Abstract
Cellular integrins were identified as human cytomegalovirus (HCMV) entry receptors and signaling mediators in both fibroblasts and endothelial cells. The goal of these studies was to determine the mechanism by which HCMV binds to cellular integrins to mediate virus entry. HCMV envelope glycoprotein B (gB) has sequence similarity to the integrin-binding disintegrin-like domain found in the ADAM (a disintegrin and metalloprotease) family of proteins. To test the ability of this region to bind to cellular integrins, we generated a recombinant soluble version of the gB disintegrin-like domain (gB-DLD). The gB-DLD protein bound to human fibroblasts in a specific, dose-dependent and saturable manner that required the expression of an intact β1 integrin ectodomain. Furthermore, a physical association between gB-DLD and β1 integrin was demonstrated through in vitro pull-down assays. The function of this interaction was shown by the ability of cell-bound gB-DLD to efficiently block HCMV entry and the infectivity of multiple in vivo target cells. Additionally, rabbit polyclonal antibodies raised against gB-DLD neutralized HCMV infection. Mimicry of the ADAM family disintegrin-like domain by HCMV gB represents a novel mechanism for integrin engagement by a virus and reveals a unique therapeutic target for HCMV neutralization. The strong conservation of the DLD across beta- and gammaherpesviruses suggests that integrin recognition and utilization may be a more broadly conserved feature throughout the Herpesviridae.
Like many other herpesviruses, human cytomegalovirus (HCMV) is an opportunistic pathogen that is able to asymptomatically infect the human population with high incidence throughout the world. Primary infection is followed by a life-long latent phase that may reactivate and cause disease during the immunosuppression experienced by AIDS patients and organ transplant recipients (14, 52). HCMV disease is also a cause of significant morbidity and mortality during primary congenital infections (66). Currently there is no effective HCMV vaccine, and HCMV antiviral therapies, such as ganciclovir, are highly toxic and unsuitable for treating pregnant women in the congenital setting (92).
HCMV disease can manifest itself in most organ systems and tissue types. Pathology from HCMV-infected individuals reveals that HCMV can infect most cell types, including fibroblasts, endothelial cells, epithelial cells, smooth muscle cells, stromal cells, monocytes/macrophages, neutrophils, neuronal cells, and hepatocytes (20, 25, 77, 83, 87). The broad intrahost organ and tissue tropism of HCMV is paralleled in vitro with the virus' ability to bind and fuse with nearly every vertebrate cell type tested (40, 62, 78). However, full productive infection is limited to secondary strains of fibroblasts and endothelial cells. The ability of HCMV to enter such a diverse range of cell types is indicative of multiple cell-specific receptors, broadly expressed receptors, or a complex entry pathway in which a combination of both cell-specific and broadly expressed cellular receptors are utilized.
The genes that encode envelope glycoprotein B (gB) and gH are essential (37), play several key roles during virus entry and egress, and are conserved throughout the Herpesviridae (reviewed in reference 80). A soluble form of gB truncated at the transmembrane domain (gBs) binds to permissive cells specifically, blocks virus entry, and is sufficient to trigger signal transduction events that result in the activation of an interferon-responsive pathway that is also activated by HCMV virions (10, 12, 13).
HCMV entry requires initial tethering of virions to cell surface heparan sulfate proteoglycans (HSPGs) (22, 80). The HCMV envelope contains at least two separate glycoprotein complexes with affinities for heparan sulfate: gB (22) and the gM/gN complex (48). The gM/gN complex is more abundant than gB within the envelope (88) and binds heparin with higher affinity (49). Thus, the gM/gN complex is thought to be the primary heparin-binding component of the HCMV envelope.
Virus-cell tethering via HSPGs is followed by a more stable interaction and subsequent signal transduction cascades. This interaction was proposed to be mediated via cell surface epidermal growth factor receptor (EGFR) (17, 95). These data, however, conflicted with more recent reports that demonstrate EGFR is not explicitly required for infection (21, 42). Platelet-derived growth factor receptor (PDGFR) has also been reported to function as an attachment receptor that functions to activate signaling cascades required for infection (79). The relative contribution of signaling and virus-host cell attachment for each of these growth factor receptors remains to be further characterized. The possibility also exists that additional attachment receptors still remain unidentified.
Integrins are expressed on the cell surfaces of all vertebrate cells, a characteristic that parallels the promiscuity of HCMV entry. Additionally, β1 integrins are capable of mediating many of the same signal transduction pathways that are triggered during HCMV entry into host cells. Upon binding and fusing with host cell surfaces, HCMV triggers changes in Ca2+ homeostasis (36) and the activation of phospholipases C and A2, as well as an increased release of arachidonic acid and its metabolites (2). Additionally, mitogen-activated protein kinase (MAPK) (44, 45), phosphatidylinositol-3-OH kinase (PI3-K) (46), and G proteins are activated (73). Indeed, it was shown that HCMV entry led to an activation of integrin signaling pathways that reorganized the actin cytoskeleton (31) and phosphorylated β1 and β3 integrin cytoplasmic domains (31), focal adhesion kinase (FAK) (31), and Src (94). Integrin antibody blocking studies in combination with HCMV infectivity assays in β1 integrin-null GD25 cells identified α2β1, α6β1, and αVβ3 integrins as HCMV “postattachment” entry receptors (31). Certain integrin signaling events could be triggered by both HCMV and a soluble version of gB and require the expression of β1 integrin, identifying this specific viral ligand in integrin engagement (31).
ADAM family members are multifunctional proteins that contain a metalloproteinase domain involved in ectodomain shedding and a disintegrin module of approximately 90 amino acids that confers RGD-independent integrin binding (43, 81, 99). The minimum component of the disintegrin module required for integrin engagement is the 12- to 13-amino-acid disintegrin loop, for which a consensus sequence has been described: RX6DLXXF (29). The 20-amino-acid stretch encompassing the gB disintegrin-like domain is highly conserved, with greater than 98% amino acid identity among HCMV clinical isolates. Additionally, this domain is present in most gammaherpesviruses and all betaherpesviruses, suggesting that integrin engagement may be a conserved feature for most of the Herpesviridae. Synthetic peptides of the gB disintegrin loop block virus fusion (tegument delivery) but not virus attachment (31). This fact suggests a disintegrin-mediated molecular mechanism of herpesvirus-integrin engagement. Glycoprotein H (gH) has also been identified as an αVβ3 integrin ligand (94). However, gH contains no previously identified integrin recognition motifs, and the αVβ3 integrin heterodimer does not typically engage ADAM family proteins.
Herein, we explore the molecular mechanism of integrin engagement by HCMV envelope gB. We provide multiple lines of evidence that demonstrate a physical interaction between the gB disintegrin module with β1 integrin. Furthermore, this interaction has significant consequences to the viral life cycle, since a soluble version of the gB disintegrin module efficiently blocks HCMV infection at a postattachment step during entry into multiple in vivo cell targets. Similarly, polyclonal antibodies directed against the gB disintegrin-like domain neutralize HCMV infectivity. These data identify the molecular mechanism of an HCMV ligand-receptor interaction required for virus-host fusion.
MATERIALS AND METHODS
Cells and viruses.
β1 integrin knockout fibroblasts (GD25), β1 integrin-restored GD25 cells (GD25β1) (97), and β1 integrin-restored GD25 expression cytoplasmic double tyrosine mutant (GD25β1-YYFF) (68, 96) cells were a generous gift from D. Mosher (University of Wisconsin, Madison). Normal human dermal fibroblasts (NHDFs), GD25, GD25β1, and GD25β1-YYFF cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (GIBCO) in a 5% CO2 atmosphere at 37°C. GD25β1 and GD25β1-YYFF were grown in medium that also contained 20 μg/ml puromyocin (Sigma). To prevent heparan sulfate biosynthesis, NHDF, GD25, GD25β1, and GD25β1-YYFF cells were grown in the presence of sulfate-deficient minimal essential medium (MEM), as previously described (23, 74, 100). Human umbilical vein endothelial cells (HUVEC; Clonetics) were maintained in endothelial cell basal medium 2 (EBM-2) supplemented with 10% fetal bovine serum and other recommended growth factors (EGM-2 SingleQuots; Cambrex) in a 5% CO2 atmosphere at 37°C. HCMV AD169 was grown and titers were determined on NHDFs as previously described (62). [3H]thymidine-labeled HCMV AD169 was grown and gradient purified and titers were determined on NHDFs as previously described (84). HCMV AD169 producing immediate-early protein 2 fused to green fluorescent protein (GFP) was a kind gift from D. Spector (University of California, San Diego) and was grown and titers were determined as previously described (69). Endothelial cell tropic HCMV strain VHL/e was grown in HUVEC and the titers were determined as previously described (90, 91). Herpes simplex virus 1 strain (HSV-1) strain HSV-1(KOS)gL86 marked with the Escherichia coli lacZ gene was a gift from R. Montgomery (University of Wisconsin, Madison) (57).
Proteins.
A fragment corresponding to amino acids 57 to 146 of gB (gB disintegrin-like domain [DLD]) was cloned from pCAGGS-gB (13) by PCR with the following primers: 5′ gB-DLD (5′-GGA ATT CCA TAT GGT AAC GTC TTC TGA AGC C-3′) and 3′gB-DLD (5′-CGG GAT CCT TAA ACC TTT TGG TAG ACC CG-3′). The gB-DLD fragment was cloned into the NdeI and BamHI sites of the bacterial expression vector pET-28a (Novagen, Madison, WI) with an amino-terminal His6 tag fragment corresponding to amino acids 651 to 718 of gB (gB-S651-718). This fragment was also amplified from pCAGGS-gB by PCR with the following primers: OML30 (5′-CGG GAT CCA TGG ATA TCG ACC CGC TGG AA-3′) and OML31 (5′-CG AGA TCT TCG AAT TAC TAC TAC TAC TAC TAC TGA AGG AGC ACC TTG TTC GTC CGG CGA GTA CTC CAG CAG-3′). The fragment was cloned into the NcoI and HindIII sites of the bacterial expression vector pTriEx-1.1 (Novagen) with a carboxyl-terminal His6 tag. Both vectors were transformed into E. coli DH5α. To produce recombinant protein, gB-DLD and gB-S651-718 plasmids were isolated by using the FastPlasmid minikit per the manufacturer's instructions (Eppendorf, Hamburg, Germany) and transformed into E. coli BL21(DE3) strain for protein expression. E. coli containing the pET-28a-gB-DLD construct was grown at 37°C in Luria-Bertani (LB) medium containing kanamycin (50 μg/ml) and cells with the pTriEx 1.1-gB-S651-718 construct were grown in LB medium containing ampicillin (50 μg/ml) to an A600 of 0.6. To produce radiolabeled gB-DLD, E. coli containing the pET-28a-gB-DLD fusion was grown at 37°C in Vogel's medium (26) without sulfate as described above. Ten microcuries of 35S-labeled Na2SO4 was then added at the time of protein induction. To induce recombinant protein, isopropyl-β-d-thiogalactopyranoside (IPTG; Roche, Indianapolis, IN) was added to a final concentration of 1 mM and incubated for 4 h at 37°C. The cells were chilled on ice and harvested by centrifugation at 4,000 rpm for 10 min at 4°C. Protein was isolated from inclusion bodies as previously described (61). Protein was then solubilized in 8 M urea-300 mM NaCl-10 mM imidazole-50 mM Tris (pH 7.9). Affinity purification of the proteins was accomplished through nickel-nitriloacetic acid agarose (Ni-NTA) columns (Qiagen, Valencia, CA) per the manufacturer's instructions. Eluate from the Ni-NTA column was placed onto an S-200 sizing column, and 1-ml fractions were collected. Fractions containing protein were determined by measuring the absorbance of each fraction at 214 nm using a SpectraMax 190 spectrophotometer (Molecular Devices, Sunnyvale, CA). Fractions that corresponded to absorption peaks were analyzed by sodium dodecyl sulfate-polyacrylamide electrophoresis (SDS-PAGE) to determine the size of the protein. The absorption peak fractions that contained a protein of the same size as the gB-DLD or gB-S651-718 were pooled and concentrated using a Ni-NTA column as described above. The concentrated fractions of gB-DLD were dialyzed extensively against 55 mM MES (pH 5.5), 300 mM NaCl, while gB-S651-718 fractions were dialyzed against 55 mM Tris (pH 8.3), 300 mM NaCl.
Antibodies.
Monoclonal antibodies (MAb) used for Western blotting in the gB-DLD pull-down experiment that were specific for β1 integrin (MAb 1965) and β3 integrin (MAb 2008) were purchased from Chemicon (Temecula, CA). For immunoprecipitation (IP) experiments a mouse monoclonal anti-gB (Abcam), mouse monoclonal anti-integrin β3 (Abcam), or mouse polyclonal anti-integrin β1 (Millipore) antibody was used. The antibodies used for Western blotting of the immunoprecipitations were a human monoclonal anti-gB (ITC88), rabbit monoclonal anti-integrin β3 (Abcam), and rabbit polyclonal anti-integrin β1 (Millipore). Monoclonal antibody 1203, which recognizes the immediate-early gene products of HCMV, was purchased from the Rumbaugh-Goodwin Institute for Cancer Research, Inc. (Plantation, FL). A monoclonal antibody raised against the tegument protein pp65 was purchased from Advanced Biotechnologies (Columbia, MD). His probe (sc-803), a rabbit polyclonal raised against a His-tagged recombinant protein that recognizes His6-tagged proteins, was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Fluorescein-conjugated and horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Pierce (Rockford, IL). To generate rabbit polyclonal antibodies to gB-DLD, gB-DLD was cross-linked to keyhole limpet hemocyanin (KLH) by using 1-ethyl-3-dimethyl aminopropylcarbodiimide (EDC), prior to immunization. Pathogen-free, barrier-raised New Zealand White rabbits (HsdOkd:NZW) were primed by injection with gB-DLD, followed by dosing administered 28, 56, and 84 days later. Rabbit serum was collected 98, 105, and 112 days following the primary immunization (Harlan Bioproducts for Science, Madison, WI). Total IgG antibodies were purified from serum using the ImmunoPure (G) IgG purification kit per the manufacturer's instructions (Pierce, Rockford, IL).
Immunoprecipitation and immunoblotting.
NHDF cells were infected with adenovirus particles expressing full-length VR1814 gB (20 PFU/cell). Protein expression was allowed to proceed for 72 h before the cells were washed in 1× phosphate-buffered saline (PBS; 0.137 M NaCl, 0.0027 M KCl, 0.1 M Na2HPO4, 0.002 M KH2PO4, pH 7.4) and lysed (0.025 M Tris, 0.15 M NaCl, 0.001 M EDTA, 1% NP-40, 5% glycerol, pH 7.4) in the presence of protease inhibitors (Thermo Scientific). A 500-μg aliquot of lysate was incubated with 5 μg of antibody for 16 h at 4°C. Complexes were immunoprecipitated using the Classic IP kit (Pierce Biotechnology) according to the manufacturer's protocol. The complexes were washed two times in lysis buffer and two times in 1× Tris-buffered saline (0.025 M Tris, 0.15 M NaCl; pH 7.2). Samples were boiled for 5 min at 100°C. Protein was then run on 4 to 12% SDS-PAGE gels and transferred to nitrocellulose membranes. Membranes were blocked in PBST (0.137 M NaCl, 0.0027 M KCl, 0.1 M Na2HPO4, 0.002 M KH2PO4, 0.05% Tween 20; pH 7.4) containing 5% dried milk. Blots were probed with the respective antibodies, all diluted 1:1,000 in PBST containing 5% dried milk for 16 h 4°C. The membranes were then washed in PBST and incubated with the appropriate secondary antibody conjugated to HRP diluted 1:2,000 in PBST containing 5% dried milk for 1 h. HRP was detected using Amersham ECL Plus Western blotting detection reagents (GE Healthcare) according to the manufacturer's instructions. HRP signal was detected using Amersham Hyperfilm ECL (GE Healthcare) and a Kodak X-OMAT 2000A processor.
Protein-binding experiments.
NHDF, GD25, GD25β1, and GD25β1-YYFF cells were grown to confluence in 96-well plates. Cells were chilled to 4°C, washed in cold PBS, and blocked in 1 mg/ml bovine serum albumin (BSA) for 60 min. Cells were then washed with cold PBS and MES protein buffer and incubated with 10 μg/ml of the indicated protein for 30 min or during the time course experiment for the indicated times. Cells were then washed briefly in PBS and fixed with 3% paraformaldehyde. Bound His-tagged protein was detected with polyclonal antibody His probe (sc-803), horseradish peroxidase-conjugated goat anti-mouse secondary antibody, and ImmunoPure tetramethylbenzidine substrate (Pierce) with absorbance measured at 405 nm. The specific activity for radiolabeled gB-DLD was 55,000 cpm. For radiolabeled binding experiments, NHDF, GD25, GD25β1, and GD25β1-YYFF cells were grown to complete confluence in 24-well plates. Cells were chilled to 4°C, washed in cold PBS, and blocked in 1 mg/ml BSA for 60 min. Cells were then washed with cold PBS and MES protein buffer and incubated with 5 μg/ml of the indicated protein for 30 min. Cells were then briefly washed in PBS to remove unbound protein in PBS, lysed in 1% Triton X-100, and scintillation counts were determined.
Homologous competition.
NHDFs were grown to confluence on 12-well plates. Cells were chilled to 4°C, washed in cold PBS, and blocked in 1 mg/ml BSA for 60 min. Cells were then washed with cold PBS and MES protein buffer and incubated with 2 μg/ml of radiolabeled gB-DLD in the presence of increasing amounts of unlabeled gB-DLD for 30 min. Cells were then washed three times with PBS to remove unbound protein and lysed in 1% Triton X-100, and radioactivity (in cpm) was determined for by scintillation.
Virus infectivity and entry assays.
To assay for HCMV infectivity, NHDFs were incubated with gB-DLD or gB-651 for 60 min at 37°C. Cells were washed with PBS and incubated with HCMV AD169-GFP at an approximate multiplicity of infection (MOI) of 0.5 PFU per cell for 60 min at 37°C. Nonpenetrated virus was inactivated with low-pH citrate buffer and removed with PBS washes. At 24 h postinfection flow cytometry was performed to quantitate GFP-positive versus total NHDF cells. Cell viability was simultaneously measured by propidium iodide exclusion. To assay for entry by pp65 nuclear localization, NHDFs were plated on glass coverslips and incubated with gB-DLD or gB-651 as indicated for 60 min at 37°C. Cells were washed with PBS and incubated with virus at an approximate MOI of 0.5 PFU per cell for 3 h at 37°C. Immunofluorescence was performed to detect nuclear-localized pp65, as previously described (31). For endothelial infectivity experiments, HUVEC plated on glass coverslips were incubated with gB-DLD or gB-651 for 30 min at 37°C. Cells were washed with PBS and incubated with HCMV VHL/e at an approximate MOI of 0.1 PFU per cell for 120 min at 37°C. Nonpenetrated virus was inactivated with low-pH citrate buffer (40 mM citric acid-10 mM KCl-135 mM NaCl; pH 3.0), and 24 h later, immunofluorescence was performed to detect immediate early proteins (16). For the HSV entry assay, gB-DLD or gB-651 was incubated with NHDFs for 60 min at 37°C and challenged with HSV-1(KOS)gL86. Any nonpenetrated virus was either removed with washes or inactivated with low-pH citrate buffer. Cells were incubated 6 h at 37°C before lysis in 1% Triton X-100. β-Galactosidase activity was measured by addition of o-nitrophenyl-β-d-galactoside, and the absorbance was monitored at 420 nm.
Virus attachment experiment.
NHDFs were treated with gB-DLD, gB-651, or heparin for 60 min at 4°C. Cells were washed and infected with [3H]thymidine-labeled HCMV (MOI, 100 PFU per cell) for 120 min at 4°C. Unbound virus was removed with PBS washes. Cells were then lysed in 1% Triton X-100, scraped, and counted by scintillation to measure 3H counts per minute.
RESULTS
Production and purification of glycoprotein B fragments.
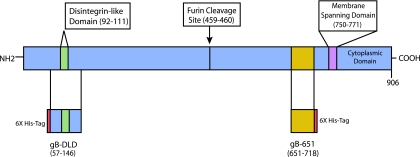
To test the cell and receptor-binding properties of the gB disintegrin module, we produced and purified two histidine-tagged gB fragments. The fragment that we termed the gB-DLD module contains the gB amino-terminal amino acids 57 to 146. This module encompasses the consensus ADAM family disintegrin loop (RX6DLXXF), the minimum motif required for ADAM-mediated, RGD-independent integrin engagement (29). The gB-DLD was subcloned, expressed in E. coli, and purified by both nickel affinity and size exclusion. A carboxy-terminal gB fragment comprised of amino acids 651 to 718 that contains no recognizable receptor-binding motifs (gB-651) was similarly produced and purified. The relationship between full-length gB, gB-DLD, and gB-651 are represented schematically in Fig. 1.
FIG. 1.
Relationships between glycoprotein B fragments used in this study. Schematic of HCMV envelope gB fragments. The amino-terminal gB disintegrin module is comprised of amino acids 57 to 146, while gB-651 represents a C-terminal gB fragment with no recognizable receptor-binding motifs.
The rapid binding of the gB disintegrin-like domain to permissive cells is specific, dose-dependent, and saturable.
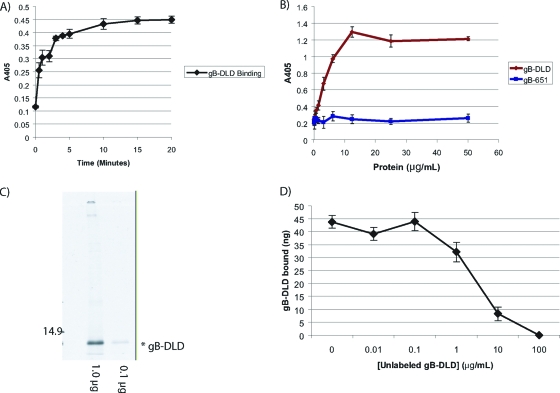
The kinetics of gB-DLD binding to permissive host cells was measured by incubating NHDF cells with a constant concentration of gB-DLD at 4°C for the indicated times. Interestingly, gB-DLD bound permissive cells with very rapid kinetics, which approached saturation in less than 15 min (Fig. 2 A). The measured kinetics were more rapid than that previously observed for HCMV-cell binding; however, they were consistent with prebound virus fusion events (12, 84). These data support the hypothesis that integrin engagement occurs at a postattachment step in the entry pathway, as previously described (31).
FIG. 2.
Binding properties of the gB disintegrin-like domain. (A) gB-DLD binds permissive human fibroblasts with rapid kinetics. NHDF cells were incubated with a constant concentration (10 μg/ml) of gB-DLD at 4°C in 96-well plates after blocking with 1 mg/ml BSA. Cells were incubated for the indicated times, washed, fixed, and probed for the gB-DLD His6 tag by cell enzyme-linked immunosorbent assay (ELISA). (B) gB-DLD binds permissive human fibroblasts in a dose-dependent and saturable manner. Increasing concentrations of gB-DLD or gB-651 were added to NHDF cells for 60 min at 4°C, and cells were washed, fixed, and assayed for bound protein by cell ELISA. (C) Audioradiograph of 35S-labeled gB-DLD. E. coli cells were grown in the absence of sulfate prior to recombinant protein induction and the addition of 35S. A sample of the prep was separated by SDS-PAGE and exposed to film overnight. A prominent band at approximately 12 kDa corresponds to the predicted mass of gB-DLD. The specific activity of this preparation was 56,207 cpm/μg. (D) Homologous competition. A constant concentration of radiolabeled gB-DLD (1 μg/ml) was added to human fibroblasts in the presence of increasing concentrations of cold gB-DLD. Nonspecific binding was determined by the addition of a 100-fold molar excess and was determined to be 19%.
We next compared the permissive cell-binding properties of gB-DLD and a C-terminal gB fragment that contains no recognizable receptor-binding motifs (gB-651). Cells treated with gB-DLD exhibited a dose-dependent binding that reached saturation at 12 μg/ml (1.1 μM), while gB-651 demonstrated only minimal cell binding (Fig. 2B).
To confirm specific binding of the gB-DLD to human fibroblasts, we performed a homologous competition experiment. In this experiment, a constant concentration of 35S-radiolabeled gB-DLD (Fig. 2C) was incubated in the presence of increasing concentrations of nonradiolabeled gB-DLD. Inoculates were then allowed to bind to NHDF cells at 4°C and washed, and the amount of radiolabeled gB-DLD that bound to cells was detected by scintillation after cell lysis. We observed a dose-dependent competition for cell surface binding sites between labeled and unlabeled gB-DLD (Fig. 2D). These data indicate that the disintegrin module of gB binds specifically to human fibroblasts.
gB-DLD binding to cell surfaces is dependent on β1 integrin expression.
Cellular integrins α2β1, α6β1, and αVβ3 have been shown to serve as HCMV entry receptors that act at a postattachment step in the entry pathway (31, 94). Since disintegrin-like domains of the ADAM family preferentially bind β1 integrins and represent the only recognizable integrin-binding motif found on HCMV envelope glycoproteins (31, 81), we tested the ability of the gB disintegrin-like domain to bind cells that either express or lack an intact β1 integrin ectodomain. Low-level gB-DLD binding to mouse fibroblast cells lacking β1 integrin (GD25) was observed compared with the dose-dependent and saturable binding to permissive human fibroblasts (NHDF), GD25 cells with β1 integrin expression restored (GD25-β1), or GD25 cells restored with a signaling-defective β1 integrin (GD25-β1YYFF) (Fig. 3 A). These data confirm that β1 integrin is required for gB-DLD cell surface binding. Furthermore, the presence of dose-dependent binding to the GD25-B1YYFF mutant suggests that gB-DLD binding does not require certain integrin-specific signaling cascades.
FIG. 3.
gB-DLD binding to cell surfaces is dependent on β1 integrin expression. (A) Increasing concentrations of gB-DLD were added to NHDF, GD25, GD25-β1, or GD25-β1YYFF cells, which rendered them unable to activate certain integrin-specific signal transduction cascades. Cells were then washed, fixed, and probed for the gB-DLD His6 tag by cell enzyme-linked immunosorbent assay. (B) A constant concentration of radiolabeled gB-DLD (1.0 μg/ml) was added to the indicated cell lines grown in DMEM (blue) or in sodium chlorate MEM (red) to inhibit heparan sulfate formation. The specific activity of the preparation was 56,207 cpm/μg.
HCMV infection begins with a tethering interaction between two distinct viral glycoprotein complexes: glycoprotein M/N complex and glycoprotein B with cell surface HSPGs (12, 22, 48). While the number and precise location of gB heparin-binding domains remains uncertain, synthetic peptides to a linear motif found adjacent to the disintegrin loop have been shown to bind heparin and block virus attachment (75). To test whether gB-DLD cell binding requires cell surface HSPG, we assayed for the ability of radiolabeled gB-DLD to bind NHDF, GD25, GD25-β1, and GD25-β1YYFF cells grown in the absence of sulfate. Cells grown in medium containing sodium chlorate fail to produce cell surface HSPGs (23, 74, 100), a characteristic confirmed by a greater than 95% reduction in HCMV entry compared to the same cell line grown in DMEM (data not shown). Interestingly, we observed a reduction in gB-DLD binding to all cell lines that lack HSPGs (Fig. 3B). These data support the hypothesis that gB-DLD may possess partial heparan sulfate-binding abilities but that HSPGs are not required for gB-DLD host cell binding. Confirming the results above, gB-DLD was unable to bind the GD25 cells that lack an intact β1 integrin ectodomain.
gB-DLD physically associates with β1 integrin.
To confirm a physical association between full-length gB and β1 integrin we performed immunoprecipitation experiments. NHDF cells were transduced with an adenovirus that expresses gB. While gB, β1, and β3 integrin were all present in NHDF cells, immunoprecipitation of gB pulled down β1, but not β3 integrin (Fig. 4 A). Conversely, immunoprecipitation of β1 integrin pulled down gB and not β3 integrin. Immunoprecipitation of β3 integrin did not pull down gB or β1 integrin. To further confirm the specificity of the β1 integrin-gB interaction, we observed a cell surface protein unrelated to integrins, the discoidin domain receptor 1 (DDR1), which was present in the lysate but was not pulled down in any immunoprecipitation test. These data confirm that full-length gB interacts with β1 but not β3 integrin.
FIG. 4.
gB-DLD physically associates with β1 integrin. (A) NHDFs were infected with adenovirus particles expressing full-length gB at an MOI of 20 PFU/cell. Protein expression was allowed to proceed for 72 h before the cells were washed and lysed. Complexes were immunoprecipitated, reduced, boiled, and separated by SDS-PAGE. Samples were then Western blotted as indicated. (B) NHDFs were lysed and incubated in the presence or absence of gB-DLD or gB-651 for 4 h, prior to the addition of Ni-NTA for 16 h. Beads were washed, resuspended in reducing sample buffer, boiled, and separated by SDS-PAGE. Samples were then separately blotted for either β1 integrin, β3 integrin, or His6 tag.
To confirm that it is the gB disintegrin-like domain that is responsible for the gB-β1 integrin interaction, we performed in vitro pull-down assays. NHDF cells were incubated with either gB-DLD or gB-651, and lysates were precipitated with Ni-NTA agarose beads. Western blot assays were performed for either β1 or β3 integrin to demonstrate a physical interaction, or with a His6 probe to ensure efficient pull down of each protein fragment. Antibodies directed against a histidine tag detected bands at approximately 12 kDa and 10 kDa, representing an efficient pull down of both gB-DLD and gB-651, respectively (Fig. 4B). Additionally, nickel-NTA beads alone did not interact with either β1 or β3 integrin. However, while both β1 and β3 integrin were detected in the lysate, gB-DLD pulled down β1 integrin but not β3 integrin. gB-651 did not precipitate either integrin (Fig. 4B). These data demonstrate the first physical association between gB and an integrin subunit and confirm the ability of the gB disintegrin-like domain to bind β1 integrin.
Cells bound with gB-DLD block HCMV infectivity at the level of postattachment entry.
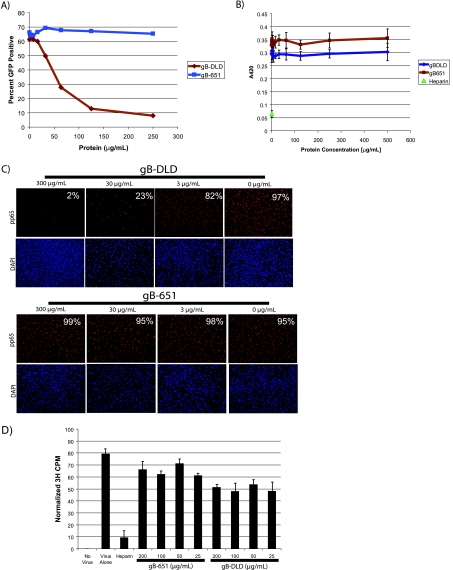
We next performed a series of experiments to identify the functional significance of gB-DLD-integrin binding. To measure the effect of these fragments on HCMV infectivity, NHDF cells were bound with the indicated concentrations of either gB-DLD or gB-651, washed, and infected with an HCMV-GFP reporter virus (IE2-GFP) (69). Flow cytometry was performed 24 h postinfection to quantitate GFP-positive (infected) versus total cells. Additionally, cell viability for each sample was assayed simultaneously via propidium iodide exclusion. All doses had greater than 90% viability (data not shown). While gB-651 did not inhibit HCMV infectivity at any tested dose, we observed a dose-dependent block to HCMV infectivity in cells bound to gB-DLD with a 50% inhibitory concentration (IC50) of 50 μg/ml or 4.7 μM (Fig. 5 A).
FIG. 5.
gB-DLD binding to host cells blocks HCMV infection at a postattachment step of virus entry. (A and B) Infectivity assays. gB-DLD or gB-651 was allowed to bind to permissive human fibroblasts, followed by washes and infection with HCMV-GFP or HSV-1(KOS)gL86 for 60 min. Cells were then citrate washed to remove unbound virus, and infection was allowed to proceed for 24 h followed by flow cytometry to assay for GFP-positive cells (HCMV) (A) or for 6 h followed by assaying for β-galactosidase activity (HSV-1) (B). (C) Entry assay. Human fibroblasts were incubated with increasing concentrations of gB-DLD or gB-651. Unbound protein was washed, and cells were infected with HCMV for 3 h. Virus was then washed off, cells were fixed, and an immunofluorescence assay was performed to visualize nuclear localization of HCMV tegument pp65. The percent pp65-positive nuclei per total 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei is shown for each panel. (D) HCMV-binding assay. Human fibroblasts were incubated with gB-DLD, gB-651, or heparin, followed by challenge with 3H-labeled HCMV at 4°C. Unbound virus was washed, cells were lysed, and scintillation counting was performed.
To eliminate the possibility that the inhibition of HCMV infectivity in cells bound to gB-DLD was nonspecific, we tested the effect of gB-DLD or gB-651 on the infectivity of HSV-1, a related virus that lacks a gB disintegrin-like domain. Here, we saw no effect on HSV-1 infectivity in cells treated with either gB-DLD or gB-651; however, infectivity could be blocked with soluble heparin, as previously described, indicating that the block to HCMV infectivity imposed by gB-DLD was not due to nonspecific steric hindrance (Fig. 5B). Furthermore, these data also indicate that the HSPG binding observed with radiolabeled gB-DLD in Fig. 3B does not lead to a functional inhibition of HCMV infectivity.
A block to HCMV infectivity could be due to one of several steps prior to HCMV gene expression, including virus-cell attachment, fusion, or nucleocapsid uncoating and trafficking to the nucleus. To discern between these possible mechanisms, we bound both gB fragments to NHDFs and infected the cells with HCMV for 3 h prior to fixation and immunofluorescence for nuclear-localized HCMV tegument phosphoprotein of 65 kDa (pp65). Within 2 h of virus-cell fusion, the highly abundant pp65 (88) translocates to and accumulates within the nucleus (70), where it blocks the induction of certain antiviral genes (15). A dose-dependent decrease of pp65-positive NHDF nuclei was observed in cells treated with gB-DLD but not gB-651 (Fig. 5C). These data indicate that gB-DLD was blocking infection at or before virus-cell fusion. To test whether the block to HCMV infectivity occurred at the level of virus attachment, we assayed for the ability of [3H]thymidine-labeled HCMV to bind to NHDFs treated with either gB-DLD or gB-651. We observed a reduction in the ability of HCMV to bind to host cells that were bound to gB-DLD; however, this effect was not dose dependent and was less than 50% reduced at doses that completely block HCMV infectivity (Fig. 5D). Combined, these data confirm the role of β1 integrins as postattachment entry receptors. Cells bound and saturated with gB-DLD are resistant to HCMV infectivity. Furthermore, the block to infection occurs after virus attachment but before the delivery of pp65 to the cytosol.
Polyclonal antibodies generated against gB-DLD neutralize HCMV infectivity.
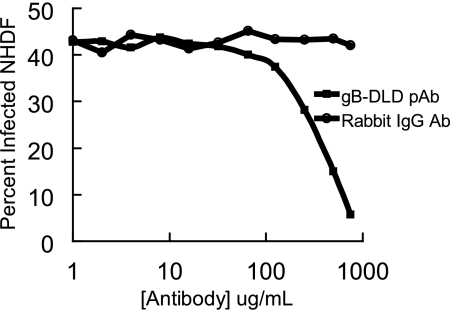
We next tested whether antibodies generated against the gB disintegrin-like domain could neutralize HCMV infectivity by binding to this domain on virions during the entry pathway and preventing β1 integrin engagement. HCMV was incubated with increasing concentrations of isotype control rabbit IgG or total rabbit IgG purified from gB-DLD-immunized rabbit sera. A dose-dependent neutralization of HCMV infectivity was demonstrated in viruses treated with gB-DLD rabbit polyclonal during infection, while no effect was observed in isotype control-treated virus (Fig. 6). These data further point to the importance of the gB disintegrin-like domain during the HCMV entry pathway.
FIG. 6.
Polyclonal antibodies generated against gB-DLD neutralize HCMV infectivity. Rabbits were immunized with gB-DLD, and serum was collected and IgG purified. Either control rabbit IgG or gB-DLD rabbit IgG was incubated with HCMV-GFP prior to infection. After 60 min, unpenetrated HCMV was removed with citrate washes, and the infection was allowed to proceed for 24 h. To measure infectivity, cells were collected and flow cytometry was performed.
The gB disintegrin-like domain mediates entry into other physiologically relevant cell types.
HCMV can infect a broad range of cell types in vivo, including fibroblasts, endothelial cells, and monocytes/macrophages (3, 25, 40, 76, 84, 91). It was previously reported that the 20 amino acids encompassing the gB disintegrin-like domain was greater-than-98% identical at the amino acid level among 44 HCMV clinical isolates (31). To test the importance of the gB disintegrin-like domain in HCMV entry of another physiologically relevant cell type, we infected HUVEC with a nonfibroblast-adapted strain of HCMV, VHL/e (90, 91). At 24 h postinfection cells were fixed and immunofluorescence performed to visualize immediate-early viral protein synthesis. We observed a dose-dependent block in HCMV infectivity when endothelial cells were treated with gB-DLD, but not with gB-651 (Fig. 7 A). The percentage of IE-positive cells per total cells was counted for each treatment and is presented relative to the percent infection of untreated cells. gB-DLD inhibited VHL/e infection with an IC50 of 3 μg/ml (0.3 μM) (Fig. 7B). Similarly, the gB-DLD fragment blocked epithelial cell and fibroblast infection of the clinical isolate VR1814, with IC50 values that ranged from 1 to 5 μM (data not shown). These data support a role for a gB-β1 integrin-dependent entry pathway in endothelial and epithelial cells and potentially other physiologically relevant cell types.
FIG. 7.
gB-DLD blocks the infectivity of endothelial cell-tropic HCMV. (A) HUVEC were incubated with increasing concentrations of gB-DLD or gB-651, washed, and infected with endothelial cell-tropic VHL/e. After 120 min, unpenetrated virus was removed with washes and infection was allowed to proceed for 24 h. Infection was assessed via immunofluorescence to HCMV immediate-early proteins and 4′,6-diamidino-2-phenylindole (DAPI) staining (total cells). (B) To quantify these data, numbers of immediate-early protein-positive nuclei per total DAPI-stained cells were counted and are represented as the percent infectivity. Data are shown relative to percent infectivity without any protein treatment.
DISCUSSION
For many years the molecules that mediate HCMV entry have remained elusive. Recently, the discovery that α2β1, α6β1, and αVβ3 integrin heterodimers functioned as HCMV entry receptors (31) has fueled further study toward identifying the molecular mechanism of integrin engagement by HCMV envelope glycoproteins. Herein, we demonstrate that engagement of β1 integrins is mediated by a specific region on the amino terminus of gB. Binding studies revealed that the gB disintegrin module binds to cells specifically and that this binding requires the expression of β1 integrin. Furthermore, a direct physical association between the gB disintegrin module and β1 integrin was established through in vitro pull-down assays. Indeed, this interaction was physiologically relevant, since both permissive human fibroblasts and endothelial cells bound to and saturated with the gB disintegrin module were refractory to HCMV infection at the level of postattachment entry.
Additionally, it was previously proposed that gH could function as a ligand for αVβ3 integrin (94). However, the precise molecular mechanism of αVβ3 engagement by gH is unknown, since this glycoprotein lacks any previously identified integrin recognition motif. The data presented in this study, combined with the reports that αVβ3 integrin is also important for entry (31) and interacts with gH (94), suggest that HCMV interacts with both β1 and β3 integrin heterodimers during the entry pathway to mediate HCMV entry to permissive human fibroblasts.
In addition to gB- and gH-mediated integrin engagement, HCMV entry has been shown to phosphorylate EGFR and PDGFR (79, 95). Both growth factor receptors have been shown to perform a similar function and trigger PI-3 kinase and Akt activation coincident with virus entry. The relative contribution of each growth factor receptor with respect to signaling virus-cell attachment remain to be further explored. Indeed, complex cross talk pathways (65) and physical associations (101) exist between integrins and growth factor receptors (11, 24, 67, 71, 82). For example, it has previously been demonstrated that growth factor receptor phosphorylation can lead to inside-out integrin activation, resulting in a change in integrin conformation and affinity for integrin ligand (50, 85). Conversely, integrin binding can activate classic outside-in signaling, whereby the integrin ligand can stimulate growth factor phosphorylation in the absence of cognate growth factor (55, 56, 58, 59). Future work will elucidate the contributions of each of these receptors in HCMV entry-induced signal transduction pathways.
Interesting parallels can be drawn between disintegrin-like domain-mediated mammalian cell-cell fusion events and those of HCMV and other viruses. The interaction of the disintegrin-like domain of the ADAM family with integrins has been implicated in the coordination of many pH-neutral mammalian cell-cell fusion events, including sperm-egg fusion (9, 30, 102), osteoclast- (1, 19) and macrophage-derived multinucleated giant cell formation (1, 54), trophoblast syncytialization (38, 47, 72, 93), and myogenesis (33, 51). The molecular mechanism of most ADAM integrin-mediated fusion events remains poorly understood. However, in myoblast fusion, it is thought that the ADAM12 cysteine-rich domain (adjacent to the disintegrin module) binds the heparan sulfate proteoglycan (syndecan-4) with low affinity, and that interaction triggers conformational changes that expose the ADAM12 disintegrin-like domain to allow for integrin binding (39). Integrins then bind the disintegrin-like domain and make firm cell-cell attachments that allow for membrane fusion. A similar mechanism can be envisaged for HCMV engagement of integrins during virus-cell fusion, since gB possesses both HSPG and β1 integrin-binding capabilities. Similar to ADAM12-mediated myoblast fusion, the role of the required HSPG binding step in triggering the exposure of gB-DLD during HCMV entry remains to be explored.
Parallels also exist between integrin-mediated HCMV fusion and ADAM-mediated sperm-egg attachment. The first ADAM family member identified, PH-30 (now known as fertilin-β, or ADAM-2), was found on the surface of sperm cells and was first characterized due to its similarities with classic viral fusion proteins (9, 60, 98). Sequence similarity between the amino terminus of PH-30 with the high-affinity integrin-binding snake venom disintegrin proteins led investigators to coin the term disintegrin-like domain. This protein was found on the sperm cell surface and contains a putative integrin-binding disintegrin-like domain on the amino terminus along with a highly hydrophobic stretch that is predicted to function as a fusion peptide. It was determined that sperm glycoprotein PH-30 could bind to β1 integrin (via a disintegrin-like domain) (5). While subsequent work has shown that the integrin-ADAM interaction is not strictly required for sperm-egg binding and fusion, in vivo evidence supports its role for enhancing the rate of sperm-egg binding (8).
The molecular mechanism of membrane fusion remains a largely unknown facet of herpesvirology. Thus, by identifying envelope glycoprotein ligands and their cognate receptors we can begin to understand the molecular mechanism of herpesvirus fusion. Unlike orthomyxoviruses, filoviruses, and retroviruses, which use a single glycoprotein for membrane fusion, herpesviruses typically employ multicomponent fusion machines that frequently consist minimally of gB, gH, and gL (80). Despite the complexity of multicomponent fusion machines, it is very likely the fusion process bears strong parallels to single-component fusion proteins. α-Helical coiled-coil domains are critical structures involved in both mammalian intracellular fusion events and virus-cell fusion that function to drive the energetic folding of membranes together. In viral systems, fusion is a regulated process that requires a conformational change in the fusogenic protein to expose these coiled-coil motifs. By using an algorithm to detect potential coiled-coils, heptad repeat regions in gB and gH were predicted to form α-helical coiled-coils (28, 53). Synthetic peptides to these motifs inhibited HCMV entry, including virion content (tegument and capsids) delivery, suggesting that these motifs play a fundamental role in membrane fusion. Mutational analysis of both the gB disintegrin loop and coiled-coils in the context of HCMV virions will be required to further analyze the importance of these domains during HCMV entry. Furthermore, recent data indicate that gB plays a critical role during cell-cell fusion (41, 86). Future work will also explore the significance of the gB-DLD-integrin interaction in mediating this interaction to promote the transmission of virus genetic material to adjacent uninfected cells.
The rapid kinetics of gB-DLD binding compared with HCMV-cell binding suggests that the integrin-binding disintegrin-like domain may be hidden on virion gB and may then be exposed after receptor engagements to allow for integrin binding and fusion. Currently, there is no crystal structure of HCMV gB, so the location of the gB-DLD with respect to other HCMV gB domains and relative exposure is unknown. The structure of glycoprotein B from both HSV and Epstein-Barr virus (EBV) has been resolved (7, 35). However, the orientation of individual domains differs between the structures, and it is hypothesized that the resolved structures for both proteins represent the postfusion form of the protein. The fusion process likely requires significant conformational changes, and recent data suggest that gB may interact directly with gH and that gB conformational changes may be driven by the UL131 locus gene products that associate with gH (64).
Herein, we report that the gB disintegrin module from the fibroblast-tropic HCMV AD169 strain can potently block infectivity of the endothelium-tropic VHL/e strain. This is not surprising, given the high conservation of the gB disintegrin module among clinical isolates; the 20-residue stretch of the disintegrin-like domain is greater than 98% identical at the amino acid level. Future work will focus on identifying a role for specific cellular integrin heterodimers in mediating HCMV entry into other physiologically relevant cell types, such as endothelial cells and monocytes.
The conservation of the gB disintegrin-like domain throughout the beta- and gammaherpesviruses suggests that this domain may bind integrins to mediate the entry of these viruses as well. Kaposi's sarcoma-associated herpesvirus (KSHV) has been shown to utilize an RGD sequence on gB to bind α3β1, αVβ3, and αVβ5 integrin heterodimers to mediate virus entry (4, 32). Recently, vascular endothelial growth factor receptor (VEGFR) was also shown to be activated by KSHV gB (103). The coordination of growth factor receptor activation, integrin engagement, and virus entry is beginning to emerge as a common theme in herpesvirus entry (89). Furthermore, integrins have also been shown to be important for the entry of memory B cells and epithelial cells (18, 27). While it is the BMRF2 and gH/gL glycoproteins that have been implicated in the integrin interactions mediating these EBV entry events, it remains possible that the gB disintegrin-like domain may also play a role in coordinating integrin engagement, growth factor signaling, and virus entry.
Finally, understanding the molecular mechanism of integrin engagement by HCMV could identify promising new therapeutic avenues and research areas to explore. Envelope glycoproteins (gB), tegument protein pp65, and HCMV dense body (defective particles consisting of tegument and envelope glycoproteins) subunit vaccines have been developed to induce strong humoral and cell-mediated immune responses (6, 34). These recent attempts have not resulted in an effective vaccine against HCMV, but encouraging progress toward this goal is under way. In future studies, an antigen affinity-purified rabbit polyclonal gB-DLD antibody may be a valuable reagent in further exploring the gB disintegrin loop in virus entry. Furthermore, humanized monoclonal antibodies generated against the fusion protein of respiratory syncytial virus have proven to be an effective therapeutic when administered to RSV-infected neonates (63). Similarly, humanized monoclonal antibodies to the disintegrin loop may be a valuable therapeutic agent to block herpesvirus entry in immunocompromised populations.
Acknowledgments
This research was funded by U.S. Public Health Service grant R01 AI034998 (to T.C.).
We are grateful to Deane Mosher and Rebecca Montgomery for kind gifts of cells and virus, Heidi Koss for her initial work on this project, and to all the members of the Compton lab for critical review of the manuscript.
Footnotes
Published ahead of print on 21 July 2010.
REFERENCES
- 1.Abe, E., H. Mocharla, T. Yamate, Y. Taguchi, and S. C. Manolagas. 1999. Meltrin-alpha, a fusion protein involved in multinucleated giant cell and osteoclast formation. Calcif. Tissue Int. 64:508-515. [DOI] [PubMed] [Google Scholar]
- 2.AbuBakar, S., I. Boldogh, and T. Albrecht. 1990. Human cytomegalovirus stimulates arachidonic acid metabolism through pathways that are affected by inhibitors of phospholipase A2 and protein kinase C. Biochem. Biophys. Res. Commun. 166:953-959. [DOI] [PubMed] [Google Scholar]
- 3.Adler, B., and C. Sinzger. 2009. Endothelial cells in human cytomegalovirus infection: one host cell out of many or a crucial target for virus spread? Thromb. Haemost. 102:1057-1063. [DOI] [PubMed] [Google Scholar]
- 4.Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2002. Integrin α3β1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell 108:407-419. [DOI] [PubMed] [Google Scholar]
- 5.Almeida, E. A., A. P. Huovila, A. E. Sutherland, L. E. Stephens, P. G. Calarco, L. M. Shaw, A. M. Mercurio, A. Sonnenberg, P. Primakoff, D. G. Myles, et al. 1995. Mouse egg integrin alpha 6 beta 1 functions as a sperm receptor. Cell 81:1095-1104. [DOI] [PubMed] [Google Scholar]
- 6.Arvin, A. M., P. Fast, M. Myers, S. Plotkin, and R. Rabinovich. 2004. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 39:233-239. [DOI] [PubMed] [Google Scholar]
- 7.Backovic, M., R. Longnecker, and T. S. Jardetzky. 2009. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proc. Natl. Acad. Sci. U. S. A. 106:2880-2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baessler, K. A., Y. Lee, and N. S. Sampson. 2009. β1 integrin is an adhesion protein for sperm binding to eggs. ACS Chem. Biol. 4:357-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blobel, C. P., T. G. Wolfsberg, C. W. Turck, D. G. Myles, P. Primakoff, and J. M. White. 1992. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature 356:248-252. [DOI] [PubMed] [Google Scholar]
- 10.Boehme, K. W., J. Singh, S. T. Perry, and T. Compton. 2004. Human cytomegalovirus elicits a coordinated cellular antiviral response via envelope glycoprotein B. J. Virol. 78:1202-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borges, E., Y. Jan, and E. Ruoslahti. 2000. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J. Biol. Chem. 275:39867-39873. [DOI] [PubMed] [Google Scholar]
- 12.Boyle, K. A., and T. Compton. 1998. Receptor-binding properties of a soluble form of human cytomegalovirus glycoprotein B. J. Virol. 72:1826-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyle, K. A., R. L. Pietropaolo, and T. Compton. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol. Cell. Biol. 19:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brantsaeter, A. B., K. Liestol, A. K. Goplen, O. Dunlop, and J. N. Bruun. 2002. CMV disease in AIDS patients: incidence of CMV disease and relation to survival in a population-based study from Oslo. Scand. J. Infect. Dis. 34:50-55. [DOI] [PubMed] [Google Scholar]
- 15.Browne, E. P., and T. Shenk. 2003. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc. Natl. Acad. Sci. U. S. A. 100:11439-11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlson, C., W. J. Britt, and T. Compton. 1997. Expression, purification, and characterization of a soluble form of human cytomegalovirus glycoprotein B. Virology 239:198-205. [DOI] [PubMed] [Google Scholar]
- 17.Chan, G., M. T. Nogalski, and A. D. Yurochko. 2009. Activation of EGFR on monocytes is required for human cytomegalovirus entry and mediates cellular motility. Proc. Natl. Acad. Sci. U. S. A. 106:22369-22374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chesnokova, L. S., S. L. Nishimura, and L. M. Hutt-Fletcher. 2009. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins αvβ6 or αvβ8. Proc. Natl. Acad. Sci. U. S. A. 106:20464-20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi, S. J., J. H. Han, and G. D. Roodman. 2001. ADAM8: a novel osteoclast stimulating factor. J. Bone Miner. Res. 16:814-822. [DOI] [PubMed] [Google Scholar]
- 20.Cinque, P., R. Marenzi, and D. Ceresa. 1997. Cytomegalovirus infections of the nervous system. Intervirology 40:85-97. [DOI] [PubMed] [Google Scholar]
- 21.Cobbs, C. S., L. Soroceanu, S. Denham, W. Zhang, W. J. Britt, R. Pieper, and M. H. Kraus. 2007. Human cytomegalovirus induces cellular tyrosine kinase signaling and promotes glioma cell invasiveness. J. Neurooncol. 85:271-280. [DOI] [PubMed] [Google Scholar]
- 22.Compton, T., D. M. Nowlin, and N. R. Cooper. 1993. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology 193:834-841. [DOI] [PubMed] [Google Scholar]
- 23.Conrad, H. E. 2001. Inhibition of heparan sulfate synthesis by chlorate. Methods Mol. Biol. 171:325-328. [DOI] [PubMed] [Google Scholar]
- 24.Cooke, M. E., T. Sakai, and D. F. Mosher. 2000. Contraction of collagen matrices mediated by α2β1A and αvβ3 integrins. J. Cell Sci. 113:2375-2383. [DOI] [PubMed] [Google Scholar]
- 25.Dankner, W. M., J. A. McCutchan, D. D. Richman, K. Hirata, and S. A. Spector. 1990. Localization of human cytomegalovirus in peripheral blood leukocytes by in situ hybridization. J. Infect. Dis. 161:31-36. [DOI] [PubMed] [Google Scholar]
- 26.Davis, R., and F. de Serres. 1970. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 17A:79-143. [Google Scholar]
- 27.Dorner, M., F. Zucol, D. Alessi, S. K. Haerle, W. Bossart, M. Weber, R. Byland, M. Bernasconi, C. Berger, S. Tugizov, R. F. Speck, and D. Nadal. 2010. β1 integrin expression increases susceptibility of memory B cells to Epstein-Barr virus infection. J. Virol. 84:6667-6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.English, E. P., R. S. Chumanov, S. H. Gellman, and T. Compton. 2005. Rational development of beta-peptide inhibitors of human cytomegalovirus entry. J. Biol. Chem. 281:2661-2667. [DOI] [PubMed] [Google Scholar]
- 29.Eto, K., C. Huet, T. Tarui, S. Kupriyanov, H. Z. Liu, W. Puzon-McLaughlin, X. P. Zhang, D. Sheppard, E. Engvall, and Y. Takada. 2002. Functional classification of ADAMs based on a conserved motif for binding to integrin α9β1: implications for sperm-egg binding and other cell interactions. J. Biol. Chem. 277:17804-17810. [DOI] [PubMed] [Google Scholar]
- 30.Evans, J. P. 1999. Sperm disintegrins, egg integrins, and other cell adhesion molecules of mammalian gamete plasma membrane interactions. Front. Biosci. 4:D114-D131. [DOI] [PubMed] [Google Scholar]
- 31.Feire, A. L., H. Koss, and T. Compton. 2004. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. U. S. A. 101:15470-15475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrigues, H. J., Y. E. Rubinchikova, C. M. Dipersio, and T. M. Rose. 2008. Integrin αVβ3 binds to the RGD motif of glycoprotein B of Kaposi's sarcoma-associated herpesvirus and functions as an RGD-dependent entry receptor. J. Virol. 82:1570-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilpin, B. J., F. Loechel, M. G. Mattei, E. Engvall, R. Albrechtsen, and U. M. Wewer. 1998. A novel, secreted form of human ADAM 12 (meltrin alpha) provokes myogenesis in vivo. J. Biol. Chem. 273:157-166. [DOI] [PubMed] [Google Scholar]
- 34.Gonczol, E., and S. Plotkin. 2001. Development of a cytomegalovirus vaccine: lessons from recent clinical trials. Expert Opin. Biol. Ther. 1:401-412. [DOI] [PubMed] [Google Scholar]
- 35.Heldwein, E. E., H. Lou, F. C. Bender, G. H. Cohen, R. J. Eisenberg, and S. C. Harrison. 2006. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 313:217-220. [DOI] [PubMed] [Google Scholar]
- 36.Himpens, B., P. Proot, J. Neyts, H. De Smedt, E. De Clercq, and R. Casteels. 1995. Human cytomegalovirus modulates the Ca2+ response to vasopressin and ATP in fibroblast cultures. Cell. Calcium 18:111-119. [DOI] [PubMed] [Google Scholar]
- 37.Hobom, U., W. Brune, M. Messerle, G. Hahn, and U. H. Koszinowski. 2000. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: mutational analysis of human cytomegalovirus envelope glycoprotein genes. J. Virol. 74:7720-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huovila, A. P., E. A. Almeida, and J. M. White. 1996. ADAMs and cell fusion. Curr. Opin. Cell Biol. 8:692-699. [DOI] [PubMed] [Google Scholar]
- 39.Iba, K., R. Albrechtsen, B. Gilpin, C. Frohlich, F. Loechel, A. Zolkiewska, K. Ishiguro, T. Kojima, W. Liu, J. K. Langford, R. D. Sanderson, C. Brakebusch, R. Fassler, and U. M. Wewer. 2000. The cysteine-rich domain of human ADAM 12 supports cell adhesion through syndecans and triggers signaling events that lead to β1 integrin-dependent cell spreading. J. Cell Biol. 149:1143-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ibanez, C. E., R. Schrier, P. Ghazal, C. Wiley, and J. A. Nelson. 1991. Human cytomegalovirus productively infects primary differentiated macrophages. J. Virol. 65:6581-6588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isaacson, M. K., and T. Compton. 2009. Human cytomegalovirus glycoprotein B is required for virus entry and cell-to-cell spread but not for virion attachment, assembly, or egress. J. Virol. 83:3891-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isaacson, M. K., A. L. Feire, and T. Compton. 2007. Epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling. J. Virol. 81:6241-6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jia, L. G., K. Shimokawa, J. B. Bjarnason, and J. W. Fox. 1996. Snake venom metalloproteinases: structure, function and relationship to the ADAMs family of proteins. Toxicon 34:1269-1276. [DOI] [PubMed] [Google Scholar]
- 44.Johnson, R. A., S. M. Huong, and E. S. Huang. 2000. Activation of the mitogen-activated protein kinase p38 by human cytomegalovirus infection through two distinct pathways: a novel mechanism for activation of p38. J. Virol. 74:1158-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson, R. A., X. L. Ma, A. D. Yurochko, and E. S. Huang. 2001. The role of MKK1/2 kinase activity in human cytomegalovirus infection. J. Gen. Virol. 82:493-497. [DOI] [PubMed] [Google Scholar]
- 46.Johnson, R. A., X. Wang, X. L. Ma, S. M. Huong, and E. S. Huang. 2001. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. J. Virol. 75:6022-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jungers, K. A., C. Le Goff, R. P. Somerville, and S. S. Apte. 2005. Adamts9 is widely expressed during mouse embryo development. Gene Expr. Patterns 5:609-617. [DOI] [PubMed] [Google Scholar]
- 48.Kari, B., and R. Gehrz. 1992. A human cytomegalovirus glycoprotein complex designated gC-II is a major heparin-binding component of the envelope. J. Virol. 66:1761-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kari, B., and R. Gehrz. 1993. Structure, composition and heparin binding properties of a human cytomegalovirus glycoprotein complex designated gC-II. J. Gen. Virol. 74:255-264. [DOI] [PubMed] [Google Scholar]
- 50.Klemke, R. L., M. Yebra, E. M. Bayna, and D. A. Cheresh. 1994. Receptor tyrosine kinase signaling required for integrin alpha v beta 5-directed cell motility but not adhesion on vitronectin. J. Cell Biol. 127:859-866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lafuste, P., C. Sonnet, B. Chazaud, P. A. Dreyfus, R. K. Gherardi, U. M. Wewer, and F. J. Authier. 2005. ADAM12 and α9β1 integrin are instrumental in human myogenic cell differentiation. Mol. Biol. Cell 16:861-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ljungman, P. 1996. Cytomegalovirus infections in transplant patients. Scand. J. Infect. Dis. Suppl. 100:59-63. [PubMed] [Google Scholar]
- 53.Lopper, M., and T. Compton. 2004. Coiled-coil domains in glycoproteins B and H are involved in human cytomegalovirus membrane fusion. J. Virol. 78:8333-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNally, A. K., and J. M. Anderson. 2002. β1 and β2 integrins mediate adhesion during macrophage fusion and multinucleated foreign body giant cell formation. Am. J. Pathol. 160:621-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miranti, C. K. 2002. Application of cell adhesion to study signaling networks. Methods Cell Biol. 69:359-383. [DOI] [PubMed] [Google Scholar]
- 56.Miyamoto, S., H. Teramoto, J. S. Gutkind, and K. M. Yamada. 1996. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 135:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 58.Moro, L., L. Dolce, S. Cabodi, E. Bergatto, E. B. Erba, M. Smeriglio, E. Turco, S. F. Retta, M. G. Giuffrida, M. Venturino, J. Godovac-Zimmermann, A. Conti, E. Schaefer, L. Beguinot, C. Tacchetti, P. Gaggini, L. Silengo, G. Tarone, and P. Defilippi. 2002. Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J. Biol. Chem. 277:9405-9414. [DOI] [PubMed] [Google Scholar]
- 59.Moro, L., M. Venturino, C. Bozzo, L. Silengo, F. Altruda, L. Beguinot, G. Tarone, and P. Defilippi. 1998. Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 17:6622-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Myles, D. G., L. H. Kimmel, C. P. Blobel, J. M. White, and P. Primakoff. 1994. Identification of a binding site in the disintegrin domain of fertilin required for sperm-egg fusion. Proc. Natl. Acad. Sci. U. S. A. 91:4195-4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagai, K., and T. H. C. 1987. Synthesis and sequence-specific proteolysis of hybrid proteins produced in Escherichia coli. Methods Enzymol. 153:461-481. [DOI] [PubMed] [Google Scholar]
- 62.Nowlin, D. M., N. R. Cooper, and T. Compton. 1991. Expression of a human cytomegalovirus receptor correlates with infectibility of cells. J. Virol. 65:3114-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Null, D., Jr., B. Pollara, P. H. Dennehy, J. Steichen, P. J. Sanchez, L. B. Givner, D. Carlin, B. Landry, F. H. Top., Jr., and E. Connor. 2005. Safety and immunogenicity of palivizumab (Synagis) administered for two seasons. Pediatr. Infect. Dis. J. 24:1021-1023. [DOI] [PubMed] [Google Scholar]
- 64.Patrone, M., M. Secchi, E. Bonaparte, G. Milanesi, and A. Gallina. 2007. Cytomegalovirus UL131-128 products promote gB conformational transition and gB-gH interaction during entry into endothelial cells. J. Virol. 81:11479-11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Plopper, G. E., H. P. McNamee, L. E. Dike, K. Bojanowski, and D. E. Ingber. 1995. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol. Biol. Cell 6:1349-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramsay, M. E., E. Miller, and C. S. Peckham. 1991. Outcome of confirmed symptomatic congenital cytomegalovirus infection. Arch. Dis. Child. 66:1068-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riemenschneider, M. J., W. Mueller, R. A. Betensky, G. Mohapatra, and D. N. Louis. 2005. In situ analysis of integrin and growth factor receptor signaling pathways in human glioblastomas suggests overlapping relationships with focal adhesion kinase activation. Am. J. Pathol. 167:1379-1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sakai, T., R. Jove, R. Fassler, and D. F. Mosher. 2001. Role of the cytoplasmic tyrosines of beta 1A integrins in transformation by v-src. Proc. Natl. Acad. Sci. U. S. A. 98:3808-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanchez, V., C. L. Clark, J. Y. Yen, R. Dwarakanath, and D. H. Spector. 2002. Viable human cytomegalovirus recombinant virus with an internal deletion of the IE2 86 gene affects late stages of viral replication. J. Virol. 76:2973-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmolke, S., P. Drescher, G. Jahn, and B. Plachter. 1995. Nuclear targeting of the tegument protein pp65 (UL83) of human cytomegalovirus: an unusual bipartite nuclear localization signal functions with other portions of the protein to mediate its efficient nuclear transport. J. Virol. 69:1071-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schneller, M. 2001. Identification of a candidate integrin-fraction associated with the activated form of the PDGF-receptor. Biochem. Biophys. Res. Commun. 281:595-602. [DOI] [PubMed] [Google Scholar]
- 72.Shi, Z., W. Xu, F. Loechel, U. M. Wewer, and L. J. Murphy. 2000. ADAM 12, a disintegrin metalloprotease, interacts with insulin-like growth factor-binding protein-3. J. Biol. Chem. 275:18574-18580. [DOI] [PubMed] [Google Scholar]
- 73.Shibutani, T., T. M. Johnson, Z. X. Yu, V. J. Ferrans, J. Moss, and S. E. Epstein. 1997. Pertussis toxin-sensitive G proteins as mediators of the signal transduction pathways activated by cytomegalovirus infection of smooth muscle cells. J. Clin. Invest. 100:2054-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shieh, M. T., D. WuDunn, R. I. Montgomery, J. D. Esko, and P. G. Spear. 1992. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J. Cell Biol. 116:1273-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Silvestri, M. E., and V. A. Sundqvist. 2001. An investigation into the heparin-binding properties of a synthetic peptide deduced from the antigenic domain 2 of human cytomegalovirus glycoprotein B. Scand. J. Immunol. 53:282-289. [DOI] [PubMed] [Google Scholar]
- 76.Sinzger, C., M. Digel, and G. Jahn. 2008. Cytomegalovirus cell tropism. Curr. Top. Microbiol. Immunol. 325:63-83. [DOI] [PubMed] [Google Scholar]
- 77.Sinzger, C., and G. Jahn. 1996. Human cytomegalovirus cell tropism and pathogenesis. Intervirology 39:302-319. [DOI] [PubMed] [Google Scholar]
- 78.Sinzger, C., M. Kahl, K. Laib, K. Klingel, P. Rieger, B. Plachter, and G. Jahn. 2000. Tropism of human cytomegalovirus for endothelial cells is determined by a post-entry step dependent on efficient translocation to the nucleus. J. Gen. Virol. 81:3021-3035. [DOI] [PubMed] [Google Scholar]
- 79.Soroceanu, L., A. Akhavan, and C. S. Cobbs. 2008. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature 455:391-395. [DOI] [PubMed] [Google Scholar]
- 80.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stone, A. L., M. Kroeger, and Q. X. Sang. 1999. Structure-function analysis of the ADAM family of disintegrin-like and metalloproteinase-containing proteins (review). J. Protein Chem. 18:447-465. [DOI] [PubMed] [Google Scholar]
- 82.Sundberg, C., and K. Rubin. 1996. Stimulation of β1 integrins on fibroblasts induces PDGF independent tyrosine phosphorylation of PDGF beta-receptors. J. Cell Biol. 132:741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sweet, C. 1999. The pathogenicity of cytomegalovirus. FEMS Microbiol. Rev. 23:457-482. [DOI] [PubMed] [Google Scholar]
- 84.Taylor, H. P., and N. R. Cooper. 1989. Human cytomegalovirus binding to fibroblasts is receptor mediated. J. Virol. 63:3991-3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trusolino, L., G. Serini, G. Cecchini, C. Besati, F. S. Ambesi-Impiombato, P. C. Marchisio, and R. De Filippi. 1998. Growth factor-dependent activation of αvβ3 integrin in normal epithelial cells: implications for tumor invasion. J. Cell Biol. 142:1145-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vanarsdall, A. L., B. J. Ryckman, M. C. Chase, and D. C. Johnson. 2008. Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans. J. Virol. 82:11837-11850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Den Pol, A. N., E. Mocarski, N. Saederup, J. Vieira, and T. J. Meier. 1999. Cytomegalovirus cell tropism, replication, and gene transfer in brain. J. Neurosci. 19:10948-10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Varnum, S. M., D. N. Streblow, M. E. Monroe, P. Smith, K. J. Auberry, L. Pasa-Tolic, D. Wang, D. G. Camp II, K. Rodland, S. Wiley, W. Britt, T. Shenk, R. D. Smith, and J. A. Nelson. 2004. Identification of proteins in human cytomegalovirus (HCMV) particles: the HCMV proteome. J. Virol. 78:10960-10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Veettil, M. V., S. Sadagopan, N. Sharma-Walia, F. Z. Wang, H. Raghu, L. Varga, and B. Chandran. 2008. Kaposi's sarcoma-associated herpesvirus forms a multimolecular complex of integrins (αVβ5, αVβ3, and α3β1) and CD98-xCT during infection of human dermal microvascular endothelial cells, and CD98-xCT is essential for the postentry stage of infection. J. Virol. 82:12126-12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Waldman, W. J., W. H. Roberts, D. H. Davis, M. V. Williams, D. D. Sedmak, and R. E. Stephens. 1991. Preservation of natural endothelial cytopathogenicity of cytomegalovirus by propagation in endothelial cells. Arch. Virol. 117:143-164. [DOI] [PubMed] [Google Scholar]
- 91.Waldman, W. J., J. M. Sneddon, R. E. Stephens, and W. H. Roberts. 1989. Enhanced endothelial cytopathogenicity induced by a cytomegalovirus strain propagated in endothelial cells. J. Med. Virol. 28:223-230. [DOI] [PubMed] [Google Scholar]
- 92.Walmsley, S., and A. Tseng. 1999. Comparative tolerability of therapies for cytomegalovirus retinitis. Drug Saf. 21:203-224. [DOI] [PubMed] [Google Scholar]
- 93.Wang, H. X., Y. G. Zhao, H. M. Wang, Q. Yang, H. Y. Lin, Q. X. Sang, and C. Zhu. 2005. Expression of adamalysin 19/ADAM19 in the endometrium and placenta of rhesus monkey (Macaca mulatta) during early pregnancy. Mol. Hum. Reprod. 11:429-435. [DOI] [PubMed] [Google Scholar]
- 94.Wang, X., D. Y. Huang, S. M. Huong, and E. S. Huang. 2005. Integrin αvβ3 is a coreceptor for human cytomegalovirus. Nat. Med. 11:515-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang, X., S. M. Huong, M. L. Chiu, N. Raab-Traub, and E. S. Huang. 2003. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 424:456-461. [DOI] [PubMed] [Google Scholar]
- 96.Wennerberg, K., A. Armulik, T. Sakai, M. Karlsson, R. Fassler, E. M. Schaefer, D. F. Mosher, and S. Johansson. 2000. The cytoplasmic tyrosines of integrin subunit β1 are involved in focal adhesion kinase activation. Mol. Cell. Biol. 20:5758-5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wennerberg, K., L. Lohikangas, D. Gullberg, M. Pfaff, S. Johansson, and R. Fassler. 1996. Beta 1 integrin-dependent and -independent polymerization of fibronectin. J. Cell Biol. 132:227-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wolfsberg, T. G., J. F. Bazan, C. P. Blobel, D. G. Myles, P. Primakoff, and J. M. White. 1993. The precursor region of a protein active in sperm-egg fusion contains a metalloprotease and a disintegrin domain: structural, functional, and evolutionary implications. Proc. Natl. Acad. Sci. U. S. A. 90:10783-10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wolfsberg, T. G., P. D. Straight, R. L. Gerena, A. P. Huovila, P. Primakoff, D. G. Myles, and J. M. White. 1995. ADAM, a widely distributed and developmentally regulated gene family encoding membrane proteins with a disintegrin and metalloprotease domain. Dev. Biol. 169:378-383. [DOI] [PubMed] [Google Scholar]
- 100.WuDunn, D., and P. G. Spear. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu, X., S. Miyamoto, and E. Mekada. 2000. Integrin alpha 2 beta 1-dependent EGF receptor activation at cell-cell contact sites. J. Cell Sci. 113:2139-2147. [DOI] [PubMed] [Google Scholar]
- 102.Yuan, R., P. Primakoff, and D. G. Myles. 1997. A role for the disintegrin domain of cyritestin, a sperm surface protein belonging to the ADAM family, in mouse sperm-egg plasma membrane adhesion and fusion. J. Cell Biol. 137:105-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang, X., J. F. Wang, B. Chandran, K. Persaud, B. Pytowski, J. Fingeroth, and J. E. Groopman. 2005. Kaposi's sarcoma-associated herpesvirus activation of vascular endothelial growth factor receptor 3 alters endothelial function and enhances infection. J. Biol. Chem. 280:26216-26224. [DOI] [PubMed] [Google Scholar]