Abstract
Defining the earliest virologic events following human immunodeficiency virus type 1 (HIV-1) transmission may be critical for the design of vaccine strategies aimed at blocking acquisition of HIV-1 infection. In particular, the length of the eclipse phase and the number of transmitted virus variants may define the window in which a prophylactic vaccine must act. Here we show that the dose of the virus inoculum affects these key virologic parameters following intrarectal simian immunodeficiency virus (SIV) infection of rhesus monkeys. Low-dose SIV infection resulted in a lengthened eclipse phase, fewer transmitted virus variants, and decreased innate immune activation compared with these parameters in high-dose SIV infection. These data suggest a mechanism by which it may be considerably easier for a vaccine to protect against low-risk HIV-1 transmission than against high-risk HIV-1 transmission. These findings have implications for the design and interpretation of HIV-1 vaccine efficacy studies.
Mucosal human immunodeficiency virus type 1 (HIV-1) transmission in humans and simian immunodeficiency virus (SIV) infection in rhesus monkeys are characterized by a limited number of transmitted/founder virus variants (5, 6). A vaccine aimed at preventing the acquisition of infection would need to block these infecting virus variants in the mucosa during the eclipse phase of infection prior to systemic viremia in order to prevent the establishment of permanent virus reservoirs. Determining the length and characteristics of the eclipse phase is therefore critical in defining the window of vulnerability of the virus to vaccine-elicited humoral and cellular immune responses. In this study, we assessed the effect of the dose of the virus inoculum on the length of the eclipse phase, the number of transmitted virus variants, and the innate and adaptive immune responses following atraumatic intrarectal SIV infection of rhesus monkeys.
Materials and methods.
Outbred adult rhesus monkeys (4 to 16 years old) that did not express the major histocompatibility complex (MHC) class I alleles Mamu-A*01, Mamu-B*08, and Mamu-B*17 were housed at New England Primate Research Center (NEPRC), Southborough, MA. Animals were inoculated once by the intrarectal route with a 1:1, 1:10, 1:100, or 1:1,000 dilution of our SIVmac251 challenge stock (n = 6/group). This challenge stock was derived by expanding a previously described virus stock (6, 8) in human peripheral blood mononuclear cells (PBMC) stimulated with concanavalin A and interleukin-2 (IL-2). The genotypic diversity of the two stocks was indistinguishable (data not shown). The new challenge stock had a concentration of 1 × 109 SIV RNA copies/ml and a 50% tissue culture infective dose (TCID50) titer in TZM-bl cells of 9.3 × 105/ml. The virus was diluted by serial 10-fold dilutions in RPMI containing 10% fetal bovine serum. A 1-ml inoculation was administered atraumatically by the intrarectal route to anesthetized animals, using a 3-ml syringe and a flexible catheter. Plasma SIV RNA levels were determined on days 0, 1, 2, 4, 7, 10, 14, 21, and 28 and then every other week following infection (Siemans Diagnostics). All animal studies were approved by the Harvard Medical School Institutional Animal Care and Use Committee (IACUC).
Transmitted/founder viruses and their progeny were identified by single-genome amplification (SGA) of plasma SIV RNA, direct amplicon sequencing, and phylogenetic analysis within the context of a model of random virus evolution (5-7). SGA was performed by extracting SIV RNA from plasma or culture supernatant and performing limiting-dilution PCR of newly synthesized cDNA. Although the inoculum sequences proportionally represent the challenge stock, they do not represent a comprehensive sampling of the challenge stock. A total of 525 full-length gp160 env sequences (range, 26 to 33, and median, 29 sequences per animal) were generated from the 18 productively infected monkeys. Twenty-seven full-length gp160 env sequences were also generated from the challenge stock. Transmitted/founder virus lineages were identified by low-diversity sequence lineages as previously described (5, 6) and by single sequences with unique mutations that exceeded the number predicted by mathematical modeling (>4 mutations per 2,600 bp of env, or >0.15%) and measured empirically to occur within the first 10 days of infection. In animals infected by larger numbers of viruses, recombination may have confounded the identification of certain transmitted/founder virus lineages. Phylogenetic trees were generated by the neighbor-joining method using ClustalW or PAUP* and were evaluated for significance by bootstrapping.
Twenty-three cytokines were measured in serum using a nonhuman primate cytokine Milliplex kit (Millipore) according to the manufacturer's instructions. The cytokines included IL-1β, IL-1Rα, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12/23 (p40), IL-13, IL-15, IL-17, IL-18, gamma interferon (IFN-γ), granulocyte colony-stimulating factor (G-CSF), granulocyte-macrophage (GM)-CSF, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, tumor necrosis factor alpha (TNF-α), transforming growth factor β (TGF-β), sCD40L, and vascular endothelial growth factor (VEGF). Serum samples were incubated with antibody-coupled beads overnight, followed by incubation with a biotinylated detection antibody and streptavidin-phycoerythrin (PE). Each sample was assayed in duplicate, and cytokine standards supplied by the manufacturer were run on each plate. Multianalyte profiling was performed using a Luminex-100 system, and data were analyzed using BioPlex manager software, version 4.1 (Bio-Rad). IFN-α was measured using a human IFN-α enzyme-linked immunosorbent assay (ELISA) kit (PBL Interferon Source). The median levels of each analyte per group are reported.
SIV-specific cellular immune responses were assessed by IFN-γ enzyme-linked immunospot (ELISPOT) assays utilizing pooled SIV Gag, Pol, Nef, and Env peptides essentially as described previously (11). Flow cytometric assessments of T lymphocyte subsets utilized the following monoclonal antibodies (MAbs): anti-CD3-Alexa Fluor 700 (SP34), anti-CD4- AmCyan (L200), anti-CD8-antigen-presenting cell (APC)-Cy7 (SK1), anti-CD28-peridinin chlorophyll protein (PerCP)-Cy5.5 (L293), anti-CD95-APC (DX2), anti-CCR5-PE (3A9), anti-HLA-DR-PE-Cy7 (L243), and anti-Ki67-fluorescein isothiocyanate (FITC) (B56).
SIV-specific humoral IgG responses were evaluated by a Luminex assay to measure binding antibodies as previously described (19). Briefly, SIVmac239 Gag p55 (Protein Sciences) and Env gp140 (provided by Bing Chen, Children's Hospital, Boston, MA) were coupled to microspheres (Bio-Rad) and incubated with serial dilutions of serum, and specific antibody binding was detected using a biotinylated anti-monkey IgG (Rockland). Positive and negative monkey serum controls were used in each assay, and the midpoint titer (50% effective concentration [EC50]) of each sample was calculated using the four-parameter logistic model (4PL) fit. The median titers per group are reported.
Statistical analyses were conducted using GraphPad Prism, version 5. The comparisons of eclipse phase length, transmitted/founder variants, and innate and adaptive immune parameters utilized the 2-sided Wilcoxon rank-sum test. P values of <0.05 were considered significant.
Results and discussion.
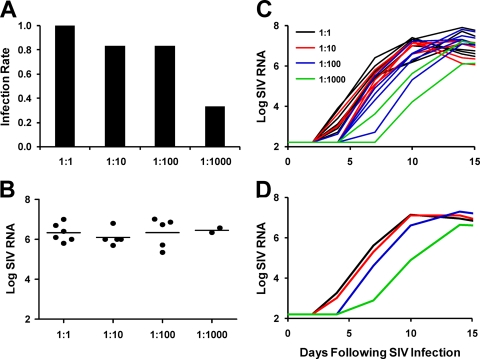
Twenty-four naïve adult rhesus monkeys (n = 6/group) received a single intrarectal inoculation with 1 ml of a 1:1, 1:10, 1:100, or 1:1,000 dilution of our SIVmac251 challenge stock, reflecting virus doses of 109, 108, 107, or 106 SIV RNA copies (9.3 × 105, 9.3 × 104, 9.3 × 103, or 9.3 × 102 TCID50), respectively. This virus stock was derived by expanding a previously described challenge stock (6, 8) in human PBMC, and the genotypic diversity of the two stocks was indistinguishable (data not shown). As expected, decreasing the virus inoculum dose resulted in reduced infectivity. The 1:1 dilution resulted in 100% (6/6) infectivity, the 1:10 and 1:100 dilutions resulted in 83% (5/6) infectivity, and the 1:1,000 dilution resulted in 33% (2/6) infectivity after a single intrarectal inoculation (Fig. 1A). To characterize the length of the eclipse phase, plasma SIV RNA levels were determined on days 0, 1, 2, 4, 7, 10, 14, and 21 following infection. All infected monkeys exhibited comparable levels of plasma SIV RNA on day 21, indicating robust virus replication in all groups (Fig. 1B). However, we observed substantial differences in the length of the eclipse phase, which we defined as the time to the first positive plasma SIV RNA. Monkeys infected by the 1:1, 1:10, 1:100, or 1:1,000 dilution of virus exhibited their first positive peripheral plasma SIV RNA levels (>50 copies/ml) after a median of 4, 4, 7, or 8.5 days, respectively (Fig. 1C and D and Table 1). In particular, monkeys infected by the 1:100 and 1:1,000 dilutions exhibited significantly longer eclipse phases than monkeys infected by the 1:1 and 1:10 dilutions (P = 0.01, two-sided Wilcoxon rank-sum test). Following the eclipse phase, virus replication kinetics appeared comparable among all groups, with parallel slopes of increasing SIV RNA levels and similar peak SIV RNA levels (Fig. 1D), comparable with the results of previous studies (8, 11, 20, 21).
FIG. 1.
Replication kinetics of acute SIV infection. Rhesus monkeys (n = 6/group) were inoculated by the intrarectal route with 1 ml of a 1:1, 1:10, 1:100, or 1:1,000 dilution of our SIVmac251 stock. (A) Fraction of monkeys infected in each group. (B) SIV RNA levels on day 21 following infection. (C and D) SIV RNA levels of all monkeys (C) and median SIV RNA levels in each group from day 0 to day 14 (D).
TABLE 1.
Virologic characteristics of acute intrarectal SIV infection in rhesus monkeys
| Dose | No. of SIV RNA copies | Infection rate (%) | Median eclipse period (days) | Median T/Fa virus variants |
|---|---|---|---|---|
| 1:1 | 109 | 6/6 (100) | 4 | >10 |
| 1:10 | 108 | 5/6 (83) | 4 | >10 |
| 1:100 | 107 | 5/6 (83) | 7 | 2 |
| 1:1,000 | 106 | 2/6 (33) | 8.5 | 1 |
T/F, transmitted/founder.
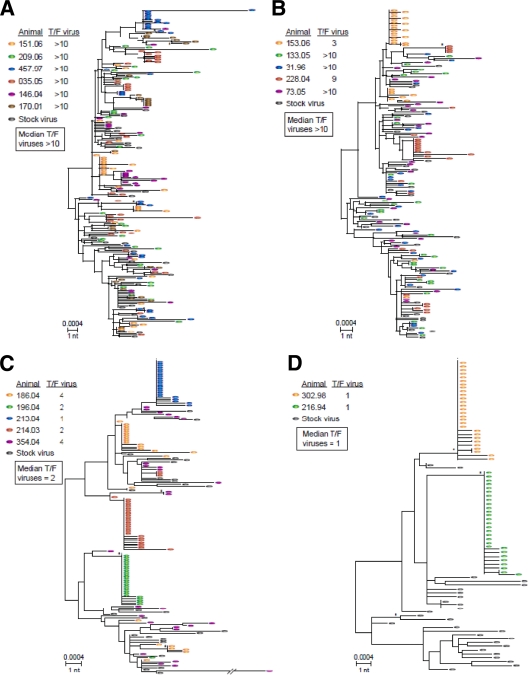
We next determined the number of transmitted/founder virus variants that established infection by assessing the phylogenetic patterns of env diversity in each animal on day 10 following infection, utilizing single-genome amplification (SGA) and direct amplicon sequencing (5, 6, 15, 16). Monkeys infected by the 1:1 and 1:10 dilutions of virus were characterized by a median of more than 10 transmitted/founder virus variants with lineages found throughout the challenge inoculum tree (Fig. 2A and B and Table 1). Precise estimation of the numbers of transmitted/founder viruses in these groups was not possible due to their large numbers, similar to previous findings with intravenously inoculated animals (6). In contrast, monkeys infected by the 1:100 dilution of virus exhibited a median of 2 transmitted/founder virus variants, and the two animals infected by the 1:1,000 dilution each showed only a single transmitted/founder virus variant, with 95% confidence of detecting a minor variant representing at least 5% of the population (Fig. 2C and D and Table 1). These data demonstrate that monkeys that received the 1:100 and 1:1,000 dilutions were infected by significantly fewer transmitted/founder virus variants than monkeys infected by the 1:1 and 1:10 dilutions (P = 0.0002, two-sided Wilcoxon rank-sum test). Virus diversification conformed to a model of random evolution, and transmitted/founder virus sequences could be identified unambiguously in the lower dose groups. Analysis of transmitted lineages from each animal compared to the inoculum revealed no preferential transmission regardless of dose. Since the number of variants ranged from many more than 10 down to single variant, these data suggest that susceptible target cells in the colorectal mucosa were not a limiting factor for virus transmission. Furthermore, these data suggest that the challenge inoculum can be limited to control the number of transmitted variants, thus providing a unique opportunity to probe early viral and immune events.
FIG. 2.
Neighbor-joining phylogenetic trees of transmitted/founder (T/F) viruses and their progeny. (A to D) Env sequences from monkeys infected by a 1:1 (A), 1:10 (B), 1:100 (C), or 1:1,000 (D) dilution of virus are shown from day 10 following infection, along with the SIVmac251 inoculum sequences. Transmitted/founder virus lineages are most clearly evident in animals inoculated with the two lower doses of virus. Numbers of transmitted/founder viruses for each animal and group medians are shown. Bootstraps significant at 90% are indicated by asterisks. Low-diversity transmitted/founder lineage sequences were significantly clustered when sequences from individual animals were analyzed with stock sequences.
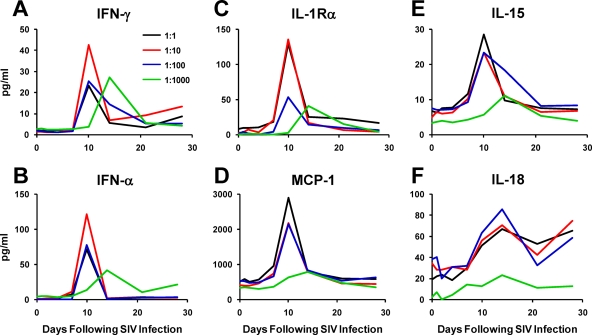
We evaluated serum cytokine and chemokine levels following infection by performing ELISA and Luminex assays (17). Analytes that increased during acute SIV infection (IFN-γ, IFN-α, IL-1Rα, MCP-1, IL-15, and IL-18) showed trends toward lower and delayed median peak levels in the lower dose groups compared with the trends in the higher dose groups (Fig. 3). In particular, monkeys infected by the 1:1,000 dilution of virus exhibited median peak levels of these cytokines on day 14, whereas animals infected by the 1:1 and 1:10 dilutions of virus demonstrated median peak levels on day 10. These kinetic differences presumably reflected, in part, the increased length of the eclipse phase in the lower dose groups, but the magnitudes of these analytes were also reduced despite similar peak SIV RNA levels (Fig. 1D). These data suggest lower levels of innate immune activation in the groups that received the lower doses of virus. The results for the cytokines and chemokines that showed minimal changes during acute SIV infection (G-CSF, GM-CSF, IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, MIP-1α, MIP-1β, sCD40L, TGF-β, TNF-α, and VEGF) were comparable among groups (data not shown).
FIG. 3.
Cytokine and chemokine levels during acute SIV infection. (A to F) Serum levels of IFN-γ (A), IFN-α (B), IL-1Rα (C), MCP-1 (D), IL-15 (E), and IL-18 (F) in monkeys infected by a 1:1, 1:10, 1:100, or 1:1,000 dilution of virus were determined. Median levels of each analyte are shown for each group.
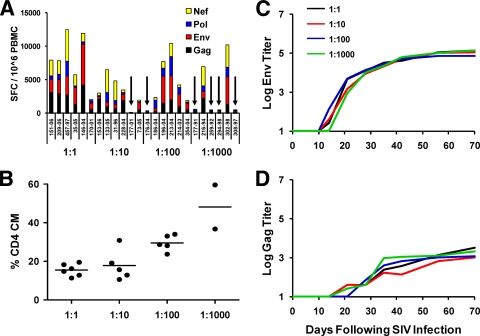
The adaptive SIV-specific cellular immune responses to Gag, Pol, Nef, and Env, as determined by IFN-γ ELISPOT assays at week 4 following infection, appeared comparable in all infected monkeys regardless of dose (11) (Fig. 4A) (not significant). However, monkeys infected by the 1:100 and 1:1,000 dilutions of virus exhibited higher levels of CD28+ CD95+ central and transitional memory CD4+ T lymphocytes than monkeys infected by the 1:1 and 1:10 dilutions of virus (Fig. 4B) (P = 0.001, two-sided Wilcoxon rank-sum test). SIV-specific humoral IgG responses (19) to Env gp140 (Fig. 4C) and Gag p55 (Fig. 4D) also appeared comparable in all infected monkeys regardless of dose, although the kinetics of the induction of Env-specific antibody responses was slightly delayed in the animals that received the 1:1,000 dilution of virus (Fig. 4C).
FIG. 4.
Adaptive immune responses during acute SIV infection. (A) SIV Gag-, Pol-, Nef-, and Env-specific cellular immune responses were determined at week 4 following infection with a 1:1, 1:10, 1:100, or 1:1,000 dilution of virus by performing IFN-γ ELISPOT assays. Arrows indicate animals that did not become infected. SFC, spot-forming cells. (B) Percentages of CD28+ CD95+ central and transitional memory CD4+ T lymphocytes (CD4+ CM) at week 4 in infected animals. (C and D) SIV Env gp140-specific (C) and Gag p55-specific (D) antibody responses following infection were determined with a Luminex binding antibody assay. Median IgG responses are shown for each group.
Our data demonstrate that the dose of the virus inoculum affects critical virologic parameters of acute intrarectal SIV infection of rhesus monkeys. In particular, lower doses of virus resulted in a longer eclipse phase, fewer transmitted/founder virus variants, and reduced innate immune activation (Table 1). These virologic parameters of acute infection may critically affect the capacity of a vaccine to block acquisition of infection, since the eclipse period defines the window of time in which vaccine-elicited immune responses must act and the number of transmitted/founder variants defines the diversity of viruses that must be blocked. In particular, it may be considerably easier for a vaccine to block a single transmitted virus in a longer time frame than a swarm of genetically diverse viruses in a shorter time frame (10). For example, if vaccine-elicited antibodies could neutralize a fraction of circulating viruses in a given population, then such a vaccine would be far more likely to protect against a single virion than against multiple genetically diverse virus variants. We acknowledge, however, that SIV RNA levels, numbers of transmitted/founder virus variants, and cytokine levels in plasma may differ in the mucosa as compared with the blood. Moreover, the present studies were conducted by intrarectal SIV infection, and thus, the generalizability of the conclusions to intravaginal SIV infection remains to be determined (18).
In the recently completed RV144 study, the ALVAC-AIDSVAX vaccine regimen afforded 31% protection against the acquisition of HIV-1 infection in a community-based efficacy study in Thailand (14). Subgroup analyses revealed that the point estimate of protective efficacy was 40 to 48% in low- and medium-risk subjects but only 4% in high-risk subjects (14). Although RV144 was not formally statistically powered for such subgroup analyses, these observations nevertheless raise the important hypothesis that a vaccine may be far more effective at blocking low-risk HIV-1 transmission than high-risk HIV-1 transmission. Consistent with these observations, AIDSVAX alone, as well as an unrelated recombinant adenovirus serotype 5 vector-based vaccine, proved ineffective at blocking high-risk HIV-1 transmission in intravenous drug users and in men who have sex with men (3, 4, 12). High-risk HIV-1 transmission is characterized not only by increased frequencies of HIV-1 exposure but also by higher per-exposure transmission risk and higher virus doses per exposure (2, 13), as well as increased numbers of transmitted/founder virus variants (1, 9). We speculate that the results of the present studies may offer a potential mechanistic basis for why an HIV-1 vaccine candidate may afford greater protective efficacy in low-risk than in high-risk populations. Taken together, our data suggest that key differences in the early virologic events of acute infection may critically affect the capacity of an HIV-1 vaccine candidate to block acquisition of infection.
Nucleotide sequence accession numbers.
All sequences were deposited in GenBank under accession numbers GU998976 to GU999527.
Acknowledgments
We thank S. Clark, A. La Porte, D. Lynch, K. O'Brien, A. Riggs, N. Simmons, F. Stephens, J. Schmitz, L. Eslamizar, M. Lifton, V. Ashley, Y. Lin, B. Chen, J. White, and A. Perelson for generous advice, assistance, and reagents.
We acknowledge support from the National Institutes of Health Center for HIV/AIDS Vaccine Immunology (AI067854), as well as from the National Institutes of Health (AI066305, AI066924, AI078526, AI084794, AI087383, RR000168, and HHSN266200400088C). The authors declare no competing financial interests.
J.L. prepared the challenge stock and performed and analyzed the cellular immunologic assays. B.F.K., H.L., B.H.H., and G.M.S. performed and analyzed the transmitted/founder virus assays. S.K. and P.J.N. performed and analyzed the cytokine assays. A.C. and K.G.M. led the animal work. G.D.T. performed and analyzed the humoral immunologic assays. D.K.-G. performed the TCID50 assay. B.F.H., N.L.L., B.H.H., G.M.S., and D.H.B. designed the study. D.H.B. led the study. All coauthors discussed the data and contributed to writing the manuscript.
Footnotes
Published ahead of print on 4 August 2010.
The authors have paid a fee to allow immediate free access to this article.
REFERENCES
- 1.Bar, K. J., H. Li, A. Chamberland, C. Tremblay, J. P. Routy, T. Grayson, C. Sun, S. Wang, G. H. Learn, C. J. Morgan, J. E. Schumacher, B. F. Haynes, B. F. Keele, B. H. Hahn, and G. M. Shaw. 2010. Wide variation in the multiplicity of HIV-1 infection among injection drug users. J. Virol. 84:6241-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boily, M. C., R. F. Baggaley, L. Wang, B. Masse, R. G. White, R. J. Hayes, and M. Alary. 2009. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect. Dis. 9:118-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, and M. N. Robertson. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert, P. B., M. L. Peterson, D. Follmann, M. G. Hudgens, D. P. Francis, M. Gurwith, W. L. Heyward, D. V. Jobes, V. Popovic, S. G. Self, F. Sinangil, D. Burke, and P. W. Berman. 2005. Correlation between immunologic responses to a recombinant glycoprotein 120 vaccine and incidence of HIV-1 infection in a phase 3 HIV-1 preventive vaccine trial. J. Infect. Dis. 191:666-677. [DOI] [PubMed] [Google Scholar]
- 5.Keele, B. F., E. E. Giorgi, J. F. Salazar-Gonzalez, J. M. Decker, K. T. Pham, M. G. Salazar, C. Sun, T. Grayson, S. Wang, H. Li, X. Wei, C. Jiang, J. L. Kirchherr, F. Gao, J. A. Anderson, L. H. Ping, R. Swanstrom, G. D. Tomaras, W. A. Blattner, P. A. Goepfert, J. M. Kilby, M. S. Saag, E. L. Delwart, M. P. Busch, M. S. Cohen, D. C. Montefiori, B. F. Haynes, B. Gaschen, G. S. Athreya, H. Y. Lee, N. Wood, C. Seoighe, A. S. Perelson, T. Bhattacharya, B. T. Korber, B. H. Hahn, and G. M. Shaw. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. U. S. A. 105:7552-7557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keele, B. F., H. Li, G. H. Learn, P. Hraber, E. E. Giorgi, T. Grayson, C. Sun, Y. Chen, W. W. Yeh, N. L. Letvin, J. R. Mascola, G. J. Nabel, B. F. Haynes, T. Bhattacharya, A. S. Perelson, B. T. Korber, B. H. Hahn, and G. M. Shaw. 2009. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206:1117-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, H. Y., E. E. Giorgi, B. F. Keele, B. Gaschen, G. S. Athreya, J. F. Salazar-Gonzalez, K. T. Pham, P. A. Goepfert, J. M. Kilby, M. S. Saag, E. L. Delwart, M. P. Busch, B. H. Hahn, G. M. Shaw, B. T. Korber, T. Bhattacharya, and A. S. Perelson. 2009. Modeling sequence evolution in acute HIV-1 infection. J. Theor. Biol. 261:341-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li, H., K. J. Bar, S. Wang, J. M. Decker, Y. Chen, C. Sun, J. F. Salazar-Gonzalez, M. G. Salazar, G. H. Learn, C. J. Morgan, J. E. Schumacher, P. Hraber, E. E. Giorgi, T. Bhattacharya, B. T. Korber, A. S. Perelson, J. J. Eron, M. S. Cohen, C. B. Hicks, B. F. Haynes, M. Markowitz, B. F. Keele, B. H. Hahn, and G. M. Shaw. 2010. High multiplicity infection by HIV-1 in men who have sex with men. PLoS Pathog. 6:e1000890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, Q., P. J. Skinner, S. J. Ha, L. Duan, T. L. Mattila, A. Hage, C. White, D. L. Barber, L. O'Mara, P. J. Southern, C. S. Reilly, J. V. Carlis, C. J. Miller, R. Ahmed, and A. T. Haase. 2009. Visualizing antigen-specific and infected cells in situ predicts outcomes in early viral infection. Science 323:1726-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu, J., K. L. O'Brien, D. M. Lynch, N. L. Simmons, A. La Porte, A. M. Riggs, P. Abbink, R. T. Coffey, L. E. Grandpre, M. S. Seaman, G. Landucci, D. N. Forthal, D. C. Montefiori, A. Carville, K. G. Mansfield, M. J. Havenga, M. G. Pau, J. Goudsmit, and D. H. Barouch. 2009. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature 457:87-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pitisuttithum, P., P. Gilbert, M. Gurwith, W. Heyward, M. Martin, F. van Griensven, D. Hu, J. W. Tappero, and K. Choopanya. 2006. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J. Infect. Dis. 194:1661-1671. [DOI] [PubMed] [Google Scholar]
- 13.Powers, K. A., C. Poole, A. E. Pettifor, and M. S. Cohen. 2008. Rethinking the heterosexual infectivity of HIV-1: a systematic review and meta-analysis. Lancet Infect. Dis. 8:553-563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rerks-Ngarm, S., P. Pitisuttithum, S. Nitayaphan, J. Kaewkungwal, J. Chiu, R. Paris, N. Premsri, C. Namwat, M. de Souza, E. Adams, M. Benenson, S. Gurunathan, J. Tartaglia, J. G. McNeil, D. P. Francis, D. Stablein, D. L. Birx, S. Chunsuttiwat, C. Khamboonruang, P. Thongcharoen, M. L. Robb, N. L. Michael, P. Kunasol, and J. H. Kim. 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N. Engl. J. Med. 361:2209-2220. [DOI] [PubMed] [Google Scholar]
- 15.Salazar-Gonzalez, J. F., E. Bailes, K. T. Pham, M. G. Salazar, M. B. Guffey, B. F. Keele, C. A. Derdeyn, P. Farmer, E. Hunter, S. Allen, O. Manigart, J. Mulenga, J. A. Anderson, R. Swanstrom, B. F. Haynes, G. S. Athreya, B. T. Korber, P. M. Sharp, G. M. Shaw, and B. H. Hahn. 2008. Deciphering human immunodeficiency virus type 1 transmission and early envelope diversification by single-genome amplification and sequencing. J. Virol. 82:3952-3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salazar-Gonzalez, J. F., M. G. Salazar, B. F. Keele, G. H. Learn, E. E. Giorgi, H. Li, J. M. Decker, S. Wang, J. Baalwa, M. H. Kraus, N. F. Parrish, K. S. Shaw, M. B. Guffey, K. J. Bar, K. L. Davis, C. Ochsenbauer-Jambor, J. C. Kappes, M. S. Saag, M. S. Cohen, J. Mulenga, C. A. Derdeyn, S. Allen, E. Hunter, M. Markowitz, P. Hraber, A. S. Perelson, T. Bhattacharya, B. F. Haynes, B. T. Korber, B. H. Hahn, and G. M. Shaw. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 206:1273-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stacey, A. R., P. J. Norris, L. Qin, E. A. Haygreen, E. Taylor, J. Heitman, M. Lebedeva, A. DeCamp, D. Li, D. Grove, S. G. Self, and P. Borrow. 2009. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J. Virol. 83:3719-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone, M., B. F. Keele, Z. M. Ma, E. Bailes, J. Dutra, B. H. Hahn, G. M. Shaw, and C. J. Miller. 2010. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J. Virol. 84:7083-7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomaras, G. D., N. L. Yates, P. Liu, L. Qin, G. G. Fouda, L. L. Chavez, A. C. Decamp, R. J. Parks, V. C. Ashley, J. T. Lucas, M. Cohen, J. Eron, C. B. Hicks, H. X. Liao, S. G. Self, G. Landucci, D. N. Forthal, K. J. Weinhold, B. F. Keele, B. H. Hahn, M. L. Greenberg, L. Morris, S. S. Karim, W. A. Blattner, D. C. Montefiori, G. M. Shaw, A. S. Perelson, and B. F. Haynes. 2008. Initial B-cell responses to transmitted human immunodeficiency virus type 1: virion-binding immunoglobulin M (IgM) and IgG antibodies followed by plasma anti-gp41 antibodies with ineffective control of initial viremia. J. Virol. 82:12449-12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson, N. A., B. F. Keele, J. S. Reed, S. M. Piaskowski, C. E. MacNair, A. J. Bett, X. Liang, F. Wang, E. Thoryk, G. J. Heidecker, M. P. Citron, L. Huang, J. Lin, S. Vitelli, C. D. Ahn, M. Kaizu, N. J. Maness, M. R. Reynolds, T. C. Friedrich, J. T. Loffredo, E. G. Rakasz, S. Erickson, D. B. Allison, M. Piatak, Jr., J. D. Lifson, J. W. Shiver, D. R. Casimiro, G. M. Shaw, B. H. Hahn, and D. I. Watkins. 2009. Vaccine-induced cellular responses control simian immunodeficiency virus replication after heterologous challenge. J. Virol. 83:6508-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson, N. A., J. Reed, G. S. Napoe, S. Piaskowski, A. Szymanski, J. Furlott, E. J. Gonzalez, L. J. Yant, N. J. Maness, G. E. May, T. Soma, M. R. Reynolds, E. Rakasz, R. Rudersdorf, A. B. McDermott, D. H. O'Connor, T. C. Friedrich, D. B. Allison, A. Patki, L. J. Picker, D. R. Burton, J. Lin, L. Huang, D. Patel, G. Heindecker, J. Fan, M. Citron, M. Horton, F. Wang, X. Liang, J. W. Shiver, D. R. Casimiro, and D. I. Watkins. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 80:5875-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]