Abstract
Toll-like receptor (TLR) ligands are critical activators of innate immunity and are being developed as vaccine adjuvants. However, their utility in conjunction with viral vector-based vaccines remains unclear. In this study, we evaluated the impact of a variety of TLR ligands on antigen-specific CD8+ T lymphocyte responses elicited by a recombinant adenovirus serotype 26 (rAd26) vector expressing simian immunodeficiency virus Gag in mice. The TLR3 ligand poly(I:C) suppressed Gag-specific cellular immune responses, whereas the TLR4 ligands lipopolysaccharide and monophosphoryl lipid A substantially augmented the magnitude and functionality of these responses by a MyD88- and TRIF-dependent mechanism. These data demonstrate that TLR ligands can modulate the immunogenicity of viral vaccine vectors both positively and negatively. Moreover, these findings suggest the potential utility of TLR4 ligands as adjuvants for rAd vector-based vaccines.
Toll-like receptors (TLRs) are critical sensors of infection with a fundamental role in the activation of innate immune responses and the subsequent modulation of pathogen-specific adaptive immunity (2). TLR ligands have therefore emerged as potential vaccine adjuvants, particularly in the context of peptide, protein, and DNA vaccines (17). In particular, TLR agonists are widely reported to modulate antibody and T helper lymphocyte responses, and in some cases CD8+ T lymphocyte responses, elicited by protein-based vaccines (5, 19, 33, 41). However, far less is known about the impact of TLR ligands on the immunogenicity of viral vector-based vaccines.
Compared with DNA vaccines, viral vectors are typically more immunogenic, presumably as a result of the activation of innate immunity via multiple TLRs or other pattern recognition receptors (29). Viral vectors elicit robust T lymphocyte responses and thus are attractive vaccine candidates for pathogens such as human immunodeficiency virus type 1 (HIV-1) and malaria (10). Whether the addition of exogenous TLR agonists might further enhance the immunogenicity of viral vectors, however, remains unclear. The few studies that have explored the utility of TLR adjuvants with viral vectors have typically shown no or mild enhancement of antibody and T lymphocyte responses (7, 26). We therefore sought to determine systematically whether TLR ligands can modulate cellular immune responses elicited by a recombinant adenovirus serotype 26 (rAd26) vector in mice.
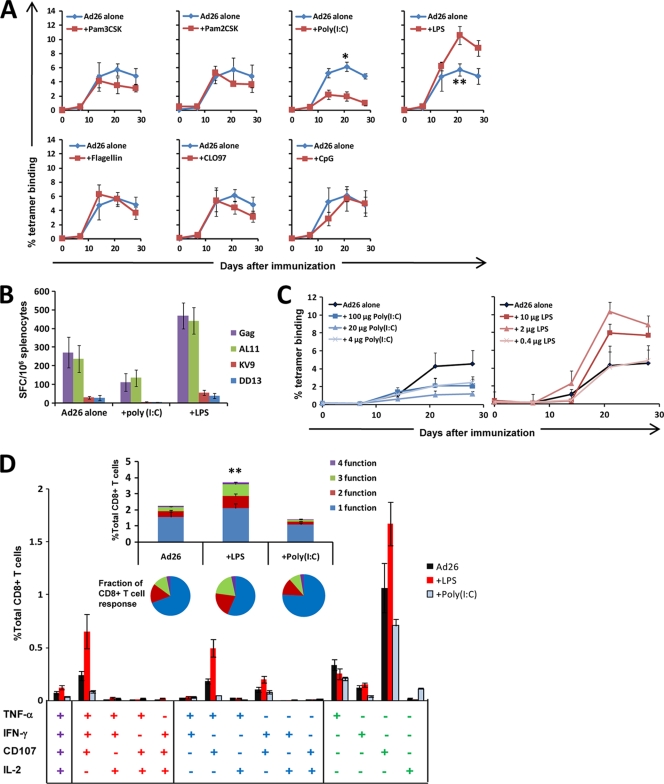
C57BL/6 mice (n = 7 to 8/group) were immunized with a single injection of 3 × 108 viral particles (vp) rAd26-Gag alone or combined with various TLR ligands (1). Vectors were mixed with soluble TLR agonists 1 h prior to intramuscular (i.m.) injection into both quadriceps muscles. Cellular immune responses were assessed by Db/AL11 tetramer binding assays (3, 6), gamma interferon (IFN-γ) enzyme-linked immunospot (ELISPOT) assays (6), and multiparameter intracellular cytokine staining (ICS) assays (14). As shown in Fig. 1 A, immunization with rAd26-Gag plus either 20 μg Pam3CSK (TLR1/2 ligand) (25), 20 μg Pam2CSK (TLR2/6 ligand) (9, 20), 10 μg flagellin (TLR5 ligand) (5, 8), 100 μg CLO97 (TLR7 ligand) (41), or 40 μg CpG (TLR9 ligand) (40) (all obtained from InvivoGen, San Diego, CA) elicited AL11-specific tetramer-positive responses (3, 6) that were similar to those detected in the unadjuvanted groups.
FIG. 1.
Antigen-specific CD8+ T cell responses elicited by rAd26-Gag are modulated by soluble TLR ligands. (A) C57BL/6 mice (n = 7 to 8 mice/group) were immunized once with 3 × 108 vp rAd26-Gag alone or 3 × 108 vp rAd26-Gag combined with the following TLR ligands: 20 μg synthetic triacylated lipoprotein (Pam3CSK; TLR1/2 ligand), 20 μg synthetic diacylated lipoprotein (Pam2CSK; TLR 2/6 ligand), 100 μg poly(I:C) (TLR3 ligand), 10 μg LPS (TLR4 ligand), 10 μg flagellin (TLR5 ligand), 100 μg CLO97 (TLR7 ligand), or 40 μg unmethylated CpG-oligodeoxynucleotides (CpG; TLR9 ligand). Gag-specific cellular immune responses were assayed by Db/AL11 tetramer binding assays at multiple time points following injection. (B) At week 4 following immunization, functional immune responses from mice immunized with rAd26 vaccine alone or with 10 μg LPS or 100 μg poly(I:C) were assessed by IFN-γ ELISPOT assays in response to pooled Gag peptides, the CD8+ T lymphocyte epitopes AL11 and KV9, and the CD4+ T lymphocyte epitope DD13. (C) Assessment of the dose response of LPS (10 μg, 2 μg, 0.4 μg) and poly(I:C) (100 μg, 20 μg, 4 μg) with rAd26-Gag (n = 4 mice/group) by Db/AL11 tetramer binding assays. (D) Mice were immunized once i.m. with 3 × 108 vp rAd26-Gag alone, rAd26-Gag with 2 μg LPS, or rAd26-Gag with 20 μg poly(I:C) (n = 4 to 8 mice/group), and Gag-specific CD8+ T cell responses in splenocytes were assessed 4 weeks after vaccination by intracellular cytokine assays for IFN-γ, TNF-α, IL-2, and CD107. Responses to pooled Gag peptides are presented for each individual combination of functions and collated as the number of functions elaborated as a percent of total CD8+ T lymphocytes (insert; bar graph) and as the fraction of Gag-specific CD8+ T lymphocytes (insert; pie charts). Mean responses with standard errors are shown (*, P < 0.001; **, P < 0.05; two-tailed t test).
The TLR3 ligand poly(I:C) (InvivoGen, San Diego, CA), however, markedly suppressed responses to the rAd26-Gag vaccine (Fig. 1A). This finding contrasts with prior reports demonstrating its adjuvanticity for protein antigen vaccines (22, 34, 37). By day 28, mice that received the vaccine plus 100 μg poly(I:C) developed Gag-specific CD8+ T lymphocyte responses that were significantly lower (1.7%) than those of mice that received the vaccine alone (5.4%; P < 0.001; two-tailed t test). Similarly, IFN-γ ELISPOT responses in mice that received poly(I:C) were lower than those observed in the unadjuvanted group (Fig. 1B) (6). In a dose response study (Fig. 1C), 100-μg, 20-μg, and 4-μg doses of poly(I:C) all resulted in diminished tetramer-positive responses.
In contrast, the TLR4 ligand lipopolysaccharide (LPS) (Ultrapure LPS from Escherichia coli 0111:B4; InvivoGen, San Diego, CA) substantially enhanced Gag-specific CD8+ T lymphocyte responses elicited by the rAd26-Gag vaccine (Fig. 1A). At day 28, tetramer-positive responses in mice that received the vaccine plus 10 μg LPS (9.6%) were significantly higher than those in the unadjuvanted group (5.4%; P = 0.04). Moreover, IFN-γ ELISPOT responses (6, 21) to pooled Gag peptides, the CD8+ T lymphocyte epitopes AL11 and KV9, and the CD4+ T lymphocyte epitope DD13 were greater in mice that received the vaccine with LPS than in mice that received the vaccine alone at week 4 after immunization (P = 0.02) (Fig. 1B). To further quantify this effect, mice were immunized once i.m. (n = 4 mice/group) with rAd26-Gag with various doses of LPS (10 μg, 2 μg, 0.4 μg). Tetramer-positive responses were enhanced by 10 μg and 2 μg LPS but not by 0.4 μg LPS (Fig. 1C), indicating that this LPS effect was dose dependent. No overt clinical toxicities were observed by using these doses of LPS in mice.
We next evaluated the functionality of CD8+ T lymphocyte responses by multiparameter ICS assays that assessed IFN-γ, tumor necrosis factor alpha (TNF-α), interleukin-2 (IL-2), and the cytotoxic degranulation marker CD107 expression at week 4 following immunization with rAd26-Gag alone, rAd26-Gag with 2 μg LPS, or rAd26-Gag with 20 μg poly(I:C) (n = 4 to 8 mice/group) (15). As shown in Fig. 1D, the addition of LPS significantly enhanced not only the overall magnitude of Gag-specific CD8+ T lymphocyte responses (P = 0.04) but also the fraction of Gag-specific CD8+ T lymphocytes that expressed two or more effector functions (P = 0.04). In particular, the LPS-adjuvanted group induced higher levels of single-function CD107+, 2-function TNF-α+ CD107+, as well as 3-function IFN-γ+ TNF-α+ CD107+ CD8+ T lymphocytes than mice that received rAd26-Gag alone. These data show that LPS enhanced both the magnitude and functionality of antigen-specific cellular responses elicited by rAd26-Gag. In contrast, the addition of poly(I:C) diminished both the overall magnitude of Gag-specific responses and the fraction of these responses that were multifunctional.
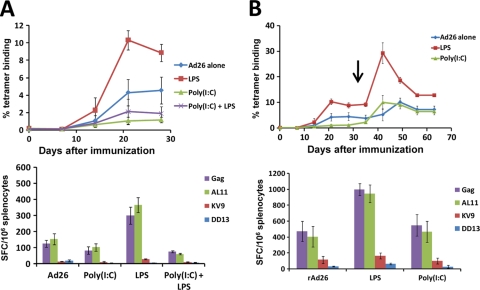
We further characterized the opposing effects of poly(I:C) and LPS by administering the rAd26-Gag vaccine with both poly(I:C) and LPS. C57BL/6 mice (n = 4 mice/group) were immunized with a single injection of rAd26-Gag alone or with 10 μg LPS, 60 μg poly(I:C), or both TLR ligands. As shown in Fig. 2 A, administration of both TLR ligands resulted in reduced Gag-specific responses, suggesting that the suppressive effect of poly(I:C) was dominant over the enhancing effect of LPS. To determine the durability of the effects of poly(I:C) and LPS, C57BL/6 mice were primed with rAd26-Gag alone or with 2 μg LPS or 20 μg poly(I:C) (n = 4 mice/group) and were boosted on day 35 with a single i.m. injection of the heterologous vector rAd5HVR48(1-7) also expressing simian immunodeficiency virus (SIV) Gag (32). As shown in Fig. 2B, the mice that received poly(I:C) with the priming immunization responded to the boosting immunization with Gag-specific responses that were comparable to those observed in the mice that received rAd26-Gag alone. In contrast, mice that received LPS with the priming immunization exhibited sustained enhanced Gag-specific tetramer and ELISPOT responses, demonstrating the proliferative potential of antigen-specific CD8+ T lymphocytes elicited by the LPS-adjuvanted rAd26-Gag vaccine.
FIG. 2.
Dominant suppressive effect of poly(I:C) over LPS with the rAd26-Gag vaccine. (A) Mice were immunized once i.m. with 3 × 108 vp rAd26-Gag alone or with 20 μg poly(I:C), 2 μg LPS, or both poly(I:C) and LPS (n = 4 mice/group). Gag-specific CD8+ T lymphocyte responses were assessed by Db/AL11 tetramer binding assays and IFN-γ ELISPOT assays 4 weeks after immunization. (B) Mice were primed once with 3 × 108 vp rAd26-Gag alone or with 2 μg LPS or 20 μg poly(I:C) and then boosted (↓) with 3 × 108 vp rAd5HVR48(1-7) at week 5. Gag-specific cellular immune responses were assessed by Db/AL11 tetramer binding assays and by IFN-γ ELISPOT responses at week 4 postboost. Mean responses with standard errors are shown.
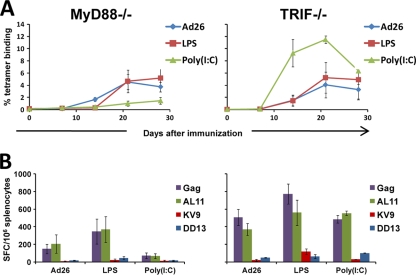
We next investigated whether the mechanism underlying the immunomodulatory effects of LPS and poly(I:C) involved the expected TLR signaling pathways. Although LPS and poly(I:C) are chiefly considered TLR ligands, poly(I:C) can also signal through the intracellular sensor MDA-5 (14), and both LPS and poly(I:C) may activate inflammasomes through Nalp3 (12, 28). To explore whether the effects of LPS and poly(I:C) involved TLR signaling, we utilized C57BL/6 mice lacking TRIF (Jackson Laboratory, Bar Harbor, ME), which is utilized by TLR3, or C57BL/6 mice lacking MyD88 (provided by S. Akira and B. Pulendran), which is utilized by the majority of TLRs. In particular, TLR4 signals through both TRIF and MyD88. Wild-type, MyD88−/−, and TRIF−/− mice (n = 4 mice/group) were immunized with rAd26-Gag vaccine alone or with 2 μg LPS or 20 μg poly(I:C). As shown in Fig. 3, the adjuvant activity of LPS was abrogated in both MyD88−/− and TRIF−/− mice (Fig. 3A and B), suggesting that the adjuvanticity of the TLR4 ligand LPS was dependent on both MyD88 and TRIF, as expected. In contrast, the suppressive effect of poly(I:C) was observed in MyD88−/− mice but not in TRIF−/− mice (Fig. 3A and B), indicating that the suppressive effect of the TLR3 ligand poly(I:C) was dependent on TRIF, rather than MDA-5 or nonspecific effects (14, 39). These data confirm that the immunomodulatory effects of LPS and poly(I:C) were dependent on the expected TLR signaling pathways.
FIG. 3.
The immunomodulatory effects of poly(I:C) and LPS are TLR dependent. MyD88−/− and TRIF−/− mice (n = 4 mice/group) were immunized once i.m. with 3 × 108 vp rAd26-Gag alone or with 2 μg LPS or 20 μg poly(I:C). (A) Db/AL11 tetramer binding assays were performed at multiple time points following injection, and (B) IFN-γ ELISPOT responses were assessed 4 weeks after immunization. Mean responses with standard errors are shown.
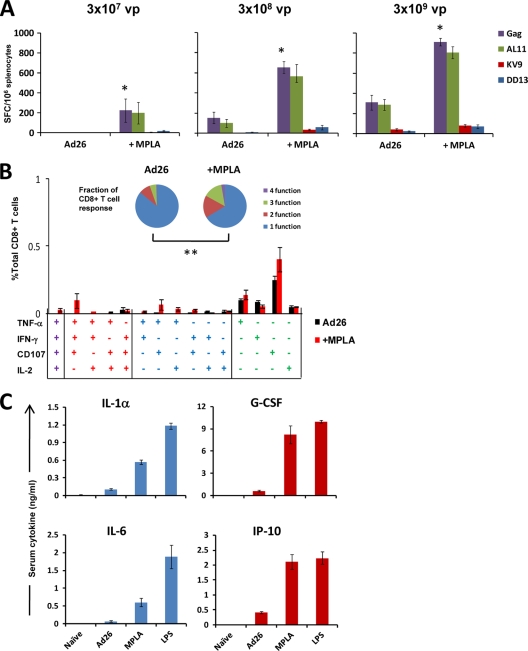
LPS is not a likely adjuvant for clinical development as a result of its toxicities, and alternative TLR4 ligands have been developed for potential clinical use. In particular, monophosphoryl lipid A (MPLA) is an LPS derivative that retains the immunologically active lipid A portion of the parent molecule (23, 27). The reduced toxicity of MPLA is attributed to the preferential recruitment of TRIF upon TLR4 activation, resulting in decreased induction of inflammatory cytokines (18). To determine if MPLA can similarly adjuvant cellular immune responses elicited by rAd26-Gag, C57BL/6 mice were immunized with 3 × 107, 3 × 108, or 3 × 109 vp rAd26-Gag alone or with 5 μg MPLA (derived from Salmonella enterica serovar Minnesota R595 LPS; InvivoGen, San Diego, CA) (n = 4 mice/group). This optimal dose of MPLA was selected by dose response studies (data not shown). As shown in Fig. 4 A, Gag-specific IFN-γ ELISPOT responses to the lowest dose of vector were essentially undetectable in the unadjuvanted group, consistent with prior observations (1). In contrast, clear responses were observed in the mice that received 3 × 107 vp rAd26-Gag with MPLA (P < 0.01; two-tailed t test). Mice that received the 3 × 108 vp and 3 × 109 vp doses of rAd26-Gag with MPLA also exhibited higher Gag-specific cellular immune responses than the unadjuvanted groups (P < 0.01). Functionality of these Gag-specific CD8+ T lymphocyte responses, as measured by multiparameter ICS assays assessing IFN-γ, TNF-α, IL-2, and CD107 expression, was also greater in mice that received rAd26-Gag with MPLA compared with rAd26-Gag (P < 0.05 for the lowest dose group) (Fig. 4B). Thus, the TLR4 ligand MPLA also augmented antigen-specific CD8+ T lymphocyte responses elicited by rAd26-Gag.
FIG. 4.
The TLR4 ligand MPLA augments the immunogenicity of rAd26-Gag. C57BL/6 mice (n = 4 mice/group) were immunized once i.m. with 3 × 107, 3 × 108, or 3 × 109 vp rAd26-Gag with or without 5 μg MPLA. Gag-specific cellular immune responses were assessed 4 weeks after immunization by IFN-γ ELISPOT responses (*, P < 0.01 for responses to pooled Gag peptides; two-tailed t test) (A) and by ICS for IFN-γ, TNF-α, IL-2, and CD107 (B). Responses to pooled Gag peptides in mice immunized with 3 × 107 vp rAd26-Gag with or without 5 μg MPLA are presented for each individual combination of functions and collated as the number of functions as a fraction of the total Gag-specific CD8+ T lymphocyte response (insert; pie charts) (**, P < 0.05). (C) Cytokine levels were measured in sera of mice 8 h after immunization with 3 × 108 vp rAd26-Gag alone or 3 × 108 vp rAd26-Gag with 5 μg MPLA or 2 μg LPS (n = 4 mice/group). Mean responses with standard errors are shown.
To explore differences in acute inflammatory responses following MPLA and LPS administration, serum levels of IL-1α, IL-6, granulocyte colony-stimulating factor (G-CSF), and IP-10 were assessed 8 h after vaccination in duplicate using multiplexed fluorescent bead-based immunoassays (Millipore, Billerica, MA) and analyzed on the Luminex 100 IS (Luminex, Austin, TX). As shown in Fig. 4C, mice that received MPLA had lower levels of the MyD88-associated acute proinflammatory cytokines IL-1α and IL-6 than mice that received LPS, as expected. Levels of IP-10 and G-CSF, which are associated with TRIF activation (18), were comparable (Fig. 4B). These data confirm that MPLA resulted in lower levels of systemic inflammatory cytokine secretion than LPS.
Optimization of the immunogenicity of viral vectors is an important research priority. However, there have been few reports addressing the potential use of adjuvants together with viral vectors. Combining alum with rAd35 elicited improved antibody responses to a malaria antigen (24), and the addition of TLR9 agonists (CpGs) resulted in paradoxically diminished immune responses elicited by a rAd5 vector but improved protection against a cancer antigen (13). Most recently, Appledorn et al. reported enhanced antigen-specific T lymphocyte responses with the coadministration of a rAd vector engineered to express a novel TLR5 agonist (4). Our study extends these findings and represents the first systematic investigation of the capacity of a panel of soluble TLR ligands to modulate rAd-elicited CD8+ T lymphocyte responses.
The TLR agonists that modulated vaccine-elicited immune responses in this study included poly(I:C), LPS, and MPLA. These ligands have all been reported to augment CD8+ T lymphocyte responses elicited by peptide or protein vaccines (11, 22, 31, 33, 42), presumably through enhanced cross-presentation (34, 35). TLR signaling has been shown to be important for virus-elicited CD8+ T lymphocyte responses (38), often through activation of multiple TLRs or other pattern recognition receptors (30). The activation of TLR4 by LPS or MPLA with a viral vector most likely provides an additive or synergistic signal, probably resulting in enhanced APC maturation in the appropriate cytokine milieu. Moreover, immunization of the viral vector and LPS at different sites abrogated the observed adjuvanticity (data not shown), indicating that TLR4 adjuvanticity involves a local mechanism of action. However, the mechanism by which a TLR3 agonist suppresses immunogenicity of a viral vector remains unclear. It is possible that the high levels of type I interferon elicited by poly(I:C) (data not shown) may limit expression from the rAd26 vector. Alternatively, poly(I:C) has been reported to elicit IL-10 secretion, and this suppressive cytokine may limit CD8+ T cell proliferation (22, 36). The unexpected suppressive activity of poly(I:C) illustrates the inherent complexity of viral vectors compared to protein-based vaccines (16, 37).
Our data demonstrate that antigen-specific CD8+ T lymphocyte responses elicited by a rAd26-Gag vaccine vector can be both positively and negatively modulated by soluble TLR ligands, and the mechanism underlying these observations involves the expected TRIF and MyD88 signaling pathways. In particular, the TLR4 ligands LPS and MPLA substantially augmented the magnitude and functionality of antigen-specific cellular immune responses elicited by this vaccine vector. These findings suggest that TLR ligands, particularly MPLA, deserve further exploration as potential adjuvants to improve the immunogenicity and protective efficacy of viral vaccine vectors.
Acknowledgments
We thank S. Akira, A. Hovav, M. Cayabyab, and A. Riggs for generous advice, assistance, and reagents.
We acknowledge support from the Bill and Melinda Gates Foundation grant no. 38614 (D.H.B.) and from NIH grants AI078526 (D.H.B.), AI066924 (D.H.B.), and AI066305 (D.H.B.).
Footnotes
The authors have paid a fee to allow immediate free access to this article.
Published ahead of print on 14 July 2010.
REFERENCES
- 1.Abbink, P., A. A. Lemckert, B. A. Ewald, D. M. Lynch, M. Denholtz, S. Smits, L. Holterman, I. Damen, R. Vogels, A. R. Thorner, K. L. O'Brien, A. Carville, K. G. Mansfield, J. Goudsmit, M. J. Havenga, and D. H. Barouch. 2007. Comparative seroprevalence and immunogenicity of six rare serotype recombinant adenovirus vaccine vectors from subgroups B and D. J. Virol. 81:4654-4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. [DOI] [PubMed] [Google Scholar]
- 3.Altman, J. D., P. A. Moss, P. J. Goulder, D. H. Barouch, M. G. McHeyzer-Williams, J. I. Bell, A. J. McMichael, and M. M. Davis. 1996. Phenotypic analysis of antigen-specific T lymphocytes. Science 274:94-96. [DOI] [PubMed] [Google Scholar]
- 4.Appledorn, D. M., Y. A. Aldhamen, W. Depas, S. S. Seregin, C. J. Liu, N. Schuldt, D. Quach, D. Quiroga, S. Godbehere, I. Zlatkin, S. Kim, J. J. McCormick, and A. Amalfitano. 2010. A new adenovirus based vaccine vector expressing an Eimeria tenella derived TLR agonist improves cellular immune responses to an antigenic target. PLoS One 5:e9579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bargieri, D. Y., D. S. Rosa, C. J. Braga, B. O. Carvalho, F. T. Costa, N. M. Espindola, A. J. Vaz, I. S. Soares, L. C. Ferreira, and M. M. Rodrigues. 2008. New malaria vaccine candidates based on the Plasmodium vivax merozoite surface protein-1 and the TLR-5 agonist Salmonella Typhimurium FliC flagellin. Vaccine 26:6132-6142. [DOI] [PubMed] [Google Scholar]
- 6.Barouch, D. H., M. G. Pau, J. H. Custers, W. Koudstaal, S. Kostense, M. J. Havenga, D. M. Truitt, S. M. Sumida, M. G. Kishko, J. C. Arthur, B. Korioth-Schmitz, M. H. Newberg, D. A. Gorgone, M. A. Lifton, D. L. Panicali, G. J. Nabel, N. L. Letvin, and J. Goudsmit. 2004. Immunogenicity of recombinant adenovirus serotype 35 vaccine in the presence of pre-existing anti-Ad5 immunity. J. Immunol. 172:6290-6297. [DOI] [PubMed] [Google Scholar]
- 7.Belyakov, I. M., D. Isakov, Q. Zhu, A. Dzutsev, D. Klinman, and J. A. Berzofsky. 2006. Enhancement of CD8+ T cell immunity in the lung by CpG oligodeoxynucleotides increases protective efficacy of a modified vaccinia Ankara vaccine against lethal poxvirus infection even in a CD4-deficient host. J. Immunol. 177:6336-6343. [DOI] [PubMed] [Google Scholar]
- 8.Braga, C. J., L. M. Massis, M. E. Sbrogio-Almeida, B. C. Alencar, D. Y. Bargieri, S. B. Boscardin, M. M. Rodrigues, and L. C. Ferreira. 2010. CD8+ T cell adjuvant effects of Salmonella FliCd flagellin in live vaccine vectors or as purified protein. Vaccine 28:1373-1382. [DOI] [PubMed] [Google Scholar]
- 9.Buwitt-Beckmann, U., H. Heine, K. H. Wiesmuller, G. Jung, R. Brock, S. Akira, and A. J. Ulmer. 2005. Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur. J. Immunol. 35:282-289. [DOI] [PubMed] [Google Scholar]
- 10.Draper, S. J., and J. L. Heeney. 2010. Viruses as vaccine vectors for infectious diseases and cancer. Nat. Rev. Microbiol. 8:62-73. [DOI] [PubMed] [Google Scholar]
- 11.Geurtsen, J., F. Fransen, R. J. Vandebriel, E. R. Gremmer, L. J. de la Fonteyne-Blankestijn, B. Kuipers, J. Tommassen, and P. van der Ley. 2008. Supplementation of whole-cell pertussis vaccines with lipopolysaccharide analogs: modification of vaccine-induced immune responses. Vaccine 26:899-906. [DOI] [PubMed] [Google Scholar]
- 12.Kanneganti, T. D., N. Ozoren, M. Body-Malapel, A. Amer, J. H. Park, L. Franchi, J. Whitfield, W. Barchet, M. Colonna, P. Vandenabeele, J. Bertin, A. Coyle, E. P. Grant, S. Akira, and G. Nunez. 2006. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 440:233-236. [DOI] [PubMed] [Google Scholar]
- 13.Karan, D., A. M. Krieg, and D. M. Lubaroff. 2007. Paradoxical enhancement of CD8 T cell-dependent anti-tumor protection despite reduced CD8 T cell responses with addition of a TLR9 agonist to a tumor vaccine. Int. J. Cancer. 121:1520-1528. [DOI] [PubMed] [Google Scholar]
- 14.Kato, H., O. Takeuchi, S. Sato, M. Yoneyama, M. Yamamoto, K. Matsui, S. Uematsu, A. Jung, T. Kawai, K. J. Ishii, O. Yamaguchi, K. Otsu, T. Tsujimura, C. S. Koh, C. Reis e Sousa, Y. Matsuura, T. Fujita, and S. Akira. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101-105. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman, D. R., M. Bivas-Benita, N. L. Simmons, D. Miller, and D. H. Barouch. 2010. Route of adenovirus-based HIV-1 vaccine delivery impacts the phenotype and trafficking of vaccine-elicited CD8+ T-lymphocytes. J. Virol. 84:5986-5996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar, H., S. Koyama, K. J. Ishii, T. Kawai, and S. Akira. 2008. Cutting edge: cooperation of IPS-1- and TRIF-dependent pathways in poly IC-enhanced antibody production and cytotoxic T cell responses. J. Immunol. 180:683-687. [DOI] [PubMed] [Google Scholar]
- 17.Manicassamy, S., and B. Pulendran. 2009. Modulation of adaptive immunity with Toll-like receptors. Semin. Immunol. 21:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mata-Haro, V., C. Cekic, M. Martin, P. M. Chilton, C. R. Casella, and T. C. Mitchell. 2007. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 316:1628-1632. [DOI] [PubMed] [Google Scholar]
- 19.Morefield, G. L., L. D. Hawkins, S. T. Ishizaka, T. L. Kissner, and R. G. Ulrich. 2007. Synthetic Toll-like receptor 4 agonist enhances vaccine efficacy in an experimental model of toxic shock syndrome. Clin. Vaccine Immunol. 14:1499-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakao, Y., K. Funami, S. Kikkawa, M. Taniguchi, M. Nishiguchi, Y. Fukumori, T. Seya, and M. Matsumoto. 2005. Surface-expressed TLR6 participates in the recognition of diacylated lipopeptide and peptidoglycan in human cells. J. Immunol. 174:1566-1573. [DOI] [PubMed] [Google Scholar]
- 21.Nanda, A., D. M. Lynch, J. Goudsmit, A. A. Lemckert, B. A. Ewald, S. M. Sumida, D. M. Truitt, P. Abbink, M. G. Kishko, D. A. Gorgone, M. A. Lifton, L. Shen, A. Carville, K. G. Mansfield, M. J. Havenga, and D. H. Barouch. 2005. Immunogenicity of recombinant fiber-chimeric adenovirus serotype 35 vector-based vaccines in mice and rhesus monkeys. J. Virol. 79:14161-14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ngoi, S. M., M. G. Tovey, and A. T. Vella. 2008. Targeting poly(I:C) to the TLR3-independent pathway boosts effector CD8 T cell differentiation through IFN-alpha/beta. J. Immunol. 181:7670-7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okemoto, K., K. Kawasaki, K. Hanada, M. Miura, and M. Nishijima. 2006. A potent adjuvant monophosphoryl lipid A triggers various immune responses, but not secretion of IL-1beta or activation of caspase-1. J. Immunol. 176:1203-1208. [DOI] [PubMed] [Google Scholar]
- 24.Ophorst, O. J., K. Radosevic, J. M. Klap, J. Sijtsma, G. Gillissen, R. Mintardjo, M. J. van Ooij, L. Holterman, A. Companjen, J. Goudsmit, and M. J. Havenga. 2007. Increased immunogenicity of recombinant Ad35-based malaria vaccine through formulation with aluminum phosphate adjuvant. Vaccine 25:6501-6510. [DOI] [PubMed] [Google Scholar]
- 25.Ozinsky, A., D. M. Underhill, J. D. Fontenot, A. M. Hajjar, K. D. Smith, C. B. Wilson, L. Schroeder, and A. Aderem. 2000. The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. U. S. A. 97:13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkins, S. D., A. J. Williams, L. M. O'Brien, T. R. Laws, and R. J. Phillpotts. 2008. CpG used as an adjuvant for an adenovirus-based Venezuelan equine encephalitis virus vaccine increases the immune response to the vector, but not to the transgene product. Viral Immunol. 21:451-457. [DOI] [PubMed] [Google Scholar]
- 27.Persing, D. H., R. N. Coler, M. J. Lacy, D. A. Johnson, J. R. Baldridge, R. M. Hershberg, and S. G. Reed. 2002. Taking toll: lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 10:S32-S37. [DOI] [PubMed] [Google Scholar]
- 28.Pétrilli, V., C. Dostert, D. A. Muruve, and J. Tschopp. 2007. The inflammasome: a danger sensing complex triggering innate immunity. Curr. Opin. Immunol. 19:615-622. [DOI] [PubMed] [Google Scholar]
- 29.Pulendran, B. 2009. Learning immunology from the yellow fever vaccine: innate immunity to systems vaccinology. Nat. Rev. Immunol. 9:741-747. [DOI] [PubMed] [Google Scholar]
- 30.Querec, T., S. Bennouna, S. Alkan, Y. Laouar, K. Gorden, R. Flavell, S. Akira, R. Ahmed, and B. Pulendran. 2006. Yellow fever vaccine YF-17D activates multiple dendritic cell subsets via TLR2, 7, 8, and 9 to stimulate polyvalent immunity. J. Exp. Med. 203:413-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quintilio, W., F. S. Kubrusly, D. Iourtov, C. Miyaki, M. A. Sakauchi, F. Lucio, C. Dias Sde, C. S. Takata, E. N. Miyaji, H. G. Higashi, L. C. Leite, and I. Raw. 2009. Bordetella pertussis monophosphoryl lipid A as adjuvant for inactivated split virion influenza vaccine in mice. Vaccine 27:4219-4224. [DOI] [PubMed] [Google Scholar]
- 32.Roberts, D. M., A. Nanda, M. J. Havenga, P. Abbink, D. M. Lynch, B. A. Ewald, J. Liu, A. R. Thorner, P. E. Swanson, D. A. Gorgone, M. A. Lifton, A. A. Lemckert, L. Holterman, B. Chen, A. Dilraj, A. Carville, K. G. Mansfield, J. Goudsmit, and D. H. Barouch. 2006. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441:239-243. [DOI] [PubMed] [Google Scholar]
- 33.Salem, M. L., S. A. El-Naggar, A. Kadima, W. E. Gillanders, and D. J. Cole. 2006. The adjuvant effects of the toll-like receptor 3 ligand polyinosinic-cytidylic acid poly(I:C) on antigen-specific CD8+ T cell responses are partially dependent on NK cells with the induction of a beneficial cytokine milieu. Vaccine 24:5119-5132. [DOI] [PubMed] [Google Scholar]
- 34.Schulz, O., S. S. Diebold, M. Chen, T. I. Naslund, M. A. Nolte, L. Alexopoulou, Y. T. Azuma, R. A. Flavell, P. Liljestrom, and C. Reis e Sousa. 2005. Toll-like receptor 3 promotes cross-priming to virus-infected cells. Nature 433:887-892. [DOI] [PubMed] [Google Scholar]
- 35.Schwarz, K., T. Storni, V. Manolova, A. Didierlaurent, J. C. Sirard, P. Rothlisberger, and M. F. Bachmann. 2003. Role of Toll-like receptors in costimulating cytotoxic T cell responses. Eur. J. Immunol. 33:1465-1470. [DOI] [PubMed] [Google Scholar]
- 36.Sel, S., M. Wegmann, S. Sel, S. Bauer, H. Garn, G. Alber, and H. Renz. 2007. Immunomodulatory effects of viral TLR ligands on experimental asthma depend on the additive effects of IL-12 and IL-10. J. Immunol. 178:7805-7813. [DOI] [PubMed] [Google Scholar]
- 37.Stahl-Hennig, C., M. Eisenblatter, E. Jasny, T. Rzehak, K. Tenner-Racz, C. Trumpfheller, A. M. Salazar, K. Uberla, K. Nieto, J. Kleinschmidt, R. Schulte, L. Gissmann, M. Muller, A. Sacher, P. Racz, R. M. Steinman, M. Uguccioni, and R. Ignatius. 2009. Synthetic double-stranded RNAs are adjuvants for the induction of T helper 1 and humoral immune responses to human papillomavirus in rhesus macaques. PLoS Pathog. 5:e1000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takeuchi, O., and S. Akira. 2009. Innate immunity to virus infection. Immunol. Rev. 227:75-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang, Y., M. Cella, S. Gilfillan, and M. Colonna. 2010. Cutting edge: polyinosinic:polycytidylic acid boosts the generation of memory CD8 T cells through melanoma differentiation-associated protein 5 expressed in stromal cells. J. Immunol. 184:2751-2755. [DOI] [PubMed] [Google Scholar]
- 40.Wille-Reece, U., B. J. Flynn, K. Lore, R. A. Koup, R. M. Kedl, J. J. Mattapallil, W. R. Weiss, M. Roederer, and R. A. Seder. 2005. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. Proc. Natl. Acad. Sci. U. S. A. 102:15190-15194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wille-Reece, U., C. Y. Wu, B. J. Flynn, R. M. Kedl, and R. A. Seder. 2005. Immunization with HIV-1 Gag protein conjugated to a TLR7/8 agonist results in the generation of HIV-1 Gag-specific Th1 and CD8+ T cell responses. J. Immunol. 174:7676-7683. [DOI] [PubMed] [Google Scholar]
- 42.Zhu, Q., C. Egelston, S. Gagnon, Y. Sui, I. M. Belyakov, D. M. Klinman, and J. A. Berzofsky. 2010. Using 3 TLR ligands as a combination adjuvant induces qualitative changes in T cell responses needed for antiviral protection in mice. J. Clin. Invest. 120:607-616. [DOI] [PMC free article] [PubMed] [Google Scholar]