Abstract
Background
Few studies have examined the effects of in utero smoke exposure (IUS) on lung function in children with asthma, and there are no published data on the impact of IUS on treatment outcomes in asthmatic children.
Objectives
To explore whether IUS exposure is associated with increased airway responsiveness among children with asthma, and whether IUS modifies the response to treatment with inhaled corticosteroids (ICS).
Methods
To assess the impact of parent-reported IUS exposure on airway responsiveness in childhood asthma we performed a repeated-measures analysis of methacholine PC20 data from the Childhood Asthma Management Program (CAMP), a four-year, multicenter, randomized double masked placebo controlled trial of 1041 children ages 5–12 comparing the long term efficacy of ICS with mast cell stabilizing agents or placebo.
Results
Although improvement was seen in both groups, asthmatic children with IUS exposure had on average 26% less of an improvement in airway responsiveness over time compared to unexposed children (p=.01). Moreover, while children who were not exposed to IUS who received budesonide experienced substantial improvement in PC20 compared to untreated children (1.25 fold-increase, 95% CI 1.03, 1.50, p=.02) the beneficial effects of budesonide were attenuated among children with a history of IUS exposure (1.04 fold-increase, 95% CI 0.65, 1.68, p=.88).
Conclusions
IUS reduces age-related improvements in airway responsiveness among asthmatic children. Moreover, IUS appears to blunt the beneficial effects of ICS use on airways responsiveness. These results emphasize the importance of preventing this exposure through smoking cessation counseling efforts with pregnant women.
Keywords: asthma, in utero smoke exposure, airway responsiveness, inhaled corticosteroids
Introduction
Exposure to cigarette smoke during fetal development is harmful to children. In utero smoke exposure (IUS) increases risk of obstetric complications (e.g., abruptio placentae, premature birth, and intrauterine growth restriction); and sudden infant death syndrome (SIDS). Prenatal smoke exposure has also been shown to be associated with decreased cognitive development and school performance, and an increased risk of behavioral and psychiatric difficulties1, 2.
There is compelling evidence that IUS results in significant respiratory sequelae as well, which can manifest early in life. Reductions in forced expiratory flows, tidal flow-volume ratios, and respiratory compliance have been observed in healthy newborns of mothers who smoked during pregnancy3–5. Similar reductions in peak expiratory flows6 and small airway flows6, 7 have been observed in school-aged children, suggesting a long-term impact of IUS on pulmonary function. An analysis of pooled data from several international studies demonstrated an increased risk of poor lung function among children exposed to IUS, even after adjustment for current exposure to environmental tobacco smoke (ETS)7. IUS has also been associated with an increased risk of recurrent wheeze8 and incident asthma at school-age 9–11 and in adulthood.12.
In contrast to numerous population-based studies about the effects of IUS on lung function in infants and children, only one study has specifically examined the effects of IUS on the lung function of children already diagnosed with asthma. Data from 5,933 participants in the California Children’s Health Study showed that children and adolescents who were diagnosed with asthma before age 5 years and who had been exposed to IUS had greater deficits in FEV1/FVC, FEF25–75, and FEF25–75/FVC than unexposed asthmatic children13. Importantly, there are no published data regarding the impact of IUS on airway responsiveness or treatment outcomes in asthmatic children.
Here we assess the relationship between IUS and subsequent airway responsiveness in a cohort of children aged 5–12 years with well-established mild to moderate persistent asthma who participated in a randomized, placebo controlled trial that evaluated the long term efficacy and safety of inhaled anti-inflammatory medications. This study design provides a unique opportunity to explore whether in utero tobacco smoke exposure attenuated the beneficial effect of inhaled steroids that was seen overall among study participants14. We demonstrate that IUS has a substantial detrimental impact on responsiveness to inhaled corticosteroid therapy.
Methods
Details of the Childhood Asthma Management Program (CAMP) trial have been described elsewhere15, 16. Briefly, CAMP is a multicenter, randomized, double-masked clinical trial to compare the long-term effectiveness and safety of three inhaled treatments for asthma: budesonide, nedocromil, and placebo. CAMP enrolled 1041 children ages 5–12 years with mild-moderate asthma at eight clinical centers between December 1993 and September 1995. Asthma was defined by the presence of asthma symptoms or the use of an inhaled bronchodilator at least twice per week, or use of a daily asthma medication for the 6 months prior to the screening interview. All participants had increased airway responsiveness to methacholine (a provocation concentration causing a 20% reduction in FEV1 [PC20] ≤ 12.5mg/ml) at study entry. Children were excluded if they had severe asthma or other active sinopulmonary disease. Each parent or guardian signed a consent form, and each participant 7 years of age and older signed an assent form approved by each clinical center’s institutional review board.
Information regarding in utero tobacco smoke exposure was obtained during the baseline medical interview, which was nearly always completed by the mothers of CAMP participants. Children were considered to have IUS exposure if respondents answered affirmatively to the question “Did this child’s mother smoke while she was pregnant with this child?” Information about current environmental tobacco smoke exposure was obtained during follow-up interviews with the question “Do any caretakers of the child currently smoke cigarettes?”
Of the 1,041 participating children, 311 were assigned to receive budesonide (200 µg twice daily[Turbuhaler, AstraZeneca]), 312 were assigned to receive nedocromil sodium (8mg twice daily[Tilade, Rhone-Poulenc Rorer, Collegeville, PA]), and 418 were assigned to receive a matching placebo. Albuterol (two 90 µg actuations from a metered-dose inhaler [Ventolin, Glaxo Wellcome, Research Triangle Park, N.C]) was used as needed for symptoms of asthma and to prevent exercised-induced bronchospasm.
Spirometry, both before and after the administration of a bronchodilator, was performed twice per year. A methacholine challenge was performed at baseline (after a 28-day washout period during which no participants were taking inhaled steroids) and then annually during the treatment phase, using the Wright nebulizer-tidal breathing technique, at least 4 hours after the last use of a short-acting bronchodilator and at least 24 hours after the last use of a long-acting bronchodilator. After a control diluent challenge, nine doubling doses of methacholine were nebulized for 2 minutes each at 5-minute intervals. Spirometry was performed 90 seconds after each challenge until FEV1 had fallen by 20% or more. Methacholine challenge was not performed within 28 days of an upper respiratory tract infection or the use of prednisone for exacerbations of asthma. The child’s height and weight were recorded at every visit.
The distribution of PC20 at each visit was skewed, with a long right tail. PC20 was therefore natural log (ln)-transformed for all regression analyses. Longitudinal regression was performed to test the association between IUS and PC20 before and after adjustment for potential confounders and to distinguish inter-individual changes in PC20 from within-subject change. We developed random-effects models using SAS PROC MIXED (SAS Institute, Cary, NC), related log-transformed PC20 to the aforementioned effects, and accounted for associated time observations within a patient. Components of variability were a random intercept supplemented with a serial correlation. Longitudinal analysis using PROC MIXED was also used to test the effect of treatment group assignment (budesonide vs. nedocromil or placebo) on PC20 after stratification by IUS status.
Results
All children with asthma in CAMP (n=1041) had baseline methacholine challenge tests. Methacholine challenge testing was completed for 92, 90, 85, and 82% of the cohort at 8, 20, 32, and 44 months after randomization, respectively. Baseline characteristics were generally similar between children with and without methacholine challenge data at 44 months. Children who were missing PC20 measurements were more likely to have been recruited from three of the clinical sites (Albuquerque, Boston, and Seattle), to have come from families with lower household income, and to have mothers with a history of either asthma or smoking during pregnancy. Baseline median loge PC20 was similar between groups (Wilcoxon rank sum p values >.30)17.
Of the 1,041 participants, 5 subjects were missing IUS data and were therefore excluded from analyses. 150 (14%) had reported exposure to IUS (Table 1). 109 children (73% of those exposed) had IUS exposure during all three trimesters. Exposed children were more likely to have mothers without any college education (p<.001) and subsequent exposure to environmental tobacco smoke than children not exposed to IUS (p<.001). The characteristics of the two groups at baseline, including spirometry and PC20, were otherwise similar.
Table I.
Characteristics of the study populationa
| Not exposed to in utero smoke (n=886) N (%) |
Exposed to in utero smoke (n=150) N (%) |
P Valueb | |
|---|---|---|---|
| Sex (female) | 356 (40.2) | 61 (40.7) | .91 |
| “Current” ETS exposure | |||
| 8 months post-randomization | 177 (20.6) | 101 (69.7) | <.0001 |
| 20 months post-randomization | 170 (20.1) | 106 (73.6) | <.0001 |
| 32 months post-randomization | 160 (19.1) | 95 (68.8) | <.0001 |
| 44 months post-randomization | 152 (18.2) | 93 (69.9) | <.0001 |
| Maternal history of asthma | 232 (26.8) | 30 (20.8) | .13 |
| Paternal history of asthma | 182 (21.6) | 26 (20.0) | .67 |
| Assigned to treatment group | .26 | ||
| Budesonide | 271 (30.6) | 39/150 (26) | |
| Nedocrimil/Placebo | 615 (69.4) | 111/150 (74) | |
| Clinic Site | .41 | ||
| Albequerque | 103 (11.6) | 17 (11.3) | |
| Baltimore | 106 (12.0) | 22 (14.7) | |
| Boston | 105 (11.9) | 19 (12.7) | |
| Denver | 118 (13.3) | 26 (17.3) | |
| San Diego | 111 (12.5) | 11 (7.3) | |
| Seattle | 119 (13.4) | 21 (14.0) | |
| St. Louis | 119 (13.4) | 14 (9.3) | |
| Toronto | 105 (11.9) | 20 (13.3) | |
| Income <$30,000 | 186 (21.9) | 55 (37.9) | <.0001 |
| Maternal Education: ≥ some college | 756 (85.4) | 95 (63.3) | <.0001 |
| Median (IQR) Values at Baseline | |||
| No IUS | IUS | P Valuec | |
| Age at study entrance (years) | 8.9 (3.4) | 8.7 (3.4) | .39 |
| FEV1 (% predicted) | 94 (18) | 95 (16) | .52 |
| FEV1/FVC | 81 (11) | 81 (9) | .14 |
| Serum total IgE (IU) | 460 (1057) | 368 (921) | .05 |
| PC20 (mg/ml) | 1.1 (2.3) | 1.1(1.8) | .89 |
| Height (cm) | 132.8 (20.7) | 131.2 (21.3) | .13 |
ETS=environmental tobacco smoke; FEV1=forced expiratory volume in 1st second; FVC=forced vital capacity; PC20= provocation concentration of methacholine causing a 20% reduction in FEV1;
Numbers and percentages may vary because of missing values for some variables.
Chi square p value
Kruskal Wallis p value
A repeated-measures analysis of methacholine PC20 data collected over the 44 months of the CAMP clinical trial found, as previously reported,17 that parental history of asthma, total serum IgE, and lung function were associated with increased airway responsiveness, both at baseline (prior to treatment randomization) and over the 44 months of observation. While current ETS exposure was associated with increased airway responsiveness at baseline, this relationship did not remain significant over time. Baseline PC20 (prior to the initiation of treatment) did not differ between children who were and were not exposed to IUS (p=.89), and there was improvement in the median PC20 in all treatment groups over time, regardless of IUS exposure. However, children in all treatment groups exposed to IUS demonstrated significantly less improvement in airways hyperresponsiveness than children who were not exposed, demonstrating a mean change in PC20 that was 26% lower than that of non-exposed children (95% CI 6% – 42%; p=0.01). Similar results were obtained after adjustment for environmental tobacco exposure, height, and lung function (both at baseline and over the course of the trial), suggesting an independent effect of IUS on airway responsiveness (Table 2). Although there were significant differences in household income and maternal education level between those exposed and unexposed to IUS, inclusion of these terms in multivariable models did not change the results; these terms were not included in final models.
Table II.
Effects of IUS on post-randomization repeated measures of methacholine PC20a
| Fold-change in PC20 (95%CI)b | P value | |
|---|---|---|
| IUS (yes) | 0.74 (0.58, 0.94) | .01 |
| Age (per year increase) | 0.91 (0.86, 0.95) | .04 |
| Female gender (yes) | 0.94 (0.79, 1.10) | .40 |
| Maternal history of asthma (yes) | 0.83 (0.69, 0.99) | .04 |
| Paternal history of asthma (yes) | 0.80 (0.66, 0.97) | .02 |
| Environmental tobacco smoke exposure (yes)a | 0.99 (0.87, 1.13) | .89 |
| Treatment with budesonide (yes) | 1.26 (1.06, 1.50) | .01 |
| FEV1 (per Liter increase)a | 2.90 (2.44, 3.46) | <.001 |
| Height (per cm increase)a | 0.98 (0.97, 0.99) | <.001 |
| Total serum IgE at baseline (per log10 IU increase) |
0.45 (0.40, 0.51) | <.001 |
Measured at each study visit
Fold-change in methacholine PC20 as determined from exponential of β-estimate derived from multivariate linear regression, adjusting for other covariates in table.
Multivariable mixed models assumed an unstructured, autoregressive covariance and were also adjusted for study center.
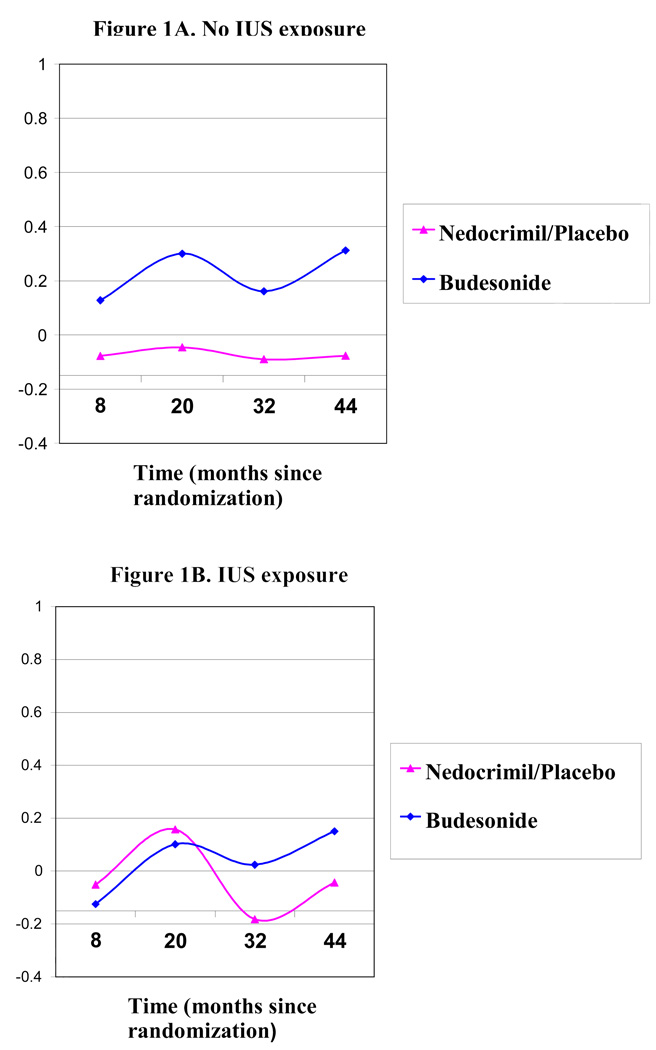
As previously reported,14,17 children randomized to budesonide (an inhaled corticosteroid) demonstrated a substantial improvement in airway hyperresponsiveness over the course of the CAMP clinical trial compared to children randomized to either inhaled nedocromil or placebo. The association between IUS and greater airway hyperresponsiveness following, but not prior to (at baseline), treatment randomization suggested that IUS may alter responsiveness to inhaled corticosteroids – the primary recommended treatment for persistent asthma18. To explore this possibility, we assessed the impact of inhaled corticosteroids on airway responsiveness in an analysis stratified by IUS status. Whereas children who had no history of IUS derived a substantial benefit from budesonide treatment on airway responsiveness over time compared to nedocrimil or placebo (25% increase [i.e. improvement] in PC20 from baseline, 95% CI 350%, p = .02, Table 3, Figure 1a), children exposed to IUS had a severely blunted response to inhaled budesonide, with no discernible difference in improvement in PC20 as compared to nedocromil or placebo (p = .88, Table 3, Figure 1b). This effect persisted after adjustment for height, current lung function and current environmental tobacco smoke exposure. We attempted to test the statistical significance of this differential effect of IUS on treatment response by repeating the original longitudinal analysis with the inclusion of an interaction term for treatment arm by IUS over time. The three-way interaction term of IUS*treatment group*time was significant (p-value test for interaction 0.02), though upon inclusion of second order terms (IUS*treatment, IUS*time, and treatment*time), this interaction term was no longer significant (p=.78), most likely due to the small number of subjects exposed to IUS that were randomized to the budesonide treatment arm (n=39).
Table III.
The impact of IUS exposure on responsiveness of methacholine PC20 to treatment with inhaled budesonidee.
| IUS =No (n=886) | IUS=Yes (n=150) | |||
|---|---|---|---|---|
| Fold-change in PC20 (95% CI)c |
P value | Fold-change in PC20 (95% CI)c |
P value | |
| Treatment with budesonide (yes) | 1.25 (1.03, 1.50) | .02 | 1.04 (0.65, 1.68) | .88 |
| FEV1 (per Liter increase)a | 2.97 (2.46, 3.59) | <.001 | 3.16 (1.98, 5.02) | <.001 |
| Height (per cm increase)a | 0.97 (0.97, 0.98) | <.001 | 0.96 (0.94, 0.98) | <.001 |
| Maternal history of asthma (yes) | 0.82 (0.68, 1.00) | .05 | 0.82 (0.48, 1.4) | .64 |
| Paternal history of asthma (yes) | 0.72 (0.59, 0.89) | .003 | 1.72 (1.02, 2.92) | .07 |
| Total serum IgE at baseline (per log10 IU increase) |
0.43 (.030, 0.61) | <.001 | 0.44 (0.32, 0.61) | <.001 |
| Clinic of origin | <.001 b | .05b | ||
| Current ETS exposure (yes) | 0.96 (0.83, 1.11) | .65 | 0.96 (0.70, 1.32) | .81 |
Assessed at each study visit
F test p value
Fold-change in methacholine PC20 as determined from exponential of β-estimate derived from multivariate linear regression, adjusting for other covariates in table.
Multivariable mixed models assumed an unstructured, autoregressive covariance.
P value for 3-way interaction IUS*Treatment with Budesonide*Time=.78. Model also includes interaction terms for IUS* Treatment with Budesonide, IUS* Time, Treatment with Budesonide*Time.
Figure 1.
Effects on longitudinal methacholine PC20 attributable to treatment with either budesonide or nedocrimil/placebo post-randomization.
Discussion
Inhaled corticosteroids are both commonly prescribed and efficacious for the treatment of asthma in children and adults. They have been shown to reduce airway responsiveness, asthma symptoms, need for breakthrough bronchodilator therapy, and exacerbation rates14, 19. However, there is considerable between-subject variability in clinical response to inhaled corticosteroids, including patients who manifest partial to substantial steroid resistance necessitating escalation of treatment dose20. Though there is evidence that a proportion of this variability has a genetic basis21, other factors may be at play.
We here demonstrate for the first time that in utero tobacco smoke exposure may be an important determinant of responsiveness to inhaled corticosteroids. In our study, a history of IUS conferred near complete resistance to inhaled corticosteroid treatment, whereas children without such exposure demonstrated marked improvements. We also found a differential effect for paternal history of asthma between IUS exposed and unexposed groups (p=.005). The importance of a paternal history of asthma on airway responsiveness has been reported in a prior manuscript17. Our current findings may be indicative of additional gene by environment effects, however we are unable to examine this further in multivariable models because of sample size limitations.
Though the precise mechanisms underlying our findings are not yet known, the observation that airway responsiveness was similar between exposure groups prior to treatment but different thereafter suggests that the initiation of therapy with inhaled corticosteroids can differentiate clinical subgroups of asthmatic children with underlying differences in lung structure that result from IUS. Studies of animal and human lung morphology support the notion that IUS causes structural changes in the lung. Sekhon and colleagues have demonstrated that nicotine crosses the placenta and induces increased connective tissue expression within pulmonary vessels,22 resulting in notable impairment of lung function in newborn rhesus monkeys.23 This group also found decreased alveolar airspace complexity and increased expression of nicotinic cholinergic receptors in airway epithelial cells, smooth muscle cells, and blood vessels in rhesus monkeys exposed to IUS. Nicotinic receptor expression was strongly correlated with increased collagen deposition in cartilaginous airways of these monkeys24. Prenatal nicotine exposure stimulates branching morphogenesis in murine lung explants, potentially contributing to disynaptic lung growth25. Postmortem studies in infants provide further evidence of the adverse effects of IUS on airway morphology. In a study of airway morphology in 38 infants who died from SIDS (half of whom were exposed to IUS), IUS was associated with increased inner airway wall thickness26. Similar studies have demonstrated an association between IUS and increased alveolar attachments distance27, a finding previously associated with reduced elastic recoil28.
In addition to affecting lung structure, the effects of in utero tobacco smoke exposure on airway responsiveness may be due to changes in airway smooth muscle physiology. Singh and colleagues29 demonstrated airway hyperresponsiveness in mice exposed to IUS that was not due to differences in airway inflammation. Lavage fluid from these mice contained decreased levels of cyclic adenosine monophosphate (cAMP) – a potent airway smooth muscle relaxant - compared to IUS-unexposed mice. IUS-induced reductions in cAMP could also influence response to inhaled corticosteroids in other ways, as cAMP is an inhibitor of T-cell chemotaxis and may inhibit airway remodeling. It is also possible that the impact of cigarette smoke exposure on subsequent glucocorticoid responsiveness is indirectly mediated through increased susceptibility to lower respiratory tract infections in early life, which in turn contribute to airway remodeling, as proposed by Piedimonte and others30, 31. Testing of this hypothesis in prospective birth cohorts with accurate assessments of early-life respiratory infections would help clarify this possibility.
The CAMP study was primarily designed to assess the primary effects of inhaled anti-inflammatory medication on lung function in asthmatic children, not to specifically evaluate the effects of in utero exposures on treatment response. Thus, it is important to consider whether our findings could be explained by design-related biases. Three major issues of concern are statistical power, exposure misclassification, and residual confounding by both current environmental tobacco smoke exposure and differences in adherence between IUS exposed and unexposed children. Of the 150 children with reported IUS, only 39 were randomized to budesonide. Therefore, we first assessed whether our inability to detect improvement in airway responsiveness among children exposed to IUS was due to low statistical power. This is unlikely because although low power may limit our ability to detect a statistical difference between treatment arms, the observed mean difference in response in the exposed group (as reflected in the fold changes in PC20 in Table 3) was a fraction of that observed in the unexposed group. Furthermore, there was a protocol in place for open-label rescue use of inhaled beclomethosone. By the end of the clinical trial, both the nedocromil and placebo groups had significantly more rescue use of beclomethasone than the budesonide groups14. This disproportionate use of supplemental ICS by the non-budesonide groups would be expected to reduce the likelihood that we would detect a difference between treatment arms. With regard to exposure misclassification, we recognize that IUS designation was based on self-report. Though several studies support the higher accuracy of self-reported smoking during pregnancy compared to other detection methods (i.e. serum cotinine32, urine cotinine33, 34, and exhaled carbon monoxide35), others suggest that self-report underestimates the true prevalence of smoking during pregnancy36–38. It is therefore possible that exposure misclassification was underestimated, particularly given that questionnaires were administered 5–12 years post-partum. If so, we may have underestimated the true effect of IUS on airway responsiveness.
Could our results be due to residual confounding by environmental tobacco smoke exposure rather than primary in utero effects? Because the majority of subjects with a history of IUS also report current exposure to cigarette smoke, it is not possible to fully exclude this possibility. However, in our study, models that do not adjust for IUS fail to demonstrate a significant association of current exposure with PC20 (p=.89 in multivariable models adjusted for IUS and .38 in multivariable models not adjusted for IUS). While this is somewhat reassuring, we recognize that confirmation of our findings in other populations with sufficient numbers of subjects with in utero, but not current, smoke exposure is required. We also note that we were unable to explore a possible dose-response relationship because for most IUS exposed subjects, the exposure was present for all three trimesters. An additional outstanding question is whether children exposed to IUS were also more likely to be non-compliant with daily inhaled corticosteroids. Unfortuntely, we do not have sufficient data available to us to compare adherence between the IUS exposed and unexposed groups. However, we note that we did not see significant differences between those subjects exposed to ETS and those without such exposure with regards to treatment effect on airway hyperresponsiveness. One might expect that adherence would similarly be a confounder among that subgroup as well.
In summary, this study demonstrates for the first time that IUS has the potential to attenuate the beneficial effect of inhaled corticosteroids on airways responsiveness in children with asthma. These results provide further reasons for physicians to emphasize the importance of preventing this exposure through smoking cessation counseling efforts with pregnant women. Further study is needed to define the mechanism of action of this exposure as well as to elucidate the effect of IUS on other measures of airway responsiveness including response to bronchodilators.
Key Messages
In utero smoke exposure (IUS) has been associated with increased prevalence of asthma and reduced lung function in healthy children. There is little data about the impact of IUS on lung function of children diagnosed with asthma
IUS is associated with less of an improvement in airway responsiveness over time among children with asthma
IUS may attenuate the beneficial effect of inhaled corticosteroids among children with asthma
Acknowledgements
The Childhood Asthma Management Program is supported by contracts NO1-HR-16044, 16045, 16046, 16047, 16048, 16049, 16050, 16051, and 16052 with the National Heart, Lung, and Blood Institute and General Clinical Research Center grants M01RR00051, M01RR0099718-24, M01RR02719-14, and RR00036 from the National Center for Research Resources. B.A.R. is a recipient of a Mentored Clinical Scientist Development Award from NHLBI/NIH (K08 HL074193).
Abbreviations
- IUS
in utero smoke
- SIDS
sudden infant death syndrome
- FEV1
forced expiratory volume in 1 second
- FVC
forced vital capacity
- FEF 25–75
forced expiratory flow between the 25%–75% of the FVC maneuver
- CAMP
Childhood Asthma Management Program
- PC20
provocation concentration of methacholine causing a 20% reduction in FEV1
- cAMP
cyclic adenosine monophosphate
Members of the CAMP Research Group
Clinical centers
ASTHMA, Inc, Seattle, WA: Gail G. Shapiro, MD (Director); Thomas R. DuHamel, PhD (Co-Director); Mary V. Lasley, MD (Co-Director); Tamara Chinn, MSN, ARNP (Coordinator). Michele Hinatsu, MSN, ARNP; Clifton T. Furukawa, MD; Leonard C. Altman, MD; Frank S. Virant, MD; Paul V. Williams, MD; Michael S. Kennedy, MD; Jonathan W. Becker, MD; Grace White. C. Warren Bierman, MD (1992–1997); Dan Crawford, RN (1996–2002); Heather Eliassen, BA (1996–1999); Babi Hammond (1996–1999); Dominick A. Minotti, MD (1992–2003); Chris Reagan (1992–2003); Marian Sharpe, RN (1992–1994); Timothy G. Wighton, PhD (1994–1998).
Brigham & Women's Hospital, Boston, MA: Scott Weiss, MD, MS (Director); Anne Fuhlbrigge, MD (Principal Investigator); Anne Plunkett, NP, MS (Coordinator). Nancy Madden, RN, BSN; Peter Barrant, MD; Christine Darcy; Kelly Thompson, MD. Walter Torda, MD (Co-Investigator Director, 1993–2003); Martha Tata, RN (1993–2002); Sally Babigian, RN (1997–1999); Linda Benson (1998–2004); Jose Caicedo (1998–1999); Tatum Calder (1998–2001); Anthony DeFilippo (1994–2000); Cindy Dorsainvil (1998–2001); Julie Erickson (1998–1999); Phoebe Fulton (1997); Mary Grace, RN (1994–1996); Jennifer Gilbert (1997–1998); Dirk Greineder, MD (1993–2000); Stephanie Haynes (1993–1998); Margaret Higham, MD (1996–1998); Deborah Jakubowski (1999); Susan Kelleher (1993–1997); Jay Koslof, PhD (1993–1995); Dana Mandel (1996–1998); Patricia Martin (2001–2003); Agnes Martinez (1994–1997); Jean McAuliffe (1994–1995); Erika Nakamoto (2002–2004); Paola Pacella (1993–1998); Paula Parks (1993–1995); Johanna Sagarin (1998–1999); Kay Seligsohn, PhD (1995–2004); Susan Swords (2003–2005); Meghan Syring (1998–2001); June Traylor, MSN, RN (1996–1998); Melissa Van Horn, PhD (1996–1999); Carolyn Wells, RN (1993–1995); Ann Whitman, RN (1994–1996).
The Hospital for Sick Children, Toronto, Ontario, Canada: Ian MacLusky, MD, FRCP(C) (Director); Joe Reisman, MD, FRCP(C), MBA (Director, 1996–1999); Henry Levison, MD, FRCP(C) (Director, 1992–1996); Anita Hall, RN (Coordinator). Jennifer Chay; Melody Miki, RN, BScN; Renée Sananes, PhD. Yola Benedet (1994–1999); Susan Carpenter, RN (1998–2001); Michelle Collinson, RN (1994–1998); Jane Finlayson-Kulchin, RN (1994–1998); Kenneth Gore, MA (1993–1999); Noreen Holmes, RRT (1998–1999); Sharon Klassen, MA(1999–2000); Joseé Quenneville, MSc (1993–1995); Christine Wasson, PhD (1999).
Johns Hopkins Asthma & Allergy Center, Baltimore, MD: N. Franklin Adkinson, Jr, MD (Director); Peyton Eggleston, MD (Co-Director); Elizabeth H. Aylward, PhD; Karen Huss, DNSc (Co-Investigator); Leslie Plotnick, MD (Co-Investigator); Margaret Pulsifer, PhD (Co-Investigator); Cynthia Rand, PhD (Co-Investigator); Nancy Bollers, RN (Coordinator). Deborah Bull, LPN; Robert Hamilton, PhD; Kimberly Hyatt; Susan Limb, MD; Mildred Pessaro; Stephanie Philips, RN; Barbara Wheeler, RN, BSN.
National Jewish Medical and Research Center, Denver, CO: Stanley Szefler, MD (Director); Harold S. Nelson, MD (Co-Director); Bruce Bender, PhD (Co-Investigator); Ronina Covar, MD (Co-Investigator); Andrew Liu, MD (Co-Investigator); Joseph Spahn, MD (Co-Investigator); D Sundström (Coordinator). Melanie Phillips; Michael P. White. Kristin Brelsford (1997–1999); Jessyca Bridges (1995–1997); Jody Ciacco (1993–1996); Michael Eltz (1994–1995); Jeryl Feeley, MA (Coordinator, 1992–1995); Michael Flynn (1995–1996); Melanie Gleason, PA-C (1992–1999); Tara Junk-Blanchard (1997–2000); Joseph Hassell (1992–1998); Marcia Hefner (1992–1994); Caroline Hendrickson, RN (1995–1998; Coordinator, 1995–1997); Daniel Hettleman, MA (1995–1996); Charles G. Irvin, PhD (1992–1998); Jeffrey Jacobs, MD (1996–1997); Alan Kamada, PharmD (1994–1997); Sai Nimmagadda, MD (1993–1996); Kendra Sandoval (1995–1997); Jessica Sheridan (1994–1995); Trella Washington (1993–1997); Eric Willcutt, MA (1996–1997). We also thank the pediatric allergy and immunology fellows for their participation (Kirstin Carel, MD; Neal Jain, MD; Harvey Leo, MD; Beth Macomber, MD; Chris Mjaanes, MD; Lora Stewart, MD; Ben Song, MD).
University of California, San Diego and Kaiser Permanente Southern California Region, San Diego, CA: Robert S. Zeiger, MD, PhD (Director); Noah Friedman, MD (Co-Investigator); Michael H. Mellon, MD (Co-Investigator); Michael Schatz, MD (Co-Investigator); Kathleen Harden, RN (Coordinator). Elaine M. Jenson; Serena Panzlau; Eva Rodriguez, RRT. James G. Easton, MD (Co-Director, 1993–1994); M. Feinberg (1997–1998); Linda L. Galbreath (1991–2002); Jennifer Gulczynski (1998–1999); Ellen Hansen (1995–1997); Al Jalowayski, PhD (Co-Investigator, 1991–2005); Alan Lincoln, PhD (Co-Investigator, 1991–2003); Jennie Kaufman (1994); Shirley King, MSW (1992–1999); Brian Lopez (1997–1998); Michaela Magiari-Ene, MA (1994–1998); Kathleen Mostafa, RN (1994–1995); Avraham Moscona (1994–1996); Catherine A. Nelle, RN (1991–2005); Jennifer Powers (2001–2003); Karen Sandoval (1995–1996); Nevin W. Wilson, MD (Co-Director, 1991–1993).
University of New Mexico, Albuquerque, NM: H. William Kelly, PharmD (Director); Aaron Jacobs (Co-Investigator); Mary Spicher, RN (Coordinator). Hengameh H. Raissy. Robert Annett, PhD (Co-Investigator, 1993–2004); Teresa Archibeque (1994–1999); Naim Bashir, MD (Co-Investigator, 1998–2005); H. Selda Bereket (1995–1998); Marisa Braun (1996–1999); Shannon Bush (2002–2006); Michael Clayton, MD (Co-Investigator, 1999–2001); Angel Colon-Semidey, MD (Co-Investigator, 1997–2000); Sara Devault (1993–1997); Roni Grad, MD (Co-Investigator, 1993–1995); David Hunt, RRT (1995–2004); Jeanne Larsson, RN (1995–1996); Sandra McClelland, RN (Coordinator, 1993–1995); Bennie McWilliams, MD (Co-Investigator, Director, 1992–1998); Elisha Montoya (1997–2000); Margaret Moreshead (1996–1999); Shirley Murphy, MD (Co-Investigator, 1992–1994); Barbara Ortega, RRT (1993–1999); David Weers (1997–1998); Jose Zayas (1995–1996).
Washington University, St. Louis, MO: Robert C. Strunk, MD (Director); Leonard Bacharier, MD (Co-Investigator); Gordon R. Bloomberg, MD (Co-Investigator); James M. Corry, MD (Co-Investigator); Denise Rodgers, RFPT (Coordinator). Lila Kertz, MSN, RN, CPNP; Valerie Morgan, RRT; Tina Oliver-Welker, CRTT; Deborah K. White, RPFT, RRT.
Resource centers
Chair's Office, National Jewish Medical and Research Center, Denver, CO: Reuben Cherniack, MD (Study Chair).
Coordinating Center, The Johns Hopkins University, Baltimore, MD: James Tonascia, PhD (Director); Curtis Meinert, PhD (Co-Director). Patricia Belt; Karen Collins; Betty Collison; Ryan Colvin, MPH; John Dodge; Michele Donithan, MHS; Judith Harle; Rosetta Jackson; Hope Livingston; Jill Meinert; Kapreena Owens; Michael Smith; Alice Sternberg, ScM; Mark Van Natta, MHS; Margaret Wild; Laura Wilson, ScM; Robert Wise, MD; Katherine Yates, ScM.
Project Office, National Heart, Lung, and Blood Institute, Bethesda, MD: Virginia Taggart, MPH (Project Officer); Lois Eggers; James Kiley, PhD; Gang Zheng, PhD. Paul Albert, PhD (1991–1999); Suzanne Hurd, PhD (1991–1999); Sydney Parker, PhD (1991–1994); Pamela Randall (1992–2003); Margaret Wu, PhD (1991–2001).
Committees
Data and Safety Monitoring Board: Howard Eigen, MD (Chair); Michelle Cloutier, MD; John Connett, PhD; Leona Cuttler, MD; David Evans, PhD; Meyer Kattan, MD; Rogelio Menendez, MD; F. Estelle R. Simons, MD. Clarence E. Davis, PhD (1993–2003); Sanford Leikin, MD (1993–1999).
Executive Committee: Reuben Cherniack, MD (Chair);Robert Strunk, MD; Stanley Szefler, MD; Virginia Taggart, MPH; James Tonascia, PhD. Curtis Meinert, PhD (1992–2003).
Steering Committee: Reuben Cherniack, MD (Chair); Robert Strunk, MD (Vice-Chair); N. Franklin Adkinson, MD; Robert Annett, PhD (1992–1995, 1997–1999); Bruce Bender, PhD (1992–1994, 1997–1999); Mary Caesar, MHS (1994–1996); Thomas R. DuHamel, PhD (1992–1994, 1996–1999); H. William Kelly, PharmD; Henry Levison, MD (1992–1996); Alan Lincoln, PhD (1994–1995); Ian MacLusky, MD; Bennie McWilliams, MD (1992–1998); Curtis L. Meinert, PhD; Sydney Parker, PhD (1991–1994); Joe Reisman, MD, FRCP(C), MBA (1991–1999); Denise Rodgers (2003–2005); Kay Seligsohn, PhD (1996–1997); Gail G. Shapiro, MD; Marian Sharpe (1993–1994); D Sundström (1998–1999); Stanley Szefler, MD; Virginia Taggart, MPH; Martha Tata, RN (1996–1998); James Tonascia, PhD; Scott Weiss, MD, MS; Barbara Wheeler, RN, BSN (1993–1994); Robert Wise, MD; Robert Zeiger, MD, PhD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113:1007–1015. [PubMed] [Google Scholar]
- 2.Hofhuis W, de Jongste JC, Merkus PJ. Adverse health effects of prenatal and postnatal tobacco smoke exposure on children. Arch Dis Child. 2003;88:1086–1090. doi: 10.1136/adc.88.12.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanrahan JP, Tager IB, Segal MR, Tosteson TD, Castile RG, Van Vunakis H, et al. The effect of maternal smoking during pregnancy on early infant lung function. Am Rev Respir Dis. 1992;145:1129–1135. doi: 10.1164/ajrccm/145.5.1129. [DOI] [PubMed] [Google Scholar]
- 4.Stick SM, Burton PR, Gurrin L, Sly PD, LeSouef PN. Effects of maternal smoking during pregnancy and a family history of asthma on respiratory function in newborn infants. Lancet. 1996;348:1060–1064. doi: 10.1016/s0140-6736(96)04446-7. [DOI] [PubMed] [Google Scholar]
- 5.Lodrup Carlsen KC, Jaakkola JJ, Nafstad P, Carlsen KH. In utero exposure to cigarette smoking influences lung function at birth. Eur Respir J. 1997;10:1774–1779. doi: 10.1183/09031936.97.10081774. [DOI] [PubMed] [Google Scholar]
- 6.Gilliland FD, Berhane K, McConnell R, Gauderman WJ, Vora H, Rappaport EB, et al. Maternal smoking during pregnancy, environmental tobacco smoke exposure and childhood lung function. Thorax. 2000;55:271–276. doi: 10.1136/thorax.55.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moshammer H, Hoek G, Luttmann-Gibson H, Neuberger MA, Antova T, Gehring U, et al. Parental smoking and lung function in children: an international study. Am J Respir Crit Care Med. 2006;173:1255–1263. doi: 10.1164/rccm.200510-1552OC. [DOI] [PubMed] [Google Scholar]
- 8.Lannero E, Wickman M, Pershagen G, Nordvall L. Maternal smoking during pregnancy increases the risk of recurrent wheezing during the first years of life (BAMSE) Respir Res. 2006;7:3. doi: 10.1186/1465-9921-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilliland FD, Li YF, Peters JM. Effects of maternal smoking during pregnancy and environmental tobacco smoke on asthma and wheezing in children. Am J Respir Crit Care Med. 2001;163:429–436. doi: 10.1164/ajrccm.163.2.2006009. [DOI] [PubMed] [Google Scholar]
- 10.Pattenden S, Antova T, Neuberger M, Nikiforov B, De Sario M, Grize L, et al. Parental smoking and children's respiratory health: independent effects of prenatal and postnatal exposure. Tob Control. 2006;15:294–301. doi: 10.1136/tc.2005.015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaakkola JJ, Kosheleva AA, Katsnelson BA, Kuzmin SV, Privalova LI, Spengler JD. Prenatal and postnatal tobacco smoke exposure and respiratory health in Russian children. Respir Res. 2006;7:48. doi: 10.1186/1465-9921-7-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skorge TD, Eagan TM, Eide GE, Gulsvik A, Bakke PS. The adult incidence of asthma and respiratory symptoms by passive smoking in uterus or in childhood. Am J Respir Crit Care Med. 2005;172:61–66. doi: 10.1164/rccm.200409-1158OC. [DOI] [PubMed] [Google Scholar]
- 13.Gilliland FD, Berhane K, Li YF, Rappaport EB, Peters JM. Effects of early onset asthma and in utero exposure to maternal smoking on childhood lung function. Am J Respir Crit Care Med. 2003;167:917–924. doi: 10.1164/rccm.200206-616OC. [DOI] [PubMed] [Google Scholar]
- 14.The Childhood Asthma Management Program Research Group. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343:1054–1063. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 15.Childhood Asthma Management Program Research Group. The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Control Clin Trials. 1999;20:91–120. [PubMed] [Google Scholar]
- 16.Weiss ST, Van Natta ML, Zeiger RS. Relationship between increased airway responsiveness and asthma severity in the childhood asthma management program. Am Respir Crit Care Med. 2000;162:50–56. doi: 10.1164/ajrccm.162.1.9811005. [DOI] [PubMed] [Google Scholar]
- 17.Raby BA, Van Steen K, Celedon JC, Litonjua AA, Lange C, Weiss ST. Paternal history of asthma and airway responsiveness in children with asthma. Am J Respir Crit Care Med. 2005;172:552–558. doi: 10.1164/rccm.200501-010OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin Immunol. 2007;120:S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 19.Zeiger RS, Szefler SJ, Phillips BR, Schatz M, Martinez FD, Chinchilli VM, et al. Response profiles to fluticasone and montelukast in mild-to-moderate persistent childhood asthma. J Allergy Clin Immunol. 2006;117:45–52. doi: 10.1016/j.jaci.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 20.Szefler SJ, Martin RJ, King TS, Boushey HA, Cherniack RM, Chinchilli VM, et al. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109:410–418. doi: 10.1067/mai.2002.122635. [DOI] [PubMed] [Google Scholar]
- 21.Tantisira KG, Lake S, Silverman ES, Palmer LJ, Lazarus R, Silverman EK, et al. Corticosteroid pharmacogenetics: association of sequence variants in CRHR1 with improved lung function in asthmatics treated with inhaled corticosteroids. Hum Mol Genet. 2004;13:1353–1359. doi: 10.1093/hmg/ddh149. [DOI] [PubMed] [Google Scholar]
- 22.Sekhon HS, Proskocil BJ, Clark JA, Spindel ER. Prenatal nicotine exposure increases connective tissue expression in foetal monkey pulmonary vessels. Eur Respir J. 2004;23:906–915. doi: 10.1183/09031936.04.00069604. [DOI] [PubMed] [Google Scholar]
- 23.Sekhon HS, Keller JA, Benowitz NL, Spindel ER. Prenatal nicotine exposure alters pulmonary function in newborn rhesus monkeys. Am J Respir Crit Care Med. 2001;164:989–994. doi: 10.1164/ajrccm.164.6.2011097. [DOI] [PubMed] [Google Scholar]
- 24.Sekhon HS, Jia Y, Raab R, Kuryatov A, Pankow JF, Whitsett JA, et al. Prenatal nicotine increases pulmonary alpha7 nicotinic receptor expression and alters fetal lung development in monkeys. J Clin Invest. 1999;103:637–647. doi: 10.1172/JCI5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wongtrakool C, Roser-Page S, Rivera HN, Roman J. Nicotine alters lung branching morphogenesis through the alpha7 nicotinic acetylcholine receptor. Am J Physiol Lung Cell Mol Physiol. 2007;293:L611–L618. doi: 10.1152/ajplung.00038.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elliot J, Vullermin P, Robinson P. Maternal cigarette smoking is associated with increased inner airway wall thickness in children who die from sudden infant death syndrome. Am J Respir Crit Care Med. 1998;158:802–806. doi: 10.1164/ajrccm.158.3.9709055. [DOI] [PubMed] [Google Scholar]
- 27.Elliot JG, Carroll NG, James AL, Robinson PJ. Airway alveolar attachment points and exposure to cigarette smoke in utero. Am J Respir Crit Care Med. 2003;167:45–49. doi: 10.1164/rccm.2110005. [DOI] [PubMed] [Google Scholar]
- 28.Saetta M, Ghezzo H, Kim WD, King M, Angus GE, Wang NS, et al. A morphometric correlate of lung function impairment. Loss of alveolar attachments in smokers. Am Rev Respir Dis. 1985;132:894–900. doi: 10.1164/arrd.1985.132.4.894. [DOI] [PubMed] [Google Scholar]
- 29.Singh SP, Barrett EG, Kalra R, Razani-Boroujerdi S, Langley RJ, Kurup V, et al. Prenatal cigarette smoke decreases lung cAMP and increases airway hyperresponsiveness. Am J Respir Crit Care Med. 2003;168:342–347. doi: 10.1164/rccm.200211-1262OC. [DOI] [PubMed] [Google Scholar]
- 30.Piedimonte G. Pathophysiological mechanisms for the respiratory syncytial virus-reactive airway disease link. Respir Res. 2002;3 Suppl 1:S21–S25. doi: 10.1186/rr185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gern JE, Rosenthal LA, Sorkness RL, Lemanske RF., Jr Effects of viral respiratory infections on lung development and childhood asthma. J Allergy Clin Immunol. 2005;115:668–674. doi: 10.1016/j.jaci.2005.01.058. quiz 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wagenknecht LE, Burke GL, Perkins LL, Haley NJ, Friedman GD. Misclassification of smoking status in the CARDIA study: a comparison of self-report with serum cotinine levels. Am J Public Health. 1992;82:33–36. doi: 10.2105/ajph.82.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pickett KE, Rathouz PJ, Kasza K, Wakschlag LS, Wright R. Self-reported smoking, cotinine levels, and patterns of smoking in pregnancy. Paediatr Perinat Epidemiol. 2005;19:368–376. doi: 10.1111/j.1365-3016.2005.00660.x. [DOI] [PubMed] [Google Scholar]
- 34.Klebanoff MA, Levine RJ, Morris CD, Hauth JC, Sibai BM, Ben Curet L, et al. Accuracy of self-reported cigarette smoking among pregnant women in the 1990s. Paediatr Perinat Epidemiol. 2001;15:140–143. doi: 10.1046/j.1365-3016.2001.00321.x. [DOI] [PubMed] [Google Scholar]
- 35.Christensen AE, Tobiassen M, Jensen TK, Wielandt H, Bakketeig L, Host A. Repeated validation of parental self-reported smoking during pregnancy and infancy: a prospective cohort study of infants at high risk for allergy development. Paediatr Perinat Epidemiol. 2004;18:73–79. doi: 10.1111/j.1365-3016.2003.00520.x. [DOI] [PubMed] [Google Scholar]
- 36.England LJ, Grauman A, Qian C, Wilkins DG, Schisterman EF, Yu KF, et al. Misclassification of maternal smoking status and its effects on an epidemiologic study of pregnancy outcomes. Nicotine Tob Res. 2007;9:1005–1013. doi: 10.1080/14622200701491255. [DOI] [PubMed] [Google Scholar]
- 37.Parna K, Rahu M, Youngman LD, Rahu K, Nygard-Kibur M, Koupil I. Self-reported and serum cotinine-validated smoking in pregnant women in Estonia. Matern Child Health J. 2005;9:385–392. doi: 10.1007/s10995-005-0022-6. [DOI] [PubMed] [Google Scholar]
- 38.Jedrychowski W, Whyatt RM, Cooper TB, Flak E, Perera FP. Exposure misclassification error in studies on prenatal effects of tobacco smoking in pregnancy and the birth weight of children. J Expo Anal Environ Epidemiol. 1998;8:347–357. [PubMed] [Google Scholar]