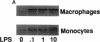Abstract
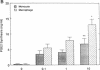
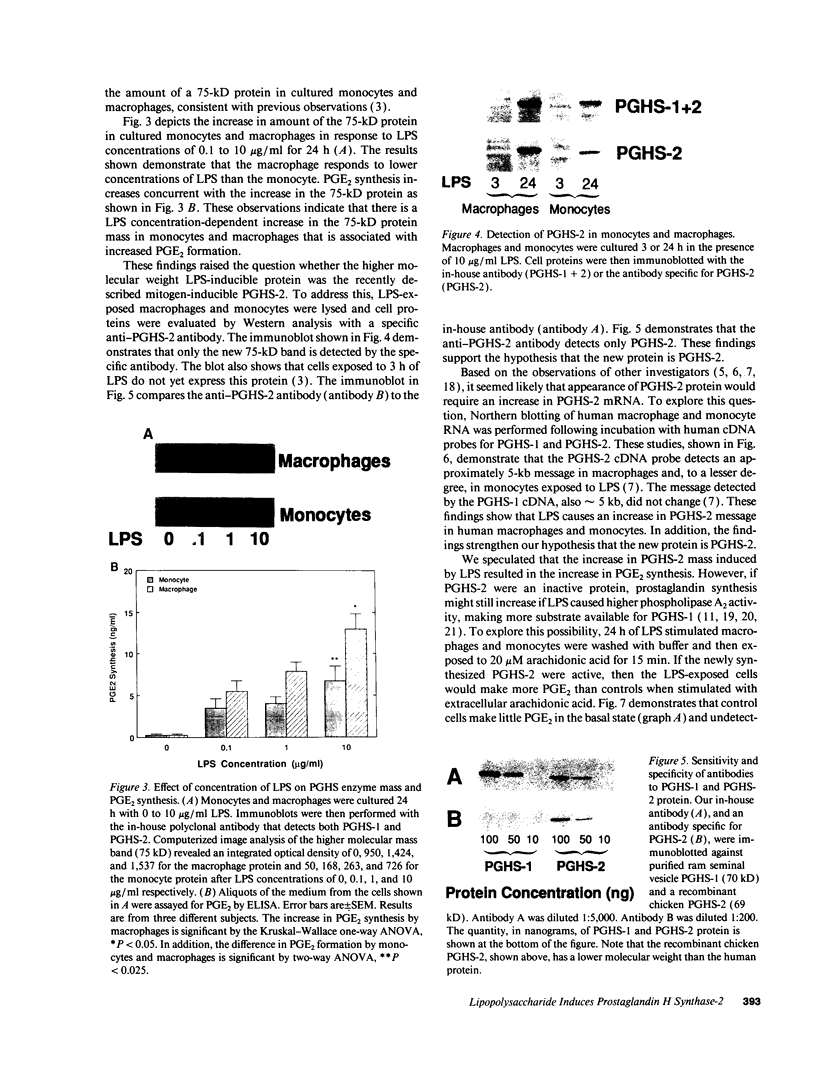
We and others have previously demonstrated that human alveolar macrophages produce more PGE2 in response to lipopolysaccharide (LPS) than do blood monocytes. We hypothesized that this observation was due to a greater increase in prostaglandin H synthase-2 (PGHS-2) enzyme mass in the macrophage compared to the monocyte. To evaluate this hypothesis, alveolar macrophages and blood monocytes were obtained from healthy nonsmoking volunteers. The cells were cultured in the presence of 0 to 10 micrograms/ml LPS. LPS induced the synthesis of large amounts of a new 75-kD protein in human alveolar macrophages, and a lesser amount in monocytes. Synthesis of this protein required more than 6 h and peaked in 24 to 48 h; the protein reacted with an anti-PGHS-2 antibody prepared against mouse PGHS-2. Associated with synthesis of the protein was a marked increase in LPS-stimulated and arachidonic acid-stimulated synthesis of PGE2 by alveolar macrophages compared to monocytes. Cells not exposed to LPS contained only PGHS-1 and synthesized very little PGE2 during culture or in response to exogenous arachidonic acid. An LPS-induced mRNA, which hybridized to a human cDNA probe for PGHS-2 mRNA, was produced in parallel with production of this new protein and was produced in much greater amounts by alveolar macrophages compared to blood monocytes. This mRNA was not detectable in cells not exposed to LPS. In contrast, both types of cells contain mRNA, which hybridizes to a cDNA probe for PGHS-1. This mRNA did not increase in response to LPS. LPS also had no effect on PGHS-1 protein. These data demonstrate that PGE2 synthesis in human alveolar macrophages and blood monocytes correlates to the mass of PGHS-2 in the cell. We conclude that the greater ability of the macrophage to synthesize PGE2 in response to LPS is due to greater synthesis of PGHS-2 by the macrophage.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. Role of monocytes and interstitial cells in the generation of alveolar macrophages II. Kinetic studies after carbon loading. Lab Invest. 1980 May;42(5):518–524. [PubMed] [Google Scholar]
- Aderem A. A., Cohen D. S., Wright S. D., Cohn Z. A. Bacterial lipopolysaccharides prime macrophages for enhanced release of arachidonic acid metabolites. J Exp Med. 1986 Jul 1;164(1):165–179. doi: 10.1084/jem.164.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachwich P. R., Chensue S. W., Larrick J. W., Kunkel S. L. Tumor necrosis factor stimulates interleukin-1 and prostaglandin E2 production in resting macrophages. Biochem Biophys Res Commun. 1986 Apr 14;136(1):94–101. doi: 10.1016/0006-291x(86)90881-8. [DOI] [PubMed] [Google Scholar]
- Balter M. S., Toews G. B., Peters-Golden M. Different patterns of arachidonate metabolism in autologous human blood monocytes and alveolar macrophages. J Immunol. 1989 Jan 15;142(2):602–608. [PubMed] [Google Scholar]
- Barchowsky A., Kent R. S., Whorton A. R. Recovery of porcine aortic endothelial cell prostaglandin synthesis following inhibition by sublethal concentrations of hydrogen peroxide. Biochim Biophys Acta. 1987 Mar 11;927(3):372–381. doi: 10.1016/0167-4889(87)90102-9. [DOI] [PubMed] [Google Scholar]
- Bigby T. D., Holtzman M. J. Enhanced 5-lipoxygenase activity in lung macrophages compared to monocytes from normal subjects. J Immunol. 1987 Mar 1;138(5):1546–1550. [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y. Role of monocytes and interstitial cells in the generation of alveolar macrophages I. Kinetic studies of normal mice. Lab Invest. 1980 May;42(5):511–517. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brotherton A. F., Hoak J. C. Prostacyclin biosynthesis in cultured vascular endothelium is limited by deactivation of cyclooxygenase. J Clin Invest. 1983 Oct;72(4):1255–1261. doi: 10.1172/JCI111081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. P., Monick M. M., Hunninghake G. W. Human alveolar macrophage arachidonic acid metabolism. Am J Physiol. 1988 Jun;254(6 Pt 1):C809–C815. doi: 10.1152/ajpcell.1988.254.6.C809. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- DeWitt D. L., el-Harith E. A., Smith W. L. Molecular cloning of prostaglandin G/H synthase. Adv Prostaglandin Thromboxane Leukot Res. 1989;19:454–457. [PubMed] [Google Scholar]
- Diaz A., Reginato A. M., Jimenez S. A. Alternative splicing of human prostaglandin G/H synthase mRNA and evidence of differential regulation of the resulting transcripts by transforming growth factor beta 1, interleukin 1 beta, and tumor necrosis factor alpha. J Biol Chem. 1992 May 25;267(15):10816–10822. [PubMed] [Google Scholar]
- Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [PubMed] [Google Scholar]
- Goerig M., Habenicht A. J., Zeh W., Salbach P., Kommerell B., Rothe D. E., Nastainczyk W., Glomset J. A. Evidence for coordinate, selective regulation of eicosanoid synthesis in platelet-derived growth factor-stimulated 3T3 fibroblasts and in HL-60 cells induced to differentiate into macrophages or neutrophils. J Biol Chem. 1988 Dec 25;263(36):19384–19391. [PubMed] [Google Scholar]
- Han J. W., Sadowski H., Young D. A., Macara I. G. Persistent induction of cyclooxygenase in p60v-src-transformed 3T3 fibroblasts. Proc Natl Acad Sci U S A. 1990 May;87(9):3373–3377. doi: 10.1073/pnas.87.9.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Lands W. E. Evidence for a peroxide-initiated free radical mechanism of prostaglandin biosynthesis. J Biol Chem. 1980 Jul 10;255(13):6253–6261. [PubMed] [Google Scholar]
- Hempel S. L., Haycraft D. L., Hoak J. C., Spector A. A. Reduced prostacyclin formation after reoxygenation of anoxic endothelium. Am J Physiol. 1990 Nov;259(5 Pt 1):C738–C745. doi: 10.1152/ajpcell.1990.259.5.C738. [DOI] [PubMed] [Google Scholar]
- Hla T., Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman M. J., Turk J., Shornick L. P. Identification of a pharmacologically distinct prostaglandin H synthase in cultured epithelial cells. J Biol Chem. 1992 Oct 25;267(30):21438–21445. [PubMed] [Google Scholar]
- Iwamoto G. K., Monick M. M., Burmeister L. F., Hunninghake G. W. Interleukin 1 release by human alveolar macrophages and blood monocytes. Am J Physiol. 1989 May;256(5 Pt 1):C1012–C1015. doi: 10.1152/ajpcell.1989.256.5.C1012. [DOI] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmerich B., Rossing T. H., Pennington J. E. Comparative oxidative microbicidal activity of human blood monocytes and alveolar macrophages and activation by recombinant gamma interferon. Am Rev Respir Dis. 1987 Aug;136(2):266–270. doi: 10.1164/ajrccm/136.2.266. [DOI] [PubMed] [Google Scholar]
- Kujubu D. A., Fletcher B. S., Varnum B. C., Lim R. W., Herschman H. R. TIS10, a phorbol ester tumor promoter-inducible mRNA from Swiss 3T3 cells, encodes a novel prostaglandin synthase/cyclooxygenase homologue. J Biol Chem. 1991 Jul 15;266(20):12866–12872. [PubMed] [Google Scholar]
- Lee S. H., Soyoola E., Chanmugam P., Hart S., Sun W., Zhong H., Liou S., Simmons D., Hwang D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992 Dec 25;267(36):25934–25938. [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Lin A. H., Bienkowski M. J., Gorman R. R. Regulation of prostaglandin H synthase mRNA levels and prostaglandin biosynthesis by platelet-derived growth factor. J Biol Chem. 1989 Oct 15;264(29):17379–17383. [PubMed] [Google Scholar]
- Maier J. A., Hla T., Maciag T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J Biol Chem. 1990 Jul 5;265(19):10805–10808. [PubMed] [Google Scholar]
- Monick M., Glazier J., Hunninghake G. W. Human alveolar macrophages suppress interleukin-1 (IL-1) activity via the secretion of prostaglandin E2. Am Rev Respir Dis. 1987 Jan;135(1):72–77. doi: 10.1164/arrd.1987.135.1.72. [DOI] [PubMed] [Google Scholar]
- Murakami M., Kudo I., Inoue K. Molecular nature of phospholipases A2 involved in prostaglandin I2 synthesis in human umbilical vein endothelial cells. Possible participation of cytosolic and extracellular type II phospholipases A2. J Biol Chem. 1993 Jan 15;268(2):839–844. [PubMed] [Google Scholar]
- Newman S. L., Henson J. E., Henson P. M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J Exp Med. 1982 Aug 1;156(2):430–442. doi: 10.1084/jem.156.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nüsing R., Ullrich V. Regulation of cyclooxygenase and thromboxane synthase in human monocytes. Eur J Biochem. 1992 May 15;206(1):131–136. doi: 10.1111/j.1432-1033.1992.tb16910.x. [DOI] [PubMed] [Google Scholar]
- O'Banion M. K., Winn V. D., Young D. A. cDNA cloning and functional activity of a glucocorticoid-regulated inflammatory cyclooxygenase. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4888–4892. doi: 10.1073/pnas.89.11.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan M. G., Chilton F. H., Huggins E. M., Jr, McCall C. E. Lipopolysaccharide priming of alveolar macrophages for enhanced synthesis of prostanoids involves induction of a novel prostaglandin H synthase. J Biol Chem. 1992 Jul 25;267(21):14547–14550. [PubMed] [Google Scholar]
- O'Sullivan M. G., Huggins E. M., Jr, Meade E. A., DeWitt D. L., McCall C. E. Lipopolysaccharide induces prostaglandin H synthase-2 in alveolar macrophages. Biochem Biophys Res Commun. 1992 Sep 16;187(2):1123–1127. doi: 10.1016/0006-291x(92)91313-f. [DOI] [PubMed] [Google Scholar]
- Pueringer R. J., Hunninghake G. W. Lipopolysaccharide stimulates de novo synthesis of PGH synthase in human alveolar macrophages. Am J Physiol. 1992 Jan;262(1 Pt 1):L78–L85. doi: 10.1152/ajplung.1992.262.1.L78. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Moss J., Oberpriller J., Hom B., Stier L., Ozaki T., Crystal R. G. Fibroblasts: important producers and targets of inflammatory prostaglandins in the lungs. Chest. 1983 May;83(5 Suppl):92S–93S. doi: 10.1378/chest.83.5_supplement.92s. [DOI] [PubMed] [Google Scholar]
- Rinehart J. J., Wuest D., Ackerman G. A. Corticosteroid alteration of human monocyte to macrophage differentiation. J Immunol. 1982 Oct;129(4):1436–1440. [PubMed] [Google Scholar]
- Rosen G. D., Birkenmeier T. M., Raz A., Holtzman M. J. Identification of a cyclooxygenase-related gene and its potential role in prostaglandin formation. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1358–1365. doi: 10.1016/0006-291x(89)91819-6. [DOI] [PubMed] [Google Scholar]
- Shimokawa T., Smith W. L. Prostaglandin endoperoxide synthase. The aspirin acetylation region. J Biol Chem. 1992 Jun 15;267(17):12387–12392. [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Rolfe M. W., Evanoff H. L., Allen R. M., Strieter R. M. Regulation of human alveolar macrophage- and blood monocyte-derived interleukin-8 by prostaglandin E2 and dexamethasone. Am J Respir Cell Mol Biol. 1992 Jan;6(1):75–81. doi: 10.1165/ajrcmb/6.1.75. [DOI] [PubMed] [Google Scholar]
- Stevenson H. C., Katz P., Wright D. G., Contreras T. J., Jemionek J. F., Hartwig V. M., Flor W. J., Fauci A. S. Human blood monocytes: characterization of negatively selected human monocytes and their suspension cell culture derivatives. Scand J Immunol. 1981 Sep;14(3):243–256. doi: 10.1111/j.1365-3083.1981.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. D., Ramberg R. E., Sale G. E., Sparkes R. S., Golde D. W. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science. 1976 Jun 4;192(4243):1016–1018. doi: 10.1126/science.775638. [DOI] [PubMed] [Google Scholar]
- Willems C., De Groot P. G., Pool G. A., Gonsalvez M. S., Van Aken W. G., Van Mourik J. A. Arachidonate metabolism in cultured human vascular endothelial cells. Evidence for two prostaglandin synthetic pathways sensitive to acetylsalicylic acid. Biochim Biophys Acta. 1982 Dec 13;713(3):581–588. doi: 10.1016/0005-2760(82)90318-6. [DOI] [PubMed] [Google Scholar]
- Wu K. K., Hatzakis H., Lo S. S., Seong D. C., Sanduja S. K., Tai H. H. Stimulation of de novo synthesis of prostaglandin G/H synthase in human endothelial cells by phorbol ester. J Biol Chem. 1988 Dec 15;263(35):19043–19047. [PubMed] [Google Scholar]
- Xie W. L., Chipman J. G., Robertson D. L., Erikson R. L., Simmons D. L. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2692–2696. doi: 10.1073/pnas.88.7.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]