Abstract
Human rhinoviruses (HRVs) are a highly prevalent and diverse group of respiratory viruses. Although HRV-A and HRV-B are traditionally detected by virus isolation, a series of unculturable HRV variants have recently been described and assigned as a new species (HRV-C) within the picornavirus Enterovirus genus. To investigate their genetic diversity and occurrence of recombination, we have performed comprehensive phylogenetic analysis of sequences from the 5′ untranslated region (5′ UTR), VP4/VP2, VP1, and 3Dpol regions amplified from 89 HRV-C-positive respiratory samples and available published sequences. Branching orders of VP4/VP2, VP1, and 3Dpol trees were identical, consistent with the absence of intraspecies recombination in the coding regions. However, numerous tree topology changes were apparent in the 5′ UTR, where >60% of analyzed HRV-C variants showed recombination with species A sequences. Two recombination hot spots in stem-loop 5 and the polypyrimidine tract in the 5′ UTR were mapped using the program GroupingScan. Available HRV-C sequences showed evidence for additional interspecies recombination with HRV-A in the 2A gene, with breakpoints mapping precisely to the boundaries of the C-terminal domain of the encoded proteinase. Pairwise distances between HRV-C variants in VP1 and VP4/VP2 regions fell into two separate distributions, resembling inter- and intraserotype distances of species A and B. These observations suggest that, without serological cross-neutralization data, HRV-C genetic groups may be equivalently classified into types using divergence thresholds derived from distance distributions. The extensive sequence data from multiple genome regions of HRV-C and analyses of recombination in the current study will assist future formulation of consensus criteria for HRV-C type assignment and identification.
A series of studies published in 2006 to 2007 described a novel group of human rhinoviruses (HRVs), referred to as A2 or group C (HRV-C), genetically distinct from existing rhinoviruses (3, 21, 23, 25, 27, 33, 42). These and subsequent investigations have shown the proposed new species HRV-C to be remarkably prevalent, widely distributed geographically, and frequently associated with severe respiratory disease, both following primary infections, particularly in young children (24, 25, 29, 32, 59), and as an exacerbating factor in asthma and other chronic obstructive airway diseases (20, 25, 29, 36). On the other hand, HRV-C has also been abundant in specimens collected from both children and adults with milder cases of respiratory disease (47, 48).
The HRV-C species is a member of the Enterovirus genus within the family Picornaviridae (53) and shares many features of its genome organization and structure with other picornaviruses and, more specifically, with other members of the Enterovirus genus. This includes an approximately 7,100-base genome containing a single reading frame; this encodes four capsid proteins and a series of functionally conserved nonstructural proteins involved in virus replication. HRV-C, in common with other members of the Enterovirus genus, possesses a type I internal ribosomal entry site (IRES) that enables initiation of translation from an internal methionine codon several hundreds of bases from the 5′ end of the genome.
Although no more closely related to each other than they are to other Enterovirus species phylogenetically, the three species of human rhinoviruses (A, B, and C) share a number of biological and genetic attributes. Most prominent is their transmission route and primary tropism for the respiratory tract, associated with the known acid lability of species A and B that is traditionally believed to prevent their colonization of the gastrointestinal tract. The HRV genome possesses a lower G+C content than genomes of other enteroviruses, and it is often speculated that this represents an adaptation for replication at the lower temperatures found in upper airways. HRV-C differs from other species, however, in other aspects, including the siting of the cis-replicating element in VP2 (11) instead of in 2A (species A) (13) or VP1 (species B) (34). Species C has so far proven refractory to all attempts at in vitro culture, despite the use of a wide variety of cell lines and primary cell cultures (25, 33). This restriction has hindered investigations of its replication, receptor use, and antigenic diversity.
Like most picornavirus groups, human rhinoviruses show substantial genetic heterogeneity that underlies the existence of a large number of antigenically distinct variants. In the case of species A and B, a total of 74 and 25 different serotypes, respectively, have been defined using cross-neutralization assays in cell culture (14, 18, 19). Available nucleotide sequences from the capsid region of HRV-C reveal similar or even greater diversity than is found between species A and B serotypes, but its lack of culturability currently precludes an equivalent classification of HRV-C into serotypes.
Although coding regions of HRV-C are genetically distinct from those of species A and B rhinoviruses and other enteroviruses, both the initial detection and subsequent genetic analyses of HRV-C have been complicated by the similarity of most (>60%) sequences of the 5′ untranslated region (UTR) to those of species A (49), with the remainder being phylogenetically distinct. It has been proposed that one or more interspecies recombination events between species C and species A rhinoviruses have occurred to generate these chimeric sequences (15, 17, 49, 59). Despite these differences in genetic composition, no differences in clinical presentations or epidemiology have been detected between HRV-C variants with species A-like and species C-like 5′ UTR (HRV-Ca and HRV-Cc, respectively) sequences (17, 59).
In the current study we have generated comparative sequence data from several genome regions (5′ UTR, VP4/VP2, VP1, and 3Dpol) from a large number of HRV-C-positive clinical specimens. Combined with published sequence information from these regions, we have investigated the occurrence, frequency, and location of recombination events within this rhinovirus species. The information gained on comparative phylogenies and sequence divergence in different regions provides data that will assist in the eventual classification of the current plethora of HRV-C sequences into a number of genetically determined types.
MATERIALS AND METHODS
Genetic characterization of HRV-C variants.
A total of 89 HRV-C-positive samples from the Edinburgh Specialist Virology Centre (SVC) respiratory sample archive (60) were selected for genetic characterization; these represent a subset of the 144 samples previously characterized in the VP4/partial VP2 region (59). RNA was extracted from clinical samples as previously described (60). Sequences were amplified from four different genomic regions using the methods described below.
(i) VP4/VP2.
The VP4 and 5′ end of VP2 were amplified using nested primers (see Table S1 posted at http://www.virus-evolution.org/Downloads/JVI00962-10/) as previously described (59).
(ii) VP1.
Nested reverse transcription-PCRs (RT-PCRs) were performed as previously described (35) but using primers shown in Table S1 (posted at the URL mentioned above). A total of 72 from the 89 samples amplifiable in other genome regions were successfully amplified in two VP1 regions. The two sequence fragments overlapped by 105 bases, allowing a composite 777-base sequence to be generated for phylogenetic analysis.
(iii) 3Dpol and 5′ UTR.
RNA extracted from respiratory samples was reverse transcribed into cDNA using the Promega reverse transcription system (Promega, United Kingdom) as per the manufacturer's instructions except that 5 μl of extracted RNA template was used. Amplification of the 3Dpol region and the 5′ UTR region from cDNA used nested primers (see Table S1 posted at the URL mentioned above) and previously described PCR conditions (59) but with an annealing temperature of 48°C in the second round. Samples negative on initial amplification were amplified using the nested PCR strategy described for VP1 above. A 3Dpol amplicon of 470 nucleotides in length was produced. For the 5′ UTR, two sequence fragments with a 61-base overlap allowed a 680-base composite 5′ UTR sequence overlapping the VP4/VP2 amplicon to be generated. A subset of HRV-C variants (n = 12) was further amplified using heminested, combined species A and C primers (5′ UTR set 1 in Table S1, posted at http://www.virus-evolution.org/Downloads/JVI00962-10/) to generate an amplicon from nucleotide position 1 to 355 (27 to 335, excluding primer sequences).
All amplicons were sequenced using a BigDye Terminator kit (Applied Biosystems, Warrington, United Kingdom). Prior to sequencing reactions, all samples underwent a PCR product cleanup procedure using EXOSAP-IT (GE Healthcare, United Kingdom).
Sequence analysis.
All available VP4/VP2 region sequences were downloaded from GenBank on 26 April 2010. Sequences that were <90% complete across the region from position 615 to 1043 were discarded (sequence positions are numbered throughout the manuscript using the sequence 024 [accession number EF582385]; the current GenBank entry for the QPM prototype sequence [EF186077] is incomplete at the 5′ end). These comprised 538 sequences along with a further 16 previously unpublished sequences from the SVC archive. The sequence data set was supplemented with the 10 complete genome sequences of HRV-C currently available on public databases as well as all available complete genome sequences of species A (n = 82) and B (n = 25) of defined serotypes.
Phylogenetic trees were constructed using the MEGA package, version 4.0 (54), by the neighbor-joining method (44) from 100 bootstrap resampled sequence alignments of maximum composite likelihood distances (MCL) (55) with pairwise deletion for missing data. Distributions of pairwise sequence distances for identification of type thresholds were calculated using the program SequenceDist in the Simmonics package.
Recombination breakpoints in HRV-Ca 5′ UTR sequences were determined using the program GroupingScan (51), using complete genome sequences of species A (n = 82), B (n = 25), and nonrecombinant species C (Cc) sequences (n = 29) as control groups. Trees were constructed using a fragment size of 200 with incremental steps of 10 bases, a bootstrap value of 70% to define phylogenetically supported groups, and 10 sequence relabelings (default) to calculate control values. A second analysis of sequences extending to the extreme 5′ end of the genome was performed to investigate further recombination events around the replication structures (eight HRV-Cc control sequences). Recombination breakpoints were interpolated as the nucleotide positions where the grouping values switched from the A group to the C group.
5′ UTR RNA structure prediction.
Structure prediction for HRV-C was based on the established model for HRV-A (6) and previous comparative analyses of HRV-A and -B sequences (43, 56). Mfold analysis of a selection of HRV-Ca and HRV-Cc sequences produced minimum-energy secondary structures for HRV-C variants that closely matched the HRV-A2 structure (data not shown).
Nucleotide sequence accession numbers.
All newly generated sequences were submitted to GenBank and were assigned accession numbers HM236897 to HM236968 (VP1), HM352737 to HM352752 (VP4), HM485468 to HM485556 (3Dpol), and HM581802 to HM581888 (5′ UTR).
RESULTS
Phylogenies of HRV-C 5′ UTR, VP4/VP2, VP1, and 3Dpol regions.
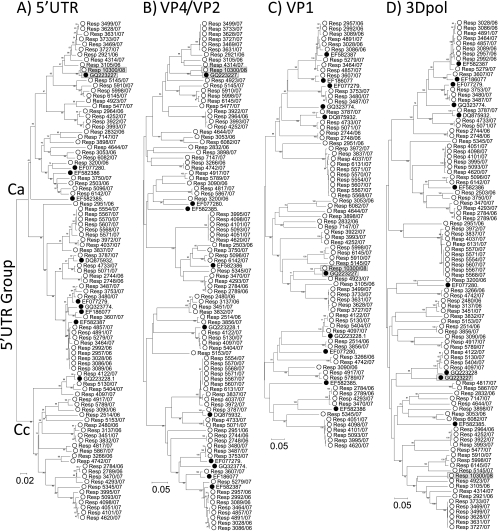
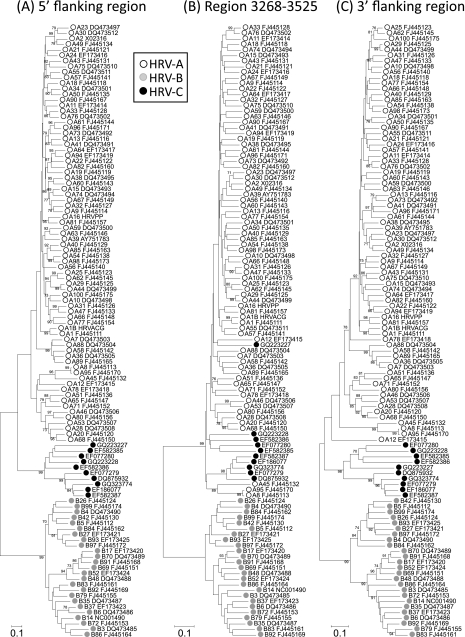
To investigate sequence relationships of HRV-C in different regions of the genome, 89 study samples were sequenced in the 5′ UTR (nucleotide position 185 to 614; n = 87), VP4 and partial VP2 (615 to 1043), whole VP1 (2348 to 3125; n = 72), and partial 3Dpol regions (6384 to 6854) and aligned with the 10 available complete genome sequences of HRV-C (Fig. 1). On phylogenetic analysis, sequences in each region fell into a number of clearly defined, bootstrap-supported phylogenetic groups, with the majority containing several variants and others represented by single sequences. The small subset of variants for which we were unable to obtain VP1 sequences were scattered throughout the 5′ UTR, VP4/VP2, and 3Dpol trees. They therefore did not constitute an identifiable genetic subset of sequences whose omission would have biased the analysis of VP1 sequence diversity.
FIG. 1.
Phylogenetic analysis of study sequences and corresponding regions from complete genome sequences in the 5′ UTR (position 185 to 614, numbered according to the reference sequence EF582385) (A), VP4/VP2 (616 to 1043) (B), VP1 (2348 to 3125) (C), and 3Dpol (6384 to 6854) (D). Trees were constructed by neighbor joining of pairwise maximum composite likelihood distance implemented in the program MEGA (54); branches showing at least 70% bootstrap support are indicated. Complete genome sequences were labeled using filled symbols. The putative recombinant sequence N4 (GQ223227) and the other group 7 sequence Resp_10300/08 are indicated by shaded boxes.
The phylogenies of the three coding regions (VP4/VP2, VP1, and 3Dpol) were remarkably congruent, with identical grouping patterns for all but one of the sequences analyzed. Using a bootstrap value of 70% or greater to define clades, there was only one phylogeny violation between trees constructed from coding region sequences (Fig. 1B, C, and D). This involved the previously characterized complete genome sequence, N4 (GQ223227) (Fig. 1, shaded box), which changed tree position between VP1 and 3Dpol regions. Our sample collection contained a single variant (Resp_10300/08) that grouped with N4 in the 5′ UTR, VP4/VP2, and VP1 regions. In contrast to N4, its 3Dpol sequence maintained a similar phylogenetic position relative to other sequences, as was observed in the 5′ UTR, VP4/VP2, and VP1 regions (Fig. 1). In investigating this anomaly, we found on closer inspection of the N4 sequence in the 3Dpol region that its 5′ end (position 6384 to 6645) was similar to that of Resp_10300/08 (pairwise distance, 0.08), while its 3′ end (position 6646 to 6854) was nearly identical to another sequence, N10 (GQ223228), originating from the same laboratory (sequence divergence, 0.01, compared to 0.35 divergence from Resp_10300/08). The chimeric nature of this sequence is evident from the change in tree position of 5′- and 3′-end fragments of the 3Dpol region (see Fig. S1 and supplementary data posted at http://www.virus-evolution.org/Downloads/JVI00962-10/); N4 grouped with Resp_10300/08 at the 5′ half of the fragment (comparable to other genome regions) (Fig. 1B and C) but clustered very closely with N10 (GQ223228) at the 3′ end. Possible underlying explanations for the anomalous phylogeny of the N4 sequence in the 3Dpol region are discussed below.
In contrast to the identity of coding region phylogenies, a minimum of 12 phylogeny violations were observed in the species C sequence data set on comparison of phylogenetic trees from the 5′ UTR and VP4/VP2. Members of the previously described HRV-Cc 5′ UTR group, distinct from the species A-like 5′ UTR sequences of the majority of HRV-C sequences (labeled in Fig. 1), were drawn from several coding region lineages. These observations are consistent with the occurrence of multiple recombination events between the 5′ UTR and the rest of the genome.
Pairwise distance distributions.
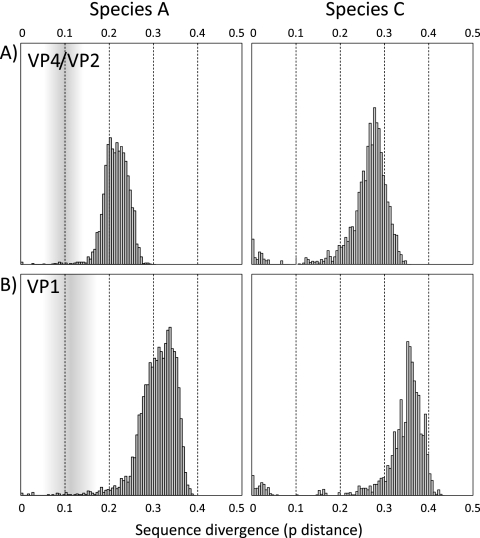
Distributions of pairwise distances between HRV-C sequences in VP4/VP2 and VP1 regions were computed to investigate whether they contained clear sequence divergence thresholds that might be used to define intertype and intratype divergence values (Fig. 2B). These distributions were compared with those of species A in the same genome regions (Fig. 2A). They were also similar to those of species B, but in the latter case there were insufficient numbers of classified sequences in either genome region for a conclusive analysis.
FIG. 2.
Distribution of pairwise p distances between HRV-A sequences (A) and available sequences of study samples and complete genome sequences of HRV-C (B) in the VP4 and VP1 regions. The shaded area represents the zone between inter- and intraserotype divergence values in species A.
The set of HRV-C VP4/VP2 and VP1 pairwise distance values (Fig. 2B) contained a first distribution (maximum values of 6.9% and 8%, respectively) that corresponded to pairwise distances between variants within the same phylogenetic clades (Fig. 1B and C). These were comparable to the majority of (intraserotype) distances between the small number of available HRV-A sequences of the same serotype (Fig. 2A). A second, nonoverlapping distribution, with minimum values of 10.9% in VP4 and 14.7% in VP1, corresponded to the set of pairwise distances between HRV-C clades. There was a comparable division of pairwise distances between VP4/VP2 and VP1 sequences of species A (Fig. 2A) and species B (data not shown) into two largely separate distributions. The previously proposed VP1 divergence thresholds of 12% and 13% for serotype assignment (22) correspond closely to the low point in the distribution of HRV-A and HRV-B VP1 pairwise distances. With the currently available sequence data, a threshold around this divergence value in VP1 and 10% in VP4/VP2 would appear appropriate for HRV-C. Observations of the separate distributions of pairwise distances in VP1 and phylogenetic evidence for marked phylogenetic clustering of HRV-C in all three coding regions analyzed support the idea that HRV-C variants might be usefully classified into a number of (genetically determined) groups that correspond to serotypes of other rhinoviruses. Such assignments might be made even though we lack serological evidence for antigenic distinctiveness of HRV-C variants.
As a preparatory step toward a future genotypic classification of HRV-C, we have tabulated all available HRV-C variants into groups containing members that show >0.13 sequence divergence from each other in VP1 or, if not available, >0.10 in VP4/VP2 (Table 1). Table 1 has been split into two sections, one for which complete genome sequences are available (genetic groups numbered 1 to 11) and a second containing genetic groups for which VP4/VP2 sequences are available, with or without VP1, 3Dpol, and 5′ UTR sequences. Combining the assignments, if sequences grouped in this way are considered the equivalent of (sero)types in other rhinoviruses, then the current data set of sequences can be grouped into a total of 60 genetically determined types.
TABLE 1.
Division of HRV-C into genetic groups based on divergence in VP1 or VP4/VP2
| HRV-C genetic groupa | Accession no.b | Identifier | 5′ UTR groupc | Submission date (mo/day/yr)d | Referencee |
|---|---|---|---|---|---|
| Groups containing complete genomes | |||||
| 1 | EF077279 | NAT001 | Ca | 10/20/06 | 21 |
| 2 | EF077280 | NAT045 | Ca | 10/20/06 | 21 |
| 3 | EF186077 | QPM | Ca | 12/14/06 | 33 |
| 4 | EF582385 | 024 | Ca | 4/27/07 | 25 |
| 5 | EF582386 | 025 | Ca | 4/27/07 | 25 |
| 6 | EF582387 | 026 | Ca | 4/27/07 | 25 |
| 7 | DQ875932 | NY-074 | Ca | 7/14/08 | 23 |
| 8 | GQ223227 | N4 | Ca | 5/29/09 | 17 |
| 9 | GQ223228 | N10 | Cc | 5/29/09 | 17 |
| 10 | GQ323774 | QCE | Ca | 6/29/09 | 2 |
| 11 | FJ392317f | CL170085 | Ca | 10/17/08 | 58 |
| Groups containing partial genomes | |||||
| 12 | EF077264 | NAT083 | Ca | 10/20/06 | 21 |
| 13 | EU081795 | tu403 | Ca | 8/03/07 | 42 |
| 14 | EU081796 | 06-445 | Cc | 8/03/07 | 42 |
| 15 | EU081800 | 06-20 | Ca | 8/03/07 | 42 |
| 16 | EU081808 | g2-4 | Ca | 8/03/07 | 42 |
| 17 | EU081809 | 06-582 | Ca | 8/03/07 | 42 |
| 18 | EU590074 | PNC41788 | Ca | 3/25/08 | 48 |
| 19 | EU697850 | 7316563 | Cc | 5/05/08 | 7 |
| 20 | EU697851 | DK-1 | Cc | 5/05/08 | 7 |
| 21 | EU752377 | RV471 | Ca | 5/26/08 | 36 |
| 22 | EU752381 | RV541 | Ca | 5/26/08 | 36 |
| 23 | EU752424 | RV64 | Ca | 5/26/08 | 36 |
| 24 | EU752426 | RV408 | Ca | 5/26/08 | 36 |
| 25 | EU752427 | RV1123 | Ca | 5/26/08 | 36 |
| 26 | EU752441 | RV177 | Ca/Ccg | 5/26/08 | 36 |
| 27 | GQ223122 | N22 | Ca/Cc | 1/09/09 | 17 |
| 28 | GQ223134 | N46 | Ca | 1/09/09 | 17 |
| 29 | FJ615699 | 201882 | Cc | 1/09/09 | 37 |
| 30 | GQ476669 | Resp_3898 | Ca | 8/13/09 | 60 |
| 31 | GU294380 | Resp_3776 | Ca | 12/04/09 | 59 |
| 32 | GU294466 | Resp_5613 | Ca | 12/04/09 | 59 |
| 33 | GU294480 | Resp_6157 | Cc | 12/04/09 | 59 |
| 34 | EF077256 | NAT069 | Cc | 10/20/06 | 21 |
| 35 | EF077260 | NAT069 | Cc | 10/20/06 | 21 |
| 36 | EU081790 | 06-739 | Cc | 8/03/07 | 42 |
| 37 | EU081791 | tu304 | Ca | 8/03/07 | 42 |
| 38 | EU081799 | g2-11 | 8/03/07 | 42 | |
| 39 | EU081802 | g2-25 | Ca | 8/03/07 | 42 |
| 40 | EU081803 | g2-23 | 8/03/07 | 42 | |
| 41 | EU081805 | g2-28 | Ca | 8/03/07 | 42 |
| 42 | EU081807 | 06-230 | 8/03/07 | 42 | |
| 43 | EU590054 | PNC86718 | Cc | 3/25/08 | 48 |
| 44 | EU590061 | PNC40168 | 3/25/08 | 48 | |
| 45 | EU590064 | PNC40449 | 3/25/08 | 48 | |
| 46 | EU697839 | IN-36 | 5/05/08 | 7 | |
| 47 | EU697852 | SA365412 | 5/05/08 | 7 | |
| 48 | EU743925 | CO-1368 | Cc | 5/22/08 | 11a |
| 49 | EU752358 | RV1250 | 5/26/08 | 36 | |
| 50 | EU752398 | RV1039 | 5/26/08 | 36 | |
| 51 | EU752412 | RV546 | Cc | 5/26/08 | 36 |
| 52 | FJ598096 | GDFY100 | Cc | 12/29/08 | Unpublished |
| 53 | FJ615722 | 202511 | 1/09/09 | 37 | |
| 54 | FJ615737 | 202092 | 1/09/09 | 37 | |
| 55 | FJ615745 | 202642 | 1/09/09 | 37 | |
| 56 | FJ841957 | S05986 | 3/17/09 | 9 | |
| 57 | FJ869923 | KR1868 | 3/27/09 | 15 | |
| 58 | FJ869950 | KR2315 | Cc | 3/27/09 | 15 |
| 59 | GQ466482 | K1091 301104 | 8/07/09 | 47 | |
| 60 | GU214340 | PV68 | Ca | 11/18/09 | 41 |
Genetic groups showing >0.10 sequence divergence in VP4/VP2. Groups for which VP4/VP2 sequences are available, with or without VP1, 3DPol, and 5′ UTR sequences, were considered partial.
Accession number of the first member of the assigned genetic group.
Identity of the 5′ UTR group (Ca or Cc) associated with viruses of this group (59).
Submission date of the first sequence of the assigned genetic group in GenBank/EMBL/DDBJ.
Citation for the first submitted member of the genetic group.
Accession number FJ392317 refers to a previously published VP1 sequence (58). The full genome of this variant has now been completed (C. Tapparel, personal communication). In order to preserve the numbering of genetic groups, sequences grouping with this complete genome in VP4/VP2 have been assigned as genetic group 11.
Variants with Ca and Cc 5′ UTR sequences are found in these genetic groups (59).
Sequence divergence of HRV-C in different genome regions.
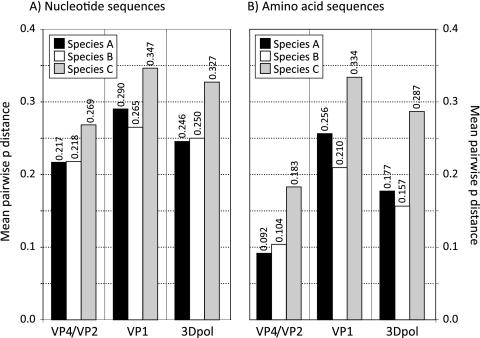
Using the genetic groups in the previous section, we compared the degree of sequence divergence in the analyzed genome regions with divergence in regions of different serotypes of HRV-A and HRV-B species (Fig. 3). Both at the nucleotide and amino acid level, VP1 was the most variable region between types and serotypes, followed by 3Dpol and VP4/VP2. However, HRV-C genetic groups were consistently more diverse than serotypes of other rhinovirus species, showing, for example, 35% (nucleotide) and 33% (amino acid) divergence compared to 25% and 21 to 26% in HRV-A and -B serotypes. Even more marked differences in 3Dpol and VP4/VP2 regions were observed, with amino acid divergence between species C groups almost twice that of species A and B.
FIG. 3.
Mean pairwise (uncorrected) p distances of nucleotide (A) and amino acid (B) sequences in the VP4/VP2, VP1, and 3Dpol regions between serotypes of HRV-A and HRV-B and between identified genetic groups of HRV-C (values shown above each bar).
Mapping positions of recombination between the 5′ UTR and VP4/VP2.
Combining sequences from overlapping 5′ UTR and VP4/VP2 amplicons provided a continuous sequence from position 185 to 1043 that enabled the recombination breakpoint(s) to be determined in each of the Ca recombinant variants identified to date. The combined set of 10 available complete genomes, 87 samples from the current study, and 34 5′ UTR sequences described by Huang et al. (17) represented 45 of the 60 sequence groups listed in Table 1. Twenty-nine HRV-C sequence groups had 5′ UTR sequences that grouped within the species A 5′ UTR clade (labeled Ca in Fig. 1), two contained sequences from both the Ca and Cc groups, and the remainder were phylogenetically distinct, falling in the Cc clade that contained the N10 complete genome sequence (GQ223228).
The program Grouping Scan (51) was used to identify recombination breakpoints in the Ca 5′ UTR sequences. This program was chosen in preference to bootscanning methods because it scores the extent of grouping within predefined control groups rather than simply the bootstrap support for the grouping of a query sequence with a group consensus sequence. As discussed previously (51), the latter method can lead to false assignment in cases where a query sequence is not closely affiliated to any of the control groups. Furthermore, by simply condensing the often large amount of comparative sequence data within control groups to a single consensus sequence, bootscanning additionally discards informative data on sequence diversity within groups that are of value in assessing phylogeny relationships. The available set of HRV-A and HRV-B complete genome sequences were used as control groups while the HRV-Cc control group (nonrecombinant Cc sequences) was assembled from the N10 sequence and Cc variants identified in the current study (n = 29).
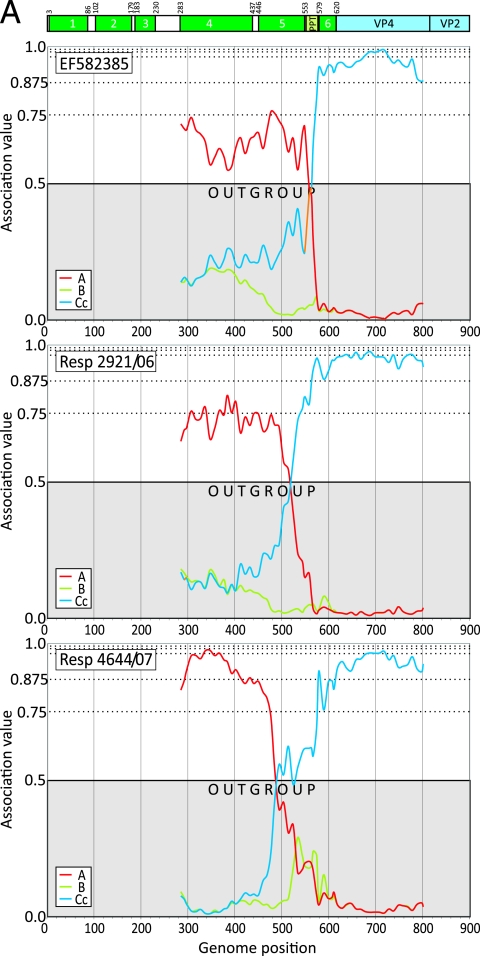
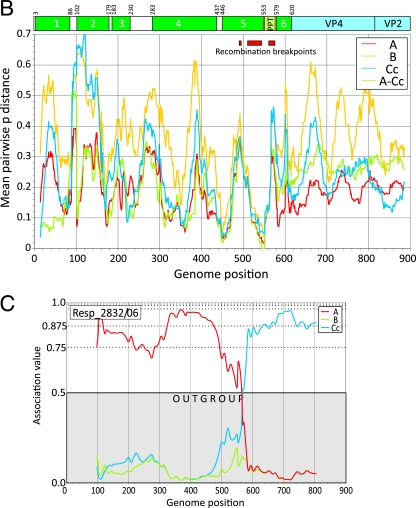
Each of the complete genome and study sequences from the Ca 5′ UTR clade was scanned between position 185 and 903 (three representative results depicting different breakpoints are shown in Fig. 4A). Superimposed is a diagrammatic summary of the RNA secondary structure elements comprising the replication structures (stem-loop 1 cloverleaf) and IRES (stem-loops 2 to 6, the latter containing the AUG start codon of the HRV-C open reading frame at position 615).
FIG. 4.
(A) Mapping of recombination breakpoints of three representative published or study HRV-Ca sequences using the program Grouping Scan with HRV-A, -B, and HRV-Cc sequences as control groups, as indicated on the figure. Above the graphs is a diagrammatic summary of 5′ UTR RNA structure elements (stem-loops 1 to 6 [1] and the PPT). (B) Variability scan of the 5′ UTR, showing mean pairwise interserotype/genetic group distances of HRV-A, -B, and -Cc sequences averaged over a 30-base window and, separately, the interspecies divergence between HRV-A and HRV-Cc (A-Cc). The position of 5′ UTR structures and range of identified recombination breakpoints (Table 2) are indicated. (C) Extension of Grouping Scan analysis to the 5′ end of the HRV genome using the subset of control sequences complete from position 27 onwards and an HRV-Ca query sequence.
Recombination breakpoints identified in HRV-Ca variants were invariably positioned similarly among variants in the same genetic group (Table 2). Two recombination hot spots were identified, one occurring in the polypyrimidine tract (PPT) between stem-loops 5 and 6 (position 561 to 576; mean, 565) and a second around the terminal loop of stem-loop 5 (position 508 to 544; mean, 523), while two HRV-C genetic groups showed a more 5′ site (position 479 to 489; mean, 481). Sequences of the stem-loop stem regions either side of the recombination hot spot centering on position 523 were highly conserved (Fig. 4B). The HRV-Ca recombinants with a 5′ stem sequence derived from species A and a 3′ sequence from species C showed predicted pairings similar to those of HRV-A and nonrecombinant HRV-Cc sequences (data not shown) and would therefore accommodate a recombination event without destabilizing stem-loop 5.
TABLE 2.
Position of recombination breakpoints in different HRV-Ca groups
| Genetic group | Total no. of sequences analyzed | Breakpoint (mean nucleotide position [range]) |
|---|---|---|
| 1 | 5 | 520 (519-523) |
| 2 | 1 | 564 |
| 3 | 2 | 526 (526-527) |
| 4 | 1 | 561 |
| 5 | 3 | 563 (563-564) |
| 6 | 10 | 524 (524-526) |
| 7 | 2 | 574 (574) |
| 8 | 4 | 565 (561-570) |
| 10 | 1 | 525 |
| 11 | 2 | 554 (552-557) |
| 12 | 4 | 508 (508) |
| 13 | 1 | 523 |
| 15 | 3 | 483 (479-489) |
| 16 | 3 | 520 (518-526) |
| 17 | 1 | 567 |
| 18 | 8 | 528 (518-544) |
| 21 | 6 | 572 (570-575) |
| 22 | 2 | 576 (576) |
| 23 | 3 | 562 (552-570) |
| 24 | 1 | 560 |
| 25 | 1 | 558 |
| 27 | 3 | 535 (522-559) |
| 28 | 2 | 543 (536-550) |
| 30 | 1 | 561 |
| 31 | 1 | 528 |
| 32 | 9 | 524 (523-527) |
| 37 | 2 | 555 (555) |
| 39 | 1 | 560 |
| 41 | 1 | 479 |
| 60 | 1 | 516 |
Finally, the occurrence of further recombination events at the extreme 5′ end of the 5′ UTR was investigated using a subset of 11 HRV-Ca sequences with sequences complete from position 27. All query sequences grouped within the HRV-A clade, with association values substantially above the 0.5 outgroup position score (an example is shown in Fig. 4C). Although the resolution of this method is limited by the 200-base fragment size used for analysis, visual inspection of trees constructed from shorter sequence fragments (50 and 100 bases) at the extreme 5′ end of the genome provides no evidence for a change in the phylogenetic position of any Ca variants to a position outside the HRV-A clade (data not shown).
Interspecies recombination in the 2A region.
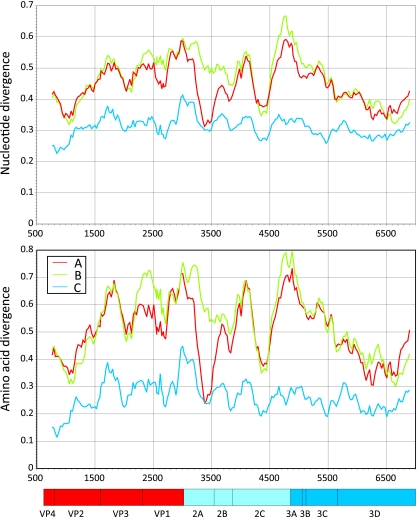
To investigate whether other genome regions of HRV-C showed evidence for interspecies recombination, we performed a scan of sequence divergence between complete genome sequences and those of species A and B rhinoviruses (Fig. 5). Between-species divergence was substantially greater than the mean pairwise distance within species C sequences except for an approximately 260-base region in 2A. This region (position 3268 to 3525) showed a markedly different phylogeny from flanking regions of the same length (Fig. 6). More specifically, species C sequences grouped within the HRV-A clade in a manner similar to that observed in the 5′ UTR. As found in the latter region, single HRV-C sequences or groups of HRV-C sequences became scattered among the HRV-A sequences, implying the occurrence of separate recombination events. Remarkably, the bootstrap-supported grouping of five HRV-C sequences (accession numbers EF077279, DQ875932, EF582387, EF186077, and GQ323774) in 2A was similarly monophyletic in the 5′ UTR (Fig. 1A). The main difference from the 5′ UTR phylogeny was the position of N10 (GQ223228). This formed part of the Cc 5′ UTR clade (Fig. 1A) but contained a 2A sequence embedded within the HRV-A clade (Fig. 6). A change in the phylogenetic position of N4 (GQ223227) was additionally apparent in trees from the two regions.
FIG. 5.
Divergence scan, showing mean nucleotide and amino acid sequence divergence between complete genome sequences of HRV-C with those of HRV-A and -B and within-species diversity of HRV-C. The scan used fragment lengths of 300 nucleotides, incrementing by 30 bases across the HRV coding sequence. The genome diagram below the graph shows positions of different HRV-C genes, using annotation supplied with the sequence EF582385.
FIG. 6.
Comparison of the phylogeny of HRV-A, -B, and -C sequences in the putative recombinant region between position 3268 and 3525 with those of 5′ and 3′ flanking regions (positions 3009 to 3267 and 3526 to 3784). See the legend of Fig. 1 for tree construction method, labels, and symbols. For clarity, the accession numbers of species A and B variants have been prefixed by the species letter and serotype designation (e.g., A8 FJ445113 and B14 NC001490).
DISCUSSION
Recombination in HRV-C.
This study has investigated the occurrence and sites of recombination within the genomes of the recently described species C human rhinoviruses. The most striking finding was the almost identical phylogenetic trees of the three coding regions analyzed, including gene regions at the extreme 5′ and 3′ ends of the open reading frame (VP4/VP2 and 3Dpol). The absence of recombination implied by these observations is consistent with previous comparisons of VP4/VP2 and 3Dpol region phylogenies of HRV species A and B (46), where only minimal changes in branching order were observed among the full set of 101 classified serotypes. These findings together with the data obtained here for species C contrast dramatically with the rampant and ongoing recombination process between structural and nonstructural gene regions in other species within the Enterovirus genus, particularly in species A, B, and C human enteroviruses (10, 28, 30, 38, 52). Recombination is also extensively documented in aphthoviruses (16, 50), cardioviruses (5, 12), and parechoviruses (4, 8). For these, the concept of separate, modular evolution of structural and nonstructural regions of picornavirus genomes has been developed (28, 31, 50).
Potentially underlying this difference in recombination frequency in rhinoviruses is their different pattern of sequence divergence in structural and nonstructural regions from most other picornaviruses. Species C variants along with HRV-A and HRV-B show substantial sequence divergence throughout the coding region. For example, mean intergenetic group pairwise distances in the 3Dpol region of HRV-C (33% nucleotide; 29% amino acid) were similar to the divergence of VP1 (35% and 33%, respectively). This contrasts markedly with human enteroviruses and several other picornavirus groups where nonstructural gene regions are much less divergent between serotypes (<10% at the amino acid level). As previously proposed (50), this restricted variability increases the likelihood of recombinants with breakpoints in the nonstructural regions being viable biologically. In contrast, the highly divergent sequences in equivalent regions of HRV may be functionally incompatible with each other and effectively isolate each rhinovirus serotype into a separate evolutionary path.
One HRV-C sequence showed different phylogenetic relationships in different regions of the coding region (N4; GQ223227). Although we cannot exclude the possibility of a natural recombination site within the 3D region as an explanation for the hybrid nature of its 3Dpol sequence (see Fig. S1 and the supplementary data posted at http://www.virus-evolution.org/Downloads/JVI00962-10/), its close resemblance of the 3′ end to the N10 sequence originating from the same laboratory suggests the possibility of laboratory contamination during assembly of the complete genome sequence. Another group 7 variant (Resp_10300/08) showed consistent phylogenetic relationships to other sequences in all three coding regions analyzed, including 3Dpol (Fig. 1B, C, and D). It would be of value if the laboratory from which the N4 sequence originated (17) were able to perform additional amplification and sequencing to rule out contamination/assembly errors and correct the GenBank entry if necessary.
Evidence for a different, likely more evolutionarily ancient, pattern of recombination in HRV-A has been previously obtained, manifested by differences in sequence relatedness between sequences in different genome regions (39, 57). For example, HRV-53 was substantially more similar to HRV-46 in the nonstructural region than anticipated by their sequence relationship in the capsid-encoding region; conversely HRV-78 and HRV-12 were more divergent. In these and other specific examples, changes in the phylogeny relationship usually occurred at the P1/P2 boundary, implying a limited degree of compatibility between structural and nonstructural gene modules derived from different serotypes. As described above, this pattern of recombination is quite distinct from the multiple recombination sites within nonstructural gene regions of enteroviruses and other picornaviruses. No evidence for its occurrence was documented among the data set of HRV-C sequences assembled in the current study, where both small-scale (genetic group) and larger sequence groupings were preserved across the genome. Potentially, the much greater amino acid sequence divergence between HRV-C sequence groups (Fig. 3) increases the likelihood of biological incompatibility and restricts further the occurrence of recombination.
Despite the limited evidence for recombination in the coding regions of rhinoviruses, this study confirmed its frequent occurrence between VP4 (and the rest of the genome downstream) and the 5′ UTR (17, 49). The generation of a large number of 5′ UTR Ca and Cc sequences permitted a detailed investigation of the positions where recombination occurred. The distribution of recombination hot spots centered around position 565 (in the PPT), 523 (within stem-loop 5 of the IRES), and two groups showing evidence of a breakpoint around position 481. This is a much more restricted distribution than previously described in an analysis using a smaller data set and a different scanning method (17). Nor could we confirm the second recombination event at the extreme 5′ end of the genome where Ca variants remained grouped with HRV-A sequences.
As suggested from the analysis of recombination in the coding regions, the occurrence and positions of recombination events in the 5′ UTR may be governed by biological compatibility restrictions. For example, the conservation of sequences within stem-loop 5 and potentially independent modular functions of stem-loops 5 and 6 may favor the creation of viable recombinants. Similarly, the high degree of sequence conservation of the 5′ end of the genome between rhinovirus species may facilitate the interaction between the species C replication complex and the HRV-A-derived cloverleaf replication structure (stem-loop 1) (56). In the future, in vitro insertion of the HRV-C-derived IRES and adjacent coding sequences into species A replicons or infectious clones will be of value in functionally mapping these compatibility restrictions.
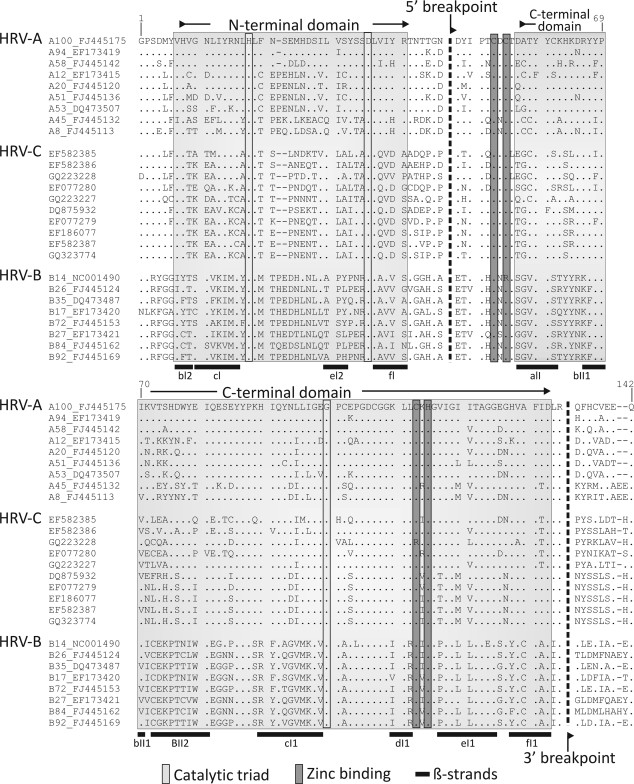
A new and unexpected finding in the current study was the second site of HRV-A/HRV-C interspecies recombination in the 2A region (Fig. 6 and 7). Although a previous bootscanning analysis showed the N4 sequence to group with the HRV-A consensus sequence in this region (17), this observation was misinterpreted. As demonstrated by phylogenetic analysis of this and flanking regions, all HRV-C variants genetically characterized in this region to date show evidence for recombination (Fig. 6) in a pattern remarkably similar to that observed in the 5′ UTR (with the noted exceptions of N10 and N4). The 2A protein of rhinoviruses and enteroviruses is a chymotrypsin-like proteinase of 142 amino acids. The region between positions 3268 to 3525 (residues 47 to 133) identified as recombinant corresponds precisely to the boundaries of the previously C-terminal structural domain of 2A, being comprised of six β-sheets that coordinate a zinc ion adjacent to the catalytic site of the protease (Fig. 7) (40). Our observations of recombination in the HRV-C 2A gene suggest at least some degree of modularity of its two structural domains. Investigation of the enzymatic properties of the HRV-C chimeric protein and the influence of the more variable N terminus of 2A encoded by HRV-C sequences on its function and specificity may provide insights into potential biological differences between HRV species. Finally, the proposed recombination junction at position 3268 coincides almost exactly with the 3′ end of the species A CRE (cis-acting RNA element) region (mapped to position 3226 to 3270 [13]). The absence of a homologous structure in the N′ terminal domain of species C variants is consistent with current predictions for its localization in VP2 (11).
FIG. 7.
Annotation of the secondary structure elements in the 2A gene of HRV-2 (40) superimposed on an alignment of the most genetically divergent HRV-A (n = 9) and HRV-B (n = 8) sequences and all available HRV-C 2A sequences and the inferred positions of the recombination breakpoints (dotted lines; nucleotide position 3268 to 3525). β-Strands and catalytic and zinc binding residues are as previously proposed (40). The positions of the N-terminal and C-terminal structural domains are indicated in shaded boxes. Numbering of amino acid residues follows that of the HRV-2 sequence X02316. For clarity, the accession numbers of species A and B variants have been prefixed by the species letter and serotype designation (as described in the legend of Fig. 6).
The evolutionary events underlying the inferred 5′ UTR and 2A recombination events remain unclear. The existence of multiple recombination breakpoints in the 5′ UTR and the scattering of HRV-Ca genetic groups in the VP4/VP2 tree (17, 59) imply that recombination in the 5′ UTR has occurred several times independently. Similarly, the existence of multiple groupings of HRV-C sequences in the 2A clade of HRV-A suggests that more than one recombination event of that specific fragment occurred. What is remarkable is the evidence that recombination in these two regions is at least partly linked, with the same grouping of five of the HRV-C variants observed in both genomic regions. We are currently sequencing the 89 study samples in the 2A region to obtain additional information on the occurrence and linkage of recombination at these two sites in order to obtain a better understanding of the process and constraints under which these interspecies recombination events occur.
Genetic diversity of HRV-C.
HRV-C variants showed substantially greater diversity than HRV-A or HRV-B serotypes (Fig. 3) but showed similar clustering of genetic groups into a number of well-defined (bootstrap-supported) clades (Fig. 1C) to other rhinovirus species. There were additionally two well-separated distributions of pairwise distances in VP4/VP2 and VP1 regions (Fig. 2B), suggesting that genetically defined types of HRV-C might be readily defined and demarcated for classification purposes. This genetic approach may be necessitated by the absence of HRV-C isolates with which to investigate serological interrelationships, as has been performed previously for HRV-A and -B species.
For other HRV species, there exists a small number of pairs of variants with genetic distances at an intermediate position in the distributions (the gray zone marked in Fig. 2 for HRV-A). For both HRV-A and -B, while there is a good correlation between VP1 sequence divergence and serological relationships (22, 26, 45), the latter are of limited value in defining a precise nucleotide sequence divergence threshold to separate inter- from intraserotype divergence values (22, 26). These discrepancies likely reflect the somewhat variable relationship between sequence divergence, epitope exposure, and antigenicity and the possibility that much of the sequence divergence between serotypes is immunologically driven.
For species C, however, the current data set of VP1 (and VP4/VP2) sequences shows a marked absence of variants showing intermediate distances in this sequence divergence range, and all can be defensibly assigned into different genetic groups using the 0.13 and 0.10 upper divergence thresholds for the VP1 and VP4/VP2 regions and into the same group if below. Also potentially facilitating a possible future genetically based classification of HRV-C is the lack of observed recombination between the VP4/VP2 and VP1 regions (Fig. 1B and C). This suggests that, once classified, HRV-C types may be readily identified using either VP4/VP2 sequences or those from VP1. This would be particularly helpful, given the preponderance of VP4/VP2 sequence data and the ease of amplification of this region for typing purposes. Remarkably, within 5 years of discovery of HRV-C, currently available sequences would correspond to a total of 60 types if classified in this way (Table 1).
Future classification proposals and the development of robust, well-defined type assignment criteria for HRV-C require discussion and consensus from an expert group, likely affiliated to the Picornavirus Study Group of the International Committee for the Taxonomy of Viruses. The data generated in the current study will be of value in future formulation of divergence thresholds in different genome regions for type assignment if this is to be adopted for classification purposes, and a consensus paper with formal proposals for type assignment based on the genetic groups defined in Table 1 has now been published (52a). Information on recombination frequency will similarly assist the interpretation of sequence data from other genomic regions and substantiate the proposed classification of Ca and Cc 5′ UTR variants (17) that we have extended in the current study. Other issues specific to HRV-C and which require consideration in formulating classification proposals include the lack of virus isolates or type strains equivalent to those of other rhinoviruses that currently precludes their serological and genetic/biological characterization.
Acknowledgments
We are grateful to Elly Gaunt, Heli Harvala, Kate Templeton, Peter McCullough, Julie White, Mary Notman, Eleanor Leslie, and Carol Thomson for providing samples, data, and other virus testing results from the respiratory sample archive. We thank David J. Evans for valuable discussion of the manuscript.
Chloe McIntyre's Ph.D. studentship was funded by the Medical Research Council.
Footnotes
Published ahead of print on 28 July 2010.
REFERENCES
- 1.Anderson, E. C., S. L. Hunt, and R. J. Jackson. 2007. Internal initiation of translation from the human rhinovirus-2 internal ribosome entry site requires the binding of Unr to two distinct sites on the 5′ untranslated region. J. Gen. Virol. 88:3043-3052. [DOI] [PubMed] [Google Scholar]
- 2.Arden, K. E., C. E. Faux, N. T. O'Neill, P. McErlean, A. Nitsche, S. B. Lambert, M. D. Nissen, T. P. Sloots, and I. M. Mackay. 2010. Molecular characterization and distinguishing features of a novel human rhinovirus (HRV) C, HRVC-QCE, detected in children with fever, cough and wheeze during 2003. J. Clin. Virol. 47:219-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arden, K. E., P. McErlean, M. D. Nissen, T. P. Sloots, and I. M. Mackay. 2006. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J. Med. Virol. 78:1232-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benschop, K. S., C. H. Williams, K. C. Wolthers, G. Stanway, and P. Simmonds. 2008. Widespread recombination within human parechoviruses: analysis of temporal dynamics and constraints. J. Gen. Virol. 89:1030-1035. [DOI] [PubMed] [Google Scholar]
- 5.Blinkova, O., A. Kapoor, J. Victoria, M. Jones, N. Wolfe, A. Naeem, S. Shaukat, S. Sharif, M. M. Alam, M. Angez, S. Zaidi, and E. L. Delwart. 2009. Cardioviruses are genetically diverse and cause common enteric infections in South Asian children. J. Virol. 83:4631-4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borman, A., and R. J. Jackson. 1992. Initiation of translation of human rhinovirus RNA: mapping the internal ribosome entry site. Virology 188:685-696. [DOI] [PubMed] [Google Scholar]
- 7.Briese, T., N. Renwick, M. Venter, R. G. Jarman, D. Ghosh, S. Kondgen, S. K. Shrestha, A. M. Hoegh, I. Casas, E. V. Adjogoua, C. Akoua-Koffi, K. S. Myint, D. T. Williams, G. Chidlow, B. R. van den, C. Calvo, O. Koch, G. Palacios, V. Kapoor, J. Villari, S. R. Dominguez, K. V. Holmes, G. Harnett, D. Smith, J. S. Mackenzie, H. Ellerbrok, B. Schweiger, K. Schonning, M. S. Chadha, F. H. Leendertz, A. C. Mishra, R. V. Gibbons, E. C. Holmes, and W. I. Lipkin. 2008. Global distribution of novel rhinovirus genotype. Emerg. Infect. Dis. 14:944-947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvert, J., T. Chieochansin, K. Benschop, E. C. McWilliam-Leitch, J. F. Drexler, K. Grywna, H. da Costa Ribeiro, C. Drosten, H. Harvala, Y. Poovorawan, K. Wolthers, and P. Simmonds. 2010. The recombination dynamics of human parechoviruses; investigation of type-specific differences in frequency and epidemiological correlates. J. Gen. Virol. 91:1229-1238. [DOI] [PubMed] [Google Scholar]
- 9.Calvo, C., M. Luz Garcia, F. Pozo, N. Reyes, P. Perez-Brena, and I. Casas. 2009. Role of rhinovirus C in apparently life-threatening events in infants, Spain. Emerg. Infect. Dis. 15:1506-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chevaliez, S., A. Szendroi, V. Caro, J. Balanant, S. Guillot, G. Berencsi, and F. Delpeyroux. 2004. Molecular comparison of echovirus 11 strains circulating in Europe during an epidemic of multisystem hemorrhagic disease of infants indicates that evolution generally occurs by recombination. Virology 325:56-70. [DOI] [PubMed] [Google Scholar]
- 11.Cordey, S., D. Gerlach, T. Junier, E. M. Zdobnov, L. Kaiser, and C. Tapparel. 2008. The cis-acting replication elements define human enterovirus and rhinovirus species. RNA 14:1568-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Dominguez, S. R., T. Briese, G. Palacious, J. Hui, J. Villari, V. Kapoor, R. Tokarz, M. P. Glodé, M. S. Anderson, C. C. Robinson, K. V. Homes, and W. I. Lipkin. 2008. Multiplex MassTag PCR for respiratory pathogens in pediatric nasopharyngeal washes negative by conventional diagnostic testing shows a high prevalence of viruses belonging to a newly recognized picornavirus clade. J. Clin. Virol. 43:219-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drexler, J. F., S. Baumgarte, L. K. Souza Luna, A. Stocker, P. S. Almeida, T. C. Ribeiro, N. Petersen, P. Herzog, C. Pedroso, C. Brites, R. H. da Costa, Jr., A. Gmyl, C. Drosten, and A. Lukashev. 2010. Genomic features and evolutionary constraints in Saffold-like Cardioviruses. J. Gen. Virol. 91:1418-1427. [DOI] [PubMed] [Google Scholar]
- 13.Gerber, K., E. Wimmer, and A. V. Paul. 2001. Biochemical and genetic studies of the initiation of human rhinovirus 2 RNA replication: identification of a cis-replicating element in the coding sequence of 2Apro. J. Virol. 75:10979-10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hamparian, V. V., R. J. Colonno, M. K. Cooney, E. C. Dick, J. M. Gwaltney, Jr., J. H. Hughes, W. S. Jordan, Jr., A. Z. Kapikian, W. J. Mogabgab, A. Monto, C. A. Philips, R. R. Rueckert, J. H. Schieble, E. J. Stott, and D. A. J. Tyrrell. 1987. A collaborative report: rhinoviruses—extension of the numbering system from 89 to 100. Virology 159:191-192. [DOI] [PubMed] [Google Scholar]
- 15.Han, T. H., J. Y. Chung, E. S. Hwang, and J. W. Koo. 2009. Detection of human rhinovirus C in children with acute lower respiratory tract infections in South Korea. Arch. Virol. 154:987-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heath, L., W. E. van der, A. Varsani, and D. P. Martin. 2006. Recombination patterns in aphthoviruses mirror those found in other picornaviruses. J. Virol. 80:11827-11832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang, T., W. Wang, M. Bessaud, P. Ren, J. Sheng, H. Yan, J. Zhang, X. Lin, Y. Wang, F. Delpeyroux, and V. Deubel. 2009. Evidence of recombination and genetic diversity in human rhinoviruses in children with acute respiratory infection. PLoS One 4:e6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapikian, A. Z., R. M. Conant, V. V. Hamparian, R. M. Chanock, P. J. Chapple, E. C. Dick, J. D. Fenters, J. M. Gwaltney, Jr., D. Hamre, J. C. Holper, W. S. Jordan, E. H. Lennette, J. L. Melnick, W. J. Mogabgab, M. A. Mufson, C. A. Phillips, J. H. Schieble, and D. A. J. Tyrell. 1967. Rhinoviruses: a numbering system. Nature 213:761-762.4291698 [Google Scholar]
- 19.Kapikian, A. Z., R. M. Conant, V. V. Hamparian, R. M. Chanock, E. C. Dick, J. M. Gwaltney, Jr., D. Hamre, W. S. Jordan, G. E. Kenny, E. H. Lennette, J. L. Melnick, W. J. Mogabgab, C. A. Phillips, J. H. Schieble, E. J. Stott, and D. A. J. Tyrell. 1971. A collaborative report: rhinoviruses—extension of the numbering system. Virology 43:524-526.5543842 [Google Scholar]
- 20.Khetsuriani, N., X. Lu, W. G. Teague, N. Kazerouni, L. J. Anderson, and D. D. Erdman. 2008. Novel human rhinoviruses and exacerbation of asthma in children. Emerg. Infect. Dis. 14:1793-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kistler, A., P. C. Avila, S. Rouskin, D. Wang, T. Ward, S. Yagi, D. Schnurr, D. Ganem, J. L. DeRisi, and H. A. Boushey. 2007. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J. Infect. Dis. 196:817-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laine, P., C. Savolainen, S. Blomqvist, and T. Hovi. 2005. Phylogenetic analysis of human rhinovirus capsid protein VP1 and 2A protease coding sequences confirms shared genus-like relationships with human enteroviruses. J. Gen. Virol. 86:697-706. [DOI] [PubMed] [Google Scholar]
- 23.Lamson, D., N. Renwick, V. Kapoor, Z. Liu, G. Palacios, J. Ju, A. Dean, K. St George, T. Briese, and W. I. Lipkin. 2006. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004-2005. J. Infect. Dis. 194:1398-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau, S. K., C. C. Yip, A. W. Lin, R. A. Lee, L. Y. So, Y. L. Lau, K. H. Chan, P. C. Woo, and K. Y. Yuen. 2009. Clinical and molecular epidemiology of human rhinovirus C in children and adults in Hong Kong reveals a possible distinct human rhinovirus C subgroup. J. Infect. Dis. 200:1096-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau, S. K., C. C. Yip, H. W. Tsoi, R. A. Lee, L. Y. So, Y. L. Lau, K. H. Chan, P. C. Woo, and K. Y. Yuen. 2007. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J. Clin. Microbiol. 45:3655-3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ledford, R. M., N. R. Patel, T. M. Demenczuk, A. Watanyar, T. Herbertz, M. S. Collett, and D. C. Pevear. 2004. VP1 sequencing of all human rhinovirus serotypes: insights into genus phylogeny and susceptibility to antiviral capsid-binding compounds. J. Virol. 78:3663-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, W. M., C. Kiesner, T. Pappas, I. Lee, K. Grindle, T. Jartti, B. Jakiela, R. F. Lemanske, Jr., P. A. Shult, and J. E. Gern. 2007. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS One 2:e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindberg, A. M., P. Andersson, C. Savolainen, M. N. Mulders, and T. Hovi. 2003. Evolution of the genome of human enterovirus B: incongruence between phylogenies of the VP1 and 3CD regions indicates frequent recombination within the species. J. Gen. Virol. 84:1223-1235. [DOI] [PubMed] [Google Scholar]
- 29.Linsuwanon, P., S. Payungporn, R. Samransamruajkit, N. Posuwan, J. Makkoch, A. Theanboonlers, and Y. Poovorawan. 2009. High prevalence of human rhinovirus C infection in Thai children with acute lower respiratory tract disease. J. Infect. 59:115-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lukashev, A. N., V. A. Lashkevich, O. E. Ivanova, G. A. Koroleva, A. E. Hinkkanen, and J. Ilonen. 2003. Recombination in circulating enteroviruses. J. Virol. 77:10423-10431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukashev, A. N., V. A. Lashkevich, O. E. Ivanova, G. A. Koroleva, A. E. Hinkkanen, and J. Ilonen. 2005. Recombination in circulating Human enterovirus B: independent evolution of structural and non-structural genome regions. J. Gen. Virol. 86:3281-3290. [DOI] [PubMed] [Google Scholar]
- 32.McErlean, P., L. A. Shackelton, E. Andrews, D. R. Webster, S. B. Lambert, M. D. Nissen, T. P. Sloots, and I. M. Mackay. 2008. Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C). PLoS One 3:e1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McErlean, P., L. A. Shackelton, S. B. Lambert, M. D. Nissen, T. P. Sloots, and I. M. Mackay. 2007. Characterisation of a newly identified human rhinovirus, HRV-QPM, discovered in infants with bronchiolitis. J. Clin. Virol. 39:67-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McKnight, K. L., and S. M. Lemon. 1998. The rhinovirus type 14 genome contains an internally located RNA structure that is required for viral replication. RNA 4:1569-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McWilliam Leitch, E. C., J. Bendig, M. Cabrerizo, J. Cardosa, T. Hyypia, O. E. Ivanova, A. Kelly, A. C. Kroes, A. Lukashev, A. Macadam, P. McMinn, M. Roivainen, G. Trallero, D. J. Evans, and P. Simmonds. 2009. Transmission networks and population turnover of echovirus 30. J. Virol. 83:2109-2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, E. K., K. M. Edwards, G. A. Weinberg, M. K. Iwane, M. R. Griffin, C. B. Hall, Y. Zhu, P. G. Szilagyi, L. L. Morin, L. H. Heil, X. Lu, and J. V. Williams. 2009. A novel group of rhinoviruses is associated with asthma hospitalizations. J. Allergy Clin. Immunol. 123:98-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miller, E. K., N. Khuri-Bulos, J. V. Williams, A. A. Shehabi, S. Faouri, J. Al, I., Q. Chen, L. Heil, Y. Mohamed, L. L. Morin, A. Ali, and N. B. Halasa. 2009. Human rhinovirus C associated with wheezing in hospitalised children in the Middle East. J. Clin. Virol. 46:85-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oprisan, G., M. Combiescu, S. Guillot, V. Caro, A. Combiescu, F. Delpeyroux, and R. Crainic. 2002. Natural genetic recombination between co-circulating heterotypic enteroviruses. J. Gen. Virol. 83:2193-2200. [DOI] [PubMed] [Google Scholar]
- 39.Palmenberg, A. C., D. Spiro, R. Kuzmickas, S. Wang, A. Djikeng, J. A. Rathe, C. M. Fraser-Liggett, and S. B. Liggett. 2009. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science 324:55-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen, J. F., M. M. Cherney, H. D. Liebig, T. Skern, E. Kuechler, and M. N. James. 1999. The structure of the 2A proteinase from a common cold virus: a proteinase responsible for the shut-off of host-cell protein synthesis. EMBO J. 18:5463-5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piralla, A., F. Rovida, G. Campanini, V. Rognoni, A. Marchi, F. Locatelli, and G. Gerna. 2009. Clinical severity and molecular typing of human rhinovirus C strains during a fall outbreak affecting hospitalized patients. J. Clin. Virol. 45:311-317. [DOI] [PubMed] [Google Scholar]
- 42.Renwick, N., B. Schweiger, V. Kapoor, Z. Liu, J. Villari, R. Bullmann, R. Miething, T. Briese, and W. I. Lipkin. 2007. A recently identified rhinovirus genotype is associated with severe respiratory-tract infection in children in Germany. J. Infect. Dis. 196:1754-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rivera, V. M., J. D. Welsh, and J. V. Maizel. 1988. Comparative sequence analysis of the 5′ noncoding region of the enteroviruses and rhinoviruses. Virology 165:42-50. [DOI] [PubMed] [Google Scholar]
- 44.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 45.Savolainen, C., S. Blomqvist, M. N. Mulders, and T. Hovi. 2002. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J. Gen. Virol. 83:333-340. [DOI] [PubMed] [Google Scholar]
- 46.Savolainen, C., P. Laine, M. N. Mulders, and T. Hovi. 2004. Sequence analysis of human rhinoviruses in the RNA-dependent RNA polymerase coding region reveals large within-species variation. J. Gen. Virol. 85:2271-2277. [DOI] [PubMed] [Google Scholar]
- 47.Savolainen-Kopra, C., S. Blomqvist, S. Kaijalainen, U. Juonio, R. Juvonen, A. Peitso, A. Saukkoriipi, O. Vainio, T. Hovi, and M. Roivainen. 2010. All known human rhinovirus species are present in sputum specimens of military recruits during respiratory infection. Viruses 1:1178-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Savolainen-Kopra, C., S. Blomqvist, T. Kilpi, M. Roivainen, and T. Hovi. 2009. Novel species of human rhinoviruses in acute otitis media. Pediatr. Infect. Dis. J. 28:59-61. [DOI] [PubMed] [Google Scholar]
- 49.Savolainen-Kopra, C., S. Blomqvist, T. Smura, M. Roivainen, T. Hovi, D. Kiang, I. Kalra, S. Yagi, J. K. Louie, H. Boushey, J. Boothby, and D. P. Schnurr. 2009. 5′ Noncoding region alone does not unequivocally determine genetic type of human rhinovirus strains. J. Clin. Microbiol. 47:1278-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Simmonds, P. 2006. Recombination and selection in the evolution of picornaviruses and other mammalian positive-stranded RNA viruses. J. Virol. 80:11124-11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simmonds, P., and S. Midgley. 2005. Recombination in the genesis and evolution of hepatitis B virus genotypes. J. Virol. 79:15467-15476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simmonds, P., and J. Welch. 2006. Frequency and dynamics of recombination within different species of human enteroviruses. J. Virol. 80:483-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52a.Simmonds, P., C. L. McIntyre, C. Savolainen-Kopra, C. Tapparel, I. M. Mackay, and T. Hovi. 21 July 2006. Proposals for the classification of human rhinovirus species C into genotypically-assigned types. J. Gen. Virol. doi: 10.1099/vir.0.023994-0. [DOI] [PubMed]
- 53.Stanway, G., F. Brown, P. Christian, T. Hovi, T. Hyypia, A. M. Q. King, N. J. Knowles, S. M. Lemon, P. D. Minor, M. A. Pallansch, A. C. Palmenberg, and T. Skern. 2005. Family Picornaviridae, p. 757-778. In C. M. Fauquet, M. A. Mayo, J. Maniloff, U. Desselberger, and L. A. Ball (ed.), Virus taxonomy: classification and nomenclature of viruses. Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier/Academic Press, London, United Kingdom.
- 54.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 55.Tamura, K., M. Nei, and S. Kumar. 2004. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. U. S. A. 101:11030-11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tapparel, C., T. Junier, D. Gerlach, S. Cordey, S. Van Belle, L. Perrin, E. M. Zdobnov, and L. Kaiser. 2007. New complete genome sequences of human rhinoviruses shed light on their phylogeny and genomic features. BMC Genomics 8:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tapparel, C., T. Junier, D. Gerlach, S. Van Belle, L. Turin, S. Cordey, K. Muhlemann, N. Regamey, J. D. Aubert, P. M. Soccal, P. Eigenmann, E. Zdobnov, and L. Kaiser. 2009. New respiratory enterovirus and recombinant rhinoviruses among circulating picornaviruses. Emerg. Infect. Dis. 15:719-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tapparel, C., A. G. L'Huillier, A. L. Rougemont, M. Beghetti, C. Barazzone-Argiroffo, and L. Kaiser. 2009. Pneumonia and pericarditis in a child with HRV-C infection: a case report. J. Clin. Virol. 45:157-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wisdom, A., A. Kutkowska, E. C. McWilliam-Leitch, E. Gaunt, K. Templeton, H. Harvala, and P. Simmonds. 2009. Genetics, recombination and clinical features of human rhinovirus species C (HRV-C) infections; interactions of HRV-C with other respiratory viruses. PLoS One 4:e8518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wisdom, A., C. McWilliam Leitch, E. Gaunt, H. Harvala, and P. Simmonds. 2009. Screening respiratory samples for human rhinoviruses (HRV) and enteroviruses: comprehensive VP4/2-typing reveals high incidence and genetic diversity of HRV species C. J. Clin. Microbiol. 47:3958-3967. [DOI] [PMC free article] [PubMed] [Google Scholar]