Abstract
A large number of cardiology clinical trials have mortality as an endpoint unless adequate surrogate endpoints are available. Although there are nine classes of agents used in the treatment of diabetes mellitus, none have shown a mortality benefit in clinical trials. The United Kingdom Prospective Diabetic Study was the first to suggest that metformin given for diabetes mellitus had a trend toward lowering mortality. The accidental discovery of peroxisome proliferator-activated receptors (PPARs) led to the introduction of the thiazolidinediones (TZD), a PPAR agent with a suggestion of a promise for the future. As the incidence of cardiovascular complications related to diabetes mellitus increases, there is a sense of urgency to produce antidiabetic medications that achieve not only nontoxic glycemic control but also improved cardiovascular outcomes. The goal of this review is to aid the clinician to appropriately assess the benefits and risks of TZD use when prescribing for patients.
Introduction
It is well known that microvascular disease in type 2 diabetes mellitus can be halted with aggressive glycemic control. Even with nine classes of antidiabetic agents currently on the market, only the biguanide metformin has shown a trend toward decreasing macrovascular disease. The goal so far, understandably, has been focused on glycemic control. However, with the abundance of hypoglycemic agents on the market, medications will have to be chosen to not only achieve glycemic control but also decrease cardiovascular mortality.
The thiazolidinediones (TZDs) are the first group of antidiabetic medications that attempted to scale this pinnacle of reducing cardiovascular mortality within a highly competitive arena. In July 2008, the US Food and Drug Administration (FDA) convened a meeting to discuss the question of whether there should be a requirement that any antidiabetic medications without a concerning cardiovascular safety signal during early-phase clinical trials be further studied in a long-term cardiovascular trial.1 The answer was resoundingly in the affirmative for the need to have future antidiabetic medications achieve a beneficial cardiovascular mortality profile before FDA approval is given.
TZDs are a class of medications currently approved by the FDA to treat type 2 diabetes mellitus. However, there is significant debate surrounding its safety. Troglitazone, the first TZD to be approved by the FDA to treat type 2 diabetes mellitus was withdrawn in the year 2000 because of idiosyncratic hepatotoxicity. Currently there are two TZDs on the market: rosiglitazone (Avandia) and pioglitazone (Actos). Because they improve insulin sensitivity2 and carry a low risk of causing hypoglycemia, they have been quickly incorporated into clinical practice and represent as much as 25% of total prescriptions for oral hypoglycemia medications.3 However, the TZDs—specifically, rosiglitazone—have faced a great deal of criticism because of the discovery of worrisome adverse affects. This has affected TZD prescribing patterns within Kaiser Permanente Northern California (KPNC) (Table 1). The most debated side effect is whether rosiglitazone causes heart attacks. The aim of this review is to shed light on the overall understanding of TZDs. Subsequently, we hope that it provokes a healthy discussion regarding the appropriate use and placement of TZDs (specifically, pioglitazone) within the KPNC PHASE (Prevent Heart Attack and Stroke Everyday) program.
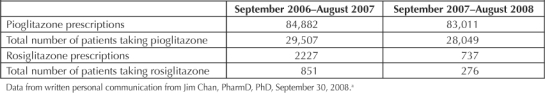
Table 1.
Crude thiazolidinedione (pioglitazone and rosiglitazone) use within Kaiser Permanente Northern California in two contiguous years
Biology of Peroxisome Proliferator–Activated Receptors
Peroxisome proliferator-activated receptors (PPARs) are a family of intracellular receptors for fatty acids and fatty-acid derivatives. Three types of PPARs are expressed in a variety of metabolic tissues: PPAR-α, PPAR-β/δ, and PPAR-γ. PPARs, unlike other receptors, are located within the cell nucleus, where they are thought to exert their effect of regulating gene transcription directly within the cell. Each receptor has unique locations and functions.
PPAR-α is expressed in metabolically active tissues such as the liver and plays a large role in lipid and lipoprotein metabolism and also in suppressing vascular and systemic inflammation. Fenofibrate and gemfibrozil are some important examples of ligands for this receptor. PPAR-δ is the most widely distributed PPAR. Its exact role is yet unclear, but it too plays a role in lipid metabolism; it also plays a role in cholesterol homeostasis in macrophages, embryo implantation, and cell proliferation. PPAR-γ is mostly expressed in adipose tissue (adipocytes). It is also found in skeletal muscle, hepatocytes, intestinal tissue, endothelial cells, cardiac muscle, the renal collecting duct, and in macrophages.
The primary role of PPAR-γ appears to be in regulating adipogenesis along with glucose and lipid metabolism. PPAR-γ is thought to enhance the actions of insulin and decrease resistance to insulin. Ligands for PPAR-γ include free fatty acids, certain prostaglandin derivatives, non-steroidal anti-inflammatory agents, and TZDs. All TZDs have varying selectivity for each PPAR receptor and thus have a variety of effects on the human body besides their primary action3–7 (Table 2).
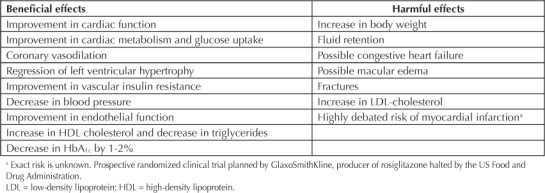
Table 2.
The beneficial and harmful effects of thiazolidinediones
Effects of Thiazolidinediones on Diabetes Mellitus, Lipids, and Adipocytes
TZDs have additional effects besides their primary role as antihypoglycemics. They typically reduce glycated HbA1c by 1% to 2% when compared with placebo. This is similar to the hypoglycemic effects of the sulfonylureas and metformin.8 They do this primarily by increasing skeletal muscle glucose uptake and less by decreasing hepatic production of glucose. They also are thought to preserve β-cell function; this effect has been shown in animal models as well as in human studies.
They have varying effects on lipid metabolism. Both TZDs increase high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol. A variation between the two TZDs has been noted in respect to their effects on LDL particle concentration and size, producing an overall shift to a larger, more buoyant LDL particle. Triglyceride levels are also decreased with both TZDs, with there being a larger decrease with pioglitazone. These effects may be related to pioglitazone's effect on hepatic PPAR-α.
TZDs also increase body weight by differentiation of preadipocytes to adipocytes and increasing adipocyte mass. Although it is known that increased levels of adiposity increase the propensity of cardiovascular risk, other features of TZDs are thought to perhaps attenuate this risk. One such example is redistribution of fat from visceral to subcutaneous depots, a pattern that is thought to be associated with decreased risk for cardiovascular disease (CVD).3 This pattern of change is also associated with increased adiponectin and decreased tissue-necrosis factor–α levels. Both are associated with favorable changes in CVD risk profile. TZDs also decrease circulating free fatty acids, with resultant favorable effects on the liver and skeletal muscle.3
Effects of Thiazolidinediones on Inflammation and Endothelial Function
Vascular inflammation is a fundamental component in the process of atherosclerosis. This process, with subsequent thrombosis, is lengthy and complicated, developing usually over decades. The final rupture of the atherosclerotic cap, with spillage of the highly thrombogenic infracap contents into the coronary vessel lumen, is the explanation for most fatal coronary thromboses. However, for coronary plaque progression to occur, continued inflammation is needed. Numerous mediators of inflammation are expressed, such as adhesion molecules and growth factors, whereas release of chemoattractants and elaboration of cytokines weaken the fibrous atherosclerotic cap. It is thought that transcription factor nuclear factor (NF)-κB mediates many of the inflammatory processes that occur during the development of atherosclerosis. Multiple studies have suggested that PPAR activation favorably modulates NF-κB action.3
TZDs also favorably affect coronary and peripheral vasodilation, along with minimally improving blood pressure. These effects are thought to be mediated by increasing endothelial release of nitric oxide, increased expression of vascular endothelial growth factor, and decreased expression of endothelin-1. TZDs also partially inhibit voltagegated L-type calcium channels. These channels are the mechanisms of action on the nondihydropyridine calcium-channel blockers. Although the effect of blood pressure reduction is minimal, epidemiologic estimates suggest that this small change may provide a significant decrease in the risk of stroke and myocardial infarctions.3
Data Favoring Thiazolidinediones (the Good or Neutral)
The Diabetes Control and Complications Trial (DCCT) conclusively demonstrated that tight glucose control in persons with type 1 diabetes significantly decreased microvascular complications such as retinopathy, nephropathy, and neuropathy.9 After a follow-up period of 7 to 9 years of 1205 persons with well-controlled type 1 diabetes who were involved in the DCCT study, the Epidemiology of Diabetes Interventions and Complications study showed decreased macrovascular complications (coronary calcification).10
A decrease in microvascular complications in persons with type 2 diabetes mellitus is thought to be backed up by reasonably strong data.11,12 Although it was shown in 1982 that intensive glycemic control decreases microvascular complications in type 1 diabetes mellitus, there is yet no conclusive proof that a current FDA-approved treatment can reduce the risk of macrovascular complications in persons with type 2 diabetes mellitus. The University Group Diabetes Program actually showed that tolbutamide increased cardiovascular mortality.8 The United Kingdom Prospective Diabetes Study was the first study to suggest that a diabetic medication had a favorable CVD risk profile. It showed a nonsignificant reduction (p = 0.052) in myocardial infarction in patients treated intensively with insulin or sulfonylureas. It also showed a reduction in diabetes-related death and all-cause mortality in a substudy of 342 overweight persons given metformin.12 In a 10-year post-trial observational follow-up assessment, the reduction in these CVD events became statistically significant.13
Numerous studies have assessed the role of TZDs in persons with type 2 diabetes mellitus. Small controlled studies using surrogate markers such as carotid intimal-media thickness (IMT) have shown a decrease in the progression of carotid IMT in persons treated with a TZD. Protective effects against restenosis after percutaneous intervention in TZD-treated patients have also been noted.3 Large randomized, controlled trials that have assessed the effects of TZDs on major CVD events that are completed or ongoing are described in the following paragraphs. In evaluating the results of all these trials, one should distinguish those that compare TZDs with placebo as an add-on therapy to those that compare TZDs with other hypoglycemic drugs.
PROactive14 (the PROspective pioglitAzone Clinical Trial In macroVascular Events) was the first study that assessed the effect of an antidiabetic medication on cardiovascular outcomes. PROactive was a well-run prospective randomized-controlled trial in 5238 patients, designed to assess whether pioglitazone titrated to a maximum dose of 45 mg/d, compared with placebo in addition to the usual standard of glycemic therapy care, decreased macrovascular events. The average follow-up period was 34.5 months. The results showed a statistically nonsignificant reduction in the primary endpoint (all-cause mortality, nonfatal myocardial infarction, stroke, acute coronary syndrome, endovascular or surgical intervention in the coronary or leg arteries, or above-the-knee amputation) in the pioglitazone group. This was despite an adequate number of events in both arms of the study (514 of 2605 in the pioglitazone group and 572 of 2633 in the placebo group; p = 0.095). However, the secondary endpoint that was predefined (all-cause mortality, myocardial infarction, or stroke) was significantly reduced in the pioglitazone group (301 patients in the pioglitazone group and 358 in the placebo group; p = 0.027). It is noteworthy that the time to permanent insulin use was significantly decreased in the pioglitazone group.
ADOPT15 (A Diabetes Outcome Progression Trial) was a multicenter, randomized, double-blind, controlled clinical trial that sought to answer whether monotherapy with either rosiglitazone, metformin, or glyburide was sufficient to maintain euglycemia in persons in whom type 2 diabetes mellitus had recently been diagnosed and who had not taken any diabetes medications before. The primary outcome was the time to monotherapy failure (plasma glucose >180 mg/dL after an overnight fast). Analysis of the outcomes showed a cumulative incidence of monotherapy failure at 5 years of 15% with rosiglitazone, 21% with metformin, and 34% with glyburide. This was a 32% greater risk reduction for rosiglitazone compared with metformin, and a 63% greater risk reduction compared with glyburide (p < 0.0001 for both comparisons). However, some important limitations of the study should be mentioned: 1) the study was an efficacy and safety trial and not a primary cardiovascular endpoint trial; 2) there was a large withdrawal rate; and 3) patients with type 2 diabetes mellitus were in a very early stage in this study, and thus they may not represent the general population of patients with type 2 diabetes mellitus. Preliminary cardiovascular safety findings had not detected a significant difference in cardiac ischemic event rates between rosiglitazone and metformin or glyburide, but many believe that an increased risk cannot be ruled out.1 There were understandably more patients with heart-failure events with rosiglitazone than with metformin or glyburide.
The DREAM16 (Diabetes REduction Assessment with ramipril and rosiglitazone Medication) trial was a primary prevention study to assess whether rosiglitazone would prevent type 2 diabetes mellitus in persons at high risk for developing the disease. The inclusion criteria included either impaired fasting glucose (fasting plasma glucose of 110–126 mg/dL and 2-hour plasma glucose <200 mg/dL during the oral glucose tolerance test) or impaired glucose tolerance (either fasting plasma glucose <126 mg/dL and 2-hour plasma glucose of 140–200 mg/dL). The exclusion criteria were a history of type 2 diabetes mellitus, CVD, or intolerance of either medication. For the study, 24,592 patients were screened (18,784 were excluded), with a total of 5269 patients randomized to treatments (2635 to the rosiglitazone arm; 2634 to the placebo arm) and followed for a median of 3.0 years. Rosiglitazone was titrated to a maximum dose of 8 mg/d and ramipril was titrated to a maximum dose of 15 mg/d in a 2 × 2 factorial design. The primary outcome was a composite of incident type 2 diabetes mellitus or death. The results of the study showed that fewer individuals experienced the composite primary outcome in the rosiglitazone group compared with the placebo group [306 (11.6%), 686 (26.0%); p < 0.0001]. One-half of the individuals in the rosiglitazone group and approximately one-third of the placebo group achieved normoglycemia [1330 (50.5%), 798 (30.3%); p < 0.0001]. Also among individuals with impaired fasting glucose or impaired glucose tolerance, taking ramipril for 3 years significantly increased regression to normoglycemia but did not significantly decrease the incidence of type 2 diabetes mellitus or death.
The ACT NOW17 trial, presented at the American Diabetes Association 2008 meeting, randomized participants to placebo or pioglitazone titrated to 45 mg/d. Pioglitazone decreased the rate of progression to type 2 diabetes mellitus (1.5% per year) compared with placebo (6.8% per year; hazard ratio [HR], 0.19; p < 0.00001). The risk of fracture, heart failure, and other adverse events was similar except for a higher rate of edema in the pioglitazone group compared with placebo (22% vs 15%).
… the unknown risk of myocardial infarction with rosiglitazone is the biggest and most heated debate.
In April 2008, results of the PERISCOPE18 (Comparison of Pioglitazone versus Glimepiride on Progression of Coronary Atherosclerosis in Patients with Type 2 Diabetes) trial were published. PERISCOPE was a coronary intravascular ultrasound (IVUS) study in 547 patients with type 2 diabetes mellitus who underwent coronary angiography for clinical indications with a “target vessel” for IVUS that had a stenosis of <50% in an area of 40 mm or longer. The primary endpoint was change in percent atheroma volume (PAV) from baseline. They were then randomized to receive either glimepiride or pioglitazone, which was then titrated to a maximum tolerated dose. If a patient required cardiac catheterization for a clinical indication at a point between 12 and 18 months, a follow-up IVUS study was performed. Only 181 patients in the glimepiride group and 179 patients in the pioglitazone group were included in the primary analysis (66% of the initial cohort). The least-squares mean of the PAV increased in patients taking glimepiride and decreased in patients taking pioglitazone (0.73 vs −0.16; p = 0.002).
RECORD (Rosiglitazone Evaluated for Cardiovascular Outcomes in oRal agent combination therapy for type 2 Diabetes) was a multicenter, open-label, noninferiority trial that randomized persons who had inadequate glycemic control with metformin or sulfonylurea to either receive add-on rosiglitazone or not. An interim analysis19 to assess for increased rates of myocardial infarction was not conclusive. The final analysis20 again noted no differences in the primary endpoint, with 321 events in the rosiglitazone group and 323 events in the control group (HR, 0.99; 95% confidence interval [CI], 0.85–1.16). As suspected, there was an increased risk of congestive heart failure (HR, 2.10; 95% CI, 1.35–3.27) and fractures (HR, 1.57; 95% CI, 1.12–2.19).
… no TZD has shown a harmful effect on cardiac structure or function.
The APPROACH21 (Assessment on the Prevention of Progression by ROsiglitazone on Atherosclerosis in diabetes patients with Cardiovascular History) trial, the results of which were published recently, was an IVUS trial that randomized patients presenting to a cardiac catheterization laboratory who had at least one area in their epicardial coronary arterial system that contained an atherosclerotic plaque that was not intervened upon prior with a stenosis of 10–50%. This trial noted no change in the primary endpoint (PAV), whereas one secondary outcome, normalized total atheroma volume, was significantly reduced (−5.1 mm3; 95% CI, −10.0 to −0.3; p = 0.04).
Recently published findings of three clinical trials—Action to Control CardiOvascular Risk in Diabetes (ACCORD),22 the Veterans Administration Diabetes Study,23 and the Bypass Angioplasty Revascularization Investigation in Type 2 Diabetes (BARI 2D)24—showed no firm causal association between rosiglitazone and ischemic heart disease (IHD) events.
Data Not Favoring Thiazolidinediones (the Bad)
Known adverse effects that occur with the use of TZDs are discussed in the following sections. Some of these are thought be unique to a particular TZD, and others may be thought of as a class effect. Some have a more robust backing of scientific data; others have less. The most-recognized adverse effects include peripheral edema, heart failure, macular edema, and fractures. The overall medical community now is well aware of the effects of TZDs on peripheral edema and heart failure. However, the unknown risk of myocardial infarction with rosiglitazone is the biggest and most heated debate.
Edema
The incidence of new or worsening edema is noted to occur in 2.5% to 16.2% of persons with type 2 diabetes mellitus. This risk increases with increasing age, higher doses, female sex, and increasing creatinine levels, with concomitant use of insulin. Two mechanisms are thought to contribute to this problem with TZDs. The first is increasing sodium retention and plasma volume expansion because of the presence of PPAR-γ in the epithelium of the renal collecting duct. There is some thought that amiloride or spironolactone could decrease this effect. The second is the similarity of TZDs to perhaps the dihydropyridine type of calcium-channel blockers (eg, nifedipine, nicardipine, amlodipine) that exert their effects through L-type calcium channels that may cause an increased fluid permeability.3,25
Heart Failure
The second and more serious problem of heart failure is thought to occur much less frequently, in 0.25% to 0.45% of persons with type 2 diabetes mellitus per year.3,25 In May 2007, the FDA recommended, on the basis of clinical data, that TZD use in patients with any degree of heart failure be avoided. It is of interest, however, that no TZD has shown a harmful effect on cardiac structure or function. In fact, some small studies have shown improvement in hemodynamic values such as stroke volume index and cardiac index. One small randomized study assessed the effect of rosiglitazone versus placebo in patients with New York Heart Association class I and II heart failure and with a left ventricular ejection fraction of <45%. This trial showed an increase in peripheral edema in the rosiglitazone group compared with the placebo group (25.5% vs 8.8%; p = 0.037). There was no deterioration of systolic function and perhaps an improvement in diastolic function.3 In the PROactive study, nonadjudicated heart-failure events were more common in the pioglitazone group, but no evidence of increase in heart failure mortality was noted.14 A similar finding was noted in the interim analysis of the RECORD study.19 These studies' results suggest an overall low but distinct risk of hospitalizations for heart failure.
Macular Edema
Although there have been case reports,26 and retrospective studies27–30 in the literature suggesting the association of macular edema with use of a TZD, the overall evidence either proving or disproving it is fair at best. However, most experts in the field believe that TZDs probably exacerbate macular edema and that with discontinuation of TZDs, macular edema may decrease or abate completely. A case example is given in Figure 1.
Figure 1.
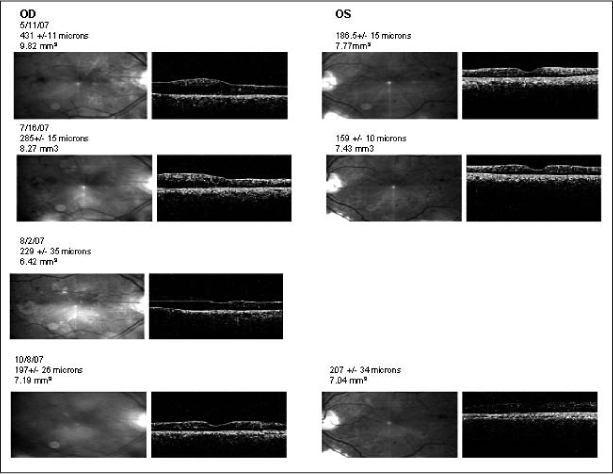
Results of optical coherence tomography (OCT) and fluorescein angiography in a man with diabetes.
aFoveal thickness and macular volume are noted for each date. On August 2, 2007, only the right eye was scanned. Rosiglitazone was stopped after the visit of May 11, 2007. Because of residual symptoms, the right eye was treated by laser on August 2, 2007; follow-up evaluation on October 8, 2007, showed significant resolution. OCT uses a laser in a technique similar to ultrasound to obtain information about the macula. Laser light reflected from the retina is detected, and because of the partial transparency of the retina, different layers reflect differing amounts of laser light. A computer-reconstructed scan is produced that allows very accurate measurements of macular contour and thickness. OCT is extremely useful in the diagnosis and evaluation of diabetic macular edema and can be used to monitor the effect of treatment on retinal thickness. OD = ocular dextra (right eye); OS = ocular sinistra (left eye).
a Figure available in color at: www.thepermanentejournal.org/images/Fall2010/p69.jpg.
Bone Loss
Another important aspect of TZDs is its effect on bone. Early basic science and preclinical work have shown that TZDs decrease osteoblast differentiation and increase osteoclast formation, suggesting overall bone loss. The mechanism of TZD effect on bone has not been completely elucidated but again appears to be due to its effect on PPAR-γ There is hope that eventually selective PPAR-γ modulators may overcome the undesirable extraglycemic effects. In 2006, the ADOPT group published a separate analysis of the fracture risk associated with rosiglitazone in comparison with metformin and glyburide. Rosiglitazone had an increased relative risk (RR) of 1.81 compared with metformin and 2.13 compared with glyburide. A sex-based association of risk was noted, with women having an increased risk for both upper and lower limb factures; men did not have an increased risk in this study. The risk ratios calculated showed the largest increases in fracture risk for the foot (RR = 3.3), the hand (RR = 2.6), and the proximal humerus (RR > 8). There was an insufficient number of fractures of the hip and spine to assess the risks for these fractures.31,32 Pioglitazone carried a similar risk for all clinical fractures (1.9 per 100 person-years). Significant research is needed in many areas in this field. Specifically, there is a need to further define which subgroups are at high risk and also to determine the effects of osteoporosis treatment in persons with type 2 diabetes mellitus who are taking a TZD.
The Ugly?
The possible association of TZDs with myocardial infarction came to light after a Peto fixed-effects meta-analysis, published in 2007, of 42 clinical trials concerning rosiglitazone use in approximately 28,000 patients suggested an odds ratio (OR) of 1.43, or a 43% greater risk, for myocardial infarction and an OR of 1.64, or a 64% greater risk, for cardiovascular death compared with placebo or other antidiabetics.33 A subsequent editorial suggested that there were numerous limitations to this meta-analysis, including possible misclassification, ascertainment errors, and a variability of entry criteria and outcome definitions among the original studies. The Peto fixed-effects model that was used for analysis was also thought to be more favorable for obtaining statistical significance. The conclusion of the editorial was that “the risk for myocardial infarction or death from cardiovascular patients taking rosiglitazone is uncertain: neither increased nor decreased risk is established.”34
Whether any risk is due to an individual drug or a class effect is not known. Another meta-analysis of randomized trials concerning pioglitazone use was undertaken that suggested that pioglitazone decreased rather than increased adverse CVD events. This study evaluated 19 clinical trials, with a total participant enrollment of 16,390. The duration of treatment was between 4 months and 3.5 years. Death, myocardial infarction, or stroke occurred in 4.4% (375 of 8554) of participants receiving pioglitazone and 5.7% (450 of 7836) of participants receiving control therapy (HR, 0.82; 95% CI, 0.72–0.94; p = 0.005).35
An excellent overview of the safety of TZDs in relation to IHD risk is provided in a recent scientific advisory reported by Kaul et al.36 The advisory statement addressed 1) rosiglitazone and IHD risk, 2) pioglitazone and IHD risk, and 3) pioglitazone versus rosiglitazone and IHD risk. Their conclusions were that 1) an association between rosiglitazone and IHD outcomes has not yet been firmly established, but sufficient safety signals have emerged to raise concerns; 2) the majority of published study findings do not positively correlate an increased risk for IHD in patients treated with pioglitazone, and hence there has been no black-box warning issued for pioglitazone; and 3) current evidence suggests that TZDs should not be used with the expectation of benefit with respect to IHD events.
Update
In June of 2010, Nissen and Wolksi published yet another meta-analysis: Rosiglitazone Revisited.37 The conclusion of this meta-analysis including data from the RECORD study noted an increased risk of myocardial infarction (OR = 1.28; 95% CI 1.02–1.63, p = 0.04) but not cardiovascular mortality (OR = 1.03; 95% CI 0.78–1.36; p = 0.86). Excluding the RECORD trial yielded qualitatively similar results but quantitatively higher odds ratio disfavoring rosiglitazone.37 The firestorm over TZDs has continued and led to an FDA advisory committee meeting again on July 14, 2010 to decide the fate of Avandia. Numerous presentations were made from many leaders in the academic community as well from GlaxoSmith-Kline.38 Two decisions were made. The first was to keep Avandia on the market but recommend stricter warning labels. The second was that the postmarketing trial known as TIDE (Thiazolidinedione Intervention with vitamin D Evaluation) be placed on partial clinical hold.39 Under the partial clinical hold no new patients may be enrolled into the trial until further notice from the FDA. Patients already enrolled in the trial will be allowed to continue to participate.
Conclusion
In medicine, as in many other areas of innovation, initial enthusiasm is usually tempered with the realities of subsequent knowledge. The evolution of TZD development is a prime example. Once seen as holding a promise of mortality reduction, TZDs are currently used with a focus on additional glycemic control, with careful patient selection to avoid possible toxicities.
The primary prevention of type 2 diabetes mellitus and cardiovascular mortality in persons with type 2 diabetes mellitus is of the utmost importance. The current PHASE program at KPNC addresses this exact need of improving the outcome in these high-risk patients. Without current data suggesting any benefits of prescribing a TZD except for improving glycemic control, care should be taken to avoid subgroups of patients who may have a higher risk of developing edema, congestive heart failure, fractures, and possibly macular edema. These subgroups may include patients of advancing age, those taking higher doses of a TZD, women, those with renal insufficiency, and those who also take insulin. The type 2 diabetes mellitus treatment algorithm currently proposed may need to be further refined to balance adequate glycemic control, costs, and expected future risks in individuals (Figure 2–4).40
Figure 2.
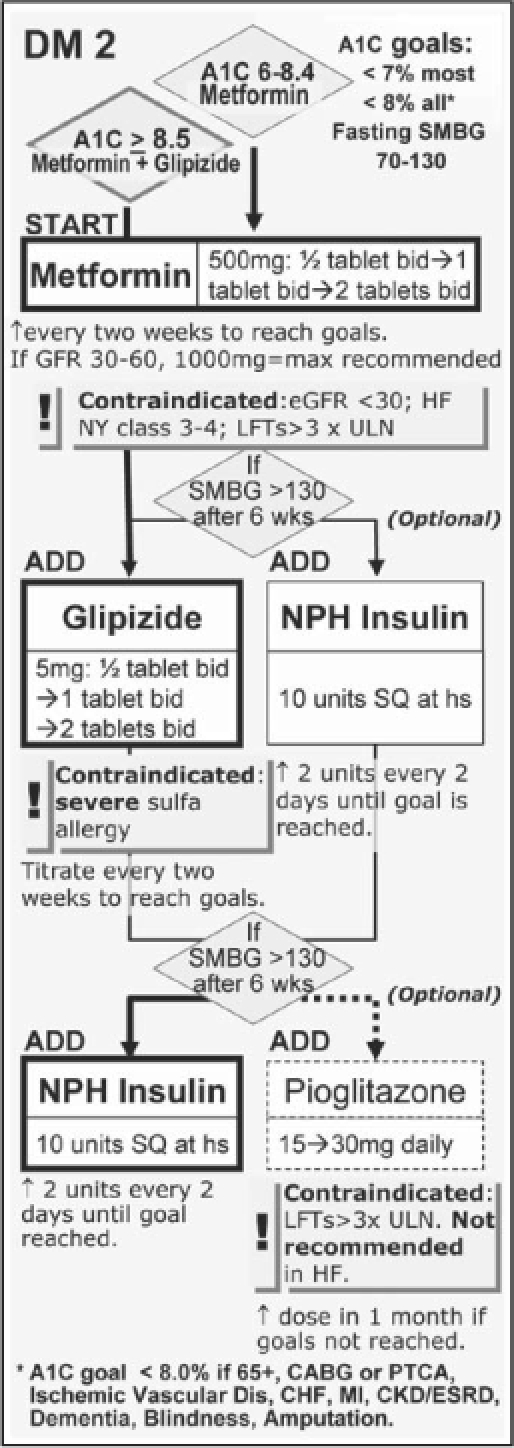
The diabetes mellitus portion of the current Kaiser Permanente Northern California PHASE (Prevent Heart Attack and Stroke Everyday) program.
Cr = creatinine; HF = heart failure; LFT = liver function test; NPH = neutral protamine Hagedorn (insulin); SMBG = self-monitoring of blood glucose; SQ = subcutaneous; ULN = upper limit of normal; bid = twice daily; hs = at bedtime; q2days = every 2 days.
Figure 3.
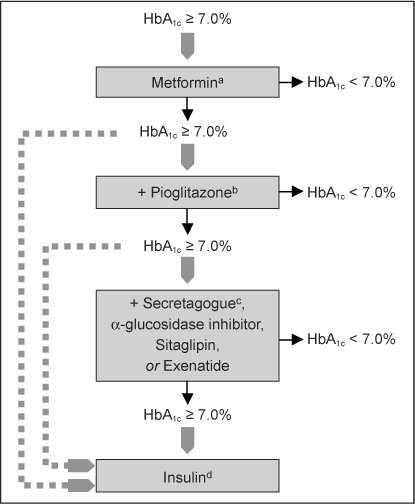
Proposed antihyperglycemic strategy in the patient with type 2 diabetes mellitus and coronary artery disease.
a Because of the risk of lactic acidosis, metformin should be avoided in patients whose coronary artery disease is complicated by acute or unstable heart failure.
b Because of the risk of fluid retention, pioglitazone should be avoided in patients whose coronary artery disease is complicated by heart failure; it is contraindicated in those with New York Heart Association class III to IV symptoms. Because of recent concerns regarding the increased risk of myocardial infarction with rosiglitazone, this drug is best avoided in coronary artery disease patients until further safety data become available.
c Secretagogues include the sulfonylureas and the nonsulfonylurea glinides. Certain sulfonylureas (eg, glyburide) may impair ischemic preconditioning and are probably best avoided in patients with active coronary insufficiency.
d Insulin can be added to or substituted for oral agents at any point in the disease course. When more advanced regimens are used, insulin secretagogues traditionally are discontinued. Reprinted with permission from Inzucchi SE, McGuire DK. New drugs for treatment of diabetes: part II: Incretin-based therapy and beyond. Circulation 2008 Jan 29;117(4):574–84; Figure 1.
Figure 4.
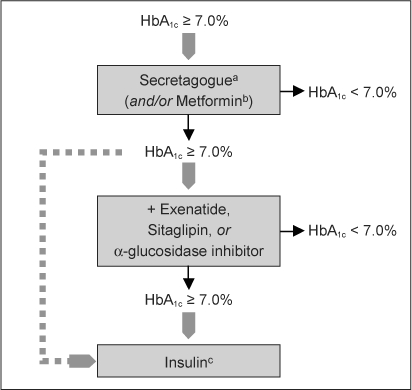
Proposed antihyperglycemic strategy in the patient with type 2 diabetes mellitus and heart failure.
a Secretagogues include the sulfonylureas and the nonsulfonylurea glinides. Certain sulfonylureas (eg, glyburide) may impair ischemic preconditioning and probably are best avoided in patients with active coronary insufficiency.
b Metformin is no longer contraindicated in this setting and may be used cautiously, but only in stable, compensated heart failure patients with normal renal function and acid/base status.
c Insulin can be added to or substituted for oral agents at any point in the disease course. When more advanced regimens are used, insulin secretagogues traditionally are discontinued. Because of the sodium-retaining properties of insulin, the lowest effective dose should be used, and the dose should be titrated carefully. Reprinted with permission from Inzucchi SE, McGuire DK. New drugs for treatment of diabetes: part II: Incretin-based therapy and beyond. Circulation 2008 Jan 29;117(4):574–84; Figure 2.
Aleglitazar, a promising novel dual PPAR agent that is currently being tested in a phase III clinical trial, again brings hope to this field. We will await the results of this and other ongoing studies of diabetes medications that can now enter the market only if a favorable cardiovascular risk profile is attained.
Disclosure
The author(s) have no conflicts of interest to disclose.
Acknowledgments
We sincerely thank Saul Genuth, MD, Principal Investigator and Director of the Diabetes Management Center of the BARI 2D study, Case Western Reserve, Cleveland, Ohio, for reviewing our manuscript.
Katharine O'Moore-Klopf, ELS, of KOK Edit provided editorial assistance.
Footnotes
a Pharmacy Outcomes Research Group, KPNC
References
- Joffe HV. Silver Spring, MD: Endocrinologic and Metabolic Drugs Advisory Committee, Diabetes Drug Group, US Food and Drug Administration; 2008. Cardiovascular assessment in the pre-approval and post-approval settings for drugs and biologics developed for the treatment of type 2 diabetes mellitus [PowerPoint presentation on the Internet from meeting on 2008 Jul 1–2] [cited 2010 Jul 15]. Available from: www.fda.gov/ohrms/dockets/ac/08/slides/2008-4368s1-08-FDA-Joffe.ppt. [Google Scholar]
- Yki-Järvinen H. Thiazolidinediones. N Engl J Med. 2004 Sep 9;351(11):1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- McGuire DK, Inzucchi SE. New drugs for the treatment of diabetes mellitus: part I: Thiazolidinediones and their evolving cardiovascular implications. Circulation. 2008 Jan 22;117(3):440–9. doi: 10.1161/CIRCULATIONAHA.107.704080. [DOI] [PubMed] [Google Scholar]
- Wang CH, Weisel RD, Liu PP, Fedak PW, Verma S. Glitazones and heart failure: critical appraisal for the clinician. Circulation. 2003 Mar 18;107(10):1350–4. doi: 10.1161/01.cir.0000054675.30348.9a. [DOI] [PubMed] [Google Scholar]
- Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002 Jan 16;287(3):360–72. doi: 10.1001/jama.287.3.360. [DOI] [PubMed] [Google Scholar]
- Levine TB, Levine AB. Metabolic syndrome and cardiovascular disease. Philadelphia, PA: WB Saunders; 2006. [Google Scholar]
- Little PJ, Topliss DJ, Madaliar S, Law RE, editors. Diabetes and cardiovascular disease: integrating science and clinical medicine. Baltimore, MD: Williams & Wilkins; 2004. [Google Scholar]
- Effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. VIII. Evaluation of insulin therapy: final report. Diabetes. 1982 Nov;31(Suppl 5):1–81. [PubMed] [Google Scholar]
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993 Sep 30;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- Cleary PA, Orchard TJ, Genuth S, et al. DCCT/EDIC Research Group The effect of intensive glycemic treatment on coronary artery calcification in type 1 diabetic participants of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study. Diabetes. 2006 Dec;55(12):3556–65. doi: 10.2337/db06-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837–53. Erratum in: Lancet 1999 Aug 14;354(9178):602. [PubMed] [Google Scholar]
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):854–65. Erratum in: Lancet 1998 Nov 7;352(9139):1558. [PubMed] [Google Scholar]
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008 Oct 9;359(15):1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- Dormandy JA, Charbonnel B, Eckland DJ, et al. PROactive Investigators Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005 Oct 8;366(9493):1279–89. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- Kahn SE, Haffner SM, Heise MA, et al. ADOPT Study Group Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006 Dec 7;355(23):2427–43. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- DREAM Trial Investigators. Bosch J, Yusuf S, Gerstein HC, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006 Oct 12;355(15):1551–62. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]
- Phend C. ADA: diabetes prevention hopes revived for thiazolidinedione [monograph on the Internet] MedPage Today. 2008 [cited 2010 Jul 12]. Available from: www.medpagetoday.com/MeetingCoverage/ADA/9784. [Google Scholar]
- Nissen SE, Nicholls SJ, Wolski K, et al. PERISCOPE Investigators Comparison of pioglitazone vs glimepiride on progression of coronary atherosclerosis in patients with type 2 diabetes: the PERISCOPE randomized controlled trial. JAMA. 2008 Apr 2;299(13):1561–73. doi: 10.1001/jama.299.13.1561. [DOI] [PubMed] [Google Scholar]
- Home PD, Pocock SJ, Beck-Nielsen H, et al. RECORD Study Group Rosiglitazone evaluated for cardiovascular outcomes—an interim analysis. N Engl J Med. 2007 Jul 5;357(1):28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- Home PD, Pocock SJ, Beck-Nielsen H, et al. RECORD Study Team Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009 Jun 20;373(9681):2125–35. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- Gerstein HC, Ratner RE, Cannon CP, et al. APPROACH Study Group Effect of rosiglitazone on progression of coronary atherosclerosis in patients with type 2 diabetes mellitus and coronary artery disease: the assessment on the prevention of progression by rosiglitazone on atherosclerosis in diabetes patients with cardiovascular history trial. Circulation. 2010 Mar 16;121(10):1176–87. doi: 10.1161/CIRCULATIONAHA.109.881003. [DOI] [PubMed] [Google Scholar]
- Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545–59. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth W, Abraira C, Moritz T, et al. VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009 Jan 8;360(2):129–39. doi: 10.1056/NEJMoa0808431. Erratum in: N Engl J Med 2009 Sep 3;361(10):1028; N Engl J Med 2009 Sep 3;361(10);1024–5. [DOI] [PubMed] [Google Scholar]
- BARI 2D Study Group. Frye RL, August P, Brooks MM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009 Jun 11;360(24):2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesto RW, Bell D, Bonow RO, et al. American Heart Association; American Diabetes Association Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation. 2003 Dec 9;108(23):2941–8. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- Kendall C, Wooltorton E. Rosiglitazone (Avandia) and macular edema. CMAJ. 2006 Feb 28;174(5):623. doi: 10.1503/cmaj.060074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatti P, Arrigoni F, Longobardi A, Costanza F, Di Blasi P, Merante D. Retrospective analysis of rosiglitazone and macular oedema in patients with type 2 diabetes mellitus. Clin Drug Investig. 2008;28(5):327–32. doi: 10.2165/00044011-200828050-00006. [DOI] [PubMed] [Google Scholar]
- Ryan EH, Jr, Han DP, Ramsay RC, et al. Diabetic macular edema associated with glitazone use. Retina. 2006 May–Jun;26(5):562–70. doi: 10.1097/00006982-200605000-00011. [DOI] [PubMed] [Google Scholar]
- Liazos E, Broadbent DM, Beare N, Kumar N. Spontaneous resolution of diabetic macular oedema after discontinuation of thiazolidinediones. Diabet Med. 2008 Jul;25(7):860–2. doi: 10.1111/j.1464-5491.2008.02491.x. [DOI] [PubMed] [Google Scholar]
- Fong DS, Contreras R. Glitazone use associated with diabetic macular edema. Am J Ophthalmol. 2009 Apr;147(4):583–6.e1. doi: 10.1016/j.ajo.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Evans R, Grey A, Clarke BL. Three perspectives on thiazolidine and bone health. Endocrine News. 2008 Sep:14–21. [Google Scholar]
- Schwartz AV. TZDs and bone: a review of the recent clinical evidence. PPAR Res [serial on the Internet] 2008 [2010 Jul 12]:297893 [about 6 pages]. Available from: www.hindawi.com/journals/ppar/2008/297893.html. [DOI] [PMC free article] [PubMed]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007 Jun 14;356(24):2457–71. doi: 10.1056/NEJMoa072761. Erratum in: N Engl J Med 2007 Jul 5;357(1):100. [DOI] [PubMed] [Google Scholar]
- Diamond GA, Bax L, Kaul S. Uncertain effects of rosiglitazone on the risk for myocardial infarction and cardiovascular death. Ann Intern Med. 2007 Oct 16;147(8):578–81. doi: 10.7326/0003-4819-147-8-200710160-00182. [DOI] [PubMed] [Google Scholar]
- Singh S, Loke YK, Furberg CD. Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA. 2007 Sep 12;298(10):1189–95. doi: 10.1001/jama.298.10.1189. [DOI] [PubMed] [Google Scholar]
- Kaul S, Bolger AF, Herrington D, Giugliano RP, Eckel RH. Thiazolidinedione drugs and cardiovascular risks. a science advisory from the American Heart Association and American College of Cardiology Foundation. Circulation. 2010 Apr 27;121(16):1868–77. doi: 10.1161/CIR.0b013e3181d34114. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Rosiglitazone revisited: An updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med. 2010 Jun 28. [Epub ahead of print.] [DOI] [PubMed]
- GlaxoSmithKline. Advisory Committee briefing document: Cardiovascular safety of rosiglitazone [monograph on the Internet] Silver Spring, MD: US Food and Drug Administration: Endocrinologic and Metabolic Drugs Advisory Committee, Drug Safety and Risk Management Advisory Committee; 2010 Jul 13–14. [cite 2010 Aug 2]. Available from: www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM218492.pdf. [Google Scholar]
- FDA statement on Avandia TIDE trial [monograph on the Internet] Silver Spring, MD: US Food and Drug Administration; updated 2010 Jul 21 [cited 2010 Aug 2]. Available from: www.fda.gov/Drugs/DrugSafety/ucm219780.htm. [Google Scholar]
- Inzucchi SE, McGuire DK. New drugs for treatment of diabetes: part II: Incretin-based therapy and beyond. Circulation. 2008 Jan 29;117(4):574–84. doi: 10.1161/CIRCULATIONAHA.107.735795. [DOI] [PubMed] [Google Scholar]


