Abstract
Background
Several actin nucleators, including Arp2/3 and various formins, control numerous cytoskeletal-based functions in vivo.
Results
We investigated the relative roles of these nucleators. As a model system, we used natural killer (NK) lymphocytes, which display a wide range of cytoskeletal-based functions that culminate in the lysis of target cells. NK cells lacking either Arp2/3 or the formin hDia1 were ineffective in target cell lysis, but for distinct reasons. Loss of Arp2/3 function led to defects in cells adhesion and actin assembly at the junction with the target cell (the lytic synapse). In contrast, loss of hDia1 did not disrupt actin assembly at the lytic synapse. Instead, loss of hDia1 led to perturbations in the microtubule cytoskeleton, including the targeting of microtubules to the lytic synapse.
Conclusions
These studies reveal novel distinctions and relationships among the functions of Arp2/3, formins and microtubules in cells. Notably, a formin mediates the capture of microtubules at the cell periphery.
INTRODUCTION
Within cells, regulated actin assembly is initiated by two major classes of actin nucleators, the Arp2/3 complex and the formins [1, 2]. Arp2/3 complex binds to the side of an existing “mother” filament and promotes the formation of a new “daughter” filament, creating a dendritic actin array. Arp2/3 generally requires activation by a nucleation-promoting factor (NPF) from one or more of the following families: the Wiskott-Aldrich Syndrome Protein (WASp) family, the WASp family-Verprolin homologous protein (WAVE), and the cortactin family, which includes hematopoietic lineage cell specific protein (HS1).
In contrast to Arp2/3, formins interact with the barbed end of actin filaments and promote incorporation of new actin monomers [3, 4]. Formin-mediated actin assembly generally leads to the formation of elongated parallel bundles of straight filaments like those in filopodia [3, 5]. Formins are a large and diverse group of proteins, with many representatives in mammalian systems. They differ in the degrees to which they promote actin assembly at barbed ends, inhibit capping and nucleate new filament formation. Most striking, they contain different regulatory regions, and they show widely divergent cell and tissue expression patterns [6].
We wish to understand the relative roles of Arp2/3- and formin-mediated actin assembly in cells. As a model system, we chose Natural Killer lymphocytes because they display a range of cytoskeletal functions in their ultimate role of killers of target cells, including cancer cells and virus-infected cells. The processes by which natural killer (NK) cells interact with and kill potential target cells include extravasation, chemotactic migration, and binding to potential target cells, which depend on actin and microtubule functions [7-9].
NK cell motility is initiated by environmental signals that promote adhesion between NK and vascular endothelial cells, resulting in attachment and transmigration. Adhesion and migration involve the regulated assembly and cross-linking of actin filaments that support leading-edge membrane protrusions, in coordination with cycles of integrin-based cell-substrate attachment and detachment, actin disassembly behind the leading edge, cell body contraction, and retraction of the uropod.
NK cells interact with potential target cells through multiple cell-surface receptors for different ligands on potential target cells. Depending upon the receptor/ligand repertoires of both the NK and target cells, NK cells establish either a tight, stable “lytic synapse,” resulting in lysis of the target cell, or a transient synapse, resulting in release of the target cell with no lysis. During a transient, non-cytolytic interaction, the NK cell shows little actin reorganization. In contrast, during a stable cytolytic interaction, the NK cell actin cytoskeleton reorganizes to become highly polarized towards the target cell, which is essential for formation of a tight lytic synapse and cytolysis of the target cell [7, 8, 10]. Microtubules (MTs) also have an important role in the lytic activity of cytotoxic cells. Lytic granules move along MTs, mediated by kinesins in cytotoxic T lymphocytes (CTLs) [11] In CTLs, the MT-organizing center (MTOC) moves toward the lytic synapse [12].
In addition to their role in actin assembly, recent evidence has linked formins to the regulation of MT dynamics, most notably in migrating fibroblasts [13-15]. In T cells, the loss of the formins FMNL1 or mDia1 leads to a partial disruption of lytic synapse formation as well as a loss of MTOC localization [16]. The connection between formins and MTs appears to be mediated by the interaction between the MT end-binding proteins EB1 and APC, which have been shown to bind to the mouse formin mDia1 [17]. Therefore, formins may provide a link between the actin and MT cytoskeletons.
Here we compared the contributions of Arp2/3 complex and the formin hDia1 to cytoskeletal regulation during distinct NK cell functions. Arp2/3 was required for actin assembly at the lytic synapse and for mature lytic synapse formation. Integrin and NK receptor signaling were unaffected by the loss of Arp2/3. In contrast, the loss of hDia1 expression did not disrupt actin assembly or lytic synapse formation, but instead impaired the targeting of the MT network required for polarization and secretion of lytic granules. Thus, we found that the Arp2/3 complex and formins regulate distinct cell functions required for NK-mediated cytotoxicity, and that formins link the functions of the actin and MT cytoskeletons.
RESULTS
Arp2/3 and hDia1 localize to the lytic synapse at distinct stages
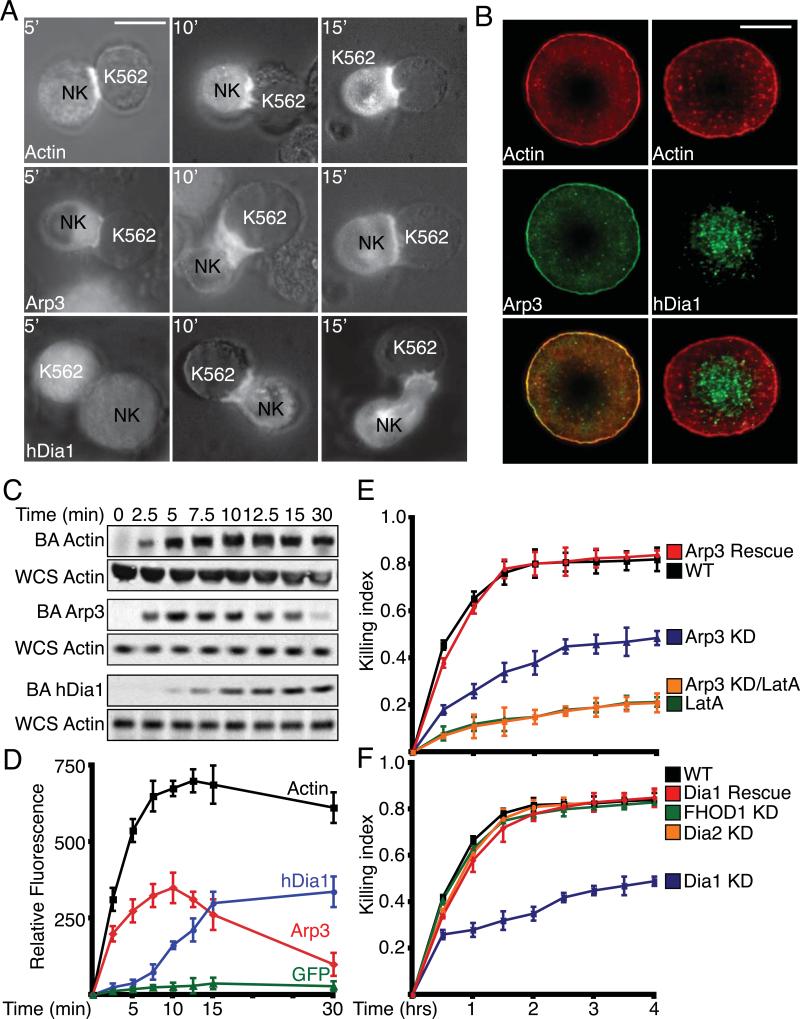
The actin cytoskeleton is critical for lytic synapse (LS) formation [7, 10], so we determined the temporal and spatial regulation of the actin nucleators Arp2/3 and hDia1 at the LS. Upon adhesion to target cells, GFP-Actin and GFP-Arp3 localized to the LS within 5 min (Figure 1A). In contrast, GFP-hDia1 did not localize to the LS until 10 min. We next wanted to determine the spatial regulation of Arp2/3 and hDia1 within the LS. Primary NK cells were allowed to adhere to the ligand, UL16-binding protein (ULBp), for the activating receptor NKG2D and the ligand, intracellular adhesion molecule 1 (ICAM-1), for αLβ2 integrin (LFA-1). Arp3 was localized peripherally to a cortical ring with punctate staining within the cell interior with a high degree of actin co-localization (Figure 1B). In contrast, punctate hDia1 staining was localized centrally within the LS with little actin co-localization.
Figure 1. Actin, Arp2/3 and hDia1 localization during LS formation and their role in cytolysis.
(A) Fluorescence localization: primary NK cells expressing GFP-Actin , GFP-Arp3 or GFP-hDia1 were incubated with K562 target cells for indicated times. (Scale Bar: 10μm). (B) Localization of Arp3 and hDia1 within the LS. Primary NK cells were adhered to ICAM-1 and ULBp coated glass bottom dishes for 15 min. Cells were fixed, stained with Alexa Fluor 568 phalloidin or specific antibodies, and imaged for fluorescence. (Scale Bar:5μm) (C) Primary NK cells were incubated with Dynabeads coated with ICAM-1/ULBp. At indicated times, magnetically restrained beads were pulse sonicated, subjected to SDS-PAGE and indicated proteins were immunoblotted (BA: Bead Associated). Whole cell sonicates (WCS) were run separately and blotted for total actin. Blots shown are representative of at least three separate experiments (MW: Actin 42kDa; Arp3 47kDa; hDia1 198kDa) (D) Primary NK cells expressing GFP-Actin, GFP-Arp3 or GFP-hDia1 were incubated with Dynabeads coated with ICAM-1 and ULBp. At indicated times, magnetically restrained beads were pulse sonicated and bead-associated GFP-fluorescence was measured. (E and F) Cytolysis assays with knockdown of (E) Arp2/3 and (F) Formins: Primary NK and K562 target cells were incubated together in 96-well round-bottom dishes for indicated times at 37°C. The killing index, based on release of adenylate kinase into the media, is plotted. Values plotted are mean ± SEM for at least three separate experiments. For all above and subsequent Western blots, equal protein was loaded into each well as determined by Bradford assay and Ponceau staining. Blots shown are representative of at least three separate experiments.
To test a defined synthetic target and examine endogenous proteins, we incubated primary NK cells with Dynabeads coated with ULBp and ICAM-1. As seen with target cells, actin was recruited to the bead within 2.5 min and persisted for at least 30 min (Figure 1C). Arp3 was also recruited to the bead within 2.5 min. However, Arp2/3 recruitment decreased after 10 min with little Arp2/3 remaining at 30 min. In contrast, bead recruitment of hDia1 did not occur until 7.5 min but persisted for at least 30 min.
To quantitate recruitment, we incubated primary NK cells expressing GFP-actin, GFP-Arp3 or GFP-hDia1 with ULBp/ICAM-1 coated beads and quantified bead-associated fluorescence over time. As observed with endogenous proteins, GFP-Arp3 and GFP-actin were associated with the beads within 2.5 min. GFP-actin remained bead-associated for up to 30 min but GFP-Arp3 association began to decrease after 7.5 min. As seen with immunoblots, GFP-hDia1 bead recruitment did not peak until 10 min and persisted for at least 30 min (Figure 1D). We observed very little bead-associated GFP fluorescence with primary NK cells expressing GFP alone.
Inducible Knockdown of Arp2/3 and Formins
To investigate the function of Arp2/3 in NK cells, we knocked down Arp2/3 with Tet-inducible shRNA expression with two different Arp3 targeting constructs (Arp3A and Arp3B). Arp3 and Arp2 protein levels were decreased by greater than 95% in NKL cells and 91% in primary NK cells at 36 hrs post addition of doxycycline (Figure S1A). A non-targeting version of Arp3A (Arp3sc) had no effect on Arp3 expression. Rescue with shRNA resistant non-tagged Arp3 or GFP-tagged Arp3 produced levels of exogenously expressed Arp3 comparable to endogenous Arp3 in control cells (Figure S1C).
To investigate the role of formins, we knocked down hDia1, hDia2 and FHOD1 using Tet-inducible shRNA expression. These Dia formins were chosen based upon their roles in immune cell adhesion and migration [16, 18, 19]. FHOD1 was chosen because it also has been shown to play a role in cell adhesion and migration, however it is structurally distinct from the Dia formins [20-22]. Two different hDia1 targeting constructs, (hDia1A and hDia1B), decreased hDia1 protein levels by greater than 98% in NKL cells and 93% in primary NK cells at 36 hrs (Figure S1B). A non-targeting version of hDia1A (hDia1sc) had no effect on hDia1 expression. In addition, hDia2 expression was unaffected by the loss of hDia1 expression. This was a concern as it has been observed that loss of hDia1 in knockout mice has led to an increase in hDia2 expression [23]. Rescue with shRNA resistant non-tagged hDia1 or GFP-tagged hDia1 produced levels of exogenously expressed Arp3 comparable to endogenous hDia1 in control cells (Figure S1C). (Figure S1D). In addition we were able to knock down hDia2 and FHOD1 expression by greater than 98% (Figure S1E and F).
Role of Arp2/3 and Formins in Cytolysis
To assess the role of Arp2/3 and formins in NK cell functions, we first assayed NK-mediated cytotoxicity. Primary NK cells with decreased Arp2/3 expression displayed a significant decrease (41%) in cytolytic activity (Figure 1E, S2A). However, Arp2/3 knockdown did not inhibit cytotoxicity to the same degree as Latrunculin A (LatA) treatment did (80%). As the knockdown of Arp2/3 was not complete, low levels of Arp2/3 may be sufficient to mediate low levels of cytotoxicity, or alternative pathways may substitute.
Decreased hDia1 expression also inhibited cytolytic activity (40%) (Figure 1F, S2A). In primary NK cells lacking hDia2 or FHOD1, we observed no effect on cytotoxicity; therefore, we did not further investigate hDia2 or FHOD1. The rate of cytolysis by primary hDia1-knockdown cells was initially similar to control cells, and then decreased after 30 min. To test whether this effect could be attributed to a less complete knockdown observed in primary NK cells, we measured cytotoxicity with NKL cells, in which there was almost complete knockdown (>98%). With NKL cells, cytotoxicity was decreased immediately, from the initial time point, and the level remained low over time (Figure S2B).
Adhesion to ICAM-1 and Fibronectin
NK cells must first exit the vasculature in order to interact with potential target cells. The interaction of NK cells with vascular smooth muscle cells (VSM cells) is mediated by interaction of NK-expressed LFA-1 (αLβ2) with VSM-cell-expressed ICAM-1. Once extravasation occurs, the NK cells also utilize the integrins VLA-4/5 (α4/5β1) to mediate adhesion to fibronectin (Fn) within the extra-cellular matrix. To assess the role of Arp2/3 and hDia1 in adhesion, we performed adhesion assays in which primary NK cells were allowed to adhere to ICAM-1 or Fn. The loss of Arp2/3 expression inhibited adhesion to both ICAM-1 (49%) and Fn (28%) (Figure S3A). Adhesion to both ligands was rescued with re-expression of Arp3. In contrast, adhesion to ICAM-1 was not affected by the loss of hDia1 expression (Figure S3B); however, loss of hDia1 did inhibit adhesion to Fn (>40%) and was rescued by re-expression of hDia1 (Figure S3B).
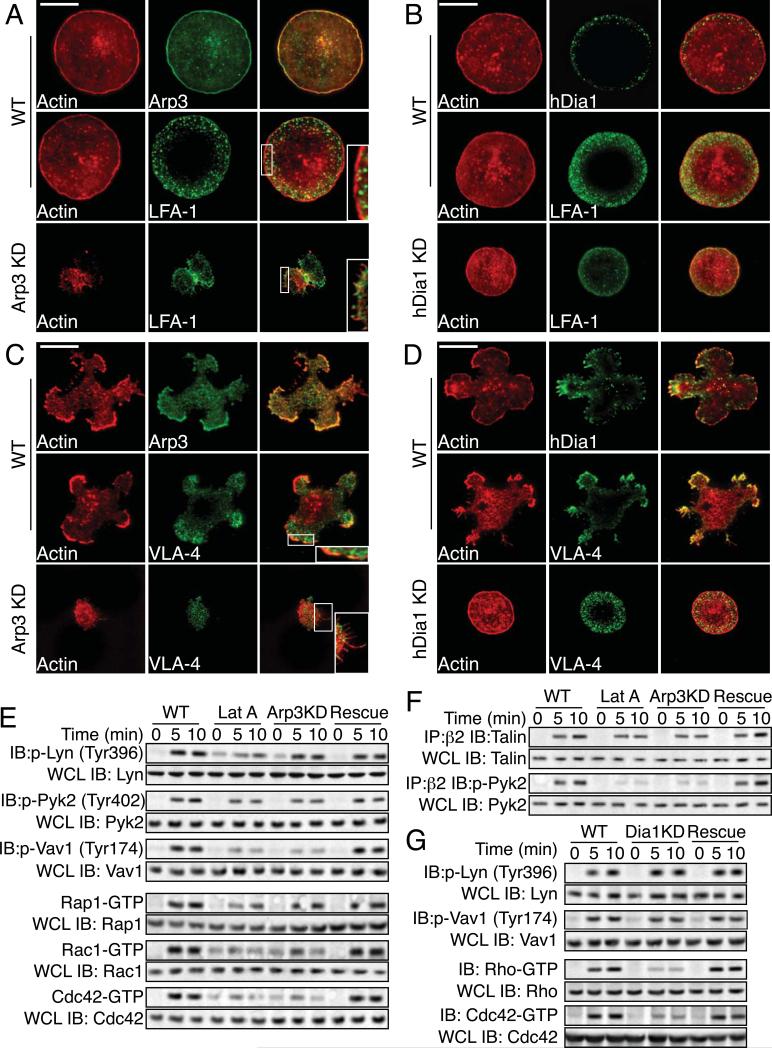
To assess the state of the actin cytoskeleton and localization of integrins during cell adhesion, primary NK cells were allowed to adhere to ICAM-1 or Fn, stained with fluorescent phalloidin and specific antibodies and imaged by TIRF microscopy. Control cells on ICAM-1 spread evenly and displayed an F-actin-rich cortical ring and central punctate F-actin staining (Figure 2A). At the cortex, Arp3 co-localized with F-actin (Figure 2A). LFA-1 staining was localized to a peripheral ring with little central LFA-1 staining. Loss of Arp2/3 resulted in little cell spreading, more diffuse actin staining with little or no cortical actin accumulation and the appearance of numerous filopodia (Figure 2A inset). LFA-1 staining in Arp2/3 knockdown-cells was punctate but not localized to a peripheral ring. The control phenotype was rescued with expression of Arp3 (Figure S3C). On ICAM-1, hDia1 was localized to the cell periphery but unlike Arp2/3, was not highly co-localized with F-actin (Figure 2B). In contrast to Arp2/3, we did not observe any significant change in actin cytoskeleton morphology with the loss of hDia1 expression on ICAM-1; however, hDia1-knockdown cells (mean diameter: 5.9±1.3 μm) were less spread as compared to control cells (8.6±1.6 μm)(p<.01). Although hDia1 knockdown cells were less spread, LFA-1 and actin staining were still properly localized.
Figure 2. Arp2/3 and hDia1 are required for adhesion to distinct ligands.
(A and B) Morphology of adhering cells on ICAM-1. Primary NK cells were allowed to adhere to ICAM-1 in the presence of SDF-1α. Cells were fixed, stained for F-actin with Alexa Fluor 568 phalloidin, or specific antibodies, and imaged for fluorescence. (Scale Bar:5μm) (C and D) Morphology of adhering cells on Fn. Primary NK cells were allowed to adhere to Fn in the presence of SDF-1α. Cells were fixed, stained with Alexa Fluor 568 phalloidin or specific antibodies, and imaged for fluorescence. (Scale Bar:5μm) (E) Signaling; Primary cells were allowed to adhere to ICAM-1 for indicated times at 37°C. Immunoblots of cell lysates were probed with indicated phospho-specific Abs. Whole cell lysates (WCL) are 1/10 total lysates and were run separately and probed with indicated Abs. For Rho-family GTPase signaling, primary cells were allowed to adhere to ICAM-1 for indicated times (min) at 37°C, and GTP-bound Rac1, Cdc42 or Rap1 were pulled down from cell lysates using CRIB pull down assays, then subjected to SDS-PAGE with detection by immunoblot. Total Rac1, Cdc42 or Rap1 were blotted separately using WCL as above. (MW: Lyn 26kDa; Pyk2 106kDa; Vav1 95kDa; Rap1/Rac1/Cdc42 24kDa) (F) Association of specific signaling proteins with integrin β2. Primary cells were allowed to adhere to ICAM-1 for indicated times at 37°C. Cell lysates were immunoprecipitated with anti-β2-coated Dynabeads and blotted with mAbs for indicated proteins. Total proteins were blotted separately using WCL as above. (MW: Talin 235kDa) (G). Signaling. Primary cells were allowed to adhere to Fn for indicated times at 37°C. Immunoblots of cell lysates were probed with phospho-specific Abs. Total proteins were blotted separately using WCL as above. For Rho-family GTPase signaling, primary cells were allowed to adhere to Fn for indicated times (min) at 37°C. GTP-bound Rho and Cdc42 were pulled down as above and detected by immunoblot. (MW: Rho 24kDa).
On Fn, primary NK cells spread more unevenly and displayed numerous F-actin-rich pseudopodia and filopodia with cortical F-actin staining (Figure 2C). Again, Arp3 co-localized with F-actin (Figure 2C) and VLA-4/5 localized to a peripheral ring and little central staining but with more actin co-localization than with LFA-1 on ICAM-1 (Figure 2C). The loss of Arp2/3 resulted in little cell spreading with numerous filopodial like projections remaining (Figure 2C inset) and VLA-4/5 staining was punctate, but not localized to a peripheral ring. Cell spreading, morphology, and VLA-4/5 localization were rescued with expression of Arp3 (Figure S3C). For hDia1 on Fn, we observed more co-localization of hDia1 with F-actin, especially at the tips of the pseudopodia and filopodia (Figure 2D). The loss of hDia1 resulted in decreased cell spreading with little or no filopodial or pseudopodial projections. The mean diameter was 5.1±0.7μm in hDia1-knockdown cells and 9.7±1.8 μm in control cells (p<.01) and hDia1-knockdown cells on Fn resembled NK cells adhered to ICAM-1 with less cell spreading. In contrast to the loss of Arp2/3, peripheral localization of VLA-4/5 was observed even with the loss of hDia1 (Figure 2D). Cell spreading was rescued with re-expression of hDia1 (Figure S3C). The observed inhibition of adhesion with the loss of Arp2/3 or hDia1 expression was not due to an inhibition of integrin activation. Loss of Arp2/3 or hDia1 did not inhibit conversion of LFA-1 or VLA-4/5 to the high affinity state, as assayed by mAb24 and 9G2 staining respectively (Table S1).
Thus, Arp2/3-mediated actin assembly is required for firm adhesion to and cell spreading on ICAM-1 and Fn and the proper localization of integrins that are mediating cell adhesion. hDia1 is required for complete cell spreading on ICAM-1 and for adhesion to Fn as well as the formation of filopodia and pseudopodial extensions, but hDia1 is not required for integrin localization.
Integrin Signaling
Because the loss of Arp2/3 did not decrease integrin activation; therefore, we did not expect significant defects in integrin-mediated signaling. As expected, loss of Arp2/3 only marginally inhibited Lyn (8%) and Pyk2 (9%) (Figure 2E) as well as Syk (11%) and PI3 Kinase (13%) (Figure S3D) activation at 10 min on ICAM-1. However, Vav1 activation was significantly decreased Vav1 (>65%) (Figure 2E). We then assessed proteins associated with LFA-1 and observed no significant decrease in the association of talin (Figure 2F) or cytohesin-1 (Figure S3D). However, although we did not observe a significant decrease in Pyk2 activation (9%), we observed a significant decrease in the association of activated Pyk2 with LFA-1 (>85%) (Figure 2F).
Because we did not observe a significant inhibition of adhesion on ICAM-1 with the loss of hDia1, we did not assay LFA-1-mediated signaling. On Fn, loss of hDia expression did not inhibit Lyn (Figure 2G) Pyk2 or PI3K activation (Figure S3E) and only moderately inhibited Vav1 activation (15%) (Figure 2G). In addition, loss of hDia1 expression did not disrupt association of talin or Pyk2 with VLA 4 (Figure S3E).
Rho-family GTPases are critical for the actin assembly needed for integrin-mediated adhesion, so we examined the role of Arp2/3 and hDia1 in Rho-family GTPase activation. Control primary NK cells adhered to ICAM-1 showed activation of Rac1, Cdc42 and Rap1 that began at 5 min and peaked at 10 min post adhesion, based on CRIB-pulldown assays (Figure 2E). Arp2/3 knockdown diminished Rac1 (42%) and Cdc42 (61%) activation at 10 min while Rap1 activation was largely unaffected (11%). We next examined the role of hDia1-mediated actin assembly in Rho-family GTPase activation in NK cells on Fn. In control primary NK cells on Fn, activation of Rho, Rac and Cdc42 was observed within 5 min and peaked at 10 min (Figure 2G and S3E). The loss of hDia1 did not diminish the activation of Rac1 (Figure S3E); however, the activation of Cdc42 (66%) and RhoA (72%) was inhibited at 10 min (Figure 2G) and this inhibition was rescued with re-expression of hDia1.
Arp2/3 and hDia1 are required for chemotaxis
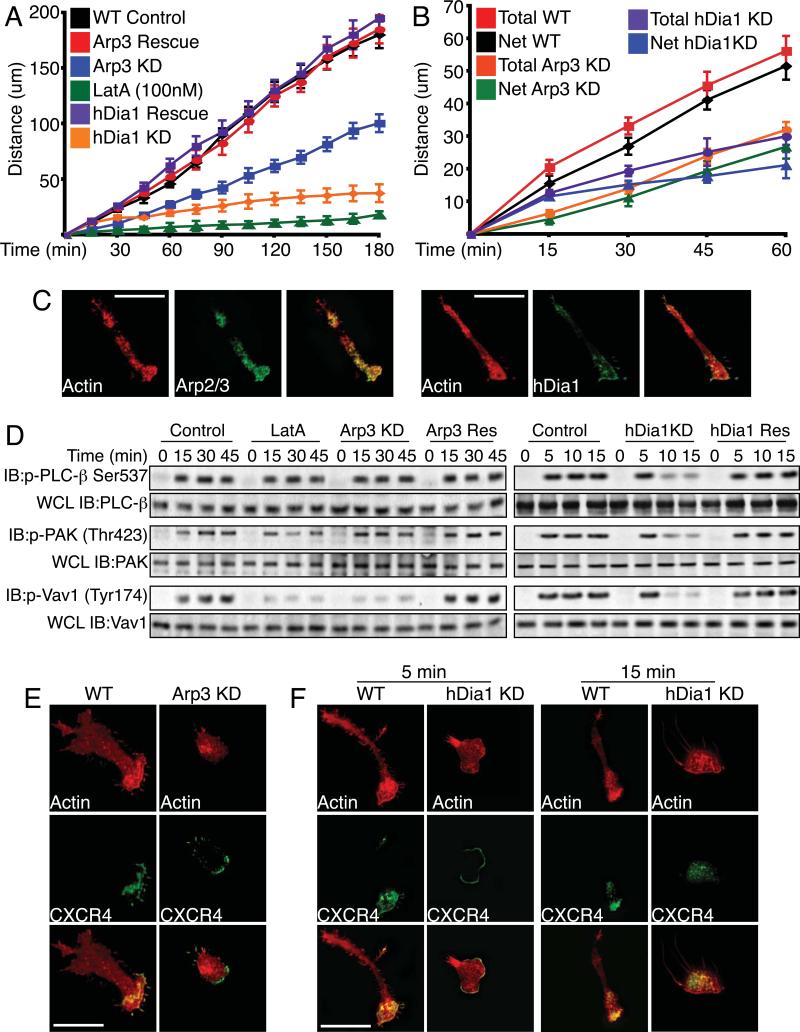
Following extravasation, NK cells chemotax towards sites of inflammation or tumorigenesis. To assay chemotaxis, we examined primary NK cells on Fn in the presence of an SDF-1α gradient and quantified the rate of migration. Treatment of primary NK cells with Lat A completely disrupted the ability of the cells to migrate. Arp2/3-knockdown cells showed a significantly slower rate (0.22 μm/min) of migration compared to WT cells (0.4μm/min) (Figure 3A). This defect was rescued with re-expression of Arp3. We observed no increase in the rate of migration as the cells migrated closer to the chemotactic source. hDia1-knockdown cells also showed a significantly slower rate of migration compared to control cells (Figure 3A). The rate of migration (0.21μm/min) up to 30 min was similar to that of Arp2/3-knockdown cells (0.18μm/min); however, after 30 min the rate of migration decreased (0.11μm/min) for the remainder of the assay.
Figure 3. Arp2/3 is required for efficient chemotactic cell migration.
(A) Chemotaxis assays. Primary NK cells were allowed to adhere to Fn-coated glass bottom dishes on one side of the dish. SDF-1α was placed on the opposite side of dish in an agarose pellet. At indicated times the distance that the cell front migrated was determined by crystal violet staining. Plotted are means ± SEM for at least three separate experiments. (B) Cells were treated as in (A), except that live time-lapse images of cells were taken every 30 seconds. Individual cell tracks were plotted over time. Total displacement was determined as the total path length traveled between time points independent of direction. Net displacement was determined as the single vectorial displacement over time, from beginning to end. Plotted are means ± SEM for 100 cells. (C) Localization of Arp2/3 and hDia1 during chemotaxis. Cells were treated as in (A). At 15 min, cells were fixed and stained with Alexa Fluor 568 phalloidin and anti-Arp3 or anti-hDia1 and imaged for fluorescence. (D) Activity of signaling proteins. Primary cells adhered to Poly-L-Lysine were treated with SDF-1α as in (A). Cell lysates were subjected to SDS-PAGE and immunoblotting using indicated mAbs. Totals were determined using 1/10 whole cell lysates (WCL) run separately and probed with indicated Abs. (MW: PLC-β 130kDa; PAK1 65kDa; Vav1 95kDa) (E and F) Localization of SDF-1α receptor during chemotaxis. Primary NK cells were treated as in (A). At 15 min for Arp3 and 5 and 15 min for hDia1, cells were fixed, stained with Alexa Fluor 568 phalloidin and anti-CXCR4 and imaged for fluorescence.
In order to determine if Arp2/3 or hDia1-deficient NK cells are able to migrate in a persistent direction and we determined that NK cells lacking Arp2/3 or hDia1 expression were as efficient in maintaining persistence of movement as WT cells (Figure 3B).
During chemotaxis both Arp2/3 and hDia1 were localized at the leading edge. Arp2/3 also localized centrally within the cell, while hDia1 displayed additional localization at the rear of the cell (Figure 3C).
Chemokine (SDF-1α ligation of its receptor (CXCR4) results in the activation of multiple signaling pathways, including the phosphorylation and activation of phospholipase-C-β (PLCβ), PAK1 and PI3K. NK cells were adhered to PLL instead of Fn to remove integrin-mediated signaling and exposed to SDF-1α. Loss of Arp2/3 did not disrupt PLCβ (11%), PAK1 (6%) (Figure 3D) and PI3K (8%) (Figure S4A) activation but Vav1 activation was greatly diminished (85%) (Figure 3D). In contrast, the loss of hDia1 disrupted PLCβ, PAK1 and PI3K activation at 10min, although the initial activation at 5 min was unaffected (Figure 3D)
We next examined the role of Arp2/3 or hDia1 in CXCR4 localization. In control NK cells, CXCR4 was localized at the leading edge and highly co-localized with F-actin (Figure 3E). While not as spread, Arp2/3-knockdown cells were still oriented towards the SDF-1α, and CXCR4 was still localized in the direction of migration (Figure 3E). However, we observed CXCR4 localization at the rear of the cell in Arp2/3-knockdown cells. Cell spreading and CXCR4 localization was restored with the re-expression of Arp3 (Figure S4B).
Because SDF-1α-mediated signaling was disrupted with the loss of hDia1 after 5 min, we evaluated CXCR4 localization at 5 and 15 min and observed that, unlike Arp2/3-knockdown cells, the CXCR4 receptor was not localized to the leading edge at 15 min but was localized within the interior of the cell (Figure 3F). Localization of the CXCR4 receptor was restored with re-expression of hDia1 (Figure S4B).
Lytic synapse architecture is disrupted with loss of Arp2/3
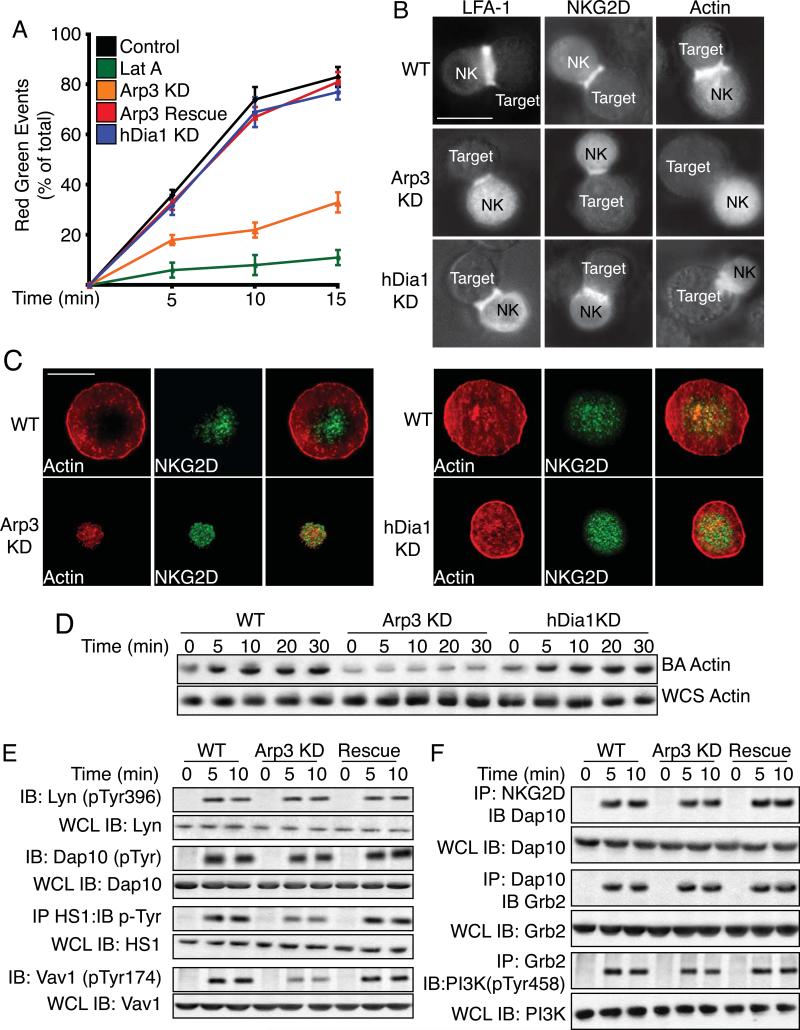
Upon reaching a site of inflammation or tumorigenesis, an NK cell interacts with potential target cells to form a stable lytic synapse (LS). The LS creates a tight seal between the NK and target cell that facilitates the transfer of lytic granules. Therefore, we evaluated the ability of Arp2/3 or hDia1 knockdown cells to form stable LSs. LatA treatment abolished stable conjugate formation (Figure 4A) and Arp3-knockdown cells formed far fewer stable cell conjugates (p<.001) while hDia1-knockdown cells were unaffected.
Figure 4. Arp2/3 is required for stable lytic synapse formation.
(A) Time course of cell-cell conjugate formation by primary NK cells. Target K562 cells were stained with the red dye PKH26 and incubated with primary NK cells expressing GFP-actin for indicated times at 37°C. The percentage of red-green events as compared to total events was determined by two-color flow cytometry. Plotted are means ± SEM for at least three separate experiments. (B) Primary NK cells expressing or not expressing Arp2/3 or hDia1 were incubated with target K562 cells for 15 min at 37°C. For actin fluorescence, cells were expressing GFP-actin. For LFA-1 and NKG2D localization, cells were fixed and stained with fluorescent mAbs. Representative images of fixed cells are shown. (C) Morphology and co-localization. Primary NK cells were allowed to adhere to ICAM-1 and ULBp-coated glass bottom dishes. Cells were fixed, stained for F-actin with Alexa Fluor 568 phalloidin, specific antibodies, and imaged for fluorescence. (D) Actin recruitment to the LS. Primary NK cells were incubated with Dynabeads coated with ICAM-1 and ULBp. At indicated times, magnetically restrained beads were pulse sonicated and then subjected to SDS-PAGE and actin was immunoblotted (BA: Bead Associated). Total actin was blotted from whole cell sonicates (WCS) run separately. (MW: Actin 42kDa) (E) Activation of signaling proteins. Primary NK cells were allowed to adhere to dishes coated with ICAM-1 and ULBp for indicated times at 37°C. For DAP10 and HS1, cell lysates were immunoprecipitated with anti-DAP10 or anti-HS1 and probed with anti-pY 4G10. For other proteins, immunoblots of cell lysates were probed with phospho-specific Abs. Lysate totals are from 1/10 whole cell lysate (WCL) run separately. (MW: Lyn 26kDa; Dap10 39 kDa; HS1 75kDa; Vav1 95 kDa) (F). Association of proteins with the NKG2D receptor complex. Primary NK cells were incubated with Dynabeads coated with ICAM-1 and ULBp; lysates were immunoprecipitated with anti-NKG2D, anti-DAP10 or anti-Grb2 and immunoblotted with mAbs for indicated proteins. Whole cell lysate (WCL) totals are from 1/10 total lysates run separately. (MW: Dap10 39kDa; Grb2 28kDa; PI3K 85kDa)
NK cells stably attach to potential target cells by engagement of the integrin LFA-1 and activating NK receptors, including NKG2D, so we assessed the ability of LFA-1 and NKG2D to localize to the LS. For Arp2/3-knockdown cells, LFA-1 and NKG2D were localized to the LS, but actin was not (Figure 4B). In addition, the diameter of the LS, using LFA-1 fluorescence, was significantly smaller, 2.3μm, in Arp2/3-knockdown cells as compared to 4.8μm in control cells or 4.1μm in Arp2/3 rescue cells (Figure S5A). We observed no loss of actin, NKG2D, or LFA-1 LS localization with hDia1-knockdown cells (Figure 4B) and LSs were the same diameter as control cells (data not shown).
NK cells were then evaluated for fluorescence intensity of proteins localized to the LS (Figure S5B). In control cells, 47% of the fluorescent actin was localized to the LS, compared to 9% in Arp2/3-knockdown cells. In control cell conjugates, 67% of the LFA-1 and 76% of the NKG2D fluorescence intensity was localized to the LS as compared to 31% and 36% of the LFA-1 and NKG2D fluorescence in cells lacking Arp2/3 expression, respectively.
We next examined LS organization by TIRF microscopy on ICAM-1 and ULBp. Actin staining formed a cortical ring with less staining within the central region in a percentage of cells (32%) (Figure 4C). LFA-1 staining formed a peripheral ring that began just inside of the cortical F-actin ring (Figure S5C), while NKG2D localized centrally within the cell (Figure 4C). Upon depletion of Arp2/3 and we observed punctate LFA-1 and NKG2D staining over the entire LS (Figure 4C and S5C). Localization of NKG2D and LFA-1 was restored with re-expression of Arp3 (Figure S5C). In contrast, loss of hDia1 expression did not disrupt LFA-1 (Figure S5C) or NKG2D (Figure 4C) localization, even though hDia1-knockdown cells were not as spread as control cells. In addition, using bead assays, we observed a significant loss of actin accumulation at the LS with the loss of Arp2/3 (88% at 10min) (Figure 4D) but not with the loss of hDia1 expression. Together, these data suggest that Arp2/3 plays a major role in actin assembly at the LS, while hDia1 does not and effects on the LS cannot account for the decrease in NK-mediated cytotoxicity displayed by hDia1-knockdown cells.
Because knockdown of Arp2/3 impaired F-actin localization to the LS, we investigated the protein composition of the LS. Using the ICAM-1/ULBp bead assay, we observed that actin and several actin regulatory proteins localized to the LS in a specific temporal fashion (Figure S5D. In Arp2/3-deficient NK cells, recruitment of actin, CP, WASp, and cofilin were all impaired. The low levels of recruitment of these proteins throughout the assay are indicative of multiple transient interactions occurring.
Arp2/3 and NK receptor Signaling
We next investigated whether Arp2/3 was important for signaling pathways downstream of NK receptors required for cytolysis. LS localization NKG2D still occurred in Arp2/3-knockdown cells. Using ICAM-1/ULBp coated beads we observed that activation of early signaling events, including Lyn (7%) and Dap10 (9%) was unaffected in Arp2/3-knockdown cells at 10 min (Figure 4E). However sustained activation of HS1 (37%) and Vav1 (82%) was inhibited at 10 min by the loss of Arp2/3, and was restored by re-expression of Arp3.
Recruitment of signaling molecules including Dap10 (8%) and Grb2 (18%) to the NKG2D receptor was largely unaffected by the loss of Arp2/3 (Figure 4F). However, the association of PI3K with Grb2 was moderately decreased at 10 min (38%) with the loss of Arp2/3, and this defect was rescued with re-expression of Arp3.
We next examined RhoGTPase signaling which is downstream of the GEF Vav1 and critical for NK-mediated cytolysis. Using ICAM-1/ULBp coated beads we observed that in control NK cells Rac1 and Cdc42 activation started within 5 min and peaked at 15 min (Figure S5E). Knockdown of Arp2/3 diminished the sustained activation of both Rac1 and Cdc42, and this defect was rescued by expression of Arp3.
Effect of hDia1 on NK-mediated cytotoxicity is regulated through MT dynamics
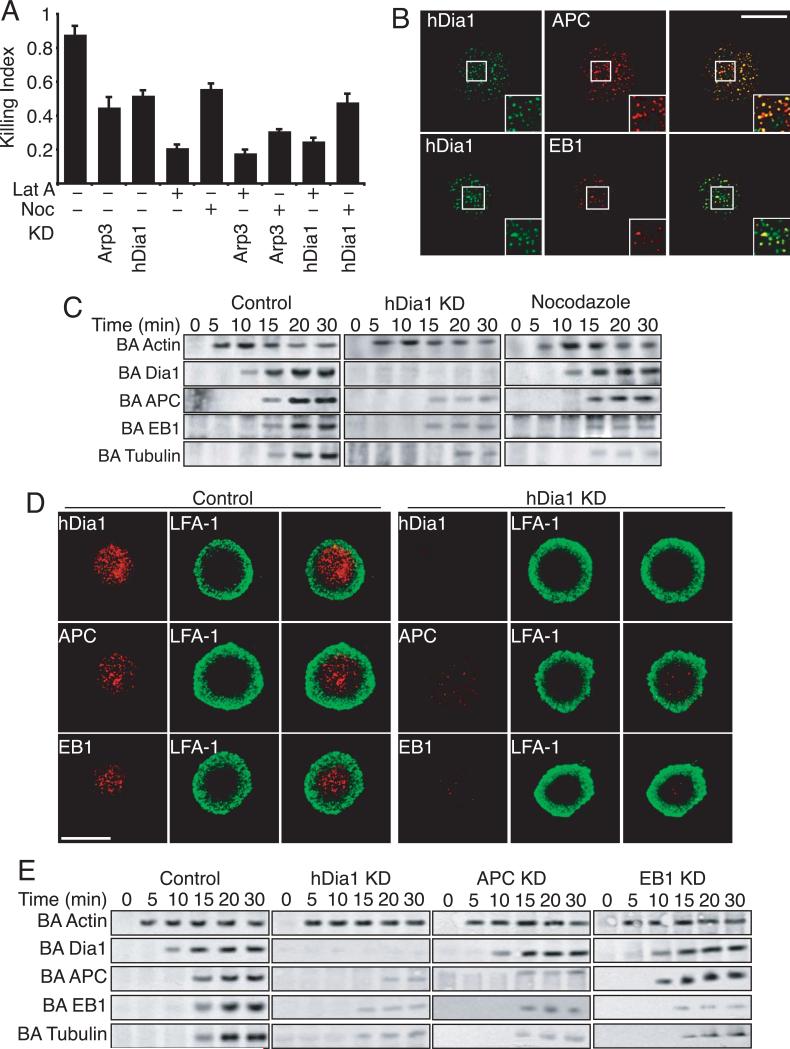
Microtubule (MT) dynamics play a critical role in the movement and release of lytic granules in NK cells and cytoxic T lymphocytes (CTLs) [11, 24]. Formins, including mDia1 and mDia2, can interact with and regulate MT dynamics in a number of cell types including T cells [15, 16, 25]. To determine whether the role that hDia1 is playing in NK-mediated cytotoxicity is through the regulation of MT dynamics, we first performed chemical epistasis experiments. In addition to the knockdown of Arp2/3 or hDia1, we treated primary NK cells with LatA or nocodazole (Noc) to disrupt the actin or MT cytoskeleton, respectively. The loss of either Arp2/3 or hDia1, in conjunction with Lat A treatment, inhibited NK-mediated cytolysis to the same extent as Lat A alone (Figure 5A). In contrast, Arp2/3-knockdown cells treated with Noc were inhibited more (p<.01) than with Arp2/3-knockdowns or Noc treatment alone, indicating that Arp2/3-mediated actin assembly and MT dynamics are acting in separate pathways. In contrast, hDia1-knockdown cells treated with Noc did not show enhanced inhibition of cytolysis, suggesting that hDia1 is regulating cytotoxicity in a pathway that includes MTs.
Figure 5. hDia1 regulates microtubule localization to lytic synapse.
(A) Chemical epistasis. Primary NK cells were incubated with target K562 cells for 4 hours at 37°C. The killing index, based on release of adenylate kinase into the media, is plotted. Control, Arp2/3 or hDia1-knockdown cells were incubated with Latrunculin A (Lat A) to disrupt the actin cytoskeleton or nocodazole (Noc) to disrupt MTs. Plotted are means ± SEM for at least three separate experiments. (B) Co-localization of hDia1 with APC and EB1. Primary NK cells were allowed to adhere to ICAM-1/ULBp-coated glass bottom dishes for 15 min at 37°C. Cells were fixed and stained with indicated antibodies and imaged for fluorescence. (C) Recruitment of MTs and associated proteins to with the LS. Primary control, hDia1-knockdown or Nocodazole-treated cells were incubated with ICAM-1/ULBp-coated Dynabeads for indicated times. While magnetically restrained, beads were pulse sonicated. Beads were then subjected to SDS-PAGE and immunoblotted with indicated Abs. (MW: Actin 42kDa; hDia1 198kDa; kDa; APC 310kDa; EB1 29kDa; Tubulin 55kDa) (D) LS localization of APC and EB1 requires hDia1. Control or hDia1-knockdown primary NK cells were allowed to adhere to ICAM-1/ULBp coated glass bottom dishes in the presence of SDF-1α for 15 min at 37°C. Cells were fixed and stained with indicated antibodies and imaged for fluorescence. (E) Recruitment of MT associated proteins with the LS over time. Control, hDia1, APC or EB1-knockdown primary NK cells were incubated with ICAM-1/ULBp coated Dynabeads for indicated times and while magnetically restrained beads were pulse sonicated. Beads were then subjected to SDS-PAGE and immunoblotted with indicated Abs. Shown are representative blots from at least three separate experiments. (MW: Actin 42kDa; hDia1 198kDa; kDa; APC 310kDa; EB1 29kDa; Tubulin 55kDa)
MTs are dynamic structures that undergo regulated assembly and disassembly. Stable MTs can be found localized at the leading edge of migrating cells, apparently captured at their plus ends. EB1 and APC bind to the plus ends of MTs and stabilize them [26]. mDia1 and mDia2 can bind to EB1 and APC, as well as to CLIP170, acting in concert with these proteins to stabilize MTs at the leading edge of migrating cells [17, 27]. We therefore asked what role hDia1, EB1 and APC might play in regulation of the MT cytoskeleton during cytolysis.
First, we visualized the LS by TIRFM with NK cells plated on ICAM-1/ULBp. We observed that hDia1 co-localized with both APC and EB1 (Figure 5B) to a high degree. At high magnification (Fig. 5B, inset) some foci contained APC or hDia1 alone, but EB1 and hDia1 foci were almost completely overlapping. We observed that 68% (100 cells) of all APC foci were co-localized with hDia1 while 88% (100 cells) of all EB1 foci were co-localized with hDia1.
We next examined MT-related protein recruitment using bead assays with primary NK cells. hDia1 was recruited to the beads within 10 min and this interaction persisted for at least 30 min (Figure 5C). APC, EB1 and tubulin were recruited to the beads at 15 min, and persisted for at least 30 min. The loss of hDia1 led to a loss (>90%) of recruitment of the MT-associated proteins EB1 and APC (Figure 5C). In contrast, Noc treatment did not lead to a loss of APC recruitment, but bead-associated EB1 and tubulin were significantly reduced (>85%).
To examine EB1 and APC recruitment further, we visualized the LS by TIRFM in NK cells plated on ICAM-1/ULBp. hDia1 was abundant at the cell / substrate interfaces and localized within the peripheral ring of LFA-1 (Figure 5D). EB1 and APC localization were similarly restricted inside the ring of LFA-1. In hDia1-knockdown cells the formation of the LFA-1 ring was unaffected; however, the localization of both EB1 and APC within the ring of LFA-1 was greatly reduced.
To further evaluate the hierarchy of recruitment to the LS, we knocked down APC and EB1 (Figure S6A and B). Loss of either APC or EB1 inhibited NK-mediated cytotoxicity to the same degree as the loss of hDia1 (Figure S6C). Second, in bead assays, hDia-knockdown cells displayed a significant loss in bead-associated APC, EB1 and tubulin. In contrast, the knockdown of APC led to a loss of EB1 and tubulin recruitment but not that of hDia1 while the knockdown of EB1 expression only decreased tubulin recruitment (Figure 5E). Taken together these data indicate that recruitment of hDia1 to the LS is required for the subsequent recruitment of APC, which is necessary for recruitment of EB1 and MTs.
Dia1 is required for lytic granule polarization and secretion
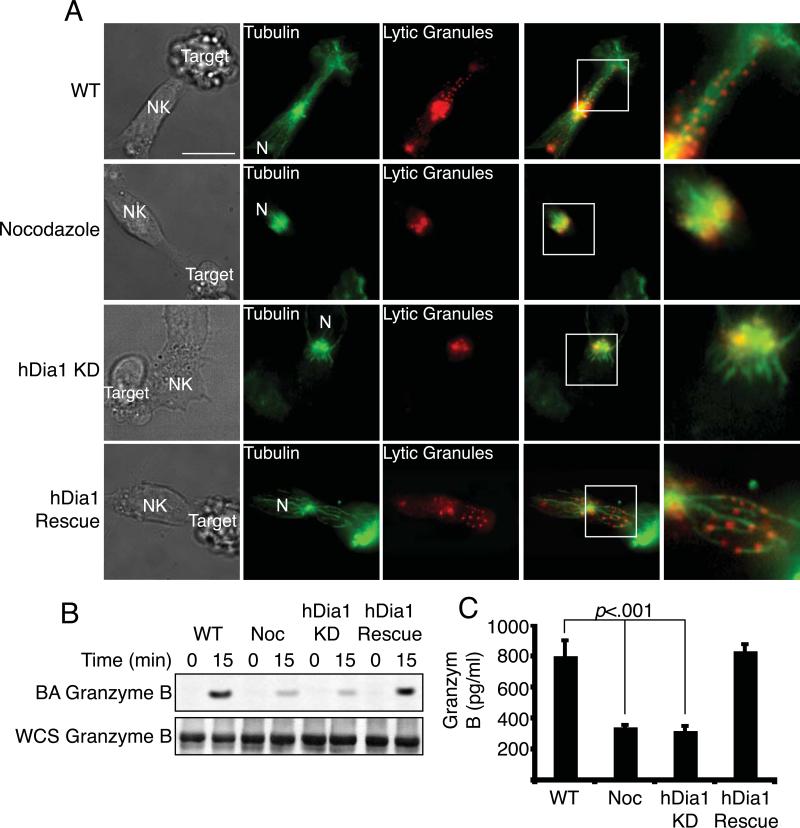
The final stage in NK-mediated cytotoxicity is the polarization and exocytosis of lytic granules towards the target cell, allowing for the release of cytolytic enzymes including perforin and granzyme B. We evaluated how MT-dependent lytic granule localization and release depend on hDia1. In control cells, the lytic granules were localized along MTs and directed towards the target cell (Figure 6A). Treatment with Noc completely disrupted MTs, and lytic granules were found only at the MTOC. In hDia1-knockdown cells, we observed only short MTs radiating from the MTOC that did not contact the LS and lytic granules remained localized at the MTOC, however the MTOC was still polarized in front of the nucleus (N). This defect was rescued with re-expression of hDia1.
Figure 6. hDia1 regulates lytic granule localization and release.
(A) MT and lytic granule localization. Control, hDia1-knockdown, Noc treated or hDia1-rescue primary NK cells were incubated with Lysotracker Red (red panels) to stain lytic granules and then incubated with target K562 cells for 30 min at 37°C while cells were adhered to Fn. Cells were fixed and stained for tubulin (Alexa 488 anti-tubulin, green panels) and imaged for fluorescence. (N) denotes nucleus of NK cell. (B) Lytic granule recruitment to the LS. Control, hDia1-knockdown, Noc-treated or hDia1-rescue primary NK cells were allowed to adhere to ICAM-1/ULBp coated Dynabeads for 15 min at 37°C. While magnetically restrained, the beads were pulse sonicated. Beads were then subjected to SDS-PAGE and immunoblotted with anti-Granzyme B. Whole cell sonicate (WCS) was run separately and probed with anti-granzyme B. (MW: Granzyme B 37kDa) (C) Release of lytic granule contents into the medium. Control, Noc-treated, hDia1-knockdown or hDia1-rescue primary NK cells were allowed to adhere to ICAM-1/ULBp-coated 96-well plates for 1 hour at 37°C. Supernatant was collected, and Granzyme B was measured by ELISA. Shown are means ± SEM for three separate experiments.
Next, we asked whether lytic granules arrived at the LS with a biochemical assay. Using ICAM-1/ULBp-coated Dynabeads we observed that Granzyme B was recruited to the beads in control NK cells, but not in Noc-treated or hDia1-knockdown cells (Figure 6B). This defect was rescued with re-expression of hDia1. In addition, we assayed for the release of granzyme B into the medium by ELISA. hDia1-knockdown and Noc-treated NK cells released less Granzyme B than did control cells, again rescued with re-expression of hDia1 (Figure 6C).
We next examined MT stability by staining for postranslational modifications. Stabilized MTs have elevated levels of detyrosinated α-tubulin (i.e. Glu MTs), as well as acetylated MTs [28-30]. We observed a dramatic decrease in the amount of both forms of modified tubulin in hDia1-knockdown cells, compared to control cells (Figure S7A and B). Taken together, these data indicate that hDia1 plays a critical role in NK-mediated cytotoxicity, and that hDia1 acts through the targeting and stabilization of MTs, via APC and EB1.
DISCUSSION
Two major classes of actin nucleators, the Arp2/3 complex and formins, promote actin assembly in cells. Here, we sought to understand their individual roles, including how their roles relate to cell signaling. Our study made several important discoveries. First, Arp2/3 is necessary for many of the processes essential to NK cell function, including adhesion to specific ligands, chemotaxis, and lytic synapse assembly. Second, while many of the early steps of receptor-based signaling are independent of Arp2/3, the response of Vav1 and small GTPases and Vav1 do depend on Arp2/3-mediated actin assembly, suggesting the existence of positive feedback regulation. Third, the formin hDia1 is not required for stable lytic synapse formation, but is required to capture dynamic MTs, which is necessary to target lytic granules.
Adhesion
The first steps in NK cell function are based on adhesion, to vascular smooth muscle cells during extravasation or then to extracellular matrix during chemotaxis. We observed that the loss of Arp2/3 or the formin hDia1 inhibited adhesion and spreading on specific ligands in distinct ways. On ICAM-1 and Fn, the loss of Arp2/3 inhibited adhesion, spreading and proper localization of the integrins LFA-1 and VLA-4. Arp2/3 is critical for generating the protrusive forces needed to change cell shape and generate lamellipodia in fibroblasts [31-33] and T cells [34]. Therefore, we were not surprised to find that the loss of Arp2/3 led to defects in cell adhesion and spreading, including the loss of lammellipodial protrusions. In addition, the loss of Arp2/3 also led to the formation of numerous filopodia. The loss of hDia1 led to an inhibition of adhesion on Fn but not ICAM-1. The hDia1-knockdown cells did display less cell spreading on either ICAM-1 or Fn. Formins, in contrast to Arp2/3, bind to the barbed end of existing actin filaments and generate long straight actin filaments that are observed in filopodia [6]. Here, the loss of hDia1 led to a decrease in filpodial and pseudopodial protrusions on Fn.
Chemotaxis
After exiting the vasculature, NK cells migrate to the site of inflammation or tumorigenesis guided by chemotactic cues. Here, Arp2/3 and hDia1 were both required for efficient chemotaxis by NK cells, but through different mechanisms. Arp2/3-deficient cells migrated more slowly, and examining the actin cytoskeleton, we found Arp2/3-knockdown cells to be defective in spreading with a decrease in lamellipodia in the direction of migration. Loss of hDia1 also led to slower migration during chemotaxis. As with the loss of Arp2/3, hDia-knockdown cells were less spread, but they maintained a leading edge in the direction of migration. The loss of hDia1 led to a loss of filopodial protrusions, similar to what we observed with the loss hDia1 during adhesion to Fn. These results are consistent with defects in chemotaxis observed in neutrophils and T cells from mDia1 knockout mice [18, 19, 35].
Signaling
NK cell biology displays a complex set of interactions between the cytoskeleton and signaling pathways. Signals from membrane receptors direct assembly of the actin cytoskeleton, but one can also imagine that the cytoskeleton helps to form a scaffold on which signaling pathways are amplified and integrated. In NK cells, signaling occurs downstream of integrin, chemokine and activating NK receptors, and these pathways are important for adhesion, chemotaxis and lytic synapse formation, respectively.
Here, we observed that the early steps of receptor-mediated signaling from integrins, chemokines and activating NK receptors were largely unaffected by the loss of Arp2/3 or hDia1. In contrast, later steps in each of these signaling pathways were inhibited. For example, in Arp2/3-knockdown cells, in terms of integrin signaling downstream of LFA-1 ligation, the activation of Lyn, Pyk2, Syk and PI3K was unaffected, while activation of the GEF Vav1 and the Rho family GTPases were inhibited. This latter result was somewhat surprising because Rho family GTPases have generally been considered to be “upstream” of and therefore required for the activation of formins and of WASp/Wave proteins, which activate Arp2/3. However, such a result is not without precedent because the loss of mDia1 or mDia2 has been observed to inhibit Rho and / or Cdc42 in neutrophils and glioma cells [19, 36]. With regard to chemokine-based signaling, the loss of hDia1 significantly disrupted downstream elements of the signaling pathway as well as proper localization of the chemokine receptor. Both of these observations are in contrast to what was observed for loss of Arp2/3. Thus, the cellular actin structures that result from these different actin nucleators may be responsible for distinct functions during chemotaxis. Alternatively, the loss of hDia1 may mediate this function by some process other than actin assembly including MT based localization of adhesive and signaling proteins to the leading edge.
Taken together, the signaling pathway results here suggest that actin assembly is not merely the effector of the signaling cascades that operate during NK cell function, but that actin assembly also helps facilitate signaling pathways in a dynamic feedback loop. This general conclusion was also reached in our previous study of the role of the cortactin homologue HS1 in NK cells [37].
Lytic Synapse Formation and Cytolysis
Once at the site of inflammation or tumorigenesis, an NK cell interacts with potential target cells and, if appropriate, forms a lytic synapse (LS), which requires regulated actin assembly. Here, we observed that actin accumulated at the cell-cell contact zone within minutes of contact between an NK cell and a potential target cell and that actin assembly persisted at the LS for up to 30 min. The localization of Arp2/3 and hDia1 were quite distinct, both spatially and temporally. Arp2/3 appeared at the LS early, coincident with actin accumulation, while hDia1 did not appear at the LS until late. Arp2/3 recruitment decreased over time, while that of hDia1 did not. In addition, utilizing TIRF microscopy, Arp2/3 and hDia1 localized to distinct areas of the LS, with Arp2/3 concentrated at the cortex and in peripheral zone while hDia1 was in a non-overlapping central zone.
NK cells are innate immune cells and they interact with and kill target cells without the need for prior activation. This is due in part to the constitutive surface expression of both activating NK receptors as well as integrins. Upon contact with potential target cells these receptors become engaged and help initate actin cytoskeletal reorganization required for stable LS formation and cytotoxicity. Partitioning of the NK receptors to the central core of the LS and the integrins to the periphery requires Arp2/3-mediated actin assembly, as knockdown of Arp2/3 led to a loss of actin accumulation at the LS and a dramatic loss in the formation of stable NK/target cell conjugates. Proper recruitment and localization of actin associated proteins, as well as signaling and adhesion proteins, to the LS, were also impaired. In contrast, stable NK/target cell conjugates formed, and actin and actin-associated proteins were recruited normally in hDia1-knockdown cells. This result is consistent with observations in T cells, where the loss of mDia1 did not disrupt actin accumulation at the immune synapse [16]. Because hDia1 did not appear to be regulating NK-mediated cytotoxicity through actin assembly during lytic synapse formation, we examined MTs and lytic granules.
hDia1 and Microtubules
MT dynamics are known to play a critical role in NK-mediated cytotoxicity [24]. Formins can interact with and regulate MT dynamics in a number of cell types [15, 16, 25]. Of note, the actin-nucleating activity of mDia2 is not required for its association with MTs [15]. Therefore, we considered whether hDia1 might have an effect on MTs here, independent of actin. Indeed, we observed that the loss of hDia1 disrupted the ability of the LS to capture MTs and to recruit the MT-associated proteins APC and EB1 to the lytic synapse. During LS formation, hDia1 was recruited first, before the MT-associated proteins. In addition, the presence of hDia1 at the LS was required for the subsequent recruitment of APC, EB1, and MTs. Knockdown of APC did not affect hDia1 recruitment but did inhibit EB1 and MT recruitment. Knockdown of EB1 disrupted MT recruitment to the lytic synapse, without effects on hDia1 or APC. Thus, hDia1 appears to recruit APC, which then recruits EB1 and captures MTs. How hDia1 is recruited to the LS remains an open question.
The details of the method by which cytolytic granules are secreted onto the target cells is largely unknown. In cytotoxic T lymphocytes, which are similar to but distinct from NK cells, the MTOC becomes intimately associated with the lytic synapse [12], and granules are closely associated with the MTOC. Thus, the granules appear to have a very short distance to travel in order to fuse with the plasma membrane near the target cell. Here, in striking contrast, we did not observe intimate contact between the MTOC and the target cell. In control cells, the MTOC was an appreciable distance away from the LS, and MTs were directed from the MTOC towards the LS. Cytolytic granules were distributed along these MTs, consistent with the granules using the MTs as tracks for polarized secretion. These MTs oriented toward the LS were stained with markers for stabilized MTs, confirming that the MT plus ends are captured at the LS. In contrast, in hDia1-knockdown NK cells, MTs did not appear to be captured by the LS. The MTs were short and did not extend very far away from the MTOC. Accordingly, the lytic granules remained near the MTOC, along the MTs. The MTOC was generally still oriented towards the target cell, which may be a finding distinct for NK cells, in that T cells with loss of mDia1 exhibited loss of polarity of the MTOC [16].
While a difference in cell types, namely T cell and CTL versus NK cell, may explain the differences in our results here compared to others in the literature [12, 38], another alternative explanation may be the way in which the assays were performed. We visualized NK cells in contact with target cells on a surface and in the context of an adhesive environment. The NK cells were required to migrate along the surface to find a target cell. In contrast, previous studies have used cells in suspension, centrifuging them together to bring them in contact.
Taken together, our results demonstrate that Arp2/3 and the formin hDia1 contribute in different ways to the variety of NK cell functions. In addition, Arp2/3 and formin hDia1 are not merely downstream effectors of signaling cascades, but instead appear to contribute, in distinct ways, to the propagation and maintenace of signaling. The fact that both Arp2/3 and formins appear to be necessary for the activity of Rho-family GTPases but can be activated by Rho-family GTPases suggests that positive feedback loops may be critical to the activation and maintenace of both actin assembly and signaling pathways.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by NIH grants GM 38542 and NRSA AI071429. We acknowledge the Siteman Cancer Center High Speed Sorter Core Facility, which is supported in part by NCI grant P30 CA91842.
LITERATURE CITED
- 1.Higgs HN, Pollard TD. Regulation of actin filament network formation through ARP2/3 complex: activation by a diverse array of proteins. Annu Rev Biochem. 2001;70:649–676. doi: 10.1146/annurev.biochem.70.1.649. [DOI] [PubMed] [Google Scholar]
- 2.Pollard TD. Regulation of actin filament assembly by arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–477. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- 3.Li F, Higgs HN. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr Biol. 2003;13:1335–1340. doi: 10.1016/s0960-9822(03)00540-2. [DOI] [PubMed] [Google Scholar]
- 4.Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- 5.Yang C, Czech L, Gerboth S, Kojima S, Scita G, Svitkina T. Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 2007;5:e317. doi: 10.1371/journal.pbio.0050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9:1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- 7.Orange JS, Ramesh N, Remold-O'Donnell E, Sasahara Y, Koopman L, Byrne M, Bonilla FA, Rosen FS, Geha RS, Strominger JL. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci U S A. 2002;99:11351–11356. doi: 10.1073/pnas.162376099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orange JS, Harris KE, Andzelm MM, Valter MM, Geha RS, Strominger JL. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc Natl Acad Sci U S A. 2003;100:14151–14156. doi: 10.1073/pnas.1835830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirwan SE, Burshtyn DN. Regulation of natural killer cell activity. Curr Opin Immunol. 2007;19:46–54. doi: 10.1016/j.coi.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Carpen O, Virtanen I, Lehto VP, Saksela E. Polarization of NK cell cytoskeleton upon conjugation with sensitive target cells. J Immunol. 1983;131:2695–2698. [PubMed] [Google Scholar]
- 11.Burkhardt JK, McIlvain JMJ, Sheetz MP, Argon Y. Lytic granules from cytotoxic T cells exhibit kinesin-dependent motility on microtubules in vitro. J Cell Sci. 1993;104:151–162. doi: 10.1242/jcs.104.1.151. [DOI] [PubMed] [Google Scholar]
- 12.Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- 13.Ishizaki T, Morishima Y, Okamoto M, Furuyashiki T, Kato T, Narumiya S. Coordination of microtubules and the actin cytoskeleton by the Rho effector mDia1. Nat Cell Biol. 2001;3:8–14. doi: 10.1038/35050598. [DOI] [PubMed] [Google Scholar]
- 14.Eng CH, Huckaba TM, Gundersen GG. The formin mDia regulates GSK3beta through novel PKCs to promote microtubule stabilization but not MTOC reorientation in migrating fibroblasts. Mol Biol Cell. 2006;17:5004–5016. doi: 10.1091/mbc.E05-10-0914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartolini F, Moseley JB, Schmoranzer J, Cassimeris L, Goode BL, Gundersen GG. The formin mDia2 stabilizes microtubules independently of its actin nucleation activity. J Cell Biol. 2008;181:523–536. doi: 10.1083/jcb.200709029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gomez TS, Kumar K, Medeiros RB, Shimizu Y, Leibson PJ, Billadeau DD. Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse. Immunity. 2007;26:177–190. doi: 10.1016/j.immuni.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, Wallar BJ, Alberts AS, Gundersen GG. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol. 2004;6:820–830. doi: 10.1038/ncb1160. [DOI] [PubMed] [Google Scholar]
- 18.Eisenmann KM, West RA, Hildebrand D, Kitchen SM, Peng J, Sigler R, Zhang J, Siminovitch KA, Alberts AS. T cell responses in mammalian diaphanous-related formin mDia1 knock-out mice. J Biol Chem. 2007;282:25152–25158. doi: 10.1074/jbc.M703243200. [DOI] [PubMed] [Google Scholar]
- 19.Shi Y, Zhang J, Mullin M, Dong B, Alberts AS, Siminovitch KA. The mDial formin is required for neutrophil polarization, migration, and activation of the LARG/RhoA/ROCK signaling axis during chemotaxis. J Immunol. 2009;182:3837–3845. doi: 10.4049/jimmunol.0803838. [DOI] [PubMed] [Google Scholar]
- 20.Takeya R, Taniguchi K, Narumiya S, Sumimoto H. The mammalian formin FHOD1 is activated through phosphorylation by ROCK and mediates thrombin-induced stress fibre formation in endothelial cells. EMBO J. 2008;27:618–628. doi: 10.1038/emboj.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gasteier JE, Schroeder S, Muranyi W, Madrid R, Benichou S, Fackler OT. FHOD1 coordinates actin filament and microtubule alignment to mediate cell elongation. Exp Cell Res. 2005;306:192–202. doi: 10.1016/j.yexcr.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Gasteier JE, Madrid R, Krautkramer E, Schroder S, Muranyi W, Benichou S, Fackler OT. Activation of the Rac-binding partner FHOD1 induces actin stress fibers via a ROCK-dependent mechanism. J Biol Chem. 2003;278:38902–38912. doi: 10.1074/jbc.M306229200. [DOI] [PubMed] [Google Scholar]
- 23.Eisenmann KM, Harris ES, Kitchen SM, Holman HA, Higgs HN, Alberts AS. Dia-interacting protein modulates formin-mediated actin assembly at the cell cortex. Curr Biol. 2007;17:579–591. doi: 10.1016/j.cub.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Carpen O. The role of microtubules in human natural killer cell-mediated cytotoxicity. Cell Immunol. 1987;106:376–386. doi: 10.1016/0008-8749(87)90180-8. [DOI] [PubMed] [Google Scholar]
- 25.Goulimari P, Knieling H, Engel U, Grosse R. LARG and mDia1 link Galpha12/13 to cell polarity and microtubule dynamics. Mol Biol Cell. 2008;19:30–40. doi: 10.1091/mbc.E06-11-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morrison EE. Action and interactions at microtubule ends. Cell Mol Life Sci. 2007;64:307–317. doi: 10.1007/s00018-007-6360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewkowicz E, Herit F, Le Clainche C, Bourdoncle P, Perez F, Niedergang F. The microtubule-binding protein CLIP-170 coordinates mDia1 and actin reorganization during CR3-mediated phagocytosis. J Cell Biol. 2008;183:1287–1298. doi: 10.1083/jcb.200807023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao G, Gundersen GG. Kinesin is a candidate for cross-bridging microtubules and intermediate filaments. Selective binding of kinesin to detyrosinated tubulin and vimentin. J Biol Chem. 1998;273:9797–9803. doi: 10.1074/jbc.273.16.9797. [DOI] [PubMed] [Google Scholar]
- 29.Hammond JW, Cai D, Verhey KJ. Tubulin modifications and their cellular functions. Curr Opin Cell Biol. 2008;20:71–76. doi: 10.1016/j.ceb.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schulze E, Asai DJ, Bulinski JC, Kirschner M. Posttranslational modification and microtubule stability. J Cell Biol. 1987;105:2167–2177. doi: 10.1083/jcb.105.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Machesky LM, Reeves E, Wientjes F, Mattheyse FJ, Grogan A, Totty NF, Burlingame AL, Hsuan JJ, Segal AW. Mammalian actin-related protein 2/3 complex localizes to regions of lamellipodial protrusion and is composed of evolutionarily conserved proteins. Biochem J. 1997;328:105–112. doi: 10.1042/bj3280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bailly M, Ichetovkin I, Grant W, Zebda N, Machesky LM, Segall JE, Condeelis J. The F-actin side binding activity of the Arp2/3 complex is essential for actin nucleation and lamellipod extension. Curr Biol. 2001;11:620–625. doi: 10.1016/s0960-9822(01)00152-x. [DOI] [PubMed] [Google Scholar]
- 33.Weaver AM, Karginov AV, Kinley AW, Weed SA, Li Y, Parsons JT, Cooper JA. Cortactin promotes and stabilizes Arp2/3-induced actin filament network formation. Curr Biol. 2001;11:370–374. doi: 10.1016/s0960-9822(01)00098-7. [DOI] [PubMed] [Google Scholar]
- 34.Nicholson-Dykstra SM, Higgs HN. Arp2 depletion inhibits sheet-like protrusions but not linear protrusions of fibroblasts and lymphocytes. Cell Motil Cytoskeleton. 2008;65:904–922. doi: 10.1002/cm.20312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sakata D, Taniguchi H, Yasuda S, Adachi-Morishima A, Hamazaki Y, Nakayama R, Miki T, Minato N, Narumiya S. Impaired T lymphocyte trafficking in mice deficient in an actin-nucleating protein, mDia1. J Exp Med. 2007;204:2031–2038. doi: 10.1084/jem.20062647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D'Agati V, Schmidt AM. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem. 2008;283:34457–34468. doi: 10.1074/jbc.M801465200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butler B, Kastendieck DH, Cooper JA. Differently phosphorylated forms of the cortactin homolog HS1 mediate distinct functions in natural killer cells. Nat Immunol. 2008;9:887–897. doi: 10.1038/ni.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuhn JR, Poenie M. Dynamic polarization of the microtubule cytoskeleton during CTL-mediated killing. Immunity. 2002;16:111–121. doi: 10.1016/s1074-7613(02)00262-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.