GEIKNC motif in the C′ terminus).
It is interesting that the narrow cross-neutralizing antibody responses elicited by our soluble gp140 immunogens were due to antibodies whose epitopes were mostly absent from D368R gp120, potentially targeting elements of the CD4-bs (or the gp120 “core”), or epitopes that are absent from soluble gp120 but present on the virion-associated Env spike. Antibodies that recognize “quaternary” epitopes on the SF162 Env have been isolated from a human infected with a heterologous HIV-1 virus (22) and from rhesus macaques infected with SHIVSF162P4 (39). It is possible that immunization with soluble SF162 gp140-derived proteins also results in the generation of antibodies that recognize quaternary epitopes that are common between SF162 and HxB2. These two Envs are derived from viruses that are easy to neutralize, and thus these Envs may express quaternary epitopes not present on Envs derived from difficult-to-neutralize primary HIV-1 isolates (such as YU2 or JRFL). Defining the epitope specificities of such antibodies is important not only for understanding why these antibodies are effective against HxB2 and not the other heterologous Envs examined here but also for designing future Env-based immunogens.
Acknowledgments
This work was supported by grant R01AI47708 and the ASM Robert D. Watkins Graduate Fellowship (grant 39769). We also acknowledge the support of the J. B. Pendleton Charitable Trust and the M. J. Murdock Charitable Trust.
We acknowledge all those who generously contributed reagents. We acknowledge G. Sellhorn for purifying and biophysically characterizing the soluble gp140 immunogens employed here.
Footnotes
Published ahead of print on 21 July 2010.
REFERENCES
- 1.Barnett, S. W., S. Lu, I. Srivastava, S. Cherpelis, A. Gettie, J. Blanchard, S. Wang, I. Mboudjeka, L. Leung, Y. Lian, A. Fong, C. Buckner, A. Ly, S. Hilt, J. Ulmer, C. T. Wild, J. R. Mascola, and L. Stamatatos. 2001. The ability of an oligomeric human immunodeficiency virus type 1 (HIV-1) Envelope antigen to elicit neutralizing antibodies against primary HIV-1 isolates is improved following partial deletion of the second hypervariable region. J. Virol. 75:5526-5540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beddows, S., M. Franti, A. Dey, M. Kirschner, S. Iyer, D. Fisch, T. Ketas, E. Yuste, R. Desrosiers, P. Klasse, P. Maddon, W. Olson, and J. Moore. 2007. A comparative immunogenicity study in rabbits of disulfide-stabilized, proteolytically cleaved, soluble trimeric human immunodeficiency virus type 1 gp140, trimeric cleavage-defective gp140 and monomeric gp120. Virology 360:329-340. [DOI] [PubMed] [Google Scholar]
- 3.Beddows, S., N. Schulke, M. Kirschner, K. Barnes, M. Franti, E. Michael, T. Ketas, R. W. Sanders, P. J. Maddon, W. C. Olson, and J. P. Moore. 2005. Evaluating the immunogenicity of a disulfide-stabilized, cleaved, trimeric form of the envelope glycoprotein complex of human immunodeficiency virus type 1. J. Virol. 79:8812-8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binley, J. 2009. Specificities of broadly neutralizing anti-HIV-1 sera. Curr. Opin. HIV AIDS 4:364-372. [DOI] [PubMed] [Google Scholar]
- 5.Binley, J., R. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. Anselma, P. Maddon, W. Olson, and J. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Binley, J. M., E. A. Lybarger, E. T. Crooks, M. S. Seaman, E. Gray, K. L. Davis, J. M. Decker, D. Wycuff, L. Harris, N. Hawkins, B. Wood, C. Nathe, D. Richman, G. D. Tomaras, F. Bibollet-Ruche, J. E. Robinson, L. Morris, G. M. Shaw, D. C. Montefiori, and J. R. Mascola. 2008. Profiling the specificity of neutralizing antibodies in a large panel of plasmas from patients chronically infected with human immunodeficiency virus type 1 subtypes B and C. J. Virol. 82:11651-11668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binley, J. M., T. Wrin, B. Korber, M. B. Zwick, M. Wang, C. Chappey, G. Stiegler, R. Kunert, S. Zolla-Pazner, H. Katinger, C. J. Petropoulos, and D. R. Burton. 2004. Comprehensive cross-clade neutralization analysis of a panel of anti-human immunodeficiency virus type 1 monoclonal antibodies. J. Virol. 78:13232-13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckner, C., L. G. Gines, C. J. Saunders, L. Vojtech, I. Srivastava, A. Gettie, R. Bohm, J. Blanchard, S. W. Barnett, J. T. Safrit, and L. Stamatatos. 2004. Priming B cell-mediated anti-HIV envelope responses by vaccination allows for the long-term control of infection in macaques exposed to a R5-tropic SHIV. Virology 320:167-180. [DOI] [PubMed] [Google Scholar]
- 9.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thorton, P. H. I. Parren, L. S. W. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, M. Lamacchia, E. Garratty, E. R. Stiehm, Y. J. Bryson, Y. Cao, J. P. Moore, D. D. Ho, and C. F. I. Barbas. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 10.Center, R., B. Kemp, and P. Poumbourios. 1997. Human immunodeficiency virus type 1 and 2 envelope glycoproteins oligomerize through conserved sequences. J. Virol. 71:5706-5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman, B. S., R. M. Thayer, K. A. Vincent, and N. L. Haigwood. 1991. Effect of intron A from human cytomegalovirus (Towne) immediate-early gene on heterologous expression in mammalian cells. Nucleic Acids Res. 19:3979-3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ching, L. K., G. Vlachogiannis, K. A. Bosch, and L. Stamatatos. 2008. The first hypervariable region of the gp120 Env glycoprotein defines the neutralizing susceptibility of heterologous human immunodeficiency virus type 1 isolates to neutralizing antibodies elicited by the SF162 gp140 immunogen. J. Virol. 82:949-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conley, A. J., M. K. Gorny, J. A. Kessler, 2nd, L. J. Boots, M. Ossorio-Castro, S. Koenig, D. W. Lineberger, E. A. Emini, C. Williams, and S. Zolla-Pazner. 1994. Neutralization of primary human immunodeficiency virus type 1 isolates by the broadly reactive anti-V3 monoclonal antibody, 447-52D. J. Virol. 68:6994-7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corti, D., J. Langedijk, A. Hinz, M. Seaman, F. Vanzetta, B. Fernandez-Rodriguez, C. Silacci, D. Pinna, D. Jarrossay, S. Balla-Jhagjhoorsingh, B. Willems, M. Zekveld, H. Dreja, E. O'Sullivan, C. Pade, C. Orkin, S. Jeffs, D. Montefiori, D. Davis, W. Weissenhorn, A. McKnight, J. Heeney, F. Sallusto, Q. Sattentau, R. Weiss, and A. Lanzavecchia. 2010. Analysis of memory B cell responses and isolation of novel monoclonal antibodies with neutralizing breadth from HIV-1-infected individuals. PLoS One 5:e8805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. Sutton, C. Hill, C. Davis, S. Peiper, T. Schall, D. Littman, and N. Landau. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 16.Derby, N., S. Gray, E. Wayner, D. Campogan, G. Vlahogiannis, Z. Kraft, S. Barnett, I. Srivastava, and L. Stamatatos. 2007. Isolation and characterization of monoclonal antibodies elicited by trimeric HIV-1 Env gp140 protein immunogens. Virology 366:433-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Derby, N. R., Z. Kraft, E. Kan, E. T. Crooks, S. W. Barnett, I. K. Srivastava, J. M. Binley, and L. Stamatatos. 2006. Antibody responses elicited in macaques immunized with human immunodeficiency virus type 1 (HIV-1) SF162-derived gp140 envelope immunogens: comparison with those elicited during homologous simian/human immunodeficiency virus SHIVSF162P4 and heterologous HIV-1 infection. J. Virol. 80:8745-8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dey, B., M. Pancera, K. Svehla, Y. Shu, S. H. Xiang, J. Vainshtein, Y. Li, J. Sodroski, P. D. Kwong, J. R. Mascola, and R. Wyatt. 2007. Characterization of human immunodeficiency virus type 1 monomeric and trimeric gp120 glycoproteins stabilized in the CD4-bound state: antigenicity, biophysics, and immunogenicity. J. Virol. 81:5579-5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhillon, A. K., H. Donners, R. Pantophlet, W. E. Johnson, J. M. Decker, G. M. Shaw, F. H. Lee, D. D. Richman, R. W. Doms, G. Vanham, and D. R. Burton. 2007. Dissecting the neutralizing antibody specificities of broadly neutralizing sera from human immunodeficiency virus type 1-infected donors. J. Virol. 81:6548-6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Earl, P., L, B. Moss, and R. W. Doms. 1991. Folding, interaction with GRP78-BiP, assembly, and transport of the human immunodeficiency virus type 1 envelope protein. J. Virol. 65:2047-2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Earl, P., and B. Moss. 1993. Mutational analysis of the assembly domain of the HIV-1 envelope glycoprotein. AIDS Res. Hum. Retroviruses 9:589-594. [DOI] [PubMed] [Google Scholar]
- 22.Gorny, M. K., L. Stamatatos, B. Volsky, K. Revesz, C. Williams, X. H. Wang, S. Cohen, R. Staudinger, and S. Zolla-Pazner. 2005. Identification of a new quaternary neutralizing epitope on human immunodeficiency virus type 1 virus particles. J. Virol. 79:5232-5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gray, E. S., M. C. Madiga, P. L. Moore, K. Mlisana, S. S. Abdool Karim, J. M. Binley, G. M. Shaw, J. R. Mascola, and L. Morris. 2009. Broad neutralization of human immunodeficiency virus type 1 mediated by plasma antibodies against the gp41 membrane proximal external region. J. Virol. 83:11265-11274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gray, E. S., P. L. Moore, I. A. Choge, J. M. Decker, F. Bibollet-Ruche, H. Li, N. Leseka, F. Treurnicht, K. Mlisana, G. M. Shaw, S. S. Karim, C. Williamson, and L. Morris. 2007. Neutralizing antibody responses in acute human immunodeficiency virus type 1 subtype C infection. J. Virol. 81:6187-6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray, E. S., N. Taylor, D. Wycuff, P. L. Moore, G. D. Tomaras, C. K. Wibmer, A. Puren, A. DeCamp, P. B. Gilbert, B. Wood, D. C. Montefiori, J. M. Binley, G. M. Shaw, B. F. Haynes, J. R. Mascola, and L. Morris. 2009. Antibody specificities associated with neutralization breadth in plasma from human immunodeficiency virus type 1 subtype C-infected blood donors. J. Virol. 83:8925-8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grundner, C., Y. Li, M. Louder, J. Mascola, X. Yang, J. Sodroski, and R. Wyatt. 2005. Analysis of the neutralizing antibody response elicited in rabbits by repeated inoculation with trimeric HIV-1 envelope glycoproteins. Virology 331:33-46. [DOI] [PubMed] [Google Scholar]
- 27.Johnson, W. E., H. Sanford, L. Schwall, D. R. Burton, P. W. Parren, J. E. Robinson, and R. C. Desrosiers. 2003. Assorted mutations in the envelope gene of simian immunodeficiency virus lead to loss of neutralization resistance against antibodies representing a broad spectrum of specificities. J. Virol. 77:9993-10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kraft, Z., K. Strouss, W. F. Sutton, B. Cleveland, F. Y. Tso, P. Polacino, J. Overbaugh, S. L. Hu, and L. Stamatatos. 2008. Characterization of neutralizing antibody responses elicited by clade A envelope immunogens derived from early transmitted viruses. J. Virol. 82:5912-5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, Y., S. A. Migueles, B. Welcher, K. Svehla, A. Phogat, M. K. Louder, X. Wu, G. M. Shaw, M. Connors, R. T. Wyatt, and J. R. Mascola. 2007. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat. Med. 13:1032-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li, Y., K. Svehla, N. Mathy, G. Voss, J. Mascola, and R. Wyatt. 2006. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J. Virol. 80:1414-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCaffrey, R., C. Saunders, M. Hensel, and L. Stamatatos. 2004. N-linked glycosylation of the V3 loop and the immunologically silent face of gp120 protects human immunodeficiency virus type 1 SF162 from neutralization by anti-gp120 and anti-gp41 antibodies. J. Virol. 78:3279-3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montefiori, D. 2009. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol. Biol. 485:395-405. [DOI] [PubMed] [Google Scholar]
- 33.Nandi, A., C. L. Lavine, P. Wang, I. Lipchina, P. A. Goepfert, G. M. Shaw, G. D. Tomaras, D. C. Montefiori, B. F. Haynes, P. Easterbrook, J. E. Robinson, J. G. Sodroski, and X. Yang. 2010. Epitopes for broad and potent neutralizing antibody responses during chronic infection with human immunodeficiency virus type 1. Virology 396:339-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pancera, M., J. Lebowitz, A. Schon, P. Zhu, E. Freire, P. D. Kwong, K. H. Roux, J. Sodroski, and R. Wyatt. 2005. Soluble mimetics of human immunodeficiency virus type 1 viral spikes produced by replacement of the native trimerization domain with a heterologous trimerization motif: characterization and ligand binding analysis. J. Virol. 79:9954-9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park, I., H. Koziel, W. Hatch, X. Li, B. Du, and J. Groopman. 1999. CD4 receptor-dependent entry of human immunodeficiency virus type-1 env-pseudotypes into CCR5-, CCR3-, and CXCR4-expressing human alveolar macrophages is preferentially mediated by the CCR5 coreceptor. Am. J. Respir. Cell Mol. Biol. 20:864-871. [DOI] [PubMed] [Google Scholar]
- 36.Picard, L., D. Wilkinson, A. McKnight, P. Gray, J. Hoxie, P. Clapham, and R. Weiss. 1997. Role of the amino-terminal extracellular domain of CXCR-4 in human immunodeficiency virus type 1 entry. Virology 231:105-111. [DOI] [PubMed] [Google Scholar]
- 37.Pietzsch, J., J. F. Scheid, H. Mouquet, M. S. Seaman, C. C. Broder, and M. C. Nussenzweig. 2010. Anti-gp41 antibodies cloned from HIV-infected patients with broadly neutralizing serologic activity. J. Virol. 84:5032-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polonis, V., B. Brown, A. Rosa Borges, S. Zolla-Pazner, D. Dimitrov, M. Zhang, S. Barnett, R. Ruprecht, G. Scarlatti, E. Fenyö, D. Montefiori, F. McCutchan, and N. Michael. 2008. Recent advances in the characterization of HIV-1 neutralization assays for standardized evaluation of the antibody response to infection and vaccination. Virology 375:315-320. [DOI] [PubMed] [Google Scholar]
- 39.Robinson, J. E., K. Franco, D. H. Elliott, M. J. Maher, A. Reyna, D. C. Montefiori, S. Zolla-Pazner, M. K. Gorny, Z. Kraft, and L. Stamatatos. 2010. Quaternary epitope specificities of anti-HIV-1 neutralizing antibodies generated in rhesus macaques infected by the simian/human immunodeficiency virus SHIVSF162P4. J. Virol. 84:3443-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rubinstein, N., I. Mayrose, D. Halperin, D. Yekutieli, J. Gershoni, and T. Pupko. 2008. Computational characterization of B-cell epitopes. Mol. Immunol. 45:3477-3489. [DOI] [PubMed] [Google Scholar]
- 41.Sanders, R. W., M. Venturi, L. Schiffner, R. Kalyanaraman, H. Katinger, K. O. Lloyd, P. D. Kwong, and J. P. Moore. 2002. The mannose-dependent epitope for neutralizing antibody 2G12 on human immunodeficiency virus type 1 glycoprotein gp120. J. Virol. 76:7293-7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sather, D. N., J. Armann, L. K. Ching, A. Mavrantoni, G. Sellhorn, Z. Caldwell, X. Yu, B. Wood, S. Self, S. Kalams, and L. Stamatatos. 2009. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J. Virol. 83:757-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saunders, C. J., R. A. McCaffrey, I. Zharkikh, Z. Kraft, S. E. Malenbaum, B. Burke, C. Cheng-Mayer, and L. Stamatatos. 2005. The V1, V2, and V3 regions of the human immunodeficiency virus type 1 envelope differentially affect the viral phenotype in an isolate-dependent manner. J. Virol. 79:9069-9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scanlan, C. N., R. Pantophlet, M. R. Wormald, E. Ollmann Saphire, R. Stanfield, I. A. Wilson, H. Katinger, R. A. Dwek, P. M. Rudd, and D. R. Burton. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1→2 mannose residues on the outer face of gp120. J. Virol. 76:7306-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulke, N., M. S. Vesanen, R. W. Sanders, P. Zhu, M. Lu, D. J. Anselma, A. R. Villa, P. W. Parren, J. M. Binley, K. H. Roux, P. J. Maddon, J. P. Moore, and W. C. Olson. 2002. Oligomeric and conformational properties of a proteolytically mature, disulfide-stabilized human immunodeficiency virus type 1 gp140 envelope glycoprotein. J. Virol. 76:7760-7776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sellhorn, G., Z. Caldwell, C. Mineart, and L. Stamatatos. 2009. Improving the expression of recombinant soluble HIV envelope glycoproteins using pseudo-stable transient transfection. Vaccine 28:430-436. [DOI] [PubMed] [Google Scholar]
- 47.Si, Z., N. Phan, E. Kiprilov, and J. Sodroski. 2003. Effects of HIV type 1 envelope glycoprotein proteolytic processing on antigenicity. AIDS Res. Hum. Retroviruses 19:217-226. [DOI] [PubMed] [Google Scholar]
- 48.Simmons, G., D. Wilkinson, J. Reeves, M. Dittmar, S. Beddows, J. Weber, G. Carnegie, U. Desselberger, P. Gray, R. Weiss, and P. Clapham. 1996. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J. Virol. 70:8355-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Srivastava, I. K., L. Stamatatos, E. Kan, M. Vajdy, Y. Lian, S. Hilt, L. Martin, C. Vita, P. Zhu, K. H. Roux, L. Vojtech, C. M. D., J. Donnelly, J. B. Ulmer, and S. W. Barnett. 2003. Purification, characterization, and immunogenicity of a soluble trimeric envelope protein containing a partial deletion of the V2 loop derived from SF162, an R5-tropic human immunodeficiency virus type 1 isolate. J. Virol. 77:11244-11259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srivastava, I. K., L. Stamatatos, H. Legg, E. Kan, A. Fong, S. R. Coates, L. Leung, M. Wininger, J. J. Donnelly, J. B. Ulmer, and S. W. Barnett. 2002. Purification and characterization of oligomeric envelope glycoprotein from a primary r5 subtype B human immunodeficiency virus. J. Virol. 76:2835-2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stamatatos, L., and C. Cheng-Mayer. 1998. An envelope modification that renders a primary, neutralization-resistant clade B human immunodeficiency virus type 1 isolate highly susceptible to neutralization by sera from other clades. J. Virol. 72:7840-7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stamatatos, L., M. Lim, and C. Cheng-Mayer. 2000. Generation and structural analysis of soluble oligomeric envelope proteins derived from neutralization-resistant and neutralization-susceptible primary HIV-1 isolates. AIDS Res. Hum. Retroviruses 16:981-994. [DOI] [PubMed] [Google Scholar]
- 53.Stamatatos, L., M. Wiskerchen, and C. Cheng-Mayer. 1998. Effect of major deletions in the V1 and V2 loops of a macrophage-tropic HIV-1 isolate on viral envelope structure, cell-entry and replication. AIDS Res. Hum. Retroviruses 14:1129-1139. [DOI] [PubMed] [Google Scholar]
- 54.Stein, B., and E. Engleman. 1990. Intracellular processing of the gp160 HIV-1 envelope precursor. Endoproteolytic cleavage occurs in a cis or medial compartment of the Golgi complex. J. Biol. Chem. 265:2640-2649. [PubMed] [Google Scholar]
- 55.Truett, M., R. Blacher, R. Burke, D. Caput, C. Chu, D. Dina, K. Hartog, C. Kuo, F. Masiarz, and J. Merryweather. 1985. Characterization of the polypeptide composition of human factor VIII:C and the nucleotide sequence and expression of the human kidney cDNA. DNA 4:333-349. [DOI] [PubMed] [Google Scholar]
- 56.Wu, L., Z. Y. Yang, L. Xu, B. Welcher, S. Winfrey, Y. Shao, J. R. Mascola, and G. J. Nabel. 2006. Cross-clade recognition and neutralization by the V3 region from clade C human immunodeficiency virus-1 envelope. Vaccine 24:4995-5002. [DOI] [PubMed] [Google Scholar]
- 57.Yang, X., V. Tomov, S. Kurteva, L. Wang, X. Ren, M. K. Gorny, S. Zolla-Pazner, and J. Sodroski. 2004. Characterization of the outer domain of the gp120 glycoprotein from human immunodeficiency virus type 1. J. Virol. 78:12975-12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang, X., R. Wyatt, and J. Sodroski. 2001. Improved elicitation of neutralizing antibodies against primary human immunodeficiency viruses by soluble stabilized envelope glycoprotein trimers. J. Virol. 75:1165-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zolla-Pazner, S. 2005. Improving on nature: focusing the immune response on the V3 loop. Hum. Antibodies 14:69-72. [PubMed] [Google Scholar]
- 60.Zwick, M. B., R. Jensen, S. Church, M. Wang, G. Stiegler, R. Kunert, H. Katinger, and D. R. Burton. 2005. Anti-human immunodeficiency virus type 1 (HIV-1) antibodies 2F5 and 4E10 require surprisingly few crucial residues in the membrane-proximal external region of glycoprotein gp41 to neutralize HIV-1. J. Virol. 79:1252-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zwick, M. B., A. F. Labrijn, M. Wang, C. Spenlehauer, E. O. Saphire, J. M. Binley, J. P. Moore, G. Stiegler, H. Katinger, D. R. Burton, and P. W. Parren. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J. Virol. 75:10892-10905. [DOI] [PMC free article] [PubMed] [Google Scholar]