Abstract
A serine/threonine (S/T) kinase encoded by the US3 gene of herpes simplex virus type 1 (HSV-1) is conserved in varicella-zoster virus (VZV) and pseudorabies virus (PRV). Expression of US3 kinase in cells transformed with US3 expression plasmids or infected with each virus results in hyperphosphorylation of histone deacetylase 2 (HDAC2). Mapping studies revealed that each US3 kinase phosphorylates HDAC2 at the same unique conserved Ser residue in its C terminus. HDAC2 was also hyperphosphorylated in cells infected with PRV lacking US3 kinase, indicating that hyperphosphorylation of HDAC2 by PRV occurs in a US3-independent manner. Specific chemical inhibition of class I HDAC activity increases the plaquing efficiency of VZV and PRV lacking US3 or its enzymatic activity, whereas only minimal effects are observed with wild-type viruses, suggesting that VZV and PRV US3 kinase activities target HDACs to reduce viral genome silencing and allow efficient viral replication. However, no effect was observed for wild-type or US3 null HSV-1. Thus, we have demonstrated that while HDAC2 is a conserved target of alphaherpesvirus US3 kinases, the functional significance of these events is virus specific.
The serine/threonine (S/T) kinase encoded by the US3 gene is conserved among all known alphaherpesviruses, including the human and animal pathogens varicella-zoster virus (VZV), herpes simplex virus type 1 (HSV-1) and HSV-2, bovine herpesvirus type 1 (BHV-1), equine herpesvirus type 1 (EHV-1), and pseudorabies virus (PRV) (6, 33, 37). Genetic disruption of US3 in VZV, HSV-1, HSV-2, PRV, and Marek's disease virus (MDV) (52) revealed that US3 protein is not essential for viral replication in tissue culture; however, US3 mutants are impaired for growth in certain cell types and have significantly impaired viral replication and virulence in animal models (1, 10, 14, 28, 38, 47-51, 55, 58, 61).
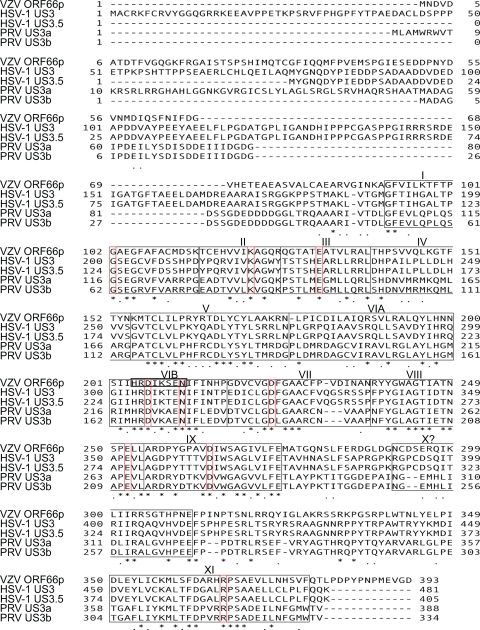
The HSV-1 and PRV US3 genes each encode two transcripts, and each transcript produces different proteins in the same reading frame, but utilizing alternative ATGs. For HSV-1, the long and short transcripts produce US3 and US3.5, respectively (Fig. 1) (44) whereas in PRV, the longer transcript produces US3a and the shorter transcript produces US3b (Fig. 1) (60). The shorter US3 proteins all display kinase activity like their longer counterparts; however, compared to the longer forms, the truncated HSV-1 and PRV kinases differ with respect to the range of functions they exhibit (3, 29, 36, 41, 44). VZV US3 kinase (ORF66p) is encoded by open reading frame 66 (ORF66), and no alternative truncated forms have been identified (Fig. 1). Alignment of US3 homologs demonstrates a low level of amino acid identity between the proteins, with the highest level of identity located within the catalytic domain, which is comprised of 12 subdomains conserved within the eukaryotic protein kinase superfamily (Fig. 1) (25). All US3 kinases contain a high ratio of acidic residues within their amino termini, whose precise role remains unclear.
FIG. 1.
Alignment of US3 kinases. The amino acid sequences of VZV ORF66p, HSV-1 US3 and US3.5, PRV US3a, and US3b were aligned, and positions marked with asterisks identify identical amino acids conserved in all species. Positions marked with dots identify similarities. A Kalign (http://www.ebi.ac.uk/Tools/kalign/index.html) alignment was performed between VZV ORF66p and the cellular kinases PKA and CDK2 to identify 12 kinase subdomains (VIA and VIB are independent domains) conserved within the eukaryotic protein kinase superfamily (25). Vertical red boxes identify invariant residues conserved in all eukaryotic kinases. The black horizontal box identifies the known catalytic loop region of VZV ORF66p.
In vitro biochemical studies of PRV US3 kinase identified a consensus target sequence as RnX(S/T)YY, where n is greater than or equal to 2; X can be Arg, Ala, Val, Pro, or Ser; and Y can be any amino acid except an acidic residue (31, 32, 46). The target site specificities of US3 homologs encoded by VZV, HSV-1, and HSV-2 appear to be broadly similar to that of PRV US3 (8, 11, 46). US3 kinases play roles in many diverse functions, including protection of cells from virus-induced apoptosis, alteration of the host cytoskeleton, egress of nucleocapsids from the nucleus, regulation of viral gene expression, major histocompatibility complex class I (MHC-I) surface presentation, interferon (IFN) signaling, and modulation of host and viral protein localization (reviewed in reference 13). To date, few targets of the kinases as a group have been identified, and not all functions of US3 appear to be shared with each homolog. Thus, there is considerable interest in which functions are virus specific and which may be shared for different alphaherpesviruses.
Histone deacetylases (HDACs) are an ancient family of enzymes that deacetylate lysine residues on histone tails, resulting in chromatin condensation and repression of gene expression (20). Eleven HDAC isoforms have been identified in mammalian genomes, and these are classified into four different families: class I (HDAC1, -2, -3, and -8), class II, (HDAC4, -5, -6, -7, -9, and -10), and sirtuin class III and class IV (HDAC11) (24, 54). The HDACs have diverse roles in many cellular functions, and as a result their activities are regulated tightly by an array of mechanisms, including posttranslational modification. Phosphorylation of HDACs is the most extensively studied modification, and reports have shown that enzymatic activity, cellular localization, and protein-protein interactions of HDACs are regulated in this manner (reviewed in reference 18). HDAC1 and HDAC2 are hyperphosphorylated during HSV-1, HSV-2, and VZV infection in a US3 kinase-dependent manner (26, 39, 43, 61). In addition, autonomous expression of HSV-1 US3 and US3.5 resulted in enhanced expression of viral or cellular genes, similar to what occurs in cells treated with sodium butyrate, a pan-tropic HDAC inhibitor (43, 45). Treatment of cells with sodium butyrate also partially complements the growth defect associated with a VZV mutant lacking ORF66p kinase activity (61). These studies suggest a mechanism whereby US3 kinase-mediated hyperphosphorylation of HDAC1 and HDAC2 inhibits their repressive activity to allow efficient gene expression and viral replication.
VZV ORF66p phosphorylates HDAC1 and HDAC2 at a conserved Ser residue within the C terminus of each protein, and this occurs by an indirect mechanism (61). Even though HDAC1 and HDAC2 are modified after expression of HSV-1 and HSV-2 US3 kinases (26, 39, 43), it is unknown if nonhuman alphaherpesviruses also target HDAC1 and -2 or if the site of VZV ORF66p-mediated phosphorylation is a conserved target of all US3 homologs.
The aim of this study was to further characterize phosphorylation of HDACs by US3 kinases from VZV, HSV-1, and PRV. We demonstrate that HDAC2 is hyperphosphorylated during VZV, HSV-1, and PRV infections by each alphaherpesvirus US3 kinase at a conserved Ser residue within the C termini of both proteins. Surprisingly, HDAC2 was also hyperphosphorylated in cells infected with mutant PRV lacking US3 kinase and pretreatment of cells with pan- or class-specific HDAC inhibitors greatly increases plaquing efficiency of VZV and PRV lacking US3 or its enzymatic activity, whereas minimal effects are observed with wild-type (WT) viruses. These data suggest that a viral growth-related function of VZV and PRV US3 kinases is to inhibit HDAC-mediated viral genome silencing and allow efficient viral replication. Interestingly, no effect was observed for wild-type or US3 null HSV-1, suggesting that while HDAC2 is a conserved target of alphaherpesvirus US3 kinases, the functional significance of these events is virus and also likely host cell type specific.
MATERIALS AND METHODS
Mammalian cells.
Human melanoma cells (MeWo), green monkey kidney cells (Vero), and 293A cells were maintained as described previously (53, 62). At 24 h prior to transformation or infection, cells were seeded into culture dishes.
Transformation of cells.
293A cells were transformed with DNA using Fugene HD (Roche, Indianapolis, IN).
Viruses. (i) VZV.
Wild-type (WT) and ORF66p kinase-deficient (ORF66KD) VZV were described previously (12, 14). Cell-associated and cell-free virus stocks were prepared and titrated as previously described (30).
(ii) HSV-1.
Wild-type HSV-1 (Glasgow strain 17), used for Fig. 2, was described previously (56). Recombinant wild-type (containing US3 with an N-terminal green fluorescent protein [GFP] tag) and US3-deleted HSV-1 were generated in an HSV-1 RE strain background. In brief, a recombinant with US3 GFP tagged (WT) or deleted with concurrent GFP insertion (US3 null) in the HSV-1 RE background was derived by amplifying the HSV-1 RE genomic template using the GC-Rich PCR proofreading system (Roche, Indianapolis, IN) under hot-start conditions and primer US3F (5′-GGGAATTCATGGCCTGTCGTAAGTTTTGTCGCGTTTAC-3′) or US3.5F (5′-GGGAATTCATGTACGGAAACCAGGACTAC-3′), each in conjunction with US3R (5′-GGAAGATCTTCATTTCTGTTGAAACAGCGGCAA-3′). The PCR products were digested with BglII and EcoRI and ligated to BamHI/EcoRI-digested pEGFP-C (downstream from and in frame with enhanced GFP [EGFP]). These represent cytomegalovirus (CMV) immediate-early (IE) promoter-driven US3 (pEGFP-US3) and US3.5 (pEGFP-US3.5). To derive US3 null HSV-1, the US3.5 construct was collapsed with XhoI to remove the N-terminal part of the US3 coding sequence encoding residues 1 to 169. The remainder of the sequence lacks an ATG, is out of frame with respect to EGFP, and does not retain kinase activity. For derivation of recombinant viruses, the CMV IE promoter in both functional and US3 null constructs was replaced with DNA containing the US3 promoter following amplification using primers US3PF (5′-GCGCCCTAGGGCTAGCTCGCCGCACCGTGAGTGCCA-3′) and US3PR (5′-GCCATTAATATTAATGCCGCGAACGGCGATCAGAGGGTCAGT-3′.) This PCR product was digested with AseI and NheI and ligated to XhoI-collapsed constructs (digested with AseI and NheI). The resulting constructs contained EGFP flanked by the US3 promoter and either intact US3 or the distal portion of US3. Viruses were selected from cotransfections of HSV-1 RE viral DNA with linearized plasmid and were identified and plaque purified based on gain of EGFP fluorescence. Insertion of GFP and the integrity or deletions of US3 coding sequences were confirmed by Southern blot analysis of viral DNA.
FIG. 2.
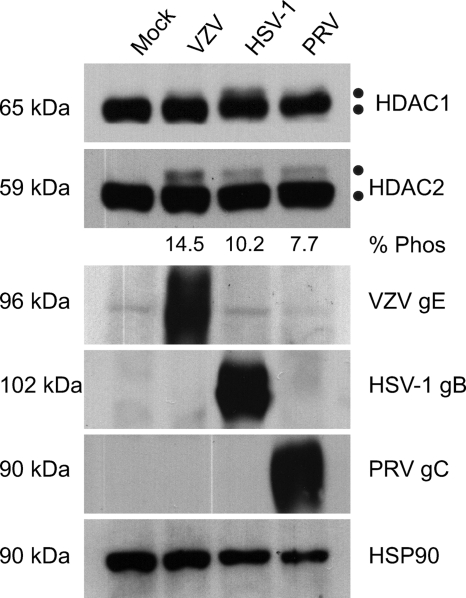
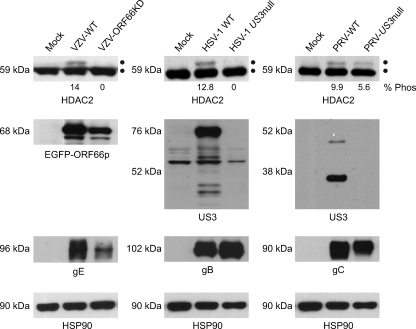
Analysis of HDAC1 and HDAC2 phosphorylation during alphaherpesvirus infection. 293A cells were mock infected or infected with cell-associated wild-type VZV at an MOI of 0.025 or with wild-type HSV-1 and PRV at an MOI of 0.1. Cells were harvested at between 48 and 72 hpi, when all of the cells were infected as determined by phase microscopy and/or ORF66p-GFP expression. Twenty micrograms of total protein from each sample was analyzed by Western blotting using antisera specific for HDAC1, HDAC2, VZV gE, HSV-1 gB, PRV gC, and HSP90. For each virus the percentage of hyperphosphorylated HDAC2 (% Phos) induced during infection relative to the total amount of HDAC2 was quantified using ImageJ software. Because of extremely low levels of HDAC1 hyperphosphorylation and our inability to clearly separate the two species, we were unable to make similar calculations for HDAC1.
(iii) PRV.
Wild-type PRV (Becker strain), used for Fig. 2, was described previously (53). A US3 null PRV (PRV645) was constructed by cotransfecting purified PRV DNA (Kaplan strain; kindly provided by T. C. Mettenleiter, Federal Centre for Virus Diseases of Animals, Insel Riems, Germany) and SpeI-linearized plasmid pML7 (36) into PK15 cells. Virus produced after cotransfection was plated on PK15 cells, and plaques expressing EGFP were identified using a Nikon TE200 inverted epifluorescence microscope. Virus was isolated from EGFP-expressing plaques and subjected to three rounds of purification. Southern blot analysis was performed on PRV645 genomic DNAs to verify that the appropriate recombination events had occurred. US3 protein expression was examined in PRV645- and wild-type PRV Kaplan strain (WT PRV)-infected cell lysates by Western blotting to confirm the absence or presence of US3 expression, respectively.
Plasmid construction. (i) EGFP plasmids.
pEGFP-C1 was purchased from Clontech Laboratories, Inc. (Mountain View, CA). pEGFP-ORF66, encoding VZV ORF66p, was previously described (12). pEGFP-US3 and pEGFP-US3.5, encoding HSV-1 US3 kinases, were generated as described above. Plasmids expressing PRV US3 long (US3a) and short (US3b) kinases with N-terminal GFP tags were generated as follows. Sequences encoding US3a and US3b were PCR amplified using the GC-Rich PCR proofreading system (Roche, Indianapolis, IN) under hot-start conditions and primer PUS3LF1 (5′-GCGGAATTCAACATGTTGGCGATGTGGAGATGC-3′) or PUS3SF1 (5′-GCGGAATTCAACATGGCCGACGCCGGAATCCC-3′) and PUS3end (5′-GAGAGATCTTTATACGGTCCACATTTC-3′). PCR products were digested with EcoRI and BglII and cloned in frame into pEGFP-C to generate pEGFP-US3a and pEGFP-US3b, respectively.
(ii) HDAC plasmids.
pFLAG-HDAC2 and pFLAG-S407A were previously described (61).
Dephosphorylation assay.
Lysates from Vero cells mock infected or infected with PRV (WT and US3 null) and HSV-1 (WT and US3 null) were subjected to dephosphorylation with calf intestinal alkaline phosphatase (CIAP) as previously described (61).
SDS-PAGE and Western blotting.
Infected and transformed cells were processed for SDS-PAGE and Western blot analysis as previously described (62) using radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris HCl [pH 8.0], 150 mM NaCl, 0.5% NP-40, 50 mM NaF) plus Complete protease inhibitor cocktail (Roche, Mannheim, Germany) and Halt phosphatase inhibitor cocktail (Pierce, Rockford, IL). Unless otherwise stated, all samples were electrophoretically separated on 7.5% polyacrylamide denaturing gels.
Drug treatment.
Sodium butyrate (C4H7O2Na) and histone deacetylase inhibitor VII, 106 (C20H25N3O2) were purchased from EMD Biosciences (La Jolla, CA) and used as indicated in the figure legends. The drugs were reconstituted in water and dimethyl sulfoxide (DMSO), respectively.
Antibodies.
All primary antibodies for Western blot analysis were used at dilutions of between 1:1,000 and 1:5,000. Mouse monoclonal antibody against VZV gE was described previously (35). Rabbit polyclonal antibody against HSV-1 US3 was a kind gift from Bernard Roizman, University of Chicago, Chicago, IL. Rabbit polyclonal antibody against HSV-1 gB was a gift from G. Cohen, University of Pennsylvania, Philadelphia, PA. Mouse monoclonal antibody against PRV US3 and goat polyclonal antibody against gC were gifts from Lynn Enquist, Princeton University, Princeton, NJ. Mouse monoclonal anti-FLAG M2 antibody and mouse monoclonal actin antibody were purchased from Sigma (St. Louis, MO). Mouse monoclonal antibody to HSP90, rabbit polyclonal antibody to HDAC1, and rabbit polyclonal HDAC2 antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibody to GFP was purchased from Clontech Laboratories, Inc. (Mountain View, CA). Goat anti-rabbit, goat anti-mouse, and rabbit anti-goat antibodies conjugated to horseradish peroxidase for immunoblotting were purchased from KPL (Gaithersburg, MD).
RESULTS
HDAC2 is hyperphosphorylated in alphaherpesvirus-infected cells.
HDACs are important regulators of chromatin activity and as such are targeted during viral infection to block host gene-silencing activities. HDAC activity is controlled by posttranslational modifications (9, 18, 40, 57), and studies with HSV-1, HSV-2, and VZV showed that HDAC1 and HDAC2 are hyperphosphorylated during virus infection (26, 39, 43, 61). To determine if phosphorylation of HDAC1 and -2 is conserved after infection with a nonhuman alphaherpesvirus, 293A cells were either mock infected or infected with wild-type VZV, HSV-1, or PRV. Because of the inherently low titers of cell-free VZV, all infections were done at low multiplicities of infection (MOI) and cells were harvested at between 48 and 72 h postinfection (hpi), when all cells were infected. The electrophoretic mobilities of HDAC1 and HDAC2 were examined by Western blotting (Fig. 2). Blots were probed for VZV gE, HSV-1 gB, or PRV gC as an indicator of infection and for HSP90 as a loading control. Two closely migrating HDAC2 species of approximately 59 kDa and 62 kDa were detected, with the larger species present only in virus-infected cells. Based on previous studies, the slower-migrating form of HDAC2 is virus induced and hyperphosphorylated (43, 61). The level of HDAC2 phosphorylated in response to virus infection is presented below the gel as percentage of total HDAC2. In these analyses we also monitored for changes in HDAC1; however, hyperphosphorylation of HDAC1 is not as vigorous and the modified species is as not as easily separated from its normal counterpart as for HDAC2. Accordingly, this study addresses changes only in HDAC2. The novel finding that HDACs are hyperphosphorylated during PRV infection demonstrates that they are targeted specifically during alphaherpesvirus infection by both human and nonhuman viruses.
Autonomous expression of alphaherpesvirus US3 kinases induces HDAC2 hyperphosphorylation.
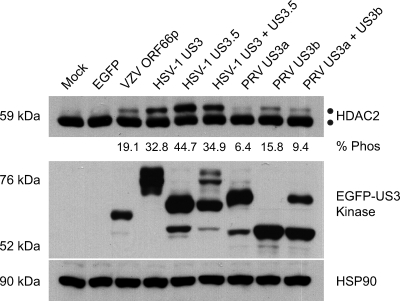
The S/T kinase activity encoded by alphaherpesvirus US3 genes is essential for hyperphosphorylation of HDAC1 and HDAC2 during HSV-1, HSV-2, and VZV infection (26, 39, 43, 61). In addition, autonomous expression of HSV-1 and VZV US3 kinases is sufficient to induce modification of HDAC1 and HDAC2 (45, 61). To investigate if expression of the PRV US3 kinases induces hyperphosphorylation of HDAC2, each PRV kinase was cloned as an amino-terminally tagged EGFP fusion protein (pEGFP-US3a and pEGFP-US3b). As positive controls, HSV-1 and VZV kinases were also cloned as amino-tagged EGFP fusion proteins (pEGFP-US3, pEGFP-US3.5, and pEGFP-ORF66, respectively). 293A cells were either mock transformed or transformed with a plasmid expressing EGFP alone (pEGFP-C1) or an EGFP-tagged US3 kinase. At 24 h posttransformation, cells were harvested and lysed and the electrophoretic mobility of endogenous HDAC2 examined (Fig. 3). EGFP and HSP90 were analyzed to confirm expression of each US3 kinase and equal loading of protein for each sample. Analysis of HDAC2 from US3 kinase-expressing cells revealed a novel higher-molecular-weight species indicative of hyperphosphorylation. This novel species was absent from cells mock transformed or expressing EGFP alone. We repeatedly found that the HDAC2 was hyperphosphorylated to the largest extent in response to expression of HSV-1 US3 kinases. In contrast, the PRV kinases were less efficient in inducing hyperphosphorylation of HDAC2. VZV ORF66p had an intermediate phenotype. Interestingly, coexpression of either of the two forms of HSV-1 and PRV US3 kinases did not significantly alter the level of HDAC hyperphosphorylation, suggesting that their effects are not additive. These data confirm that expression of VZV ORF66p (61) and both forms of HSV-1 US3 (45) results in modification of HDAC2 (45) and demonstrate for the first time that both forms of PRV US3 kinases do the same.
FIG. 3.
Analysis of HDAC2 hyperphosphorylation in cells expressing US3 kinases. 293A cells were mock transformed or transformed with 2 μg of pEGFP-C1, pEGFP-ORF66, pEGFP-US3, pEGFP-US3.5, pEGFP-US3a, or pEGFP-US3b. When the kinases were expressed in tandem, 1 μg of each plasmid was transformed. After 24 h, cells were harvested and 20 μg of total protein from each sample was analyzed by Western blotting using antisera specific for HDAC2, GFP, and HSP90. For each US3 kinase the percentage of hyperphosphorylated HDAC2 (% Phos) induced by expression relative to the total amount of HDAC2 was quantified using ImageJ software.
Expression of alphaherpesvirus US3 kinases results in hyperphosphorylation of HDAC2 at a conserved phosphorylation site within its C terminus.
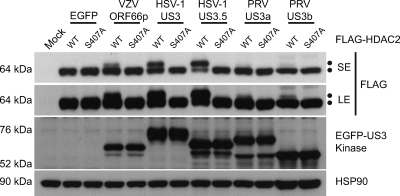
HDAC1 and HDAC2 are highly conserved proteins sharing 85% amino acid identity (19). Cellular proteins posttranslationally modify both HDAC1 and HDAC2 to play a role in regulating their function (9, 18, 40, 57). Using alanine substitution mutagenesis to block phosphorylation at potential target sites, we previously demonstrated that VZV ORF66p targets phosphorylation of FLAG-tagged HDAC1 and HDAC2 at Ser 406 and Ser 407, respectively, by an indirect mechanism (61). Therefore, we next asked if other alphaherpesvirus US3 kinases target this conserved site to phosphorylate HDAC2. 293A cells were cotransformed with pEGFP-C1 or a plasmid expressing an EGFP-tagged US3 kinase in conjunction with pFLAG-HDAC2 or pFLAG-S407A. After 24 h, cells were harvested and FLAG-HDAC2 electrophoretic mobility was examined (Fig. 4). In cells cotransformed with EGFP and wild-type or mutant FLAG-HDAC2, only a 64-kDa protein species was detected. When coexpressed with ORF66p, a 66-kDa band suggestive of a hyperphosphorylated species was detected. As expected, this species failed to accumulate in cells cotransformed with HDAC2 S407A, demonstrating that Ser 407 was the residue targeted in cells expressing ORF66p. Coexpression of HSV-1 US3 kinases with wild-type and mutant FLAG-HDAC2 produced results identical to that observed with VZV. For PRV we were able to detect only a very low level of FLAG-HDAC2 modification in the presence of each US3 kinase; however, this failed to accumulate in cells cotransformed with HDAC2 S407A. Therefore, we conclude that HDAC2 Ser 407 is a conserved target for alphaherpesvirus US3-mediated in vivo phosphorylation. Again, the highest level of HDAC2 hyperphosphorylation was observed with HSV-1 US3, VZV was intermediate, and PRV showed the lowest level.
FIG. 4.
Identification of a Ser target in HDAC2 that is phosphorylated in response to US3 kinase expression. To map the US3 kinase target phosphorylation site in HDAC2, 293A cells were mock transformed or cotransformed with 1 μg of either pFLAG-HDAC2 or pFLAG-S407A and 1 μg of pEGFP-C1, pEGFP-ORF66, pEGFP-US3, pEGFP-US3.5, pEGFP-US3a, or pEGFP-US3b. After 24 h, cells were harvested and 20 μg of total protein from each sample was analyzed by Western blotting using antisera specific for FLAG, GFP, and HSP90. SE and LE represent short and long exposures, respectively.
Hyperphosphorylation of HDAC2 during infection with PRV lacking US3.
We next asked if US3 kinase was also essential for hyperphosphorylation of HDAC2 during PRV infection, as seen in HSV-1, HSV-2, and VZV infections (26, 39, 43, 61). For these experiments, US3 deletion (US3 null) mutants of HSV-1 and PRV were constructed. 293A cells were either mock infected or infected with wild-type VZV, ORF66KD VZV, wild-type HSV-1, US3 null HSV-1, wild-type PRV, or US3 null PRV and harvested at between 48 and 72 hpi, when all cells were infected. Cell lysates were prepared, and HDAC2 electrophoretic mobilities were examined by Western blotting (Fig. 5). US3 expression was monitored from each virus, and as before, VZV gE, HSV-1 gB, PRV gC, and HSP90 were examined as surrogates for viral infection and equal loading, respectively. As expected, VZV ORF66p was detected in cells infected with both WT VZV and ORF66KD VZV. For HSV-1 and PRV US3, the kinases were only seen in 293A cells infected with wild-type viruses and not in US3 null HSV-1 or US3 null PRV infections, as each has part of the ORF deleted. Staining for HSV-1 gB and PRV gC demonstrated that both wild-type and US3 null viruses replicated in 293A cells. HDAC2 was hyperphosphorylated in cells infected with wild-type VZV, HSV-1, and PRV. Consistent with previous reports, deletion of US3 or abolishment of kinase activity prevents HDAC hyperphosphorylation in cells infected with HSV-1 and VZV (43, 61). However, for PRV, posttranslationally modified forms of HDAC2, identical in mobility to those appearing after infection with VZV and HSV-1, were detected even in 293A cells infected with US3 null PRV. Infection of Vero cells produced identical results (data not shown). These results indicate that during PRV infection, HDAC2 is posttranslationally modified in an additional US3 kinase-independent manner.
FIG. 5.
Analysis of HDAC2 hyperphosphorylation in cells infected with wild-type and US3 kinase-deficient viruses. 293A cells were mock infected or infected with wild-type (VZV-WT) or ORF66p kinase-deficient (VZV-ORF66KD) VZV at an MOI of 0.025 or with wild-type (HSV-1 WT) or US3-deleted (HSV-1 US3 null) HSV-1 or wild-type (PRV-WT) or US3-deleted (PRV-US3 null) PRV at an MOI of 0.1. Cells were harvested at between 48 and 96 hpi, when all of the cells were infected as determined by phase microscopy and GFP expression. Twenty micrograms of total protein from each sample was analyzed by Western blotting using antisera specific for HDAC2, VZV gE, EGFP, HSV-1 US3, HSV-1 gB, PRV US3, PRV gC, and HSP90. The percentage of hyperphosphorylated HDAC2 (% Phos) that accumulated during infection with each virus, relative to the total amount of HDAC2, was quantified using ImageJ software.
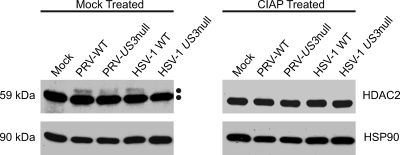
Utilizing dephosphorylation assays, we previously showed that HDAC1 and HDAC2 were hyperphosphorylated during VZV infection (61). To establish that the slower-migrating species seen in PRV-infected cells was indeed a hyperphosphorylated form, we characterized posttranslational modification of HDAC2 after infection with PRV and US3 null PRV. As a positive control, cells were infected with HSV-1 and US3 null HSV-1. At 48 h, infected Vero cells were harvested and lysates were either mock treated or treated with calf intestinal alkaline phosphatase (CIAP) as described in Materials and Methods. These lysates were subjected to Western blot analysis to examine changes in protein mobility (Fig. 6). HSP90 was analyzed to demonstrate equal loading of protein and to act as an indicator of any nonspecific protease activity associated with CIAP. In lysates from mock-infected and US3 null HSV-1-infected cells, HDAC2 migrated as a single species. When lysates from wild-type PRV-, US3 null PRV-, and wild-type HSV-1-infected cells were treated with CIAP, the slower-migrating species of HDAC2 was lost. Therefore, the additional form of PRV- and HSV-1-induced modification of HDAC2 was a result of virus-induced hyperphosphorylation. These data support our supposition that HDAC2 is hyperphosphorylated during PRV infection in a US3-independent manner. Thus, during HSV-1 (and VZV) infections a fraction of HDAC2 is hyperphosphorylated in a US3-dependent manner, and in PRV infections hyperphosphorylation occurs in both US3-dependent and -independent manners. At present it is unknown if the hyperphosphorylated form of HDAC2 induced after infection with US3 null PRV is identical to what is found following expression of PRV US3 kinase activity in transformed cells.
FIG. 6.
Analysis of HDAC2 in infected cell extracts following incubation with calf intestine alkaline phosphatase. Vero cells were mock infected or infected with, wild-type PRV (PRV-WT), US3 null PRV (PRV-US3 null), wild-type HSV-1 (HSV-1 WT), or US3 null HSV-1 (HSV-1 US3 null) at an MOI of 0.1 and harvested at 48 hpi, when all cells were infected. Lysates from mock- and virus-infected cells were reacted with dephosphorylation buffer alone (mock treated) or calf intestinal alkaline phosphatase (CIAP treated) as described in Materials and Methods. Twenty micrograms of total protein for each sample was analyzed by Western blotting using antisera specific for HDAC2 and HSP90.
Histone deacetylase activity differentially affects plaquing efficiency of US3 mutant viruses.
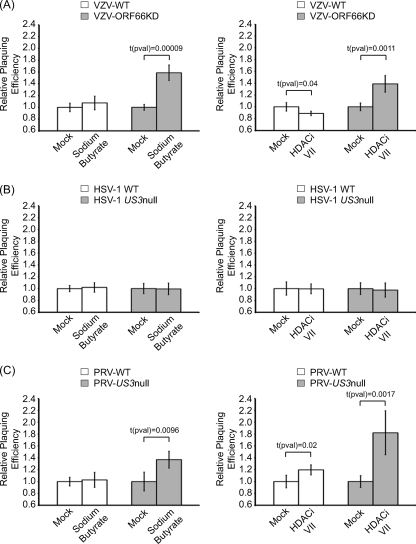
While the VZV, HSV-1, and PRV US3 genes are not essential for viral replication in tissue culture, all virus US3 mutants show impaired growth in certain cell types and have significantly impaired viral replication and virulence in animal models (10, 14, 28, 38, 39, 47-51, 55, 58, 61). Growth impairment resulting from loss of US3 activity could be associated with one of the many functions attributed to the kinase (reviewed in reference 13). However, inhibition of HDAC activity by sodium butyrate partially complements the growth defect associated with an ORF66p kinase-deficient VZV (61). This study suggests a mechanism whereby ORF66p-mediated hyperphosphorylation of HDACs inhibits their repressive activity to allow efficient gene expression and viral replication. A caveat of this work is that sodium butyrate is a pan-tropic HDAC inhibitor and not specific for HDAC1 and HDAC2. Therefore, we could not unequivocally state that the observed complementation resulted from inhibition of class I HDAC activities. Recently, histone deacetylase inhibitor VII, 106 (HDACi VII), a specific HDAC inhibitor that inhibits only class I HDACs (specifically HDAC1, HDAC2, and HDAC3), became available (4). We therefore asked what effect specific inhibition of class I HDACs had on plaquing efficiency of wild-type and ORF66p/US3 kinase-deficient VZV, HSV-1, and PRV. Confluent monolayers of MeWo cells were mock treated or treated with 5 mM sodium butyrate or 50 μM HDACi VII at 6 h before infection. Following pretreatment, cells were infected with serial dilutions of cell-free VZV and monolayers were maintained in medium without drug. At several days postinfection, monolayers were fixed and stained and plaques counted. The relative plaquing efficiency was calculated as number of plaques in the presence of drug/number of plaques in the absence of drug (Fig. 7 A and Table 1). Pretreatment of MeWo cells with sodium butyrate had no significant effect on the plaquing efficiency of wild-type virus. In contrast, pretreatment reproducibly increased the plaquing efficiency of VZV-ORF66KD by 1.6-fold. While modest, this increase was determined to be significant [t(pval) = 0.00009]. These data are consistent with our previous findings (61). Pretreatment with HDACi VII caused a small (0.9-fold) but statistically significant [t(pval) = 0.04] decrease in the plaquing efficiency of wild-type virus while reproducibly increasing the plaquing efficiency of VZV-ORF66KD by 1.4-fold. Again, this modest effect was determined to be significant [t(pval) = 0.0011]. These data confirm that inhibition of class I HDACs (specifically HDAC1, HDAC2, and HDAC3) results in increased plaquing efficiency of VZV-ORF66KD.
FIG. 7.
Plaquing efficiency of US3 kinase-deficient viruses in cells treated with HDAC inhibitors. MeWo (VZV and HSV-1) or Vero (PRV) cells were mock pretreated or pretreated with 5 mM sodium butyrate or 50 μM HDAC inhibitor VII (HDACi VII) for 6 h. Following treatment, cells were infected with serial dilutions of cell-free wild-type (VZV-WT) or ORF66p kinase-deficient (VZV-ORF66KD) VZV (A), wild-type (HSV-1 WT) or US3-deleted (HSV-1 US3 null) HSV-1 (B), or wild-type (PRV-WT) or US3-deleted (PRV-US3 null) PRV (C). Plaques were allowed to develop under 1.5% methylcellulose in the absence of inhibitor. At 3 (HSV-1 and PRV) or 6 (VZV) days postinfection, monolayers were fixed and stained and plaques counted. Relative plaquing efficiency was calculated as described in the text. Each bar represents the average from two independent experiments performed in triplicate, and error bars delineate standard deviations.
TABLE 1.
Plaquing efficiencies of wild-type and ORF66p kinase-deficient VZV in MeWo cells treated with HDAC inhibitors
| Virus | Expt no. | HDAC inhibitor treatment | No. of plaques |
|
|---|---|---|---|---|
| Avg | SD | |||
| WT VZV | 1 | None | 71.7 | 4.9 |
| Sodium butyrate | 81.3 | 3.2 | ||
| 2 | None | 109.0 | 9.5 | |
| Sodium butyrate | 110.0 | 14.8 | ||
| 1 | None | 86.7 | 1.5 | |
| HDAC inhibitor VII | 76.0 | 2.0 | ||
| 2 | None | 113.7 | 12.7 | |
| HDAC inhibitor VII | 103.0 | 5.3 | ||
| ORF66KD VZV | 1 | None | 28.3 | 2.1 |
| Sodium butyrate | 47.7 | 2.9 | ||
| 2 | None | 63.7 | 1.5 | |
| Sodium butyrate | 94.3 | 3.8 | ||
| 1 | None | 35.0 | 1.0 | |
| HDAC inhibitor VII | 52.0 | 3.0 | ||
| 2 | None | 70.7 | 6.8 | |
| HDAC inhibitor VII | 91.0 | 7.9 | ||
Similar studies with cells infected by wild-type and US3 null HSV-1 and treated with sodium butyrate or HDACi VII (Fig. 7B and Table 2) revealed that HDAC inhibitors had no significant effect on the plaquing efficiency of either virus. Indeed, identical results were also observed in Vero cells and human embryonic lung fibroblasts (HELFs) (data not shown). Therefore, US3 kinase activity is not required to overcome HDAC-mediated silencing of HSV-1 replication in MeWo, Vero, and HELF cells.
TABLE 2.
Plaquing efficiencies of wild-type and US3 kinase null HSV-1 in MeWo cells treated with HDAC inhibitors
| Virus | Expt no. | HDAC inhibitor treatment | No. of plaques |
|
|---|---|---|---|---|
| Avg | SD | |||
| WT HSV-1 | 1 | None | 42.3 | 1.5 |
| Sodium butyrate | 43.0 | 4.6 | ||
| 2 | None | 70.7 | 5.1 | |
| Sodium butyrate | 72.3 | 4.9 | ||
| 1 | None | 48.0 | 7.8 | |
| HDAC inhibitor VII | 46.7 | 3.1 | ||
| 2 | None | 72.3 | 4.9 | |
| HDAC inhibitor VII | 73.7 | 7.8 | ||
| US3 null HSV-1 | 1 | None | 51.3 | 6.7 |
| Sodium butyrate | 51.3 | 7.1 | ||
| 2 | None | 53.7 | 2.5 | |
| Sodium butyrate | 53.0 | 3.5 | ||
| 1 | None | 49.0 | 4.0 | |
| HDAC inhibitor VII | 48.0 | 9.2 | ||
| 2 | None | 57.7 | 7.8 | |
| HDAC inhibitor VII | 56.3 | 2.3 | ||
The plaquing efficiencies of wild-type and US3 null PRV in Vero cells (Fig. 7C and Table 3) revealed a phenotype similar to that seen for VZV. That is, the plaquing efficiency of US3 null PRV was increased 1.4-fold [t(pval) = 0.00009] by sodium butyrate and 1.8-fold [t(pval) = 0.0017] with HDACi VII, while effects on wild-type viruses were much less dramatic. Therefore, US3 null PRV behaves like ORF66KD VZV and has increased plaquing efficiency in cells treated with HDAC inhibitors. These data reveal that class I HDACs (specifically HDAC1, HDAC2, and HDAC3) play a role in restricting replication of some alphaherpesviruses, notably VZV and PRV, and that US3 kinase activity is required to inhibit their repressive activity.
TABLE 3.
Plaquing efficiencies of wild-type and US3 kinase null PRV in Vero cells treated with HDAC inhibitors
| Virus | Expt no. | HDAC inhibitor treatment | No. of plaques |
|
|---|---|---|---|---|
| Avg no. | SD | |||
| WT PRV | 1 | None | 48.7 | 5.1 |
| Sodium butyrate | 49.7 | 6.7 | ||
| 2 | None | 55.0 | 2.6 | |
| Sodium butyrate | 57.0 | 7.9 | ||
| 1 | None | 59.7 | 6.8 | |
| HDAC inhibitor VII | 72.3 | 2.5 | ||
| 2 | None | 57.7 | 7.1 | |
| HDAC inhibitor VII | 68.0 | 7.2 | ||
| US3 null PRV | 1 | None | 48.7 | 1.5 |
| Sodium butyrate | 68.3 | 10.6 | ||
| 2 | None | 53.7 | 13.3 | |
| Sodium butyrate | 71.7 | 0.6 | ||
| 1 | None | 53.0 | 6.1 | |
| HDAC inhibitor VII | 110.0 | 20.0 | ||
| 2 | None | 51.7 | 5.5 | |
| HDAC inhibitor VII | 81.0 | 4.6 | ||
DISCUSSION
Following infection of a host cell, herpesvirus genomes enter the nucleus to initiate viral transcription and subsequent replication. However, to block or impair viral replication, mammalian cells have developed varied mechanisms to silence transcription (and subsequent DNA replication) from viral DNA. Members of the HDAC family silence viral genomes as a consequence of deacetylating lysine residues on histone tails, resulting in chromatin condensation and subsequent repression of gene expression (5, 20). Herpesviruses have evolved diverse mechanisms to block silencing of HDAC repressor complexes (5, 21, 23, 42, 43, 61). For alphaherpesviruses such as HSV-1, HSV-2, and VZV, one aspect of this strategy involves phosphorylation of HDAC1 and HDAC2 in response to expression of their US3 kinases (26, 39, 43, 61). This phosphorylation allows for efficient viral gene expression and robust replication. Until this study, it was not known whether nonhuman alphaherpesviruses such as PRV possessed the capacity to subvert HDAC-mediated restriction or whether phosphorylation of HDAC1 and HDAC2 is a conserved function of US3 kinases.
In this report, we show that modification of HDACs during alphaherpesvirus infection occurs for all three of these representatives and that for VZV and PRV, this is US3 kinase mediated. A novel, slower-migrating species of HDAC2 appeared in cells infected with all of these viruses, and this represented a hyperphosphorylated form of HDAC2 (61). Dephosphorylation assays confirmed these findings (Fig. 6). Thus, PRV, like VZV and HSV-1, also induces hyperphosphorylation of HDAC2. Based on the data presented in Fig. 2 and the high degree of homology between the two proteins, we believe that this conclusion can be extended to HDAC1. S/T kinase activity from alphaherpesvirus US3 proteins is essential and sufficient for hyperphosphorylation of HDAC1 and HDAC2 by HSV-1, HSV-2, and VZV (26, 39, 43, 45, 61). We demonstrated that autonomous expression of VZV, HSV-1 and -2, and PRV kinases induced hyperphosphorylation of HDAC2 and that both HSV-1 and PRV US3 kinases target the same site, although PRV-mediated hyperphosphorylation is very low (Fig. 4). Infection of cells with HSV-1 and VZV mutants lacking US3 or its enzymatic activity revealed that US3 is essential for hyperphosphorylation of HDAC2 (Fig. 5). However, for PRV, HDAC2 was hyperphosphorylated in cells infected with a US3 null PRV (Fig. 5 and 6), indicating that unlike its HSV-1 and VZV homologs, PRV US3 kinase was not essential for HDAC2 hyperphosphorylation during infection. Nevertheless, it is possible that the US3-independent hyperphosphorylated species are unique and result from phosphorylation of alternative sites within HDACs. If this is correct, we postulate that US3-independent hyperphosphorylation of HDAC2 may serve a function different from that mediated by US3 kinase activity. Our initial attempts to map the sites of US3-dependent and -independent phosphorylation within HDAC2 during PRV infection proved fruitless, as FLAG-tagged exogenously expressed HDACs were not as efficiently targeted as endogenous HDACs were during infection. Accordingly, the site of HDAC2 hyperphosphorylation during PRV infection by US3-dependent and -independent means has not been resolved. How might HDAC2 become hyperphosphorylated during PRV infection in a US3-independent manner? It is possible that additional PRV proteins serve to activate an unknown cellular kinase that directs hyperphosphorylation of HDAC2 or induces different signaling pathways that mediate this process. PRV has a second kinase, encoded by UL13, that is present in all herpesviruses (7, 27), and PRV UL13 may have evolved unique functions to mediate hyperphosphorylation of HDAC2 that are not shared by VZV and HSV UL13.
In vitro kinase assays suggested that HDAC2 is not a direct substrate for VZV ORF66p (61). Therefore, VZV ORF66p-dependent phosphorylation of HDAC2 (and most likely HDAC1) occurs through an indirect mechanism. Based on data presented here, we posit that both HSV-1 and PRV kinases also indirectly phosphorylate HDAC2. We infer that alphaherpesvirus US3 kinase activates a cellular kinase or pathway leading to hyperphosphorylation of HDACs. The differences observed between the levels of HDAC hyperphosphorylation induced by each US3 kinase may correlate with their ability to activate a specific cellular kinase or pathway. Previous studies have demonstrated that US3 kinases activate cellular kinases. For instance, HSV-1 US3 kinase activates cellular protein kinase A (PKA) (2), and PRV US3 kinases activate p21 kinase 1 (PAK1) and PAK2 (59). The conserved phosphorylation targets of alphaherpesvirus US3 kinases (from HSV and VZV), in both HDAC1 and HDAC2 (Ser 406 and Ser 407, respectively), fit consensus sites for several cellular protein kinases, including PKA, PKC (HDAC2 only), and PKG (57). However, studies on the phosphorylation of murine HDAC2 indicate that PKA is not one of the primary kinases that target this protein (57). Therefore, future studies will concentrate on identification of cellular kinases involved in US3 kinase-dependent phosphorylation of HDAC1 and HDAC2.
US3 proteins of VZV, HSV-1, and PRV are not essential for viral replication in tissue culture; however, US3 mutants are impaired for growth in certain cell types and animal models (10, 14, 28, 38, 39, 47-51, 55, 58, 61). Previously, we demonstrated that broad inhibition of HDAC activity with sodium butyrate partially complements plaquing efficiency and growth of a VZV ORF66p kinase-deficient virus (61). Here, we confirm this result using plaquing efficiency analysis and a highly specific class I HDAC inhibitor (Fig. 7A and Table 1). As expected, sodium butyrate had no effect on the plaquing efficiency of wild-type VZV, whereas the plaquing efficiency of ORF66KD VZV increased by 1.6-fold. Specific inhibition of HDAC1, -2, and -3 activity by HDACi VII resulted in a small decrease in the plaquing efficiency of wild-type VZV (0.9-fold), while the plaquing efficiency of ORF66KD VZV increased 1.4-fold. Thus, inhibition of class I HDAC activity increases the frequency of successful initiation of infection of ORF66KD VZV, further supporting a role for ORF66p kinase in inhibiting HDAC during viral infection. Similar results were observed for PRV. Sodium butyrate had no effect on wild-type PRV plaquing efficiency, whereas the plaquing efficiency of US3 null PRV increased by 1.4-fold in the presence of the drug. Following pretreatment with HDACi VII, the plaquing efficiency of wild-type PRV increased 1.2-fold and that of US3 null PRV increased 1.8-fold. These results were intriguing, as a small fraction of HDAC2 is still hyperphosphorylated during infection with US3 null PRV, and therefore we would not have expected this effect. However, these data suggest that US3-independent hyperphosphorylation of HDACs is distinct from that induced by US3 kinase activity and serves a unique function during PRV infection rather than inhibiting HDAC-mediated silencing of virus replication.
Because inhibition of HDAC activity (with sodium butyrate or HDACi VII) had no effect on the plaquing efficiency of either wild-type or US3 null HSV-1, we surmise that HSV-1 US3 kinase activity is not required to overcome HDAC-mediated silencing of viral replication to establish a productive infection.
The involvement of HSV-1 ICP0 in dislodging HDAC1 and HDAC2 from their corepressors may explain why HDAC inhibitors failed to affect the plaquing efficiency of a US3 null HSV-1. In addition, ICP0 promotes both histone removal and acetylation on viral DNA during lytic infection (5). Thus, it is plausible that ICP0 is sufficient to subvert HDAC-mediated silencing of viral genomes, compensating for lack of US3. While there are ICP0 orthologs that share some biological features in both VZV and PRV, it is unknown whether they provide this ICP0 function (16). Based on plaquing efficiency data, we postulate that kinase-directed modulation of HDAC activities by these viruses might be more important. Our findings reveal that class I HDACs restrict replication of alphaherpesviruses and that for VZV and PRV, US3 kinase activity inhibits their repressive activity, resulting in efficient gene expression and viral replication.
Aside from identifying the cellular kinase(s) or pathway utilized by US3 kinases to indirectly phosphorylate HDACs, a question remaining is how HDAC-mediated silencing is blocked by US3-dependent hyperphosphorylation of only a small fraction of these proteins. One hypothesis is that US3-mediated phosphorylation of class I HDACs may regulate their enzymatic activity, as observed with cellular kinases (17, 40, 57). It is not known if phosphorylation at the conserved Ser residues regulates HDAC1 and -2 activities, but their proximity to other sites of phosphorylation, which are known to regulate activity (18), suggests that targeting these sites may have a similar role. Alternatively, hyperphosphorylation may regulate interaction of HDACs with other members of the corepressor complexes in which they reside. Studies with HSV-1 demonstrated that in addition to phosphorylation of HDAC1 and HDAC2 by US3 kinases, HDACs are dislodged from their corepressors LSD1/CoREST/REST in an ICP0-dependent process (21-23, 43). Following these events, a portion of HDAC1, HDAC2, LSD1, CoREST, and REST translocates to the cytoplasm by an unknown mechanism (21-23). It was suggested that these events play a central role in HDAC-mediated silencing of viral gene expression and subsequent replication. However, silencing of CoREST does not substantively affect HSV-1 infection (15), and inhibition of LSD-1 activity or its depletion blocks HSV-1 gene expression (34). Biochemical fractionation of VZV-infected cells showed a modest increase in cytoplasmic accumulation of hyperphosphorylated HDAC1 and HDAC2 and of the corepressors CoREST and SIN3A as infection progressed (M. S. Walters and S. Silverstein, unpublished data). Therefore, a similar sequence of events may occur in cells infected with VZV. At present it is unknown whether similar events occur during PRV infection.
In conclusion, we demonstrated that HDAC2 and likely HDAC1 are conserved targets of alphaherpesviruses and are hyperphosphorylated during VZV, HSV-1, and PRV infection. Autonomous expression of each alphaherpesvirus US3 kinase is sufficient to induce HDAC hyperphosphorylation, and each kinase targets phosphorylation of HDAC2 at a conserved Ser residue within its C terminus. Moreover, specific chemical inhibition of class I HDAC activity greatly increases the plaquing efficiency of VZV and PRV lacking US3 or its enzymatic activity, whereas only minimal effects are observed with wild-type viruses. These data suggest that VZV and PRV US3 kinase activities target HDAC2, and likely HDAC1 as well, to inhibit viral genome silencing and allow efficient viral replication. However, no effect was observed for wild-type or US3 null HSV-1. Thus, although HDAC2 (and by inference HDAC1) is a conserved target of alphaherpesvirus US3 kinases, the functional significance of phosphorylation is virus specific.
Acknowledgments
This study was supported by the following grants from the NIH Public Health Service: AI024021 (S.J.S.), NS064022 (P.R.K.), EY08098 (P.R.K.), and AI48626 (B.W.B.). The work was also supported by funds from the Eye & Ear Foundation of Pittsburgh and Research to Prevent Blindness, Inc. (P.R.K.).
We thank Angela Erazo for help with assembling the US3 kinase alignment.
Footnotes
Published ahead of print on 21 July 2010.
REFERENCES
- 1.Arii, J., M. Uema, T. Morimoto, H. Sagara, H. Akashi, E. Ono, H. Arase, and Y. Kawaguchi. 2009. Entry of herpes simplex virus 1 and other alphaherpesviruses via the paired immunoglobulin-like type 2 receptor alpha. J. Virol. 83:4520-4527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benetti, L., and B. Roizman. 2004. Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis. Proc. Natl. Acad. Sci. U. S. A. 101:9411-9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calton, C. M., J. A. Randall, M. W. Adkins, and B. W. Banfield. 2004. The pseudorabies virus serine/threonine kinase Us3 contains mitochondrial, nuclear and membrane localization signals. Virus Genes 29:131-145. [DOI] [PubMed] [Google Scholar]
- 4.Chou, C. J., D. Herman, and J. M. Gottesfeld. 2008. Pimelic diphenylamide 106 is a slow, tight-binding inhibitor of class I histone deacetylases. J. Biol. Chem. 283:35402-35409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cliffe, A. R., and D. M. Knipe. 2008. Herpes simplex virus ICP0 promotes both histone removal and acetylation on viral DNA during lytic infection. J. Virol. 82:12030-12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colle, C. F., III, C. C. Flowers, and D. J. O'Callaghan. 1992. Open reading frames encoding a protein kinase, homolog of glycoprotein gX of pseudorabies virus, and a novel glycoprotein map within the unique short segment of equine herpesvirus type 1. Virology 188:545-557. [DOI] [PubMed] [Google Scholar]
- 7.Coller, K. E., and G. A. Smith. 2008. Two viral kinases are required for sustained long distance axon transport of a neuroinvasive herpesvirus. Traffic 9:1458-1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daikoku, T., Y. Yamashita, T. Tsurumi, K. Maeno, and Y. Nishiyama. 1993. Purification and biochemical characterization of the protein kinase encoded by the US3 gene of herpes simplex virus type 2. Virology 197:685-694. [DOI] [PubMed] [Google Scholar]
- 9.David, G., M. A. Neptune, and R. A. DePinho. 2002. SUMO-1 modification of histone deacetylase 1 (HDAC1) modulates its biological activities. J. Biol. Chem. 277:23658-23663. [DOI] [PubMed] [Google Scholar]
- 10.Deruelle, M., N. De Corte, J. Englebienne, H. Nauwynck, and H. W. Favoreel. 2010. Pseudorabies virus US3-mediated inhibition of apoptosis does not affect infectious virus production. J. Gen. Virol. 91:1127-1132. [DOI] [PubMed] [Google Scholar]
- 11.Eisfeld, A. J., S. E. Turse, S. A. Jackson, E. C. Lerner, and P. R. Kinchington. 2006. Phosphorylation of the varicella-zoster virus (VZV) major transcriptional regulatory protein IE62 by the VZV open reading frame 66 protein kinase. J. Virol. 80:1710-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisfeld, A. J., M. B. Yee, A. Erazo, A. Abendroth, and P. R. Kinchington. 2007. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J. Virol. 81:9034-9049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erazo, A., and P. R. Kinchington. Varicella-zoster virus open reading frame 66 protein kinase and its relationship to alphaherpesvirus US3 kinases. Curr. Top. Microbiol. Immunol., in press. [DOI] [PMC free article] [PubMed]
- 14.Erazo, A., M. B. Yee, N. Osterrieder, and P. R. Kinchington. 2008. Varicella-zoster virus open reading frame 66 protein kinase is required for efficient viral growth in primary human corneal stromal fibroblast cells. J. Virol. 82:7653-7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett, R. D. 2010. Depletion of CoREST does not improve the replication of ICP0 null mutant herpes simplex virus type 1. J. Virol. 84:3695-3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everett, R. D., C. Boutell, C. McNair, L. Grant, and A. Orr. 2010. Comparison of the biological and biochemical activities of several members of the alphaherpesvirus ICP0 family of proteins. J. Virol. 84:3476-3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galasinski, S. C., K. A. Resing, J. A. Goodrich, and N. G. Ahn. 2002. Phosphatase inhibition leads to histone deacetylases 1 and 2 phosphorylation and disruption of corepressor interactions. J. Biol. Chem. 277:19618-19626. [DOI] [PubMed] [Google Scholar]
- 18.Gallinari, P., S. Di Marco, P. Jones, M. Pallaoro, and C. Steinkuhler. 2007. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res. 17:195-211. [DOI] [PubMed] [Google Scholar]
- 19.Gregoretti, I. V., Y. M. Lee, and H. V. Goodson. 2004. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J. Mol. Biol. 338:17-31. [DOI] [PubMed] [Google Scholar]
- 20.Grozinger, C. M., and S. L. Schreiber. 2002. Deacetylase enzymes: biological functions and the use of small-molecule inhibitors. Chem. Biol. 9:3-16. [DOI] [PubMed] [Google Scholar]
- 21.Gu, H., Y. Liang, G. Mandel, and B. Roizman. 2005. Components of the REST/CoREST/histone deacetylase repressor complex are disrupted, modified, and translocated in HSV-1-infected cells. Proc. Natl. Acad. Sci. U. S. A. 102:7571-7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gu, H., and B. Roizman. 2009. Engagement of the lysine-specific demethylase/HDAC1/CoREST/REST complex by herpes simplex virus 1. J. Virol. 83:4376-4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu, H., and B. Roizman. 2007. Herpes simplex virus-infected cell protein 0 blocks the silencing of viral DNA by dissociating histone deacetylases from the CoREST-REST complex. Proc. Natl. Acad. Sci. U. S. A. 104:17134-17139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haberland, M., R. L. Montgomery, and E. N. Olson. 2009. The many roles of histone deacetylases in development and physiology: implications for disease and therapy. Nat. Rev. Genet. 10:32-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanks, S. K., and T. Hunter. 1995. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 9:576-596. [PubMed] [Google Scholar]
- 26.Kato, A., M. Tanaka, M. Yamamoto, R. Asai, T. Sata, Y. Nishiyama, and Y. Kawaguchi. 2008. Identification of a physiological phosphorylation site of the herpes simplex virus 1-encoded protein kinase Us3 which regulates its optimal catalytic activity in vitro and influences its function in infected cells. J. Virol. 82:6172-6189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaguchi, Y., and K. Kato. 2003. Protein kinases conserved in herpesviruses potentially share a function mimicking the cellular protein kinase cdc2. Rev. Med. Virol. 13:331-340. [DOI] [PubMed] [Google Scholar]
- 28.Kimman, T. G., N. De Wind, T. De Bruin, Y. de Visser, and J. Voermans. 1994. Inactivation of glycoprotein gE and thymidine kinase or the US3-encoded protein kinase synergistically decreases in vivo replication of pseudorabies virus and the induction of protective immunity. Virology 205:511-518. [DOI] [PubMed] [Google Scholar]
- 29.Klupp, B. G., H. Granzow, and T. C. Mettenleiter. 2001. Effect of the pseudorabies virus US3 protein on nuclear membrane localization of the UL34 protein and virus egress from the nucleus. J. Gen. Virol. 82:2363-2371. [DOI] [PubMed] [Google Scholar]
- 30.Kyratsous, C. A., and S. J. Silverstein. 2009. Components of nuclear domain 10 bodies regulate varicella-zoster virus replication. J. Virol. 83:4262-4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leader, D. P. 1993. Viral protein kinases and protein phosphatases. Pharmacol. Ther. 59:343-389. [DOI] [PubMed] [Google Scholar]
- 32.Leader, D. P., A. D. Deana, F. Marchiori, F. C. Purves, and L. A. Pinna. 1991. Further definition of the substrate specificity of the alpha-herpesvirus protein kinase and comparison with protein kinases A and C. Biochim. Biophys. Acta 1091:426-431. [DOI] [PubMed] [Google Scholar]
- 33.Leung-Tack, P., J. C. Audonnet, and M. Riviere. 1994. The complete DNA sequence and the genetic organization of the short unique region (US) of the bovine herpesvirus type 1 (ST strain). Virology 199:409-421. [DOI] [PubMed] [Google Scholar]
- 34.Liang, Y., J. L. Vogel, A. Narayanan, H. Peng, and T. M. Kristie. 2009. Inhibition of the histone demethylase LSD1 blocks alpha-herpesvirus lytic replication and reactivation from latency. Nat. Med. 15:1312-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lungu, O., C. A. Panagiotidis, P. W. Annunziato, A. A. Gershon, and S. J. Silverstein. 1998. Aberrant intracellular localization of varicella-zoster virus regulatory proteins during latency. Proc. Natl. Acad. Sci. U. S. A. 95:7080-7085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lyman, M. G., G. L. Demmin, and B. W. Banfield. 2003. The attenuated pseudorabies virus strain Bartha fails to package the tegument proteins Us3 and VP22. J. Virol. 77:1403-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGeoch, D. J., and A. J. Davison. 1986. Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 14:1765-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meignier, B., R. Longnecker, P. Mavromara-Nazos, A. E. Sears, and B. Roizman. 1988. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology 162:251-254. [DOI] [PubMed] [Google Scholar]
- 39.Morimoto, T., J. Arii, M. Tanaka, T. Sata, H. Akashi, M. Yamada, Y. Nishiyama, M. Uema, and Y. Kawaguchi. 2009. Differences in the regulatory and functional effects of the Us3 protein kinase activities of herpes simplex virus 1 and 2. J. Virol. 83:11624-11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pflum, M. K., J. K. Tong, W. S. Lane, and S. L. Schreiber. 2001. Histone deacetylase 1 phosphorylation promotes enzymatic activity and complex formation. J. Biol. Chem. 276:47733-47741. [DOI] [PubMed] [Google Scholar]
- 41.Poon, A. P., L. Benetti, and B. Roizman. 2006. U(S)3 and U(S)3.5 protein kinases of herpes simplex virus 1 differ with respect to their functions in blocking apoptosis and in virion maturation and egress. J. Virol. 80:3752-3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poon, A. P., H. Gu, and B. Roizman. 2006. ICP0 and the US3 protein kinase of herpes simplex virus 1 independently block histone deacetylation to enable gene expression. Proc. Natl. Acad. Sci. U. S. A. 103:9993-9998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poon, A. P., Y. Liang, and B. Roizman. 2003. Herpes simplex virus 1 gene expression is accelerated by inhibitors of histone deacetylases in rabbit skin cells infected with a mutant carrying a cDNA copy of the infected-cell protein no. 0. J. Virol. 77:12671-12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poon, A. P., and B. Roizman. 2005. Herpes simplex virus 1 ICP22 regulates the accumulation of a shorter mRNA and of a truncated US3 protein kinase that exhibits altered functions. J. Virol. 79:8470-8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poon, A. P., and B. Roizman. 2007. Mapping of key functions of the herpes simplex virus 1 U(S)3 protein kinase: the U(S)3 protein can form functional heteromultimeric structures derived from overlapping truncated polypeptides. J. Virol. 81:1980-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Purves, F. C., A. D. Deana, F. Marchiori, D. P. Leader, and L. A. Pinna. 1986. The substrate specificity of the protein kinase induced in cells infected with herpesviruses: studies with synthetic substrates [corrected] indicate structural requirements distinct from other protein kinases. Biochim. Biophys. Acta 889:208-215. [DOI] [PubMed] [Google Scholar]
- 47.Purves, F. C., R. M. Longnecker, D. P. Leader, and B. Roizman. 1987. Herpes simplex virus 1 protein kinase is encoded by open reading frame US3 which is not essential for virus growth in cell culture. J. Virol. 61:2896-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryckman, B. J., and R. J. Roller. 2004. Herpes simplex virus type 1 primary envelopment: UL34 protein modification and the US3-UL34 catalytic relationship. J. Virol. 78:399-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sagou, K., T. Imai, H. Sagara, M. Uema, and Y. Kawaguchi. 2009. Regulation of the catalytic activity of herpes simplex virus 1 protein kinase Us3 by autophosphorylation and its role in pathogenesis. J. Virol. 83:5773-5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaap, A., J. F. Fortin, M. Sommer, L. Zerboni, S. Stamatis, C. C. Ku, G. P. Nolan, and A. M. Arvin. 2005. T-cell tropism and the role of ORF66 protein in pathogenesis of varicella-zoster virus infection. J. Virol. 79:12921-12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaap-Nutt, A., M. Sommer, X. Che, L. Zerboni, and A. M. Arvin. 2006. ORF66 protein kinase function is required for T-cell tropism of varicella-zoster virus in vivo. J. Virol. 80:11806-11816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schumacher, D., C. McKinney, B. B. Kaufer, and N. Osterrieder. 2008. Enzymatically inactive U(S)3 protein kinase of Marek's disease virus (MDV) is capable of depolymerizing F-actin but results in accumulation of virions in perinuclear invaginations and reduced virus growth. Virology 375:37-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schwartz, J. A., E. E. Brittle, A. E. Reynolds, L. W. Enquist, and S. J. Silverstein. 2006. UL54-null pseudorabies virus is attenuated in mice but productively infects cells in culture. J. Virol. 80:769-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schwer, B., and E. Verdin. 2008. Conserved metabolic regulatory functions of sirtuins. Cell Metab. 7:104-112. [DOI] [PubMed] [Google Scholar]
- 55.Soong, W., J. C. Schultz, A. C. Patera, M. H. Sommer, and J. I. Cohen. 2000. Infection of human T lymphocytes with varicella-zoster virus: an analysis with viral mutants and clinical isolates. J. Virol. 74:1864-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stow, N. D., and E. C. Stow. 1986. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J. Gen. Virol. 67:2571-2585. [DOI] [PubMed] [Google Scholar]
- 57.Tsai, S. C., and E. Seto. 2002. Regulation of histone deacetylase 2 by protein kinase CK2. J. Biol. Chem. 277:31826-31833. [DOI] [PubMed] [Google Scholar]
- 58.Van den Broeke, C., M. Deruelle, H. J. Nauwynck, K. E. Coller, G. A. Smith, J. Van Doorsselaere, and H. W. Favoreel. 2009. The kinase activity of pseudorabies virus US3 is required for modulation of the actin cytoskeleton. Virology 385:155-160. [DOI] [PubMed] [Google Scholar]
- 59.Van den Broeke, C., M. Radu, M. Deruelle, H. Nauwynck, C. Hofmann, Z. M. Jaffer, J. Chernoff, and H. W. Favoreel. 2009. Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group A p21-activated kinases. Proc. Natl. Acad. Sci. U. S. A. 106:8707-8712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Zijl, M., H. van der Gulden, N. de Wind, A. Gielkens, and A. Berns. 1990. Identification of two genes in the unique short region of pseudorabies virus; comparison with herpes simplex virus and varicella-zoster virus. J. Gen. Virol. 71:1747-1755. [DOI] [PubMed] [Google Scholar]
- 61.Walters, M. S., A. Erazo, P. R. Kinchington, and S. Silverstein. 2009. Histone deacetylases 1 and 2 are phosphorylated at novel sites during varicella-zoster virus infection. J. Virol. 83:11502-11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walters, M. S., C. A. Kyratsous, S. Wan, and S. Silverstein. 2008. Nuclear import of the varicella-zoster virus latency-associated protein ORF63 in primary neurons requires expression of the lytic protein ORF61 and occurs in a proteasome-dependent manner. J. Virol. 82:8673-8686. [DOI] [PMC free article] [PubMed] [Google Scholar]