Abstract
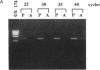
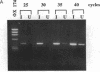
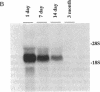
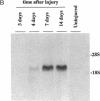
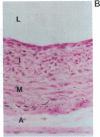
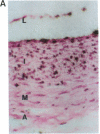
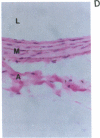
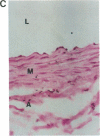
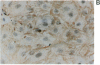
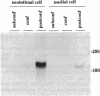
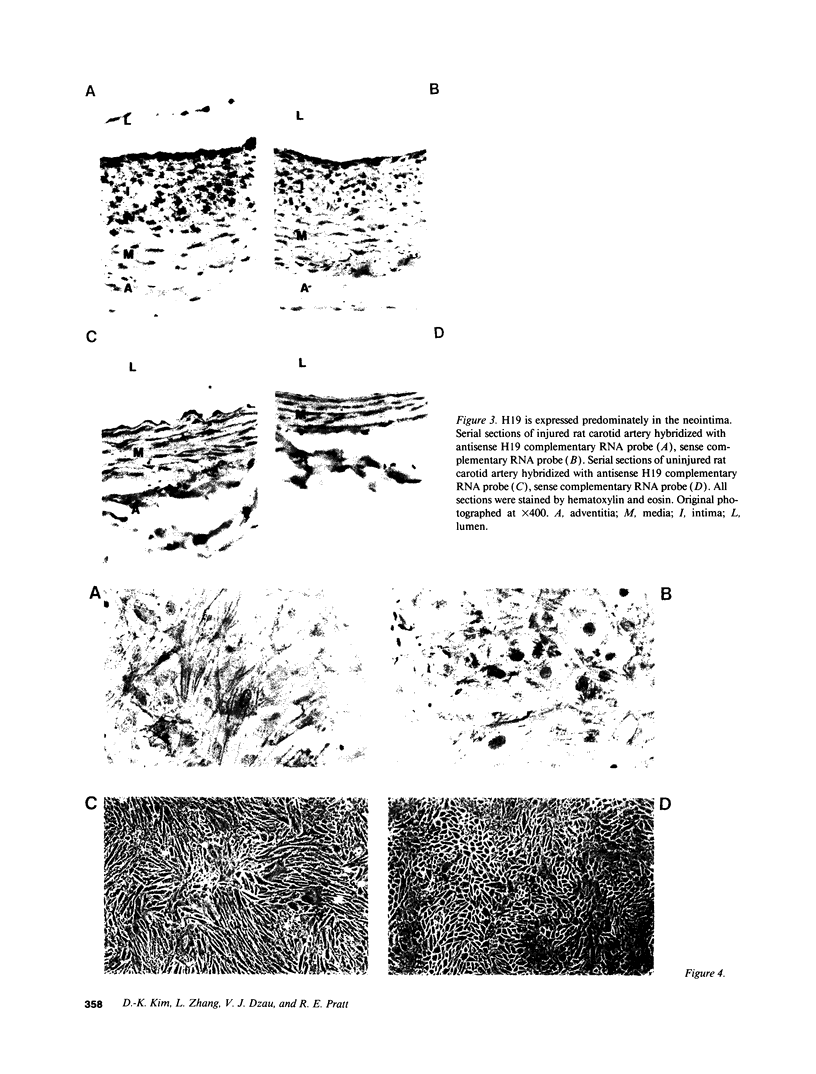
Vascular smooth muscle cell migration, proliferation, and differentiation are central to blood vessel development. Since neointimal formation after vascular injury may require the reexpression of a smooth muscle developmental sequence, we examined the expression of H19, a developmentally regulated gene, in rat blood vessels. Expression of the H19 gene is associated with the differentiation process that takes place during development of many tissues. Consistent with this, H19 was highly expressed in the 1-d-old rat aorta but was undetectable in the adult. H19 transcripts were only minimally detected in uninjured carotid artery but were abundant at 7 and 14 d after injury and were localized by in situ hybridization, primarily to the neointima. H19 transcript were undetectable in proliferating neointimal cells in culture but became highly abundant in postconfluent, differentiated neointimal cells. H19 transcripts were only minimally expressed in adult medial smooth muscle cells grown under the identical conditions. Thus, H19 may play an important role in the normal development and differentiation of the blood vessel and in the phenotypic changes of the smooth muscle cells, which are associated with neointimal lesion formation. The vascular injury model may be a useful system to use in examining the function of H19.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benditt E. P., Benditt J. M. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan C. I., Dees E. C., Ingram R. S., Tilghman S. M. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990 Jan;10(1):28–36. doi: 10.1128/mcb.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunkow M. E., Tilghman S. M. Ectopic expression of the H19 gene in mice causes prenatal lethality. Genes Dev. 1991 Jun;5(6):1092–1101. doi: 10.1101/gad.5.6.1092. [DOI] [PubMed] [Google Scholar]
- Clowes A. W., Reidy M. A., Clowes M. M. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983 Sep;49(3):327–333. [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Fingerle J., Johnson R., Clowes A. W., Majesky M. W., Reidy M. A. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8412–8416. doi: 10.1073/pnas.86.21.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman J. A., Ryan G. B., Karnovsky M. J. Endothelial regeneration in the rat carotid artery and the significance of endothelial denudation in the pathogenesis of myointimal thickening. Lab Invest. 1975 Mar;32(3):339–351. [PubMed] [Google Scholar]
- Giachelli C., Bae N., Lombardi D., Majesky M., Schwartz S. Molecular cloning and characterization of 2B7, a rat mRNA which distinguishes smooth muscle cell phenotypes in vitro and is identical to osteopontin (secreted phosphoprotein I, 2aR). Biochem Biophys Res Commun. 1991 Jun 14;177(2):867–873. doi: 10.1016/0006-291x(91)91870-i. [DOI] [PubMed] [Google Scholar]
- Goldberg I. D., Stemerman M. B., Ransil B. J., Fuhro R. L. In vivo aortic muscle cell growth kinetics. Differences between thoracic and abdominal segments after intimal injury in the rabbit. Circ Res. 1980 Aug;47(2):182–189. doi: 10.1161/01.res.47.2.182. [DOI] [PubMed] [Google Scholar]
- Grünwald J., Fingerle J., Hämmerle H., Betz E., Haudenschild C. C. Cytocontractile structures and proteins of smooth muscle cells during the formation of experimental lesions. Exp Mol Pathol. 1987 Feb;46(1):78–88. doi: 10.1016/0014-4800(87)90032-3. [DOI] [PubMed] [Google Scholar]
- Guyton J. R., Rosenberg R. D., Clowes A. W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circ Res. 1980 May;46(5):625–634. doi: 10.1161/01.res.46.5.625. [DOI] [PubMed] [Google Scholar]
- Han D. K., Liau G. Identification and characterization of developmentally regulated genes in vascular smooth muscle cells. Circ Res. 1992 Sep;71(3):711–719. doi: 10.1161/01.res.71.3.711. [DOI] [PubMed] [Google Scholar]
- Hanke H., Strohschneider T., Oberhoff M., Betz E., Karsch K. R. Time course of smooth muscle cell proliferation in the intima and media of arteries following experimental angioplasty. Circ Res. 1990 Sep;67(3):651–659. doi: 10.1161/01.res.67.3.651. [DOI] [PubMed] [Google Scholar]
- Kocher O., Gabbiani F., Gabbiani G., Reidy M. A., Cokay M. S., Peters H., Hüttner I. Phenotypic features of smooth muscle cells during the evolution of experimental carotid artery intimal thickening. Biochemical and morphologic studies. Lab Invest. 1991 Oct;65(4):459–470. [PubMed] [Google Scholar]
- Kocher O., Skalli O., Cerutti D., Gabbiani F., Gabbiani G. Cytoskeletal features of rat aortic cells during development. An electron microscopic, immunohistochemical, and biochemical study. Circ Res. 1985 Jun;56(6):829–838. doi: 10.1161/01.res.56.6.829. [DOI] [PubMed] [Google Scholar]
- Leibovitch M. P., Nguyen V. C., Gross M. S., Solhonne B., Leibovitch S. A., Bernheim A. The human ASM (adult skeletal muscle) gene: expression and chromosomal assignment to 11p15. Biochem Biophys Res Commun. 1991 Nov 14;180(3):1241–1250. doi: 10.1016/s0006-291x(05)81329-4. [DOI] [PubMed] [Google Scholar]
- Leimgruber P. P., Roubin G. S., Hollman J., Cotsonis G. A., Meier B., Douglas J. S., King S. B., Jr, Gruentzig A. R. Restenosis after successful coronary angioplasty in patients with single-vessel disease. Circulation. 1986 Apr;73(4):710–717. doi: 10.1161/01.cir.73.4.710. [DOI] [PubMed] [Google Scholar]
- Liu M. W., Roubin G. S., King S. B., 3rd Restenosis after coronary angioplasty. Potential biologic determinants and role of intimal hyperplasia. Circulation. 1989 Jun;79(6):1374–1387. doi: 10.1161/01.cir.79.6.1374. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Benditt E. P., Schwartz S. M. Expression and developmental control of platelet-derived growth factor A-chain and B-chain/Sis genes in rat aortic smooth muscle cells. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1524–1528. doi: 10.1073/pnas.85.5.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Giachelli C. M., Reidy M. A., Schwartz S. M. Rat carotid neointimal smooth muscle cells reexpress a developmentally regulated mRNA phenotype during repair of arterial injury. Circ Res. 1992 Oct;71(4):759–768. doi: 10.1161/01.res.71.4.759. [DOI] [PubMed] [Google Scholar]
- Naftilan A. J., Zuo W. M., Inglefinger J., Ryan T. J., Jr, Pratt R. E., Dzau V. J. Localization and differential regulation of angiotensinogen mRNA expression in the vessel wall. J Clin Invest. 1991 Apr;87(4):1300–1311. doi: 10.1172/JCI115133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens G. K., Loeb A., Gordon D., Thompson M. M. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986 Feb;102(2):343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pachnis V., Brannan C. I., Tilghman S. M. The structure and expression of a novel gene activated in early mouse embryogenesis. EMBO J. 1988 Mar;7(3):673–681. doi: 10.1002/j.1460-2075.1988.tb02862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier F., Chan C. T., Timmons P. M., Robertson E. J., Evans M. J., Rigby P. W. The murine H19 gene is activated during embryonic stem cell differentiation in vitro and at the time of implantation in the developing embryo. Development. 1991 Dec;113(4):1105–1114. doi: 10.1242/dev.113.4.1105. [DOI] [PubMed] [Google Scholar]
- Roubin G. S., King S. B., 3rd, Douglas J. S., Jr Restenosis after percutaneous transluminal coronary angioplasty: the Emory University Hospital experience. Am J Cardiol. 1987 Jul 31;60(3):39B–43B. doi: 10.1016/0002-9149(87)90482-6. [DOI] [PubMed] [Google Scholar]
- Schwartz S. M., Campbell G. R., Campbell J. H. Replication of smooth muscle cells in vascular disease. Circ Res. 1986 Apr;58(4):427–444. doi: 10.1161/01.res.58.4.427. [DOI] [PubMed] [Google Scholar]
- Walker L. N., Bowen-Pope D. F., Ross R., Reidy M. A. Production of platelet-derived growth factor-like molecules by cultured arterial smooth muscle cells accompanies proliferation after arterial injury. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7311–7315. doi: 10.1073/pnas.83.19.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waller B. F., Gorfinkel H. J., Rogers F. J., Kent K. M., Roberts W. C. Early and late morphologic changes in major epicardial coronary arteries after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1984 Jun 15;53(12):42C–47C. doi: 10.1016/0002-9149(84)90744-6. [DOI] [PubMed] [Google Scholar]
- Wiles M. V. Isolation of differentially expressed human cDNA clones: similarities between mouse and human embryonal carcinoma cell differentiation. Development. 1988 Nov;104(3):403–413. doi: 10.1242/dev.104.3.403. [DOI] [PubMed] [Google Scholar]
- Zanellato A. M., Borrione A. C., Tonello M., Scannapieco G., Pauletto P., Sartore S. Myosin isoform expression and smooth muscle cell heterogeneity in normal and atherosclerotic rabbit aorta. Arteriosclerosis. 1990 Nov-Dec;10(6):996–1009. doi: 10.1161/01.atv.10.6.996. [DOI] [PubMed] [Google Scholar]