Abstract
The immunogenicity and durability of genetic vaccines are influenced by the composition of gene inserts and choice of delivery vector. DNA vectors are a promising vaccine approach showing efficacy when combined in prime-boost regimens with recombinant protein or viral vectors, but they have shown limited comparative efficacy as a stand-alone platform in primates, due possibly to suboptimal gene expression or cell targeting. Here, regimens using DNA plasmids modified for optimal antigen expression and recombinant adenovirus (rAd) vectors, all encoding the glycoprotein (GP) gene from Angola Marburg virus (MARV), were compared for their ability to provide immune protection against lethal MARV Angola infection. Heterologous DNA-GP/rAd5-GP prime-boost and single-modality rAd5-GP, as well as the DNA-GP-only vaccine, prevented death in all vaccinated subjects after challenge with a lethal dose of MARV Angola. The DNA/DNA vaccine induced humoral responses comparable to those induced by a single inoculation with rAd5-GP, as well as CD4+ and CD8+ cellular immune responses, with skewing toward CD4+ T-cell activity against MARV GP. Vaccine regimens containing rAd-GP, alone or as a boost, exhibited cellular responses with CD8+ T-cell dominance. Across vaccine groups, CD8+ T-cell subset dominance comprising cells exhibiting a tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) double-positive functional phenotype was associated with an absence or low frequency of clinical symptoms, suggesting that both the magnitude and functional phenotype of CD8+ T cells may determine vaccine efficacy against infection by MARV Angola.
The filoviruses Marburgvirus (MARV) and Ebolavirus (EBOV) are endemic primarily to central Africa and cause a severe form of viral hemorrhagic fever. Of all the filovirus strains or species, the Angola strain of MARV is associated with the highest mortality rate (90%) in humans observed to date (26). An increase in natural filovirus outbreak frequency over the past decade and the potential for use to cause deliberate human mortality have focused attention on the need for therapeutics and vaccines against filoviruses. While regulatory pathways have been proposed to facilitate licensing of a preventive vaccine against potently lethal pathogens such as these, there is as yet no licensed vaccine for use in humans, and efforts remain targeted to the optimization of vaccine performance in nonhuman primates (NHP) since this animal model recapitulates many aspects of disease pathogenesis observed in humans.
Genetic vaccines are a promising approach for immunization against pathogens that are rapidly changing due to natural evolution, cross-species transmission, or intentional modification. Gene-based vaccines are produced rapidly and can be delivered by a variety of vectors. DNA vectors are advantageous because they are inherently safe and stable and can be used repeatedly without inducing antivector immune responses. However, while filovirus DNA vaccines have demonstrated efficacy in small animal models, efforts to induce protective immunity by injection of plasmid DNA alone into NHP have yielded less encouraging results. EBOV DNA vectors generate immune protection in mice and guinea pigs, but this has not been demonstrated in NHP unless DNA immunization is boosted with a viral vector vaccine (23). MARV DNA fully protects mice and guinea pigs but provides only partial protection in NHP (17). The discordant results between rodent and primate species may be due to the use of slightly modified infectious challenge viruses in rodent models or may reflect underlying differences in vaccine performance and the mechanisms of immune protection between rodents and NHP.
In the current study, we examined whether DNA plasmid-based vaccines could be improved to increase potency in NHP and compared immunogenicity of this vaccine modality with those of viral vector and prime-boost approaches. DNA-vectored vaccines were modified by codon optimizing gene target inserts for enhanced expression in primates. These vectors induced antigen-specific cellular and humoral immune responses similar to immunization using a recombinant adenoviral vector and provided protection after lethal challenge with MARV Angola. However, macaques vaccinated with DNA vectors exhibited clinical symptoms associated with MARV hemorrhagic fever (MHF) that were absent in NHP receiving a single inoculation with recombinant adenovirus (rAd) vectors, suggesting qualitative differences in the immune responses elicited by the different modalities.
MATERIALS AND METHODS
Vaccines.
The cDNA insert encoding MARV Angola glycoprotein (GP) was synthesized by using human-preferred codons as described by GeneArt (Regensburg, Germany) and inserted into mammalian expression vector CMV/R as an XbaI and BglII fragment. This vector is a cytomegalovirus (CMV)-driven plasmid that has been modified to contain the human CMV immediate early (IE) enhancer/promoter, followed by the human T-cell leukemia virus type 1 (HTLV-1) R region and a 123-bp fragment of the CMV IE 3′ intron, resulting in higher-level gene expression and enhanced immunogenicity in vivo (1). MARV GP plasmids were transfected into 293 cells to confirm protein expression by Western blot analysis with serum from mice immunized with DNA vectors containing the MARV GP native virus sequence. The rAd type 5 (rAd5) vaccine expressing MARV GP was constructed using the codon-modified insert and grown and purified as described previously (21). Briefly, the codon-optimized MARV Angola GP insert was cloned into a plasmid containing the left 5 kb of the Ad5 genome with a CMV promoter and simian virus 40 (SV40) polyadenylation sequence inserted into the E1 region, which was completely removed. Cotransfection of this plasmid with a cosmid containing the remaining Ad5 sequence (E3 deleted) to PER.C6 cells yielded an E1/E3-deleted replication-deficient recombinant Ad5 virus. The virus was plaque purified, and one plaque was expanded up to a production scale of approximately 2.4 liters. A two-step cesium chloride gradient ultracentrifugation procedure was used to purify the Ad5 MARV vector. The purified Ad5 MARV vaccine was stored as single-use aliquots below −65°C. Virus particle titers were determined by anion-exchange high-pressure liquid chromatography (HPLC) based on previously described procedures (8). Infectivity was assessed by 50% tissue culture infective dose (TCID50) using 911 cells (9). Adenovirus-mediated MARV GP expression was assessed by infection of A549 cells followed by analysis of culture lysates on a Western blot as described above. The identity of the purified vectors was confirmed by PCR.
Animal challenge study and safety.
Animal experiments were conducted in full compliance with all relevant federal guidelines and NIH policies. Fifteen cynomolgus macaques (Macaca fascicularis) with weights and ages between 2 and 3 kg and 3 and 5 years, respectively, were obtained from Covance for this immunization and challenge study. Monkeys were housed individually and given enrichment regularly as recommended by the Guide for the Care and Use of Laboratory Animals (14a; DHEW number NIH 86-23). Animals were anesthetized with ketamine prior to blood sampling or vaccination. Each vaccine group in this study (DNA MARV GP only, rAd5 MARV GP only, and DNA MARV GP + rAd5 MARV GP) contained four cynomolgus macaques, and the control, unvaccinated group contained three. All animals were transferred to the Maximum Containment Laboratory (biosafety level 4 [BSL-4]) for infection with 1,000 PFU of MARV Angola isolated from a patient from the 2005 outbreak in Angola (26). Infectious challenge was conducted with the same virus stock in two experiments; the first study (I) contained animals vaccinated with DNA-GP or DNA-GP/rAd5-GP and included two unvaccinated controls and the second study (II) contained animals vaccinated with rAd5-GP and one unvaccinated control. While in the BSL-4 facility, the monkeys were fed and checked daily. During the MARV challenge study, blood was collected from the NHP on days 0, 3, 6, 10, 14, and 28 for hematological, biochemical, and virological analyses.
Animal studies performed in BSL-4 biocontainment at USAMRIID were approved by the USAMRIID Institutional Animal Care and Use Committee. Animal research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to the principles stated in the Guide for the Care and Use of Laboratory Animals (14a). The facilities used are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Animal immunization.
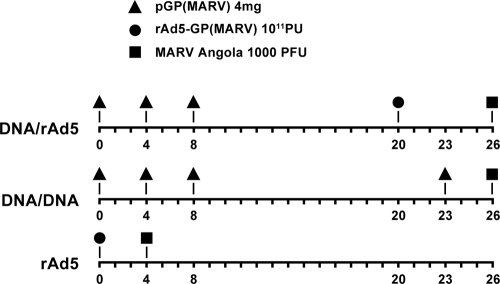
Each monkey in the DNA vaccine group (n = 4) was injected intramuscularly with 4 mg MARV Angola GP plasmid at 0, 4, 8, and 23 weeks by Biojector. These animals were exposed to a target dose of 1,000 PFU of MARV Angola by the intramuscular route 3 weeks after the fourth DNA immunization. Four cynomolgus macaques in the rAd5-only group were injected intramuscularly by needle and syringe with 1011 particle units (PU) of rAd5 MARV Angola GP. Four weeks postvaccination, animals were challenged with a target dose of 1,000 PFU of MARV Angola by the intramuscular route. Four cynomolgus macaques each received 3 injections of MARV GP DNA plasmid at 0, 4, and 8 weeks as described above. Twelve weeks after the third DNA injection (at 20 weeks), each NHP was given a boost with 1011 PU of rAd5 MARV GP administered intramuscularly by needle injection. These animals were infected with 1,000 PFU of MARV Angola by the intramuscular route 6 weeks after the final immunization.
Anti-MARV GP IgG ELISA.
Polyvinyl chloride enzyme-linked immunosorbent assay (ELISA) plates (Dynatech, Vienna, VA, or Nunc, Rochester, NY) were coated with 100 μl of antigen per well and incubated at 4°C overnight. Subsequent incubations were performed at room temperature. Transmembrane-deleted MARV GP (MARV GPΔTM) generated by calcium phosphate-mediated transient transfection of 293T cells served as the antigen. Plates were washed six times with phosphate-buffered saline (PBS) containing Tween 20 after antigen coating. Test sera were serially diluted to 7 concentrations ranging from 1:50 to 1:50,000 and added to the antigen-coated wells for 60 min. The plates were washed six times followed by incubation with the detection antibody, goat anti-human IgG (heavy plus light chain [H+L]; Chemicon/Millipore, Billerica, MA) conjugated to horseradish peroxidase. Sigma Fast o-phenylenediamine dihydrochloride (Sigma, St. Louis, MO) substrate was added to the wells, and the optical density was determined (450 nm). A panel of prevaccination sera for each animal was run every time that the assay was performed. Background-subtracted ELISA titers are expressed as 90% effective concentrations (EC90), reciprocal optical density values, which represent the dilution at which there is a 90% decrease in antigen binding.
Intracellular cytokine staining (ICS).
Whole-blood samples from cynomolgus macaques were subjected to density gradient centrifugation over Ficoll to isolate peripheral blood mononuclear cells (PBMC). Approximately 1 × 106 cells were stimulated in 100 μl RPMI medium containing 10% heat-inactivated fetal calf serum for 6 h at 37°C with anti-CD28 (clone CD28.2) and anti-CD49d (clone L25) antibodies (BD Biosciences), brefeldin A (Sigma-Aldrich, St. Louis, MO), and either dimethyl sulfoxide (DMSO) or a pool of peptides spanning the entire MARV (Angola strain) GP open reading frame. The peptides were 15-mers overlapping by 11 amino acids reconstituted in fresh sterile DMSO at a final concentration of 2.5 μg/ml. For each sample an equivalent aliquot was stimulated with staphylococcal enterotoxin B (SEB) as a positive control. After the 6-h stimulation, PBMC were stained with a mixture of antibodies against lineage markers (CD3-Cy7-allophycocyanin [APC], clone SP34-2 [BD Biosciences]; CD4-QD605, clone M-T477 [BD Biosciences]; CD8-TRPE, clone RPA-T8 [X]; CD95-Cy5-phycoerythrin [PE], clone DX2 [BD Biosciences]; CD45RA-QD655, clone 5H3 [X]) at room temperature for 20 min. After 2 washes the cells were fixed and permeabilized with Cytofix/Cytoperm (BD Biosciences) followed by staining with antibodies against cytokines (tumor necrosis factor alpha [TNF-α]-APC, clone monoclonal antibody 11 [MAb11; BD Biosciences]; gamma interferon [IFN-γ]-Cy7-PE, clone B27 [BD Biosciences]; and interleukin-2 [IL-2]-PE, clone MQ17H12 [BD Biosciences]). The viability dye ViViD (Invitrogen) was included to allow discrimination between live and dead cells (15). Samples were acquired on an LSR II cytometer (BD Biosciences) collecting up to 500,000 events and analyzed using FlowJo 8.8.5 and SPICE 5.0 software (Tree Star). Cytokine-positive cells were defined as a percentage within CD4+ and CD8+ T-cell memory subsets. Boolean combinations of the three cytokine gates were determined in FlowJo and imported into SPICE to evaluate functional phenotypes of single-, double-, and triple-cytokine-producing cells and proportional representation in pie charts.
Hematology and serum biochemistry.
Total white blood cell counts, white blood cell differentials, red blood cell counts, platelet counts, hematocrit values, total hemoglobin, mean cell volume, mean corpuscular volume, and mean corpuscular hemoglobin concentration were determined from blood samples collected in tubes containing EDTA, by using a laser-based hematologic analyzer (Coulter Electronics, Hialeah, FL). Serum samples were tested for concentrations of alanine aminotransferase (ALT) and aspartate aminotransferase (AST), by using a Piccolo point-of-care blood analyzer (Abaxis, Sunnyvale, CA).
Detection of MARV.
Virus titration was performed by plaque assay on Vero cells from all blood samples. Briefly, increasing 10-fold dilutions of the samples were adsorbed to Vero monolayers in duplicate wells (0.2 ml per well); thus, the limit for detection was 25 PFU/ml.
Statistics.
Comparison of anti-GP ELISA IgG titers and intracellular cytokine production by T-cell memory subsets was done using a one-tailed t test in GraphPad Prism software.
RESULTS
Single-injection and prime-boost immunization of cynomolgus macaques with genetic vaccines.
It has been shown for some antigens that combination prime-boost vaccines using DNA and rAd5 vectors for gene delivery generate higher-magnitude immune responses than do either DNA or rAd vectors alone (19, 23). In this study we tested the comparative immunogenicity and protection elicited by DNA and/or rAd vectors encoding the GP of MARV Angola. Vaccination against MARV using DNA vectors delivered by gene gun was shown previously to provide some protection to NHP against challenge with MARV (Musoke strain), but breakthrough mortality occurred, possibly owing to suboptimal antigen expression (17). Therefore, the plasmid vectors used in the present study were optimized for antigen expression in primates using two approaches. First, the GP gene inserts were modified for optimal codon usage in primates. Second, antigen gene inserts were cloned into plasmid vectors containing enhanced promoter regulatory elements that have been shown to increase gene expression and in vivo immune responses when injected into macaques (1). These DNA plasmid vectors were modified by adding the R region regulatory sequence from the long terminal repeat (LTR) of human T-cell leukemia virus type 1 (HTLV-1), which was placed downstream of the cytomegalovirus (CMV) enhancer/promoter. Plasmids were delivered in multiple shots as homologous (DNA/DNA) or heterologous (DNA/rAd5) prime-boost vaccines, and rAd5 vaccine vectors were delivered as a single immunization or in a prime-boost series as shown in Fig. 1. The prime-boost groups received three priming immunizations with DNA vectors delivered intramuscularly by Biojector at weeks 0, 4, and 8. The final immunization followed at week 20 for the homologous DNA-GP vector boost or at week 23 for the heterologous vector rAd5-GP boost, which was delivered by needle injection. Subjects were challenged with 1,000 PFU of MARV Angola between 3 and 6 weeks after the final immunization.
FIG. 1.
Vaccination and challenge schedule. Vaccine group NHP were injected with plasmid DNA and rAd5 vectors encoding GP from MARV Angola. Individual DNA immunizations were administered 3 times at 4-week intervals, followed by final boosts with DNA at 23 weeks or rAd5 at 20 weeks. The DNA-only and DNA/rAd5 groups of NHP were exposed to MARV Angola at 26 weeks after the first vaccination. NHP immunized with rAd5 only were infected with MARV Angola at 4 weeks postvaccination.
Comparative immunogenicity of single-injection and prime-boost vaccines.
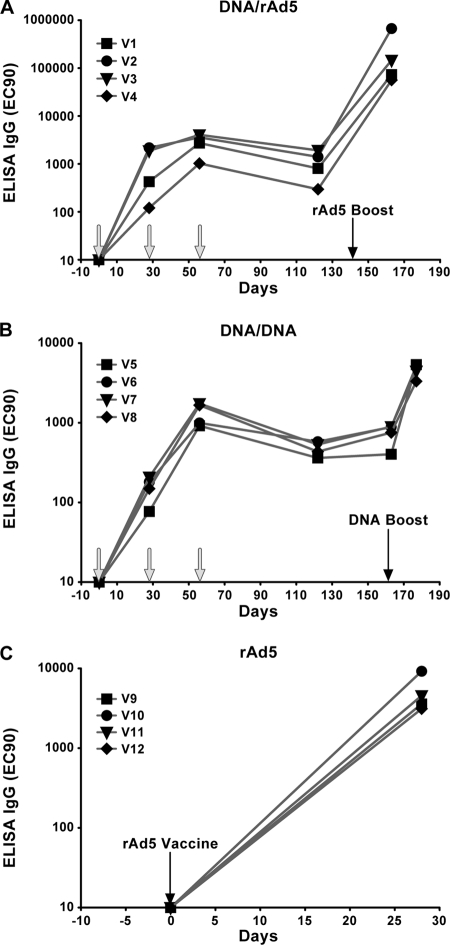
In previous studies to evaluate filovirus vaccines, we observed that high titers of IgG were induced in the sera of animals immunized with genetic vaccines expressing EBOV GP (21, 23) and that the presence of antigen-specific antibodies correlated with survival after infectious challenge (22). Therefore, to track the development of antigen-specific immunity elicited in the different groups shown in Fig. 1, the level of anti-MARV GP antibodies was assessed over the course of each immunization regimen. Samples were obtained prior to each immunization and again 1 to 3 weeks before infectious challenge with MARV Angola, and anti-MARV GP IgG titers were measured by ELISA as described in Materials and Methods. A single inoculation with DNA was immunogenic but elicited lower antibody titers than did a single shot of the rAd5 vaccine (Fig. 2). The average titer for all DNA-immunized subjects at week 4 was 1:653, about an order of magnitude lower than that for parallel samples from subjects immunized with rAd5-GP vaccines, 1:5,122 (P = 0.02). A second immunization with DNA-GP boosted titers in animals exhibiting the lowest titers preinjection (V1, V4, and V5 to V8), but the boosting effect of the second DNA injection was less pronounced in animals with higher initial titers (ca. 1:2,000; V2 and V3). A third injection with DNA-GP did not boost antibody titers beyond what was elicited after the second immunization. However, after a rest period of nearly 4 months, a significant boosting effect of subsequent DNA-GP immunization was observed. The fourth DNA injection, administered at week 163, caused antibody titers to increase from 1:734 to 1:4,590 (P = 0.002), with a final prechallenge titer comparable to that elicited by a single inoculation with the rAd5-GP vaccine. The highest antibody titers were achieved with a heterologous prime-boost vaccine. rAd5-GP boosted titers in DNA-primed animals more than 2 orders of magnitude to a final prechallenge GP ELISA IgG titer of 1:237,167. Therefore, the heterologous prime-boost modality demonstrated the greatest potency, with the highest-magnitude response being contributed by the rAd5-GP boost.
FIG. 2.
Development of vaccine-induced antibody responses. The quantity of anti-MARV GP IgG in plasma samples from vaccinated cynomolgus macaques was determined by ELISA as described in Materials and Methods. Results are shown for samples obtained at days 0, 28, 56, 122, and 163 and 0, 28, 56, 122, 163, and 177 for the DNA/rAd5 (A) and DNA-only (B) groups, respectively. Data for day 0 and 21 samples from rAd5-only-vaccinated NHP are presented in panel C. Arrows show times of vaccinations.
Induction of MARV GP cellular immune responses.
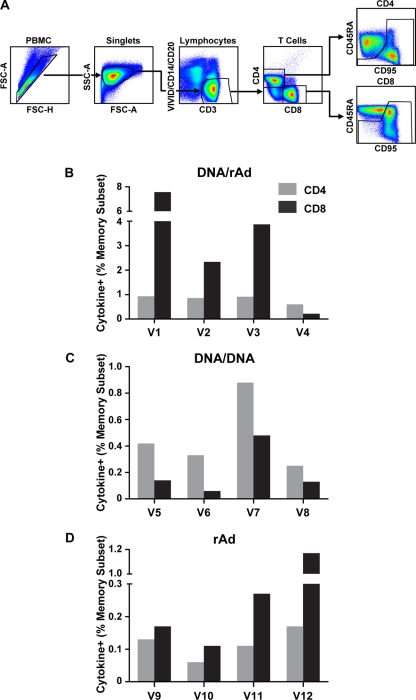
The cellular immune responses generated by each vaccine modality were assessed by measuring antigen-stimulated intracellular TNF-α, IFN-γ, and IL-2. Subject PBMC were obtained 1 week prior to infectious challenge with MARV Angola and stimulated with overlapping peptides spanning the GP open reading frame. Antigen-stimulated cells were evaluated by flow cytometric analysis for responses in CD4+ and CD8+ memory T-cell subsets using CD45RA and CD95 as phenotypic markers (Fig. 3A). All three vaccine modalities generated responses specific for MARV GP in both CD4+ and CD8+ T-cell subsets, but similarly to antibody titers, they trended higher in the heterologous prime-boost subjects (DNA/rAd) (Fig. 3). This result is consistent with previous reports in NHP that vaccination with rAd vectors provides a potent immunological boost in subjects previously primed with DNA vaccines (19, 23). On average, CD8+ responses predominated over CD4+ responses for all vaccine groups containing rAd-GP, whereas CD4+ responses dominated in the DNA/DNA group. In the group receiving a single inoculation with rAd5 vaccine, antigen-specific CD8+ T cells were higher than CD4+ responses for every subject and displayed an overall average of 0.43% of memory cells compared to 0.12 for CD4+ T cells (P = 0.13). In contrast, subjects not receiving rAd (DNA/DNA) displayed the lowest CD8+ T-cell immunity, with an average for the group equaling 0.2% of the memory subset. These subjects demonstrated cellular immunity dominated by CD4+ T cells (P = 0.08), with antigen-specific responses averaging 0.47% for the group. A CD8+ bias was also observed when rAd5-GP was used as a boost immunization for DNA-primed subjects, where there was a 4-fold excess of antigen-specific CD8+ over CD4+ T cells (P = 0.07). Notably, one subject in this group, V4, demonstrated an inverse ratio (CD4+ > CD8+), but this monkey exhibited lower responses overall. In general, the ratio of antigen-specific CD4+ to CD8+ cells induced by prime-boost vaccination was influenced primarily by the boosting immunization when the prime and boost vectors were not matched (DNA/rAd).
FIG. 3.
T-cell immune responses measured by intracellular cytokine staining. PBMC were obtained from macaques 1 week before infectious challenge and stimulated with overlapping peptides spanning the MARV GP. Antigen-specific CD4+ and CD8+ cells were enumerated in the memory cell subsets by intracellular cytokine staining and analysis by flow cytometry with gating as shown in panel A. FSC, forward scatter; SSC, side scatter. The total cytokine (TNF-α, IFN-γ, or IL-2) responses are depicted as percentages of the CD4+ (gray bars) or CD8+ (black bars) memory subset for DNA/rAd5 (B), DNA-only (C), and rAd5-only (D) groups, respectively.
Multiparameter flow cytometric analysis of T-cell quality.
In addition to the assessment of antigen-specific T-cell magnitude, the simultaneous measurement of multiple cytokines by ICS in all vaccinated subjects permitted assessment of qualitative differences in T-cell responses that are reflected in the combinations of cytokines produced, providing additional information regarding T-cell function. Cells making multiple cytokines have been described as “polyfunctional” since they produce cytokines related to both effector (IFN-γ or TNF-α) and proliferative (IL-2) potential (20), and both CD4+ and CD8+ cells exhibiting this phenotype have been associated with protection from viral, bacterial, and parasitic infections (2-4, 16).
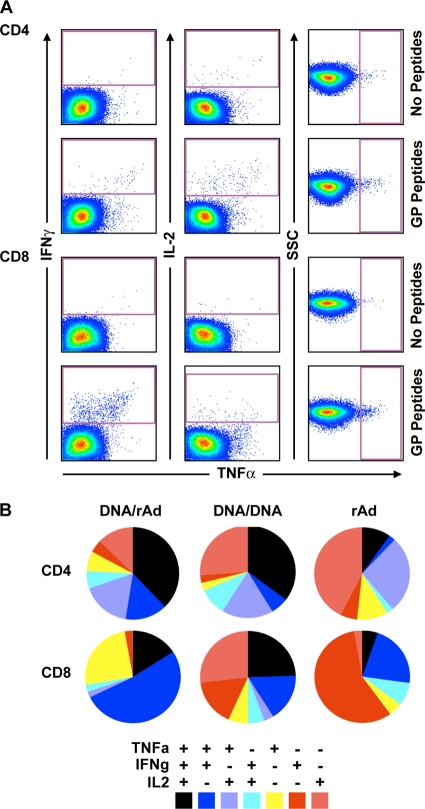
Figure 4 shows the relative proportions of CD4+ and CD8+ T cells producing the cytokines TNF-α, IFN-γ, and IL-2, alone or in combination, averaged across subjects in each vaccine group. Within the CD4+ T-cell subset, different vaccine regimens generated remarkably similar qualities, the most pronounced difference being that the rAd-GP vaccine elicited a smaller proportion of cells triple positive for TNF-α, IFN-γ, and IL-2 (black slice) compared to the groups receiving DNA-GP priming immunizations, where triple-positive cells constituted the major population. Instead, the dominant CD4+ functional phenotype in rAd-GP vaccinees was a subset of cells producing only IL-2 (pink slice). Differences in quality between vaccine groups were more pronounced in CD8+ T cells. Here, the proportion of polyfunctional cells producing two or three cytokines (black plus blue slices) was highest in the DNA/rAd group, intermediate for the DNA/DNA vaccine, and lowest in subjects receiving a single inoculation with rAd-GP. The predominant CD8+ population in the rAd group was cells producing IFN-γ only, followed by TNF-α plus IFN-γ double-positive cells, both populations representing an “effector” cell phenotype. The two vaccine groups receiving DNA-GP priming immunizations were distinguishable by their respective proportions of cells producing TNF-α plus IFN-γ, which was highest for DNA/rAd, versus IL-2 alone, which was highest in the DNA/DNA group. Notably, the IL-2 single-positive CD8+ T-cell population was almost undetectable in groups where rAd-GP was given alone or as a boost. In general, rAd-GP given as a single shot or boost for DNA-GP induced CD8+ T-cell populations with effector properties while DNA-GP immunization yielded CD4+ and CD8+ cells representative of memory cells, capable of producing IL-2 alone or in combination with other cytokines. Altogether, the total proportions of polyfunctional (triple- and double-positive) CD8+ T cells were highest in prime-boost groups (DNA/rAd5 > DNA/DNA) and lowest in subjects receiving a single-injection vaccine (rAd5-GP), suggesting that optimization for polyfunctional vaccine responses may require multiple immunizations.
FIG. 4.
Determination of T-cell polyfunctionality by multiparameter flow cytometry. CD4+ and CD8+ T-cell responses assessed for intracellular cytokine production of TNF-α, IFN-γ, and IL-2 were measured by flow cytometry. (A) Representative dot plots showing gates used to define IFN-γ, IL-2, and TNF-α expression in T-cell memory subsets. Peptide-stimulated and background responses are indicated. SSC indicates side scatter. (B) Boolean gating was used to calculate the percentages of cells making all possible combinations of one, two, or three cytokines, after subtracting background responses. The results are depicted in pie charts, where each slice represents the proportional contribution to total T-cell responses of that subset expressing the combination of cytokines specified at the bottom.
Infectious challenge of vaccinated macaques with MARV Angola.
To assess the relative potencies of the three vaccine modalities to provide immune protection against MARV infection, subjects were exposed to 1,000 PFU of MARV Angola by intramuscular injection. MARV hemorrhagic fever is accompanied by hepatic damage that results in elevated levels of circulating hepatic enzymes in humans (9, 11) and NHP (6). Therefore, infection was monitored in real time by assessment of circulating aspartate transaminase (AST) and alanine transaminase (ALT) enzymes in all subjects at time points immediately before challenge and every 3 to 4 days postexposure through day 14 (Fig. 5). Unvaccinated, control macaques displayed elevations in plasma AST and/or ALT at day 6 after exposure, indicating active infection in these subjects, while liver enzyme levels remained within normal values in all three groups of vaccinated macaques. Evaluation of liver enzymes in control subjects revealed elevations that predicted terminal infection; animals were euthanized or became moribund on days 8 and 9 (study I) and day 9 (study II), demonstrating lethal infection. To confirm MHF as the cause of mortality, plasma viral loads for MARV Angola were determined by conventional plaque assay (Fig. 5, rightmost column). Consistent with the clinical chemistry, active viral replication was detected in control subjects at day 6 post-MARV Angola exposure. Viremia remained below detectable levels in all vaccinated subjects throughout the course of study, demonstrating that single-modality DNA/DNA or rAd5, as well as heterologous prime-boost with DNA/rAd vectors, generated protective immunity in all subjects. While PCR can also be used as a sensitive assay to detect circulating virus, it, like plaque assay on Vero cells, suffers from potential false-negative detection if virus replication is primarily cell or tissue associated. Additional markers of infection were also monitored since elevations in liver enzymes or viremia can go undetected if they occur transiently between time points. Although lymphocytes are not directly infected by filoviruses, lymphopenia is frequently observed in infected subjects, possibly as a result of bystander apoptosis (7). Therefore, blood cell count monitoring was performed at each time point in the challenge study and lymphocyte numbers were determined for each subject (Table 1). Lymphocyte depletion was observed in all unvaccinated control macaques at day 6, consistent with other clinical markers of infection monitored in this study. Additionally, these subjects developed anorexia and a maculopapular rash that is characteristic for filovirus hemorrhagic fever (14). Unexpectedly, three (V5, V6, and V7) out of four DNA-GP-vaccinated animals also exhibited lymphocyte depletion on day 6 followed by rash and/or anorexia, suggesting that, while DNA vaccine-induced immunity successfully cleared virus, immune responses were suboptimal for control of virus replication to the degree necessary to prevent symptoms. These subjects all displayed ELISA IgG titers equivalent to levels observed in symptom-free subjects, and there was no significant neutralizing activity in sera from all vaccinated macaques. Similar to the DNA-only group, two subjects (V2 and V4) in the vaccine group containing DNA as a prime exhibited mild, transient symptoms apparent as lymphopenia or rash. A caveat to interpretation of vaccine efficacy results is that the infectious challenge was performed at slightly different intervals relative to the final immunization for each of the vaccine groups, and this could contribute partially to any differences observed in immune response or clinical symptoms. Nonetheless, the relationship between specific immune responses at the time of challenge and protection from illness or mortality reveals important associations. It is noteworthy, for example, that subjects exhibiting symptoms of MHF had the lowest numbers of antigen-specific T cells within their respective vaccine groups (Fig. 3) and also had lower proportions of polyfunctional CD8+ T cells exhibiting a triple-positive or double-positive TNF-α/IFN-γ phenotype compared to healthy animals within the group (data not shown). Instead, the symptomatic subjects also showed a higher proportion of single-positive cells producing IL-2, a phenotype that is unusual in the CD8+ T-cell lineage. Together, these data demonstrate that all three vaccine modalities delivering MARV GP with either DNA or rAd vectors provided protection from mortality against MARV Angola and that a single inoculation with rAd vaccine induced optimal immune responses to eliminate both symptoms and mortality from MHF.
FIG. 5.
Liver enzymes and viremia in plasma after MARV Angola challenge. Control and vaccinated animals were exposed to a target dose of 1,000 PFU of MARV Angola. Blood samples were collected before and after infection for the determination of hepatic enzymes and plasma viremia levels. AST and ALT were measured using a General Chemistry 12 reagent disk for the Piccolo analyzer (Abaxis). Plasma viremia was determined by plaque-forming assay on Vero cells. Control and vaccinated animal values are shown by green and black lines, respectively.
TABLE 1.
Clinical symptoms after MARV Angola challenge in control and vaccinated NHPa
| Subject | Study | Vaccine group | Symptom |
|
|---|---|---|---|---|
| CBC | Observation(s) | |||
| V1 | I | DNA/rAd | Normal | None |
| V2 | I | DNA/rAd | Normal | Rash (day 10) |
| V3 | I | DNA/rAd | Normal | None |
| V4 | I | DNA/rAd | Lymphopenia (day 6) | None |
| V5 | I | DNA/DNA | Lymphopenia (day 6) | None |
| V6 | I | DNA/DNA | Lymphopenia (day 6) | Anorexia (day 7), rash (day 10) |
| V7 | I | DNA/DNA | Normal | Normal |
| V8 | I | DNA/DNA | Lymphopenia (day 6) | Anorexia (days 7-10) |
| C1 | I | Control | Lymphopenia (day 6) | Anorexia, rash (days 7-8) |
| C2 | I | Control | Lymphopenia (day 6) | Anorexia, rash (days 7-8) |
| V9 | II | rAd | Normal | None |
| V10 | II | rAd | Normal | None |
| V11 | II | rAd | Normal | None |
| V12 | II | rAd | Normal | None |
| C3 | II | Control | Lymphopenia (day 6) | Anorexia (days 7-8), rash (day 8) |
Two independent infectious challenge studies were performed, and inclusion of individual subjects is denoted by Roman numerals. During the infectious challenge experiment, blood samples were obtained at days 0, 3, 6, 10, 14, and 28 for complete blood cell (CBC) measurements. Lymphopenia was noted for animals displaying a drop in lymphocyte count greater than 20% of baseline (day 0 counts) for that subject. Animals were observed for changes in behavior, food intake, and rash and were weighed at least once daily. Anorexia was noted if less than one-half of the daily food ration was consumed in a 24-h period.
DISCUSSION
The studies reported herein were undertaken to examine whether DNA vaccines could be improved to protect against lethal MARV challenge and to study the relative potency of vaccines when administered as recombinant DNA and/or viral vectors for immune protection against MARV Angola. Although methylated CpG in DNA plasmids theoretically serves as a natural adjuvant, stimulation through TLR9 appears to be less effective than activation mechanisms by viral vectors for the induction of innate responses, therefore resulting in lower immunogenicity of DNA vaccines in primates (13). Approaches to improve the potency of DNA vaccines have included methods to increase antigen expression, alter tissue targeting, and enhance immune responses through the use of adjuvants (12). In the current work we focused on two approaches to generate efficient antigen expression, promoter modification to enable efficient transcription in mammalian cells (1) and alteration of the GP gene insert for optimum codon usage (27). Partial protection against MARV (Musoke strain) was previously observed after DNA immunization at multiple sites by gene gun (17), and it was suggested that efficacy was due to gene gun targeting of the immunogen to antigen-presenting cells in skin tissue. Therefore, the use of Biojector delivery of the DNA vaccine in our studies may also have contributed to the improved efficacy for protection. Prior to this report, uniform protection from MARV mortality in NHP has been achieved only with vaccines containing viral components, as either vectors or virus-like particles (8, 10, 24, 25). The results herein demonstrate the potential utility of DNA plasmids for use as vaccine vectors in primates and suggest that improvements in antigen expression and delivery may be a valid approach for enhancing immune responses induced by DNA vaccines.
The results presented here also shed light on the nature of immune responses that are associated with vaccine protection against MARV Angola mortality. We showed previously that a prime-boost vaccine using DNA and rAd vectors protected against lethal infection with the Zaire species of EBOV, but homologous vector vaccines were not tested in that study and so the contribution of each component to protective immunity could not be assessed (23). Here, the vaccine vectors were compared singly and in combination. It is interesting that the group receiving only DNA vaccines generated levels of antibody against MARV GP similar to those of the rAd-only group and yet exhibited a reduced level of protection against illness. Riemenschneider et al. also observed a lack of correlation between antibody levels and protection after vaccination with a DNA vaccine (17). Cell-mediated immunity was not reported in that study, but the present work reveals the balance of T-cell responses (CD4/CD8) and functional phenotype to be distinguishing features between groups that became ill and those that were fully protected. Both vaccine modalities that induced skewing toward CD4+ T-cell immunity mediated incomplete protection against symptoms of MARV infection, and for the DNA/rAd vaccine, this was despite the induction of a robust CD8+ T-cell response. This observation suggests that protective immunity against MARV Angola depends on qualitative differences that are not captured by measures of the absolute magnitude of antibody or cell-mediated immune responses, as observed for other pathogens (5). Vaccine modalities using DNA to prime the immune response generated T cells with a polyfunctional quality: cells that simultaneously produce TNF-α, IFN-γ, and IL-2. Although this phenotype has defined protective immunity for a variety of pathogens (2-4, 16), the generation of polyfunctional CD4+ and CD8+ cells did not associate with the rAd-GP vaccine group that provided the most robust protection (absence of symptoms) against MARV Angola. It is noteworthy that many of the previous studies were designed to evaluate long-term memory against persistent chronic infections where it would be important to generate long-lived cells with a triple-positive cytokine phenotype. Filovirus infection kills susceptible hosts within days to weeks, and the unvaccinated NHP in this study were moribund or euthanized between days 8 and 9. Therefore, it is not surprising that the rapid generation of CD8+ T cells lacking the polyfunctional phenotype that includes IL-2 but instead possessing a dominant effector phenotype (TNF-α and IFN-γ), such as that induced by the rAd-GP vaccine, provided robust protection against infection. The relatively small number of animals used in these studies did not reveal statistically significant differences in T-cell polyfunctional quality within each vaccine group between subjects who remained healthy and those exhibiting clinical symptoms, but it is perhaps noteworthy that the single symptom-free subject in the DNA vaccine group had a higher proportion of CD8+ T cells expressing TNF-α and IFN-γ (with or without IL-2) and a lower representation of cells single positive for IL-2, indicating the dominance of T cells with greater effector potential in the healthy subject (data not shown). Similarly, in the DNA/rAd vaccine subjects that remained healthy there were higher proportions of CD8+ T cells expressing the effector cytokines TNF-α and IFN-γ.
The protection from mortality afforded by the DNA vaccine used herein suggests that plasmid vectors, previously thought to be ineffective as vaccines for filoviruses, may hold promise. The inherent safety and stability of this platform make it attractive as a vaccine to be used in areas lacking refrigeration, such as regions of Africa where filovirus outbreaks are endemic. Because of the high magnitude of antigen-specific responses achieved by heterologous prime-boost, it has been proposed that long-term immunity may be optimally achieved by priming rAd with DNA (18). This is another important consideration for vaccines used in populations where immunization programs are less well developed and subjects may have only a single opportunity to be vaccinated. The studies shown here suggest that the value of DNA priming will depend on the pathogen, antigen, and mechanism of immune protection. For MARV Angola, these studies suggest that it will be important for DNA and rAd vaccines to be optimized to skew immunity toward CD8+ T cells with effector properties, while also maintaining CD4+ and antibody responses.
Acknowledgments
We are grateful for generous support from Gary Nabel throughout the course of these studies. We thank Zhi-Yong Yang for construction of modified DNA vectors encoding MARV GP; Joshua Johnson for assistance with data management; Ati Tislerics and Brenda Hartman for help with manuscript preparation; Michael Cichanowski for graphics; J. P. Todd, Vi Dang, Srinivas Rao, and Sarah Norris for helpful discussions; and Kathryn Kenyon for editorial support.
Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the U.S. Army or the Department of Defense.
Footnotes
Published ahead of print on 21 July 2010.
REFERENCES
- 1.Barouch, D. H., Z. Y. Yang, W. P. Kong, B. Korioth-Schmitz, S. M. Sumida, D. M. Truitt, M. G. Kishko, J. C. Arthur, A. Miura, J. R. Mascola, N. L. Letvin, and G. J. Nabel. 2005. A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates. J. Virol. 79:8828-8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casazza, J. P., M. R. Betts, D. A. Price, M. L. Precopio, L. E. Ruff, J. M. Brenchley, B. J. Hill, M. Roederer, D. C. Douek, and R. A. Koup. 2006. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. J. Exp. Med. 203:2865-2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darrah, P. A., D. T. Patel, P. M. De Luca, R. W. Lindsay, D. F. Davey, B. J. Flynn, S. T. Hoff, P. Andersen, S. G. Reed, S. L. Morris, M. Roederer, and R. A. Seder. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843-850. [DOI] [PubMed] [Google Scholar]
- 5.De Rosa, S. C., F. X. Lu, J. Yu, S. P. Perfetto, J. Falloon, S. Moser, T. G. Evans, R. Koup, C. J. Miller, and M. Roederer. 2004. Vaccination in humans generates broad T cell cytokine responses. J. Immunol. 173:5372-5380. [DOI] [PubMed] [Google Scholar]
- 6.Geisbert, T. W., K. M. Daddario-DiCaprio, J. B. Geisbert, H. A. Young, P. Formenty, E. A. Fritz, T. Larsen, and L. E. Hensley. 2007. Marburg virus Angola infection of rhesus macaques: pathogenesis and treatment with recombinant nematode anticoagulant protein c2. J. Infect. Dis. 196(Suppl. 2):S372-S381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geisbert, T. W., L. E. Hensley, T. R. Gibb, K. E. Steele, N. K. Jaax, and P. B. Jahrling. 2000. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab. Invest. 80:171-186. [DOI] [PubMed] [Google Scholar]
- 8.Hevey, M., D. Negley, P. Pushko, J. Smith, and A. Schmaljohn. 1998. Marburg virus vaccines based upon alphavirus replicons protect guinea pigs and nonhuman primates. Virology 251:28-37. [DOI] [PubMed] [Google Scholar]
- 9.Johnson, E. D., B. K. Johnson, D. Silverstein, P. Tukei, T. W. Geisbert, A. N. Sanchez, and P. B. Jahrling. 1996. Characterization of a new Marburg virus isolated from a 1987 fatal case in Kenya. Arch. Virol. Suppl. 11:101-114. [DOI] [PubMed] [Google Scholar]
- 10.Jones, S. M., H. Feldmann, U. Stroher, J. B. Geisbert, L. Fernando, A. Grolla, H. D. Klenk, N. J. Sullivan, V. E. Volchkov, E. A. Fritz, K. M. Daddario, L. E. Hensley, P. B. Jahrling, and T. W. Geisbert. 2005. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 11:786-790. [DOI] [PubMed] [Google Scholar]
- 11.Kissling, R. E., F. A. Murphy, and B. E. Henderson. 1970. Marburg virus. Ann. N. Y. Acad. Sci. 174:932-945. [DOI] [PubMed] [Google Scholar]
- 12.Kutzler, M. A., and D. B. Weiner. 2008. DNA vaccines: ready for prime time? Nat. Rev. Genet. 9:776-788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu, S., S. Wang, and J. M. Grimes-Serrano. 2008. Current progress of DNA vaccine studies in humans. Expert Rev. Vaccines 7:175-191. [DOI] [PubMed] [Google Scholar]
- 14.Mahanty, S., and M. Bray. 2004. Pathogenesis of filoviral haemorrhagic fevers. Lancet Infect. Dis. 4:487-498. [DOI] [PubMed] [Google Scholar]
- 14a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 15.Perfetto, S. P., P. K. Chattopadhyay, L. Lamoreaux, R. Nguyen, D. Ambrozak, R. A. Koup, and M. Roederer. 2006. Amine reactive dyes: an effective tool to discriminate live and dead cells in polychromatic flow cytometry. J. Immunol. Methods 313:199-208. [DOI] [PubMed] [Google Scholar]
- 16.Precopio, M. L., M. R. Betts, J. Parrino, D. A. Price, E. Gostick, D. R. Ambrozak, T. E. Asher, D. C. Douek, A. Harari, G. Pantaleo, R. Bailer, B. S. Graham, M. Roederer, and R. A. Koup. 2007. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J. Exp. Med. 204:1405-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riemenschneider, J., A. Garrison, J. Geisbert, P. Jahrling, M. Hevey, D. Negley, A. Schmaljohn, J. Lee, M. K. Hart, L. Vanderzanden, D. Custer, M. Bray, A. Ruff, B. Ivins, A. Bassett, C. Rossi, and C. Schmaljohn. 2003. Comparison of individual and combination DNA vaccines for B. anthracis, Ebola virus, Marburg virus and Venezuelan equine encephalitis virus. Vaccine 21:4071-4080. [DOI] [PubMed] [Google Scholar]
- 18.Santra, S., M. S. Seaman, L. Xu, D. H. Barouch, C. I. Lord, M. A. Lifton, D. A. Gorgone, K. R. Beaudry, K. Svehla, B. Welcher, B. K. Chakrabarti, Y. Huang, Z. Y. Yang, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2005. Replication-defective adenovirus serotype 5 vectors elicit durable cellular and humoral immune responses in nonhuman primates. J. Virol. 79:6516-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santra, S., Y. Sun, B. Korioth-Schmitz, J. Fitzgerald, C. Charbonneau, G. Santos, M. S. Seaman, S. J. Ratcliffe, D. C. Montefiori, G. J. Nabel, H. C. Ertl, and N. L. Letvin. 2009. Heterologous prime/boost immunizations of rhesus monkeys using chimpanzee adenovirus vectors. Vaccine 27:5837-5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seder, R. A., P. A. Darrah, and M. Roederer. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247-258. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan, N. J., T. W. Geisbert, J. B. Geisbert, L. Xu, Z. Y. Yang, M. Roederer, R. A. Koup, P. B. Jahrling, and G. J. Nabel. 2003. Accelerated vaccination for Ebola virus haemorrhagic fever in non-human primates. Nature 424:681-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan, N. J., J. E. Martin, B. S. Graham, and G. J. Nabel. 2009. Correlates of protective immunity for Ebola vaccines: implications for regulatory approval by the animal rule. Nat. Rev. Microbiol. 7:393-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 24.Swenson, D. L., D. Wang, M. Luo, K. L. Warfield, J. Woraratanadharm, D. H. Holman, J. Y. Dong, and W. D. Pratt. 2008. Vaccine to confer to nonhuman primates complete protection against multistrain Ebola and Marburg virus infections. Clin. Vaccine Immunol. 15:460-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swenson, D. L., K. L. Warfield, T. Larsen, D. A. Alves, S. S. Coberley, and S. Bavari. 2008. Monovalent virus-like particle vaccine protects guinea pigs and nonhuman primates against infection with multiple Marburg viruses. Expert Rev. Vaccines 7:417-429. [DOI] [PubMed] [Google Scholar]
- 26.Towner, J. S., M. L. Khristova, T. K. Sealy, M. J. Vincent, B. R. Erickson, D. A. Bawiec, A. L. Hartman, J. A. Comer, S. R. Zaki, U. Stroher, F. Gomes da Silva, F. del Castillo, P. E. Rollin, T. G. Ksiazek, and S. T. Nichol. 2006. Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola. J. Virol. 80:6497-6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uchijima, M., A. Yoshida, T. Nagata, and Y. Koide. 1998. Optimization of codon usage of plasmid DNA vaccine is required for the effective MHC class I-restricted T cell responses against an intracellular bacterium. J. Immunol. 161:5594-5599. [PubMed] [Google Scholar]