Abstract
In the blood (hemolymph) of the silkworm Bombyx mori, the insect cytokine paralytic peptide (PP) is converted from an inactive precursor to an active form in response to the cell wall components of microorganisms and contributes to silkworm resistance to infection. To investigate the molecular mechanism underlying the up-regulation of host resistance induced by PP, we performed an oligonucleotide microarray analysis on RNA of blood cells (hemocytes) and fat body tissues of silkworm larvae injected with active PP. Expression levels of a large number of immune-related genes increased rapidly within 3 h after injecting active PP, including phagocytosis-related genes such as tetraspanin E, actin A1, and ced-6 in hemocytes, and antimicrobial peptide genes cecropin A and moricin in the fat body. Active PP promoted in vitro and in vivo phagocytosis of Staphyloccocus aureus by the hemocytes. Moreover, active PP induced in vivo phosphorylation of p38 mitogen-activated protein kinase (p38 MAPK) in the fat body. Pretreatment of silkworm larvae with ML3403, a pharmacologic p38 MAPK inhibitor, suppressed the PP-dependent induction of cecropin A and moricin genes in the fat body. Injection of active PP delayed the killing of silkworm larvae by S. aureus, whereas its effect was abolished by preinjection of the p38 MAPK inhibitor, suggesting that p38 MAPK activation is required for PP-dependent defensive responses. These findings suggest that PP acts on multiple tissues in silkworm larvae and acutely activates cellular and humoral immune responses, leading to host protection against infection.
Keywords: Antimicrobial Peptides, Cytokine, Innate Immunity, Insect, p38 MAPK, Phagocytosis
Introduction
The immune system is indispensable for host organisms to protect themselves against infections. Reactions that involve the production of specific antibodies are categorized as acquired immunity, and other reactions are collectively termed innate immunity. The innate immune system is the first-line of self-defense, and it triggers the activation of most immune reactions, including antibody production. In mammals, a wide variety of extracellular molecules named cytokines are produced by macrophages and lymphocytes and induce efficient clearance of microorganisms by transmitting information to other immune cells. On the other hand, excessive activation of cytokines can lead to serious pathology such as septic shock. Therefore, a better understanding of the immunologic functions of cytokines may provide clues to how to overcome infectious diseases. Because of its complexity, however, a complete picture of the cytokine network remains unclear.
Insects lack the ability to produce antibodies, so they rely completely on innate immunity to eliminate pathogenic microorganisms. The innate immune system in insects has many features in common with that in vertebrates (1). For example, blood cells (hemocytes) engulf microorganisms similar to mammalian macrophages (2). In addition, there are a number of well conserved microbicidal molecules such as lysozyme and antimicrobial peptides (AMPs)2 (3–6). Thus, insects with relatively simple biologic systems are very useful for studying innate immunity.
Recent studies on invertebrate immunity reported progress in understanding the precise mechanisms of immune responses. For example, when hemocytes engulf bacteria, several types of molecules, including cytoskeleton proteins, adhesion molecules, and intracellular signaling enzymes, act in concert (2). In insects, AMPs are produced via the Toll and Imd pathways in the fat body, the functional equivalent of the mammalian liver (7–10). For efficient clearance of pathogens, a single response is not sufficient; multiple pathways, including cellular and humoral factors, must be activated at the same time. Cytokines regulate multiple immune responses in mammals, whereas no molecule with such function has been reported in invertebrates.
In the silkworm Bombyx mori, a multifunctional cytokine-like factor named paralytic peptide (PP) is present in the blood (hemolymph) in an inactive form (11, 12). PP is activated when it is cleaved to a peptide comprising 23 amino acid residues, and the active PP induces paralysis accompanied by muscle contraction (11). PP belongs to the ENF peptide family based on its primary sequence, which is well conserved among Lepidoptera (13). In addition, the ENF family proteins have a tertiary structure that is similar to the C-terminal domain of mammalian epidermal growth factor (14). We recently reported that bacterial and fungal cell wall components induced the conversion of inactive PP to active PP in the silkworm hemolymph and that activated PP contributes to host protection against infectious pathogens (15). Although PP was speculated to stimulate several immune responses, the molecular mechanism of the up-regulation of host resistance by PP is not clear. In this study, we performed a genome-wide analysis to investigate the effect of active PP on gene expression in immune cells of silkworms. We found that active PP regulates the expression of various immune-related genes in the hemocytes and fat body. Furthermore, we demonstrated that active PP stimulates both phagocytosis of bacteria by hemocytes and production of AMPs from the fat body. These findings suggest that PP functions as an insect cytokine that regulates innate immunity by its effects on gene expression in multiple immune tissues.
EXPERIMENTAL PROCEDURES
Insects, Bacteria, and Reagents
Silkworm eggs (Hu·Yo × Tukuba·Ne) were purchased from Ehime Sanshu (Ehime, Japan). Silkworm larvae were reared on an antibiotic-free artificial diet at 27 °C. A methicillin-susceptible Staphylococcus aureus strain (MSSA-1) was harvested in tryptic soy broth (Becton, Dickinson) at 37 °C for 12–18 h. The active form of PP was chemically synthesized as described previously (12). Peptidoglycan from S. aureus, purchased from Sigma Fluka, was suspended in saline. Bovine serum albumin (BSA) (low endotoxin) was purchased from Nacalai Tesque. A pharmacologic p38 inhibitor, ML3403 ((RS)-{4-[5-(4-fluorophenyl)-2-methylsulfanyl-3H-imidazol-4-yl]pyridin-2-yl}-(1-phenylethyl)amine) (16) was purchased from Calbiochem and was solubilized in dimethyl sulfoxide (DMSO).
Oligonucleotide Microarray Analysis
Ten larvae (day 2 of fifth instar) per group were injected with 50 μl of active PP (4 μg/ml) or saline, followed by incubation at 27 °C for 3 h. Hemocytes and fat body tissues were collected in ice-cold tubes. Total RNA was extracted by RNeasy Mini kit (Qiagen), and cDNA was synthesized by reverse transcription. cRNA labeled by Cy3 or Cy5 was synthesized by the cDNA. The labeled cRNA was subjected to 4 × 44K oligonucleotide microarray system (no. 2515933; Agilent Technologies).
Quantitative Reverse Transcription (RT)-PCR Analysis
Three to 10 silkworm larvae (day 2 of fifth instar) were injected with 50 μl of saline, active PP (4 μg/ml, 1 μm), BSA (66 μg/ml, 1 μm), or peptidoglycan (1 mg/ml) followed by incubation at 27 °C for 3 h. Total RNA from hemocytes and fat body tissues was obtained as described above. After degrading the genomic DNA with RQ1 RNase-free DNase (Promega), cDNAs were synthesized by TaqMan reverse transcription reagents (Applied Biosystems) according to the manufacturer's instructions. Primers shown in supplemental Table S1, cDNAs, and a SYBR RT-PCR kit (Takara) were applied to perform real-time PCR. To test the effect of the pharmacologic p38 inhibitor, ML3403 was injected into larvae 0.5 h before the injection of active PP followed by an additional 3-h incubation at 27 °C, and gene expression was analyzed as described above.
In Vitro Phagocytosis Assay of Silkworm Hemocytes
Hemolymph of silkworm larvae (day 3–6 of fifth instar) was collected in an ice-cold tube (approximately 0.4 ml/larva) containing 1 mm benzamidine chloride dissolved in phosphate-buffered saline (PBS), pH 7.4. The hemolymph was centrifuged to separate hemocytes, and the precipitate was suspended in PBS (approximately 106 cells/ml). An overnight culture of S. aureus (1 × 109 cfu/ml) was centrifuged, and the precipitate was suspended in the same volume of PBS. The hemocyte suspension was mixed with 1/10 volume of S. aureus (the ratio of hemocytes and bacteria was 1:100), and various concentrations of active PP were added. After incubating at 27 °C for 0.5 h or 3 h, mixtures were centrifuged, and the precipitates were washed three times in PBS. To kill the extracellular bacteria, samples were further treated with Grace's insect cell culture medium (Invitrogen) containing 50 μg/ml gentamicin at 4 °C for 1 h. After two additional washes in PBS, aliquots of suspended samples were applied to a cytometer to count the number of hemocytes under a microscope. Suspensions were again centrifuged, and precipitates were added to 0.1% Triton X-100 to lyse the hemocytes. Samples were serially diluted in PBS and spread on mannitol salt agar plates to selectively isolate S. aureus. Plates were incubated at 37 °C, and cfu were determined.
In Vivo Phagocytosis of Fluorescence-labeled Bacteria by Hemocytes
Fluorescence-labeled bacteria (S. aureus, Wood strain without protein A, BioParticles Alexa Fluor 594 conjugate, S-23372) was purchased from Molecular Probes. The labeled bacteria (2 mg) was suspended in 100 μl of PBS and stored at −20 °C in the dark. In the phagocytosis assay, the suspension of the labeled bacteria was diluted 20-fold, and active PP (4 μg/ml) was added. Fifty microliters of the sample was then injected into 5 larvae (day 2 of fifth instar). After incubation at 27 °C for 3 h, hemolymph was collected in an ice-cold tube and centrifuged. Cells were fixed in 4% formaldehyde in PBS and observed under a fluorescence microscope (Leica DM4000 B). The number of fluorescent particles colocalized with hemocytes was counted in 10 microscopic fields (400–600 hemocytes).
In Vivo Phagocytosis of Live Bacteria by Hemocytes
Overnight-cultured S. aureus cells were suspended in saline (2 × 108 or 2 × 109 cfu/ml), and active PP (4 μg/ml) was added. Fifty microliters of the sample was then injected into four larvae (day 2 of fifth instar). After incubation at 27 °C for 3 h, hemolymph was collected in an ice-cold tube and centrifuged. The precipitated hemocytes were washed in PBS. As in the in vitro phagocytosis assay (see above), samples were further treated with 50 μg/ml gentamicin at 4 °C for 1 h. After two additional washes in PBS, aliquots of suspended samples were applied to a cytometer to count the number of hemocytes under a microscope. Suspensions were again centrifuged, and precipitates were added to 0.1% Triton X-100 to lyse the hemocytes. Samples were serially diluted in PBS and spread on mannitol salt agar plates to selectively isolate S. aureus. Plates were incubated at 37 °C, and cfu were determined.
In Vitro Binding Assay of Active PP to Bacteria
An overnight culture of S. aureus was centrifuged, and the precipitate was suspended in PBS to approximately 1010 cells/ml. After active PP was added (4 μg/ml), the suspension was incubated at 27 °C for 3 h. Bacterial cells were washed three times with PBS and subjected to SDS-PAGE. Detection of PP was described previously (15).
Detection of p38 and Phospho-p38 MAPK Proteins in the Silkworm Fat Body
Silkworm larvae (day 2 of fifth instar) were injected with 50 μl of BSA (66 μg/ml, 1 μm), active PP (4 μg/ml, 1 μm), or saline, followed by incubation at 27 °C. Fat body tissues were dissected and homogenized in insect physiological saline. Proteins were separated in 12.5% SDS-PAGE and transferred to Immobilon-P polyvinylidene difluoride membrane (Millipore). To detect p38 protein, 5% skim milk in TBST (Tris-buffered saline, pH 7.6, containing 0.1% Tween 20) was used as a blocking solution, and 1/1,000 diluted p38 MAPK antibody from rabbit (code 9212; Cell Signaling Technology) was used as the primary antibody. To detect phosphorylated p38 protein, 5% phosphoBLOCKERTM blocking reagent (Cell BioLabs) in TBST was used as a blocking solution, and 1/1,000 diluted phospho-p38 MAPK (Thr180/Tyr182) antibody from rabbit (code 9211; Cell Signaling Technology) was used as the primary antibody. Horseradish peroxidase-linked anti-rabbit Ig from donkey (GE Healthcare) was used as the secondary antibody in both cases. Bands were detected by Western LightningTM Chemiluminescence Reagent Plus (PerkinElmer Life Sciences) and Hyperfilm ECL (GE Healthcare).
In Vivo Melanization Assay
Silkworm larvae (day 2 of fifth instar) were injected with 50 μl of saline, active PP (4 μg/ml), or peptidoglycan from S. aureus (1 mg/ml) in the presence or absence of the p38 MAPK inhibitor ML3403 (1 mm). Three hours later, hemolymph was collected, and phenylthiourea (1 mm), an inhibitor of phenoloxidase, was added immediately to prevent further melanization after bleeding. After centrifugation, the A490 values of the supernatant fractions (hemolymph plasma) were measured by a spectrometer.
Effect of p38 MAPK Inhibitor on Host Resistance to Bacteria
Silkworm larvae (day 2 of fifth instar) were injected with 1 mm p38 MAPK inhibitor ML3403 or 10% DMSO. After 0.5 h, larvae were further injected with a 1/100 diluted suspension of an overnight culture of S. aureus (1 × 108 cfu/larva) supplemented with active PP (4 μg/ml). Survival rates of 10 larvae/group were estimated. For statistical analysis, the data were plotted with the Kaplan-Meier method using Prism 5 (GraphPad Software) and tested for significance by using the log-rank test.
RESULTS
Genome-wide Analysis of Gene Expression Regulated by Active PP
In vertebrates, cytokines produced by specific cells act on other immune cells and induce changes in gene expression. In insects, however, immunologic factors that regulate gene expression in multiple immune cells have not been identified. We previously reported that PP activation in silkworms leads to resistance against bacterial infection (15). The active form of PP induces a variety of physiologic actions such as slow and continuous muscle contraction, spreading of hemocytes, and inhibition of the release of premature hemocytes from hematopoietic organs (11, 12). We hypothesized that active PP is capable of inducing the expression of immune-related genes in the tissues that contribute to self-defense.
First, we injected silkworm larvae with active PP (200 ng/larva) and observed strong paralysis that lasted for 30–40 min. The amount of PP used in this experiment was sufficient to delay death in silkworms infected by S. aureus, as described previously (15). After 3 h, we isolated the hemocytes and fat body tissues to extract total RNA and subjected the RNA to oligonucleotide microarray analysis. Injection of active PP caused no significant change in the population or number of hemocytes, based on microscopic observation (data not shown). The microarray analysis revealed a >2-fold change in the expression levels of >400 gene clones in hemocytes and 200 gene clones in the fat body. As for the immune-related genes annotated in the database, active PP up-regulated the expression of three genes for surface molecules, four genes for cytoskeleton proteins, six genes for lipoproteins, one gene for serine protease (scolexin), and three genes for heat shock proteins in hemocytes; two genes for AMPs, two genes for lipoproteins, and one gene for peptidoglycan recognition protein (BmPGRP-S2) in the fat body. The expression of other genes involved in metabolism and development was also affected. A number of genes with altered expression profiles after injecting active PP have not yet been annotated. Some of those uncharacterized genes induced by active PP share homology with immune-related genes in other insects, like a gene (China gene model ID: BGIBMGA002396, microarray clone ID: Ka06397) that shares homology with the Drosophila spaeztle family (25% identity in the predicted protein sequence), and a gene (BGIBMGA003614, Ka05904) that possesses a tumor necrosis factor (TNF) family signature and showed 27% identity in the predicted protein sequence to eiger, a Drosophila TNF ortholog.
Quantitative Analysis of Gene Expression Levels Altered by Active PP
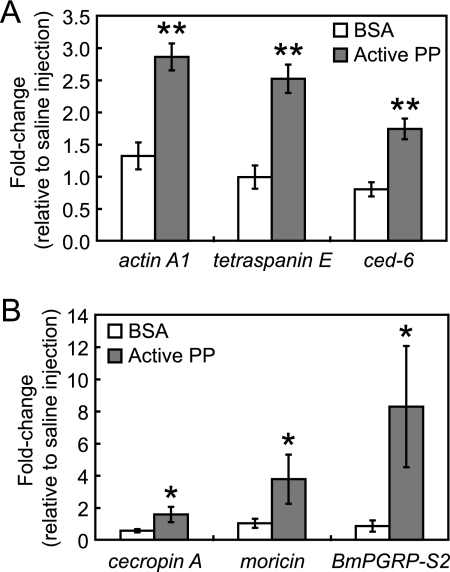
In invertebrates such as Drosophila melanogaster (17, 18) and B. mori (19), expression of genes encoding phagocytosis-associated proteins (adhesion molecules, cytoskeleton, adaptor molecules, etc.), AMPs, and pattern recognition receptors are up-regulated by infectious stimuli. Among the genes with altered expression profiles in hemocytes and the fat body after PP injection, we quantified the mRNA levels of immune-related genes by RT-PCR. In hemocytes from PP-injected silkworms, tetraspanin E, actin A1, troponin I, and ced-6 genes exhibited increased mRNA levels 2–7-fold (Table 1). In the fat body, active PP up-regulated the expression of cecropin A, moricin, and BmPGRP-S2 2–6-fold (Table 1). Furthermore, we examined the effect of BSA on the expression of these genes to determine the specificity of the PP-mediated responses. Three hours after injection of BSA or active PP into silkworm larvae, the mRNA levels of above immune-related genes (tetraspanin E, actin A1, and ced-6 in the hemocytes, and cecropin A, moricin and BmPGRP-S2 in the fat body) were analyzed by quantitative RT-PCR. In our experimental condition, the induction rates of immune-related genes in PP-injected groups were higher than those in BSA-injected groups (Fig. 1), suggesting that the effect of PP on gene expression was not due to nonspecific stimulation caused by exogenous proteins. These findings demonstrate that PP activates the expression of a distinct set of genes in hemocytes and the fat body, the major tissue involved in cellular and humoral immunity, respectively.
TABLE 1.
Quantitative RT-PCR analysis of immune-related genes
Amounts of mRNAs of immune-related genes in hemocytes and fat body 3 h after injection of saline or 4 μg/ml active PP were analyzed by quantitative RT-PCR. Values indicate fold changes in mRNA quantities of PP-injected groups relative to saline-injected groups at 3 h after injection. Data represent mean ± S.D. of six experiments.
| Tissue | Gene | Fold-change relative to saline injection |
|---|---|---|
| Hemocyte | tetraspanin E | 1.8 ± 0.1 |
| actin A1 | 5.0 ± 1.4 | |
| troponin I | 6.9 ± 1.6 | |
| ced-6 | 2.0 ± 0.2 | |
| Fat body | cecropin A | 2.1 ± 0.5 |
| moricin | 3.5 ± 0.9 | |
| BmPGRP-S2 | 6.4 ± 3.9 |
FIGURE 1.
Induction of immune-related genes by BSA or active PP. Silkworm larvae were injected with 50 μl/larva of saline, BSA (66 μg/ml, 1 μm), or active PP (4 μg/ml, 1 μm), and after 3 h RNAs were extracted from hemocytes and fat body tissues. mRNA amounts of immune-related genes in hemocytes (A) and fat body (B) were analyzed by quantitative RT-PCR. mRNA quantity of each gene was normalized to that of a control gene, elongation factor 2, whose expression is not affected by injection of the samples. Furthermore, fold changes in mRNA quantities of BSA- or PP-injected groups relative to saline-injected groups were estimated and are shown on the longitudinal axis. Data represent mean ± S.D. (error bars) of four experiments. Statistical significance was determined by Student's t test (*, p < 0.05; **, p < 0.01).
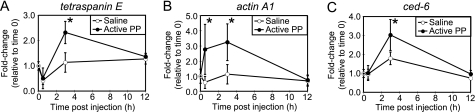
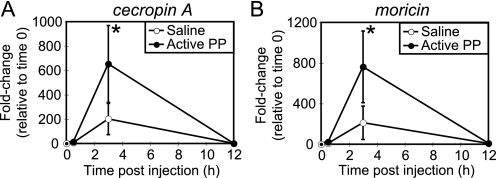
Injection of active PP into silkworm larvae induces muscle contraction and morphologic changes in hemocytes within 30 min (11, 12, 20). Therefore, we speculated that active PP is produced in the hemolymph at an early stage of infection and signals the immune systems to initiate the expression of immune-related genes. To test this hypothesis, we assessed the effects of injection of active PP on gene expression in hemocytes and fat body tissues. mRNA levels of tetraspanin E, actin A1, and ced-6 in the hemocytes (Fig. 2) and cecropin A and moricin in the fat body (Fig. 3) increased within 3 h of PP injection. The expression levels of immune genes returned to baseline by 12 h after PP injection. These findings indicate that PP rapidly induces the expression of multiple immune genes.
FIGURE 2.
Time course of induction of phagocytosis-related genes in hemocytes by active PP. mRNA amounts of phagocytosis-related genes (A, tetraspanin E; B, actin A1; C, ced-6) in hemocytes after injection of saline (open circles) or active PP (filled circles) were analyzed by quantitative RT-PCR. mRNA quantity of each gene was normalized to that of a control gene, elongation factor 2, whose expression is not affected by injection of the samples. Furthermore, fold changes in mRNA quantities relative to time 0 (no injection) were estimated and are shown on the longitudinal axis. Data represent mean ± S.D. (error bars) of four or five experiments. Statistical significance was determined by Student's t test (*, p < 0.05).
FIGURE 3.
Time course of induction of antimicrobial peptide genes in the fat body by active PP. mRNA amounts of antimicrobial peptide genes (A, cecropin A; B, moricin) in the fat body after injection of saline (open circles) or active PP (filled circles) were analyzed by quantitative RT-PCR. mRNA quantity of each gene was normalized to that of a control gene, elongation factor 2, whose expression is not affected by injection of the samples. Furthermore, fold changes in mRNA quantities relative to time 0 (no injection) were estimated and are shown on the longitudinal axis. Data represent mean ± S.D. (error bars) of four or five experiments. Statistical significance was determined by Student's t test (*, p < 0.05).
The Toll and Imd pathways are key systems for regulating insect immune responses (10). The expression of the members of the Toll and Imd pathways is up-regulated in the fat body upon bacterial infection (10, 17, 21). Because these genes were not present in the clone set on the microarray we used in this study (21), we separately analyzed their expression levels by RT-PCR. First, we injected peptidoglycan, a bacterial cell wall component that stimulates the Toll and Imd pathway, or active PP into silkworm larvae, and measured the mRNA levels of Toll and Imd pathway genes such as pelle, tube, TRAF2, IKKγ, and FADD. Injection of peptidoglycan increased the mRNA quantities of pelle, tube, IKKg, and FADD genes in the hemocytes and increased the mRNA quantities of pelle, TRAF2, IKKγ, and FADD in the fat body (Fig. 4). On the other hand, the induction rates of the above Toll and Imd pathway genes in the PP-injected groups were significantly lower than those of peptidoglycan-injected groups (Fig. 4). Based on our previous findings that peptidoglycan stimulates PP activation (15), these data suggest that the induction of Toll and Imd pathway genes does not contribute to PP-mediated immune responses.
FIGURE 4.
Induction of immune-related genes by peptidoglycan or active PP. Silkworm larvae were injected with saline, peptidoglycan, or active PP, and RNAs were extracted from hemocytes and fat body tissues 3 h later. mRNA amounts of Toll and Imd pathway genes in hemocytes (upper) and fat body (lower) were analyzed by quantitative RT-PCR. The mRNA quantity of each gene was normalized to that of a control gene, elongation factor 2. Furthermore, fold changes in mRNA quantities of peptidoglycan- or PP-injected groups relative to saline-injected groups were estimated and are shown on the longitudinal axis. Data represent mean ± S.D. (error bars) of three to five experiments. Statistical significance was determined by Student's t test (*, p < 0.05; **, p < 0.01).
Effect of Active PP on Phagocytosis of Bacteria by Silkworm Hemocytes
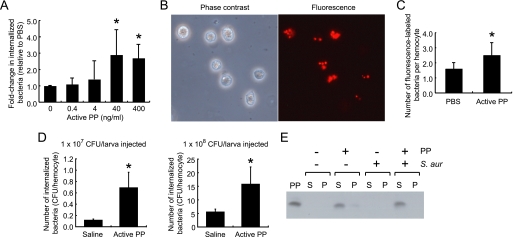
Active PP stimulates adhesion of plasmatocytes, one of the key hemocyte subtypes involved in cellular immunity, to glass slides and induces their spreading behavior (12). Microarray and quantitative RT-PCR indicated that PP induces the expression of tetraspanin E and ced-6 in silkworm hemocytes. The gene families of tetraspanin and ced encode proteins necessary for the phagocytosis of bacteria in Manduca sexta (22) and D. melanogaster (23), respectively. Based on these previous findings, we considered that PP might stimulate the phagocytotic activity of silkworm hemocytes. In our in vitro phagocytosis assay, we confirmed that the cells took up the bacteria in an actin-dependent manner because the process was blocked by low temperature and cytochalasin D treatment, which inhibit actin polymerization (supplemental Fig. S1). Using this assay, we assessed the ability of hemocytes to phagocytose S. aureus within 3 h after adding active PP. The results demonstrated that active PP promoted the in vitro uptake of S. aureus by hemocytes (Fig. 5A). The promotional effect of PP on phagocytosis was also observed after incubation for 0.5 h (data not shown). We further evaluated the effect of PP on phagocytosis in vivo by injecting either fluorescence-labeled S. aureus (Fig. 5B) or cultured live S. aureus cells. A higher number of colocalizing fluorescent particles was observed in hemocytes from PP-injected larvae than from saline-injected larvae (Fig. 5C). Furthermore, estimation of the number of bacteria internalized by hemocytes in the in vivo assay revealed higher cfu in PP-injected groups than in control groups injected with bacteria alone (Fig. 5D). Taken together, these results suggest that PP stimulates the in vitro and in vivo phagocytosis of bacteria by hemocytes. Humoral factors such as antibodies and complement stimulate phagocytosis by binding to the bacterial surface; a process called opsonization. To examine whether PP is able to opsonize bacteria, we incubated active PP with S. aureus for 3 h and centrifuged the mixture to separate the bacterial cells from the supernatant. Western blot analysis revealed active PP only in the supernatant, not in the bacterial cell fraction (Fig. 5E). These results suggest that PP stimulates phagocytosis of bacteria by silkworm hemocytes without direct opsonization.
FIGURE 5.
Effect of active PP on phagocytosis of bacteria by silkworm hemocytes. A, in vitro phagocytosis assay. Hemocytes supplied with the indicated amounts of active PP were incubated with S. aureus for 3 h. After lysing the hemocytes, cfu of S. aureus were determined and normalized to the number of hemocytes. Fold changes in cfu relative to the sample incubated without PP (0 ng/ml) are shown. Data represent mean ± S.D. (error bars) of five experiments. Statistical significance was determined by Student's t test (*, p < 0.05). B, microscope image of fluorescent (Alexa Fluor 594)-labeled S. aureus colocalized with silkworm hemocytes. Hemocytes were collected and fixed 3 h after injection of labeled bacteria. C, in vivo phagocytosis of fluorescence-labeled bacteria. Silkworms were injected with fluorescence-labeled S. aureus. After 3 h, cells were collected, and the numbers of fluorescent particles colocalized with hemocytes were counted. Values were normalized to the number of hemocytes. Statistical significance was determined by Student's t test (*, p < 0.05). D, in vivo phagocytosis of live bacteria. Silkworms were injected with the indicated numbers of live S. aureus cells. After 3 h, hemocytes were collected and lysed to determine the number of internalized bacteria; cfu normalized to the number of hemocytes are shown. Data represent mean ± S.D. of four larvae. Statistical significance was determined by Student's t test (*, p < 0.05). E, in vitro binding assay of active PP to bacteria. S. aureus was incubated with active PP in PBS for 3 h, and samples were separated into supernatant fractions (S) and precipitate fractions (P). The samples were analyzed by immunoblotting.
Effect of Active PP on in Vivo Activation of p38 MAPK in Silkworm Fat Body
Various stress-signaling pathways in host immune cells are activated by bacterial infections. For example, in mammals and nematode Caenorhabditis elegans, the p38 MAPK pathway is activated in response to bacterial infections and is required for AMP production (24–26). We previously reported that bacterial cell wall components trigger PP activation in the silkworm hemolymph (15). Thus, based on our RT-PCR data showing that PP up-regulated the expression of AMP genes, we considered that the p38 MAPK pathway might be involved in the induction of AMPs in silkworm fat body by active PP.
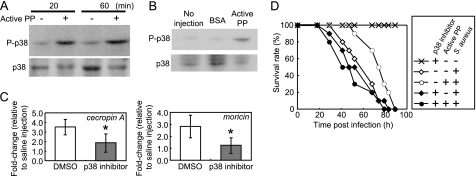
To assess the effect of PP on p38 MAPK activation, we excised the fat body from silkworm larvae injected with active PP and evaluated the amount of phosphorylated p38 (P-p38). In the fat body excised at 20 or 60 min after PP injection, the amounts of P-p38 were increased compared with those excised from saline-injected silkworms (Fig. 6A). We further evaluated the specificity of PP-triggered p38 activation using BSA as a control. Thirty minutes after injection of BSA into fat body samples prepared from silkworms, less phosphorylated p38 was observed than in the PP-injected group (Fig. 6B). These findings suggest that active PP induces an acute activation of p38 MAPK in the silkworm fat body.
FIGURE 6.
Effect of active PP on the in vivo activation of p38 MAPK in silkworm fat body. A, phosphorylation of p38 MAPK in silkworm fat body induced by active PP. Eight larvae/group were injected with saline or active PP, and after 20 or 60 min the fat body tissues were collected. Tissue samples were analyzed by immunoblot. B, effect of BSA on p38 MAPK phosphorylation in the silkworm fat body. Five larvae/group were injected with 50 μl of BSA (66 μg/ml, 1 μm) or active PP (4 μg/ml, 1 μm), and the fat body tissues were collected 30 min later. Tissue samples were analyzed by immunoblot. C, effect of a pharmacologic p38 inhibitor on the induction of AMP gene expression by active PP. Ten larvae/group were treated with 10% DMSO (a solvent) or ML3403 prior to the injection of saline or active PP, and the fat body tissues were dissected 3 h later. The mRNA amounts of cecropin A and moricin were evaluated by quantitative RT-PCR, and the values were normalized to the mRNA amounts of elongation factor 2. In each pretreatment condition (DMSO or ML3403), PP-dependent fold-changes relative to saline-injected groups in the cecropin A and moricin mRNA are shown. Data represent mean ± S.D. (error bars) of four experiments. Statistical significance was determined by Student's t test (*, p < 0.05). D, effect of ML3403, a pharmacologic p38 inhibitor, on PP-dependent up-regulation of host resistance to bacterial infection. Silkworm larvae were treated with 10% DMSO or ML3403 prior to injection of saline or active PP mixed with a suspension of S. aureus.
We then examined whether the p38 MAPK pathway is required for the expression of AMP genes induced by active PP in the larval fat body. Larvae pretreated with the solvent DMSO or ML3403 (a pharmacologic p38 inhibitor) were injected with active PP, and after 3 h fat body tissues were excised to measure the amount of expressed mRNA. In silkworms pretreated with DMSO, expression levels of cecropin A and moricin increased 3.5- and 2.8-fold, respectively, in response to active PP. On the other hand, in larvae injected with the p38 inhibitor, the two AMP genes increased only 1.9- and 1.3-fold, respectively (Fig. 6C). This result suggests that PP induces AMP gene expression via activation of the p38 MAPK pathway in the larval fat body.
We also tested the effect of a p38 inhibitor on the up-regulation of phagocytosis-related genes in silkworm hemocytes induced by active PP. ML3403 suppressed the PP-dependent induction of tetraspanin E and actin A1 in hemocytes (supplemental Fig. S2). This result suggests that induction of phagocytosis-related genes by PP involves activation of the p38 MAPK pathway.
Melanization is an immune response in insect hemolymph that is triggered by bacterial components such as peptidoglycan and followed by the activation of serine protease cascades (27). Based on our results showing PP-dependent up-regulation of some genes such as serine proteases and peptidoglycan recognition protein, we considered the possibility that PP affects the bacteria-induced melanization reaction. Injection of peptidoglycan into silkworm larvae induced melanization and increased the A490 value of the hemolymph plasma within 3 h (supplemental Fig. S3). When active PP was co-injected with peptidoglycan, the A490 value of the hemolymph plasma became higher than that of the peptidoglycan-injected group (supplemental Fig. S3). On the other hand, a pharmacologic p38 inhibitor (ML3403) did not inhibit melanization in larvae injected with peptidoglycan in the presence or absence of PP (supplemental Fig. S3). Therefore, we concluded that PP promoted bacteria-induced melanization of the hemolymph, whereas the p38 MAPK pathway played only a minor role in the melanization reaction.
Previously, we reported that injection of active PP in the hemolymph of silkworms delayed the killing effect of S. aureus (15). To clarify the contribution of the p38 MAPK pathway to the protection of the host by PP, we examined the effect of ML3403 on the PP-dependent delayed killing of larvae infected with S. aureus. Injection of active PP increased the time required to kill 50% of infected larvae by S. aureus from 57 h to 74 h (Fig. 6D, p < 0.05 using the log-rank test). In contrast, pretreatment with ML3403 abolished the lifetime prolongation effect of active PP in infected larvae (Fig. 6D). This result suggests that the p38 MAPK pathway is required for the up-regulation of bacterial resistance by active PP.
DISCUSSION
Immune reactions are classified as immediate and delayed type responses. Immediate type responses are highly important to initially prevent the explosive proliferation and systemic spreading of pathogens that can cause septic shock and organ failure. The cooperation of several immune pathways is required for efficient elimination of pathogens. Our understanding of the regulatory mechanisms of the cooperative regulation of innate immune responses, however, is insufficient. The present study demonstrated that an insect cytokine PP triggers immediate-type immune responses by inducing the acute expression of various immune-related genes in hemocytes and the fat body of silkworms (Fig. 7).
FIGURE 7.
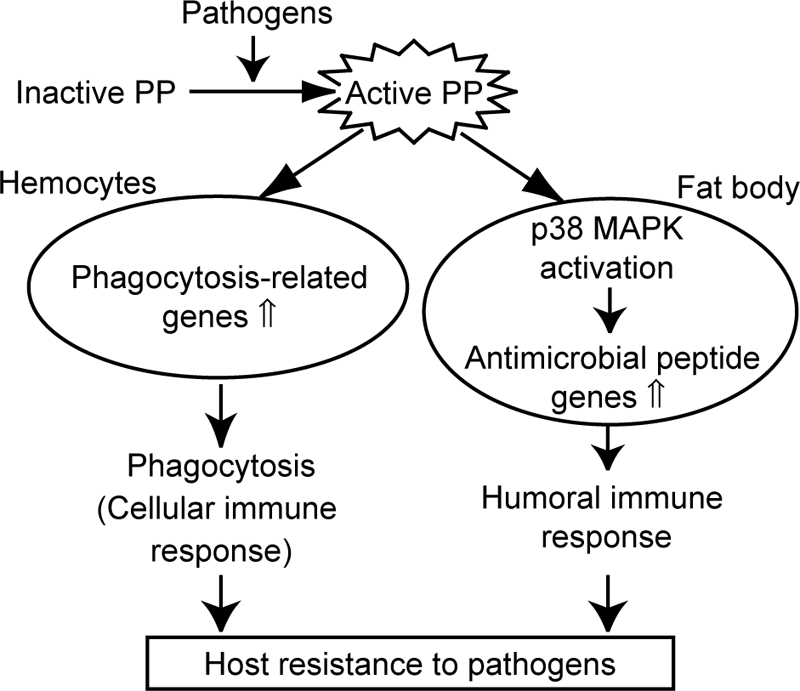
Regulation of cellular and humoral immune responses by insect cytokine PP.
Some of the outer components of microorganisms stimulate the expression of genes involved in innate immune responses in host animals. There are several reports of genome-wide analyses in flies infected by pathogens and in the hemocyte-like cell line mbn-2 treated with bacterial cell wall components (17, 18, 28, 29). Moreover, Mita et al. constructed a B. mori expressed sequence tag database (30) and analyzed the genome sequence information (31), making it possible for researchers to perform genome-wide analysis in silkworms. Recently, Tanaka et al. performed a microarray analysis to study the expression profile of the silkworm fat body and showed that the expression levels of various genes change in response to bacterial infection (21). We identified a set of genes with altered expression patterns in hemocytes and fat body tissues after injecting active PP. Some of the PP-inducible genes overlapped with those reported to be expressed in insect cells infected by pathogens (21). Although most of the genes induced by PP have yet to be characterized, some of them share homologies with immune-related genes. In D. melanogaster, a few cytokine-like factors such as Spaeztle (9), Eiger (32), and Unpaired-3 (33) are reported to be involved in innate immunity. There is no report of an insect cytokine like PP capable of affecting both the gene expression of multiple immune tissues and inducing host resistance against pathogens. Thus, this is the first genome-wide analysis of an insect cytokine in multiple tissues. Because PP-inducible genes include some other cytokine-like factors (e.g. genes with sequence similarities to spaeztle or TNF families), we would like to propose that a regulatory system corresponding to the mammalian “cytokine network” exists in insects, which lack an acquired immune system.
In our experiments, we observed significant increases in the relative amounts of mRNA for AMP genes such as cecropin A and moricin in the fat body tissues after injecting saline, the solvent for active PP (Fig. 3). On the other hand, the induction of phagocytosis-related genes in hemocytes was not so sensitive to saline injection (Fig. 2). Lipopolysaccharide (LPS) is a well known contaminant that stimulates AMP production in immune systems. Tanaka et al. (34) demonstrated that a high dose of purified LPS (1.0 μg/larva) and synthetic lipid A (a component of LPS) elicits in vivo AMP production in the silkworm fat body. The amount of endotoxin in the saline sample injected into silkworms was 0.1–0.2 pg/larva. When we injected either commercial endotoxin-free water or saline (containing <1 pg/ml endotoxin), we observed a >20-fold increase in the mRNA level of cecropin A compared with that of uninjected silkworms. These results indicated that, under our experimental conditions, there were uncontrolled factors (e.g. contamination by substances other than endotoxin, stress caused by the injection itself, and infection by a small amount of bacteria on the larval surface, etc.) that increased background AMP gene expression in the silkworm fat body. Nevertheless, when we injected active PP dissolved in endotoxin-free water or saline, the mRNA level of cecropin A increased >2-fold compared with that of silkworms injected with each endotoxin-free solvent. Therefore, the robust effect of active PP was clear.
Studies of the molecular regulation mechanisms of AMP production in the insect fat body, especially in the Toll and Imd pathways, have made great progress in the last decade. On the other hand, information regarding cellular immunity, including the mechanisms of bacterial recognition and uptake by phagocytic cells, is relatively limited. RNA interference screening of Drosophila cultured cells (35, 36) and Anopheles gambiae hemocytes (37) indicates that genes encoding pattern recognition receptors, cytoskeleton, and adhesive molecules are required for phagocytosis of microorganisms. Furthermore, in insects such as D. melanogaster and A. gambiae, thiolester-containing proteins opsonize bacteria by direct binding and promote phagocytosis (38, 39). No extracellular molecule in insects has been found that alters the ability of hemocytes to engulf bacteria. In the present study, active PP was suggested to facilitate the uptake of bacteria by inducing phagocytosis-related gene expression in larval hemocytes. There are several hemocyte subtypes in insects, and each of them has different immunologic roles (40). Because of their body size, a large number of hemocytes can be obtained from silkworms for analysis of the functions of each subtype. Nakahara et al. (41) established a method for purifying different subtypes of hemocytes in B. mori. These technical advantages of silkworms offer the prospect of identifying the specific hemocyte subtype responsible for promoting phagocytosis induced by active PP. To our knowledge, PP is the first extracellular molecule reported to be a host factor in insect that regulates cellular immunity without direct opsonization of bacteria.
Among the intracellular signaling pathways activated in infected hosts, p38 MAPK acts downstream of mammalian Toll-like receptors to produce AMPs (24). In mosquito Aedes aegypti, the p38 MAPK pathway is involved in AMP production induced by LPS (42). Here, we showed that active PP induced p38 MAPK-mediated AMP gene expression within a relatively short time. Furthermore, a p38 inhibitor abolished the in vivo therapeutic effect of active PP on silkworm larvae against S. aureus infection. Thus, we concluded that in the early stage of infection, PP triggers the activation of p38 MAPK and subsequent induction of immune-related genes, which leads to host protection against pathogens. MAPK family proteins including p38 are well conserved among various species, and their orthologs in several insects have been cloned (43–46). Some MAPK family proteins, like JNK and TAK1, are reported to be involved in innate immune responses (43–45). The roles of other members of the MAPK family in innate immunity, however, remain obscure. Further studies of PP-regulated immune responses will help unravel the importance of MAPK pathways in innate immunity.
Supplementary Material
Acknowledgments
We thank Kiyomi Kyougoku, Yumiko Matsuzawa, and Aya Yoshino for technical assistance.
This work was supported by Grant-in-aid for Young Scientists (B) 21790063 and Grant-in-aid for Japan Society for the Promotion of Science fellows (21-10519) from Japan Society for the Promotion of Science. This study was also supported by the Program for Promotion of Fundamental Studies in Health Sciences of National Institute of Biomedical Innovation and grants from Genome Pharmaceuticals Co. Ltd.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S1–S3.
- AMP
- antimicrobial peptide
- DMSO
- dimethyl sulfoxide
- PP
- paralytic peptide
- RT
- reverse transcription.
REFERENCES
- 1.Hoffmann J. A., Reichhart J. M. (2002) Nat. Immunol. 3, 121–126 [DOI] [PubMed] [Google Scholar]
- 2.Stuart L. M., Ezekowitz R. A. (2008) Nat. Rev. Immunol. 8, 131–141 [DOI] [PubMed] [Google Scholar]
- 3.Boman H. G., Faye I., Gudmundsson G. H., Lee J. Y., Lidholm D. A. (1991) Eur. J. Biochem. 201, 23–31 [DOI] [PubMed] [Google Scholar]
- 4.Natori S., Shiraishi H., Hori S., Kobayashi A. (1999) Dev. Comp. Immunol. 23, 317–328 [DOI] [PubMed] [Google Scholar]
- 5.Okada M., Natori S. (1983) Biochem. J. 211, 727–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okada M., Natori S. (1985) J. Biol. Chem. 260, 7174–7177 [PubMed] [Google Scholar]
- 7.Aggarwal K., Silverman N. (2008) BMB Rep. 41, 267–277 [DOI] [PubMed] [Google Scholar]
- 8.Lemaitre B., Nicolas E., Michaut L., Reichhart J. M., Hoffmann J. A. (1996) Cell 86, 973–983 [DOI] [PubMed] [Google Scholar]
- 9.Lemaitre B., Kromer-Metzger E., Michaut L., Nicolas E., Meister M., Georgel P., Reichhart J. M., Hoffmann J. A. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 9465–9469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Gregorio E., Spellman P. T., Tzou P., Rubin G. M., Lemaitre B. (2002) EMBO J. 21, 2568–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamimura M., Nakahara Y., Kanamori Y., Tsuzuki S., Hayakawa Y., Kiuchi M. (2001) Biochem. Biophys. Res. Commun. 286, 67–73 [DOI] [PubMed] [Google Scholar]
- 12.Nakahara Y., Kanamori Y., Kiuchi M., Kamimura M. (2003) Arch. Insect Biochem. Physiol. 52, 163–174 [DOI] [PubMed] [Google Scholar]
- 13.Strand M. R., Hayakawa Y., Clark K. D. (2000) J. Insect Physiol. 46, 817–824 [DOI] [PubMed] [Google Scholar]
- 14.Aizawa T., Hayakawa Y., Nitta K., Kawano K. (2002) Mol. Cells 14, 1–8 [PubMed] [Google Scholar]
- 15.Ishii K., Hamamoto H., Kamimura M., Sekimizu K. (2008) J. Biol. Chem. 283, 2185–2191 [DOI] [PubMed] [Google Scholar]
- 16.Laufer S. A., Wagner G. K., Kotschenreuther D. A., Albrecht W. (2003) J. Med. Chem. 46, 3230–3244 [DOI] [PubMed] [Google Scholar]
- 17.Irving P., Troxler L., Heuer T. S., Belvin M., Kopczynski C., Reichhart J. M., Hoffmann J. A., Hetru C. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 15119–15124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irving P., Ubeda J. M., Doucet D., Troxler L., Lagueux M., Zachary D., Hoffmann J. A., Hetru C., Meister M. (2005) Cell. Microbiol. 7, 335–350 [DOI] [PubMed] [Google Scholar]
- 19.Huang L., Cheng T., Xu P., Cheng D., Fang T., Xia Q. (2009) PLoS One 4, e8098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ha S. D., Nagata S., Suzuki A., Kataoka H. (1999) Peptides 20, 561–568 [DOI] [PubMed] [Google Scholar]
- 21.Tanaka H., Ishibashi J., Fujita K., Nakajima Y., Sagisaka A., Tomimoto K., Suzuki N., Yoshiyama M., Kaneko Y., Iwasaki T., Sunagawa T., Yamaji K., Asaoka A., Mita K., Yamakawa M. (2008) Insect Biochem. Mol. Biol. 38, 1087–1110 [DOI] [PubMed] [Google Scholar]
- 22.Zhuang S., Kelo L., Nardi J. B., Kanost M. R. (2007) J. Biol. Chem. 282, 22563–22572 [DOI] [PubMed] [Google Scholar]
- 23.Cuttell L., Vaughan A., Silva E., Escaron C. J., Lavine M., Van Goethem E., Eid J. P., Quirin M., Franc N. C. (2008) Cell 135, 524–534 [DOI] [PubMed] [Google Scholar]
- 24.Hussain T., Nasreen N., Lai Y., Bellew B. F., Antony V. B., Mohammed K. A. (2008) Am. J. Physiol. Lung Cell. Mol. Physiol. 295, L461–L470 [DOI] [PubMed] [Google Scholar]
- 25.Kim D. H., Feinbaum R., Alloing G., Emerson F. E., Garsin D. A., Inoue H., Tanaka-Hino M., Hisamoto N., Matsumoto K., Tan M. W., Ausubel F. M. (2002) Science 297, 623–626 [DOI] [PubMed] [Google Scholar]
- 26.Pujol N., Cypowyj S., Ziegler K., Millet A., Astrain A., Goncharov A., Jin Y., Chisholm A. D., Ewbank J. J. (2008) Curr. Biol. 18, 481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerenius L., Söderhäll K. (2004) Immunol. Rev. 198, 116–126 [DOI] [PubMed] [Google Scholar]
- 28.Boutros M., Agaisse H., Perrimon N. (2002) Dev. Cell 3, 711–722 [DOI] [PubMed] [Google Scholar]
- 29.Johansson K. C., Metzendorf C., Söderhäll K. (2005) Exp. Cell Res. 305, 145–155 [DOI] [PubMed] [Google Scholar]
- 30.Mita K., Morimyo M., Okano K., Koike Y., Nohata J., Kawasaki H., Kadono-Okuda K., Yamamoto K., Suzuki M. G., Shimada T., Goldsmith M. R., Maeda S. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14121–14126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mita K., Kasahara M., Sasaki S., Nagayasu Y., Yamada T., Kanamori H., Namiki N., Kitagawa M., Yamashita H., Yasukochi Y., Kadono-Okuda K., Yamamoto K., Ajimura M., Ravikumar G., Shimomura M., Nagamura Y., Shin-I. T., Abe H., Shimada T., Morishita S., Sasaki T. (2004) DNA Res. 11, 27–35 [DOI] [PubMed] [Google Scholar]
- 32.Schneider D. S., Ayres J. S., Brandt S. M., Costa A., Dionne M. S., Gordon M. D., Mabery E. M., Moule M. G., Pham L. N., Shirasu-Hiza M. M. (2007) PLoS Pathog. 3, e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Agaisse H., Petersen U. M., Boutros M., Mathey-Prevot B., Perrimon N. (2003) Dev. Cell 5, 441–450 [DOI] [PubMed] [Google Scholar]
- 34.Tanaka H., Sagisaka A., Fujita K., Kaneko Y., Imanishi S., Yamakawa M. (2009) Insect Mol. Biol. 18, 71–75 [DOI] [PubMed] [Google Scholar]
- 35.Stroschein-Stevenson S. L., Foley E., O'Farrell P. H., Johnson A. D. (2006) PLoS Biol. 4, e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rämet M., Manfruelli P., Pearson A., Mathey-Prevot B., Ezekowitz R. A. (2002) Nature 416, 644–648 [DOI] [PubMed] [Google Scholar]
- 37.Moita L. F., Wang-Sattler R., Michel K., Zimmermann T., Blandin S., Levashina E. A., Kafatos F. C. (2005) Immunity 23, 65–73 [DOI] [PubMed] [Google Scholar]
- 38.Levashina E. A., Moita L. F., Blandin S., Vriend G., Lagueux M., Kafatos F. C. (2001) Cell 104, 709–718 [DOI] [PubMed] [Google Scholar]
- 39.Lagueux M., Perrodou E., Levashina E. A., Capovilla M., Hoffmann J. A. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 11427–11432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lavine M. D., Strand M. R. (2002) Insect Biochem. Mol. Biol. 32, 1295–1309 [DOI] [PubMed] [Google Scholar]
- 41.Nakahara Y., Shimura S., Ueno C., Kanamori Y., Mita K., Kiuchi M., Kamimura M. (2009) Dev. Comp. Immunol. 33, 439–448 [DOI] [PubMed] [Google Scholar]
- 42.Chen-Chih Wu R., Shaio M. F., Cho W. L. (2007) Insect Mol. Biol. 16, 389–399 [DOI] [PubMed] [Google Scholar]
- 43.Davis M. M., Primrose D. A., Hodgetts R. B. (2008) Mol. Cell. Biol. 28, 4883–4895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujiwara Y., Shindome C., Takeda M., Shiomi K. (2006) Insect Biochem. Mol. Biol. 36, 47–53 [DOI] [PubMed] [Google Scholar]
- 45.Han Z. S., Enslen H., Hu X., Meng X., Wu I. H., Barrett T., Davis R. J., Ip Y. T. (1998) Mol. Cell. Biol. 18, 3527–3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shinzawa N., Nelson B., Aonuma H., Okado K., Fukumoto S., Miura M., Kanuka H. (2009) Cell Host Microbe 6, 244–252 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.