Abstract
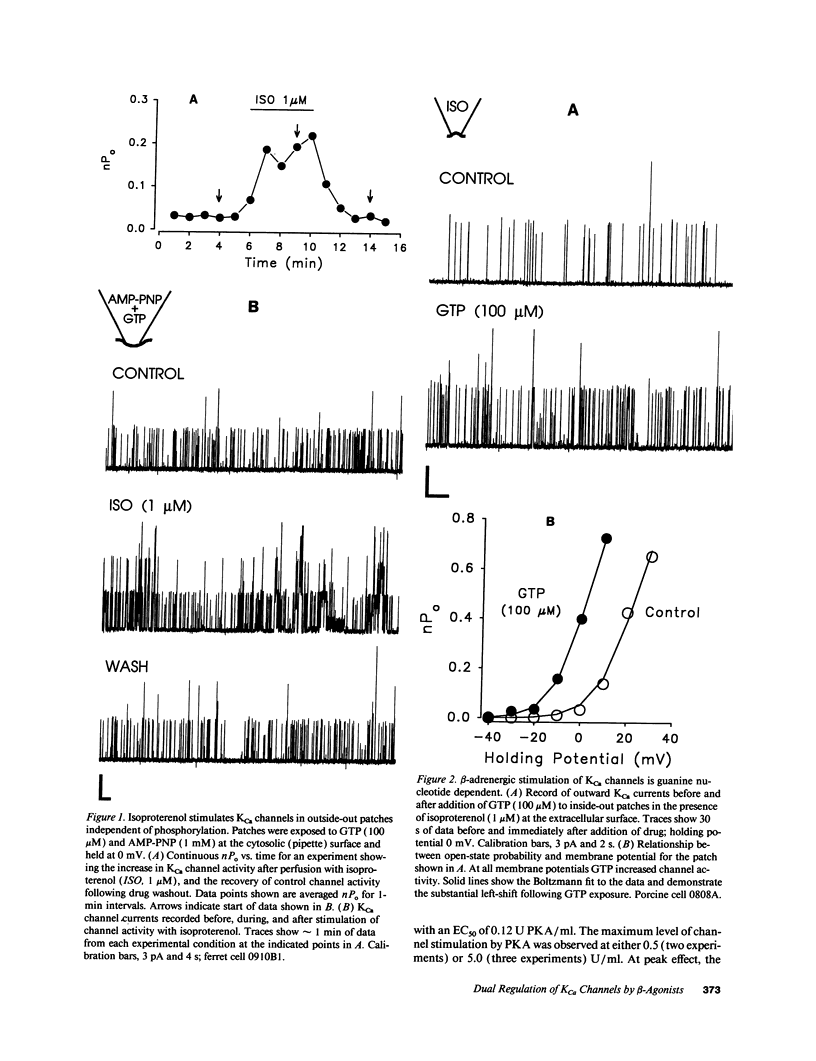
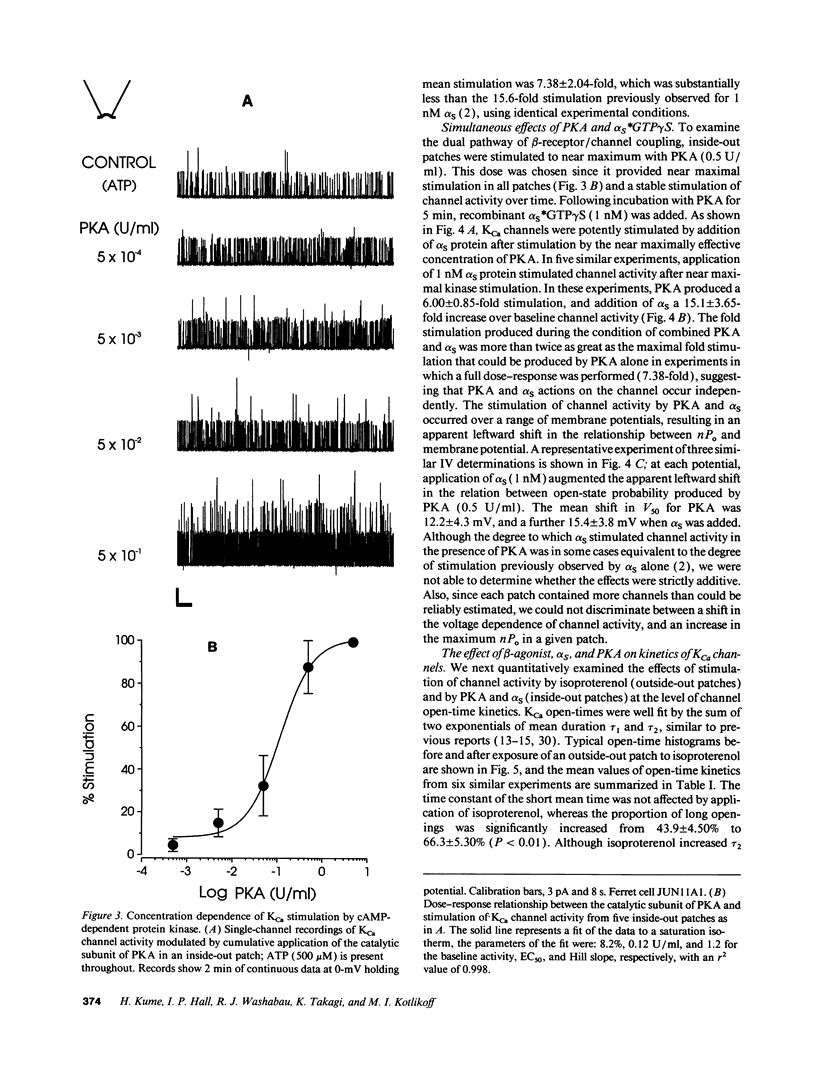
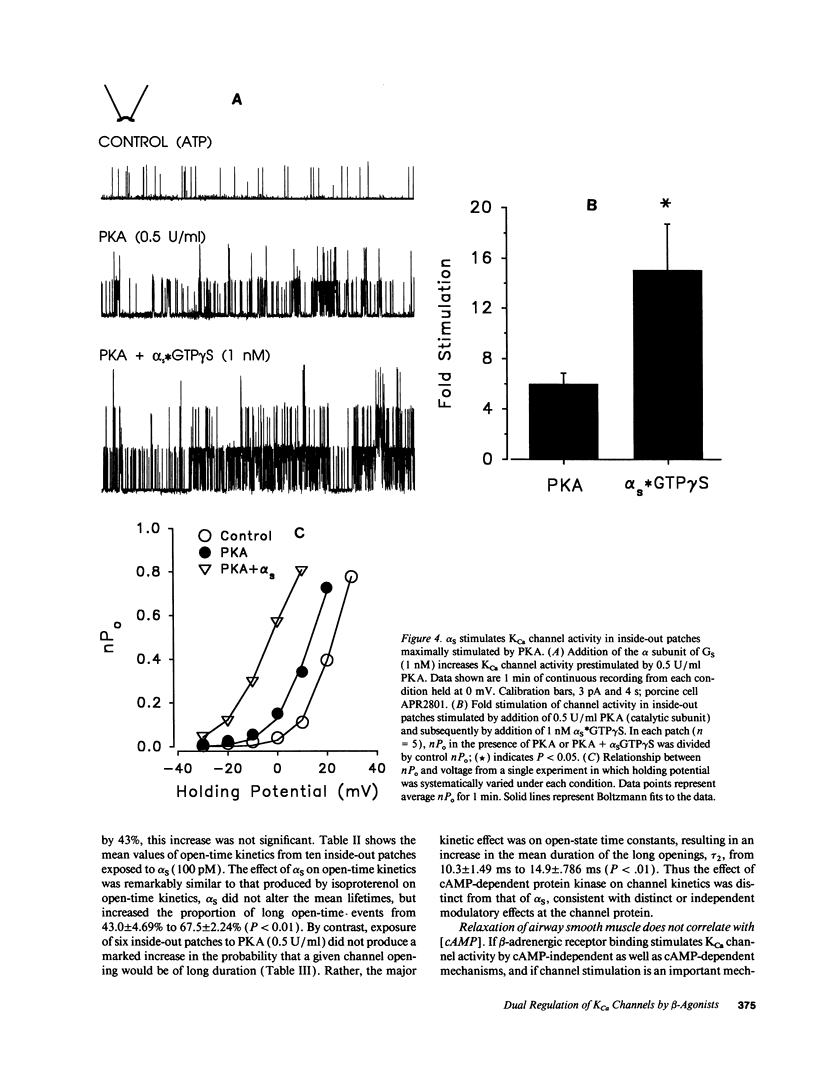
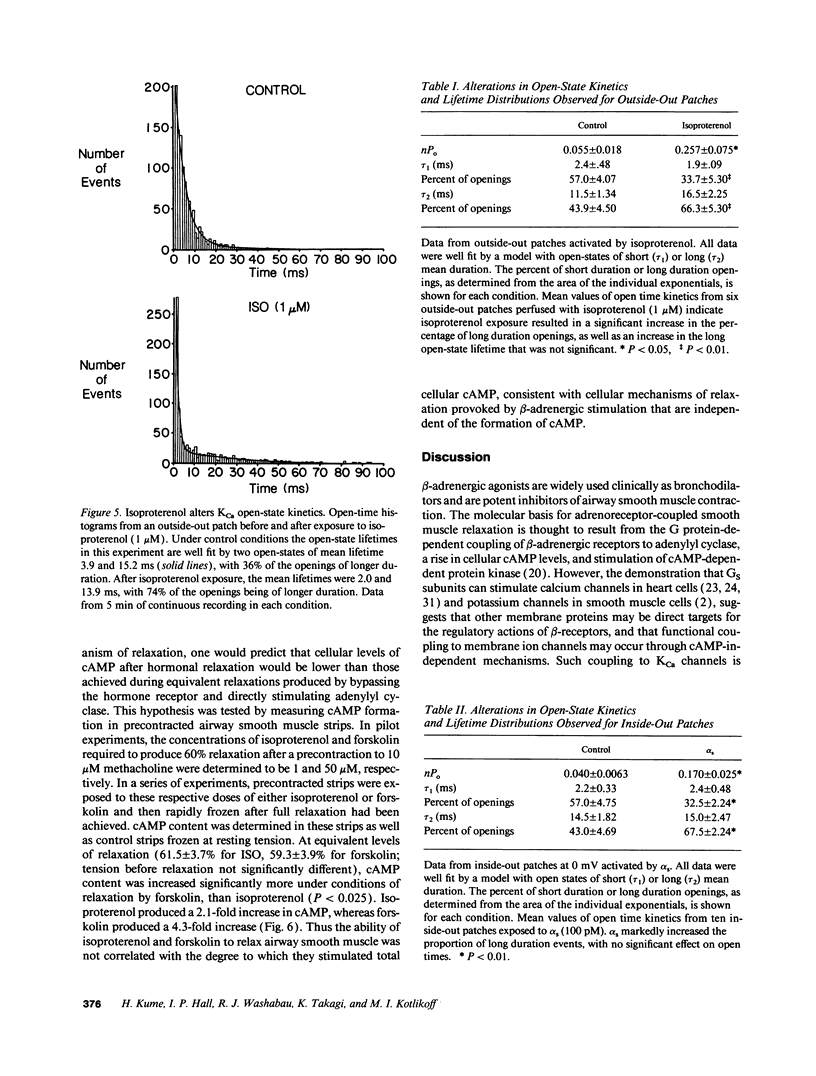
Stimulation of calcium-activated potassium (KCa) channels in airway smooth muscle cells by phosphorylation-dependent and membrane-delimited, G protein actions has been reported (Kume, H. A. Takai, H. Tokuno, and T. Tomita. 1989. Nature [Lond.]. 341:152-154; Kume, H., M. P. Graziano, and M. I. Kotlikoff. 1992. Proc. Natl. Acad. Sci. USA. 89:11051-11055). We show that beta-adrenergic receptor/channel coupling is not affected by inhibition of endogenous ATP, and that activation of KCa channels is stimulated by both alpha S and cAMP-dependent protein kinase (PKA). PKA stimulated channel activity in a dose-dependent fashion with an EC50 of 0.12 U/ml and maximum stimulation of 7.38 +/- 2.04-fold. Application of alpha S to patches near maximally stimulated by PKA significantly increased channel activity to 15.1 +/- 3.65-fold above baseline, providing further evidence for dual regulatory mechanisms and suggesting that the stimulatory actions are independent. Analysis of channel open-time kinetics indicated that isoproterenol and alpha S stimulation of channel activity primarily increased the proportion of longer duration events, whereas PKA stimulation had little effect on the proportion of short and long duration events, but resulted in a significant increase in the duration of the long open-state. cAMP formation during equivalent relaxation of precontracted muscle strips by isoproterenol and forskolin resulted in significantly less cAMP formation by isoproterenol than by forskolin, suggesting that the degree of activation of PKA is not the only determinant of tissue relaxation. We conclude that beta-adrenergic stimulation of KCa channel activity and relaxation of tone in airway smooth muscle occurs, in part, by means independent of cyclic AMP formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen S. L., Beech D. J., Foster R. W., Morgan G. P., Small R. C. Electrophysiological and other aspects of the relaxant action of isoprenaline in guinea-pig isolated trachealis. Br J Pharmacol. 1985 Dec;86(4):843–854. doi: 10.1111/j.1476-5381.1985.tb11106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwer K., Toro L., Oberti C., Stefani E., Sanborn B. M. Ca(2+)-activated K+ channels in pregnant rat myometrium: modulation by a beta-adrenergic agent. Am J Physiol. 1992 Nov;263(5 Pt 1):C1049–C1056. doi: 10.1152/ajpcell.1992.263.5.C1049. [DOI] [PubMed] [Google Scholar]
- Boyle J. P., Tomasic M., Kotlikoff M. I. Delayed rectifier potassium channels in canine and porcine airway smooth muscle cells. J Physiol. 1992 Feb;447:329–350. doi: 10.1113/jphysiol.1992.sp019005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton I. L., Brunton L. L. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J Biol Chem. 1983 Sep 10;258(17):10233–10239. [PubMed] [Google Scholar]
- Carl A., Kenyon J. L., Uemura D., Fusetani N., Sanders K. M. Regulation of Ca(2+)-activated K+ channels by protein kinase A and phosphatase inhibitors. Am J Physiol. 1991 Aug;261(2 Pt 1):C387–C392. doi: 10.1152/ajpcell.1991.261.2.C387. [DOI] [PubMed] [Google Scholar]
- Daut J., Maier-Rudolph W., von Beckerath N., Mehrke G., Günther K., Goedel-Meinen L. Hypoxic dilation of coronary arteries is mediated by ATP-sensitive potassium channels. Science. 1990 Mar 16;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Fujiwara T., Sumimoto K., Itoh T., Suzuki H., Kuriyama H. Relaxing actions of procaterol, a beta 2-adrenoceptor stimulant, on smooth muscle cells of the dog trachea. Br J Pharmacol. 1988 Jan;93(1):199–209. doi: 10.1111/j.1476-5381.1988.tb11422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Graziano M. P., Freissmuth M., Gilman A. G. Expression of Gs alpha in Escherichia coli. Purification and properties of two forms of the protein. J Biol Chem. 1989 Jan 5;264(1):409–418. [PubMed] [Google Scholar]
- Gross R. A., Uhler M. D., Macdonald R. L. The cyclic AMP-dependent protein kinase catalytic subunit selectively enhances calcium currents in rat nodose neurones. J Physiol. 1990 Oct;429:483–496. doi: 10.1113/jphysiol.1990.sp018268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hei Y. J., MacDonell K. L., McNeill J. H., Diamond J. Lack of correlation between activation of cyclic AMP-dependent protein kinase and inhibition of contraction of rat vas deferens by cyclic AMP analogs. Mol Pharmacol. 1991 Feb;39(2):233–238. [PubMed] [Google Scholar]
- Honda K., Satake T., Takagi K., Tomita T. Effects of relaxants on electrical and mechanical activities in the guinea-pig tracheal muscle. Br J Pharmacol. 1986 Apr;87(4):665–671. doi: 10.1111/j.1476-5381.1986.tb14583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. R., Charette L., Garcia M. L., Kaczorowski G. J. Selective inhibition of relaxation of guinea-pig trachea by charybdotoxin, a potent Ca(++)-activated K+ channel inhibitor. J Pharmacol Exp Ther. 1990 Nov;255(2):697–706. [PubMed] [Google Scholar]
- Kotlikoff M. I. Calcium currents in isolated canine airway smooth muscle cells. Am J Physiol. 1988 Jun;254(6 Pt 1):C793–C801. doi: 10.1152/ajpcell.1988.254.6.C793. [DOI] [PubMed] [Google Scholar]
- Kume H., Graziano M. P., Kotlikoff M. I. Stimulatory and inhibitory regulation of calcium-activated potassium channels by guanine nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):11051–11055. doi: 10.1073/pnas.89.22.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H., Takagi K., Satake T., Tokuno H., Tomita T. Effects of intracellular pH on calcium-activated potassium channels in rabbit tracheal smooth muscle. J Physiol. 1990 May;424:445–457. doi: 10.1113/jphysiol.1990.sp018076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H., Takai A., Tokuno H., Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989 Sep 14;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Levitan I. B. Phosphorylation of ion channels. J Membr Biol. 1985;87(3):177–190. doi: 10.1007/BF01871217. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Pallotta B. S. Calcium dependence of open and shut interval distributions from calcium-activated potassium channels in cultured rat muscle. J Physiol. 1983 Nov;344:585–604. doi: 10.1113/jphysiol.1983.sp014957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann J. D., Welsh M. J. Calcium-activated potassium channels in canine airway smooth muscle. J Physiol. 1986 Mar;372:113–127. doi: 10.1113/jphysiol.1986.sp016000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M., Belvisi M. G., Stretton C. D., Yacoub M. H., Barnes P. J. Role of potassium channels in bronchodilator responses in human airways. Am Rev Respir Dis. 1992 Jul;146(1):132–136. doi: 10.1164/ajrccm/146.1.132. [DOI] [PubMed] [Google Scholar]
- Murray K. J. Cyclic AMP and mechanisms of vasodilation. Pharmacol Ther. 1990;47(3):329–345. doi: 10.1016/0163-7258(90)90060-f. [DOI] [PubMed] [Google Scholar]
- Murray M. A., Berry J. L., Cook S. J., Foster R. W., Green K. A., Small R. C. Guinea-pig isolated trachealis: the effects of charybdotoxin on mechanical activity, membrane potential changes and the activity of plasmalemmal K(+)-channels. Br J Pharmacol. 1991 Jul;103(3):1814–1818. doi: 10.1111/j.1476-5381.1991.tb09868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M. T., Huang Y., Brayden J. E., Hescheler J., Standen N. B. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990 Apr 19;344(6268):770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- Sadoshima J., Akaike N., Kanaide H., Nakamura M. Cyclic AMP modulates Ca-activated K channel in cultured smooth muscle cells of rat aortas. Am J Physiol. 1988 Oct;255(4 Pt 2):H754–H759. doi: 10.1152/ajpheart.1988.255.4.H754. [DOI] [PubMed] [Google Scholar]
- Sears M. R., Taylor D. R., Print C. G., Lake D. C., Li Q. Q., Flannery E. M., Yates D. M., Lucas M. K., Herbison G. P. Regular inhaled beta-agonist treatment in bronchial asthma. Lancet. 1990 Dec 8;336(8728):1391–1396. doi: 10.1016/0140-6736(90)93098-a. [DOI] [PubMed] [Google Scholar]
- Sims S. M., Singer J. J., Walsh J. V., Jr Antagonistic adrenergic-muscarinic regulation of M current in smooth muscle cells. Science. 1988 Jan 8;239(4836):190–193. doi: 10.1126/science.2827305. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Quayle J. M., Davies N. W., Brayden J. E., Huang Y., Nelson M. T. Hyperpolarizing vasodilators activate ATP-sensitive K+ channels in arterial smooth muscle. Science. 1989 Jul 14;245(4914):177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- Vegesna R. V., Diamond J. Effects of isoproterenol and forskolin on tension, cyclic AMP levels, and cyclic AMP dependent protein kinase activity in bovine coronary artery. Can J Physiol Pharmacol. 1984 Sep;62(9):1116–1123. doi: 10.1139/y84-187. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H., Honeyman T. W., Fay F. S. Beta-adrenergic actions on membrane electrical properties of dissociated smooth muscle cells. Am J Physiol. 1988 Mar;254(3 Pt 1):C423–C431. doi: 10.1152/ajpcell.1988.254.3.C423. [DOI] [PubMed] [Google Scholar]
- Yatani A., Codina J., Imoto Y., Reeves J. P., Birnbaumer L., Brown A. M. A G protein directly regulates mammalian cardiac calcium channels. Science. 1987 Nov 27;238(4831):1288–1292. doi: 10.1126/science.2446390. [DOI] [PubMed] [Google Scholar]
- Zhou H. L., Newsholme S. J., Torphy T. J. Agonist-related differences in the relationship between cAMP content and protein kinase activity in canine trachealis. J Pharmacol Exp Ther. 1992 Jun;261(3):1260–1267. [PubMed] [Google Scholar]



