Abstract
The assembly and constriction of an actomyosin contractile ring in cytokinesis is dependent on the activation of Rho at the equatorial cortex by a complex, here termed the cytokinesis initiation complex, between a microtubule-associated kinesin-like protein (KLP), a member of the RacGAP family, and the RhoGEF Pebble. Recently, the activity of the mammalian Polo kinase ortholog Plk1 has been implicated in the formation of this complex. We show here that Polo kinase interacts directly with the cytokinesis initiation complex by binding RacGAP50C. We find that a new domain of Polo kinase, termed the intermediate domain, interacts directly with RacGAP50C and that Polo kinase is essential for localization of the KLP-RacGAP centralspindlin complex to the cell equator and spindle midzone. In the absence of Polo kinase, RacGAP50C and Pav-KLP fail to localize normally, instead decorating microtubules along their length. Our results indicate that Polo kinase directly binds the conserved cytokinesis initiation complex and is required to trigger centralspindlin localization as a first step in cytokinesis.
Keywords: Cell Cycle, Cell Division, Drosophila Genetics, Fluorescence Resonance Energy Transfer (FRET), Mitotic Spindle, Cytokinesis, Polo Kinase, Centralspindlin
Introduction
Cytokinesis is the final stage of cell division that splits a cell into two. The process initiates in anaphase with the localization of a microtubule-associated protein complex to the cell equator and the subsequent assembly and constriction of an acto-myosin-based contractile ring coordinated by the Rho signaling pathway (1). In anaphase, RhoA activation is achieved by the localization of the RhoGEF Pebble/Ect2 to the sites of cleavage furrow formation (2). This localization is mediated by RacGAP50C/RacGAP1 (2–4), which is in a complex with the microtubule-associated motor protein Pav-KLP/MKLP1 called the centralspindlin complex (5). This leads to the assembly and constriction of the contractile ring and, finally, division of the cell (6). Evidence suggests that Pebble and RacGAP50C (referred to hereafter as RacGAP) bind directly and that this binding is essential for the localization of Pebble to the equator and the subsequent activation of Rho signaling in anaphase (3). RacGAP and Pav-KLP have been shown to be interdependent in their localization to the cell equator, suggesting that a functional centralspindlin complex is required for Rho activation (7). Recent studies in mammals suggest an important role for Polo-like kinase 1 (Plk1)2 for the interaction between RacGAP and Pebble, because inhibiting the function of Plk1 in anaphase prevents the localization of Pebble/Ect2 to the equator and the subsequent ring assembly, although the centralspindlin complex still localizes correctly (8–11). The phosphorylation of RacGAP by Plk1 is necessary although not sufficient for the anaphase recruitment of Pebble/Ect2 (12, 13).
As well as the mammalian data, a number of studies in Drosophila and other models point to a role for Polo kinase in cytokinesis. Polo was first identified in Drosophila (14), and an early study showed that the activity of Polo kinase peaks in anaphase and telophase, suggesting a role in cytokinesis (15). A subsequent study examined a hypomorphic form of Polo kinase in Drosophila spermatocytes and showed early cytokinetic defects such as the formation of an abnormal spindle midzone and actin ring and the eventual failure of cytokinesis as indicated by the formation of multinucleate cells (16). The fission yeast homolog of Polo kinase, Plo1, has been shown to be essential for the formation of the actin ring during septum formation, indicating that Plo1 may play a role in cytokinesis (17). Consistent with these studies, a role for Polo kinase in regulating the centralspindlin complex in cytokinesis was also suggested by the data of Herrmann et al. (18) because they showed that polo mutant cells had a reduced central spindle. Altogether, these studies suggest an important role for Polo kinase early in cytokinesis. In this study we extend the mammalian data that suggested an interaction between Polo and RacGAP as well as providing evidence for a novel role for Polo kinase in cytokinesis. We show that Polo kinase directly interacts with the linker protein RacGAP, identify the novel interacting domains, and show that Polo is required for the localization of both RacGAP and Pav-KLP to the equator.
EXPERIMENTAL PROCEDURES
Immunohistochemistry
Brains were dissected from third instar larvae in ice-cold PBS with 4% paraformaldehyde using forceps in a two-well microscope slide. Frozen ice packs were put under the dissecting microscope stage to keep it cold. The dissected brains were then moved to a clean well on the same slide and shredded with fine needles. Four brains were done at a time, and the whole dissecting and fixing procedure was carried out within 10 min. The fix was then removed from the well without removing the brain pieces and replaced with PBS plus 0.2% Triton X-100. The brains were transferred into a microcentrifuge tube and washed with PBS plus 0.2% Triton X-100 for 2 h at room temperature and blocked in PBS plus 0.5% Triton X-100 and 10% goat serum for another 2 h at room temperature. Primary antibodies were added in the same blocking solution and incubated overnight at 4 °C, followed by a 2-h wash in PBS plus 0.2% Triton X-100. Secondary antibodies were diluted in the blocking solution and added for 4 h at room temperature followed by a 2-h wash in PBS plus 0.2% Triton X-100. Hoechst 33258 (1 μg/ml) staining was carried out for 10 min before another 30-min wash at room temperature. Brain pieces were then mounted in 80% glycerol/PBS. The samples were viewed on a Zeiss Axioplan2 upright microscope with Semrock Brightline filter sets using a 63× PlanApo (n.a. 1.4) objective. The images were acquired using an Axiocam MRm CCD camera and if necessary deconvolved using Axiovision software (Carl Zeiss). The antibodies used were: mouse anti-Polo kinase from Claudio E Sunkel (1:50), rat anti-RacGAP (1:300), rabbit anti-RacGAP (1:50), and rabbit anti-Pav-KLP (1:500). The secondary antibodies (1:200) were all “highly cross-absorbed” from Rockland or Jackson Immunochemicals. TUNEL staining to visualize degrading DNA in dying cells was carried out using a TMR-Red in situ cell death detection kit (Roche Applied Science) on fixed brain squashes following a 3-min wash in 0.1 m citrate, 0.1% Triton X-100 at room temperature and a brief wash in PBS. TUNEL labeling was carried out for 1 h at 37 °C, followed by a PBS wash and Hoechst 33342 labeling (0.5 μg/ml for 10 min). Confidence intervals for identifying a mean proportion p of a population n were calculated as 95% confidence interval = 1.96 .
FRET Analysis
Fixed third instar larval brain cells were first stained for Polo kinase and RacGAP using secondary antibodies tagged with Cy3 (donor) and Cy5 (acceptor) fluorophores, respectively, as described above. Images of first the acceptor and then the donor fluorophore were acquired using a Zeiss Axioplan2 upright microscope with a Semrock Brightline GFP filter set using a 63× PlanApo (n.a. 1.4) objective. These images are referred to in Fig. 2 as prebleach. Then the acceptor was photobleached at maximum light intensity for 30–180 s, followed by imaging of the donor and then acceptor again, and these images were referred to as post-bleach. This sequence was automated, and identical imaging conditions were used pre- and post-bleaching. Effectiveness of photobleaching was confirmed by loss of signal in the acceptor fluorescence post-bleaching. Donor fluorophore images were imported into Image J (19) and aligned to correct any lateral displacement using the StackReg plugin (20). The FRET signal was displayed using the “fire” lookup table. We did not show the results as FRET efficiency (E = 1 − pre/post) because this method has been shown to give less effective discrimination from background (21). This procedure, with the same conditions was repeated for the cross-reactivity (secondary only) and concentration (Polo kinase and Pav-KLP) controls.
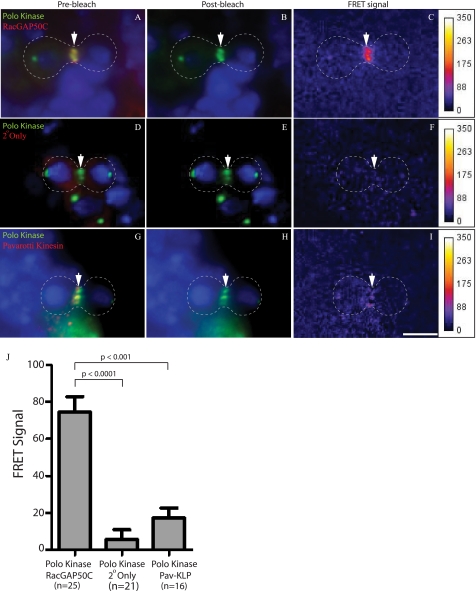
FIGURE 2.
FRET analysis of Polo kinase, RacGAP, and Pav-KLP using Cy3 donor and Cy5 acceptor fluorophores. Third instar larval brains were stained with anti-Polo kinase and a Cy3-labeled secondary antibody (green; A, B, D, E, G, and H), anti-RacGAP (A and B), or anti-Pav-KLP (G and H) and a Cy5-labeled secondary antibody (red) and Hoechst 33258 to visualize DNA (blue). A, Cy3-labeled Polo kinase and Cy5-labeled RacGAP are localized to the cell equator (arrow) before bleaching. B, after bleaching of the acceptor tag Cy5, only the Cy3 signal remains (arrow). C, a FRET signal is observed at the cell equator. D–F, staining as for A and B, except that the primary anti-RacGAP antibody is not included. D, Cy3-labeled Polo kinase is present at the cell equator, with background Cy5 staining. E, bleaching has eliminated the Cy5 signal. F, no FRET signal is observed. G–I, Pav-KLP and Polo kinase show no FRET. G, Pav-KLP and Polo kinase both localize to the cell equator. H, only the Polo kinase-Cy3 signal remains after bleaching of the Cy5-labeled Pav-KLP. I, no FRET signal is observed. Scale bar, 5 μm. J, mean FRET signal for rings from the sample (n = 25), cross-reactivity control (n = 21), and concentration control (n = 16) is shown as the mean with a 95% confidence interval. Comparisons of the means were obtained using the nonparametric Mann-Whitney test (unpaired, two tailed) (J).
Fly Stocks and Constructs
The fly stocks used were: daughterless-Gal4 (Bloomington 8641), polo10 (Szeged 2123), Deficiency (Df(3L)rgdC-co2, Bloomington 2052), and mad2 (mad2EIY1687, Bloomington 22495). polo10 mad2 and Deficiency mad2 double mutants were generated by recombining the two alleles onto the same chromosome. For immunofluorescence analysis of localization of RacGAP and Pav-KLP in the absence of Polo kinase, polo10 mad2/Deficiency mad2 flies were compared with polo10 mad2/TM6 or Deficiency mad2/TM6 controls. For live analysis of localization of RacGAP, UAS-Venus-tagged RacGAP; polo10 mad2/Deficiency mad2 da-Gal4 flies were compared with UAS-Venus-tagged RacGAP;Deficiency mad2 da-Gal4/ TM6. We made GFP-tagged Polo constructs in pUASTattB-TGW, a SphI/HpaI fragment of pTGW (Drosophila gateway collection) in pUASTattB. To inactivate the kinase domain of Polo, the mutation D166N was introduced (22).
Yeast Two-hybrid Analysis
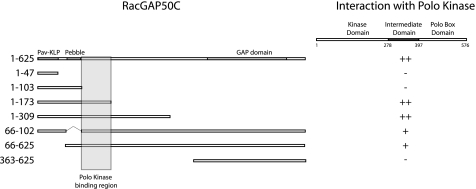
The yeast L40 strain (MATa his3Δ200 trp1-901 leu2-3112 ade2 LYS::(lexAop)4-HIS3 URA3::(lexAop)8-lacZ GAL4 gal80) and vectors pVP16 and pNLX (23) were used to detect protein-protein interactions as previously described (3). Interactions were tested by lacZ filter assay according to the Breeden lab protocol. Polo kinase was used as the bait using pNLX, and RacGAP constructs were used as the prey using pVP16. A construct of RacGAP containing amino acids 363–625 and the empty vector were used as negative controls. All of the regions of RacGAP tested are indicated in Fig. 3.
FIGURE 3.
Yeast two-hybrid analysis of the RacGAP and Polo kinase interaction. RacGAP constructs in pVP16 vector (prey) were tested against the intermediate region (amino acids 276–391) of Polo kinase (bait). RacGAP constructs containing amino acids 1–47, 1–103, and 363–625 do not show any interaction with Polo kinase, whereas RacGAP constructs including amino acids 103–173 show strong interaction. The interaction is not prevented by removal of the Pav-KLP (amino acids 11–47) or Pebble (amino acids 66–102) binding domains in RacGAP.
Live Imaging
Third instar larval brains were dissected in a 1 to 1 ratio of Halocarbon oil 700 and Halocarbon oil 27 (Sigma) and spread on coverslips using needles as described (24). Venus-tagged RacGAP was imaged in Drosophila neuroblast cells double mutant for polo mad2 every 30 s on a Zeiss Axioplan2 upright microscope with Semrock Brightline GFP filter set using a 63× PlanApo (n.a. 1.4) objective.
RESULTS
Polo Kinase Co-localizes with the Centralspindlin Complex in Cytokinesis
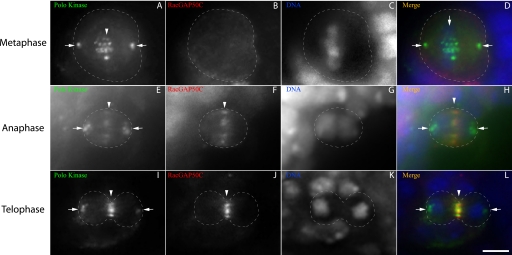
To investigate how Polo kinase may play its regulatory role at the onset of cytokinesis (10), we followed the localization of Polo kinase and the centralspindlin component RacGAP, using immunofluorescence of Drosophila larval brain cells. In metaphase, Polo kinase localizes to the spindle poles (Fig. 1, A–D) and to tight bands on the DNA, whereas RacGAP is diffusely cytoplasmic (Fig. 1, A–D). However, during anaphase, at the onset of cytokinesis, both proteins relocate to the cell equator (Fig. 1, E–H), whereas Polo kinase also maintains its presence at the poles. The equatorial co-localization is maintained during cleavage furrow ingression until the end of telophase (Fig. 1, I–L).
FIGURE 1.
RacGAP50C and Polo kinase co-localize during early cytokinesis. Third instar larval brain cells were imaged at the plane of the spindle poles for Polo kinase (A, E, and I), RacGAP (B, F, and J), and DNA (C, G, and K). Merged images (D, H, and L) show Polo kinase in green, RacGAP in red, and DNA in blue. A–D, a metaphase cell showing Polo kinase localized to the spindle poles (arrows) and to DNA at the metaphase plate (arrowhead), whereas RacGAP is diffusely cytoplasmic. E–H, an anaphase cell, showing Polo kinase and RacGAP localized to the contractile ring (arrowheads), whereas Polo kinase is also present at the poles. I–L, a telophase cell showing localization of both Polo kinase and RacGAP to the contracted central spindle (arrowheads), whereas Polo kinase is also at the poles (arrows). Scale bar, 5 μm (L).
Polo Kinase Interacts Directly with RacGAP in the Cytokinesis Initiation Complex
To test for a direct interaction between Polo kinase and RacGAP during cytokinesis, we carried out FRET by acceptor photobleaching (25). We have previously shown that this technique can be used to detect molecular interactions in situ in Drosophila brain tissue (26). FRET has been previously shown to identify proteins that are in extremely close proximity (<5 nm) and is highly correlated with a direct molecular interaction (27). Drosophila brain tissues were incubated with antibodies directed against Polo kinase and RacGAP or Pav-KLP and stained with secondary antibodies labeled with the Cy3 and Cy5 fluorophores, respectively. Upon photobleaching of Cy5 (Fig. 2, compare A and B), we saw a significant increase in the Cy3 signal (Fig. 2C) indicating FRET between the RacGAP and Polo kinase-associated fluorophores. This interaction was apparent in cells at all stages of cytokinesis but not at metaphase.3 We tested whether this FRET signal was due to cross-reactivity of the secondary antibodies by staining with the same secondary antibodies that were used to mark Polo kinase and RacGAP but in the absence of the primary antibody against RacGAP. These samples showed no significant FRET signal (Fig. 2, D–F). These results indicate that the positive FRET signal in Fig. 2C is not due to cross-reactivity.
We then tested for a FRET signal between Polo kinase and Pav-KLP, which has previously been shown to bind and co-localize with RacGAP (3, 28). As expected, Pav-KLP co-localizes with Polo kinase (Fig. 2G), but no significant FRET was observed between the Polo kinase and Pav-KLP-associated fluorophores (Fig. 2, G–I). This illustrates the specificity of our assay, in detecting the interaction between Polo kinase and RacGAP but not the Pav-KLP component of centralspindlin and shows that the FRET signal is not due to random juxtaposition of any two proteins in the central spindle. Quantification of the FRET results showed that the interaction between Polo kinase and RacGAP is highly statistically significant (p < 0.001) (Fig. 2J).
To confirm that these FRET results indicated a direct physical interaction between Polo kinase and RacGAP, we carried out a yeast two-hybrid analysis. We found that a new region of Polo kinase, between the kinase domain and the Polo Box domain, here named the intermediate domain, can physically bind a region of RacGAP between amino acids 103 and 173 of RacGAP (Fig. 3). This region of RacGAP is adjacent to its Pebble and Pav-KLP binding regions (3), and deletion of those binding regions did not prevent the interaction with Polo kinase (Fig. 3). Targeted mutagenesis of conserved residues in the Polo binding region resulted in the loss of Polo binding (supplemental Fig. S1). These mutations also reduced or removed binding to Pav-KLP, suggesting that they may affect overall protein structure. Polo kinase binding to its targets is usually mediated via its Polo Box domain, in a phospho-priming-dependent manner (29). However, binding between Polo kinase and RacGAP occurred via the intermediate region and not via the Polo Box domain. The intermediate region is quite divergent across taxa, but mutation of two conserved amino acids removes RacGAP binding, as well as preventing proper localization of Polo throughout mitosis (supplemental Fig. S1). Together, these results suggest that there is a direct physical interaction between two previously uncharacterized regions in Polo and RacGAP and that disruption of these regions has pleiotropic phenotypes, indicating that they are essential for normal protein function in anaphase.
Polo Kinase Is Required for the Localization of the Centralspindlin Complex to the Equator
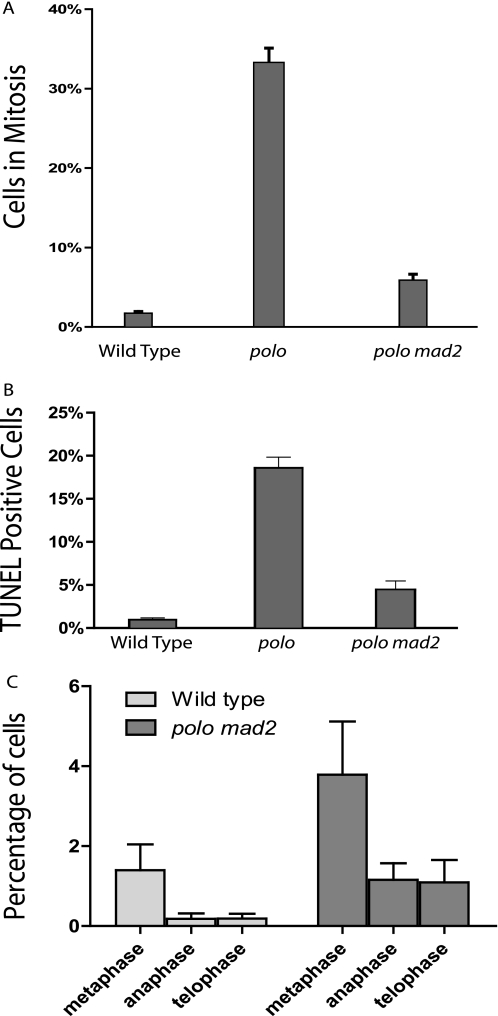
To elucidate the role Polo kinase plays in the Drosophila cytokinesis initiation complex, we examined the behavior of RacGAP in cells deficient for Polo kinase. Previous work has shown that polo mutant cells arrest in metaphase because of the activation of the spindle checkpoint, preventing analysis of cytokinesis (30). To bypass the metaphase arrest and look at the role of Polo kinase specifically in cytokinesis, we generated polo mad2 double mutant flies. Mad2 is essential for spindle checkpoint activation (31), but Mad2-deficient Drosophila have been shown to be viable and fertile, with cells dividing correctly with no apparent abnormalities (32). Removal of Mad2 therefore does not normally disrupt cell division apart from removing the checkpoint block; specifically, the process of cytokinesis is not affected (supplemental Fig. S3). To confirm that removal of mad2 in polo mutant Drosophila tissue did in fact bypass the metaphase arrest, we compared the mitotic index of polo mad2 mutant cells with that of polo mutants and wild type cells (Fig. 4A). We saw a strong rescue of the mitotic arrest of polo mutants by the mad2 mutation. To determine whether the reduction of mitotic cells was due to increased apoptosis or mitosis exit, we carried out TUNEL staining for apoptotic cells (Fig. 4B). We found that in a mad2 mutant background, the rate of apoptosis in polo mutants was reduced close to that of wild type cells, and progression through mitosis was not drastically arrested (supplemental Fig. S2). Taken together, these results indicate that the polo mad2 double mutant cells are released from metaphase arrest, allowing us to examine the requirement for Polo in cytokinesis.
FIGURE 4.
polo mad2 double mutant cells exit mitosis. A, third instar larval brains were stained with Hoechst, and the percentage of cells in mitosis was calculated for polo mutants (33%, n = 1777), polo mad2 double mutants (5.8%, n = 3082), and wild type cells (1%, n = 6575). Note that in every case the polo mutants and double mutants used were trans-heterozygous for polo10 over a deficiency (see supplemental materials). B, third instar larval brains were stained with TUNEL, and the percentage of cells in apoptosis was calculated for polo mutants (18.5%, n = 3415), polo/mad2 double mutants (4.4%, n = 1469), and wild types (0.9%, n = 4267). The number of mitotic and TUNEL-positive cells is shown as the mean with a 95% confidence interval.
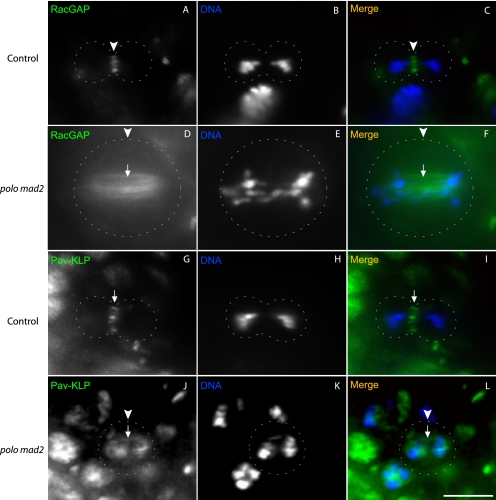
To investigate the role of Polo kinase in localization of the Pav-KLP-RacGAP centralspindlin complex, we stained polo mad2 double mutant larval brains for RacGAP or Pav-KLP (Fig. 5). We saw a complete lack of RacGAP and Pav-KLP at the cell equator and no specific localization at the spindle midzone in all mutant cells (Fig. 5, D, F, J, and L; n = 12 for RacGAP, n = 15 for Pav-KLP) compared with the control cells (Fig. 5, C and I; n = 35 for RacGAP, n = 33 for Pav-KLP). However, unlike the situation in wild type cells or mad2 mutants (supplemental Fig. S3), in polo mad2 double mutants, both Pav-KLP and RacGAP were found throughout the length of the mitotic spindle (Fig. 5, D, F, J, and L, arrows). To further examine the localization of RacGAP, we carried out live imaging of polo mad2 brains expressing Venus-tagged RacGAP. Consistent with the fixed tissue and in contrast to wild type controls (supplemental Movie S1), we saw RacGAP decorating interpolar filaments in dividing cells with no association with the equatorial region or specific localization to the midzone (supplemental Movie S2). These results suggested that Polo was necessary in the early stages of cytokinesis to ensure localization of the centralspindlin complex. We also tested whether or not the kinase function of Polo was necessary for this process by imaging mitosis in cells expressing GFP-tagged kinase-defective Polo. Our results were consistent with those observed for polo loss-of-function mutants: metaphase arrest with separated kinetochores (30) (supplemental Movie S3). To get past this arrest and to prevent any interference from endogenous Polo, we expressed tagged kinase-defective Polo in cells depleted for Mad2 and Polo (supplemental Movie S4). Only 4 of 23 observed mitoses attempted anaphase in this genotype, and all of them failed cytokinesis. The other 19 mitoses remained arrested in metaphase. As a control we expressed tagged normal Polo in cells depleted of Mad2 and Polo and showed that in this case most mitoses imaged (7 of 9) progressed through cytokinesis (supplemental Movie S5).
FIGURE 5.
The centralspindlin complex does not localize to the equatorial cortex in the absence of Polo kinase. Third instar larval brains were stained with anti-RacGAP (A and D; green in C and F) or anti-Pav-KLP (G and J; and green in I and L) antibodies and Hoechst 33258 to detect DNA (B, E, H, and K; blue in C, F, I, and L). A–C, a wild type anaphase cell showing RacGAP localization to the cell equator (arrowheads). D–F, a polo mad2 mutant anaphase cell showing RacGAP localization along the length of microtubules connecting the two poles (arrows) but no equatorial or specific localization to the spindle midzone (arrowheads). G–I, a wild type anaphase cell showing Pav-KLP localization at the cell equator (arrowheads). J–L, a polo mad2 mutant anaphase cell showing Pav-KLP localization along the length of the microtubules connecting the two poles (arrows) but not at the equator or the spindle midzone (arrowheads).
DISCUSSION
Polo Kinase Initiates Centralspindlin-driven Cell Cleavage
In the absence of Polo kinase, we have found that the centralspindlin complex remains associated with the microtubule spindle but cannot translocate to the plus ends of the microtubules at the equator and spindle midzone. This lack of normal centralspindlin localization contrasts with previous work suggesting that the mammalian homolog of Drosophila Polo kinase, Plk1, is not required for the localization of centralspindlin to the equator (8–12). In these experiments, however, chemical inhibitors to Plk1 were not added until at least 20 min after the release from metaphase arrest, which may have given Plk1 the opportunity to regulate some anaphase targets. In experiments where Plk1 activity has been removed earlier and the spindle checkpoint silenced, the localization of centralspindlin has not been tested (33, 34), leaving the question open. It remains possible that in mammalian cells Plk1 is not needed for centralspindlin localization and that this represents a point of difference in cell division between organisms, although to date the function of Polo in cytokinesis has proved to be well conserved (35).
By bypassing the metaphase arrest in Polo kinase-deficient cells, we have been able to demonstrate the need for Polo kinase in the localization of RacGAP and Pav-KLP to the cell equator. This is highly significant because it reveals a well characterized mitotic kinase as a regulator of the midzone localization of centralspindlin (3, 5, 6, 36), placing Polo at the top of the regulatory hierarchy that drives cell cleavage. It will be of considerable interest to determine the molecular mechanism by which Polo facilitates the movement of centralspindlin. From our data it appears that in mutant cells even when there are no gross defects in the anaphase spindle (supplemental Fig. S4), centralspindlin does not accumulate at the ends of microtubules. We hypothesize, therefore, that the failure is in direct molecular regulation of the complex rather than as a result of an indirect structural defect. We have tested for the role of the kinase domain of Polo, but the failure of chromosome separation induced by kinase-defective Polo is not readily bypassed even when the spindle checkpoint is depleted (supplemental Movie S4). The few cells that did separate their DNA failed cytokinesis, consistent with our model for Polo function, although further analysis will be required to determine whether this reflects the requirements for Plk1 kinase activity seen in vertebrates (12, 13). The centralspindlin localization defect we have observed when Polo is removed as well as Mad2 may be related to that of inactivated Pav-KLP, which similarly remains on the spindle without localizing to the plus ends (37). It is possible that Polo is required for the recruitment or function of other centralspindlin regulators such as cyclin-dependent kinases or phosphatases (38), although to date none of these regulators has shown a similar phenotype.
A New Domain of Polo Kinase Mediates the Initial Formation of the Cytokinesis Initiation Complex
Our studies provide evidence for a direct interaction between RacGAP and Polo kinase. RacGAP has previously been shown to bind a number of other proteins in cytokinesis such as Anillin (26), Pebble, and Pav-KLP (3). This is consistent with the important function we have shown for RacGAP in the localization and anchoring of proteins at the contractile ring early in cytokinesis (28). Recent efforts to identify Plk1 interactors by immunoprecipitation have had mixed results; some have been able to pull down a little RacGAP1 by UV cross-linking the Ect2 complex (12), whereas we3 and others did not find appreciable amounts by conventional immunoprecipitation (13). Our yeast two-hybrid interaction assay, in conjunction with our FRET data, not only makes it clear that that Polo kinase and RacGAP do in fact bind directly but also that they interact in anaphase at a time when cytokinesis initiates. We speculate that the difficulty in detecting this by immunoprecipitation is primarily a technical one, because immunohistochemistry shows most of Plk1 co-localized with centralspindlin at anaphase (12) in agreement with our results and others (39) in Drosophila. The interaction we have detected occurs via a new domain of Polo kinase with no previously known function. It will therefore be interesting to investigate whether this domain is also required for events prior to cytokinesis or whether it confers cytokinesis-specific functions on Polo; our mutagenesis of the region suggests the former (supplemental Fig. S1). Previous studies have focused on Polo binding via the Polo Box domains and have identified several metaphase targets such as Vimentin, INCENP, and Cdc25 phospho-primed by the mitotic kinase cyclin-dependent kinase 1/cyclin B (29, 40–42). Because the Polo-RacGAP interaction is not via the Polo Box regions, the possibility exists that it is not phospho-dependent, which might be better suited to maintaining interaction through anaphase, when the kinase cyclin-dependent kinase 1/cyclin B has been degraded by the APC/C (43, 44). In summary, the evidence presented here shows that Polo kinase binds RacGAP, a component of the cytokinesis initiation complex, and that it functions upstream of Pav-KLP and RacGAP localization, an event that must occur immediately after the onset of anaphase as a critical step in the cascade of regulatory events leading to cytokinesis.
Supplementary Material
Acknowledgment
We thank Claudio Sunkel for the Polo kinase antibody.
This work was supported by funding from the National Health and Medical Research Council of Australia, the Australian Research Council, the Australian National University, and the University of Adelaide.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Movies SV1–SV5 and Figs. S1–S4.
S. Ebrahimi, H. Fraval, M. Murray, R. Saint, and S. L. Gregory, unpublished observations.
- Plk1
- Polo-like kinase 1
- Pav-KLP
- Pavarotti-Kinesin-like protein.
REFERENCES
- 1.Glotzer M. (2005) Science 307, 1735–1739 [DOI] [PubMed] [Google Scholar]
- 2.Prokopenko S. N., Brumby A., O'Keefe L., Prior L., He Y., Saint R., Bellen H. J. (1999) Genes Dev. 13, 2301–2314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somers W. G., Saint R. (2003) Dev. Cell 4, 29–39 [DOI] [PubMed] [Google Scholar]
- 4.Tatsumoto T., Xie X., Blumenthal R., Okamoto I., Miki T. (1999) J. Cell Biol. 147, 921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishima M., Kaitna S., Glotzer M. (2002) Dev. Cell 2, 41–54 [DOI] [PubMed] [Google Scholar]
- 6.Nishimura Y., Yonemura S. (2006) J. Cell Sci. 119, 104–114 [DOI] [PubMed] [Google Scholar]
- 7.Jantsch-Plunger V., Gönczy P., Romano A., Schnabel H., Hamill D., Schnabel R., Hyman A. A., Glotzer M. (2000) J. Cell Biol. 149, 1391–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brennan I. M., Peters U., Kapoor T. M., Straight A. F. (2007) PLoS ONE 2, e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burkard M. E., Randall C. L., Larochelle S., Zhang C., Shokat K. M., Fisher R. P., Jallepalli P. V. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 4383–4388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petronczki M., Glotzer M., Kraut N., Peters J. M. (2007) Dev. Cell 12, 713–725 [DOI] [PubMed] [Google Scholar]
- 11.Santamaria A., Neef R., Eberspächer U., Eis K., Husemann M., Mumberg D., Prechtl S., Schulze V., Siemeister G., Wortmann L., Barr F. A., Nigg E. A. (2007) Mol. Biol. Cell 18, 4024–4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burkard M. E., Maciejowski J., Rodriguez-Bravo V., Repka M., Lowery D. M., Clauser K. R., Zhang C., Shokat K. M., Carr S. A., Yaffe M. B., Jallepalli P. V. (2009) PLoS Biol. 7, e1000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfe B. A., Takaki T., Petronczki M., Glotzer M. (2009) PLoS Biol. 7, e1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sunkel C. E., Glover D. M. (1988) J. Cell Sci. 89, 25–38 [DOI] [PubMed] [Google Scholar]
- 15.Fenton B., Glover D. M. (1993) Nature 363, 637–640 [DOI] [PubMed] [Google Scholar]
- 16.Carmena M., Riparbelli M. G., Minestrini G., Tavares A. M., Adams R., Callaini G., Glover D. M. (1998) J. Cell Biol. 143, 659–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ohkura H., Hagan I. M., Glover D. M. (1995) Genes Dev. 9, 1059–1073 [DOI] [PubMed] [Google Scholar]
- 18.Herrmann S., Amorim I., Sunkel C. E. (1998) Chromosoma 107, 440–451 [DOI] [PubMed] [Google Scholar]
- 19.Abramoff M. D., Magelhaes P. J., Ram S. J. (2004). Biophotonics Int. 11, 36–42 [Google Scholar]
- 20.Thévenaz P., Ruttimann U. E., Unser M. (1998) IEEE Trans. Image Process. 7, 27–41 [DOI] [PubMed] [Google Scholar]
- 21.König P., Krasteva G., Tag C., König I. R., Arens C., Kummer W. (2006) Lab. Invest. 86, 853–864 [DOI] [PubMed] [Google Scholar]
- 22.Lee K. S., Erikson R. L. (1997) Mol. Cell. Biol. 17, 3408–3417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollenberg S. M., Sternglanz R., Cheng P. F., Weintraub H. (1995) Mol. Cell. Biol. 15, 3813–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savoian M. S., Rieder C. L. (2002) J. Cell Sci. 115, 3061–3072 [DOI] [PubMed] [Google Scholar]
- 25.Kenworthy A. K. (2001) Methods 24, 289–296 [DOI] [PubMed] [Google Scholar]
- 26.Gregory S. L., Ebrahimi S., Milverton J., Jones W. M., Bejsovec A., Saint R. (2008) Curr. Biol. 18, 25–29 [DOI] [PubMed] [Google Scholar]
- 27.You X., Nguyen A. W., Jabaiah A., Sheff M. A., Thorn K. S., Daugherty P. S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 18458–18463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zavortink M., Contreras N., Addy T., Bejsovec A., Saint R. (2005) J. Cell Sci. 118, 5381–5392 [DOI] [PubMed] [Google Scholar]
- 29.Elia A. E., Rellos P., Haire L. F., Chao J. W., Ivins F. J., Hoepker K., Mohammad D., Cantley L. C., Smerdon S. J., Yaffe M. B. (2003) Cell 115, 83–95 [DOI] [PubMed] [Google Scholar]
- 30.Donaldson M. M., Tavares A. A., Hagan I. M., Nigg E. A., Glover D. M. (2001) J. Cell Sci. 114, 2357–2358 [DOI] [PubMed] [Google Scholar]
- 31.Li R., Murray A. W. (1991) Cell 66, 519–531 [DOI] [PubMed] [Google Scholar]
- 32.Buffin E., Emre D., Karess R. E. (2007) Nat. Cell Biol. 9, 565–572 [DOI] [PubMed] [Google Scholar]
- 33.Lénárt P., Petronczki M., Steegmaier M., Di Fiore B., Lipp J. J., Hoffmann M., Rettig W. J., Kraut N., Peters J. M. (2007) Curr. Biol. 17, 304–315 [DOI] [PubMed] [Google Scholar]
- 34.van Vugt M. A., van de Weerdt B. C., Vader G., Janssen H., Calafat J., Klompmaker R., Wolthuis R. M., Medema R. H. (2004) J. Biol. Chem. 279, 36841–36854 [DOI] [PubMed] [Google Scholar]
- 35.Archambault V., Glover D. M. (2009) Nat. Rev. Mol. Cell Biol. 10, 265–275 [DOI] [PubMed] [Google Scholar]
- 36.Zhao W. M., Fang G. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 13158–13163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minestrini G., Harley A. S., Glover D. M. (2003) Mol. Biol. Cell 14, 4028–4038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishima M., Pavicic V., Grüneberg U., Nigg E. A., Glotzer M. (2004) Nature 430, 908–913 [DOI] [PubMed] [Google Scholar]
- 39.D'Avino P. P., Archambault V., Przewloka M. R., Zhang W., Lilley K. S., Laue E., Glover D. M. (2007) PLoS One 2, e572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elia A. E., Cantley L. C., Yaffe M. B. (2003) Science 299, 1228–1231 [DOI] [PubMed] [Google Scholar]
- 41.Goto H., Kiyono T., Tomono Y., Kawajiri A., Urano T., Furukawa K., Nigg E. A., Inagaki M. (2006) Nat. Cell Biol. 8, 180–187 [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi T., Goto H., Yokoyama T., Sillje H., Hanisch A., Uldschmid A., Takai Y., Oguri T., Nigg E. A., Inagaki M. (2005) J. Cell Biol. 171, 431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clute P., Pines J. (1999) Nat. Cell Biol. 1, 82–87 [DOI] [PubMed] [Google Scholar]
- 44.Peters J. M. (2002) Mol. Cell 9, 931–943 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.