Abstract
Recent neuroimaging and neurological data implicate cerebellum in nonmotor sensory, cognitive, vegetative, and affective functions. The present study assessed cerebellar responses when the urge to breathe is stimulated by inhaled CO2. Ventilation changes follow arterial blood partial pressure CO2 changes sensed by the medullary ventral respiratory group (VRG) and hypothalamus, entraining changes in midbrain, pons, thalamus, limbic, paralimbic, and insular regions. Nearly all these areas are known to connect anatomically with the cerebellum. Using positron emission tomography, we measured regional brain blood flow during acute CO2-induced breathlessness in humans. Separable physiological and subjective effects (air hunger) were assessed by comparisons with various respiratory control conditions. The conjoint physiological effects of hypercapnia and the consequent air hunger produced strong bilateral, near-midline activations of the cerebellum in anterior quadrangular, central, and lingula lobules, and in many areas of posterior quadrangular, tonsil, biventer, declive, and inferior semilunar lobules. The primal emotion of air hunger, dissociated from hypercapnia, activated midline regions of the central lobule. The distributed activity across the cerebellum is similar to that for thirst, hunger, and their satiation. Four possible interpretations of cerebellar function(s) here are that: it subserves implicit intentions to access air; it provides predictive internal models about the consequences of CO2 inhalation; it modulates emotional responses; and that while some cerebellar regions monitor sensory acquisition in the VRG (CO2 concentration), others influence VRG to adjust respiratory rate to optimize partial pressure CO2, and others still monitor and optimize the acquisition of other sensory data in service of air hunger aroused vigilance.
For a century, the cerebellum was believed to be involved strictly in motor control functions (1–4). Over the last decade, a growing body of neuroimaging, neurological, and neurophysiological evidence suggests that the cerebellum has a more diverse role in brain function (5–8).‖ Findings now implicate it in nonmotor aspects of the perception of visual motion, speed, and direction (9), auditory pitch discrimination,** visual attention (10), temporal processing (11), olfaction (12), kinesthetic processing (13), cutaneous and tactile information processing (14), language and memory tasks (8), spatial reasoning (15), and sensory and cognitive states related to thirst (16), hunger (17), affect (18), and music (19).
Results indicating a role for the cerebellum in sensory processing and emotional states, including those with genesis in vegetative systems such as thirst and hunger, make it plausible that the cerebellum has a comparable role in other vegetative functions accompanied by compelling primal emotions. One such function is air hunger, and data from earlier studies of hypercapnia and air hunger note cerebellar activity (20, 21).
Air hunger results from increases in arterial blood partial pressure CO2 (PCO2), which is sensed by the ventral respiratory group (VRG) and Botzinger complexes in the medulla, and by the posterior hypothalamus. In response, these areas entrain a distributed pattern of activations and deactivations in other brain areas (20–23). Anatomical tracer labeling data in rat (24) indicate that a number of cerebellar areas send and/or receive projections from the VRG, which contains the structures necessary for respiratory rhythm generation (25–30). The cerebellar areas connected to the VRG are quadrangular (VI), central (III), lingula (I, II), and inferior semilunar (Crus II) lobules, as well as fastigial nucleus, interposed nucleus, and dentate nuclei, the output nuclei for the vermal, intermediate, and lateral regions of cerebellum. Moreover, cerebellum has known connectivity to the hypothalamus, thalamus, pons, and midbrain (31–33), other neural structures involved in the integrated response to increase of blood CO2. There is evidence for such reciprocal connections among these structures and the cerebellum in nonmammalian invertebrate species, suggesting that this is a phylogenetically old relationship (32, 34, 35).
The cerebellar involvement in responses to CO2 stimulation and air hunger is suggested by other kinds of data. It has been shown that the fastigial nucleus can modulate the respiratory output via influences on medullary-respiratory neurons (36). Respiratory difficulties (hyperventilation, respiratory alkalosis) have been attributed to focal tumors in areas in the quandrangle (V) and central (III) lobules of the anterior lobe of human patients (32).
The physiological mechanisms subserving the genesis of air hunger (or breathlessness) also appear to be influenced by brain areas subserving higher cognitive and affective processing. The brain areas likely supporting the cognitive and affective aspects of air hunger are in limbic, paralimbic, and association cortices. There are clear indications of reciprocal connectivity between these areas and the cerebellum (33).
Traditional theories of cerebellar function might lead one to assume that the cerebellum would be directly involved in motor aspects of respiratory responses to air hunger. However, neural output to the muscles of breathing is not particularly relevant to the genesis of air hunger with hypercapnia. Instead, breathing depends on an intact brainstem respiratory oscillator that responds to stimulation (37). Thus, mechanically ventilated curarized subjects report severe air hunger when end tidal PCO2 is increased (38). Similarly, air hunger was found with increased end tidal PCO2 in quadraplegics where the brainstem respiratory oscillator is intact but its activity cannot be transmitted via bulbo spinal fibers to the anterior horn cells of the respiratory musculature (39). Moreover, in the clinically assessed “locked in” syndrome, there is a lesion of the ventral pons and lower midbrain involving motor tracts to the rest of the body that completely removes voluntary control of muscle movement except eye elevation (40) while leaving sensation intact. Nonetheless, the breathing in such patients is normal and regular, with a normal PCO2. Voluntary effort on the part of these patients to change their breathing has no effect, although emotion will disrupt breathing, and inhalation of CO2 elicits air hunger.
Our positron emission tomography (PET) study assessed cerebellar responses to hypercapnia and the primal emotion of air hunger. Analyses of activity in brain areas outside the cerebellum are presented in separate papers (22, 23) and are summarized as follows. The rapid onset of CO2-induced air hunger was elicited when human volunteers breathed an 8% CO2, 92% O2 mixture. Our analyses delineated two different aspects of the resulting transient, intense experience of breathlessness. One set of analyses revealed neural activations due to the conjoint effects of hypercapnia and air hunger. Thus, the blood flow image for breathing the CO2 mixture through a facemask (CO2 FM) was compared with three other conditions: breathing a nitrogen/oxygen mixture in a facemask (O2 FM), voluntary paced breathing (PB) of room air (hyperventilation), and regular spontaneous breathing of room air (rest). These separate control conditions minimize effects of confounding factors such as respiratory apparatus used and associated stimulation, increased respiratory rates, and anticipatory anxiety.
In the foregoing analysis, our PET data implicated a midbrain-limbic-cortical network in the combined effects of hypercapnia and the consciousness of air hunger it evoked (22). Activity was present in the midbrain, pons, medulla, and hypothalamus and in the amygdala, hippocampus, parahippocampus, insula, claustrum, and anterior cingulate. There were also regional cerebral blood flow (rCBF) decreases in dorsal cingulate and prefrontal cortex. The pattern of activations and deactivations with CO2 FM was similar independent of which control state was used in the subtraction (22).
The second analysis of our data turned on the fact that when CO2 FM was compared with CO2 MP, the mouthpiece allowed an easier sense of breathing. Although end tidal PCO2 was essentially the same in the two circumstances, the breathlessness score was less with CO2 MP (23). Thus, subtraction analysis showed effects attributable to hunger for air independent of the extant end tidal PCO2. The analysis indicated activity in limbic areas, particularly the anterior cingulate. There were also increases in periaqueductal gray regions (ventral tegmental/substantia nigra). The latter areas are presumably associated with a heightened state of arousal. There were also parahippocampal, insula, thalamic, and hypothalamic activations. In addition to the responses in the foregoing phylogenetically ancient brain areas, there were major activations in higher cortical areas.
Methods
After giving informed consent, nine subjects were scanned twice each in the CO2 inhalation via FM, N2/O2 inhalation via FM, CO2 via MP, and at rest. One scan each was acquired during PB via MP and PB via FM. Greater detail about the subject selection, apparatus, and the various experimental paradigms for the administration of the gas mixture are described in earlier reports (22, 23). Acquired PET data were overlaid onto the anatomical MRIs acquired from each subject. Each of the primary comparisons achieved significance Z > 3.11 (P < 0.001) by a histogram-based, nonregional omnibus statistic. The significance of the activations and deactivations were tested with voxel-based Z statistic analyses.
An interregional covariation correlation also was conducted between the voxels of peak intensity in the cerebellum and all other activated voxels in the brain (using Z > 2.97, P < 0.002).
An r value was computed as a voxel-wise correlation of breathlessness intensity ratings with the PET images from the CO2 FM and CO2 MP scans for each subject. A β-2 statistic measuring kurtosis of the histogram of the difference images was used as an omnibus test to assess overall significance. Upon significance of the omnibus test, local extrema were identified within the image of correlation values. The correlation image was converted to a Z-score image, and P values were assigned from the Z distribution. Further details about our analysis procedures are in our other two papers (22, 23).
Results
Respiratory Effects.
As reported (22, 23), end tidal PCO2 was higher in the CO2 FM condition (8.8 ± 0.1%) (SEM) and CO2 MP condition (8.6 ± 0.1%) than either the rest (5.4 ± 0.1%), or N2/O2 FM condition (5.2 ± 0.2%) and lowest for the PB (4.5 ± 0.3%). Minute volume was greater during the CO2 FM (22.7 ± 3.0 liters/min) than during either the N2/O2 FM (6.6 ± 1.4 liters/min) or PB conditions (10.3 ± 1.9 liters/min). Minute volume was smaller during the O2 FM condition than the PB condition. Heart rate was not different for the CO2 FM and PB conditions, but was higher than during the O2 FM condition. Regardless of condition, recovery after each trial occurred within 30–60 s.
Perceptual and Cognitive Effects.
The breathlessness ratings after CO2 FM (78 ± 3) and CO2 MP (55 ± 4) were greater than those after N2/O2 trials (5 ± 3) or PB trials (10 ± 4), confirming that CO2 FM and CO2 MP produced air hunger. After CO2 inhalation, subjects reported other sensations. The CO2 FM trials produced variable mild lightheadedness and faintness, only occasionally a mild anxiety. Less frequent reactions included a sense of smothering, mild euphoria, a transient mild headache, and dry throat. There was no tingling of the extremities or other sensory paresthesias, nausea, or gustatory or olfactory sensations.
PET rCBF Findings.
Under the combination of hypercapnia and air hunger, there were intense, large cerebellar activations (Table 1), many with Z values above 4 and cluster sizes greater than 500 mm3. Most of these regions were activated regardless of control conditions. None of the activations described below were artifacts of the subtractions conducted; each positive activation was present in a contrast with rest.
Table 1.
Local maxima in cerebellar regions demonstrating significant rCBF increases (P < 0.001)
| Lobule or region | Lobe | Talairach*
|
Extent, mm3 | Z score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| CO2 FM–O2FM | ||||||
| Quadrangular (V) | A | 14 | −50 | −12 | 2504 | 6.77 |
| Tonsil (IX) | P | −14 | −48 | −26 | 896 | 6.51 |
| Biventer (VIIIB) | P | 24 | −48 | −44 | 776 | 5.66 |
| Inf. semilunar (Crus II) | P | 20 | −44 | −32 | 888 | 6.24 |
| Biventer (VIIIB) | P | −16 | −48 | −42 | 888 | 5.44 |
| Central lobule (III) | A | 16 | −38 | −16 | 896 | 5.64 |
| Quadrangular (VI) | P | 28 | −50 | −16 | 896 | 5.60 |
| Quadrangular (VI) | P | −26 | −68 | −12 | 648 | 5.37 |
| Quadrangular (VI) | P | −26 | −44 | −16 | 680 | 5.18 |
| Declive (vermis) (VI) | P | −6 | −66 | −12 | 656 | 5.18 |
| Declive (vermis) (VI) | P | 8 | −62 | −18 | 696 | 5.07 |
| Pyramis (vermis) (VIIIA) | P | 4 | −66 | −38 | 568 | 4.50 |
| Pyramis (vermis) (VIIIB) | P | −2 | −64 | −28 | 688 | 4.50 |
| Tonsil (IX) | P | −22 | −38 | −28 | 528 | 4.50 |
| Biventer (VIIIB) | P | 14 | −52 | −48 | 416 | 4.15 |
| Inf. semilunar (Crus II) | A | −22 | −32 | −26 | 256 | 4.32 |
| CO2 FM–PB | ||||||
| Quadrangular (V) | A | −26 | −46 | −16 | 416 | 4.90 |
| Quadrangular (IV) | A | −6 | −52 | −14 | 928 | 4.78 |
| Quadrangular (IV) | A | 12 | −52 | −12 | 872 | 4.75 |
| Lingula (I, II) | A | 0 | −44 | −16 | 848 | 4.70 |
| Culmen (IV) | A | 2 | −46 | −10 | 496 | 4.67 |
| Tonsil (IX) | P | −12 | −40 | −26 | 536 | 4.57 |
| Tonsil (IX) | P | −4 | −44 | −28 | 416 | 4.13 |
| Tuber (vermis) (VIIB) | P | −6 | −70 | −32 | 176 | 4.03 |
| Tonsil (IX) | P | 18 | −48 | −36 | 280 | 4.03 |
| Uvula (vermis) (IX) | P | 2 | −60 | −24 | 320 | 3.96 |
| Pyramis (vermis) (VIIIA) | P | 2 | −66 | −36 | 304 | 3.92 |
| Tonsil (IX) | P | −20 | −48 | −26 | 296 | 3.78 |
| Central lobule (III) | A | 18 | −38 | −18 | 304 | 3.70 |
| CO2 FM–CO2MP | ||||||
| Central lobule (III) | A | −4 | −50 | −18 | 216 | 4.33 |
| Culmen (IV) | A | −4 | −52 | −10 | 216 | 3.90 |
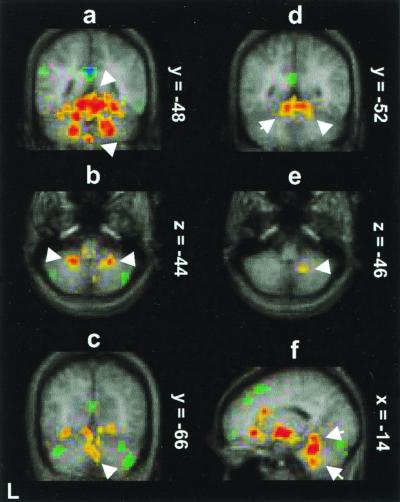
Cerebellar areas activated in common in the contrasts of CO2 FM with both O2 FM and PB include quadrangular lobule (V, VI) (Fig. 1 a and e), tonsil (IX) (Fig. 1d), central lobule (III) (Fig. 1d), biventer (VIIIB) (Fig. 1 b and f), and pyramis (vermis) (VIIIA) (Fig. 1a). These foci were distributed bilaterally and in anterior and posterior hemispheres. There were many more foci in intermediate areas from 14 to 25 mm from the midline than in vermis (midline) and very few in far lateral regions. The largest activation by far was in the right anterior quadrangular lobule (V) near the midline.
Figure 1.
PET activity (red-yellow) shown on the average MR brain of the nine subjects where breathing the CO2 mixture in a FM (CO2 FM) is compared (a–c) and (f) to breathing a nitrogen/oxygen mixture in a FM (O2 FM) or (d and e) to voluntary PB of room air (hyperventilation). (a) Activation (upper arrow) in quadrangular lobule (V, VI) and pyramis (vermis) (lower arrow) (coronal section y = −48). (b) Activation in bilateral biventer lobule (VIIIB) (transverse section z = −44). (c) Activation in bilateral declive (vermis) (VI) (coronal section y = −66). (d) Bilateral activation in quadrangular lobule (coronal section y = −52). (e) Activation in biventer lobule (transverse section z = −46). (f) Activation in central lobule (upper arrow) and tonsil (lower arrow) (sagittal section x = −14). The color coding of Z scores is shown in Fig. 2.
Activations specific to the contrast of CO2 FM with O2 FM, in which the effects of respiratory apparatus and any anticipatory anxiety are comparable, were detected in inferior semilunar lobule (Crus II), declive of vermis (VI) (Fig. 1c), and pyramis of vermis (VIIIB) (Fig. 1a). On the other hand, activations specific to the contrast of CO2 FM with PB, in both of which respiratory rate was increased, were detected in midline or vermal areas–lingula (I, II), culmen (IV), tuber (VIIB), and uvula (IX).
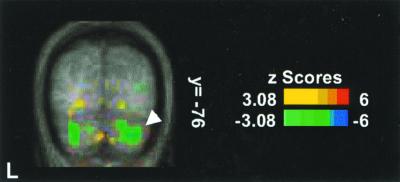
There were also deactivations in the contrast of CO2 FM with O2 FM (Table 2). There were deactivation foci distributed bilaterally in inferior semilunar (Crus II), gracile (VIIB) (Fig. 2), and biventer (VIIIA/B) lobules. The deactivations were primarily in the posterior hemisphere, ranging from 14 to 44 mm from the midline. There were no significant deactivation foci in the contrast of CO2 FM with PB.
Table 2.
Local minima in cerebellar regions demonstrating significant rCBF decreases (P < 0.001)
| Lobule or region | Lobe | Talairach*
|
Extent, mm3 | Z score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| CO2 FM–O2FM | ||||||
| Inf. semilunar (Crus II) | P | 32 | −66 | −30 | 416 | −5.43 |
| Gracile (VIIB) | P | 32 | −76 | −34 | 728 | −4.90 |
| Inf. semilunar (Crus II) | P | 16 | −80 | −24 | 496 | −4.79 |
| Inf. semilunar (Crus II) | P | 21 | −72 | −26 | 360 | −4.45 |
| Gracile (VIIB) | P | 26 | −76 | −34 | 496 | −4.41 |
| Gracile (VIIB) | P | −27 | −76 | −30 | 624 | −4.30 |
| Biventer (VIIIA) | P | −26 | −78 | −39 | 336 | −4.26 |
| Biventer (VIIIA) | P | −12 | −82 | −34 | 256 | −4.18 |
| Biventer (VIIIB) | P | −38 | −66 | −34 | 592 | −4.18 |
| Inf. semilunar (Crus II) | P | 44 | −66 | −26 | 128 | −3.84 |
| Inf. semilunar (Crus II) | P | −14 | −80 | −22 | 88 | −3.50 |
| CO2 FM–CO2MP | ||||||
| Gracile (VIIB) | P | 34 | −66 | −32 | 440 | −5.43 |
| Inf. semilunar (Crus II) | P | 20 | −70 | −24 | 624 | −5.28 |
| Gracile (VIIB) | P | −34 | −64 | −34 | 656 | −4.85 |
| Inf. semilunar (Crus II) | A | −17 | −64 | −24 | 544 | −4.73 |
| Biventer (VIIIB) | P | −28 | −40 | −38 | 200 | −4.65 |
| Gracile (VIIB) | P | 28 | −79 | −30 | 192 | −4.22 |
| Inf. semilunar (Crus II) | P | −8 | −77 | −26 | 224 | −4.11 |
Figure 2.
PET deactivations (blue-green) displayed on the average MR brain where breathing the CO2 mixture in a FM (CO2 FM) is compared with breathing a nitrogen/oxygen mixture in a FM (O2 FM). Two foci in gracile lobule (VIIB) (coronal section y = −76) are shown.
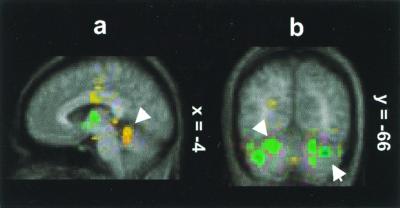
In the second analysis, which delineates brain areas involved in air hunger, the CO2 FM stimulus is compared with breathing CO2 with a MP (CO2 MP) (Table 1). In this analysis, there were significant activations in the midline regions of central (III) (Fig. 3a) and culmen (IV) lobules.
Figure 3.
PET activity displayed on the average MR brain where breathing the CO2 mixture in a FM (CO2 FM) is compared with the image of breathing CO2 with a MP (CO2 MP). (a) Activation in central lobule (III) (sagittal section x = −4). (b) Deactivation bilaterally in inferior semilunar (Crus II) (upper arrow) and gracile lobule (VIIB) (lower arrow). The color coding of Z scores is shown in Fig. 2.
There were also intense and large deactivations present when the CO2 FM condition is compared with the CO2 MP condition (Table 2). There were distinct deactivation foci primarily bilaterally in the gracile (VIIB) (Fig. 3b), inferior semilunar (Crus II) (Fig. 3b), biventer, and superior semilunar (Crus I) lobules. These foci were nearly all in the posterior lobe, from 16 to 41 mm of the midline. Note that the locations of the deactivations in this contrast are quite similar to those in the first two contrasts.
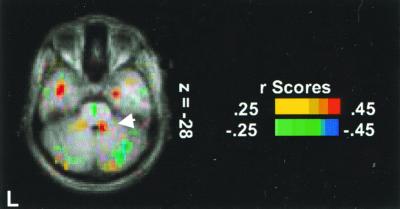
Activity in a region of central lobule (III) near the midline (x = 10, y = −44, z = −28) was positively correlated with air hunger intensity (Z = 2.96; 264 mm3) (Fig. 4). This correlated activity is consistent with the strong activation in the central lobule for the comparison of CO2 FM to CO2 MP above. Outside the cerebellum, regions of significant correlation with air hunger intensity (23) were present in the anterior cingulate cortex, insula, posterior parahippocampus, claustrum, sublenticular periamygdalar region, lingual, fusiform, and middle temporal areas. Regions of the cerebellum that were negatively correlated with air hunger intensity were in right superior semilunar (Crus I) (x = 20, y = −70, z = −20; Z = −3.27; 448 mm3), left biventer (VIIIB) (x = −31, y = −70, z = −36; Z = −3.17; 264 mm3), and left inferior semilunar lobules (Crus II) (x = −41, y = −66, z = −24; Z = −2.97; 200 mm3).
Figure 4.
The correlation of changes in cerebellar rCBF and breathlessness score. Activation in central lobule (III) (transverse section z = −28 mm) is shown.
Discussion
These data document the involvement of the cerebellum in response to CO2 stimulation and air hunger. For the conjoint effect of hypercapnia and air hunger, strong bilateral activations were observed in quadrangular (V), central (III), and lingula (I, II) lobules in the anterior hemisphere, and in very many posterior hemispheric foci in quadrangular (VI), tonsil (IX), biventer (VIIIB), declive (vermis) (VI), and inferior semilunar (Crus II) lobules. Nearly all of these foci were within 25 mm of the midline in the phylogenetically more ancient cerebellar regions.
Moreover, midline regions of the central (III) and culmen (IV) lobules of the anterior hemisphere were specifically activated in the analysis selectively highlighting the emotion of air hunger. The role of the central lobule in specific responses to the subjective state of air hunger is confirmed by the fact that activity there (near the midline) was the primary region of cerebellum correlated with ratings of air hunger intensity.
These findings are consistent with the anatomical, physiological, neurological, and evolutionary observations discussed earlier. Anterograde and retrograde tracing data in rat show that VRG connects with areas activated here: quadrangular (VI), central (III), lingula (I, II), and inferior semilunar (Crus II) lobules, as well as fastigial nucleus, interposed nucleus, and dentate nuclei (24).
The primary involvement of areas in the quandranglar (V) and central (III) lobule of anterior cerebellar hemisphere indicated here fits well with the reports of respiratory difficulties (i.e., hyperventilation, respiratory alkalosis) in patients with focal tumors in areas in or near those activated here (32).
Our findings conform to recent indications for cerebellar involvement in other interoceptor driven vegetative and autonomic functions such as receiving input from receptors that sense blood pressure in vessels (43, 44) and visceral pain (45), as well as thirst (16), and food hunger (17). Indeed, in the cases of thirst, hunger, and pain, each compelling primal emotions, the pattern of cerebellar, as well as cortical and subcortical activations, is similar to that for hypercapnia and air hunger. Thus, there is considerable similarity among regions of cerebellar involvement across hunger (lingula and quadrangular lobules), thirst (quadrangular, lingula, tonsil, biventer lobules), and hypercapnia and air hunger (quadrangular, lingula, tonsil, biventer lobules).
There were also strong deactivations in cerebellum and other brain areas. The relationship between activated and deactivated areas outside the cerebellum suggests mutually inhibitory relations between areas representing compelling primal emotions and areas subserving more general cognitive functions (23, 46). In the cerebellum, there were strong deactivations for the combined effects of hypercapnia and air hunger distributed bilaterally in the posterior hemispheres (which rely on the dentate nucleus for their output). Outside the cerebellum, there were strong deactivations in dorsal cingulate and prefrontal cortex. The dentate, the sole output nucleus for the posterior cerebellar hemispheres, projects to prefrontal cortex areas (33, 47). There appears to be considerable input from anterior cingulate (as well prefrontal, parastriatal, and parietal regions) to zona incerta (33), which in turn projects to inferior olive, the primary source of climbing fiber input to the cerebellum. The possible connectivity between deactivated regions in cortex and cerebellum here may suggest that the regions interact as a part of a larger mutually inhibitory network (48, 49).
Similar cerebellar regions were deactivated with air hunger. In this case, outside the cerebellum, deactivations were observed in inferior frontal gyrus, thalamus, medial frontal gyrus, lentiform nucleus, caudate, inferior parietal lobule, precentral gyrus, and insula. There are indications of connectivity between cerebellum and thalamus, parietal cortex, frontal cortex, and prefrontal cortex (33, 47), consistent with a supporting role for the cerebellum in a larger mutually inhibitory network.
It is important to note that during the scanning periods there was no overt motor activity, apart from spontaneous eye and respiratory movements. Indeed, there was no detected activity in neocortical motor or premotor areas in the frontal lobe in any of our task vs. intermediate control analyses. With the qualification that the vertex of the brain was not in the PET field of view, the absence of activity in motor areas is consistent with an absence of covert or preparatory motor activity (50). There were motor activations during PB minus rest in the M1 mouth region and premotor cortex and they were exactly matched by relative deactivations when the CO2 FM was contrasted with PB. These indications are consistent with the view that it is not neural output to the muscles of breathing that is relevant to the genesis of air hunger but rather an intact brainstem respiratory oscillator that responds to stimulation (37).
Thus, neocortical sensorimotor processing does not appear to be involved in responses to hypercapnia and air hunger conditions. So, the present cerebellar responses do not appear to be related to the processing of cortically mediated motoric information. Indeed, an analysis of the covariation in activity between significant cerebellar foci and other brain areas revealed no significant associations between activity in cerebellum and cerebral cortical motor systems during hypercapnia and air hunger. The cerebellum here appears to be involved in the processing of other kinds of data.
Our data (22, 23) showed that the distributed pattern of activations and deactivations that occurred with hypercapnia and air hunger was strongest in phylogenetically ancient areas of cortex, along with thalamus, amygdala, and midbrain. In conjunction with the present cerebellar data, our findings confirm that the vegetative function represented by hypercapnia and the primal emotion of air hunger is achieved via a distributed pattern of activations and deactivations with functional changes occurring primarily in phylogenetically ancient brain areas. These observations are consistent with the view that consciousness emerged phylogenetically with interoceptive initiated brain events, rather than distance (external scene) receptive events (23, 46).
It is most important to emphasize that our data do not determine the specific function performed by the cerebellum during CO2 stimulation and air hunger. Because numerous distinct regions of the cerebellum are active, it seems likely that there are different kinds of information being processed. The following hypotheses may provide a framework for interpreting and investigating these effects further.
One hypothesis is that the cerebellum is active here because air hunger is deeply, intrinsically associated with the intention to gain access to air, an intention closely related to expectations or plans for action (apart from adjusting respiratory rate). The expectation or plan for action would elicit implicit or preparatory motor activity, in turn causing activation of the neural systems supporting motor behavior (50). At that point, according to classic theories of cerebellar function, the cerebellum would be involved in its motor control capacity (2–4). Another form of preparation for movement, which is known to involve cerebellum, would be more related to sensory activity, such as preparing the muscles spindles to provide feedback during movement. Although the urge to action is certainly a sensible reaction to air hunger under typical ecological circumstances, in the present study there were no activations consistent with motor planning (22, 23, 50). Nonetheless, it remains possible that there are cerebellar processes involved in constructing abstract models of implicit intentions, incorporating environmental and somatic variables, and that these models are reflected in the pattern of cerebellar activations observed here (51).
One related hypothesis is that cerebellum forms an internal model predicting consequences of operations of control systems acting on motor, autonomic, emotional, or cognitive information involved in hypercapnia and air hunger (52). Thus, for example, such cerebellar models could conceivably support predictions about the consequences of CO2 inhalation and thereby provide internal signals to the entire respiratory system and its attendant behavioral systems.
A second, related hypothesis is that some of the more ancient regions of cerebellum, in particular, those near the midline in the anterior hemisphere, are involved in modulating emotional responses (18, 53, 54). This hypothesis is consistent with observed activations in these regions in PET studies of recall-based fear and anger (53) and with reports that damage to those regions is associated with dysfunctions of affect (18).
A different hypothesis is that some of the cerebellar regions activated here are monitoring the quality of sensory acquisition in the VRG (CO2 concentration) to ensure high-quality sensory data acquisition, whereas other cerebellar regions activated here, with which the former areas communicate, influence the VRG to adjust respiratory rate to optimize PCO2. A related hypothesis has been advanced to account for odor concentration-dependent cerebellar activations observed in functional MRI studies of olfactory tasks (12). It was hypothesized that odor concentration-dependent cerebellar activations in posterior inferior semilunar lobule (Crus II) provide accurate, rapid feedback monitoring of the concentration of an odor, whereas sniff volume-dependent anterior superior semilunar lobule (Crus I) influences motor output to adjust sniff volume to optimize the odor concentration for sensory recognition.
This process of monitoring sensory input and influencing motor structures to finely adjust motor behavior in service of sensory acquisition is also an established function of regions of cerebellum to minimize retinal slip via the vestibular-ocular reflex (55). Related interpretations fit activity in dentate nuclei for cutaneous and tactile information processing under passive sensory and activation discrimination tasks (14, 56, 57).
In fact, it has been suggested on the basis of a variety of considerations (5, 56)** that the cerebellum performs such sensory acquisition and control functions across a wide range of sensory modalities. If so, then in the face of acute air hunger and the attendant emotions, the nervous system likely has an urgent need to collect high-quality sensory data. In this state, the brain is likely to be extremely vigilant, closely monitoring all of its sensory systems. If so, then some of the cerebellar activations here would not be directly related to acute changes in respiratory state but to indirect consequences of this urgent state. A related interpretation fits the pattern of cerebellar activity for thirst, hunger, and their respective satiation. An evaluation of this proposal requires precise mapping and meta-analysis of cerebellar activity across many sense modalities and mental states.
In conclusion, although our data clearly establish some involvement of the cerebellum in hypercapnia and air hunger, further research is necessary to clarify the exact nature and function of that involvement.
Acknowledgments
We greatly appreciate the very helpful comments of James Bower, Masao Ito, and Jeremy Schmahmann. This work has been supported by the National Health and Medical Research Council of Australia, the Howard Florey Biomedical Foundation of the United States, the Robert J., Jr., and Helen C. Kleberg Foundation, the Harold G. and Leila Y. Mathers Charitable Foundation, and Grant NS37109–01A1 (to L.M.P.) from the National Institutes of Health.
Abbreviations
- PET
positron emission tomography
- rCBF
regional cerebral blood flow
- VRG
ventral respiratory group
- PCO2,
partial pressure CO2
- FM
facemask
- PB
paced breathing
- MP
mouthpiece
Footnotes
Walker, M. S., Bower, J. M. & Parsons, L. M. (2000) Soc. Neurosci. Abstr., 20.4.
Parsons, L. M., Schmahmann, J. D., Grill, S., Walker, M. S. & Bower, J. M. (2000) Soc. Neurosci. Abstr., 750.2.
References
- 1.Holmes G. Brain. 1939;62:1–30. [Google Scholar]
- 2.Thach W T, Goodkin H P, Keating J G. Annu Rev Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- 3.Houk J C, Wise S P. Cereb Cortex. 1995;5:95–110. doi: 10.1093/cercor/5.2.95. [DOI] [PubMed] [Google Scholar]
- 4.Welsh J P, Lang E J, Sugihara I, Llinas R. Nature (London) 1995;374:453–457. doi: 10.1038/374453a0. [DOI] [PubMed] [Google Scholar]
- 5.Bower J M. In: Progress in Brain Research. de Zeeuw C I, Strata P, Voogd J, editors. New York: Elsevier; 1997. pp. 483–516. [Google Scholar]
- 6.Ivry R. Hum Brain Mapp. 2000;9:115–118. doi: 10.1002/(SICI)1097-0193(200003)9:3<115::AID-HBM1>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmahmann J D, editor. The Cerebellum and Cognition. New York: Academic; 1997. [Google Scholar]
- 8.Desmond J, Fiez J. Trends Cogn Sci. 1998;2:355–362. doi: 10.1016/s1364-6613(98)01211-x. [DOI] [PubMed] [Google Scholar]
- 9.Thier P, Haarmeier T, Treue S, Barash S. Brain. 1999;122:2133–2146. doi: 10.1093/brain/122.11.2133. [DOI] [PubMed] [Google Scholar]
- 10.Allen G, Buxton R B, Wong E C, Courchesne E. Science. 1997;275:1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- 11.Ivry R B, Keele S W. J Cognit Neurosci. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- 12.Sobel N, Prabhakaran V, Hartley C A, Desmond J E, Zhao Z, Glover G H, Gabrieli J D, Sullivan E V. J Neurosci. 1998;18:8990–9001. doi: 10.1523/JNEUROSCI.18-21-08990.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tesche C D, Karhu J. Hum Brain Mapp. 2000;9:119–142. doi: 10.1002/(SICI)1097-0193(200003)9:3<119::AID-HBM2>3.0.CO;2-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao J-H, Parsons L M, Bower J M, Xiong J, Li J, Fox P T. Science. 1996;272:545–547. doi: 10.1126/science.272.5261.545. [DOI] [PubMed] [Google Scholar]
- 15.Parsons L M, Fox P T. In: The Cerebellum and Cognition. Schmahmann J D, editor. New York: Academic; 1997. pp. 255–271. [Google Scholar]
- 16.Parsons L M, Denton D, Egan G, McKinley M, Shade R, Lancaster J, Fox P T. Proc Natl Acad Sci USA. 2000;97:2332–2336. doi: 10.1073/pnas.040555497. . (First Published February 25, 2000, 10.1073/pnas.040555497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tataranni P A, Gautier J-F, Chen K, Vecker A, Bandy D, Salbe A D, Pratley R E, Lawson M, Reiman E M, Ravelssin E. Proc Natl Acad Sci USA. 1999;96:4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmahmann J D, Sherman J C. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 19.Parsons L M. In: Biological Foundations of Music. Peretz I, Zatorre R J, editors. New York: New York Acad. Sci.; 2001. , in press. [Google Scholar]
- 20.Corfield DR, Fink G R, Ramsay S C, Murphy K, Harty H R, Watson J D G, Adams L, Frackowiak R S J, Guz A. J Physiol. 1995;488:77–84. doi: 10.1113/jphysiol.1995.sp020947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gozal D, Hathout G M, Kirlew K A, Tang H, Woo M S, Zhang J, Lufkin R B, Harper R M. J Appl Physiol. 1994;76:2076–2083. doi: 10.1152/jappl.1994.76.5.2076. [DOI] [PubMed] [Google Scholar]
- 22.Brannan S, Liotti M, Egan G, Shade R, Madden L, Robillard R, Abplanalp B, Stofer K, Denton D, Fox P T. Proc Natl Acad Sci USA. 2001;98:2029–2034. doi: 10.1073/pnas.98.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liotti M, Brannan S, Egan G, Shade R, Madden L, Abplanap B, Robillard R, Lancaster J, Zamarippa F, Fox P T, Denton D. Proc Natl Acad Sci USA. 2001;98:2035–2040. doi: 10.1073/pnas.98.4.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaytan S P, Pasaro R. Brain Res Bull. 1999;47:625–642. doi: 10.1016/s0361-9230(98)00125-7. [DOI] [PubMed] [Google Scholar]
- 25.Bianchi A L, Denavit-Saugie M, Champagnat J. J Physiol Rev. 1995;75:1–10. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Ellenberger H H, Feldman J L. J Comp Neurol. 1990;294:202–211. doi: 10.1002/cne.902940205. [DOI] [PubMed] [Google Scholar]
- 27.Ezure K, Manabe M, Yamada H. Brain Res. 1988;455:262–270. doi: 10.1016/0006-8993(88)90085-6. [DOI] [PubMed] [Google Scholar]
- 28.Saether K, Hilaire G, Monteau R. Brain Res. 1987;419:87–96. doi: 10.1016/0006-8993(87)90571-3. [DOI] [PubMed] [Google Scholar]
- 29.Schwarzacher S W, Wilhelm Z, Anders K, Richter D W. J Physiol (London) 1991;435:631–611. doi: 10.1113/jphysiol.1991.sp018529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng Y, Barillot J C, Bianchi A L. Brain Res. 1991;546:261–270. doi: 10.1016/0006-8993(91)91490-r. [DOI] [PubMed] [Google Scholar]
- 31.Haines D E, Dietrichs E. J Comp Neurol. 1984;229:559–575. doi: 10.1002/cne.902290409. [DOI] [PubMed] [Google Scholar]
- 32.Haines D E, Dietrichs E, Mihailoff G A, McDonald E F. In: The Cerebellum and Cognition. Schmahmann J D, editor. San Diego: Academic; 1997. pp. 84–109. [Google Scholar]
- 33.Schmahmann J D, Pandya D N. In: The Cerebellum and Cognition. Schmahmann J D, editor. New York: Academic; 1997. pp. 31–60. [Google Scholar]
- 34.Bangma G C, Donkelaar H J. J Comp Neurol. 1982;207:255–273. doi: 10.1002/cne.902070306. [DOI] [PubMed] [Google Scholar]
- 35.Kunzle H. Exp Brain Res. 1983;49:1–12. doi: 10.1007/BF00235536. [DOI] [PubMed] [Google Scholar]
- 36.Xu F, Frazier D T. Brain Res. 1995;705:53–64. doi: 10.1016/0006-8993(95)01138-2. [DOI] [PubMed] [Google Scholar]
- 37.Guz A. Resp Physiol. 1997;109:197–203. doi: 10.1016/s0034-5687(97)00050-9. [DOI] [PubMed] [Google Scholar]
- 38.Gandevia S C, Killean K, McKenzie D R, Crawford M, Allen G M, Gorman R B, Hales J P. J Physiol (London) 1993;470:85–107. doi: 10.1113/jphysiol.1993.sp019849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banzett R B, Lansing R W, Evans C E, Shea S A. Respir Physiol. 1996;103:19–31. doi: 10.1016/0034-5687(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 40.Heywood P, Murphy K, Corfield D R, Morell M J, Howard R S, Guz A. Respir Physiol. 1996;106:13–20. doi: 10.1016/0034-5687(96)00060-6. [DOI] [PubMed] [Google Scholar]
- 41.Schmahmann J D, Doyon J A, McDonald D, Holmes C, Lavoie K, Hurwitz A, Kabani N, Toga A, Evans A, Petrides M. Neuroimage. 1999;10:233–260. doi: 10.1006/nimg.1999.0459. [DOI] [PubMed] [Google Scholar]
- 42.Larsell O. In: The Comparative Anatomy and Histology of the Cerebellum from Monotremes Through Apes. Jansen J, editor. Minneapolis: Univ. Minnesota Press; 1970. [Google Scholar]
- 43.Ghelarducci B, Sebastini L. J Auton Nerv Syst. 1996;56:149–156. doi: 10.1016/0165-1838(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 44.Nishimaru N, Katayama S. Neurosci Res. 1995;21:343–350. doi: 10.1016/0168-0102(94)00872-d. [DOI] [PubMed] [Google Scholar]
- 45.Rubia F J, Phelps J B. Pflügers Arch. 1970;314:68–85. doi: 10.1007/BF00587047. [DOI] [PubMed] [Google Scholar]
- 46.Denton D A, Shade R, Zamarippa F, Egan G, Blair-West J, McKinley M, Fox P. Proc Natl Acad Sc USA. 1999;96:2532–2537. doi: 10.1073/pnas.96.5.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Middleton F A, Strick P L. Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 48.Mayberg H S, Liotti M, Brannan S K, McGinnis S, Mahurin R K, Jerabek P A, Silva J A, Tekel J L, Martin C C, Lancaster J L, Fox P T. Am J Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- 49.Liotti M, Mayberg H S, Brannan S K, McGinnis S, Jerabek P, Fox P T. Biol Psychiatry. 2000;48:30–42. doi: 10.1016/s0006-3223(00)00874-x. [DOI] [PubMed] [Google Scholar]
- 50.Parsons L M, Fox P T. Cognit Neuropsychol. 1998;15:583–615. [Google Scholar]
- 51.Ito M. The Cerebellum and Neural Control. New York: Appleton-Century-Crofts; 1984. [Google Scholar]
- 52.Ito M. Nature (London) 2000;403:153–154. doi: 10.1038/35003097. [DOI] [PubMed] [Google Scholar]
- 53.Damasio A R, Grabowski T J, Bechara A, Damasio H, Ponto L L B, Parvizi J, Hichwa R D. Nat Neurosci. 2000;3:1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- 54.Dolan R J. Brain. 1998;121:545–546. doi: 10.1093/brain/121.4.545. [DOI] [PubMed] [Google Scholar]
- 55.Lisberger S G, Sejnowski T J. Nature (London) 1992;360:159–161. doi: 10.1038/360159a0. [DOI] [PubMed] [Google Scholar]
- 56.Parsons L M, Bower J M, Gao J H, Xiong J, Li J, Fox P T. Learn Mem. 1997;4:49–62. doi: 10.1101/lm.4.1.49. [DOI] [PubMed] [Google Scholar]
- 57.Liu Y, Pu Y, Gao J, Parsons L M, Xiong J, Liotti M, Bower J M, Fox P T. Hum Brain Mapp. 2000;10:147–159. doi: 10.1002/1097-0193(200008)10:4<147::AID-HBM10>3.0.CO;2-U. [DOI] [PMC free article] [PubMed] [Google Scholar]