Abstract
Previous studies have suggested that polyfunctional mucosal CD8+ T-cell responses may be a correlate of protection in HIV controllers. Mucosal T-cell breadth and/or specificity may also contribute to defining protective responses. In this study, rectal CD8+ T-cell responses to HIV Gag, Env, and Nef were mapped at the peptide level in four subject groups: elite controllers (n = 16; viral load [VL], <75 copies/ml), viremic controllers (n = 14; VL, 75 to 2,000 copies/ml), noncontrollers (n = 14; VL, >10,000 copies/ml), and antiretroviral-drug-treated subjects (n = 8; VL, <75 copies/ml). In all subject groups, immunodominant CD8+ T-cell responses were generally shared by blood and mucosa, although there were exceptions. In HIV controllers, responses to HLA-B27- and HLA-B57-restricted epitopes were common to both tissues, and their magnitude (in spot-forming cells [SFC] per million) was significantly greater than those of responses restricted by other alleles. Furthermore, peptides recognized by T cells in both blood and rectal mucosa, termed “concordant,” elicited higher median numbers of SFC than discordant responses. In magnitude as well as breadth, HIV Gag-specific responses, particularly those targeting p24 and p7, dominated in controllers. Responses in noncontrollers were more evenly distributed among epitopes in Gag, Env, and Nef. Viremic controllers showed significantly broader mucosal Gag-specific responses than other groups. Taken together, these findings demonstrate that (i) Gag-specific responses dominate in mucosal tissues of HIV controllers; (ii) there is extensive overlap between CD8+ T cells in blood and mucosal tissues, with responses to immunodominant epitopes generally shared by both sites; and (iii) mucosal T-cell response breadth alone cannot account for immune control.
Despite more than two decades of intensive research, the immunologic correlates of protection from human immunodeficiency virus (HIV) infection and disease progression remain incompletely understood. To date, the majority of studies of HIV-specific T-cell responses have focused on the measurement of such responses in peripheral blood lymphocytes. Nevertheless, the majority of the body's lymphocytes are housed in mucosal tissues, notably the gastrointestinal (GI) tract (18, 33, 40). The gastrointestinal mucosa also serves as a major target of HIV infection and CD4+ T-cell depletion (7, 25, 36), as well as an important site of transmission (18, 33, 40). Antigen-experienced T cells may preferentially traffic to tissue sites of infection (50), where they may also expand in an antigen-driven manner. Because of the unique role of the gastrointestinal mucosa in HIV pathogenesis, detailed studies of HIV-specific immune responses in this compartment may contribute important insights to our understanding of the disease process.
An important question is the degree to which T-cell responses in mucosal tissues are “compartmentalized” and distinct in specificity and/or clonality from those found elsewhere in the body, including in peripheral blood. Because of the technical challenges associated with obtaining large numbers of viable lymphocytes from mucosal biopsy specimen tissue, comprehensive mapping of the fine specificity of mucosal HIV-specific T-cell responses has been difficult. Relying on a polyclonal expansion approach, Ibarrondo and colleagues successfully mapped HIV-specific CD8+ T-cell responses in blood and rectal mucosa of chronically infected persons to the level of peptide pools but not to individual epitopes (29). Their studies revealed a similar pattern of responses, and nearly identical immunodominance hierarchies, in the two tissue sites.
We have focused our recent studies of mucosal immunity on a group of individuals who control HIV infection in the absence of antiretroviral therapy. These are often called “long-term nonprogressors” (LTNP) (14), referring to their ability to maintain normal CD4+ T-cell counts for more than 10 years without medication. LTNP are believed to account for 5 to 15% of the HIV-infected population. Several recent studies have used the term “HIV controllers,” defined as those who maintain undetectable plasma HIV RNA levels (“elite controllers”) and those who have persistently detectable but low plasma HIV RNA levels (“viremic controllers”). Elite controllers represent less than 1% of the HIV-infected population (14). In contrast, individuals with viral loads of >10,000 copies/ml in the absence of therapy are termed “noncontrollers.” Recently, we found that “polyfunctional” HIV-specific T cells, producing multiple antiviral factors, were significantly more abundant in gastrointestinal mucosa of HIV controllers than in those of noncontrollers or subjects on highly active antiretroviral therapy (HAART) (20). Furthermore, in many cases these strong, polyfunctional mucosal T-cell responses were not mirrored in peripheral blood, suggesting that HIV-specific T cells either preferentially traffic to or undergo expansion within mucosal tissues.
Because of these findings, we undertook a follow-up study to determine the breadth and fine specificity, to the peptide level, of mucosal CD8+ T-cell responses to HIV Gag, Env, and Nef among HIV controllers, noncontrollers, and individuals on HAART. We hypothesized that controllers might harbor an unusually broad repertoire of HIV-specific CD8+ T cells in mucosal tissues. We found a similar response breadth in mucosal tissues of all three subject groups, arguing against a critical role for mucosal T-cell response breadth in determining the extent of HIV control. In contrast, we found that high-magnitude mucosal responses directed at well-conserved regions in Gag were a strong and consistent correlate of control. Finally, concordant responses, defined as those common to blood and mucosa, were generally stronger than discordant responses, underscoring the observation that T cells responding to immunodominant epitopes are broadly distributed throughout the body in both controllers and noncontrollers.
MATERIALS AND METHODS
Subjects and tissue collection.
Subjects were enrolled through an ongoing study of chronic HIV infection based at San Francisco General Hospital and through the Center for AIDS Research, Education and Services Clinic in Sacramento, CA. Most subjects in this study have been described previously (20). However, the experimental data presented here are novel in that this study focuses on responses to three viral proteins (Gag, Env, and Nef), and includes fine mapping of peptide-specific T-cell responses in blood and rectal mucosa.
Subjects were grouped by plasma HIV RNA viral load (VL) according to the criteria outlined by Deeks and Walker (14). Elite controllers were defined as antiretroviral-untreated individuals with plasma VL of <75 copies/ml on at least three occasions. The median duration of HIV infection among elite controllers in the San Francisco SCOPE cohort was 19 years (17). Viremic controllers were defined as antiretroviral-untreated persons with plasma VL between 75 and 2,000 copies/ml on at least 3 occasions. Noncontrollers had VL consistently >10,000 copies/ml in the absence of antiretroviral therapy, and the HAART-suppressed group had plasma viremia suppressed below the limit of detection (<75 copies/ml) by antiretroviral therapy. All subjects in this study were in the chronic stage of infection, having been infected for >2 years. Written informed consent was obtained from all subjects for phlebotomy and flexible sigmoidoscopy, in accordance with the declaration of Helsinki, and with study protocols approved by the Institutional Review Board, University of California—Davis, and the Committee on Human Subjects Research, University of California—San Francisco.
Approximately 20 ml of blood was collected by sterile venipuncture into tubes containing EDTA and was processed on day of collection. Rectal biopsy specimen tissue was obtained at approximately 10 to 15 cm from the anal verge by flexible sigmoidoscopy. This procedure involves minimal discomfort and provides sufficient lymphoid cells for cellular immunology assays or polyclonal expansion (3, 4, 50-53). The sigmoidoscope was equipped with a biopsy channel, and tissues were procured with single-use biopsy forceps (Radial Jaw 3; Boston Scientific, Natick, MA). At each procedure 20 to 25 tissue pieces were collected and placed in RPMI 1640 medium supplemented with 15% fetal calf serum, penicillin (100 U/ml), streptomycin (100 μg/ml), and l-glutamine (2 mM). This medium is here referred to as R15. Specimens were immediately transported to the laboratory at the University of California—Davis for processing and analysis.
Peripheral blood and rectal biopsy specimen tissue processing.
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Hypaque (Pfizer, New York, NY) density gradient centrifugation, washed in phosphate-buffered saline (PBS), and allowed to rest overnight at 37°C and 5% CO2 in R15. Rectal mononuclear cells (RMC) were isolated from biopsy specimens following a published protocol that was optimized for high yield and viability of mucosal lymphocytes (11, 51, 53). Briefly, biopsy specimen pieces underwent three rounds of digestion using 0.5 mg/ml collagenase type II (Sigma-Aldrich, St. Louis, MO). Each digestion was followed by disruption of the tissue using a syringe with a 16-gauge blunt-end needle and subsequent passage through a 70-μm cell strainer. Strained and washed cells were pooled and centrifuged with a 35%/65% Percoll gradient (Sigma-Aldrich). RMC were collected from both interfaces to maximize cell yield. The cells were allowed to rest overnight in R15 containing 0.5 mg/ml piperacillin-tazobactam (Zosyn; Wyeth-Ayerst, Princeton, NJ) to discourage bacterial growth. Yields ranged from 4 × 106 to 21 × 106 RMC from 20 to 25 biopsy specimen pieces (mean, 10 × 106 RMC).
Polyclonal expansion of PBMC and RMC.
One to two million PBMC or RMC were polyclonally expanded in R15 with 50 U/ml human recombinant interleukin-2 (IL-2) (R&D Systems, Minneapolis, MN) and 0.62 μg/ml anti-CD3-4 bispecific antibody (generously provided by Johnson Wong, Harvard University) for CD8+ T-cell enrichment. This antibody stimulates preferential expansion of CD8+ T cells; after 2 to 3 weeks, cultures contained >90% CD8+ T cells (references 29 and 31 and data not shown). When initial attempts to culture mucosal cells (but not PBMC) gave a low success rate, we supplemented cultures with 1 ng/ml human recombinant IL-7 (R&D Systems, Minneapolis, MN), based on a previously published protocol (34). This cytokine promotes survival of memory T cells, in part by inactivating proapoptotic pathways (34, 35a). Additionally, 0.5 mg/ml Zosyn and 1.25 μg/ml amphotericin B (MP Biomedicals, Solon, OH) were added to RMC cultures to prevent the growth of potential bacterial or fungal contaminants. Two to 3 days after the initial culture, 2 to 4 million irradiated PBMC (5,000 rad) from a seronegative donor were added to the cultures as feeder cells. Cultures were expanded and refreshed with IL-2 (and IL-7 for RMC) twice per week, maintaining cells at a density of 1 to 3 × 106 cells/ml. Cultures continuing after 21 days were restimulated with 0.1 μg/ml anti-CD3 antibody (12F6, also provided by Johnson Wong, Harvard University).
HLA class l typing.
DNA was isolated from approximately 5 × 106 to 10 × 106 PBMCs using the QIAamp DNA blood minikit (Qiagen, Valencia, CA) and quantified on a spectrophotometer. Low-resolution HLA-A, -B, and -C typing was performed by PCR with sequence-specific primers using the SSP ABC Unitray kit (Invitrogen, Carlsbad, CA). High-resolution HLA-A and -B typing was performed using direct-to-high-resolution SSP-PCR kits. PCR products were resolved on a 2% agarose gel and photographed, and the patterns were analyzed with UniMatch Plus software (Invitrogen).
Peptides and peptide pools. (i) Preparation of peptides.
123 HIV subtype B Gag peptides, 211 HIV subtype B Env peptides, and 49 HIV subtype B Nef peptides (15-mers overlapping by 11 residues spanning the entire sequence) were obtained in a lyophilized state from the NIH AIDS Research and Reference Reagent Program, Rockville, MD. Each peptide was reconstituted in 50 to 150 μl of dimethyl sulfoxide (depending on solubility), kept at 4°C overnight, and the following day brought up to a total volume of 400 μl with PBS, for a concentration of 2.5 mg/ml.
(ii) Preparation of peptide pools.
Matrices of 23 Gag pools, 30 Env pools, and 14 Nef pools were designed wherein each peptide was present in exactly two pools. A 125-μl portion of each assigned peptide was added to each corresponding peptide pool, and the volume was adjusted to 3.125 ml with RPMI (Gibco), resulting in a peptide concentration of 100 μg/ml. Pools were passed through a 0.45-μm-pore-size sterile filter, aliquoted, and stored at −80°C.
Interferon gamma ELISPOT assays.
Enzyme-linked ImmunoSpot (ELISPOT) assays were performed as previously described (52). Briefly, sterile Multiscreen MAHA S4510 plates (Millipore) were coated with 50 μl/well monoclonal antibody (MAb) 1-D1K (MabTech), at 5 μg/ml in PBS overnight at 4°C. Plates were then washed with PBS and blocked with 50 μl/well R15 for at least 1 h at 37°C. Polyclonally expanded CD8+ T lymphocytes from PBMC or RMC cultures were plated at a concentration of 1 × 105 to 2 × 105 cells in 100 μl to give a total volume of 150 μl R15 per well. Pooled or individual peptides (10 μg/ml) were added in duplicate wells. Individual peptides were chosen based on results from peptide pool data utilizing the peptide matrices described above. A 4-μg/ml portion of staphylococcus enterotoxin B was used for positive-control wells, and culture medium alone was used for negative controls. Plates were incubated overnight (14 to 20 h) at 37°C and 5% CO2. Plates were developed the next day as described previously (52). The plates were read with an AID ELISPOT reader (Autoimmun Diagnostika GMBH, Strasberg, Germany). The number of peptide-specific CD8+ T cells was quantified as spot-forming cells per 106 cells (SFC/million) after subtracting negative-control values. Values of >50 SFC/million were considered positive for all analyses except for cluster maps, where only values of >100 SFC/million were utilized.
Statistical analysis.
Data and statistical analyses were done in consultation with Jerome Braun, University of California—Davis Department of Statistics. Graphing and statistical computation was performed using GraphPad Prism software, version 5 (GraphPad Software, San Diego, CA). Comparisons between two groups were made using a two-tailed Mann-Whitney test.
RESULTS
Subject characteristics.
This study included 52 HIV-infected individuals in four different subject groups: 16 elite controllers, 14 viremic controllers, 14 noncontrollers, and 8 individuals on HAART with undetectable plasma viral loads. The median viral loads for viremic controllers and noncontrollers were 286 copies/ml and 27,590 copies/ml, respectively (Table 1). The median blood CD4 counts were 746, 448, 411, and 488 for elite controllers, viremic controllers, noncontrollers, and HAART-suppressed groups, respectively (Table 1). Elite controllers had significantly higher CD4 counts than the viremic controllers and the noncontrollers (P < 0.05). A summary of major histocompatibility complex (MHC) class I genotypes is presented in Table 1.
TABLE 1.
Patient characteristics
| Patient group | n | % Male | % Caucasian | Plasma viral load (median)a | CD4 count (median)b | % Protective class I HLAc |
|---|---|---|---|---|---|---|
| Elite controller (EC) | 16 | 69 | 31 | <75 | 746* | 69 |
| Viremic controller (VC) | 14 | 71 | 57 | 286 | 448 | 64 |
| Noncontroller (NC) | 14 | 57 | 50 | 27,590 | 411 | 36 |
| HAART suppressed | 8 | 50 | 63 | <75 | 488 | 25 |
Viral RNA copies/ml.
Cells/mm3. *, P < 0.05 compared to VC and NC.
HLA-B13, -B27, -B57, -B58, and -B81.
Immunodominant responses are shared between peripheral blood and rectal mucosa.
In order to provide sufficient cells to map CD8+ T-cell responses by IFN-γ ELISPOT, CD8+ T cells were polyclonally expanded from peripheral blood mononuclear cells (PBMC) and rectal mononuclear cells (RMC). Previous studies have shown this method to expand cells nonspecifically without introducing bias toward particular peptides or T-cell receptor clonotypes (2, 29, 31) and also revealed similar targeting and magnitudes of HIV-specific T-cell responses in fresh and expanded CD8+ T cells (29).
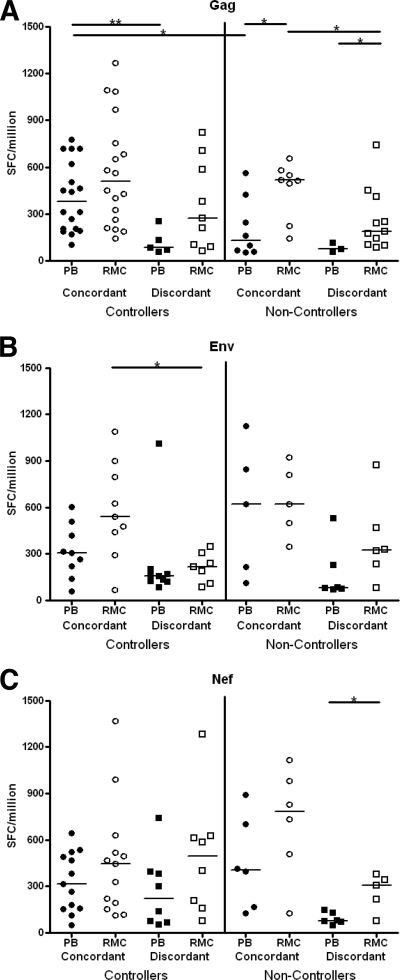
We sought to determine the extent to which responses to individual epitopes were shared between peripheral blood and rectal mucosa. Epitopes recognized by both PBMC and RMC were termed “concordant,” while those recognized by only PBMC or RMC were termed “discordant.” When the magnitudes of concordant and discordant responses were compared, concordant responses tended to be immunodominant (i.e., of higher magnitude) while discordant responses tended to be of lower magnitude (Fig. 1 ). This trend was most obvious in responses to HIV Gag, with significant differences between concordant versus discordant responses in controllers (peripheral blood, P < 0.01) and noncontrollers (rectal mucosa, P < 0.05) (Fig. 1A). Similar but weaker trends were observed for Env-specific responses (rectal mucosa in controllers, P < 0.05) (Fig. 1B). The magnitudes of concordant and discordant Nef-specific responses were similar in controllers, whereas in noncontrollers concordant responses appeared to be immunodominant (Fig. 1C).
FIG. 1.
Concordant and discordant CD8+ T-cell responses in peripheral blood and rectal mucosa of controllers and noncontrollers. Concordant (shared between peripheral blood [PB] and rectal mucosa [RMC]) and discordant (unique to PB or RMC) responses for Gag (A), Env (B), and Nef (C) as determined by IFN-γ ELISPOT assay. All data are presented in spot-forming cells per million (SFC/million). Each data point represents the median response of all concordant or discordant epitopes in a single subject. Horizontal bars represent the median response for each group. *, P < 0.05; **, P < 0.01.
A previous study by Ibarrondo et al. attributed discordant responses to culture artifacts, such as differential expansion or starting frequencies of epitope-specific cells, resulting in low or discordant responses near the limit of detection. While some discordant responses in our study were near the limit of detection for our assay (50 SFC/million), many discordant responses, particularly those in rectal mucosa, were well above this limit, with 84% of Gag responses and 85% of Env and Nef responses being 100 SFC/million or greater (Fig. 1). Another technical issue that may have contributed to discordant responses is the limitation imposed by biopsy specimen sampling due to variable localization of inductive and effector sites within the GI tract. Given the limited number of biopsies performed, it may be difficult to capture all antigen-specific T-cell populations with comparable efficiency, although this remains the best available method for acquiring tissue at this time.
HIV controllers frequently target HIV Gag p24 (capsid) and p7 (nucleocapsid).
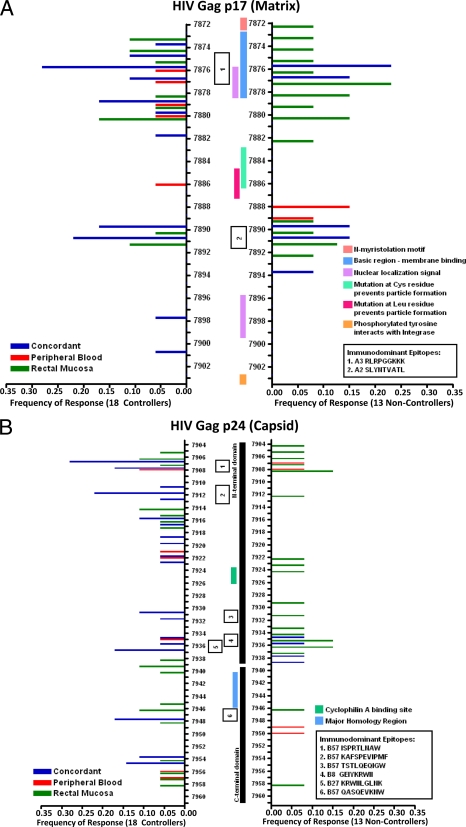
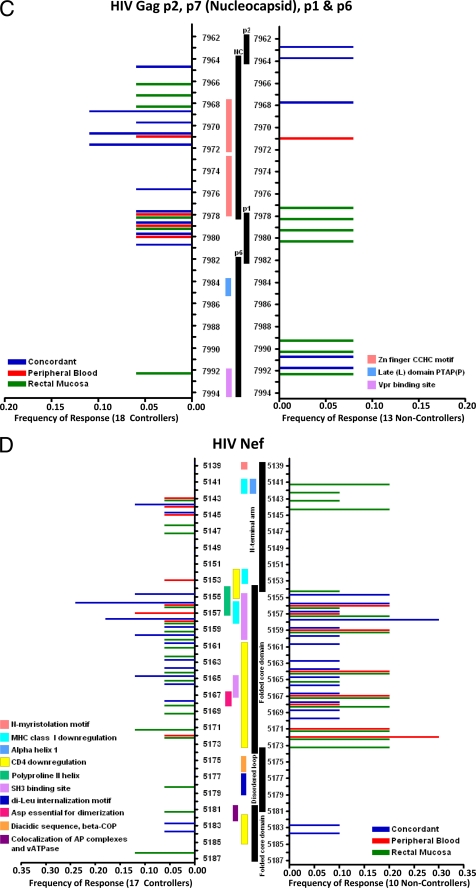
CD8+ T cells in HIV controllers may target highly conserved regions of HIV structural proteins, leading to a potentially high fitness cost associated with cytotoxic T-lymphocyte (CTL) escape mutations (8, 38, 39, 48, 49). In order to better visualize regions targeted by the mucosal CD8+ T-cell response in controllers and noncontrollers, we generated peptide cluster maps for Gag, Env, and Nef (Fig. 2A to D). We determined the percentage of subjects in each group that responded to individual peptides, irrespective of MHC restriction.
FIG. 2.
CD8+ T-cell targeting of HIV Gag and Nef. Peptide cluster maps were constructed to visualize the frequency of subjects targeting specific areas of Gag and Nef. (A) Gag p17, (B) Gag p24, (C) Gag p2, p7, p1, and p6, and (D) Nef. Each peptide numbered on the y axis is a 15-mer which overlaps by 11 amino acids with the next numbered peptide. The colored horizontal bars represent the frequency of controllers (left side) and noncontrollers (right side) with concordant (blue bars), discordant blood (red bars), or discordant mucosal (green bars) responses to a particular peptide. Vertical bars in the center of the map delineate important structural or functional features, and numbered boxes show the locations of well-characterized immunodominant epitopes.
Both controllers and noncontrollers appeared to target HIV Gag p17 (matrix) equally well and in similar locations (Fig. 2A). A higher percentage of controllers responded to p24 (capsid) peptides than noncontrollers (Fig. 2B). These responses were generally concordant and, since a majority of controllers were HLA-B57+, frequently targeted immunodominant, HLA-B57-restricted epitopes (described in detail below). Controllers also more frequently targeted the zinc finger domains of p7 (nucleocapsid) than noncontrollers (Fig. 2C). In contrast, noncontrollers preferentially targeted Nef, particularly regions in the folded core domain (Fig. 2D). HIV Env was sporadically targeted in our assays (data not shown); however, the targeting of Env peptides in these individuals may have been underestimated due to the use of consensus clade B peptides in our assay as opposed to autologous viral sequences.
Immunodominant responses to HLA-B27- and HLA-B57-restricted epitopes in rectal mucosa of HIV controllers.
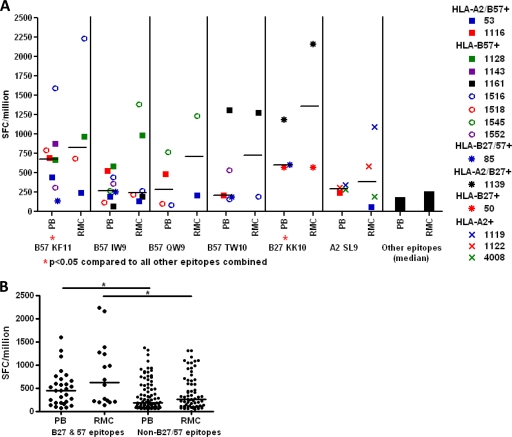
HLA-B27 and HLA-B57 are enriched in HIV controller populations (10, 15, 37). Immunodominant Gag epitopes restricted by these two alleles have been well defined: KRWIILGLNK (B27-KK10, p24 amino acids [aa] 131 to 140) (1, 9, 23, 48, 49, 54), KAFSPEVIPMF (B57-KF11, p24 aa 30 to 40) (22, 24), ISPRTLNAW (B57-IW9, p24 aa 15 to 23) (22, 30), QASQEVKNW (B57-QW9, p24 aa 176 to 184) (22), and TSTLQEQIGW (B57-TW10, p24 aa 108 to 117) (8, 22, 38, 39, 54). Our cohort included two controllers positive for HLA-B27, one positive for both HLA-B27 and HLA-B57, and 11 positive for HLA-B57.
B57-KF11 and B57-IW9 were the most frequently targeted HLA-B57-restricted epitopes among controllers in our study (Fig. 3A). B57-KF11 was also the most consistently immunodominant (Fig. 3A; Table 2), eliciting the highest median responses in blood and rectal mucosa of any HLA-B57-restricted epitope. Furthermore, both HLA-B27- and HLA-B57-restricted CD8+ T cells elicited significantly higher-magnitude responses in controllers, compared to the median responses to non-HLA-B27- or HLA-B57-restricted epitopes (P < 0.05; Fig. 3B). For comparison, the well-defined HLA-A2-restricted epitope SLYNTVATL (A2-SL9, p17 aa 77 to 85) (30, 42, 43), which is not associated with controller status, elicited responses among controllers that were similar in magnitude to those for other non-HLA-B27- and HLA-B57-restricted epitopes (Fig. 3A; Table 2).
FIG. 3.
Strength of the IFN-γ response to HLA-B27- and HLA-B57-restricted Gag epitopes in HIV controllers. (A) IFN-γ response to five immunodominant HLA-B27- and HLA-B57-restricted Gag epitopes compared to that for an immunodominant HLA-A2-restricted Gag epitope as measured by ELISPOT assay. Each symbol represents the response to a particular epitope in a single subject as indicated by the figure legend in both rectal mucosa (RMC) and peripheral blood (PB). The vertical bar graph shows the median response for all non-HLA-B27 and HLA-B57 epitopes. (B) Magnitude of five HLA-B27 and HLA-B57 epitopes compared to those of non-HLA-B27 and HLA-B57 epitopes. All data are presented in spot-forming cells per million (SFC/million). Horizontal bars represent the median magnitude for each group. *, P < 0.05.
TABLE 2.
Median IFN-γ response magnitudes for HLA-B27 and HLA-B57 epitopes
| Epitope | SFC/million in: |
|
|---|---|---|
| Peripheral blood | Rectal mucosa | |
| B27-KK10 | 597 | 1361 |
| B57-KF11 | 678 | 824 |
| B57-IW9 | 268 | 245 |
| B57-QW9 | 292.5 | 720 |
| B57-TW10 | 210 | 732.5 |
| All B27 and B57 epitopes | 443 | 626 |
| A2-SL9 | 296 | 386 |
| All other epitopes | 185 | 255 |
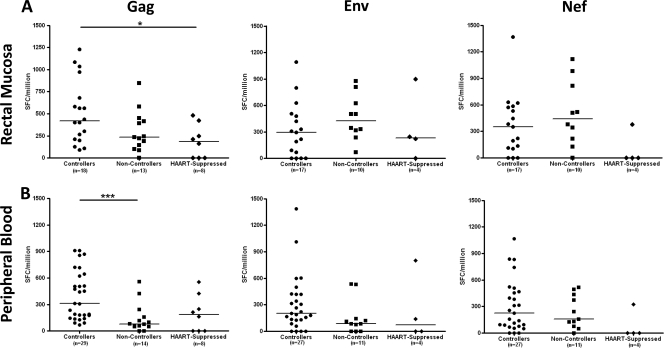
Median mucosal T-cell responses to Gag peptides are stronger in controllers than in noncontrollers, while Env and Nef responses show the opposite trend.
In general, and consistent with our earlier studies (20), controllers (elite and viremic controllers combined) had significantly higher median responses to Gag p55 in rectal mucosa than HAART-suppressed individuals (P < 0.05; Fig. 4 A). Controllers also showed a trend toward higher mucosal Gag-specific responses than noncontrollers (Fig. 4A). In PBMC, controllers had significantly higher median IFN-γ responses to HIV Gag than noncontrollers (P < 0.001, Fig. 4B).
FIG. 4.
Strength of the IFN-γ response to HIV Gag, Env, and Nef in rectal mucosa and peripheral blood. Median IFN-γ response to Gag, Env, and Nef in rectal mucosa (A) and peripheral blood (B), as measured by ELISPOT assay. All data are presented in spot-forming cells per million (SFC/million). Each data point represents the median Gag, Env, or Nef response in a single subject. Horizontal bars represent the median response for each group. *, P < 0.05; ***, P < 0.001.
The peripheral blood IFN-γ responses to Env and Nef were slightly greater in controllers than in noncontrollers or HAART-suppressed subjects (Fig. 4B). However, median Env and Nef responses in rectal mucosa were stronger in noncontrollers than either controllers or individuals on HAART (P > 0.05, Fig. 4A). Thus, mucosal responses to HIV Gag peptides tended to be stronger in controllers than in noncontrollers (in terms of IFN-γ SFC/million), while mucosal responses to Env and Nef revealed the opposite trend.
There was also a trend toward higher-magnitude Gag-, Env-, and Nef-specific responses in rectal mucosa than in PBMC in both controllers and noncontrollers (Fig. 4A and B). This trend reached significance only in Env-specific responses among noncontrollers (P < 0.05, Fig. 4A and B). This observation recalls previous findings and is a likely consequence of the higher frequency of antigen-experienced, memory T cells in gastrointestinal lamina propria than in peripheral blood (12, 20).
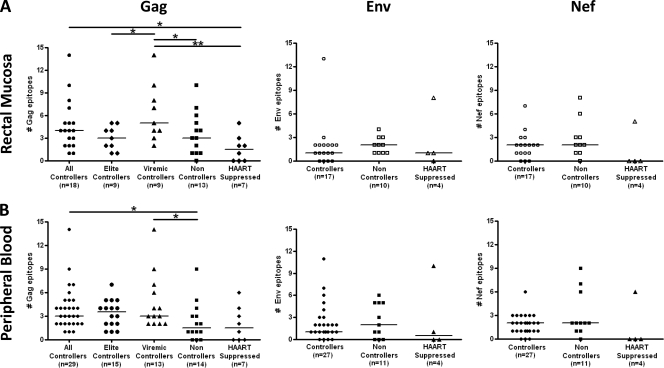
Viremic controllers show a greater breadth of response to HIV Gag than elite controllers, noncontrollers, and individuals on HAART.
The total ELISPOT response breadth for each subject was calculated by summing the number of epitopes recognized within Gag, Env, and Nef by PBMC or RMC. For these calculations, responses to overlapping peptides spanning a single known epitope were counted as a single response. Total response breadth was similar in controllers and noncontrollers (Fig. 5 A and B). However, controllers had broader Gag-specific responses in rectal mucosa than subjects on HAART (median, 4 versus 2 epitopes) (Fig. 5A). Controllers also had broader Gag-specific responses in PBMC than noncontrollers (median, 3 versus 1.5 epitopes) (Fig. 5B). Subdividing the controller group into elite (i.e., those with VL <75 copies/ml) and viremic controllers (i.e., those with VL <2,000 copies/ml), we found that viremic controllers had significantly broader Gag-specific responses in rectal mucosa than elite controllers, noncontrollers, and individuals on HAART (median, 5 versus 3, 3, and 2 epitopes, respectively; P < 0.05) (Fig. 5A), suggesting that the presence of ongoing low-level viral replication in these individuals may support continued targeting of HIV Gag.
FIG. 5.
Breadth of the CD8+ T-cell response. The number of epitopes (breadth) recognized within Gag, Env, and Nef by CD8+ T cells in rectal mucosa (A) and peripheral blood (B), as measured by IFN-γ ELISPOT assay. Horizontal bars represent the median breadth of response for each group. *, P < 0.05; **, P < 0.01.
Reduced breadth and magnitude of mucosal CD8+ T-cell responses in individuals on HAART.
Subjects on HAART with undetectable viral loads generally showed lower-magnitude CD8+ T-cell responses to Gag, Env, and Nef than HIV controllers, with many subjects on HAART showing no detectable responses at all (Fig. 4). Likewise, response breadth was also low in these individuals (Fig. 5). As a percentage of the total response in HAART-suppressed individuals (Gag, Env, and Nef combined), Gag epitopes were most frequently targeted in both rectal mucosa and blood (60 and 75% of targeted epitopes, respectively; data not shown). Env epitopes elicited fewer responses in subjects on HAART (40% and 25% of targeted epitopes in mucosa and blood, respectively) (data not shown). Only one sample from a HAART-suppressed individual in our study targeted Nef (Fig. 5).
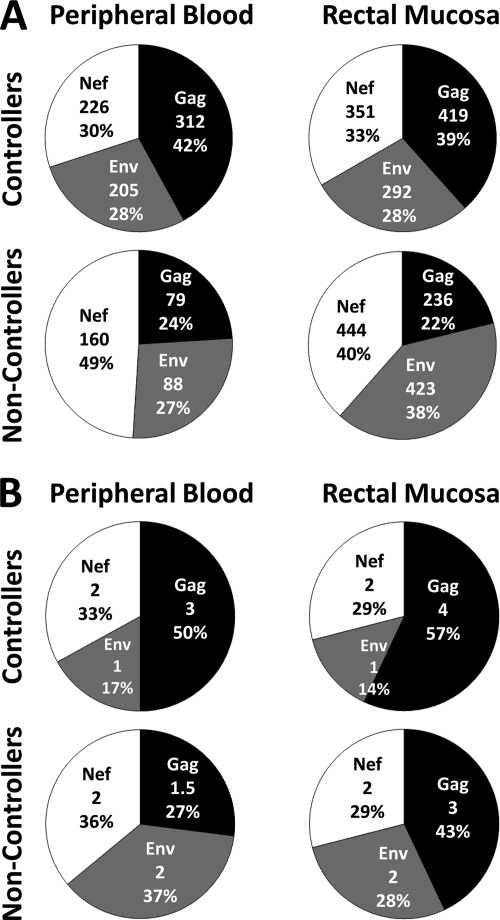
Gag-specific responses dominate in rectal mucosa of HIV controllers.
The pie charts in Fig. 6 A show median ELISPOT response magnitudes partitioned into Gag, Env, and Nef. In HIV controllers, responses in rectal mucosa and blood showed a slight bias toward Gag (39 to 42% of the total response), with the remaining 60% divided approximately evenly between Env and Nef. In contrast, in HIV noncontrollers, less than 25% of the response in either tissue was directed toward Gag, while 40% of the mucosal response and 49% of the PBMC response were directed toward Nef.
FIG. 6.
Proportion of the CD8+ T-cell response attributed to Gag, Env, or Nef. Each pie shows the proportion of the measured response magnitude (A) or breadth (B) attributed to Gag, Env, and Nef in either peripheral blood or rectal mucosa of controllers and noncontrollers. The numbers in the middle of the pies signify the median number of spot-forming cells/million (A) or the median number of epitopes targeted in each protein (B), followed by the percentage of the total response magnitude or breadth.
The pie charts in Fig. 6B show the median response breadth (number of epitopes targeted) for each tissue and subject group. In controllers, at least 50% of the epitopes targeted by CD8+ T cells in both rectal mucosa and PBMC were located within HIV Gag, compared to fewer than 20% for HIV Env (Fig. 6B). In noncontrollers, Gag, Env, and Nef were targeted at similar frequencies in blood.
Taken together, the data in Fig. 6A and B reveal that Gag responses were the largest contributor to mucosal and blood CD8+ T-cell responses in HIV controllers, while Nef and Env-specific responses dominated in HIV noncontrollers.
DISCUSSION
Concordance of T-cell responses in mucosa and blood.
Our data demonstrate a strong concordance between epitope-specific CD8+ T-cell responses in rectal mucosa and peripheral blood, suggesting broad dissemination of HIV-specific T-cell clones throughout blood and tissues in the setting of chronic HIV infection. This is in agreement with a previous study by Ibarrondo et al. showing shared responses between rectal mucosa and blood to pools of peptides spanning the HIV proteome (29). Here we expand on this previous work by mapping responses to the level of individual peptides in HIV controllers, noncontrollers, and subjects on HAART. It should be noted that during acute HIV/simian immunodeficiency virus (SIV) infection, the appearance of virus-specific CD8+ T cells in mucosal tissues may be delayed and relatively compartmentalized (46), depending upon the route of infection. Similarly, in murine vaccine studies, the route of administration and type of immunogen may determine the tissue distribution of antigen-specific T cells (5). Our data suggest a broader tissue distribution of effector T cells during long-term chronic infection than what may be observed during acute infection or postvaccination.
Dominance of HIV Gag responses in controllers.
In previous studies of individuals with chronic HIV infection, we noted that HIV Gag-specific T cells were more abundant in rectal mucosa of individuals with low viral load than in those with high viral load (11, 12); indeed, for one subject group, we observed an inverse relationship between the frequency of HIV Gag-specific IFN-γ+ mucosal T cells and plasma viral load (12). More recently, we determined that polyfunctional HIV-specific CD8+ T cells responding to Gag p55 were more abundant in rectal mucosa of HIV controllers than in that of noncontrollers or individuals on HAART (20). These complex responses were not observed in peripheral blood (20). In this study, CD8+ T cells targeting immunodominant Gag epitopes (as measured by IFN-γ SFC/million) were shared between blood and mucosa; however, the quality of those epitope-specific responses in terms of polyfunctionality was not determined.
Kiepiela et al. reported that Gag-specific CD8+ T-cell responses are immunodominant in peripheral blood of HIV-infected subjects with low viral load whereas Env- and Nef-specific responses are dominant in those with high viral load (32). Similarly, we found that mucosal responses in HIV controllers were dominated by Gag-specific CD8+ T cells whereas mucosal responses in noncontrollers were more strongly directed toward Nef and Env. Notably, mucosal responses in HIV controllers were frequently directed toward conserved regions of HIV Gag, including but not limited to immunodominant epitopes restricted by HLA-B27 and -B57.
It is important to note that technical limitations, primarily mucosal cell number, prevented us from mapping responses to the entire HIV peptidome. While it might have been possible to continue expanding T-cell cultures in order to obtain sufficient cells to analyze all HIV peptides using a matrix approach, the specificity and functionality of T cells can change with prolonged in vitro culture (our unpublished results). Instead, we chose to focus on Gag, Nef, and Env responses because (i) we had previously identified Gag responses as immunodominant in mucosal tissues (11, 12), (ii) strong Nef-specific responses are frequently present during chronic infection (21), (iii) some reports have suggested a role for Env-specific T-cell responses in mucosal tissues (41), and (iv) Env viral sequences may vary between mucosal tissues and blood (45). However, it must also be noted that clade B consensus peptides, as opposed to autologous viral peptides, were used to map these responses. Therefore, responses to autologous viral sequences that strongly diverge from the clade B consensus, particularly within the highly variable Env protein, would not have been detected in this study.
Focusing of CD8 responses on conserved regions within Gag.
By mapping mucosal responses to the peptide level, we found that HIV controllers frequently targeted the highly conserved Gag p24 as well as the zinc finger domains of Gag p7. Several studies have noted that HLA-B27- and HLA-B57-restricted responses to Gag p24 impose strong immune selection pressure on the virus (8, 38, 39, 48, 49). The resulting escape mutants show reduced viral fitness, likely due to defects such as reduced cyclophilin A binding. Additionally, de novo CD8+ T-cell responses to viral escape variants can arise, suggesting a dual mechanism of control where responses directed toward conserved structural motifs drive the evolution of less-fit escape mutants, which are then targeted by strong CD8+ T-cell responses (38). Responses directed toward Gag p7 zinc finger domains may also be important, as escape mutations may disrupt genome dimerization, RNA encapsidation, and reverse transcription (35, 47, 55). Thus, by driving viral escape, certain Gag-specific CD8+ T-cell responses may help to disrupt critical functions of highly conserved viral proteins and contribute to immune control. Our study also found response breadth to Gag, Env, and Nef to be similar among controllers and noncontrollers in both blood and tissues. Taken together, these findings suggest that the number of epitopes recognized may be less important for immune control than the targeting of structurally conserved regions that are critical for the viral replication cycle.
Role of mucosal cell-mediated immunity in HIV controllers.
Several lines of evidence argue for a “protective” role for cell-mediated immune responses in contributing to HIV control. First, certain MHC class I alleles, notably HLA-B57, are enriched in HIV controllers (17). Second, HIV controllers have strong, polyfunctional HIV-specific CD8+ T-cell responses in blood (6) and gastrointestinal mucosa (20). Polyfunctional mucosal CD4+ T-cell responses may also contribute to the maintenance of controller status (19), and many controllers have high numbers of HIV Gag-specific CD4+ and CD8+ IFN-γ+ IL-2+ T cells in blood (16, 17, 26, 27). However, as noted by several authors, there is significant heterogeneity in HIV-specific T-cell response magnitude, polyfunctionality, and breadth among HIV controllers, and some have low to undetectable HIV-specific T-cell responses (17, 44). Furthermore, many controllers lack “protective” MHC class I alleles (17). Therefore, while it is likely that host genetic factors, such as MHC class I alleles, and Gag-specific CD4+ and/or CD8+ T-cell responses contribute to immune control, additional non-T-cell factors may account for other instances of decreased viral replication. The role of innate immunity, including NK cells, in the HIV controller phenomenon should also be explored.
Even though plasma viremia in subjects on long-term HAART may be comparable to that in elite controllers (28), subjects on HAART have much lower HIV-specific T-cell responses (as defined by magnitude and breadth) in blood and tissues than controllers. One can speculate that residual viral replication in controllers (e.g., in gastrointestinal mucosa) may support continued targeting of HIV by CD4+ and CD8+ T cells. Additional studies will be required to address this question. It is interesting to note that viremic controllers, who have low but detectable plasma viremia, showed an increased breadth of response to Gag compared to those of all other groups, including elite controllers (VL, <75 copies/ml). Therefore, immune responses may operate in a Gaussian fashion driven by viral load, as previously described for antiretroviral-treated individuals with various levels of viral control (13). Subjects on antiretroviral therapy who had incomplete viral suppression, called “partial controllers on antiretroviral therapy” (PCAT), had higher levels of HIV-specific CD4+ T cells than either subjects with complete suppression or those who did not respond to treatment and maintained high viral loads (13). It will be important in future studies to determine the degree to which controllers harbor residual viral replication at mucosal sites.
Taken together, our results show that immunodominant HIV-specific CD8+ T-cell responses are broadly distributed across both blood and tissues. Additionally, Gag-specific responses, in particular those directed against conserved regions in Gag p24 and Gag p7, dominated in rectal mucosa of HIV controllers. In general, differences between patient groups in response breadth were subtle, suggesting that the total number of epitopes recognized may be less important for long-term immune control than the targeting of structurally conserved regions that are critical for the viral replication cycle. Understanding which epitopes may be most susceptible to immunity-mediated selection pressures that drive the expansion of less fit viral escape mutants will be important for future vaccine development.
Acknowledgments
This research was funded by NIH R01 AI057020 to B.L.S., grants AI069994 and AI044595 to S.G.D., grant AI065244 to P.W.H., grant AI027763, the University of California—San Francisco Gladstone Institute of Virology and Immunology Center for AIDS Research, the California HIV/AIDS Research Program (grant CH05-D-606, R.B.P.), and the University of California—San Francisco Clinical and Translational Science Institute (UL1 RR024131).
We thank Rebecca Hoh (University of California—San Francisco) and Melissa Schreiber (University of California—Davis) for assistance with subject recruitment and enrollment.
We declare no competing financial interests.
Footnotes
Published ahead of print on 28 July 2010.
REFERENCES
- 1.Altfeld, M., E. T. Kalife, Y. Qi, H. Streeck, M. Lichterfeld, M. N. Johnston, N. Burgett, M. E. Swartz, A. Yang, G. Alter, X. G. Yu, A. Meier, J. K. Rockstroh, T. M. Allen, H. Jessen, E. S. Rosenberg, M. Carrington, and B. D. Walker. 2006. HLA alleles associated with delayed progression to AIDS contribute strongly to the initial CD8(+) T cell response against HIV-1. PLoS Med. 3:e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altfeld, M., J. van Lunzen, N. Frahm, X. G. Yu, C. Schneider, R. L. Eldridge, M. E. Feeney, D. Meyer-Olson, H. J. Stellbrink, and B. D. Walker. 2002. Expansion of pre-existing, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J. Clin. Invest. 109:837-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anton, P. A., R. T. Mitsuyasu, S. G. Deeks, D. T. Scadden, B. Wagner, C. Huang, C. Macken, D. D. Richman, C. Christopherson, F. Borellini, R. Lazar, and K. M. Hege. 2003. Multiple measures of HIV burden in blood and tissue are correlated with each other but not with clinical parameters in aviremic subjects. AIDS 17:53-63. [DOI] [PubMed] [Google Scholar]
- 4.Anton, P. A., M. A. Poles, J. Elliott, S. H. Mao, I. McGowan, H. J. Lenz, and I. S. Chen. 2001. Sensitive and reproducible quantitation of mucosal HIV-1 RNA and DNA viral burden in patients with detectable and undetectable plasma viral HIV-1 RNA using endoscopic biopsies. J. Virol. Methods 95:65-79. [DOI] [PubMed] [Google Scholar]
- 5.Belyakov, I. M., J. D. Ahlers, G. J. Nabel, B. Moss, and J. A. Berzofsky. 2008. Generation of functionally active HIV-1 specific CD8+ CTL in intestinal mucosa following mucosal, systemic or mixed prime-boost immunization. Virology 381:106-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brockman, M. A., A. Schneidewind, M. Lahaie, A. Schmidt, T. Miura, I. Desouza, F. Ryvkin, C. A. Derdeyn, S. Allen, E. Hunter, J. Mulenga, P. A. Goepfert, B. D. Walker, and T. M. Allen. 2007. Escape and compensation from early HLA-B57-mediated cytotoxic T-lymphocyte pressure on human immunodeficiency virus type 1 Gag alter capsid interactions with cyclophilin A. J. Virol. 81:12608-12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buseyne, F., M. McChesney, F. Porrot, S. Kovarik, B. Guy, and Y. Riviere. 1993. Gag-specific cytotoxic T lymphocytes from human immunodeficiency virus type 1-infected individuals: Gag epitopes are clustered in three regions of the p24gag protein. J. Virol. 67:694-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrington, M., and S. J. O'Brien. 2003. The influence of HLA genotype on AIDS. Annu. Rev. Med. 54:535-551. [DOI] [PubMed] [Google Scholar]
- 11.Critchfield, J. W., D. Lemongello, D. H. Walker, J. C. Garcia, D. M. Asmuth, R. B. Pollard, and B. L. Shacklett. 2007. Multifunctional human immunodeficiency virus (HIV) Gag-specific CD8+ T-cell responses in rectal mucosa and peripheral blood mononuclear cells during chronic HIV type 1 infection. J. Virol. 81:5460-5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Critchfield, J. W., D. H. Young, T. L. Hayes, J. V. Braun, J. C. Garcia, R. B. Pollard, and B. L. Shacklett. 2008. Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status. PLoS One 3:e3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deeks, S. G., J. N. Martin, E. Sinclair, J. Harris, T. B. Neilands, H. T. Maecker, E. Hagos, T. Wrin, C. J. Petropoulos, B. Bredt, and J. M. McCune. 2004. Strong cell-mediated immune responses are associated with the maintenance of low-level viremia in antiretroviral-treated individuals with drug-resistant human immunodeficiency virus type 1. J. Infect. Dis. 189:312-321. [DOI] [PubMed] [Google Scholar]
- 14.Deeks, S. G., and B. D. Walker. 2007. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 27:406-416. [DOI] [PubMed] [Google Scholar]
- 15.den Uyl, D., I. E. van der Horst-Bruinsma, and M. van Agtmael. 2004. Progression of HIV to AIDS: a protective role for HLA-B27? AIDS Rev. 6:89-96. [PubMed] [Google Scholar]
- 16.Emu, B., E. Sinclair, D. Favre, W. J. Moretto, P. Hsue, R. Hoh, J. N. Martin, D. F. Nixon, J. M. McCune, and S. G. Deeks. 2005. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J. Virol. 79:14169-14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Emu, B., E. Sinclair, H. Hatano, A. Ferre, B. Shacklett, J. N. Martin, J. M. McCune, and S. G. Deeks. 2008. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J. Virol. 82:5398-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fackler, O. T., M. Schafer, W. Schmidt, T. Zippel, W. Heise, T. Schneider, M. Zeitz, E. O. Riecken, N. Mueller-Lantzsch, and R. Ullrich. 1998. HIV-1 p24 but not proviral load is increased in the intestinal mucosa compared with the peripheral blood in HIV-infected patients. AIDS 12:139-146. [DOI] [PubMed] [Google Scholar]
- 19.Ferre, A. L., P. W. Hunt, J. W. Critchfield, D. H. Young, J. C. Garcia, H. F. Yee, Jr., R. B. Pollard, J. N. Martin, S. G. Deeks, and B. L. Shacklett. 2009. HIV controllers with HLA-DR*13 and HLA-DQ*6 have strong, polyfunctional mucosal CD4+ T-cell responses, abstr. 214. In D. Trono, D. H. Gabuzda, and R. F. Siliciano (ed.), Keystone Symposium: HIV Immunobiology: from infection to immune control. Keystone, Symposia, Silverthrone, CO.
- 20.Ferre, A. L., P. W. Hunt, J. W. Critchfield, D. H. Young, M. M. Morris, J. C. Garcia, R. B. Pollard, H. F. Yee, Jr., J. N. Martin, S. G. Deeks, and B. L. Shacklett. 2009. Mucosal immune responses to HIV-1 in elite controllers: a potential correlate of immune control. Blood 113:3978-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frahm, N., B. T. Korber, C. M. Adams, J. J. Szinger, R. Draenert, M. M. Addo, M. E. Feeney, K. Yusim, K. Sango, N. V. Brown, D. SenGupta, A. Piechocka-Trocha, T. Simonis, F. M. Marincola, A. G. Wurcel, D. R. Stone, C. J. Russell, P. Adolf, D. Cohen, T. Roach, A. StJohn, A. Khatri, K. Davis, J. Mullins, P. J. Goulder, B. D. Walker, and C. Brander. 2004. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J. Virol. 78:2187-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goulder, P. J., M. Bunce, P. Krausa, K. McIntyre, S. Crowley, B. Morgan, A. Edwards, P. Giangrande, R. E. Phillips, and A. J. McMichael. 1996. Novel, cross-restricted, conserved, and immunodominant cytotoxic T lymphocyte epitopes in slow progressors in HIV type 1 infection. AIDS Res. Hum. Retroviruses 12:1691-1698. [DOI] [PubMed] [Google Scholar]
- 23.Goulder, P. J., R. E. Phillips, R. A. Colbert, S. McAdam, G. Ogg, M. A. Nowak, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3:212-217. [DOI] [PubMed] [Google Scholar]
- 24.Goulder, P. J., Y. Tang, S. I. Pelton, and B. D. Walker. 2000. HLA-B57-restricted cytotoxic T-lymphocyte activity in a single infected subject toward two optimal epitopes, one of which is entirely contained within the other. J. Virol. 74:5291-5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harari, A., C. Cellerai, F. B. Enders, J. Kostler, L. Codarri, G. Tapia, O. Boyman, E. Castro, S. Gaudieri, I. James, M. John, R. Wagner, S. Mallal, and G. Pantaleo. 2007. Skewed association of polyfunctional antigen-specific CD8 T cell populations with HLA-B genotype. Proc. Natl. Acad. Sci. U. S. A. 104:16233-16238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harari, A., S. Petitpierre, F. Vallelian, and G. Pantaleo. 2004. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103:966-972. [DOI] [PubMed] [Google Scholar]
- 28.Hatano, H., E. L. Delwart, P. J. Norris, T. H. Lee, J. Dunn-Williams, P. W. Hunt, R. Hoh, S. L. Stramer, J. M. Linnen, J. M. McCune, J. N. Martin, M. P. Busch, and S. G. Deeks. 2009. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J. Virol. 83:329-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibarrondo, F. J., P. A. Anton, M. Fuerst, H. L. Ng, J. T. Wong, J. Matud, J. Elliott, R. Shih, M. A. Hausner, C. Price, L. E. Hultin, P. M. Hultin, B. D. Jamieson, and O. O. Yang. 2005. Parallel human immunodeficiency virus type 1-specific CD8+ T-lymphocyte responses in blood and mucosa during chronic infection. J. Virol. 79:4289-4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson, R. P., A. Trocha, L. Yang, G. P. Mazzara, D. L. Panicali, T. M. Buchanan, and B. D. Walker. 1991. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J. Immunol. 147:1512-1521. [PubMed] [Google Scholar]
- 31.Jones, N., D. Agrawal, M. Elrefaei, A. Hanson, V. Novitsky, J. T. Wong, and H. Cao. 2003. Evaluation of antigen-specific responses using in vitro enriched T cells. J. Immunol. Methods 274:139-147. [DOI] [PubMed] [Google Scholar]
- 32.Kiepiela, P., K. Ngumbela, C. Thobakgale, D. Ramduth, I. Honeyborne, E. Moodley, S. Reddy, C. de Pierres, Z. Mncube, N. Mkhwanazi, K. Bishop, M. van der Stok, K. Nair, N. Khan, H. Crawford, R. Payne, A. Leslie, J. Prado, A. Prendergast, J. Frater, N. McCarthy, C. Brander, G. H. Learn, D. Nickle, C. Rousseau, H. Coovadia, J. I. Mullins, D. Heckerman, B. D. Walker, and P. Goulder. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 13:46-53. [DOI] [PubMed] [Google Scholar]
- 33.Kotler, D. P., S. Reka, A. Borcich, and W. J. Cronin. 1991. Detection, localization, and quantitation of HIV-associated antigens in intestinal biopsies from patients with HIV. Am. J. Pathol. 139:823-830. [PMC free article] [PubMed] [Google Scholar]
- 34.Lalvani, A., T. Dong, G. Ogg, A. A. Patham, H. Newell, A. V. Hill, A. J. McMichael, and S. Rowland-Jones. 1997. Optimization of a peptide-based protocol employing IL-7 for in vitro restimulation of human cytotoxic T lymphocyte precursors. J. Immunol. Methods 210:65-77. [DOI] [PubMed] [Google Scholar]
- 35.Laughrea, M., N. Shen, L. Jette, J. L. Darlix, L. Kleiman, and M. A. Wainberg. 2001. Role of distal zinc finger of nucleocapsid protein in genomic RNA dimerization of human immunodeficiency virus type 1; no role for the palindrome crowning the R-U5 hairpin. Virology 281:109-116. [DOI] [PubMed] [Google Scholar]
- 35a.Li, W. Q., Q. Jiang, A. R. Khaled, J. R. Keller, and S. K. Durum. 2004. Interleukin-7 inactivates the pro-apoptotic protein Bad promoting T cell survival. J. Biol. Chem. 279:29160-29166. [DOI] [PubMed] [Google Scholar]
- 36.Mehandru, S., M. A. Poles, K. Tenner-Racz, A. Horowitz, A. Hurley, C. Hogan, D. Boden, P. Racz, and M. Markowitz. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Migueles, S. A., M. S. Sabbaghian, W. L. Shupert, M. P. Bettinotti, F. M. Marincola, L. Martino, C. W. Hallahan, S. M. Selig, D. Schwartz, J. Sullivan, and M. Connors. 2000. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc. Natl. Acad. Sci. U. S. A. 97:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miura, T., M. A. Brockman, A. Schneidewind, M. Lobritz, F. Pereyra, A. Rathod, B. L. Block, Z. L. Brumme, C. J. Brumme, B. Baker, A. C. Rothchild, B. Li, A. Trocha, E. Cutrell, N. Frahm, C. Brander, I. Toth, E. J. Arts, T. M. Allen, and B. D. Walker. 2009. HLA-B57/B*5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphotye [sic] recognition. J. Virol. 83:2743-2755. (Erratum, 83:5961.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miura, T., C. J. Brumme, M. A. Brockman, Z. L. Brumme, F. Pereyra, B. L. Block, A. Trocha, M. John, S. Mallal, P. R. Harrigan, and B. D. Walker. 2009. HLA-associated viral mutations are common in human immunodeficiency virus type 1 elite controllers. J. Virol. 83:3407-3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mowat, A., and J. Viney. 1997. The anatomical basis of intestinal immunity. Immunol. Rev. 156:145-166. [DOI] [PubMed] [Google Scholar]
- 41.Musey, L., Y. Ding, J. Cao, J. Lee, C. Galloway, A. Yuen, K. R. Jerome, and M. J. McElrath. 2003. Ontogeny and specificity of mucosal and blood human immunodeficiency virus-1 specific CD8+ cytotoxic T lymphocytes. J. Virol. 77:291-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parker, K. C., M. A. Bednarek, and J. E. Coligan. 1994. Scheme for ranking potential HLA-A2 binding peptides based on independent binding of individual peptide side-chains. J. Immunol. 152:163-175. [PubMed] [Google Scholar]
- 43.Parker, K. C., M. A. Bednarek, L. K. Hull, U. Utz, B. Cunningham, H. J. Zweerink, W. E. Biddison, and J. E. Coligan. 1992. Sequence motifs important for peptide binding to the human MHC class I molecule, HLA-A2. J. Immunol. 149:3580-3587. [PubMed] [Google Scholar]
- 44.Pereyra, F., M. M. Addo, D. E. Kaufmann, Y. Liu, T. Miura, A. Rathod, B. Baker, A. Trocha, R. Rosenberg, E. Mackey, P. Ueda, Z. Lu, D. Cohen, T. Wrin, C. J. Petropoulos, E. S. Rosenberg, and B. D. Walker. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197:563-571. [DOI] [PubMed] [Google Scholar]
- 45.Poss, M., H. L. Martin, J. K. Kreiss, L. Granville, B. Chohan, P. Nyange, K. Mandaliya, and J. Overbaugh. 1995. Diversity in virus populations from genital secretions and peripheral blood from women recently infected with human immunodeficiency virus type 1. J. Virol. 69:8118-8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reynolds, M. R., E. Rakasz, P. J. Skinner, C. White, K. Abel, Z. M. Ma, L. Compton, G. Napoe, N. Wilson, C. J. Miller, A. Haase, and D. I. Watkins. 2005. CD8+ T-lymphocyte response to major immunodominant epitopes after vaginal exposure to simian immunodeficiency virus: too late and too little. J. Virol. 79:9228-9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roques, B. P., N. Morellet, H. de Rocquigny, H. Demene, W. Schueler, and N. Jullian. 1997. Structure, biological functions and inhibition of the HIV-1 proteins Vpr and NCp7. Biochimie 79:673-680. [DOI] [PubMed] [Google Scholar]
- 48.Schneidewind, A., M. A. Brockman, J. Sidney, Y. E. Wang, H. Chen, T. J. Suscovich, B. Li, R. I. Adam, R. L. Allgaier, B. R. Mothe, T. Kuntzen, C. Oniangue-Ndza, A. Trocha, X. G. Yu, C. Brander, A. Sette, B. D. Walker, and T. M. Allen. 2008. Structural and functional constraints limit options for cytotoxic T-lymphocyte escape in the immunodominant HLA-B27-restricted epitope in human immunodeficiency virus type 1 capsid. J. Virol. 82:5594-5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schneidewind, A., M. A. Brockman, R. Yang, R. I. Adam, B. Li, S. Le Gall, C. R. Rinaldo, S. L. Craggs, R. L. Allgaier, K. A. Power, T. Kuntzen, C. S. Tung, M. X. LaBute, S. M. Mueller, T. Harrer, A. J. McMichael, P. J. Goulder, C. Aiken, C. Brander, A. D. Kelleher, and T. M. Allen. 2007. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J. Virol. 81:12382-12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shacklett, B. L., C. A. Cox, J. K. Sandberg, M. A. Jacobson, N. H. Stollman, and D. F. Nixon. 2003. Trafficking of HIV-1-specific CD8+ T-cells to gut-associated lymphoid tissue during chronic infection. J. Virol. 77:5621-5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shacklett, B. L., J. W. Critchfield, and D. Lemongello. 2009. Isolating mucosal lymphocytes from biopsy tissue for cellular immunology assays. Methods Mol. Biol. 485:347-356. [DOI] [PubMed] [Google Scholar]
- 52.Shacklett, B. L., J. W. Critchfield, and D. Lemongello. 2009. Quantifying HIV-1-specific CD8 (+) T-cell responses using ELISPOT and cytokine flow cytometry. Methods Mol. Biol. 485:359-374. [DOI] [PubMed] [Google Scholar]
- 53.Shacklett, B. L., O. O. Yang, M. A. Hausner, J. Elliott, L. E. Hultin, C. Price, M. Fuerst, J. Matud, P. Hultin, C. A. Cox, J. Ibarrondo, J. T. Wong, D. F. Nixon, P. A. Anton, and B. D. Jamieson. 2003. Optimization of methods to assess human mucosal T-cell responses to HIV infection and vaccination. J. Immunol. Methods 279:17-31. [DOI] [PubMed] [Google Scholar]
- 54.Streeck, H., M. Lichterfeld, G. Alter, A. Meier, N. Teigen, B. Yassine-Diab, H. K. Sidhu, S. Little, A. Kelleher, J. P. Routy, E. S. Rosenberg, R. P. Sekaly, B. D. Walker, and M. Altfeld. 2007. Recognition of a defined region within p24 gag by CD8+ T cells during primary human immunodeficiency virus type 1 infection in individuals expressing protective HLA class I alleles. J. Virol. 81:7725-7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu, X. G., H. Shang, M. M. Addo, R. L. Eldridge, M. N. Phillips, M. E. Feeney, D. Strick, C. Brander, P. J. Goulder, E. S. Rosenberg, B. D. Walker, and M. Altfeld. 2002. Important contribution of p15 Gag-specific responses to the total Gag-specific CTL responses. AIDS 16:321-328. [DOI] [PubMed] [Google Scholar]