Abstract
Bacterial and eukaryotic transfer RNAs have been shown to contain hypermodified adenosine, 2-methylthio-N6-threonylcarbamoyladenosine, at position 37 (A37) adjacent to the 3′-end of the anticodon, which is essential for efficient and highly accurate protein translation by the ribosome. Using a combination of bioinformatic sequence analysis and in vivo assay coupled to HPLC/MS technique, we have identified, from distinct sequence signatures, two methylthiotransferase (MTTase) subfamilies, designated as MtaB in bacterial cells and e-MtaB in eukaryotic and archaeal cells. Both subfamilies are responsible for the transformation of N6-threonylcarbamoyladenosine into 2-methylthio-N6-threonylcarbamoyladenosine. Recently, a variant within the human CDKAL1 gene belonging to the e-MtaB subfamily was shown to predispose for type 2 diabetes. CDKAL1 is thus the first eukaryotic MTTase identified so far. Using purified preparations of Bacillus subtilis MtaB (YqeV), a CDKAL1 bacterial homolog, we demonstrate that YqeV/CDKAL1 enzymes, as the previously studied MTTases MiaB and RimO, contain two [4Fe-4S] clusters. This work lays the foundation for elucidating the function of CDKAL1.
Keywords: Biophysics, Chemical Modification, Enzyme Purification, Gene expression, Iron-Sulfur Protein, RNA Modification
Introduction
The methylthiotransferase (MTTase)4 family, a subclass of the large radical AdoMet enzyme superfamily, has recently received special attention (1). Indeed, its members catalyze chemically challenging reactions, in all cases involving C–H to C–SCH3 bond conversion, through a radical mechanism that remains incompletely established. Furthermore, these reactions participate in important biological processes such as tRNA or ribosomal protein modification (2–5). Prototypes for the MTTase family are two bacterial enzymes as follows: MiaB, which modifies N6-isopentenyladenosine (i6A) to its 2-methylthio derivative (ms2i6A) in tRNA, and RimO, which acts on a specific aspartate residue of the ribosomal S12 protein. Both enzymes have been shown to contain two [4Fe-4S] clusters (4–6). The first one is chelated by the three cysteines of a conserved CXXXCXXC motif that is the hallmark signature of the radical AdoMet family (Scheme 1) (7). This cluster serves to bind and reduce AdoMet into methionine and the highly reactive 5′-deoxyadenosyl radical (Ado•) (8). The latter is supposed to abstract an H atom of the substrate (tRNA or protein) selectively, thus generating an intermediate substrate radical that is amenable to C–S bond formation (9). It is believed that the thiolation step involves the second [4Fe-4S] cluster, chelated by three other conserved cysteines in the typical N-terminal UPF0004 domain (Scheme 1) (4–6). The final methylation step involves a second molecule of AdoMet (4–6). A specific signature of the methylthiotransferase subfamily not shared by the other radical AdoMet proteins is the presence of a C-terminal TRAM domain involved in substrate (tRNA or protein) recognition (Scheme 1) (5, 10).
SCHEME 1.
Amino acid sequence alignments of MiaB, RimO, and MtaB (YqeV and Cdkal-1) MTTases from T. maritima (T.m.), E. coli (E.c.), B. subtilis (B.sm.), and Homo sapiens (H.s.). The alignment was performed with ClustalW at the EBI site. Totally conserved residues are indicated by asterisks, and conserved cysteine residues are shown in boxes. The three domains UPF0004, radical AdoMet, and TRAM are shown on the right.
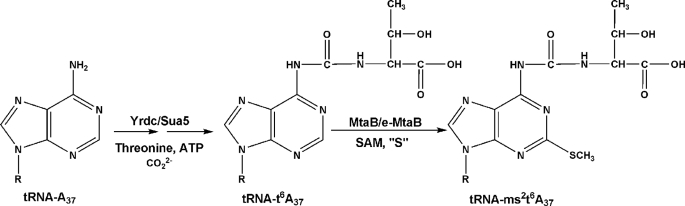
The hypermodified 2-methylthio-N6-threonylcarbamoyladenosine (ms2t6A, where ms stands for methylthio, t for threonine, and A for adenosine) is an anticodon loop modification found at position 37 of tRNAs decoding ANN codons (Scheme 2). Despite the availability of extensive data on the physiological function of this hypermodified base (11–13), its biosynthesis pathway has only been partially characterized. The prerequisite carbamoylation of threonine is an ATP-dependent process requiring threonine and carbonate, but the genes involved in this pathway have remained uncharacterized (14). A recent publication (15) demonstrates that proteins from the YrdC/Sua5 family catalyze the first step in ms2t6A biosynthesis, i.e. the addition of a threonylcarbamoyl group at the N6 nitrogen of adenosine converting adenosine 37 to t6A-37. The second step in ms2t6A-37 biosynthesis, including both sulfur insertion and methylation at position 2 of t6A-37 to yield ms2t6A-37, is likely to be catalyzed by one or more MTTases, potentially from the radical AdoMet family.
SCHEME 2.
Biosynthetic pathway for ms2t6A.
Bioinformatics analyses presented here demonstrate that five major families of homologous MTTases are encoded in vertebrate and bacterial cells. In addition to the previously characterized MiaB and RimO families, we identify three new families as follows: (i) MtaB found in eubacteria with the B. subtilis yqeV gene as its prototype; (ii) e-MtaB found in higher eukaryotes and archaebacteria with the murine CDKAL1 gene as its prototype; and (iii) MTL1 found so far exclusively in ϵ proteobacteria. CDKAL1 is of special interest because genetic polymorphisms in this gene increase the reduction of insulin secretion and increase the risk of developing type 2 diabetes (16). Using in vivo gene complementation, we show that proteins from both the MtaB and e-MtaB families are responsible for the conversion of N6-threonylcarbamoyladenosine (t6A) into 2-methylthio-N6-threonylcarbamoyladenosine (ms2t6A) in tRNA. In addition, using purified preparations of Bacillus subtilis YqeV as a representative of the MtaB family, we demonstrate that these enzymes contain two [4Fe-4S] clusters, like the MiaB and RimO families, the only other proven radical sulfur-inserting enzymes.
MATERIALS AND METHODS
Plasmids, Strains, and Growth Conditions
The Escherichia coli, B. subtilis strains and plasmids used in this study are listed in Table 1. Bacteria were routinely grown in LB medium at 37 °C.
TABLE 1.
| Genotype | Source | ||
|---|---|---|---|
| E. coli strains | |||
| DH5a | F−gyrA96(NaIr)recA1relA endA1thi-1HsdR17(rk−mk+)glnV44 | Fermentas | |
| DeoRΔ(lacZYA-argF)U169[Φ80dΔ(lacZ)M15] | |||
| BL21(DE3) | F−ompT hsdSB(rB−,mB−) gal dcm (DE3) | Invitrogen | |
| TX3346 | F−lac1378lacZ-GCGproB+/araΔ(lac-pro)xmmiaB::Tn10dCm | 29 | |
| B. subtilis strains | |||
| MGNA-001 | TrpC2 | NIG, Japan | |
| MGNA-C496 | Yqev− | NIG, Japan | |
| Plasmids | |||
| pT7-7 | ColE1 origin bla, T7 promoter | 30 | |
| pGEX6P-1 | Bla | GE Healthcare | |
| TOPO | Bla | Invitrogen | |
| pDB148-Stu | LacI Pspac ble bla kan | 20 | |
Phylogenomic Analyses
PSI-BLAST profiles (17) created via ClustalW alignment (18) of a set of MiaB and RimO homologs were used to search the human genome and the CRSH data base of proteins of likely equivalent function from 474 bacterial genomes.5 Sequences identified in this search exhibiting significant (e-value <0.05) sequence similarity to MiaB/RimO that aligned over all three domains were combined with the initial sequences to perform another PSI-BLAST search using the same significance threshold. This second search identified many proteins from other radical AdoMet enzyme families not containing the UPF0004 or TRAM domain, suggesting that all MTTases were likely to have been identified. Cladograms were reconstructed by the program MEGA 3.0 (19) based on 100 bootstrap replicates using the Unweighted Pair Group Method with Arithmetic mean with default parameters. Proteins were assigned to putative functional families via examination of the phylogenetic tree.
Cloning of the yqeV and CDKAL1 Genes and Construction of Overexpressing Plasmids
The open reading frame encoding the YqeV (MtaB1) protein (BSU25430) was PCR-amplified using B. subtilis genomic DNA, Pwo polymerase (Roche Applied Science), and the following primers: the N-terminal primer (YqeVN-ter, 5′-GAGGTGATCACATATGGCAACTGTTGCTTTC-3′) was designed to contain a unique NdeI restriction site at the predicted initiation codon and the C-terminal primer (YqeVC-ter, 5′-GTGACAGCTCTTTTATTTCTGCAGCTTACAAAC-3′) to hybridize in the 3′-untranslated region and to contain a unique PstI restriction site. The PCR fragment was purified with the High Pure PCR kit (Roche Applied Science), double-digested with NdeI and PstI (Fermentas), and gel-purified before direct cloning into the pT7-7 expression vector, leading to the pT7-mtab plasmid. DH5α cells were transformed with the pT7-mtab plasmid, and one clone containing an insert with the expected size was selected for sequencing. The cloned yqeV was confirmed to be wild type when compared with the GenBankTM accession number BSU25430. Full-length mouse CDKAL1 cDNA (BC016073) was purchased from Invitrogen. The open reading frame of the CDKAL1 gene was PCR-amplified to contain SmaI and XhoI restriction sites at the N and C termini, respectively. The PCR fragment was cloned into pCR2.1-TOPO by TOPO-TA cloning (Invitrogen). CDKAL1 fragment was excised from pCR2.1-TOPO vector by double digestion with SmaI and XhoI (New England Biolabs) and cloned into pGEX6P-1 (GE Healthcare) leading to the pGEX6P-emtab plasmid.
yqeV Cloning in pDG148-Stu Plasmid
The following primers were used to amplify yqeV gene using pT7-mtab as template: pDG148yqeVN, 5′-AAGGAGGAAGCAGGTATGGCAACTGTTGCTTTCCATACGCTTGGCTG-3′ (forward), and pDG148yqeVC, 5′-GACACGCACGAGGTTTAAGAAGACAAACGCATGTGTTCAGTTATTTC-3′ (reverse). The cloning procedure used is exactly as described previously (20). The obtained plasmid was named pDG148-mtab and sequenced to verify that no error has been introduced during the PCR experiment. The electrocompetent MGNA-C496 cells were prepared as described and transformed with pDG148-mtab by electroporation (21).
Site-directed Mutagenesis; Construction of the AXXXAXXA Triple Variant of the Radical AdoMet Motif
Triple variants of cysteine residues of the CXXXCXXC motif were constructed by PCR using the following primers: yqeVCtoA1, 5′-ATACAGGAGGGCGCCAATAATTTCGCCACATTCGCTATCATTCCGTGGGC-3′, hybridized to the noncoding strand, and yqeVCtoA2, 5′-GCCCACGGAATGATAGCGAATGTGGCGAAATTATTGGCGCCCTCCTGTAT-3′, hybridized to the coding strand; Cdkal1CtoA1, 5′-CCATCAACACGGGGGCTCTCAATGCTGCTACCTACGCCAAAACTAAACAC-3′, hybridized to the noncoding strand, and Cdkal1CtoA2, 5′-GTGTTTAGTTTTGGCGTAGGTAGCAGCATTGAGAGCCCCCGTGTTGATGG-3′, hybridized to the coding strand. Mutagenesis was carried out on plasmid pT7-mtaB and pGEX6P-emtab with QuikChangeTM site-directed mutagenesis kits from Stratagene according to the manufacturer's protocol as described previously (22). Mutations were confirmed by DNA sequencing.
Preparation and Analysis of tRNA from B. subtilis
MGNA-001 (wild type) and MGNA-C496 (yqeV−) strains were grown in LB medium at 37 °C until the A600 reached 1.0. The cells were harvested, and cell-free extract was obtained as described previously (23). Bulk tRNAs were isolated as described previously (24), and 100–200 μg of purified tRNAs were digested to nucleosides by nuclease P1 and bacterial alkaline phosphatase treatment. The resulting hydrolysate was analyzed by HPLC as described previously (24). For the complementation experiment, MGNA-C496 strain lacking the yqeV gene was transformed with the expressing plasmid pDG148-yqeV coding MtaB protein and grown in LB medium as described previously (20).
Overexpression and Purification of YqeV (MtaB) Protein
The pT7-mtab plasmid was used to transform E. coli BL21CodonPlus(DE3)-RILTM, which was grown at 37 °C in Luria Broth supplemented with 100 mg/liter ampicillin. When the A600 reached 0.6, the production of the MtaB was induced by addition of 100 μm isopropyl 1-thio-β-d-galactopyranoside, and the incubation was continued for 2 h at 30 °C. The MtaB protein was purified aerobically at 4 °C as follows. The frozen cells were thawed, broken by sonication, and centrifuged at 220,000 × g at 4 °C for 1 h. The proteins of the cell-free extract were precipitated with ammonium sulfate between 25 and 55% saturation and dialyzed two times against 60 volumes of 10 mm Tris-HCl, pH 7.5, containing 100 mm KCl (buffer A). The colored solution was then loaded on top of a 30-ml Blue-Sepharose column equilibrated with buffer A. The column was washed with 5 column volumes of buffer A and then eluted with a linear gradient of KCl (0.1–1 m) in buffer A. Fractions containing the MtaB protein were pooled, brought to 1 m ammonium sulfate in Tris buffer, and loaded to a 25-ml butyl-Sepharose FF (GE Healthcare) equilibrated in that buffer. The column was washed with 3 column volumes of buffer A, and the pure protein was eluted with a descending gradient (1–0 m) of ammonium sulfate buffer A. The fractions containing MtaB were pooled, concentrated by ultrafiltration using an Amicon cell fitted with a YM30 (Diaflo), and submitted to a final purification on an analytical Superdex-75 gel filtration column using Tris-HCl, pH 8.0, containing 0.25 m KCl and 5 mm DTT. The fractions were analyzed by SDS-PAGE (12%), and the most pure were pooled, concentrated, and stored at 77 K.
Preparation of the Apoprotein
The apo-form of MtaB protein was prepared as described previously (6).
Reconstitution of ApoMtaB
Fe-S cluster reconstitution into B. subtilis MtaB was carried out under strictly anaerobic conditions into a Jacomex NT glovebox containing less than 2 ppm O2 as described previously (6).
Analytical Methods
Quantitative amino acid analysis was used to determine extinction coefficients of purified B. subtilis MtaB: ϵ280 = 46.8 mm−1cm−1 for the apoenzyme; ϵ280 = 87.8 mm−1cm−1 and ϵ400 = 30.0 mm−1cm−1 for the holo-enzyme (i.e. an A400/A280 ratio of 0.34). Iron and inorganic sulfide were quantified as described previously (25). tRNAs were digested and analyzed by HPLC as described previously (24).
Mass Spectrometry Analysis
HPLC-tandem mass spectrometry analyses were performed with a 1100 Agilent chromatographic system coupled with an API 3000 triple quadrupolar apparatus (PerkinElmer Life Sciences) using a turbo ion spray electrospray source in the positive mode. HPLC separation was carried out on a 2 × 150-mm column containing 3 μm of octadecylsilyl silica gel (Uptisphere, Interchim Montluçon, France) using a gradient of acetonitrile in 5 mm ammonium formate. Acetonitrile content was increased from 0 to 40% over the first 20 min and then held constant for 40 min. The settings of the tandem mass spectrometer were optimized by injection of a pure solution of i6A to favor loss of the ribose unit upon collision-induced fragmentation. Mass spectrometry detection was carried out in neutral loss mode to obtain a high specificity. In this configuration, pseudo-molecular ions ([M + H]+) and fragments ([M − 132 + H]+) obtained by collision in the second quadrupole (collision) cell were analyzed in the first and third quadrupoles, respectively. Using this approach, only nucleosides losing their ribose unit were detected. The pseudo-molecular ion of the latter compounds was monitored in a 300–450 mass range.
Light Absorption Spectroscopy
UV-visible absorption spectra were recorded under anaerobic conditions in a glove box on an XL-100 Uvikon spectrophotometer equipped with optical fibers.
EPR
X-band EPR spectra were recorded on a Bruker ESP-300E EPR spectrometer operating with an ER-4116 dual-mode cavity and an Oxford Instrument ESR-9 flow cryostat.
RESULTS AND DISCUSSION
Phylogenomic Analysis of Radical AdoMet Methylthiotransferases
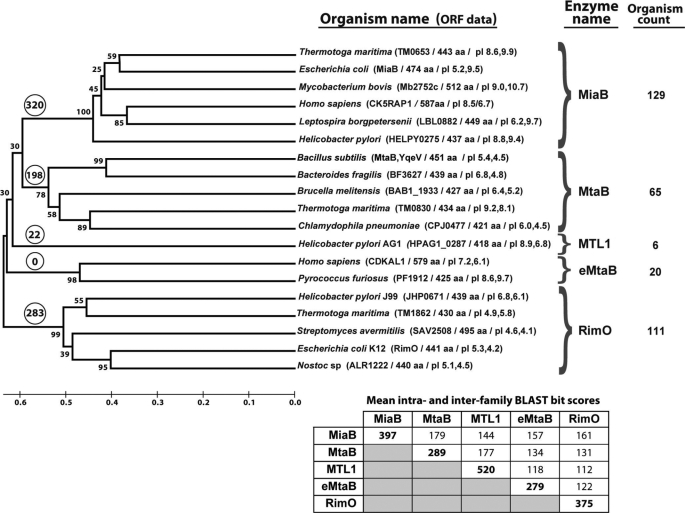
Sequence profiling techniques were used to search 474 bacterial genomes and the human genome for families of radical AdoMet MTTase. To ensure full coverage of this enzyme class, iterative expansion of the sequence profile was continued until radical AdoMet enzymes with different overall domain architecture were identified by the profile (see under “Materials and Methods”). Five distinct sequence families with representatives in at least three genomes were identified in this manner (Fig. 1). Proteins in all of these families have an N-terminal UPF0004 domain with three invariant cysteines in the CX34–36CX28–37C motif, a central radical AdoMet domain with three invariant cysteines in CX3CX2C motif, and a C-terminal TRAM domain (Scheme 1) (3, 5).
FIGURE 1.
Phylogenomic analysis of bacterial and human radical AdoMet methylthiotransferases. The cladogram shows the inferred evolutionary distances between representatives of all homologous protein families identified in a systematic search of the human genome and 474 fully sequenced bacterial genomes (see “Materials and Methods”). The MiaB and RimO families have been biochemically characterized in previous literature. This study described initial experimental characterization of representatives from the methylthiothreonylcarbamoyladenosine transferase B (MtaB) family and the eukaryotic methylthiothreonylcarbamoyladenosine transferase B (e-MtaB) family. The methYthiotransferase-like family 1 (MTL1), which is restricted to ϵ proteobacteria, has yet to be characterized experimentally. The e-MtaB family is found in archaebacteria but not eubacteria, although the other four families are found in eubacteria but not archaebacteria. The organism count indicates the number of unrelated bacterial genomes that encode a representative of each family (i.e. after correction for redundancy in genome organization). The numbers in circles at the root of each family indicate the number of times a member of one of the other families shown here is encoded simultaneously in the same genome (without redundancy correction). The numbers in a smaller font near the branch points indicate the percent of MEGA bootstrap replicates with the illustrated relative ordering of the successive branch points. Splits between the identified families confidently precede those within the families, with 100, 78, 98, and 99% confidence. Proper grouping of proteins in the MtaB family, which has the lowest 78% confidence for its first internal split, is supported by PSI-BLAST sequence profiling (data not shown) as well as the fact that proteins assigned to this family are never encoded in the same genome, even though 198 members of the other families are found encoded in the same genome as MtaB family members. The ORF data in parentheses includes the name of the protein, its amino acid sequence length, its overall pI, and the pI of its TRAM domain. The TRAM domains of bacterial MiaBs are generally basic, although those of bacterial RimOs are generally acidic. The TRAM domains of members of the other families show wider variations in pI values.
Two of the five MTTase families identified in our analyses represent the well characterized MiaB and RimO enzyme families (4–6). The human and B. subtilis genomes both encoded a member of the MiaB family (CK5RAP1 and YmcB/BSU17010, respectively) in addition to a member of another MTTase family. The third MTTase family identified in our analyses includes yqeV (BSU25430), the other MTTase encoded in the B. subtilis genome, whose enzymatic activity was characterized in this study. We have designated this family MtaB, for methylthio-threonylcarbamoyl-adenosine transferase B, because it performs the second step in the biosynthesis of this hypermodified base (Scheme 2). This nomenclature was chosen for consistency with the name of MiaB, which performs the second step in biosynthesis of methylthio-isopentenyl-adenosine. The fourth MTTase family includes enzymes of unknown substrate specificity that are found exclusively in ϵ proteobacteria, including the pathogens in the Helicobacter and Campylobacter genera. We have designated this family MTL1, for methylthiotransferase-like family-1. The fifth and final MTTase family includes CDKAL1, the other MTTase encoded in the human genome, whose enzymatic activity is also characterized in this study. We have designated this family e-MtaB for eukaryotic methylthiothreonylcarbamoyladenosine B, because it performs probably the second step in the biosynthetic pathway (Scheme 2). The e-MtaB family is not found in any eubacteria genome in our data base, but it is found in a variety of archaebacteria, as opposed to the other four MTTase families. Notably, this family is not represented in any eubacterial genome. All five families are approximately equally remote from one another, suggesting that functional differences between these families were established at an early evolutionary time.
Combining these phylogenomic results with the previously established occurrence of hypermodified adenosines ms2i6A and ms2t6A in both B. subtilis and human tRNAs (26, 27), we hypothesized that both MtaB and e-MtaB enzyme families are likely to catalyze the methylthiolation of t6A to form ms2t6A (Scheme 2). Because previously characterized MiaB family members all catalyze biosynthesis of ms2i6A (22, 28), the MiaB family members encoded in the B. subtilis and human genomes are likely to be responsible for the observed biosynthesis of ms2i6A. Because only one additional MTTase family is encoded in each of these genomes, the corresponding MtaB and e-MtaB enzymes are leading candidates to catalyze the biosynthesis of ms2t6A, the only other methylthiolated base observed in these organisms. We therefore undertook experimental studies to critically evaluate this bioinformatics-based inference.
YqeV/MtaB and CDKAL1/e-MtaB Transform t6A into ms2t6A in Vivo
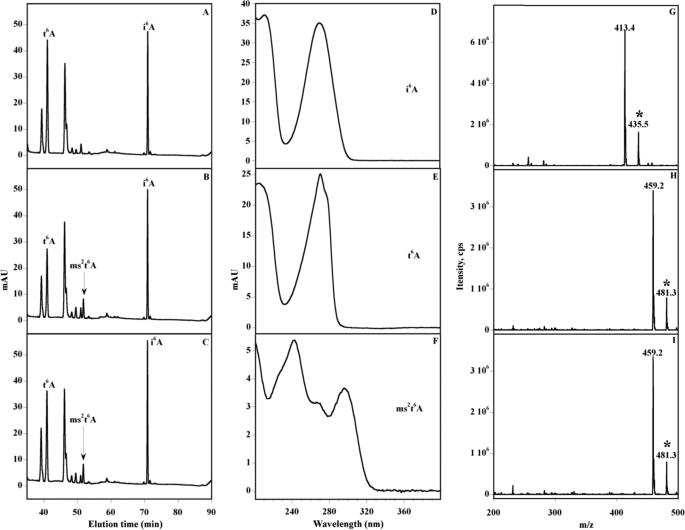
To test the function of the corresponding proteins, we assayed the influence of the yqeV and CDKAL1 genes on tRNA modification in vivo in E. coli strain TX3346, which lacks a functional miaB gene. This strain has the advantage of accumulating i6A-37, as a consequence of the inactivation of the miaB gene, and also containing t6A-37, because E. coli K12 does not encode any enzyme-catalyzing methylthiolation of this nucleoside. The TX3346 strain was transformed with either plasmid pT7-mtab expressing the B. subtilis yqeV/mtaB gene or plasmid pT7-emtab expressing the human CDKAL1/e-MtaB gene. Bulk tRNAs were isolated after growth at 37 °C, hydrolyzed, and processed for HPLC analysis of their modified nucleosides, as described previously (24). Under these conditions, chromatograms of tRNA hydrolysates from the control TX3346 strain showed, as expected, both t6A-37 and i6A-37 eluting at 41 and 71 min, respectively, with no evidence for the presence of ms2i6A-37 and ms2t6A-37 (Fig. 2A). The identity of i6A and t6A was confirmed first by their chromatographic retention times and UV-visible spectra (Fig. 2, D and E) (24) and second by coupled HPLC/mass spectrometry analysis. The latter revealed the presence of a compound eluting at 41 min that could be assigned to t6A on the basis of the m/z ratio of its protonated pseudo-molecular ion (MH+ = 413.4) (Fig. 2G), in good agreement with the theoretical value for the unprotonated molecular weight of M = 412.4. In contrast, HPLC analysis of tRNA extracted from the E. coli TX3346 transformed with either plasmid pT7-mtab or plasmid pT7-emtab clearly showed no evidence for the presence of ms2i6A in the chromatogram, with the peak corresponding to i6A remaining essentially unchanged in intensity and the presence of a new peak eluting at 52 min (Fig. 2, B and C). The elution time (Fig. 2, B and C) and UV-visible spectrum (Fig. 2F) of the new peak are identical to that of ms2t6A (24). By HPLC/MS, the corresponding protonated pseudo-molecular ions MH+ was found at m/z = 459.2 (Fig. 2, H and I), in excellent agreement with the theoretical value for the unprotonated molecular weight of M = 458.4. These results demonstrate that B. subtilis YqeV/MtaB and human CDKAL1/e-MtaB proteins are both functional in vivo and selectively involved in the conversion of t6A to ms2t6A.
FIGURE 2.
HPLC, UV-visible detection, and mass spectra of i6A, t6A, and ms2t6A modified nucleosides using E. coli. The chromatograms correspond to the analysis (45–90-min region) of bulk tRNA from the following: miaB− TX3346 E. coli strain (A), complemented with pT7-mtab (B) and pT7-emtab (C). The UV-visible spectra of the i6A (D), t6A (E) and ms2t6A (F) and the corresponding mass t6A (G) ms2t6A obtained after complementation with pT7-mtab (H), pT7-emtab (I). The experiments have been run in triplicate, and the areas have been found to be reproducible within a 5% margin error. Mass spectrometry detection was carried out in neutral loss mode to obtain a high specificity as described under “Materials and Methods.” The peak denoted with asterisk corresponds to the Na+-protonated pseudo-molecular ions for t6A (G) (MH+ = 435.5) and ms2t6A (H and I) (MH+ = 481.3). mAU, milli-arbitrary units.
Whether the predicted iron-sulfur cluster common to all members of the radical AdoMet family was required for activity was investigated by studying site-directed mutants in which the three conserved cysteines of the conserved CXXXCXXC sequence in MtaB and e-MtaB have been changed to alanine. Using the in vivo assay described above, we found that the miaB− E. coli TX3346 strain transformed with pT7-mtaB-Cys-Ala and pGEX6P-emtab-Cys-Ala was unable to produce the ms2t6A-modified nucleoside (data not shown). This provides, as expected, strong evidence that the cluster is required for activity.
In Vivo Experimental Validation of the Function of YqeV/MtaB by Using B. subtilis MGNA-C496 Strain
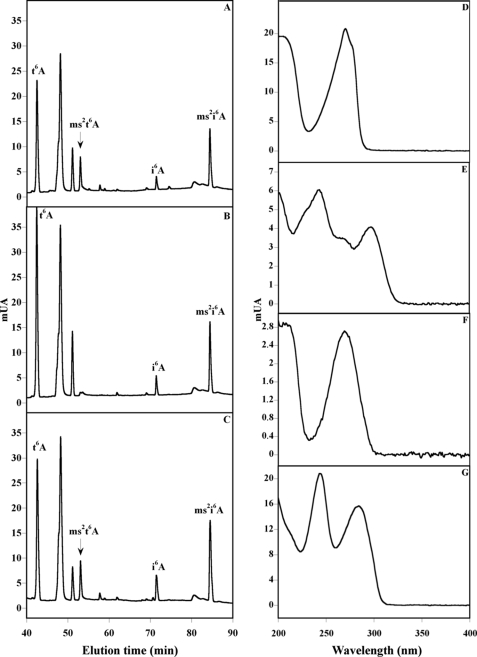
To obtain further evidence that MtaB from B. subtilis is the enzyme that is involved in the transformation of t6A into ms2t6A, we analyzed bulk tRNA extracted from the following: (i) B. subtilis wild-type strain (MGNA-A001); (ii) yqeV-strain (MGNA-C496) lacking the mtab gene; and (iii), MGNA-C496 complemented with a plasmid containing the yqeV (mtab) gene. Bulk tRNAs were isolated after growth at 37 °C, hydrolyzed, and processed for HPLC analysis of their modified nucleosides, as described previously (24). Under these conditions, chromatograms of tRNA hydrolysates from the B. subtilis wild-type MGNA-A001 strain showed, as expected, in the 45–90-min region the presence of t6A, ms2t6A, i6A, and ms2i6A that elutes at 42, 53, 71, and 85 min, respectively (Fig. 3A). The identity of the four modified nucleosides was established by their chromatographic retention times and UV-visible spectra (Fig. 3, A and D–G) (24). In Fig. 3B is shown the HPLC of tRNA hydrolysates obtained from MGNA-C496 strain. The analysis revealed that the 53-min peak detected on the UV trace in the parental strain (MGNA-A001), corresponding to the ms2t6A, disappeared in the MGNA-C496 strain, and the t6A peak increased in intensity. The ms2t6A peak was recovered when this strain was transformed with a plasmid carrying the wild-type yqeV/mtaB gene (plasmid pDB148-mtaB) confirming that the absence of ms2t6A modification in tRNAs extracted from the MGNA-C496 was due to the loss of yqeV/mtaB gene.
FIGURE 3.
HPLC and UV-visible detection of t6A, ms2t6A, i6A, and ms2i6A modified nucleosides using B. subtilis. The chromatograms correspond to the analysis (45–90-min region) of bulk tRNA from the following: MGNA-001 B. subtilis wild-type strain (A); MGNA-C496 B. subtilis yqeV− strain (B); MGNA-C496 B. subtilis yqeV− strain complemented with the pDB148-yqeV plasmid (C). The UV-visible spectra of the t6A (D), ms2t6A (E), i6A (F), and ms2i6A (G) are shown. The experiments have been run in triplicate, and the areas have been found to be reproducible within a 5% margin error. mAU, milli-arbitrary units.
In Vitro Characterization of the B. subtilis MtaB (YqeV) Protein
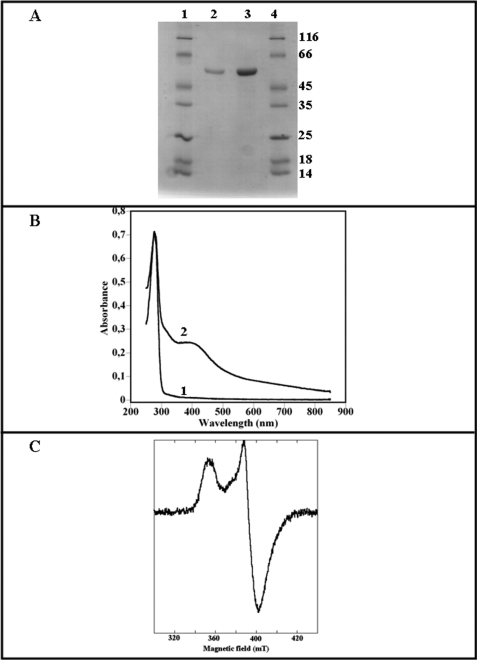
We concentrated initially on purification and in vitro characterization of the B. subtilis YqeV/MtaB protein because of the generally greater difficulty of purifying human proteins in functional form. Induction of YqeV/MtaB expression from plasmid pT7-mtab in E. coli strain BL21(DE3)RIL resulted in the overproduction of a protein migrating at ∼50,000 Da on SDS gels, in good agreement with the molecular mass deduced from amino acid sequence (51,657 Da). The expressed protein was found in the soluble fraction of cell-free extracts. After the final step of purification, the purity was evaluated by SDS-PAGE to be over 95% (Fig. 4A). The as-purified protein was light brown in color and found to contain low amounts of both iron and sulfur atoms (<0.2 iron, sulfur per monomer). This suggested the presence of a protein-bound iron-sulfur cluster, however, in substoichiometric amounts, probably as a consequence of cluster losses during aerobic purification. Anaerobic treatment of the protein solution with an excess of ferrous iron and enzymatically produced sulfide generated a form of YqeV/MtaB protein, which after desalting contained up to 7.5 ± 0.2 iron and 7.5 ± 0.5 sulfur atoms per polypeptide chain, suggesting the presence of two [4Fe-4S] clusters.
FIGURE 4.
Biochemical and spectroscopic characterization of MtaB protein. A, SDS-polyacrylamide (12%) gel of MtaB after Superdex 75 chromatography (2 and 6 μg, lanes 2 and 3, respectively). Molecular weight markers are in lanes 1 and 4. B, light absorption spectra of apo-MtaB (4 μm) (trace 1) and holo-MtaB (8 μm) (trace 2) MtaB in 50 mm Tris-Cl, pH 8.0, with 50 mm KCl. C, X-band EPR spectrum of the reduced holo-MtaB (100 μm) MtaB in 50 mm Tris-Cl, pH 8.0, with 50 mm KCl. Experimental conditions are as follows: microwave power, 100 microwatts; microwave frequency, 9.6 GHz; modulation amplitude 1 millitesla; temperature 12 K.
The UV-visible spectrum of reconstituted MtaB (Fig. 4B trace 2) displays a broad absorption band centered at around 420 nm, which is assigned to sulfur-to-iron charge transfer transitions characteristic of a [4Fe-4S]2+ cluster. This absorption band has an A420/A280 ratio of 0.34 ± 0.02 and a molar extinction coefficient at 400 nm of 30,000 mm−1 cm−1 in the lower range of most biological [4Fe-4S]2+ centers (ϵ420 = 15–17 mm−1 cm−1 on a per cluster basis) suggesting that the reconstituted protein contains slightly less than two [4Fe-4S] clusters per polypeptide chain. Upon addition of dithionite, the absorption decreased over the entire 350–700 nm range, as expected upon reduction of the chromophore to the [4Fe-4S]+ state (data not shown). EPR analysis of the resulting reduced protein confirmed the presence of S = ½, [4Fe-4S]1+ centers (Fig. 4C).
Conclusion
The physiological role of CDKAL1/e-MtaB gene in eukaryotic cells remains to be elucidated. However, its similarity to CDK5RAP1 led to the speculation that it might play an important role in insulin production under glucotoxic conditions through interaction with CDK5 that belongs to the well known large family of cyclin-dependent kinases 16). Our in vivo studies now indicate that it encodes a radical AdoMet MTTase that is involved in biosynthesis of 2-methylthio-N6-threonylcarbamoyladenosine (ms2t6A) in tRNA. We have also shown that B. subtilis yqeV/mtaB encodes a protein with equivalent biochemical activity, even though it belongs to a different MTTase sequence family. Thus, ms2t6A is synthesized through the conversion of A to t6A by the action of YrdC/Sua5 enzymes, as recently shown (15), followed by the transformation of t6A to ms2t6A by the action of either CDKAL1/e-MtaB or YqeV/MtaB.
It is now well established that the MTTase enzymes that catalyze the methylthiolation reactions belong to a class of the radical AdoMet iron-sulfur enzyme family containing two [4Fe-4S] clusters. The enzymatic reaction proceeds through the following steps: (i) AdoMet reductive cleavage promoted by the radical AdoMet [4Fe-4S]1+/2+ cluster to generate a 5′-deoxyadenosyl radical, Ado•; (ii) selective H atom abstraction at the substrate by Ado•; (iii) reaction of the resulting intermediate radical with a second [4Fe-4S]1+/2+ cluster in the N-terminal UPF0004 domain, by an unknown mechanism, to generate a thiolated intermediate; and (iv) methylation at the introduced sulfur atom most probably through the reaction with the electrophilic methyl group of a second AdoMet molecule. The observation that YqeV/MtaB is able to bind two redox-active [4Fe-4S]1+/2+ clusters is thus in full agreement with its involvement in a methylthiolation reaction.
To our knowledge five naturally occurring methylthio modifications have been reported so far; one of these occurs on a aspartic acid residue in ribosomal protein S12 and is catalyzed by RimO family (4, 5). The other four all occur on the adenosine base found at position 37 adjacent to the 3′-end of the anticodon. This modification appears to be essential for efficient and highly accurate protein translation by the ribosome (13). The 2-methylthio-N6-isopentenyladenosine (ms2i6A) is produced by the MTTase from the MiaB family (22). In this study, we demonstrate that the ms2t6A is produced by MTTases from either YqeV/MtaB or CDKAL1/e-MtaB families. There is as yet no experimental data establishing the identity of the enzymes catalyzing biosynthesis of 2-methylthio-N6-hydroxynorvalyl-carbamoyladenosine (ms2hn6A) or the 2-methylthio-N6-methyladenosine (ms2m6A). The occurrence of 2-methylthio-N6-methyladenosine remains to be confirmed because only one organism (Thermodesulfobacterium commune, not yet completely sequenced) has been reported to contain this hypermodified base. The case of ms2hn6A is more intriguing. This base has been observed in Thermotoga maritima (26), which encodes three MTTases (MiaB, MtaB, and RimO, Fig. 1) but has been shown to contain four methylthiolated derivatives as follows: Asp-89 in ribosomal protein S12, ms2i6A, ms2t6A, and ms2hn6A (5, 26, 28). Based on the likelihood that all of methylthiolation reactions are catalyzed by radical AdoMet MTTases, there are two possible explanations for the generation of ms2hn6A. One possibility, as suggested previously (3), is that YqeV/MtaB family members have broad substrate specificity and can modify both t6A and hn6A bases, which differ only by the presence of an additional methyl group in hn6A. Alternatively, a specific methylase could generate ms2hn6A from ms2t6A after production of this base by YqeV/MtaB. Further experiments are needed to distinguish between these possibilities.
The same methylthiolation reaction in tRNAs is catalyzed by different enzymes depending on the nature of the alkyl group at the N6 of A-37. Interesting questions remain unanswered concerning the mechanism by which the active sites of MTTases control the selective recognition of the modified adenine 37 on tRNA substrates. Understanding these mechanisms will require the determination of the three-dimensional structure of enzymes such as MiaB, MtaB, and e-MtaB alone and in complex with their tRNA substrates.
Acknowledgments
We thank Jon D. Luff for bioinformatics support and Serge Gambarelli for providing EPR facilities. We thank the National BioResource Project (NIG, Japan) for generously providing the B. subtilis, wild-type (MGNA-A001), and yqeV- (MGNA-C496) strains. We also thank François Denizot for the generous gifts of pDG148-Stu plasmid. We are grateful to Dr. Jean-Michel Jault, Anne-Emmanuelle Foucher, and Maria-Halima Laaberki for helpful discussion about the manipulation of B. subtilis strains.
Addendum
During the submission process of this study, a closely related paper appeared in the literature (Anton, B. P., Russell, S. P., Vertrees, J., Kasif, S., Raleigh, E. A., Limbach, P. A., and Roberts, R. J. (2010) Nucleic Acids Res., in press) that presents some results similar to ours on the B. subtilis YmcB/YqeV enzymes, in general agreement with our data. However, our study is substantially broader in the scope of both its bioinformatic and experimental analyses.
The nucleotide sequence(s) reported in this paper has been submitted to the Gen-BankTM/EBI Data Bank with accession number(s) BSU25430
S. K. Handelman and J. F. Hunt, submitted for publication.
- MTTase
- methylthiotransferase
- t6A
- N6-threonylcarbamoyladenosine
- ms2t6A
- 2-methylthio-N6-threonylcarbamoyladenosine
- AdoMet
- S-adenosylmethionine
- ms2i6A
- 2-methylthio-N6-isopentenyladenosine
- ms2hn6A
- 2-methylthio-N6-hydroxynorvalyl-carbamoyladenosine.
REFERENCES
- 1.Fontecave M., Mulliez E., Atta M. (2008) Chem. Biol. 15, 209–210 [DOI] [PubMed] [Google Scholar]
- 2.Pierrel F., Douki T., Fontecave M., Atta M. (2004) J. Biol. Chem. 279, 47555–47563 [DOI] [PubMed] [Google Scholar]
- 3.Anton B. P., Saleh L., Benner J. S., Raleigh E. A., Kasif S., Roberts R. J. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 1826–1831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee K. H., Saleh L., Anton B. P., Madinger C. L., Benner J. S., Iwig D. F., Roberts R. J., Krebs C., Booker S. J. (2009) Biochemistry 48, 10162–10174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arragain S., Garcia-Serres R., Blondin G., Douki T., Clemancey M., Latour J. M., Forouhar F., Neely H., Montelione G. T., Hunt J. F., Mulliez E., Fontecave M., Atta M. (2010) J. Biol. Chem. 285, 5792–5801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernández H. L., Pierrel F., Elleingand E., García-Serres R., Huynh B. H., Johnson M. K., Fontecave M., Atta M. (2007) Biochemistry 46, 5140–5147 [DOI] [PubMed] [Google Scholar]
- 7.Sofia H. J., Chen G., Hetzler B. G., Reyes-Spindola J. F., Miller N. E. (2001) Nucleic Acids Res. 29, 1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fontecave M., Mulliez E., Ollagnier-de-Choudens S. (2001) Curr. Opin. Chem. Biol. 5, 506–511 [DOI] [PubMed] [Google Scholar]
- 9.Fontecave M., Ollagnier-de-Choudens S., Mulliez E. (2003) Chem. Rev. 103, 2149–2166 [DOI] [PubMed] [Google Scholar]
- 10.Anantharaman V., Koonin E. V., Aravind L. (2001) FEMS Microbiol. Lett. 197, 215–221 [DOI] [PubMed] [Google Scholar]
- 11.Durant P. C., Bajji A. C., Sundaram M., Kumar R. K., Davis D. R. (2005) Biochemistry 44, 8078–8089 [DOI] [PubMed] [Google Scholar]
- 12.McCrate N. E., Varner M. E., Kim K. I., Nagan M. C. (2006) Nucleic Acids Res. 34, 5361–5368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustilo E. M., Vendeix F. A., Agris P. F. (2008) Curr. Opin. Microbiol. 11, 134–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elkins B. N., Keller E. B. (1974) Biochemistry 13, 4622–4628 [DOI] [PubMed] [Google Scholar]
- 15.El Yacoubi B., Lyons B., Cruz Y., Reddy R., Nordin B., Agnelli F., Williamson J. R., Schimmel P., Swairjo M. A., de Crécy-Lagard V. (2009) Nucleic Acids Res. 37, 2894–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinthorsdottir V., Thorleifsson G., Reynisdottir I., Benediktsson R., Jonsdottir T., Walters G. B., Styrkarsdottir U., Gretarsdottir S., Emilsson V., Ghosh S., Baker A., Snorradottir S., Bjarnason H., Ng M. C., Hansen T., Bagger Y., Wilensky R. L., Reilly M. P., Adeyemo A., Chen Y., Zhou J., Gudnason V., Chen G., Huang H., Lashley K., Doumatey A., So W. Y., Ma R. C., Andersen G., Borch-Johnsen K., Jorgensen T., van Vliet-Ostaptchouk J. V., Hofker M. H., Wijmenga C., Christiansen C., Rader D. J., Rotimi C., Gurney M., Chan J. C., Pedersen O., Sigurdsson G., Gulcher J. R., Thorsteinsdottir U., Kong A., Stefansson K. (2007) Nat. Genet. 39, 770–775 [DOI] [PubMed] [Google Scholar]
- 17.Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z., Miller W., Lipman D. J. (1997) Nucleic Acids Res. 25, 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chenna R., Sugawara H., Koike T., Lopez R., Gibson T. J., Higgins D. G., Thompson J. D. (2003) Nucleic Acids Res. 31, 3497–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S., Tamura K., Nei M. (2004) Brief. Bioinformatics 5, 150–163 [DOI] [PubMed] [Google Scholar]
- 20.Joseph P., Fantino J. R., Herbaud M. L., Denizot F. (2001) FEMS Microbiol. Lett. 205, 91–97 [DOI] [PubMed] [Google Scholar]
- 21.Yang M. M., Zhang W. W., Bai X. T., Li H. X., Cen P. L. (2009) Mol. Biol. Rep. 37, 2207–2213 [DOI] [PubMed] [Google Scholar]
- 22.Pierrel F., Björk G. R., Fontecave M., Atta M. (2002) J. Biol. Chem. 277, 13367–13370 [DOI] [PubMed] [Google Scholar]
- 23.Turgay K., Hahn J., Burghoorn J., Dubnau D. (1998) EMBO J. 17, 6730–6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gehrke C. W., Kuo K. C. (1989) J. Chromatogr. 471, 3–36 [DOI] [PubMed] [Google Scholar]
- 25.Fish W. W. (1988) Methods Enzymol. 158, 357–364 [DOI] [PubMed] [Google Scholar]
- 26.Reddy D. M., Crain P. F., Edmonds C. G., Gupta R., Hashizume T., Stetter K. O., Widdel F., McCloskey J. A. (1992) Nucleic Acids Res. 20, 5607–5615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auxilien S., Keith G., Le Grice S. F., Darlix J. L. (1999) J. Biol. Chem. 274, 4412–4420 [DOI] [PubMed] [Google Scholar]
- 28.Pierrel F., Hernandez H. L., Johnson M. K., Fontecave M., Atta M. (2003) J. Biol. Chem. 278, 29515–29524 [DOI] [PubMed] [Google Scholar]
- 29.Esberg B., Leung H. C., Tsui H. C., Björk G. R., Winkler M. E. (1999) J. Bacteriol. 181, 7256–7265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tabor S., Richardson C. C. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 1074–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]