Abstract
The ability to recollect details about past events improves during childhood. Most researchers favor the view that this improvement depends largely on the development of the prefrontal cortex, which is thought to have a protracted course of development relative to the medial temporal lobes (MTL). The primary goal of the present study was to test the hypothesis that the development of detail recollection is also associated with changes in MTL function. We collected functional magnetic resonance imaging data during an incidental encoding task in 80 participants, divided equally across four age groups: 8-year-olds, 10- to 11-year-olds, 14-year-olds, and young adults. Developmental differences in MTL activation profiles were observed. Fourteen-year-olds and adults engaged regions of the hippocampus and posterior parahippocampal gyrus selectively for subsequent detail recollection, whereas 8- and 10- to 11-year-olds did not. In 8-year-olds, these regions were recruited indiscriminately for detail recollection and item recognition; in 10- to 11-year-olds, activation in these regions did not consistently predict subsequent memory. These results suggest there are changes in the functional organization of the MTL, such that the hippocampus and posterior parahippocampal gyrus become increasingly specialized for recollection; these changes may be in part responsible for long-term memory improvements during childhood.
Introduction
Structures in the medial temporal lobe (MTL) and the prefrontal cortex (PFC) have proven critical for forming new episodic memories in adults (Davachi, 2006; Moscovitch et al., 2006; Badre and Wagner, 2007; Blumenfeld and Ranganath, 2007). Relatively little is known, however, about how these substrates mediate memory development during childhood and adolescence.
Maturation of MTL structures has been invoked to explain memory development during infancy (Bauer, 2008), but it has long been argued that changes during childhood and beyond are related to the maturation of the PFC (Cycowicz et al., 2003; Newcombe et al., 2007; Ofen et al., 2007). This account has found support in structural brain imaging studies showing protracted maturation of PFC (Paus et al., 1999; Giedd, 2004; Sowell et al., 2004). Furthermore, a functional neuroimaging study confirmed age-related increases in recruitment of PFC regions for memory encoding and stability in MTL recruitment (Ofen et al., 2007).
However, the possibility that changes in MTL function may also contribute to this development should not be prematurely dismissed, given recent evidence of protracted structural development of MTL regions well into middle childhood and beyond. That is, longitudinal research has shown that, although the overall volume of the hippocampus does not change from ages 4 to 25 years, the anterior hippocampus decreases in volume during this period, whereas the posterior hippocampus increases (Gogtay et al., 2006). Furthermore, a recent functional magnetic resonance imaging (fMRI) study has documented development in the parahippocampal place area, a region within the posterior parahippocampal gyrus. The size of this area recruited during encoding increased with age and significantly predicted subsequent recognition memory for scenes (Golarai et al., 2007).
Neuroimaging studies involving adults have shown that the hippocampus and posterior parahippocampal gyrus selectively support memory for items in association with their source or other qualitative details of encoding context (Davachi et al., 2003; Ranganath et al., 2004; Prince et al., 2005), broadly defined as episodic memory (Tulving, 1972). It is precisely this form of memory that exhibits the most pronounced behavioral improvement during childhood (Sluzenski et al., 2006; Ghetti and Angelini, 2008). Together, these observations led us to the hypothesis that changes in the function of these MTL regions might support age-related improvements in episodic memory.
To test this hypothesis, we conducted an incidental encoding fMRI study that included 80 participants divided across four age groups: 8-year-olds, 10- to 11-year-olds, 14-year-olds, and young adults. The use of an incidental task reduces the impact of developmental differences in intentional encoding strategies (Bjorklund et al., 2009). These groups were selected based on behavioral research indicating that rapid improvements in episodic memory occur during middle and late childhood (Brainerd et al., 2004; Ghetti and Angelini, 2008). We then examined blood oxygenation level-dependent activation during item encoding as a function of subsequent memory performance, that is, as a function of whether items were subsequently remembered in association with a specific detail (reflecting accurate episodic memory retrieval), recognized without accurate memory of this detail, or forgotten.
Materials and Methods
Participants
Eighty participants (20 from each of the four age groups) were included in the present study. Children received financial compensation and young adults (college students) received course credit. An equal number of male and female participants were included in each age group. Before enrollment, participants were screened for a number of conditions: low birth weight (below the 25th percentile), gestational age <36 weeks, special education placement, history of head trauma, neurological or psychiatric conditions, and previous history of attention or learning disorders. Given the specific demands of our task, individuals who were color-blind were also excluded. Finally, we relied on the standard procedures developed by the University of California (UC) Davis Research Imaging Center (IRC) for excluding individuals who should not participate in fMRI research (e.g., metal in the body, heart pace makers, insulin pumps, etc.).
Aside from the 80 participants whose data were included in the analyses, data from an additional eight participants were excluded for the following reasons: (1) two 8-year-olds, one 10-year-old, and one adult were excluded because of motion exceeding 4 mm within a scan; (2) one adult participant was excluded because a ring artifact was visible in the echo planar imaging (EPI) images; sustained volume artifacts across the entire experimental session were confirmed with Art Global (Mazaika et al., 2007); and (3) one 8-year-old, one 14-year-old, and one adult were excluded because they performed at chance on the memory task or obtained scores in the Wechsler Abbreviated Scale of Intelligence that were two or more SDs below average.
Task and procedures
Familiarization with scanning environment.
On the day of the scan session, all participants were first familiarized with the imaging procedures in an MRI mock scanner available at the UC Davis IRC. The mock scanner set-up consists of a 12-foot simulation scanner with a 6-foot tapered bore, motorized table, head coil, head stabilizer system, visual and auditory presentation systems, and equipment for monitoring participants' response to the training procedure. In the mock scanner, participants were exposed to the noises that are typical of MR and practiced lying still in the confined space. Although these procedures are geared toward training and reassuring young children, our pilot work showed that these procedures are helpful to reassure and reduce motion in adults as well. Based on these results, and to maintain methodological consistency, all participants were familiarized with the MRI environment regardless of age.
Incidental encoding task.
Next, participants were trained to perform the encoding task. They were shown a set of practice items, which consisted of 24 drawings from the same database as those used in the experiment. Drawings were presented either in green or red and participants were instructed to perform a semantic judgment for each drawing depending on the color, pressing one of two buttons on a response device. For example, when the object was presented in red, they had to indicate whether the depicted object could be found in a house; when the object was presented in green, they had to indicate whether the object was animate. The association between specific semantic judgment and color was counterbalanced. This method ensures that participants attend to the critical qualitative detail about the item, i.e., the color. Memory for the item and its color were probed in the subsequent memory test.
Once participants learned the encoding task, the scanning session began. During scanning, participants viewed 200 unambiguous line drawings that had been previously normed with child participants for familiarity and visual complexity (Cycowicz et al., 1997). Each drawing was presented for 2 s, followed by a fixation time of 2 s. Jittering was included so that each stimulus onset followed the previous stimulus 2–8 s later. To remind participants of the semantic task to be performed, a cue with the key word representing the judgment to be performed (“house?”, “alive?”) was presented above each drawing. The association between color and semantic judgment was counterbalanced, as was each item status as being viewed during encoding or being presented as a distracter. The full set of 200 drawings was presented over the course of four runs of 4:52 min each.
Recognition task.
Upon completing the scanning session, participants were trained to determine whether they had seen the drawing before and, if so, in what color. Then, participants were given a self-paced recognition test, in which they were shown line drawings rendered in black and white. The 200 drawings viewed at encoding were intermixed randomly with 100 new drawings. When an item was recognized as old, participants were also asked to indicate, with a button press, whether it had been shown in green or red. No reference to the semantic judgment was made in the recognition test instructions. This recognition test phase of the study lasted ∼45 min.
fMRI data acquisition
MRI data were collected using a high-resolution 3T Siemens scanner at the UC Davis IRC. Functional MRI data were acquired with a gradient echo EPI sequence [repetition time(TR) = 2000, echo time (TE) = 25, no interslice gap, flip angle = 90°, field of view (FOV) = 220, 144 volumes per run]. Each volume consisted of 34 contiguous 4 mm axial slices. Coplanar T2-weighted and high-resolution T1-weighted images were acquired for each participant (TR = 4000, TE = 109, FOV = 220). To minimize head movement and to ensure that participants were sufficiently comfortable to maintain focus on the task during the scan session, we placed foam and bean pillows between the head and the head coil, thereby maintaining the head in a still and comfortable position. In addition, snugly fitting headphones (MR Confon) and ear plugs dampened background scanner noise while allowing for easy communication during the session. The acquisition of structural scans was performed while participants watched cartoons (duration, ∼4:42 min). Finally, each of the four functional scans was relatively short and rest breaks were provided between scans.
fMRI data analysis
Data were preprocessed using custom routines in SPM2 (Wellcome Department of Cognitive Neurology) and MATLAB6. Images were corrected for differences in timing of slice acquisition, followed by rigid body motion correction. Structural and functional volumes were spatially normalized to T1 and EPI templates, respectively. During normalization, the volumes were resampled to 3 mm cubic voxels. Templates were based on the MNI305 stereotaxic space (Cocosco et al., 1997). These procedures have been validated for use with children aged 6 years and above (Burgund et al., 2002; Kang et al., 2003). Functional volumes were spatially smoothed with an 8 mm full-width at half-maximum isotropic Gaussian kernel.
Data for individual participants were analyzed with the general linear model in SPM. The fMRI time series data were modeled by a series of events convolved with a canonical hemodynamic response function (HRF). Although the correlations between age and the motion parameters for translation (i.e., x, y, z) and rotation (i.e., yaw, pitch, roll) did not reach conventional levels of significance (r ≤ |0.19|, p ≥ 0.08), we included these parameters as covariates of noninterest in the general linear model to account for such source of individual variability. The least-squares parameter estimates of height of the best-fitting canonical HRF for each condition were used in pairwise contrasts. Contrast images created at the individual level were submitted to group analyses. As detailed below in fMRI results, we used group data to conduct region-of-interest (ROI) analyses in regions showing memory-related activity in the MTL (with minimum cluster size of at least 10 contiguous voxels) at p < 0.001 uncorrected.
Our study design allowed for the examination of activation associated with three types of items, as follows: (1) items that were subsequently recognized along with correct recall of the item color (labeled “Color Correct” trials), (2) items that were subsequently recognized but whose color was not correctly recalled (labeled “Color Incorrect” trials), and (3) items that were subsequently forgotten (labeled “Miss” trials).
To test for differences in the pattern of MTL activation across the four age groups, we first identified clusters of at least 10 contiguous voxels that demonstrated memory-related activity in the comprehensive contrast of Color Correct > Miss at p < 0.001 uncorrected. We confined the examination of this contrast to the MTL by applying a mask created with MarsBar that included hippocampus and parahippocampal gyrus bilaterally (Table 1). We then conducted ROI analyses for the clusters that emerged within this mask.
Table 1.
Contrasts for clusters identified in the medial temporal lobes from Color Correct > Miss (all participants, p < 0.001 uncorrected) and Color Correct > Color Incorrect
| BA | MNI coordinates |
No. voxels | Peak t value | |||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Color Correct > Miss | ||||||
| L Hippocampus | −26 | −10 | −20 | 166 | 4.32 | |
| R Hippocampus | 30 | −12 | −20 | 105 | 4.01 | |
| L Posterior parahippocampal gyrus | 36 | −32 | −38 | −14 | 204 | 5.98 |
| R Posterior parahippocampal gyrus | 37 | 34 | −40 | −12 | 248 | 5.72 |
| Color Correct > Color Incorrect | ||||||
| L Hippocampus | −12 | −36 | 0 | 12 | 3.99 | |
| L Hippocampus | −24 | −10 | −20 | 24 | 3.81 | |
| L Parahippocampal gyrus | 35 | −22 | −24 | −20 | 44 | 3.95 |
| L Posterior parahippocampal gyrus | 36 | −24 | −38 | −14 | 10 | 3.73 |
| R Parahippocampal gyrus | 35 | 32 | −26 | −22 | 14 | 3.59 |
L, Left; R, right. All participants, p < 0.001, uncorrected.
In addition to the Color Correct > Miss contrast, we computed several additional contrasts. First, to identify regions engaged specifically during detail recollection, we computed the Color Correct > Color Incorrect. Second, to test whether any regions responded specifically to item recognition without detail recollection, we examined additional contrasts (i.e., Color Incorrect < Miss, Miss < Color Incorrect, and Color Incorrect > Color Correct).
Results
Behavioral results
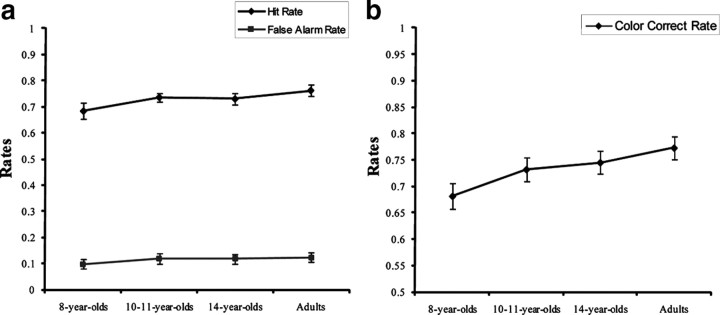
Before proceeding with analysis of behavioral data, we verified that IQ levels were comparable across age groups (F(3,76) = 1.49, p = 0.22; 8-year-olds, 115.9 ± 14.8; 10- to 11-year-olds, 114.2 ± 10.1; 14-year-olds, 108.9 ± 10.8; adults, 111.2 ± 10.1, mean ± SD). With respect to memory performance, there were no differences between groups in terms of either the rate of correct recognition of drawings that had been seen (i.e., hit rates) or the rate of incorrect recognition of new items, i.e., false-alarm rates (F(3,76) ≤ 1.95, p ≥ 0.13) (Fig. 1a). In contrast, there was a significant age difference in the rate of correct color recollection, calculated as the number of items for which the color was correctly identified divided by the total number of correctly recognized items (F(3,76) = 2.87, p < 0.05). Indeed, 8-year-olds were significantly less likely to accurately remember the color of the items they judged old than were 14-year-olds or adults (p < 0.05) (Fig. 1b); 10- to 11-year-olds did not significantly differ from the other groups.
Figure 1.
Behavioral results. a, b, Hit and false-alarm rates (a) and rates of correct color recollection (b) as a function of age group. Error bars indicate SEs.
We measured accuracy and response times for each semantic judgment performed during encoding. Accuracy of the semantic judgment did not differ by age (F(3,76) = 1.17, p = 0.34; 8-year-olds, 0.81 ± 0.10; 10- to 11-year-olds, 0.79 ± 0.11; 14-year-olds, 0.80 ± 0.11; adults, 0.84 ± 0.06, mean ± SD).
Although participants rarely failed to respond to the encoding prompt (an average rate of 0.03 of 200 encoding trials), there was a significant age difference in these rates (F(3,76) = 3.14, p < 0.05), such that 8-year-olds exhibited higher rates of failure to respond to the encoding prompt (0.04 ± 0.04, mean ± SD) than did 14-year-olds (0.02 ± 0.03, mean ± SD) or adults (0.01 ± 0.02, mean ± SD); 10- to 11-year-olds did not differ from the other groups (0.03 ± 0.04, mean ± SD). Behavioral results replicated those presented above when these trials were removed (F(3,76) = 2.96, p < 0.05). Trials in which no behavioral response was produced during encoding were excluded from fMRI analyses.
With respect to encoding response times, there was a significant effect of age (F(3,76) = 6.04, p < 0.01), such that 8-year-olds and 10- to 11-year-olds responded more slowly (1719.7 ± 330.4 ms and 1599.9 ± 215.6 ms, mean ± SD, respectively) than did 14-year-olds (1407.5 ± 181.0 ms, mean ± SD) or adults (1409.9 ± 371.9 ms, mean ± SD). However, response times did not differ as a function of whether participants recognized items and recollected their color accurately, recognized items but did not recollect their colors, or forgotten the items (F(3,152) = 0.39, p = 0.67). Thus, any age differences in the activation profile across type of trial cannot be accounted by differences in response times.
fMRI results
Following the approach described in Materials and Methods, we sought to test the hypothesis that activation profiles of the hippocampus and posterior parahippocampal gyrus become more selective during development, such that these regions are engaged specifically for color recollection in the mature state. Table 1 shows the clusters within the MTL, identified from the comprehensive contrast of Color Correct > Miss at p < 0.001 uncorrected and from the more specific detail recollection contrast Color Correct > Color Incorrect (at least 10 contiguous voxels). All brain coordinates are reported in MNI atlas space (Cocosco et al., 1997).
No cluster survived the p < 0.001 threshold for the additional contrasts (Color Incorrect < Miss, Miss < Color Incorrect, and Color Incorrect > Color Correct). For all ROIs, mean levels of activation are reported separately for Color Correct, Color Incorrect, and Miss trials.
Subsequent memory effects in the hippocampus
The comprehensive contrast of Color Correct > Miss trials, computed across all 80 participants, revealed clusters of hippocampal activation in both hemispheres. We performed ROI analyses on these clusters to test for qualitative differences in the activation profiles across age groups.
In the left hippocampal ROI (Fig. 2A), there was a significant main effect of trial type (F(2,152) = 11.65, p < 0.001), such that elevated activation was observed for Color Correct trials compared with Color Incorrect trials (p < 0.01) and Miss trials (p > 0.001). Activation did not differ significantly for Color Incorrect and Miss trials (p = 0.10). Although the overall age-by-trial-type interaction was not significant (F(6,152) = 1.81, p = 0.10), this was likely due to the robust difference between Color Correct trials and Miss trials (the contrast that yielded the functionally derived ROI) potentially obscuring the more subtle difference between Color Incorrect trials and either Color Correct or Miss trials.
Figure 2.
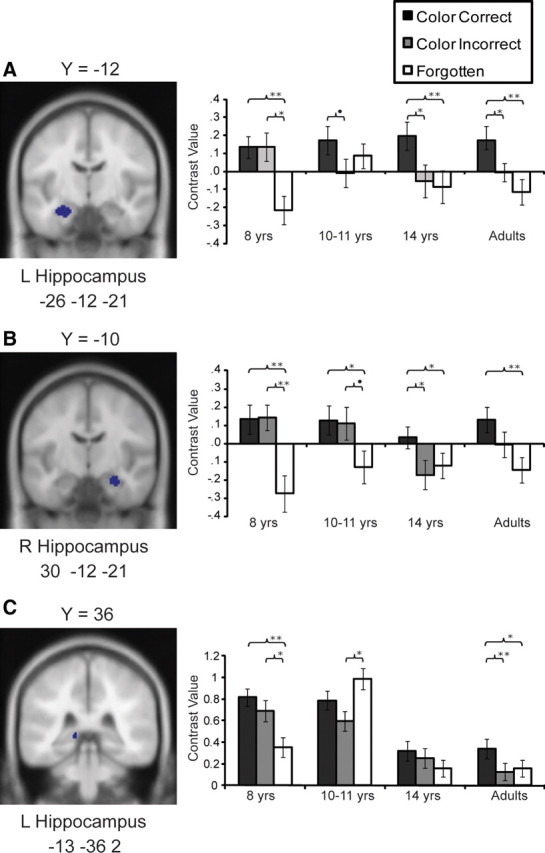
A, B, Average contrast values for each age group for ROIs in the left (L; A) and right (R; B) hippocampus, identified from the contrast Color Correct > Miss from all participants, p < 0.001. C, Contrast values for ROI in the right hippocampus from Color Correct > Color Incorrect from all participants, p < 0.001. Error bars indicate SEs. Significance levels are indicated as follows: **p < 0.01, *p < 0.05, •p < 0.10.
To test for this possibility, and to examine the effects of interest independent of the contrast defining the ROI, we examined the effects of age group and trial type in two separate ANOVAs. The first ANOVA included only Color Incorrect and Miss trials, and addressed the question of whether this hippocampal region was sensitive to item recognition (Color Incorrect > Miss trials). This analysis yielded a significant interaction between age and trial type (F(3,76) = 2.74, p < 0.05). Indeed, 8-year-olds recruited this region significantly more strongly for Color Incorrect trials than Miss trials (p < 0.05), but none of the other age groups exhibited a difference between these conditions (p > 0.26). Thus, an item recognition effect was observed in left hippocampus for 8-year-olds but not for the older age groups.
The second ANOVA included only Color Correct and Color Incorrect trials, and addressed the question of whether the left hippocampal ROI was recruited more strongly at encoding for items whose criterial detail (i.e., color) was subsequently remembered rather than forgotten. This analysis yielded a main effect of item type (F(3,76) = 8.76, p < 0.01) such that greater activation was observed for Color Correct trials compared with Color Incorrect trials; however, the difference between these two types of trials was not significant in 8-year-olds (p = 0.99) or the 10- to 11-year-olds (p = 0.08), but was significant in 14-year-olds and adults (p < 0.05 for both).
Overall, both 8-year-olds and 10- to 11-year-olds exhibited immature patterns of activation in left hippocampus. In 8-year-olds, the pattern was consistent with a response to item recognition, with a greater response for both Color Correct and Color Incorrect trials than for Miss trials. In 10- to 11-year-olds, this region was engaged similarly at encoding regardless of subsequent memory status. In 14-year-olds and adults, the pattern was consistent with a response to subsequent detail recollection, with stronger activation for Color Correct trials compared with Color Incorrect trials, and no significant difference between Color Incorrect and Miss trials.
As noted in Behavioral results, there were age differences in the rates of memory for item color. This finding raises the question of whether activation differences would still be observed if participants were matched on performance. We thus selected a subset of 14 participants in each of the four age groups who exhibited similar levels of item recognition and color recollection, and conducted all of the analyses reported with the full sample with the reduced sample of matched participants. Results concerning this region replicated those obtained in the full sample (supplemental Results and supplemental Fig. 1A, available at www.jneurosci.org as supplemental material).
In the right hippocampal ROI (Fig. 2B), results were highly similar to those in the left hippocampal region. The overall main effect of item type (F(6,152) = 12.53, p < 0.01) likely obscured the interaction between age and trial type (F(6,152) = 1.58 p = 0.16). Indeed, as in the left hippocampus, a significant age-by-item trial interaction emerged when only Color Incorrect and Miss trials were included (F(3,76) = 2.72, p < 0.05). As with the left hippocampus, 8-year-olds exhibited stronger activation, relative to Miss trials, for both Color Correct and Color Incorrect trials (p < 0.01 for both). The difference between Color Correct and Color Incorrect trials was not significant (p = 0.91). In 10- to 11-year-olds, this region exhibited stronger activation with Color Correct compared with Miss (p < 0.05), but the differences between Color Incorrect and Miss trials and Color Correct and Color Incorrect trials were not significant, (p > 0.10).
In contrast, 14-year-olds recruited this region selectively for color recollection (Color Correct > Color Incorrect trials and Color Correct > Miss, p < 0.05), whereas adults showed increased activation in the Color Correct trials compared with the Miss trials (p < 0.01), but the Color Incorrect trials did not differ significantly from either Color Correct or Miss trials (p > 0.26). This pattern of results was largely replicated with the sample of participants matched on performance (supplemental Results and supplemental Fig. 1B, available at www.jneurosci.org as supplemental material). Thus, as in the left hippocampus, in 8-year-olds, the pattern was consistent with a response to item recognition, whereas 14-year-olds exhibited a pattern consistent with detail recollection. In 10- to 11-year-olds and adults, activation indicated memory-related effects, but the patterns were less clear, with both exhibiting a gradual response.
In addition to these two hippocampal ROIs identified from Color Correct > Miss, two hippocampal regions were also identified from the more specific contrast for detail recollection (i.e., Color Correct > Color Incorrect) (Table 1). One region in the left posterior hippocampus exhibited a significant age-by-item trial interaction (F(6,152) = 3.51, p < 0.01) and exhibited selectivity for recollection only in adults. As can be seen in Figure 2C, 8-year-olds exhibited the same pattern observed in the other regions (Color Correct > Miss, p < 0.001; Color Incorrect > Miss, p < 0.05; Color Correct and Color Incorrect were not statistically different, p = 0.35). In 10- to 11-year-olds, weaker activation was observed for Color Incorrect compared with Miss trials (p < 0.05), but none of the other contrast differences were statistically significant (p > 0.14). In 14-year-olds, none of the contrast differences were significant (p > 0.16). Finally, adults exhibited a pattern clearly consistent with selective recruitment for detail recollection (Color Correct > Color Incorrect, p < 0.01; Color Correct > Miss, p = 0.05; with Color Incorrect and Miss trials not statistically different, p = 0.75). These results were virtually identical when the analyses were conducted only on the subset of participants matched for performance (supplemental Results and supplemental Fig. 1C, available at www.jneurosci.org as supplemental material).
The additional cluster in the left hippocampal region identified from Color Correct > Color Incorrect (−24, −10, −20) was completely contained in the larger cluster in the left hippocampus identified from Color Correct > Miss and analyzed earlier (Fig. 2A), and the results were highly similar. Thus, these results are only featured in the supplemental Materials (supplemental Fig. 3A, available at www.jneurosci.org as supplemental material).
In summary, 8-year-olds recruited the hippocampus bilaterally for subsequent item recognition regardless of whether the color was recalled correctly; 10- to 11-year-olds appeared to engage the hippocampus bilaterally during encoding but their activation profile did not exhibit subsequent memory. Evidence of selective recruitment of these regions for detail recollection was observed bilaterally in 14-year-olds and in the left hippocampus in adults.
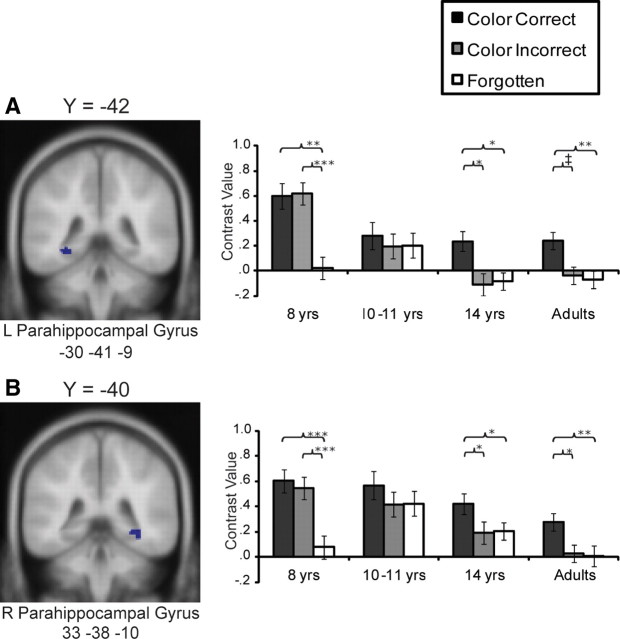
Subsequent memory effects in the posterior parahippocampal gyrus
Clusters of activation were found in bilateral posterior parahippocampal gyrus (PHG) for the Color Correct > Miss contrast. In the left posterior PHG (Fig. 3A), there was a significant interaction between age and item type (F(6,152) = 2.56, p < 0.05). In 8-year-olds, we found evidence of this region responding generally to item recognition (Color Correct > Miss, p < 0.001; Color Incorrect > Miss, p < 0.01; Color Correct and Color Incorrect did not differ, p = 0.89). In 10- to 11-year-olds, no reliable effect of item type was found (p > 0.56). In 14-year-olds and adults, the pattern of activation appeared consistent with selectivity for memory for item color (14-year-olds: Color Correct > Color Incorrect, p < 0.05; Color Correct > Miss, p < 0.05; Color incorrect ≈ Miss, p = 0.81; adults: Color Correct > Color Incorrect, p = 0.05; Color Correct > Miss, p < 0.01; Color incorrect ≈ Miss, p = 0.95). This pattern of results was confirmed when the groups were matched on performance (supplemental Results and supplemental Fig. 3B, available at www.jneurosci.org as supplemental material).
Figure 3.
A, B, Average contrast values for each age group for ROIs in the left (L; A) and right (R; B) posterior parahippocampal gyrus, identified from the contrast Color Correct > Miss from all participants, p < 0.001. Error bars indicate SEs. Significance levels are indicated as follows: ***p < 0.001, **p < 01, *p < 0.05; ‡p = 0.05.
In the right posterior PHG (Fig. 3B), there was a significant interaction between item type and age (F(6,152) = 2.16, p < 0.05). Selectivity for color recollection was not found for any of the age groups. In 8-year-olds, as in other regions, a pattern consistent with item recognition was found (Color Correct > Miss and Color Incorrect > Miss, p < 0.001; Color Correct ≈ Color Incorrect, p = 0.57). In 10- to 11-year-olds, no reliable effect of item type was found (p > 0.34).
As in the left posterior PHG, in 14-year-olds and adults, the pattern of activation was consistent with selectivity for memory for item color (14 year-olds: Color Correct > Color Incorrect, p < 0.05; Color Correct > Miss, p < 0.05; Color incorrect ≈ Miss, p = 0.86; adults: Color Correct > Color Incorrect, p < 0.05; Color Correct > Miss, p < 0.001; Color incorrect ≈ Miss, p = 0.83). The pattern of results was similar when analysis was restricted to participants matched on performance (supplemental Results and supplemental Fig. 2B, available at www.jneurosci.org as supplemental material).
Several clusters were also found from the Color Correct > Color Incorrect contrast (Table 1). One cluster in the left posterior PHG (−24, −38, −14) was completely contained in the larger cluster identified from Color Correct > Miss and analyzed earlier (Fig. 3A); the results from this smaller cluster are virtually identical to those obtained with the cluster from Color Correct > Miss (supplemental Fig. 3B, available at www.jneurosci.org as supplemental material). Finally, two additional clusters were found in more anterior regions of the parahippocampal cortex bilaterally [Brodmann area (BA) 35: −22, −24, −20; BA 35: 32, −26, −22). In neither region were age-related differences found in patterns of activation, (F(6,152) = 58, p > 0.75). On the left, the main effect of item type (F(6,152) = 8.32, p < 0.001) was consistent with selective recruitment for color recollection (Color Correct > Color Incorrect and Color Correct > Miss, p < 0.001; Color incorrect ≈ Miss, p = 0.83). On the right, the main effect of item type (F(6,152) = 12.78, p < 0.001) was consistent with a gradual response in this region (Color Correct > Color Incorrect, p < 0.001; Color Incorrect > Miss, p < 0.05; Color Correct > Miss, p < 0.001).
Overall, 8-year-olds recruited the posterior PHG bilaterally for item recognition and 10- to 11-year-olds engaged the posterior PHG indiscriminately. In 14-year-olds and adults, activation consistent with color recollection was observed in the posterior PHG bilaterally. These age differences did not extend to more anterior regions of the parahippocampal cortex.
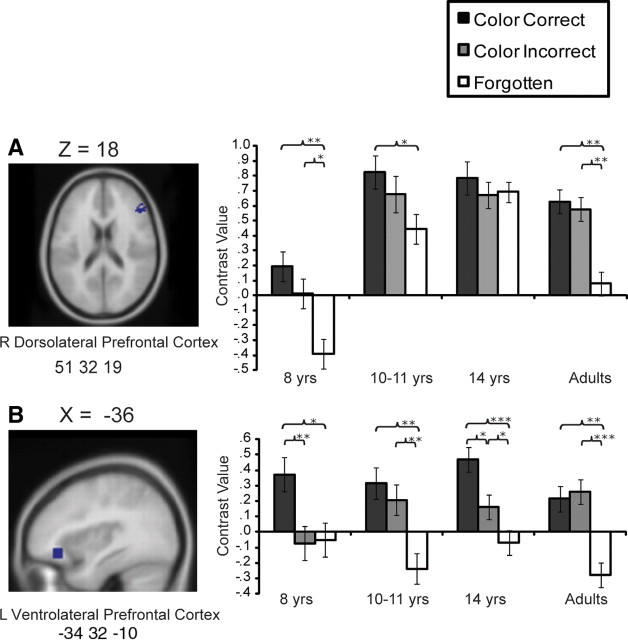
Subsequent memory effects in the PFC
Although the main focus of this study was to examine the development of the MTL, we also examined PFC recruitment during encoding. Clusters of activation in PFC from all contrasts are reported in supplemental Table 1, available at www.jneurosci.org as supplemental material.
ROI analyses were conducted on the regions identified in ventrolateral (VL) PFC and dorsolateral (DL) PFC, given prior evidence of the involvement of these regions in controlled processes supporting memory encoding (Badre and Wagner, 2007; Blumenfeld and Ranganath, 2007). One region in right DLPFC and one region in left VLPFC exhibited patterns revealing age-related differences (Fig. 4). In the right DLPFC, a significant age differences was observed (F(3,76) > 3.52, p < 0.05), such that 8-year-olds exhibited significantly lower activation than the other groups (p <0.05), which did not differ among each other, even though subsequent memory effects were present even in this younger group (Fig. 4A). When analyses were conducted with participants matched for performance, this significant effect of age was retained (F(3,52) > 2.81, p < 0.05).
Figure 4.
A, B, Average contrast values for each age group for ROIs in the right (R) DLPFC (A) and left (L) VLPFC (B) from the contrast Color Correct > Miss from all participants, p < 0.001. Error bars indicate SEs. Significance levels are indicated as follows: ***p < 0.001, **p < 01, *p < 0.05.
In the left VLPFC, an age-by-item-type interaction approached statistical significance (F(6,152) > 1.92, p = 0.08) and was significant when the interaction was examined with trend analysis (F(3,76) > 3.45, p < 0.05). As shown in Figure 4B, 8-year-olds recruited this region only for items subsequently remembered along with the color (Color Correct > Color Incorrect, p < 0.01; Color Correct > Miss, p < 0.05; Color Incorrect ≈ Miss, p = 0.89). In contrast, in each of the other age groups, both Color Correct and Color Incorrect trials recruited this region significantly more strongly than Miss trials (p < 0.05). All these differences were retained, albeit with attenuated reliability, when the analyses were conducted with the subset of the sample matched for performance (F(3,52) > 2.53, p = 0.06). This pattern was confirmed when ROI analyses were conducted with the smaller cluster in the same region (BA 47: −32, 30, −14) identified from Color Correct > Color incorrect (F(3,76) > 2.94, p < 0.05) (supplemental Table 1, available at www.jneurosci.org as supplemental material).
In all of the other PFC regions identified from Color Correct > Miss (i.e., left VLPFC; BA 45: −48, 24, 20) and Color Incorrect > Miss (left VLPFC; BA 47: −46, 30, −12), only main effects of trial types were found (F(3,156) > 9.65, p < 0.001), such that Color Correct trials recruited these regions more strongly than Miss trials across ages (p < 0.001). Thus, whereas the age-related changes in the activation profile of DLPFC with this incidental encoding task were quantitative, 8-year-olds appeared to successfully recruit the VLPFC only for items subsequently recollected, despite no overall age-difference in activation levels, whereas in the other age groups activation was observed even when color recollection was not successfully achieved in the later test.
Discussion
The goal of the present study was to test the hypothesis that the contribution of MTL regions to memory formation changes during childhood in association with the ability to remember specific details about past events. Consistent with this hypothesis, we found evidence of functional changes within the MTL pertaining to memory for item color. In 8-year-olds, activation in these regions predicted subsequent item recognition regardless of whether memory for color was correctly retrieved; in 10- to 11- year-olds, activation in these regions did not always differ based on subsequent memory performance; finally, in 14-year-olds and adults, encoding activation in regions of bilateral hippocampus and bilateral posterior PHG predicted subsequent color recollection, but not item recognition. These differences were largely replicated in a subset of the sample matched for performance. Although analyses with this sample necessarily introduce selection biases (e.g., the best performing 8-year-olds and the worse performing adults were included), their results suggest that the observed patterns could not be accounted for by differences in behavioral performance.
The ability to recollect events in association with contextual features has been associated with selective recruitment of the hippocampus and posterior PHG (Davachi et al., 2003; Ranganath et al., 2004; Diana et al., 2007). In the present study, we found age differences in the selectivity with which activation of bilateral hippocampus and posterior PGH predicts subsequent color recollection. In 8-year-olds, these regions are recruited not only for subsequent recollection, but also for subsequent item recognition. In 10- to 11-year-olds, an activation pattern similar to 8-year-olds was observed in the right hippocampus, but activation failed to exhibit a reliable pattern in the other MTL regions.
There are several plausible accounts for the developmental changes that were observed in the medial temporal lobes. First, these age differences may reflect structural changes in MTL. Longitudinal MRI research (Gogtay et al., 2006) showed that, despite the relative stability of hippocampal overall size between ages 4 and 25 years, the volume of the anterior hippocampus decreases over development, whereas that of the posterior hippocampus increases. Although the significance of these structural changes is not yet understood, there are reasons to suspect that synaptic production, pruning, and myelination play important roles (Utsunomiya et al., 1999; Nelson et al., 2003).
Second, these age differences in MTL activation may be due to increases in connectivity with PFC regions. There is evidence that the PFC–MTL network is strengthened during adolescence (Menon et al., 2005). Further, previous behavioral research has shown that a developmental improvement in recollection is driven by age-related increases in controlled operations (Ghetti and Angelini, 2008), which are known to involve PFC regions (Badre and Wagner, 2007; Blumenfeld and Ranganath, 2007). In the current study, 8-year-olds differed in DLPFC and VLPFC activation compared with the other groups. Future research should examine whether these changes play a role in the late emergence of selective MTL activation for recollection.
Changes in the PFC–MTL network may help explain the current finding that 8-year-olds exhibit more reliable activation patterns in MTL than 10- to 11-year-olds, despite this group showing subsequent memory effects in PFC and, more specifically, an activation profile comparable to that of 14-year-olds and adults in right DLPFC and left VLPFC. Preadolescence is a period of rapid change during which long-range projections begin to dominate over local ones (Fair et al., 2009); during this period, there may be temporary instability in brain networks, which might result in clearer regional patterns of activation in child groups and adults than in adolescence (Paz-Alonso et al., 2008).
Our study design and methods enhanced the probability of observing age-related differences in MTL function between middle childhood and adulthood, which have not been observed previously. Our sample included a large number of preadolescents, thereby allowing for a closer examination of development during childhood. Further, the task was specifically designed to promote recollective processes (i.e., processing of the association between items and their color; specific test of memory for item color during retrieval). These task characteristics may have hindered the probability of finding robust activation associated with item memory in the absence of memory for specific detail, thereby preventing us from exploring developmental differences in the neural substrates of this capacity.
However, these task characteristics may help explain why our results contrast those reported in the only other published event-related fMRI study on the development of encoding processes (Ofen et al., 2007), namely age-invariant levels of activation in MTL regions. Ofen et al. (2007) instructed participants to remember scenes; at retrieval, participants were asked to identify old from new items and then indicate whether the items recognized as old were subjectively experienced as Remembered or Familiar. The instructions and the materials used may have promoted global item encoding whereas encoding of specific qualitative details was not encouraged. Further, the use of scenes may have overridden subtle developmental differences in MTL recruitment, because scenes strongly engage this region (Stern et al., 1996; Brewer et al., 1998; Epstein and Kanwisher, 1998), consistent with the critical role of MTL in spatial representation (Mizumori et al., 2007; Bird and Burgess, 2008).
The differences in MTL findings between Ofen et al. (2007) and the present study raise several intriguing questions about the conditions under which differences in MTL recruitment may be observed during childhood and adolescence. Particularly interesting is the possibility that developmental changes in MTL recruitment depend on the extent to which binding between items and context features is promoted during encoding and on the extent to which retrieval operations are constrained (e.g., participants are required to remember specific details about an event versus anything about the event).
The age differences in PFC recruitment in our study were not as strong as those previously reported (Ofen et al., 2007). In our study, we used an incidental semantic encoding task to maximize similarity of encoding across ages, thereby reducing reliance on intentional strategies in older children and adults. By contrast, encoding was intentional in the study by Ofen and colleagues (2007). Whereas the use of scenes likely minimized simple labeling strategies in Ofen et al. (2007), use of other PFC-dependent strategies, known to improve with age (Bjorklund et al., 2009), was not prevented. Future research should examine whether the contribution of PFC to memory encoding can be potentiated with specific instructions and whether the resulting differences in PFC recruitment have implications for MTL contribution.
Although the present study focuses on MTL activation at encoding, we cannot discount the possibility that our neuroimaging results are in part accounted for by age differences at retrieval (Bjorklund et al., 2009). If younger participants implement retrieval processes differently than older participants, the final classification of trials (i.e., Color Correct, Color Incorrect, and Miss) and associated activation levels would be more likely to show developmental differences even assuming no true change in encoding-related activation. If this were the case, however, we should expect less systematic differences in subsequent memory effects. Instead, our results indicate a progression from activation predicting item recognition to activation predicting color recollection. Nevertheless, given that regions in MTL are recruited during retrieval (Cansino et al., 2002), the study of age differences in retrieval-related activation is a logical next step for future research (Paz-Alonso et al., 2008).
Future investigations should also take a longitudinal approach to examine the emergence of specialized recruitment of distinct MTL regions for memory. An examination of the relations between age and brain activation in cross-sectional samples, as in the present study, is informative for identifying critical transitions in development. However, it is only through the examination of within-individual trajectories that developmental change can be established with respect to the contribution of specific regions as well as networks of regions to memory formation.
Nonetheless, our findings have important implications for both theory and application. Dual-process theories contend that distinct MTL regions subserve recollection-based and familiarity-based recognition (Eichenbaum et al., 2007), whereas other theoretical frameworks emphasize that MTL regions function as a unitary system, despite some division of labor (Squire et al., 2007). Our results suggest that this functional distinction emerges during childhood. MTL regions may function as a unitary system earlier in childhood, and become increasingly differentiated in later years (de Haan et al., 2006). From an applied perspective, this study may be informative in identifying periods of increased risk of memory dysfunction. For example, it has been shown recently that some children with type-1 diabetes exhibit recollection deficits (Ghetti et al., 2010). These deficits, associated with complications putatively affecting hippocampal integrity, were more robust when these complications had occurred before age 7 years. If important transitions occur after age 8 years, subtle insults occurring earlier could have a more detrimental effect on recollection. Overall, the present study joins a slowly growing body of research that provides insights on the connection between development of brain function and changing memory performance.
Footnotes
This research was supported by a grant from the National Institute of Child Health and Human Development (HD054636-01) to S.G. We thank Carter Wendelken for assistance in data analysis.
References
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45:2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Bauer PJ. Toward a neuro-developmental account of the development of declarative memory. Dev Psychobiol. 2008;50:19–31. doi: 10.1002/dev.20265. [DOI] [PubMed] [Google Scholar]
- Bird CM, Burgess N. The hippocampus and memory: insights from spatial processing. Nat Rev Neurosci. 2008;9:182–194. doi: 10.1038/nrn2335. [DOI] [PubMed] [Google Scholar]
- Bjorklund DF, Dukes C, Brown RD. The development of memory strategies. In: Courage M, Cowan N, editors. The development of memory in infancy and childhood. Hove, UK: Psychology; 2009. pp. 145–175. [Google Scholar]
- Blumenfeld RS, Ranganath C. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization. J Neurosci. 2006;26:916–925. doi: 10.1523/JNEUROSCI.2353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainerd CJ, Holliday RE, Reyna VF. Behavioral measurement of remembering phenomenologies: so simple a child can do it. Child Dev. 2004;75:505–522. doi: 10.1111/j.1467-8624.2004.00689.x. [DOI] [PubMed] [Google Scholar]
- Brewer JB, Zhao Z, Desmond JE, Glover GH, Gabrieli JD. Making memories: brain activity that predicts how well visual experience will be remembered. Science. 1998;281:1185–1187. doi: 10.1126/science.281.5380.1185. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Cansino S, Maquet P, Dolan RJ, Rugg MD. Brain activity underlying encoding and retrieval of source memory. Cereb Cortex. 2002;12:1048–1056. doi: 10.1093/cercor/12.10.1048. [DOI] [PubMed] [Google Scholar]
- Cocosco CA, Kollokian V, Kwan RKS, Evans AC. BrainWeb: online interface to a 3D mri simulated brain database. Neuroimage. 1997;5:S425. [Google Scholar]
- Cycowicz YM, Friedman D, Rothstein M, Snodgrass JG. Picture naming by young children: norms for name agreement, familiarity and visual complexity. J Exp Child Psychol. 1997;65:171–237. doi: 10.1006/jecp.1996.2356. [DOI] [PubMed] [Google Scholar]
- Cycowicz YM, Friedman D, Duff M. Pictures and their colors: what do children remember. J Cog Neurosci. 2003;15:759–768. doi: 10.1162/089892903322307465. [DOI] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal lobe processes build item and source memories. Proc Natl Acad Sci U S A. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan M, Mishkin M, Baldeweg T, Vargha-Khadem F. Human memory development and its dysfunction after early hippocampal injury. Trends Neurosci. 2006;29:374–381. doi: 10.1016/j.tins.2006.05.008. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–386. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Power JD, Dosenbach NU, Church JA, Miezin FM, Schlaggar BL, Petersen SE. Functional brain networks develop from a “local to distributed” organization. PLoS Comput Biol. 2009;5:e1000381. doi: 10.1371/journal.pcbi.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti S, Angelini L. The development of recollection and familiarity in childhood and adolescence: evidence from dual process signal detection. Child Dev. 2008;79:339–358. doi: 10.1111/j.1467-8624.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- Ghetti S, Lee JK, Sims C, DeMaster DM, Glaser NS. Diabetic ketoacidosis and memory dysfunction in children with type 1 diabetes. J Pediatr. 2010;156:109–114. doi: 10.1016/j.jpeds.2009.07.054. [DOI] [PubMed] [Google Scholar]
- Giedd JN. Structural magnetic resonance imaging of the adolescent brain. Ann N Y Acad Sci. 2004;1021:77–85. doi: 10.1196/annals.1308.009. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Nugent TF, 3rd, Herman DH, Ordonez A, Greenstein D, Hayashi KM, Clasen L, Toga AW, Giedd JN, Rapoport JL, Thompson PM. Dynamic mapping of normal human hippocampal development. Hippocampus. 2006;16:664–672. doi: 10.1002/hipo.20193. [DOI] [PubMed] [Google Scholar]
- Golarai G, Ghahremani DG, Whitfield-Gabrieli S, Reiss A, Eberhardt JL, Gabrieli JD, Grill-Spector K. Differential development of high-level visual cortex correlates with category-specific recognition memory. Nat Neurosci. 2007;10:512–522. doi: 10.1038/nn1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Mazaika PK, Whitfield-Gabrieli S, Reiss AL. Artifact repair of fMRI data from high motion clinical subjects. Paper presented at the Annual Meeting of the Organization for Human Brain Mapping; Chicago, IL. 2007. [Google Scholar]
- Menon V, Boyett-Anderson JM, Reiss AL. Maturation of medial temporal lobe response and connectivity during memory encoding. Brain Res Cogn Brain Res. 2005;25:379–385. doi: 10.1016/j.cogbrainres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Smith DM, Puryear CB. Hippocampal and neocortical interactions during context discrimination: electrophysiological evidence from the rat. Hippocampus. 2007;17:851–862. doi: 10.1002/hipo.20317. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L, Winocur G, Gilboa A, Rosenbaum RS. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol. 2006;16:179–190. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Nelson EE, McClure EB, Monk CS, Zarahn E, Leibenluft E, Pine DS, Ernst M. Developmental differences in neuronal engagement during implicit encoding of emotional faces: an event-related fMRI study. J Child Psychol Psychiatry. 2003;44:1015–1024. doi: 10.1111/1469-7610.00186. [DOI] [PubMed] [Google Scholar]
- Newcombe NS, Lloyd ME, Ratliff KR. Development of episodic and autobiographical memory: a cognitive neuroscience perspective. Adv Child Dev Behav. 2007;35:37–85. doi: 10.1016/b978-0-12-009735-7.50007-4. [DOI] [PubMed] [Google Scholar]
- Ofen N, Kao YC, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JD. Development of the declarative memory system in the human brain. Nat Neurosci. 2007;10:1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Paz-Alonso PM, Ghetti S, Donohue SE, Goodman GS, Bunge SA. Neurodevelopmental correlates of true and false recognition. Cereb Cortex. 2008;18:2208–2216. doi: 10.1093/cercor/bhm246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: successful encoding and retrieval of semantic and perceptual associations. J Neurosci. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Sluzenski J, Newcombe NS, Kovacs SL. Binding, relational memory, and recall of naturalistic events: a developmental perspective. J Exp Psychol Learn Mem Cogn. 2006;32:89–100. doi: 10.1037/0278-7393.32.1.89. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–883. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CE, Corkin S, González RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 1996;93:8660–8665. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic; 1972. pp. 381–403. [Google Scholar]
- Utsunomiya H, Takano K, Okazaki M, Mitsudome A. Development of the temporal lobe in infants and children: analysis by MR-based volumetry. AJNR Am J Neuroradiol. 1999;20:717–723. [PMC free article] [PubMed] [Google Scholar]