Abstract
How receptors control virus infection is poorly understood. Polyomavirus (Py) binds to the sialic acid-galactose moiety on receptors to gain entry into host cells and cause infection. We previously demonstrated that the sialic acid-galactose-containing glycolipids called gangliosides GD1a and GT1b promote Py infection, in part, by sorting the virus from the endolysosomes to the endoplasmic reticulum (ER), a critical infection route. Whether these glycolipids act as Py entry receptors, however, is not clear. Additionally, as the majority of glycoproteins also harbor terminal sialic acid-galactose residues, their roles in Py infection are also not well established. Using a ganglioside-deficient cell line, we show that GD1a is the functional entry receptor for Py. GD1a binds to Py on the plasma membrane, and the receptor-virus complex is internalized and transported to the late endosomes and then the ER to initiate infection. In contrast, our findings indicate that glycoproteins act as decoy receptors, restricting the ER transport and infection of Py. Thus, glycolipids and glycoproteins, two major constituents of the plasma membrane, execute opposing functions in regulating infection by a defined virus.
The first step in successful virus infection is the binding of virus to cellular receptors. In contrast to the many viruses that rely on glycoproteins as productive entry receptors (31, 36), members of the polyomavirus family, including murine polyomavirus (Py), simian virus 40 (SV40), and the human polyomaviruses BK virus (BKV) and Merkel cell polyomavirus (MCPyV), are unusual in that they use glycolipid molecules called gangliosides as functional receptors (4, 9, 11, 12, 23, 32, 37). The primary observation leading to this conclusion is that addition of specific gangliosides to ganglioside-deficient cells stimulates virus infection. However, despite this finding, the precise mechanism by which a ganglioside promotes virus infection is unclear. Is it acting as a virus entry receptor or as an intracellular sorter that engages the virus postentry to guide the viral particles along the infectious pathway or both?
Gangliosides are lipid molecules that consist of a hydrophilic carbohydrate moiety attached to a hydrophobic ceramide domain (17, 29). These lipids are inserted into the outer leaflet of the plasma membrane. During downregulation, gangliosides are internalized and transported to the early and late endosomes and finally reach the lysosomes, where they are hydrolyzed by lysosomal enzymes. Although gangliosides can be transported back to the Golgi complex from the cell surface (29), a very low level is transported back to the endoplasmic reticulum (ER).
Structurally, Py is composed of 72 pentamers of the outer structural protein called VP1 (34), with the entire viral capsid enclosing the VP2 or VP3 internal protein (6). As VP1 directly engages the carbohydrate moiety of gangliosides, it dictates the specificity of interaction between polyomaviruses and gangliosides. For instance, Py VP1 makes contact with the sialic acid-galactose moiety on GD1a and GT1b (34, 37), BKV VP1 binds to the disialic acid motif on gangliosides GD1b and GT1b (23), and MCPyV VP1 interacts with sialic acids on both branches of GT1b (9).
Upon entry, Py is transported to the lumen of the ER. Transport to the ER is essential for infection, as inactivation of factors resident in the ER significantly blocks infection (14, 22, 25). It has previously been postulated that Py then penetrates the ER membrane to gain access to the cytosol (28, 36). From the cytosol, the virus is transported further to the nucleus, where transcription and replication of the viral DNA ensue, leading to lytic infection or cell transformation.
Understanding of how ganglioside GD1a facilitates transport of Py from the plasma membrane to the ER is nebulous. We recently demonstrated that Py is initially transferred to the low-pH endolysosomes prior to reaching the ER (27), a finding consistent with an earlier observation (21). Intriguingly, our data also indicated that GD1a acts to sort Py from the endolysosomes to the ER (27). This finding led us to speculate whether GD1a serves solely as an intracellular sorter in facilitating the endolysosome-to-ER targeting of Py or also acts as an entry receptor in mediating Py internalization from the cell surface to the endolysosomes.
In addition to the glycolipids, many glycoproteins also harbor a sialic acid-galactose motif (18) that is sufficient to engage Py on the cell surface (33, 34, 37). The functional consequence of engaging glycoproteins on the plasma membrane for Py infection, however, is unclear.
Using a combination of microscopy, cell infection, and biochemical studies, we present evidence that ganglioside GD1a functions as an entry receptor for Py. GD1a engages the virus on the cell surface, targeting the viral particles along the infectious pathway through the endolysosomes en route to the ER. Importantly, we also uncover a key role for glycoproteins during Py infection. Using loss- and gain-of-function strategies, we found that glycoproteins engage Py to divert the virus away from the ER, consequently attenuating infection. Thus, glycoproteins appear to restrict Py infection, likely by targeting the virus on nonproductive routes. Our data thus indicate that lipids and proteins serve opposing roles in controlling infection of a defined virus, a phenomenon not yet described for any other virus.
MATERIALS AND METHODS
Reagents.
Antibodies against VP1 and large T antigen and purified Py were provided by Tom Benjamin (Harvard Medical School). The CFP-heme oxygenase-2 (CFP-HO2 [an ER maker]) construct was a gift from Melissa Rolls (Penn State). The CFP-Rab7 and YFP-Rab7 plasmids were from Joel Swanson (University of Michigan). The FLAG-tagged human epidermal growth factor receptor (EGFR) construct was from John Kuriyan (University of California, Berkeley). Purified GM1 and GD1a were purchased from Matreya, Alexa Fluor 594 and BODIPY FL C5-ganglioside GM1 were purchased from Invitrogen, dithiobis succinimidyl propionate (DSP) was purchased from Thermo Scientific, proteinase K was purchased from Sigma, and PNGase F was purchased from New England BioLabs. BODIPY-GD1a was kindly provided by Julian Molotkovsky (Russian Academy of Sciences). NIH 3T3 cells stably overexpressing insulin-like growth factor type I glycoprotein receptor (IGF-1R) were from Peter Arvan (University of Michigan).
Preparation of Alexa Fluor 594-labeled Py.
Purified Py (RA strain) was labeled with Alexa Fluor 594 succinimidyl ester (1 mM), following the protocol of the manufacturer (Invitrogen). Labeled Py was separated from excess labeling reagent by the use of a Micro Bio-Spin 30 column (Bio-Rad Laboratories).
Infection assay.
NIH 3T3 cells or A1-1 cells were incubated with Py (about 100 PFU/cell or 1 × 104 particles/cell) for 1 h, washed, and incubated for 48 h at 37°C. Cells were fixed with 3.6% formaldehyde, permeabilized with 0.2% Triton X-100, and subjected to immunofluorescence (IF) analysis with an antibody (Ab) against the large T antigen. Bright-phase and fluorescence images were taken using a Nikon Eclipse TE2000-E microscope with a 20× objective. For the GD1a time course experiments, NIH 3T3 cells or A1-1 cells were treated with GD1a (80 μM) at the indicated time pre- or postinfection for at least 2 h. For the proteinase K or PNGase F experiments, cells were treated with 4 μg of proteinase K/ml for 1 h at 4°C or with 10,000 U of PNGase F with G7 buffer (New England Biolabs) in 1 ml of medium for 1 h at 37°C. For the EGFR overexpression experiments, cells were transfected using Effectene (Qiagen) with a control green fluorescent protein (GFP) construct or with a combination of GFP and EGFR constructs for 2 days prior to infection.
Immunofluorescence staining.
Cells were fixed with formaldehyde (3.6%) and permeabilized with Triton X-100 (0.2%). The cells were then incubated with an antibody against the Py large T antigen or an antibody against the Py VP1 protein for 1 to 2 h at room temperature, washed, and incubated with a fluorescently tagged secondary antibody (rhodamine-labeled donkey anti-rat antibody for the large T antigen or rhodamine-labeled donkey anti-rabbit antibody for VP1).
Image analysis.
Different color images were taken with Nikon filter cubes for Texas Red (catalog no. 96313), yellow fluorescent protein (YFP) (catalog no. 96345), and CFP (catalog no. 96341). The images for colocalization experiments were taken with a 100× objective and a Nikon TE2000-E microscope. A ECFP/DsRed filter set (catalog no. 51018; Chroma) was used to take two fluorescence images simultaneously. The dual-color image was split to two channels by the use of a Dual-View image splitter (Optical Insight) and projected to the two halves of a charge-coupled device (CCD) camera (CoolSnap EZ2; Photometric). Bilinear transformation calculation was used to correct the imaging misalignment between the different channels. The Fast Fourier Transform Bandpass Filter function in the ImageJ program (NIH) was used to define the boundaries of the ER clearly. The filtering settings were set for filtering large structures down to 15 pixels, filtering small structures up to 3 pixels, and a tolerance of direction of 5%.
Py-EGFR binding studies.
NIH 3T3 cells were incubated with Py at 4°C for 1 h, the unbound virus was removed by washing, and the resulting cell pellet was incubated with the cross-linker dithiobis succinimidyl propionate (DSP) at 4°C for 1 h. Cells were lysed with a buffer containing 150 mM KOAc, 50 mM HEPES (pH 7.4), 2 mM Mg(OAc)2, 250 mM sucrose, and 1% Triton X-100, and the resulting lysate was subjected to immunoprecipitation using either a control ribophorin I (Ribo I)- or an EGFR-specific antibody. The precipitated sample was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting with antibodies against Py VP1 and EGFR.
RESULTS
GD1a addition to a cell line lacking functional receptors stimulates Py binding, entry, ER transport, and infection.
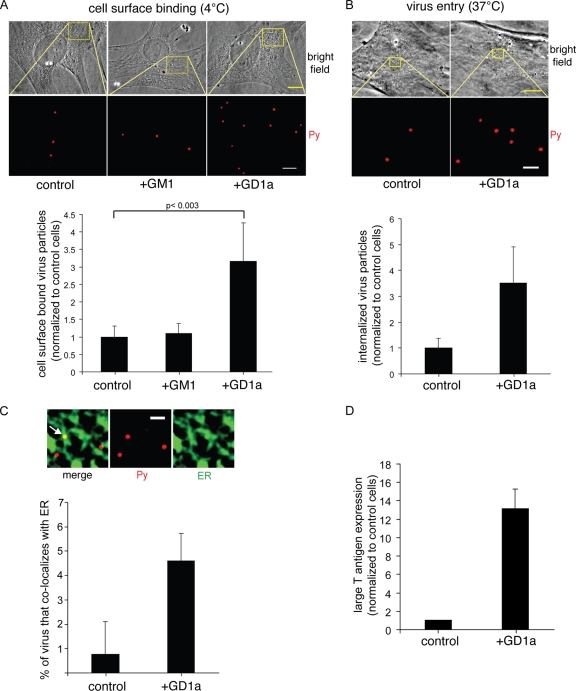
In this study, we used A1-1, a murine mammary tumor-derived cell line, which was characterized previously as devoid of ganglioside GD1a, Py's functional receptor, on the cell surface (13); addition of GD1a to this cell line stimulates Py infection (13). To test whether supplementation of GD1a to the A1-1 cells affects Py cell surface binding, control and GD1a-supplemented cells, as well as cells supplemented with ganglioside GM1, previously shown not to bind virus in vitro (37), were incubated with Py at 4°C (to prevent endocytosis) for 1 h. Unbound virus was removed by washing, the cells were fixed, and a Py VP1-specific antibody was used for immunostaining to detect surface-bound virus. We found that the number of virus particles increased in the GD1a-supplemented cells compared to control and GM1-supplemented cells (Fig. 1A, top panels; quantified in the graph at the bottom of the panels), indicating that GD1a interacts with Py on the plasma membrane. When these cells were heated to 37°C to promote virus entry, we found more Py entering the GD1a-supplemented cells compared to control cells (Fig. 1B, top panels; quantified in the graph at the bottom of the panels). Thus, GD1a promotes Py binding and internalization.
FIG. 1.
GD1a addition to a murine cell line lacking functional receptors stimulates Py binding, entry, ER transport, and infection. (A and B) Control cells, GM1-supplemented cells (shown in panel A alone), and GD1a-supplemented A1-1 cells were incubated with Py at 4°C for 1 h (A) or at 37°C for 4 h (B), washed to remove the unbound virus, and subjected to immunofluorescence with an antibody against VP1. Top panel, representative images. Scale bars, 10 μm for bright-field images and 2 μm (A) or 1 μm (B) for Py images. Bottom panel, quantification of Py binding to the plasma membrane from at least 3 cells. Data represent means ± standard deviations. A two-tailed t test was used. (C) Control and GD1a-supplemented A1-1 cells expressing CFP-HO2 were incubated with Py at 4°C for 40 min, washed to remove the unbound Py, and then incubated at 37°C for 4.5 h. Cells were subjected to immunofluorescence with an antibody against VP1. Top panels, representative images. Arrow, Py that colocalized with the ER. Scale bar, 1 μm. Bottom panel, quantification of the Py colocalizing with the ER from at least 3 cells. Data represent means ± standard deviations. (D) Control and GD1a-supplemented A1-1 cells were incubated with Py at 37°C for 48 h and subjected to immunofluorescence with an antibody against the large T antigen. Data represent means ± standard deviations of the results of at least 2 independent experiments. A total of 3 of 4,004 cells expressed T antigen in the control cells.
Postentry, Py is driven to the ER in a GD1a-dependent manner to cause infection (12, 27, 37). We tracked transport of Py to the ER in these cells by expressing CFP-HO2 and assessed the extent of Py colocalization with CFP-HO2 at 4.5 h postinfection. As the ER is highly convoluted in these cells, the ER images were filtered as previously described (27) in order to better define the boundaries of the ER. This effort enables a more accurate analysis of Py-ER colocalization. An example of a filtered image demonstrating colocalization of Py (red) with the ER (green) is shown in Fig. 1C (top panels). Using this method, we found that addition of GD1a increased the number of ER-localized virus particles (Fig. 1C; see quantification in graph at bottom of the panels), which is consistent with previous observations of other cells (12, 27, 37). Furthermore, Py infection (as measured by expression of the large T antigen) increased significantly in the GD1a-supplemented cells compared to control cells (Fig. 1D), as expected (13). Together, these findings indicate that ganglioside GD1a is an entry receptor, facilitating the binding, entry, ER trafficking, and infectivity of Py.
Py colocalizes with GD1a on the plasma membrane, the late endosomes, and the ER.
Since it is a functional entry receptor, we hypothesized that GD1a engages Py on the plasma membrane, guiding the virus through the endolysosomes en route to the ER. This scenario necessitates colocalization of GD1a with Py on the cell surface, the endolysosomes, and the ER.
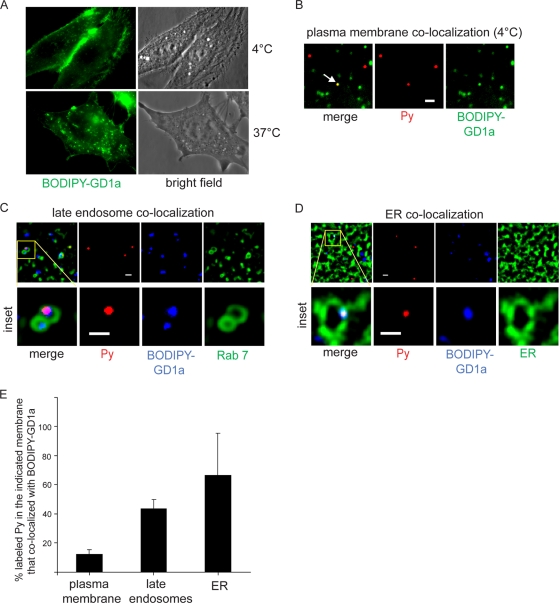
To visualize the behavior of GD1a in cells, we used a modified form of GD1a in which the BODIPY fluorophore is conjugated to the ceramide domain of GD1a (BODIPY-GD1a) (3, 16). When the A1-1 cells were incubated with BODIPY-GD1a at 4°C for 20 min, this lipid localized mostly to the plasma membrane (Fig. 2A, top panels). In contrast, when the cells were heated to 37°C for 30 min, the majority of BODIPY-GD1a localized to vesicular structures (Fig. 2A, bottom panels), indicating that this lipid was internalized.
FIG. 2.
Py colocalizes with GD1a on the plasma membrane, the late endosomes, and the ER. (A) A1-1 cells were incubated with BODIPY-GD1a at 4°C for 20 min (top panels) or at 37°C for 30 min (bottom panels). (B) Cells were incubated first with BODIPY-GD1a at 4°C for 15 min and then with labeled Py at 4°C for 30 min. Arrow, Py that colocalized with BODIPY-GD1a on the plasma membrane. Scale bar, 1 μm. (C) Cells expressing CFP-Rab7 were incubated first with BODIPY-GD1a at 4°C for 15 min and then with labeled Py at 37°C for 3 h. A representative image of Py colocalizing with BODIPY-GD1a in the Rab7-containing vesicle is shown. Scale bars, 1 μm. (D) Cells expressing CFP-HO2 were incubated first with BODIPY-GD1a at 4°C for 15 min and then with labeled Py at 37°C for 4.5 h. A representative image of Py colocalizing with BODIPY-GD1a in the ER is shown. Scale bars, 1 μm. (E) Quantification of the extent of colocalization between labeled Py and BODIPY-GD1a in the indicated membrane from at least 3 cells. Data represent means ± standard deviations.
In addition to BODIPY-GD1a, we also used Py labeled with Alexa Fluor 594 to study colocalization of Py with GD1a. The labeled Py was previously shown to recapitulate the normal cellular transport and infection processes of unlabeled Py (27). For analysis of Py-GD1a colocalization on the plasma membrane, cells were first incubated with BODIPY-GD1a at 4°C for 15 min, followed by addition of labeled Py at 4°C for 30 min. A typical image of labeled Py (red) colocalizing with BODIPY-GD1a (green) on the plasma membrane is depicted in Fig. 2B. Quantification of the extent of colocalization demonstrated that only a small amount (approximately 12%) of Py colocalized with BODIPY-GD1a on the cell surface (Fig. 2E). This finding is not surprising, as nonganglioside receptors likely compete with BODIPY-GD1a for Py binding.
To determine the level of Py colocalization with the endolysosomal system, we analyzed cells expressing CFP-Rab7 (a marker of the late endosomes) that were first incubated with BODIPY-GD1a at 4°C for 15 min and then with labeled Py at 37°C for 3 h. An example of an image of labeled Py (red) colocalizing with BODIPY-GD1a (blue) in the Rab7-containing vesicle (green) is shown in Fig. 2C (see inset). Quantification analysis showed that 43% of labeled Py in the late endosomes colocalized with BODIPY-GD1a (Fig. 2E). Hence, a higher percentage of Py colocalizes with BODIPY-GD1a in the late endosomes than in the plasma membrane.
To assess the extent of Py-GD1a colocalization in the ER, cells expressing CFP-HO2 initially incubated with BODIPY-GD1a at 4°C for 15 min and then with labeled Py at 37°C for 4.5 h were analyzed. A typical image depicting labeled Py (red) colocalizing with BODIPY-GD1a (blue) in the ER (green) is shown in Fig. 2D (see inset). When quantified, approximately 67% of Py in the ER colocalized with BODIPY-GD1a (Fig. 2E). That the percentage of Py-GD1a colocalization was highest in the ER (67%) compared to the late endosomes (43%) and the plasma membrane (12%) suggests that Py bound to GD1a on the cell surface, upon entry, is preferentially targeted to the ER.
Ligand-induced retrograde transport of gangliosides to the ER.
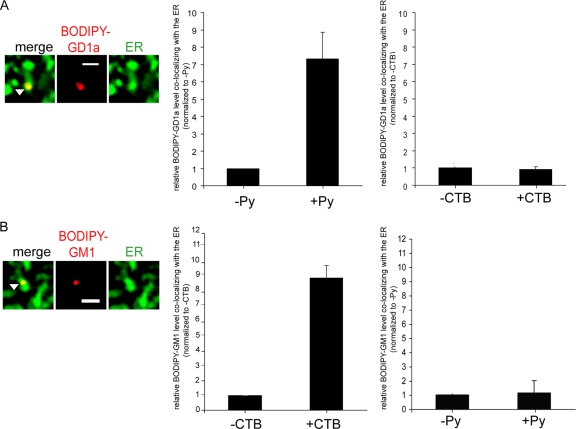
The observations that addition of GD1a stimulates Py transport to the ER (Fig. 1C) and that a majority of Py in the ER colocalizes with GD1a (Fig. 2E) suggest that Py should in turn promote transport of GD1a to the ER.
To test this prediction, we measured the BODIPY-GD1a level in the ER of cells in a virus-dependent manner. A1-1 cells expressing CFP-HO2 were incubated with BODIPY-GD1a at 4°C for 15 min followed by incubation with or without Py at 37°C for 4.5 h. A representative image of an ER-localized BODIPY-GD1a is shown in Fig. 3A. Indeed, our quantification analysis showed a significant increase in ER-localized BODIPY-GD1a levels in cells incubated with Py compared to those with no virus incubation (Fig. 3A; see quantification in the middle panel). In contrast, the cholera toxin B (CTB) subunit, which binds to ganglioside GM1, did not stimulate ER transport of BODIPY-GD1a (Fig. 3A; see quantification in the right panel). GM1 normally engages CT on the cell surface, targeting the toxin to the ER to cause intoxication of intestinal epithelial cells (20). We conclude that binding between Py and ganglioside GD1a triggers the specific retrograde transport of its receptor to the ER.
FIG. 3.
Ligand-induced retrograde transport of gangliosides to the ER. (A) A1-1 cells expressing CFP-HO2 were incubated first with BODIPY-GD1a at 4°C for 15 min and were then either incubated with Py at 37°C for 4.5 h or left untreated or incubated with CTB at 37°C for 1 h or left untreated. The picture shown is a representative image of BODIPY-GD1a colocalizing with the ER. Scale bar, 1 μm. Left graph, quantification of the extent of colocalization between BODIPY-GD1a and the ER from at least 3 control or Py-supplemented cells. Right graph, quantification of the extent of colocalization between BODIPY-GD1a and the ER from at least 3 control or CTB-supplemented cells. Data represent means ± standard deviations. (B) NIH 3T3 cells expressing CFP-HO2 were incubated first with BODIPY-GM1 at 4°C for 15 min and then either incubated with CTB at 37°C for 1 h or left untreated or incubated with Py at 37°C for 4.5 h or left untreated. The picture shown is a representative image of BODIPY-GM1 colocalizing with the ER. Scale bar, 1 μm. Left graph, quantification of the extent of colocalization between BODIPY-GM1 and the ER from at least 3 control or CTB-supplemented cells. Right graph, quantification of the extent of colocalization between BODIPY-GM1 and the ER from at least 3 control or Py-supplemented cells. Data represent means ± standard deviations.
To determine whether the observed Py-induced reverse transport of GD1a to the ER is a common mechanism, we assessed whether CTB triggers the ER transport of GM1. NIH 3T3 cells expressing CFP-HO2 were incubated with BODIPY-GM1 at 4°C for 15 min, followed by incubation with or without CTB at 37°C for 1 h or Py at 37°C for 4.5 h. We found that CTB, but not Py, stimulated the transport of GM1 to the ER (Fig. 3B; see quantification in the left and right graphs). These findings indicate that interaction between a ligand (e.g., virus or toxin) and its respective ganglioside receptor is a general mechanism driving the ganglioside to the ER. In this context, it is interesting that both Py VP1 and CTB are pentamers when assembled into their native structures (20, 34), suggesting that a multivalent ligand-ganglioside interaction may be important for transportation of this complex to the ER.
GD1a must be added before, but not after, incubation of cells with Py to stimulate infection.
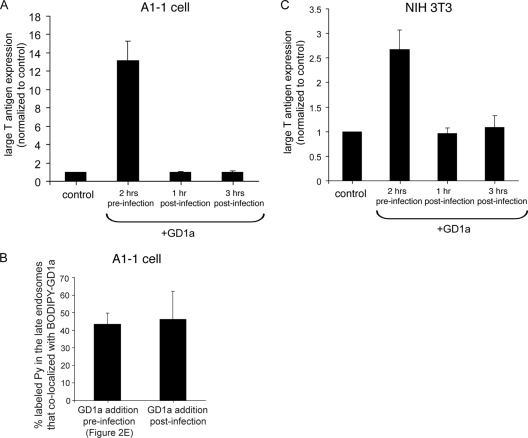
Our finding that addition of GD1a to cells increased the plasma membrane binding and entry of Py (Fig. 1) suggests that GD1a functions as the entry receptor. To further strengthen our contention that Py engages GD1a on the plasma membrane prior to entry to cause infection, we asked whether addition of GD1a after virus entry stimulates infection as well. We reasoned that, should addition of GD1a after virus entry promote Py infection, virus interaction with GD1a on the cell surface must not be a prerequisite step for stimulation of infection. In this scenario, it is possible that other sialic acid-galactose-containing receptors such as glycoproteins serve as alternative entry receptors and deliver Py from the cell surface to the endolysosomes. In the endolysosomes, Py is conceivably released from the glycoproteins to bind to GD1a. Alternatively, if addition of GD1a after virus entry fails to stimulate Py infection, Py must therefore interact with GD1a on the cell surface prior to entry to enable infection.
In the A1-1 cells, we found that Py infection was stimulated only when GD1a was added before, but not after, incubation of the cells with the virus (Fig. 4 A). A potential trivial explanation is that GD1a added subsequent to virus entry fails to reach the endolysosomes efficiently, thereby resulting in an inability to bind Py that has been delivered to this compartment via nonganglioside receptors. However, we found that the extents of colocalization between BODIPY-GD1a and labeled Py in the Rab7-positive late endosomes were similar regardless of whether BODIPY-GD1a was added before or after virus incubation (Fig. 4B; see also Fig. 2E). Hence, the inability of GD1a to stimulate infection when this glycolipid was added after virus entry cannot be attributed to a deficiency in transport of GD1a to the same vesicles harboring Py. Instead, the simplest explanation of these results is that, to cause infection, Py must bind to GD1a on the cell surface before internalization. The observation that GD1a must be added before but not after incubation with Py to stimulate infection was recapitulated in experiments with NIH 3T3 cells (Fig. 4C). These data further underscore our view that Py initiates its binding to GD1a on the plasma membrane, and not within an intracellular organelle, to infect cells. The GD1a-Py complex is then internalized and transported through the endolysosomes en route to the ER.
FIG. 4.
GD1a must be added before, and not after, incubation of cells with Py to stimulate infection. (A) A1-1 cells were treated with 80 μM GD1a at the indicated time points with respect to addition of cells with Py. At 48 h after incubation of cells with Py, cells were subjected to immunofluorescence with an antibody against the large T antigen. Data represent means ± standard deviations of the results of at least 2 independent experiments. A total of 3 of 4,004 control cells expressed T antigen. (B) A1-1 cells expressing CFP-Rab7 were incubated first with BODIPY-GD1a at 4°C for 15 min and then with labeled Py at 37°C for 3 h. GD1a (80 μM) was added pre- or postinfection. The extent of colocalization between labeled Py and BODIPY-GD1a in the CFP-Rab7 late endosomes was quantified from at least 3 cells. Data represent means ± standard deviations. (C) Data are as described for panel A, except NIH 3T3 cells were used. A total of 78 of 1,006 control cells expressed T antigen.
Removing plasma membrane glycoproteins stimulates Py infection and ER transport.
In addition to glycolipids such as gangliosides, many glycoproteins also contain the sialic acid-galactose moiety (18), a defining motif for binding to Py (33, 34, 37). Hence, in principle, glycoproteins displaying the terminal sialic acid-galactose sugars should also engage Py. What then is the functional consequence of such an interaction in controlling Py infection?
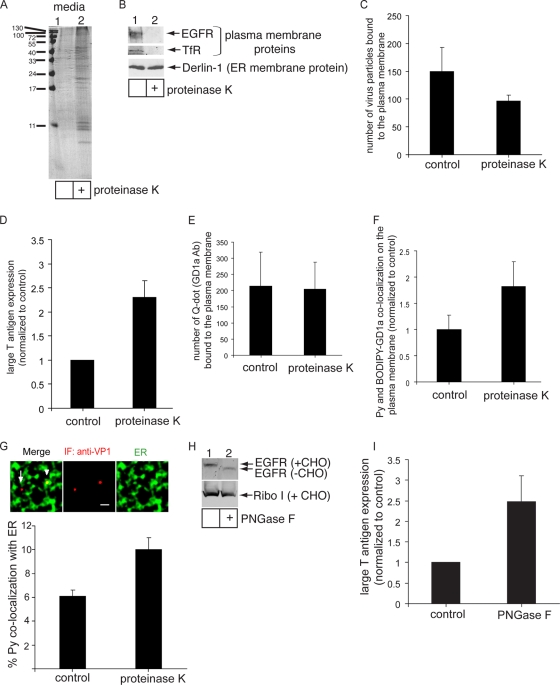
To assess the function of glycoproteins in regulating virus infection, NIH 3T3 cells were treated with or without a low concentration of the general protease proteinase K at 4°C for 1 h to remove cell surface proteins. The contents of the media from these cells were precipitated and subjected to SDS-PAGE analysis followed by Coomassie staining. Degraded protein products of various molecular weights were observed in the media derived from cells treated with proteinase K, while a very small amount was found in the media from control cells (Fig. 5A; compare lanes 2 and 1). This finding demonstrates that the protease effectively removed proteins from the cells.
FIG. 5.
Removal of plasma membrane glycoproteins stimulates Py infection and ER transport. (A) NIH 3T3 cells were treated with 4 mg of proteinase K/ml at 4°C for 1 h or left untreated. The contents of media from these cells were precipitated and subjected to SDS-PAGE analysis followed by Coomassie staining. (B) Cells were treated with 4 μg of proteinase K/ml at 4°C for 1 h or left untreated. Cell lysates were subjected to SDS-PAGE followed by immunoblotting with antibodies against the EGFR, TfR, and Derlin-1. (C) Quantification of the number of Py particles bound to the plasma membrane in control and proteinase K-treated cells. At least 3 cells in each group were analyzed. Data represent means ± standard deviations. (D) Large T antigen expression in control and proteinase K-treated cells was analyzed as described for Fig. 1D. Data represent the means ± standard deviations of the results from at least 3 independent experiments. A total of 81 of 5,404 control cells expressed large T antigen. (E) Quantification of the number of quantum dots (Q-dot) (GD1a Ab) bound to the cell surface of control and proteinase K-treated cells. (F) Quantification of Py and BODIPY-GD1a colocalization on the plasma membrane of control and proteinase K-treated A1-1 cells. Data were analyzed as described for Fig. 2. (G) Py-ER colocalization in control and proteinase K-treated cells was analyzed by immunofluorescence (IF) staining. Top panel, representative images. Arrowhead, Py that colocalized with the ER. Arrow, Py that did not colocalize with the ER. Scale bar, 1 μm. Bottom panel, quantification of Py colocalizing with the ER from at least 3 cells. Data represent means ± standard deviations. (H) Cells were treated with or without PNGase F at 37°C for 1 h. Cell lysates were subjected to SDS-PAGE followed by immunoblotting with antibodies against the EGFR and Ribo I. (I) Large T antigen expression in control and PNGase F-treated cells was analyzed as described for Fig. 1D. Data represent the means ± standard deviations of the results from at least 2 independent experiments. A total of 27 of 1,232 control cells expressed large T antigen.
To test whether the proteinase K treatment affected the integrity of cell surface proteins but not internal proteins, cell lysates from cells treated with the protease or left untreated were subjected to SDS-PAGE followed by immunoblotting with antibodies against the plasma membrane proteins, EGF receptor (EGFR), and transferrin receptor (TfR), as well as an ER membrane protein, Derlin-1. Whereas EGFR and TfR were efficiently degraded, Derlin-1 was not proteolyzed (Fig. 5B; compare lanes 2 and 1). We conclude that under the conditions used for the protease treatment, plasma membrane proteins were effectively removed without degradation of internal proteins.
We then assessed the ability of Py to interact with these cells and found that removing proteins from the cell surface decreased Py binding (Fig. 5C). This finding indicates that protein factors engage Py on the plasma membrane, as suggested by our previous finding (27). Strikingly, Py infection was stimulated in cells treated with proteinase K compared to control cells (Fig. 5D), suggesting that proteins on the cell surface act to attenuate infection normally. This effect was not due to increased cell surface GD1a expression, as cell surface binding of a quantum dot (Q-dot) conjugated to an antibody against GD1a (GD1a Ab) shown previously to bind to GD1a (27) did not increase in the proteinase K-treated cells (Fig. 5E).
Presumably, the increased infection was due to increased Py binding to GD1a. This could be because of the fact that glycoproteins bind to Py normally and their absence allows more Py to engage GD1a. Alternatively, it is also possible that glycoproteins prevent virus access to GD1a simply because they protrude from the membrane. We used fluorescently labeled Py and BODIPY-GD1a to measure their colocalization on the cell surface of A1-1 cells (Fig. 2B and E); this cell line lacks endogenous GD1a on the plasma membrane (13) that would compete with BODIPY-GD1a for virus binding. We found that removing plasma membrane glycoproteins with proteinase K increased cell surface Py-GD1a colocalization in these cells (Fig. 5F), which is consistent with the hypothesis that the increased infection observed in NIH 3T3 cells in the absence of glycoproteins was due to increased Py-GD1a binding.
As the plasma membrane-to-ER transport pathway constitutes the infectious route, one clear implication of these results is that virus trafficking to the ER should also be enhanced when plasma membrane proteins are removed. Indeed, we found an increase in colocalization of Py with the ER in cells treated with proteinase K compared to nontreated cells (Fig. 5G). This finding is consistent with the infection data and demonstrates that, in the absence of cell surface proteins, Py is preferentially transported to the ER to promote infection.
As an independent method to support these findings, NIH 3T3 cells were treated with PNGase F, an enzyme that removes the carbohydrate moiety from glycoproteins but not glycolipids, at 37°C for 1 h (PNGase F does not function effectively at 4°C). This set of conditions removed the sugar moiety from the EGFR membrane glycoprotein but not from the Ribo I ER membrane glycoprotein (Fig. 5H; compare lanes 2 and 1), indicating that PNGase F acted only on cell surface glycoproteins and not on internal glycoproteins. In similarity to cells treated with proteinase K, cells incubated with PNGase F supported more Py infection than the nontreated control cells (Fig. 5I). Thus, we conclude that glycoproteins normally act to restrict Py infection, likely by binding to and targeting Py on nonproductive pathways.
Overexpression of model glycoprotein receptors decreases Py infection.
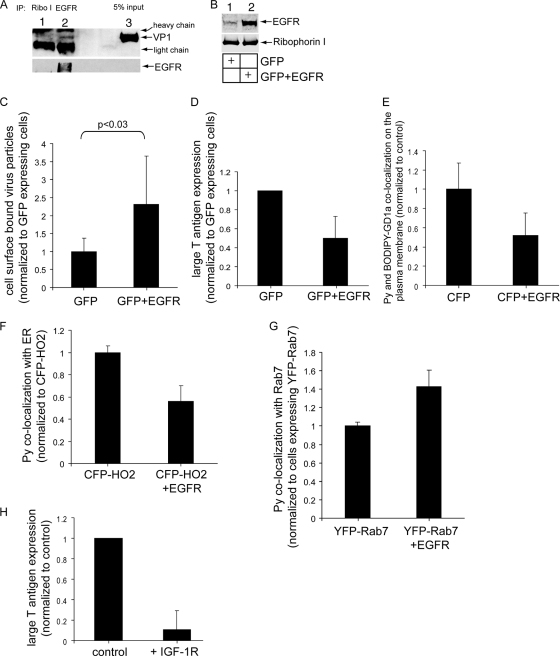
In addition to the loss-of-function approach, we investigated whether a gain-of-function strategy in which cells overexpress a model glycoprotein would result in a block in Py infection. EGFR is a classic membrane glycoprotein containing, among other sugars, terminal sialic acid-galactose residues (7). We first investigated whether EGFR interacts with Py by the use of coimmunoprecipitation analysis. NIH 3T3 cells were incubated with Py at 4°C for 1 h, the unbound virus was removed by washing, and the resulting cell pellet was incubated with the cross-linker dithiobis succinimidyl propionate (DSP) at 4°C for 1 h. Cells were lysed, and the resulting lysate was subjected to immunoprecipitation using either a control Ribo I- or an EGFR-specific antibody. The precipitated sample was subjected to SDS-PAGE followed by immunoblotting with antibodies against Py VP1 and EGFR. Using this approach, we found a low level of Py that coprecipitated with the EGFR but not with Ribo I (Fig. 6A, top panel; compare lanes 2 and 1), demonstrating that the EGFR interacts with Py.
FIG. 6.
Overexpression of model glycoprotein receptors decreases Py infection. (A) NIH 3T3 cells were incubated with Py at 4°C for 1 h and washed to remove the unbound virus; the resulting cell pellet was incubated with the cross-linker DSP at 4°C for 1 h. Cells were lysed, and the resulting lysate was subjected to immunoprecipitation (IP) using either a control Ribo I- or EGFR-specific antibody. The precipitated sample was subjected to SDS-PAGE followed by immunoblotting with antibodies against Py VP1 and EGFR. (B) Cells were transfected with the control GFP construct or with a combination of GFP and EGFR constructs. Cell lysates were subjected to SDS-PAGE followed by immunoblotting with antibodies against the EGFR and Ribo I. (C) Quantification of the number of Py particles bound to the plasma membrane in cells transfected with the control GFP construct or with a combination of GFP and EGFR constructs. At least 3 cells that expressed GFP in each group were analyzed. Data represent means ± standard deviations. (D) Large T antigen expression in cells transfected with the control GFP construct or with a combination of GFP and EGFR constructs was analyzed as described for Fig. 1D. Only those cells that expressed GFP were counted. Data represent the means ± standard deviations of the results of at least 3 independent experiments. A total of 23 of 342 control cells expressed large T antigen. (E) Quantification of Py and BODIPY-GD1a colocalization on the plasma membrane of A1-1 cells transfected with CFP or with a combination of CFP and EGFR. Only those cells expressing CFP were counted. Data were analyzed as described for Fig. 2. (F) Quantification of the Py-ER colocalization in cells transfected with the control CFP-HO2 construct or with a combination of CFP-HO2 and EGFR constructs. At least 3 cells in each group were analyzed. Data represent means ± standard deviations. (G) Quantification of Py-late endosome colocalization in cells transfected with the control YFP-Rab7 construct or with a combination of YFP-Rab7 and EGFR constructs. At least 3 cells in each group were analyzed. Data represent means ± standard deviations. (H) Large T antigen expression in control NIH 3T3 cells or NIH 3T3 cells stably overexpressing the IGF-1R was analyzed as described for Fig. 1D.
Based on this finding, we hypothesized that overexpression of EGFR competes with ganglioside GD1a in interacting with Py, potentially attenuating infection. To test this hypothesis, cells were transfected with either the control GFP construct or a combination of GFP and a FLAG-tagged human EGFR construct, and the total lysates from these cells were analyzed for EGFR expression. As expected, we found an increase in the EGFR level in cells transfected with GFP and EGFR compared to cells transfected with GFP alone (Fig. 6B). It should be noted that, as the transfection efficiency in these cells is approximately 20 to 25%, the difference in the EGFR expression levels in cells transfected with or without EGFR is likely to be even more exaggerated than that revealed in immunoblot analysis (which reflects the difference determined from the entire pool of cells).
We then analyzed the extent of Py binding to the cell surface in cells transfected with GFP or in cells cotransfected with GFP and EGFR by incubating the cells with Py at 4°C for 1 h. Only cells expressing GFP were analyzed. Our results indicated that cells cotransfected with GFP and EGFR supported more Py binding than cells transfected with GFP alone (Fig. 6C). We conclude that EGFR overexpression increases Py plasma membrane binding.
Importantly, we found a decrease in Py infection in cells that were cotransfected with GFP and EGFR compared to cells transfected with GFP alone (Fig. 6D). Thus, overexpressing EGFR attenuates Py infection. In the A1-1 cell experiments, we found that the extent of cell surface colocalization between labeled Py and BODIPY-GD1a decreased in cells overexpressing CFP and EGFR compared to cells transfected with CFP alone (Fig. 6E). Thus, the simplest explanation is that, in NIH 3T3 cells, excess EGFR competes with GD1a for Py binding, thereby decreasing infection.
We then asked whether ER transport of Py is similarly decreased in cells overexpressing EGFR. Cells were first transfected with either the ER marker CFP-HO2 alone or a combination of CFP-HO2 and EGFR. The cells were then incubated with Py, and the extent of Py-ER colocalization was assessed by immunofluorescence. Our findings show that the Py-ER colocalization was decreased in cells cotransfected with CFP-HO2 and EGFR compared to cells transfected with CFP-HO2 alone (Fig. 6F). By shifting the extent of Py binding toward glycoproteins, overexpressing EGFR prevented proper Py trafficking along the ER infection route, consequently blocking infection.
What might be the mechanism by which EGFR overexpression attenuates infection? We found a modest increase in Py colocalization with Rab7 in cells overexpressing EGFR and YFP-Rab7 compared to cells overexpressing YFP-Rab7 alone (Fig. 6G). This finding suggests that the increased level of EGFR in cells targets more Py to the late endosomes, where the viral particles are trapped and unable to sort further to the ER.
Finally, we found that Py infection markedly decreased in NIH 3T3 cells stably overexpressing the IGF-1 glycoprotein receptor (IGF-1R) compared to control cells (Fig. 6H). As IGF-1R is known to induce signaling properties different from those induced by the EGFR (35), the decrease in infection observed when either of the glycoproteins was overexpressed was not likely due to their signaling events. Instead, our results suggest that glycoproteins such as the EGFR or IGF-1R normally function to restrict Py infection by engaging the virus, leading it along nonproductive routes. Thus, glycoproteins execute an function opposite to that of the glycolipid gangliosides that act as functional entry receptors.
DISCUSSION
The plasma membrane serves as the fundamental barrier to protect host cells against virus infection. However, viruses have evolved various strategies to gain entry into and infect target cells. One primary tactic used by viruses is to identify entry portals on the plasma membrane that contain specific functional entry receptors. Engaging these receptors enables the virus particles to be internalized and transported along the productive pathway leading to infection.
To counter the tactics of these viruses, deployment of “decoy” receptors, molecules that mimic some aspects of the functional receptor, can be used to guide the virus down nonproductive pathways. Ultimately, the degree of efficiency of virus engagement with these two receptor types helps to determine the efficiency of infection. To date, identification of a functional receptor system and a decoy receptor system that play opposing roles in controlling infection of a defined virus has been elusive. In this report, we present evidence that glycolipids and glycoproteins, two major constituents of the plasma membrane, execute opposing functions in regulating Py infection (Fig. 7).
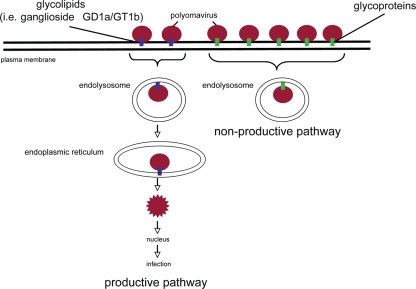
FIG. 7.
Lipids and proteins play opposing roles in mediating murine polyomavirus infection. Py that binds to the glycolipid ganglioside receptors is targeted down the infectious route. In this pathway, gangliosides first target Py to the endolysosomes and then sort the virus to the ER, where the virus then penetrates the ER membrane to reach the cytosol. From the cytosol, Py is transported further to the nucleus to initiate infection. In contrast, Py that interacts with glycoproteins such as the EGFR commits to a nonproductive route. After engaging glycoproteins, the virus is taken to endolysosomes and sequestered in this compartment. Because these viruses do not bind to glycolipids, they are not sorted to the ER and consequently do not cause infection.
Previous studies conclusively showed that gangliosides GD1a and GT1b are functional receptors for Py (12, 13, 27, 32, 37). This conclusion is based primarily on three independent findings. First, sucrose flotation experiments using liposomes that contained defined gangliosides indicated a direct interaction between Py and GD1a/GT1b (37). The terminal sugars sialic acid and galactose, which are common to both GD1a and GT1b, presumably mediate binding between those gangliosides and Py (34, 37). Second, purified GD1a added to ganglioside-deficient rat glioma C6 cells (12, 37), tumor-derived murine cells lacking functional Py receptors (13), or NIH 3T3 cells (27) stimulated Py infection. And third, cells supplemented with GD1a promoted the plasma membrane-to-ER trafficking of Py (12, 27, 37), an established Py infection route (14, 22, 25).
However, as there is no direct evidence that addition of GD1a increases cell surface binding and entry (12), coupled with the observation that GD1a plays a role in the intracellular sorting of Py from the endolysosomes to the ER (27), the function of GD1a as an Py entry receptor remains ambiguous. We now provide several lines of evidence demonstrating that GD1a serves as the Py functional entry receptor.
Glycolipid ganglioside GD1a as the functional entry receptor.
Using a murine tumor-derived A1-1 cell line resistant to Py infection (but supportive of infection when supplemented with GD1a [13]), we found that addition of GD1 increased Py binding, entry, ER transport, and infection. While the specific compositions of the gangliosides in the A1-1 cells are not known, binding studies showed that ganglioside GD1a is not expressed on the cell surface (13). Moreover, colocalization experiments using fluorescently labeled GD1a and Py demonstrated that the percentage of GD1a-Py colocalization increases as these factors are transported from the plasma membrane through the endolysosomes and then to the ER. These data suggest that the GD1a-Py complex is preferentially targeted from the cell surface to the ER.
As is consistent with this model, we found that the GD1a level in the ER in these cells was significantly stimulated by incubation of the cells with Py. Interestingly, the GM1 level in the ER also increased dramatically in response to addition of CTB. Thus, it appears that ligand binding generally triggers the retrograde transport of gangliosides from the plasma membrane to the ER. While forward transport of gangliosides from the ER to the plasma membrane constitutes the biosynthetic route of these lipid molecules, it should be noted that reverse transport of gangliosides from the cell surface to the ER is a very inefficient process. A majority of gangliosides are normally transported from the cell surface to the lysosomes, where they are degraded by lysosome-resident hydrolases. Only a very small fraction of gangliosides reaches the Golgi complex, and likely an even lower level is targeted to the ER (29).
However, in specialized circumstances where gangliosides interact with toxic molecules such as viruses or toxins, these glycolipids appear to have gained unique access to the ER. What might be the trigger for this reverse transport? One possibility is that, as both Py and CTB are pentamers, their binding to the respective gangliosides triggers the clustering of the glycolipids, generating a hydrophobic platform within the membrane that recruits other cellular components necessary to drive the lipids to the ER. Identification of the mechanism by which ligand-activated gangliosides enlist cellular factors to promote their transport to the ER requires further experimentation.
It is likely that clustering of the gangliosides impacts the underlying membrane curvature involved in virus internalization and ER transport. In fact, a very recent finding indicates that interaction of SV40 with GM1 is sufficient to induce membrane tubulation in giant unilamellar vesicles (10). This phenomenon is postulated to be the principle driving force that enables virus uptake. Whether cytosolic factors are recruited to these membrane tubules to facilitate subsequent ER transport is not known.
To strengthen further our contention that Py initially engages GD1a at the plasma membrane, we demonstrate that the timing of supplementing GD1a to cells is critical for infection. Specifically, it was only when this glycolipid was added before, but not after, the cells were incubated with Py that GD1a addition resulted in increased infection. This finding indicates that GD1a added exogenously cannot stimulate infection once Py enters the cells, supporting the hypothesis that GD1a must engage Py on the cell surface to promote infection.
Should GD1a stimulate Py infection when this lipid is added after virus entry, the proposed GD1a-Py interaction on the plasma membrane must not be required for GD1a to act as a functional receptor. Instead, GD1a might simply act as an intracellular sorter (27), receiving Py in an intracellular organelle such as the endolysosomes. In this scenario, nonganglioside receptors would target Py to the endolysosomes, where the virus is released and rebinds to a ganglioside receptor. As the efficiency with which BODIPY-GD1a reaches the endolysosome system is not affected whether this lipid is added before or after incubation of the cells with Py, the lack of virus infection stimulation seen when exogenous GD1a is added after incubation of the cells with Py cannot be attributed to the absence of added GD1a in the endolysosomes. Thus, as our data are inconsistent with this scenario and demonstrate a requirement of GD1a to bind to Py on the cell surface to promote infection, we conclude that GD1a acts as the functional entry receptor for Py.
Glycoproteins as decoy receptors.
In addition to glycolipids (such as gangliosides), glycoproteins represent the other major constituent of the plasma membrane. Because glycoproteins also contain terminal sialic acid-galactose residues (18), which represent the disaccharide moiety sufficient to bind to Py (34, 37), they can in principle interact with Py on the cell surface and compete with gangliosides for virus binding. Indeed, we found that removing proteins from the cell surface decreased Py binding, which is consistent with a previous result showing that ganglioside-deficient C6 cells resistant to Py infection nonetheless support Py binding and entry (27). The latter observation suggests that the glycoprotein binding event by itself is not sufficient to lead to productive infection. However, whether glycoproteins play a positive role in Py infection by acting as attachment factors that then deliver the virus to the GD1a entry receptor, or whether they regulate infection negatively, is not known. In this context, it was previously suggested that glycoproteins harboring O-linked branched sialyloligosaccharides or glycolipids function as pseudo- or decoy receptors for Py (2).
Using a loss-of-function approach, we found that removing glycoproteins from the cell surface of NIH 3T3 cells stimulated both Py transport to the ER and infection, suggesting that glycoproteins normally serve to restrict Py infection. As the glycoprotein integrin was previously suggested to mediate productive Py infection (5), we analyzed the level of expression of this protein in the proteolyzed cells and found that it was inefficiently degraded (not shown). Thus, it remains possible that specific glycoproteins such as integrin can act together with GD1a to promote infection in a fashion that requires further investigation. Nonetheless, our data indicate that glycoproteins in general function as decoy receptors in attenuating Py infection.
In addition to this loss-of-function approach, we employed a gain-of-function strategy to further test the hypothesis that glycoproteins negatively regulate Py infection. The EGFR, a highly glycosylated plasma membrane protein, harbors the sialic acid-galactose moiety (7). Hence, we tested whether overexpression of this model glycoprotein competes with GD1a for Py binding, leading to a block in ER transport and infection, and found that it did. Furthermore, overexpression of the glycoprotein IGF-1 receptor also attenuated infection. We conclude that, as increasing the level of glycoproteins decreases productive infection, glycoproteins likely function to attenuate Py infection, which is consistent with our loss-of-function results.
There are at least two possible hypotheses to explain the mechanism by which EGFR overexpression decreases virus infection. The excess EGFR may simply bind and trap the viral particles on the cell surface. Alternatively, the EGFR may stimulate the uptake of Py, leading the virus down a nonproductive pathway. Our observation that EGFR overexpression increases transport of Py to the endolysosomes suggests that glycoproteins target and sequester Py in nonproductive organelles such as the endolysosomes, thereby preventing the virus from reaching the ER infectious pathway (Fig. 7).
The use of multiple receptors during virus infection.
It is not uncommon for viruses to bind to multiple receptors on the cell surface during entry (1, 8). The multiple receptors may be used in a redundant fashion, with several receptors acting independently of each other to facilitate entry of the viruses. For example, entry of alphaherpesviruses can be independently mediated by nectins, herpesvirus entry mediator (HVEM), and heparin sulfate receptors without any of the receptors acting as coreceptors (15, 30).
Alternatively, the multiple receptors could function in a sequential manner. For example, the DC-SIGN functions as an attachment receptor that concentrates the dengue virus on the plasma membrane, allowing the dengue virus to interact efficiently with an unidentified receptor that is responsible for the entry of the dengue virus (24). Additionally, in the case of HIV, this virus binds to the primary CD4 receptor, subsequently interacting with one of the chemokine receptors (either CCR5 or CXCR4) for entry (19).
Our findings presented in the present report add a fresh perspective to this concept by demonstrating that multiple receptor types (i.e., glycolipids versus glycoproteins) engage the murine Py to perform completely opposite functions. Py uses the intrinsic intracellular transport properties of glycolipids, as well as signaling events that the virus induces upon interaction with the lipid molecules (26), to reach the ER and initiate infection. To counter this productive pathway, cells in turn have evolved a defensive mechanism by displaying a vast number of decoy glycoprotein receptors that prevent Py from accessing the productive route. Thus, while Py seeks to maximize its infection efficiency by imposing rather simple interaction specificity (i.e., for sialic acid-galactose) with its functional entry receptor, the presence of such a disaccharide moiety on decoy glycoprotein receptors in turn minimizes this efficiency and protects against virus infection.
Acknowledgments
Support was provided by NIH/NIAID grant AI064296 (B.T.).
Footnotes
Published ahead of print on 28 July 2010.
REFERENCES
- 1.Baranowski, E., C. M. Ruiz-Jarabo, and E. Domingo. 2001. Evolution of cell recognition by viruses. Science 292:1102-1105. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, P. H., C. Cui, W. R. Liu, T. Stehle, S. C. Harrison, J. A. DeCaprio, and T. L. Benjamin. 1999. Discrimination between sialic acid-containing receptors and pseudoreceptors regulates polyomavirus spread in the mouse. J. Virol. 73:5826-5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boldyrev, I. A., X. Zhai, M. M. Momsen, H. L. Brockman, R. E. Brown, and J. G. Molotkovsky. 2007. New BODIPY lipid probes for fluorescence studies of membranes. J. Lipid Res. 48:1518-1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campanero-Rhodes, M. A., A. Smith, W. Chai, S. Sonnino, L. Mauri, R. A. Childs, Y. Zhang, H. Ewers, A. Helenius, A. Imberty, and T. Feizi. 2007. N-glycolyl GM1 ganglioside as a receptor for simian virus 40. J. Virol. 81:12846-12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caruso, M., L. Belloni, O. Sthandier, P. Amati, and M. I. Garcia. 2003. Alpha4beta1 integrin acts as a cell receptor for murine polyomavirus at the postattachment level. J. Virol. 77:3913-3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, X. S., T. Stehle, and S. C. Harrison. 1998. Interaction of polyomavirus internal protein VP2 with the major capsid protein VP1 and implications for participation of VP2 in viral entry. EMBO J. 17:3233-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings, R. D., A. M. Soderquist, and G. Carpenter. 1985. The oligosaccharide moieties of the epidermal growth factor receptor in A-431 cells. Presence of complex-type N-linked chains that contain terminal N-acetylgalactosamine residues. J. Biol. Chem. 260:11944-11952. [PubMed] [Google Scholar]
- 8.Dimitrov, D. S. 2004. Virus entry: molecular mechanisms and biomedical applications. Nat. Rev. Microbiol. 2:109-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Erickson, K. D., R. L. Garcea, and B. Tsai. 2009. Ganglioside GT1b is a putative host cell receptor for the Merkel cell polyomavirus. J. Virol. 83:10275-10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewers, H., W. Romer, A. E. Smith, K. Bacia, S. Dmitrieff, W. Chai, R. Mancini, J. Kartenbeck, V. Chambon, L. Berland, A. Oppenheim, G. Schwarzmann, T. Feizi, P. Schwille, P. Sens, A. Helenius, and L. Johannes. 2010. GM1 structure determines SV40-induced membrane invagination and infection. Nat. Cell Biol. 12:11-18. [DOI] [PubMed] [Google Scholar]
- 11.Ewers, H., A. E. Smith, I. F. Sbalzarini, H. Lilie, P. Koumoutsakos, and A. Helenius. 2005. Single-particle tracking of murine polyoma virus-like particles on live cells and artificial membranes. Proc. Natl. Acad. Sci. U. S. A. 102:15110-15115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilbert, J., and T. Benjamin. 2004. Uptake pathway of polyomavirus via ganglioside GD1a. J. Virol. 78:12259-12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert, J., J. Dahl, C. Riney, J. You, C. Cui, R. Holmes, W. Lencer, and T. Benjamin. 2005. Ganglioside GD1a restores infectibility to mouse cells lacking functional receptors for polyomavirus. J. Virol. 79:615-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert, J., W. Ou, J. Silver, and T. Benjamin. 2006. Downregulation of protein disulfide isomerase inhibits infection by the mouse polyomavirus. J. Virol. 80:10868-10870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heldwein, E. E., and C. Krummenacher. 2008. Entry of herpesviruses into mammalian cells. Cell. Mol. Life Sci. 65:1653-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalinin, S. V., and J. G. Molotkovsky. 2001. Anion binding to lipid bilayers: a study using fluorescent lipid probes. Membr. Cell Biol. 14:831-846. [PubMed] [Google Scholar]
- 17.Kolter, T., and K. Sandhoff. 2005. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu. Rev. Cell Dev. Biol. 21:81-103. [DOI] [PubMed] [Google Scholar]
- 18.Kornfeld, R., and S. Kornfeld. 1985. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 54:631-664. [DOI] [PubMed] [Google Scholar]
- 19.Kuritzkes, D. R. 2009. HIV-1 entry inhibitors: an overview. Curr. Opin. HIV AIDS 4:82-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lencer, W. I., and B. Tsai. 2003. The intracellular voyage of cholera toxin: going retro. Trends Biochem. Sci. 28:639-645. [DOI] [PubMed] [Google Scholar]
- 21.Liebl, D., F. Difato, L. Hornikova, P. Mannova, J. Stokrova, and J. Forstova. 2006. Mouse polyomavirus enters early endosomes, requires their acidic pH for productive infection, and meets transferrin cargo in Rab11-positive endosomes. J. Virol. 80:4610-4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lilley, B. N., J. M. Gilbert, H. L. Ploegh, and T. L. Benjamin. 2006. Murine polyomavirus requires the endoplasmic reticulum protein Derlin-2 to initiate infection. J. Virol. 80:8739-8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Low, J. A., B. Magnuson, B. Tsai, and M. J. Imperiale. 2006. Identification of gangliosides GD1b and GT1b as receptors for BK virus. J. Virol. 80:1361-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lozach, P. Y., L. Burleigh, I. Staropoli, E. Navarro-Sanchez, J. Harriague, J. L. Virelizier, F. A. Rey, P. Despres, F. Arenzana-Seisdedos, and A. Amara. 2005. Dendritic cell-specific intercellular adhesion molecule 3-grabbing non-integrin (DC-SIGN)-mediated enhancement of dengue virus infection is independent of DC-SIGN internalization signals. J. Biol. Chem. 280:23698-23708. [DOI] [PubMed] [Google Scholar]
- 25.Magnuson, B., E. K. Rainey, T. Benjamin, M. Baryshev, S. Mkrtchian, and B. Tsai. 2005. ERp29 triggers a conformational change in polyomavirus to stimulate membrane binding. Mol. Cell 20:289-300. [DOI] [PubMed] [Google Scholar]
- 26.Pelkmans, L., D. Puntener, and A. Helenius. 2002. Local actin polymerization and dynamin recruitment in SV40-induced internalization of caveolae. Science 296:535-539. [DOI] [PubMed] [Google Scholar]
- 27.Qian, M., D. Cai, K. J. Verhey, and B. Tsai. 2009. A lipid receptor sorts polyomavirus from the endolysosome to the endoplasmic reticulum to cause infection. PLoS Pathog. 5:e1000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rainey-Barger, E. K., B. Magnuson, and B. Tsai. 2007. A chaperone-activated nonenveloped virus perforates the physiologically relevant endoplasmic reticulum membrane. J. Virol. 81:12996-13004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarzmann, G. 2001. Uptake and metabolism of exogenous glycosphingolipids by cultured cells. Semin. Cell Dev. Biol. 12:163-171. [DOI] [PubMed] [Google Scholar]
- 30.Shieh, M. T., D. WuDunn, R. I. Montgomery, J. D. Esko, and P. G. Spear. 1992. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J. Cell Biol. 116:1273-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith, A. E., and A. Helenius. 2004. How viruses enter animal cells. Science 304:237-242. [DOI] [PubMed] [Google Scholar]
- 32.Smith, A. E., H. Lilie, and A. Helenius. 2003. Ganglioside-dependent cell attachment and endocytosis of murine polyomavirus-like particles. FEBS Lett. 555:199-203. [DOI] [PubMed] [Google Scholar]
- 33.Stehle, T., and S. C. Harrison. 1996. Crystal structures of murine polyomavirus in complex with straight-chain and branched-chain sialyloligosaccharide receptor fragments. Structure 4:183-194. [DOI] [PubMed] [Google Scholar]
- 34.Stehle, T., Y. Yan, T. L. Benjamin, and S. C. Harrison. 1994. Structure of murine polyomavirus complexed with an oligosaccharide receptor fragment. Nature 369:160-163. [DOI] [PubMed] [Google Scholar]
- 35.Tong, G. M., T. T. Rajah, and J. T. Pento. 2000. The differential influence of EGF, IGF-I, and TGF-beta on the invasiveness of human breast cancer cells. In Vitro Cell. Dev. Biol. Anim. 36:493-494. [DOI] [PubMed] [Google Scholar]
- 36.Tsai, B. 2007. Penetration of nonenveloped viruses into the cytoplasm. Annu. Rev. Cell Dev. Biol. 23:23-43. [DOI] [PubMed] [Google Scholar]
- 37.Tsai, B., J. M. Gilbert, T. Stehle, W. Lencer, T. L. Benjamin, and T. A. Rapoport. 2003. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 22:4346-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]