Abstract
Chemokines are characterized by the homing activity of leukocytes to targeted inflammation sites. Recent research indicates that chemokines play more divergent roles in various phases of pathogenesis as well as immune reactions. The chemokine receptor, CCR1, and its ligands are thought to be involved in inflammatory bone destruction, but their physiological roles in the bone metabolism in vivo have not yet been elucidated. In the present study, we investigated the roles of CCR1 in bone metabolism using CCR1-deficient mice. Ccr1−/− mice have fewer and thinner trabecular bones and low mineral bone density in cancellous bones. The lack of CCR1 affects the differentiation and function of osteoblasts. Runx2, Atf4, Osteopontin, and Osteonectin were significantly up-regulated in Ccr1−/− mice despite sustained expression of Osterix and reduced expression of Osteocalcin, suggesting a lower potential for differentiation into mature osteoblasts. In addition, mineralized nodule formation was markedly disrupted in cultured osteoblastic cells isolated from Ccr1−/− mice. Osteoclastogenesis induced from cultured Ccr1−/− bone marrow cells yielded fewer and smaller osteoclasts due to the abrogated cell-fusion. Ccr1−/− osteoclasts exerted no osteolytic activity concomitant with reduced expressions of Rank and its downstream targets, implying that the defective osteoclastogenesis is involved in the bone phenotype in Ccr1−/− mice. The co-culture of wild-type osteoclast precursors with Ccr1−/− osteoblasts failed to facilitate osteoclastogenesis. This finding is most likely due to a reduction in Rankl expression. These observations suggest that the axis of CCR1 and its ligands are likely to be involved in cross-talk between osteoclasts and osteoblasts by modulating the RANK-RANKL-mediated interaction.
Keywords: Bone, Cell Differentiation, Cell Surface Receptor, Chemokines, Cytokines Induction, G Protein-coupled Receptors (GPCRs), Receptors, Bone Metabolism, Osteoblast Differentiation, Osteoclast Differentiation
Introduction
Chemokines are initially identified as small cytokines that direct the homing of circulating leukocytes into sites of inflammation (1). Chemokines are now recognized to be major factors in inflammation and immune development as well as tumor growth, angiogenesis, and osteolysis. Chemokine receptors are expressed in a well organized spatiotemporal manner in various types of leukocytes, including lymphocytes, granulocytes, and macrophages. They facilitate the recruitment of these cells into inflammatory sites during the appropriate phase of inflammation.
Recent findings indicate that chemokine receptors, including CCR17 and its related chemokines, CCL3 and CCL9, are involved in the pathogenesis of a variety of diseases. In particular, CCL3 (also called MIP-1α), a major pro-inflammatory chemokine produced at inflammatory sites, appears to play a crucial role in pathological osteoclastogenesis (2, 3). In osteolytic bone inflammation (e.g. rheumatoid arthritis-associated bone destruction), CCL3 induces ectopic osteoclastogenesis (4) and results in bone destruction (5). Several reports suggested that CCL3 is also produced by myeloma cells and directly stimulates bone destruction in myeloma-related bone diseases (5–7). These findings indicate the possible roles of CCL3 as a crucial chemokine for osteoclast function. Several antagonists of the chemokine ligands of CCL3, such as CCR1-specific (BX471) and CCR5-specific (TAK779) blockers, have been tested as drug candidates for the treatment of patients with rheumatoid arthritis-associated bone destruction and multiple myeloma (4, 8). The chemokine CCL9 (also called MIP-1γ), is also abundantly produced by various myeloid lineage-derived cells, including osteoclasts (9), activates osteoclastogenesis through its receptor, CCR1 (10–12). However, the exact physiological functions of CCR1 and its related chemokines in bone remodeling are still not fully characterized (12, 13).
A recent study using an ovariectomy-induced bone loss model found that the chemokine receptor CCR2 was associated with postmenopausal bone loss (14), but there are few reports on bone phenotypes in other chemokine receptor-deficient mouse models. In the present study, we demonstrated that osteopenia in Ccr1−/− mice appeared to be due to impaired osteoclast and osteoblast function. Our data also uncovered a possible role for CCR1 and its related ligands in the communication between osteoclasts and osteoblasts.
EXPERIMENTAL PROCEDURES
Mice
Standard male C57BL/6 mice (6–9 weeks of age) were obtained from CLEA Japan. Ccr1−/− mice (15) purchased from Jackson Laboratories were backcrossed for 8–10 generations on the C57BL/6 background mice. Mice were all bred and maintained under pathogen-free conditions at the animal facilities of the University of Tokyo. All experiments were performed according to the Institutional Guidelines for the Care and Use of Laboratory Animals in Research and were approved by the ethics committees of both the University of Tokyo and the Research Institute of International Medical Center of Japan.
Materials
Recombinant mouse M-CSF and RANKL were purchased from R&D Systems Inc. (Minneapolis, MN) and PeproTech Inc. (Rocky Hill, NJ), respectively. Recombinant mouse CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), CCL9 (MIP-1γ), and CCL11 (eotaxin-1) and their corresponding-neutralizing antibodies were purchased from R&D Systems. Control rat IgG was purchased from Jackson ImmunoResearch (Bar Harbor, ME). Recombinant mouse CX3CL1 (fractalkine) was purchased from R&D Systems. Hamster anti-CX3CL1-neutralizing antibody and control hamster IgG were kindly provided by Dr. Toshio Imai (Kan Research Institute, Kobe, Japan). Rabbit anti-human/mouse CCR1 polyclonal antibody and control rabbit IgG were purchased from AbCam (Cambridge, MA) and Chemicon (Temecula, CA), respectively. Secondary antibodies (Alexa488-labeled anti-rabbit IgG and Streptavidin-PE) were purchased from Molecular Probes (Eugene, OR). Rabbit anti-TRAP and anti-Cathepsin K polyclonal antibodies were both purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Osteoclast and Osteoblastic Cell Culture
Mouse bone marrow cells cultured in α-minimal essential medium were used as sources of osteoclastic and osteoblastic cell cultures. The non-adherent cells were collected for bone marrow-derived macrophage and pre-osteoclast induction, and adherent bone marrow-derived mesenchymal stromal cells were collected for osteoblast induction. Bone marrow-derived macrophages were induced with 10 ng/ml M-CSF for an additional 10 days. To generate pre-osteoclasts, non-adherent cells were passed through a column filled with Sephadex G-10 microspheres (Amersham Biosciences) and were then cultured with 10 ng/ml M-CSF and 20 ng/ml RANKL for 4 days. The mature osteoclasts were induced from pre-osteoclasts by culturing for an additional 14 days with M-CSF and RANKL. The culture media were replaced every 3 days. TRAP activity in the osteoclasts was determined by staining using an acid phosphatase leukocyte staining kit (Sigma). The contamination of stromal/osteoblastic cells was monitored using Q-PCR analysis, as a low expression level of the Osteoprotegrin gene indicates stromal/osteoblastic cells.
Osteoblastic differentiation in adherent bone marrow mesenchymal stromal cells was induced by culture in α-minimal essential medium containing 10% FBS, 200 μm ascorbic acid, 10 mm β-glycerophosphate, and 10 nm dexamethasone (16). The culture media was replaced once every 3 days in the presence or absence of chemokine-neutralizing antibodies. The cells were fixed with 4% paraformaldehyde and stained for alkaline phosphatase with naphthol AS-MX phosphate plus Fastblue-BB (Sigma) and for minerals with alizarin red. Mineral deposition was alternatively identified by von Kossa staining (Polysciences, Inc., Warrington, PA), and the mineralized areas were measured by using an Array Scan VTI HCS analyzer (Beckman Coulter).
Co-culture experiments with osteoclast precursors and osteoblasts were performed by inoculating bone marrow-derived precursors (1 × 105 cells/well) onto the layer of osteoblastic cells that had been cultured for 21 days with osteoblast-inducing media in 24-well plates. Thereafter, these cells were co-cultured for 7 days in α-minimal essential medium supplemented with 10% FBS and 10 μg/ml vitamin D3. To assess bone resorption activity, these co-culture studies were also conducted using bone slices. After fixation of the cells with 2.5% glutaraldehyde/1.6% paraformaldehyde in 0.1 m cacodylic acid (pH 7.4), the bone slices were briefly rinsed, and were completely dehydrated in an ascending series of ethanol and liquid carbon dioxide. The samples were coated with an ultrafine titanium oxide powder and observed under a scanning electron microscopy.
Immunohistochemical Staining
For the immunohistochemical staining analyses, osteoclasts were fixed with 4% paraformaldehyde, permeabilized, and stained with the indicated specific antibodies, followed by Alexa594-conjugated secondary antibodies and Alexa488-labeled phalloidin (Molecular Probes). The osteoclasts with multiple nuclei (>3) were quantified. Images were captured using an IX-81 fluorescence microscope equipped with a confocal microscopy DSU unit (Olympus, Japan) and were analyzed with the MetaMorphTM software program (Universal Imaging, Molecular Devices, Sunnyvale, CA). The formation of osteoclasts was quantified by capturing and analyzing images using the ImageJ software program (National Institutes of Health, Bethesda, MD) based on TRAP staining of 25 fields in each well, which were randomly chosen and analyzed.
Real-time PCR Analysis
Total cellular RNA from osteoclasts, osteoblasts, and bone tissues (proximal tibia after the bone marrow flush and the removal of metaphysial regions) was isolated using the RNeasy kit (Qiagen, Valencia, CA). The total RNA was then reverse-transcribed into cDNA using the Superscript III RT kit (Invitrogen). The real-time quantitative PCR analyses were performed using the ABI 7700 sequence detector system with SYBR Green (Applied Biosystems, Foster City, CA). The sequences were amplified for 40 cycles under the following conditions: denaturation at 95 °C for 15 s, annealing at 60 °C for 30 s, and extension at 72 °C for 45 s with primers for the chemokine receptors as previously reported (17). Gene expression levels were compared with Gapdh gene expression by the 2−Δ(Ct) method.
Measurement of Cytokines and Chemokines
Chemokine CCL5 and CCL9 secretion levels were determined by ELISA using the antibodies MAB4781 and BAF478 (R&D systems) and MAB463 and BAF463 (R&D systems), respectively. The reaction intensities were determined by using HRP-conjugated streptavidin (Chemicon). The cytokine production levels were quantified with a mouse 23-plex multiple cytokine detection system (Bio-Rad Corp., Hercules, CA) according to the manufacturer's instructions.
Flow Cytometry
FITC-, PE-, APC-, PerCP-Cy5.5-, PE-Cy7-, or biotin-conjugated anti-mouse mAbs to CD45.2 (104), CD115 (AFS98), and CD265/RANK (R12–31), and subclass-matched control antibodies were purchased from eBioscience (San Diego, CA). Anti-mouse mAbs to FcγR (2.4G2), Ly6C/6G (RB6–8C5), CD11b (M1/70), and CD19 (1D3) were purchased from BD Pharmingen (San Diego, CA). The flow cytometric analyses were performed using an LSR II flow cytometer with the FACS diva software program (BD Biosciences) and were analyzed with the FlowJo software program (TreeStar, Ashland, OR). Dead cells were excluded on the basis of the forward and side scatter profiles and propidium iodide staining.
Microcomputed Tomography and Peripheral Quantitative Computed Tomography
Micro-computed tomography (microCT) scanning was performed on proximal tibiae by μCT-40 (SCANCO Medical AG) with a resolution of 12 μm, and the microstructure parameters were three-dimensionally calculated as previously described (18). The bone scores were measured by peripheral quantitative CT using the XCT Research SA+ system (Stratec Medizintechnik GmbH, Pforzheim, Germany). The bone scores and density were measured and analyzed at 1.2 mm below the epiphyseal plate of distal femora. The scores were defined according to the American Society for Bone and Mineral Research standards.
Bone Histomorphometry
The unilateral proximal tibiae fixed with ethanol were embedded in glycol methacrylate, and the blocks were cut in 5-μm-thick sections. The structural parameters were analyzed at the secondary spongiosa. For the assessment of dynamic histomorphometric indices, calcein (at a dose of 20 mg/kg body weight) was injected twice (72-h interval) to wild-type and Ccr1-deficient mice, respectively. The sections were stained with toluidine blue and analyzed using a semi-automated system (Osteoplan II, Zeiss). The nomenclature, symbols, and units used in the present study are those recommended by the Nomenclature Committee of the American Society for Bone and Mineral Research (19).
Measurement of TRAP, BALP, and Collagen-type I N-telopeptides (NTx)
Tartrate-resistant acid phosphatases (TRAP5b) in serum and culture supernatant were measured by the mouse TRAP EIA assay kit (Immunodiagnostic system, Fountain Hills, AZ). In brief, the culture supernatant or diluted serum was applied to an anti-TRAP5b-coated microplate, according to the manufacturer's instruction. The enzymatic activities of bound TRAP were determined with chromogenic substrates. Bone-specific alkaline phosphatase (BALP) levels were measured using the mouse BALP ELISA kit (Cusabio Biotech Co. Ltd., Wilmington, DE). Collagen-type I NTx were measured by ELISA (SRL, Tokyo).
Collagen-based Zymography
Collagen digestion activity was measured by using modified methods, which were based on gelatin-based zymography (20), with some modification for type-I collagen (21, 22). In brief, the osteoclasts were gently digested with lysis buffer (150 mm NaCl, 50 mm HEPES, 5 mm EDTA, and 10% Nonidet P-40 with Halt protease inhibitor mixture, pH 7.5). The lysates were separated by SDS-PAGE on a 10% polyacrylamide gel with porcine type-I collagen (1 mg/ml, Nitta Gelatin Inc., Osaka, Japan) under chilled conditions. The gel was washed with denaturation buffer (Tris-buffered saline (150 mm NaCl, 25 mm Tris-HCl, pH 7.4, supplemented with 2.5% Triton X-100) and then subjected to zymography for 18–24 h at 37 °C in zymography developing buffer (Tris-buffered saline, supplemented with 1 mm CaCl2, 1 μm ZnCl2, and 0.05% Brij-35). The signals were detected using Coomassie Brilliant Blue solution (Wako Pure Chemicals, Osaka, Japan).
Immunoblot Analysis
Total cell lysates were isolated, separated by SDS-PAGE, and electrotransferred onto Immobilon-P PVDF membranes (Millipore). The membrane was blocked by 5% BSA in TBST (150 mm NaCl, 25 mm Tris-HCl (pH 7.4) supplemented with 0.1% Tween 20) and incubated with rabbit anti-ATF4 polyclonal antibody (1/2,000), followed by HRP-conjugated anti-rabbit IgG (1/10,000). The signals were detected using an ECL chemiluminescence substrate (Amersham Biosciences). The quantitative analysis of blots was normalized using the lumino image analyzer LAS-4000 (Fujifilm Corp., Japan).
Statistics
Data are presented as the mean ± S.E. for the indicated number of independent experiments. Statistical significance was determined with a post-hoc test of one-factor factorial analysis of variance (Figs. 3E, 6D, 7B, and 7C), the Wilcoxon Mann-Whitney U test (non-parametric analysis, Fig. 2C, and Fig. 6C), and Student's t test (other figures) using the KaleidaGraph® 4.0 programs (Synergy Software, Reading, PA). Differences with a p value of <0.05 was considered statistically significant (* and # indicate up-regulation and down-regulation, respectively; NS indicates not significant).
FIGURE 3.
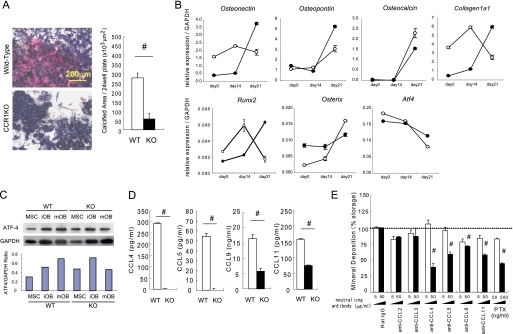
Impaired mineralized nodule formation in CCR1-deficient osteoblastic cells. In A, osteoblastic cells were cultured from the bone marrow of wild-type and Ccr1−/− mice, and then minerals were stained with alizarin red and BALP with chromogenic reagents (shown in “blue”) (magnification ×100, left). Mineral deposition was determined by von Kossa staining (n = 6, right). In B, total RNAs were isolated from osteoblastic cells isolated from wild-type (open circles) and Ccr1−/−mice (filled circles). The real-time Q-PCR analyses examined the relative expression levels of osteoblast-related transcriptional factor mRNAs (Runx-2, Osterix, and Atf4) and osteoblast-related marker mRNAs (Osteonectin, Osteopontin, Osteocalcin, and Collagen1α1). Data are expressed as the copy numbers of these markers normalized to Gapdh expression (mean ± S.E., n = 8). In C, the protein expression levels of the transcriptional factor ATF4 by wild-type and Ccr1−/− osteoblastic cells were measured by a Western blot analysis. Osteoblast lysates (10 μg of protein per lane) was loaded and separated by SDS-PAGE. The expression levels of ATF4 were normalized to GAPDH expression. In D, the production of CCR1-related chemokine ligands in the culture media of wild-type and Ccr1−/− osteoblastic cells was measured by ELISA (n = 5). #, significantly different from wild-type controls, p < 0.05. In E, osteoblastic cells were cultured with the indicated neutralizing antibodies against chemokines. The mineral deposition rate was measured by von Kossa staining (n = 4). Stained cells cultured with control rat IgG were set as 100%. #, significantly different from between different concentrations of each antibody, p < 0.05. PTX, pertussis toxin.
FIGURE 6.
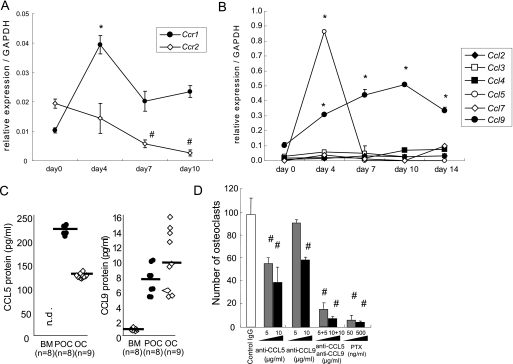
CCR1 signaling is involved in osteoclast differentiation. Osteoclastic cells and macrophages were cultured from the bone marrow of wild-type and Ccr1−/− mice. Total RNAs were isolated from the cultured cells. The relative mRNA expression levels of chemokine receptors Ccr1, Ccr2 (A) and chemokine ligands (B) during osteoclastogenesis were measured by real-time Q-PCR (mean ± S.E., n = 5). * and #, significantly different from day 0 of Ccr1 and Ccr2, respectively, p < 0.05 in A. *, significantly different from day 0 of culture in each ligand expression, p < 0.05 in B. In C, chemokine levels during osteoclastogenesis were measured by ELISA. BM, bone marrow-derived macrophage; POC, pre-osteoclast (day 4); and OC, osteoclast (day14). Bars indicate the mean. In D, the number of osteoclasts after neutralization of CCL5, CCL9, and their combination in the osteoclastic cultures were scored (mean ± S.E., n = 3). #, significantly different between two distinct concentrations of each antibody, p < 0.05. PTX, pertussis toxin.
FIGURE 7.
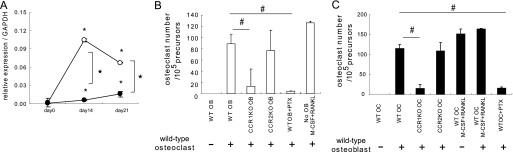
CCR1 is involved in the RANK–RANKL axis and induces the impaired osteoclastogenesis. In A, osteoblastic cells were cultured from the bone marrow of wild-type and Ccr1−/− mice. Relative expression levels of Rankl by Ccr1−/− osteoblasts as measured by real-time Q-PCR (mean ± S.E., n = 3). #, significantly different from wild-type controls, p < 0.05. In B and C, the number of TRAP+ multinuclear osteoclasts induced by co-culture with osteoblasts. Co-culture with osteoblastic cells isolated from wild-type or Ccr1−/− mice (mean ± S.E., duplicated, n = 2, B), and with osteoclast precursors isolated from wild-type or Ccr1−/− mice (mean ± S.E., duplicated, n = 2, C). Osteoclast cultures with M-CSF and RANKL without osteoblasts were set as positive control. #, significantly different from co-culture of osteoclasts with wild-type osteoblasts, p < 0.05.
FIGURE 2.
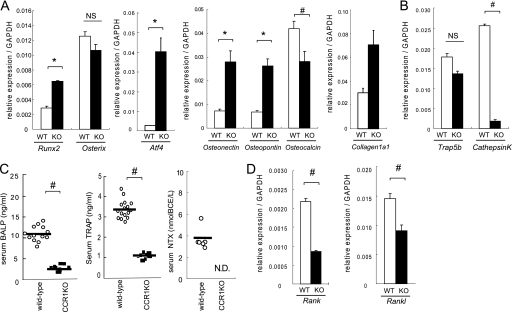
Expression of markers related to osteoblasts and osteoclasts in bones and sera in wild-type and CCR1−/− mice. In A, B, and D, total RNAs were isolated form the proximal tibia of wild-type and Ccr1−/− male mice at 8 weeks of age. Real-time Q-PCR revealed the relative expression levels of osteoblast-related mRNAs (Runx-2, Osterix, Atf4, Osteonectin, Osteopontin, Osteocalcin, and Collagen1α1, A), osteoclast-related mRNA (Trap5a and Cathepsin K, B), and RANK–RANKL axis (Rank and Rankl, D). Data are expressed as the copy numbers of these markers normalized to Gapdh expression (mean ± S.E., n = 8). In C, the levels of serum BALP, TRAP, and serum collagen-type1 N-telopeptides (NTx) were measured by ELISA. The bars indicate the mean ± S.E. Each sample was duplicated. Wild-type and Ccr1−/− male mice at 9 weeks of age (n = 10 and 6, respectively) were subjected to BALP and TRAP. Wild-type and Ccr1−/− male mice at 9–13 weeks of age (n = 8 and 6, respectively) were assayed for NTx. #, significantly different from wild-type controls, p < 0.05. N.D., not detected.
RESULTS
CCR1-deficient Mice Exhibit Osteopenia
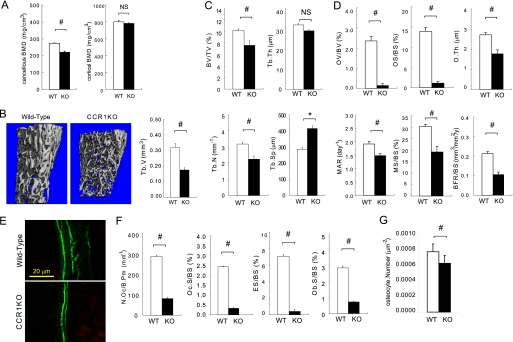
To understand the functions of CCR1 in bone metabolism, we investigated the bone mineral density in Ccr1−/− mice. A peripheral quantitative CT analysis showed a significant reduction in bone mineral density in cancellous bone in Ccr1−/− mice compared with wild-type mice (Fig. 1A). There were no significant differences between bone mineral density in the cortical bone at the metaphysial (Fig. 1A) and diaphysial regions (data not shown) between Ccr1-deficient and wild-type mice. In Ccr1−/− mice, a microCT analysis indicated decreased cancellous bone tissue at the metaphysical region (Fig. 1B). An analysis of bone histomorphometrics confirmed a significant decrease of bone volume (BV/TV) at the metaphysial region of Ccr1−/− mice. This was associated with a diminished number of trabeculae (Tb.N), increased trabecular bone separation (Tb.Sp), and no significant changes in trabecular bone thickness (Tb.Th), thus indicating that Ccr1-deficient mice have sparse trabeculae (Fig. 1C). We examined the effect of Ccr1 deficiency on the function of osteoblasts and osteoclasts in bone morphometry (Fig. 1, D–F). The morphological analyses revealed that Ccr1−/− mice have a significantly reduced number of osteoblasts (Ob.S./BS) (Fig. 1F). Ccr1−/− mice exhibited extremely low values of osteoid surface (OS/BS) and osteoid volume (OV/BV) compared with wild-type mice (Fig. 1D). Notably, Ccr1−/− mice showed a significant decreases in the mineral apposition rate (MAR), mineralized surface (MS/BS), and bone formation rate (BFR/BS) (Fig. 1D), which were calculated based on calcein administration (representative pictures are shown in Fig. 1E). In addition, the number of osteocytes per area was significantly reduced in Ccr1−/− mice (Fig. 1G). These results indicate that Ccr1−/− mice have impaired bone formation. Fig. 1F summarizes the bone morphometric parameters associated with bone resorption. Ccr1−/− mice have significantly decreased osteoclast numbers (N.Oc./B.Pm) and osteoclast surface area (Oc.S./BS), and an eroded surface (ES/BS). These findings indicate that Ccr1−/− mice have diminished osteoclast function. Taken together, the morphometric analyses suggest that the bone phenotype in Ccr1-deficient mice exhibit osteopenia with low bone turnover, which is most likely due to the diminished function of osteoblasts and osteoclasts.
FIGURE 1.
Bone morphometric analyses of CCR1−/− mice. A shows the bone mineral density of trabecular and cortical bones in distal femurs as measured by peripheral quantitative CT. B shows the microCT images and the quantitative measurements of trabecular bones (Tb.V.) in the distal femurs of wild-type and Ccr1−/− mice (n = 10). In C–F, the bone histomorphometric analyses of distal femurs in wild-type and CCR1−/− mice were carried out as described under “Experimental Procedures.” Parameters relating to the trabecular structure (in C): bone volume per tissue volume (BV/TV), trabecular number (Tb.N.), and trabecular separation (Tb.Sp.). Parameters relating to bone formation (in D): osteoid volume to bone volume (OV/BV), osteoid surface/bone surface (OS/BS), osteoid thickness (O.Th.), formation rate referenced to bone surface (BFR/BS), mineral apposition rate (MAR), and mineralizing surface per bone surface (MS/BS). The immunofluorescence images of calcein labeling in wild-type and Ccr1−/− mice (in E). Parameters relating to bone resorption (in F): osteoclast number per bone perimeter (N.Oc./B.Pm), osteoclast surface per bone surface (Oc.S./BS), eroded surface per bone surface (ES/BS), and osteoblast surface per bone surface (Ob.S./BS). The bone histomorphometric analysis data are represented as the mean ± S.E. obtained from six mice in each group. #, significantly different from wild-type controls, p < 0.05. In G, osteocyte numbers per area are represented as the mean ± S.E. obtained from three mice in each group.
Impaired Osteogenesis and Osteoclastogenesis in the Bone Tissue of Ccr1-deficient Mice
To elucidate the status of osteoblasts and osteoclasts in bones of Ccr1−/− mice, we compared the transcriptional levels of osteoclast- and osteoblast-related markers in the proximal tibiae of wild-type and Ccr1−/− mice. The analyses of osteoblast-related markers, such as bone-specific transcriptional factors (Runx-2, Atf4, and Osterix) (23–25) and bone matrix proteins (Collagen1a1, Osteonectin, Osteopontin, and Osteocalcin), revealed that the expression levels of Runx2 and Atf4 were dramatically up-regulated in Ccr1−/− mice than in wild-type mice (Fig. 2A). However, there were no significant changes in the expression levels of Osterix. Early markers for osteoblast differentiation, including Collagen1a1, Osteonectin, and Osteopontin, were significantly up-regulated. Osteocalcin expression, a marker for mature osteoblasts, was significantly down-regulated in Ccr1−/− mice. These results suggest that osteoblasts in Ccr1-deficient mice are retained in an immature state due to the overexpression of Runx-2 and Atf4 by osteoblasts, which is also consistent with the significant reduction in number of osteocytes in Ccr1−/− mice. Constitutive Runx-2 overexpression in osteoblasts results in maturation arrest in osteoblasts and in a reduced number of osteocytes (25). The serum levels of BALP in Ccr1-deficient mice were significantly decreased (Fig. 2C).
The expression levels of markers related to osteoclast differentiation, revealed attenuated transcription levels of TRAP5b and cathepsin K in Ccr1−/− mice (Fig. 2B). In addition, Ccr1−/− mice exhibited significantly decreased levels of serum TRAP (26) and collagen-type I NTx (27, 28) (Fig. 2C). This finding is consistent with diminished osteoclastic bone resorption in Ccr1−/− mice. These observations led us to assess the RANK-RANKL axis, a key signaling pathway in osteoblast-osteoclast interactions that regulates osteoclast differentiation and function. Interestingly, the analyses revealed that both Rank and Rankl were down-regulated (Fig. 2D), thus implying that CCR1 is involved in the regulation of the RANK-RANKL axis. Considering the fact that Ccr1−/− mice exhibit osteopenia with low bone turnover, these bone cell marker expression levels suggest that CCR1 is heavily involved in the differentiation and function of osteoblasts and osteoclasts as well as in the cellular interactions between these cell types.
CCR1 Signaling Is Important in the Maturation and Function of Osteoblasts
To further corroborate the necessity of CCR1 in osteoblast maturation and function, we examined the formation of mineralized nodules in vitro by osteoblastic cells isolated from bone marrow of wild-type and Ccr1−/− mice. Mineralized nodule formation in osteoblastic cells isolated from Ccr1−/− mice was markedly abrogated compared with wild-type osteoblastic cells (Fig. 3A). We next investigated the time-course expression profiles of osteoblastic markers in this in vitro culture system and compared them between wild-type and Ccr1−/− mice (Fig. 3B). In wild-type mice, Runx2 exhibited the highest levels of expression at day 14, but was drastically down-regulated at day 21, during the mineralization stage. However, an inverse Runx2 expression pattern was observed in CCR1-deficient osteoblastic cells, in which the levels of expression were markedly suppressed in the early stages (days 0 and 14), and was then significantly up-regulated at day 21, reaching the levels present in wild-type mice. Osterix expression was highly up-regulated at day 21 in wild-type mice, whereas its expression in CCR1-deficient osteoblastic cells was sustained at an intermediate level between the lowest and the highest levels in wild-type mice, overall resulting in a lower expression levels than in wild-type mice at day 21. These inverted expression patterns were also consistently observed, especially at day 21, with other osteoblastic markers, including Atf4, Caollagen1a1, Osteonectin, Osteopontin, and Osteocalcin. Similarly, the expression pattern of ATF4 was also confirmed by a Western blot analysis (Fig. 3C). These observations indicated that CCR1 deficiency severely affected the temporal expression of osteoblastic markers, resulting in the impaired differentiation and maturation of osteoblasts. Because CCR1 signaling is activated by several cross-reactive chemokines (CCL4, CCL5, CCL9, and CCL11), we next compared the levels of these chemokines in wild-type and CCR1-deficient osteoblastic cells. We observed significantly diminished expression levels of these chemokines in CCR1-deficient osteoblastic cells (Fig. 3D). A test on the effects of neutralizing antibodies against various chemokines, including CCR1 ligands, revealed the role of each chemokine in mineralized nodule formation by osteoblastic cells. The neutralizing antibodies against CCL4, CCL5, CCL9, and CCL11 significantly reduced the number of mineralized nodules in osteoblastic cells, although the antibodies against CCL2 and CCL3 did not inhibit the numbers completely (Fig. 3E). Pertussis toxin (PTX), an inhibitor of Gi protein-coupled receptors involved in chemokine signaling, inhibited mineralized nodule formation in a dose-dependent manner. In further support of these findings, we observed similar temporal changes in the transcriptional levels of osteoblastic markers in wild-type osteoblastic cultures treated with an anti-CCL9 antibody, compared with Ccr1−/− osteoblastic cells (supplemental Fig. 2). These results suggest that CCR1 signaling mediated by its ligands (CCL4, CCL5, CCL 9, and CCL11) plays an essential role in mineralized nodule formation.
Lack of Chemokine Receptor CCR1 Causes Impaired Osteoclast Differentiation and Bone-resorbing Activity
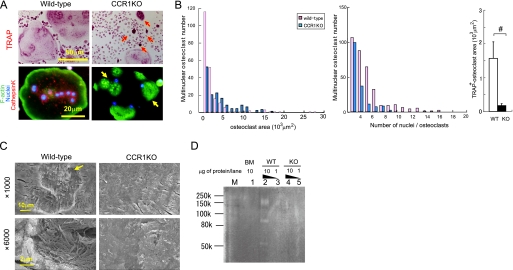
To elucidate the roles of CCR1 in osteoclast differentiation, we analyzed the differentiation potency of osteoclast precursors derived from Ccr1−/− mice (Fig. 4A). Osteoclast precursors from Ccr1-deficient mice markedly abrogated multinucleation with defective actin ring formation (Fig. 4A, yellow arrows) compared with precursors from wild-type mice, which generated a large numbers of osteoclasts with multinucleation and well organized actin ring formation at the cell periphery. The histograms of the osteoclast area and number of nuclei per cell as well as TRAP-positive areas reveal the presence of impaired cellular fusion and differentiation in Ccr1-deficient osteoclasts (Fig. 4B). We further investigated the activity of bone resorption in Ccr1-deficient osteoclasts (Fig. 4C). Few resorption pits were observed in Ccr1−/− osteoclasts by scanning electron microscopic examination, in contrast to obvious resorption pits with well digested collagen fibers detected in wild-type osteoclasts. This observation was also confirmed by collagen zymography demonstrating that Ccr1−/− osteoclasts failed to digest type-I collagens (Fig. 4D).
FIGURE 4.
Essential roles of CCR1 in multinucleation and bone-resorbing activity. Pre-osteoclastic cells were cultured from the bone marrow of wild-type and Ccr1−/− mice. Osteoclasts were induced from the pre-osteoclastic cells by M-CSF and RANKL treatment. In A, the formation of multinuclear osteoclasts by wild-type and Ccr1−/− precursors was visualized by TRAP chromogenic staining (magnification ×400, upper panels). Immunohistochemical staining was carried out using an anti-cathepsin K antibody conjugated with Alexa594 (red). F-actin and nuclei were counterstained by phalloidin-AlexaFluor 488 (green) and Hoechst 33258 (blue), respectively (magnification ×640, bottom panels). The yellow arrow indicates multinuclear giant cells with an impaired actin ring rearrangement, and the red arrows indicate TRAP accumulation. In B, histograms of the area distribution of multinuclear osteoclasts delimited with phalloidin, and of the number of multinuclear osteoclasts in A. Area comprises TRAP-positive multinuclear (>3 nuclei) giant cells shown in A (mean ± S.E., n = 3). In C, pit formation by wild-type and Ccr1−/− osteoclasts on bone slice observed by scanning electron microscopy (magnification: ×1000 (top) and ×6000 (bottom), respectively). In D, collagen digestion activity by wild-type and Ccr1−/− osteoclasts was measured by collagen-based zymography. Lanes M, 1, 2–3, and 4–5 indicate the molecular markers, bone marrow-derived macrophage lysates (10 μg of protein/lane), wild-type osteoclast lysates (1 and 10 μg of protein/lane), and Ccr1−/− osteoclasts lysates (1 and 10 μg of protein/each lane), respectively.
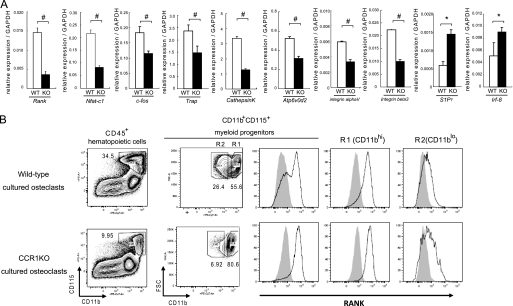
Furthermore, the transcriptional levels of osteoclastic differentiation markers were investigated in the osteoclast culture system. Rank and its downstream targets Nfat-c1, other markers such as c-fos, Trap, CathepsinK, Atp6v0d2, integrin αV, and integrin β3 were markedly down-regulated in Ccr1-deficient cells, whereas S1P1 and Irf-8 were up-regulated (Fig. 5A). We next examined whether the down-regulation in RANK expression in vivo (see Fig. 2D) and in vitro (Fig. 5A) directly correlated with the reduction in RANK-expressing osteoclast precursors. The cellular profiles of osteoclast precursors by a flow cytometric analysis revealed that the Ccr1−/− mice had lower numbers of CD45+CD11b+CD115+ myeloid-lineage precursors compared with wild-type mice (Fig. 5B). In addition, the subpopulations of osteoclast precursors, which are categorized into CD11bhi (R1) and CD11blo (R2), were marked reduced in the R2 subpopulation in CCR1-deficient cells. Because the R1 and R2 subpopulations reportedly express higher and lower levels of RANK, respectively (29), a reduction in the R2 subpopulation likely contributed to reduced expression of osteoclast markers in CCR1-deficient osteoclastic cells. Importantly, our observation is also consistent with a previous work reporting that RANKlo precursors are required for cellular fusion (29).
FIGURE 5.
Osteoclastic impairment by CCR1 deficiency is due to the changes in osteoclastic precursor population. Pre-osteoclastic cells were cultured from the bone marrow of wild-type and Ccr1−/− mice. Osteoclasts were induced from the pre-osteoclastic cells by M-CSF and RANKL treatment. In A, relative expression levels of the osteoclastic differentiation markers (Rank, Nfatc1 transcription factor, c-fos, Trap, CathepsinK protease, H+-ATPase subunit ATP6v0d2, integrins αV and β3, S1P1, and Irf-8) on wild-type (open column) and Ccr1−/− (filled column) osteoclasts were measured by a real-time Q-PCR analysis at day 4 after culture (mean ± S.E., n = 5). #, significantly different from wild-type controls, p < 0.05. In B, expression analysis of RANK in CD45+CD11b+CD115+ pre-osteoclastic cells isolated from the bone marrows of wild-type and Ccr1−/− mice after 4 days in culture were analyzed by flow cytometry.
CCR1 Signaling Is Involved in Osteoclast Differentiation
To further explore the role of CCR1 signaling in osteoclast differentiation, we next examined the expression levels of chemokine receptors during osteoclastogenesis using an in vitro culture system. CCR1 was expressed in the course of the osteoclastogenesis, with the highest levels of expression at day 4 after culture (10–12), whereas other chemokine receptor CCR2 was gradually down-regulated during this culture period (30) (Fig. 6A). Immunohistochemical staining revealed that CCR1 was highly expressed on the multinuclear osteoclasts (supplemental Fig. 3). The expression profiles of CCR ligands in this in vitro osteoclast culture system revealed that ligands specific for CCR1, such as Ccl5 and Ccl9, had a relatively higher levels of expression than other ligands, and appeared to be regulated depending on the maturation stages of the osteoclasts. Ccl5 was preferentially expressed at day 4, a stage of mononuclear pre-osteoclasts, whereas multinuclear osteoclasts predominantly produced Ccl9 at later times (Fig. 6B). These regulated transcriptional patterns of Ccl5 and Ccl9 were also confirmed by the analysis of protein expression levels in cultured media (Fig. 6C). These observations suggested that the interaction between CCR1 and its ligands, CCL5 and CCL9, could be involved in osteoclast differentiation.
We verified this hypothesis by culturing osteoclast precursors in the presence of neutralizing antibodies against CCL5 and CCL9. Blockade of either ligand resulted in a partial inhibition of osteoclast formation in a dose-dependent manner. Similarly, simultaneous treatment with neutralizing antibodies against CCL5 and CCL9 induced synergistic inhibitory effects (Fig. 6D). Furthermore, PTX treatment blocked osteoclastogenesis to the basal levels. Notably, we found no CCL3 production by ELISA or any inhibitory osteoclastogenesis effects using an anti-CCL3 antibody (data not shown), although CCL3 is thought to play an essential role in inflammation-related osteoclastogenesis in humans (4, 7, 31, 32). These findings indicate that CCR1 is essential for osteoclast differentiation, and CCL5 and CCL9 are the likely candidate ligands that participate in the CCR1 axis.
CCR1 Is Involved in the RANK–RANKL Axis and Induces the Impaired Osteoclastogenesis
Because osteoclast differentiation is critically regulated by the signals through the RANK–RANKL axis, we investigated the transcriptional level of Rankl in Ccr1−/− osteoblastic cells. The cells expressed significantly lower levels of RANKL compared with wild-type osteoblastic cells (Fig. 7A). We next performed co-cultures of pre-osteoclasts with layers of osteoblastic cells by reciprocal combinations of these two cell populations from wild-type and Ccr1−/− mice. As expected from the reduced Rankl expression, a significantly reduced number of osteoclasts were formed from co-culture with Ccr1−/− osteoblastic cells compared with wild-type osteoblastic cells (Fig. 7B). In the presence of PTX, wild-type osteoblastic cells also failed to generate substantial numbers of osteoclasts (Fig. 7B). Ccr1−/− osteoclast precursors did not form differentiated osteoclasts even in the presence of wild-type-derived osteoblasts (Fig. 7C), as is consistent with our observations in Fig. 4. These observations suggest that the CCR1 chemokine receptor, which is expressed by both osteoblasts and osteoclasts, plays a critical role on osteoblast-osteoclast communication through the regulation of the RANK and RANKL expression.
DISCUSSION
Pathological findings postulate that chemokines and chemokine receptors are involved in bone remodeling (9–13). Among these receptors, CCR1 appears to be an important molecule involved in bone metabolism (9). We used Ccr1−/− mice to investigate whether CCR1 affects bone metabolism. Our findings have demonstrated that a CCR1-deficiency affects the differentiation and function of both osteoblasts and osteoclasts, and also causes osteopenia.
Our bone histomorphometric study in Ccr1−/− mice clearly demonstrated impaired osteoblast differentiation and function (Fig. 1, D–G). The bone tissues in Ccr1−/− mice exhibited down-regulation of osteocalcin, which is a marker for mature osteoblasts, whereas the expression of Osteonectin and Osteopontin, which are markers for early osteoblasts, were up-regulated in the bones of these mice (Fig. 2A). Significantly, Ccr1−/− osteoblastic cells exhibited much less potency to generate mineralized tissues (Fig. 3A). These results suggest that the deficiency of CCR1 results in arrested osteoblast maturation and defective osteoblast function. Previous reports have demonstrated that the sustained expression of Runx2 in osteoblasts inhibits their terminal maturation and causes osteopenia with a reduction in the number of osteocytes (25, 33). Consistent with these findings, bone tissue specimens from Ccr1−/− mice exhibited a higher expression level of Runx2 and a reduced number of osteocytes (Fig. 3G). These findings suggest that osteopenia in Ccr1−/− mice is due to impaired osteoblastic function via Runx2 up-regulation. Our findings in Ccr1−/− osteoblastic culture supportively demonstrated that an inverse temporal expression level of osteoblastic transcriptional factors, such as Runx2, Atf4, and Osterix could be related to the disordered expressions of bone matrix proteins, thus resulting in impaired bone mineral deposition (Fig. 3B).
Furthermore, treatment with neutralizing antibodies against CCR1 ligands (e.g. CCL4, CCL5, CCL9, and CCL11) significantly inhibited mineral deposition (Fig. 3E) and osteoblastic protein expression (supplemental Fig. 2) in osteoblastic cells isolated from wild-type mice. These observations indicate that CCR1-mediated signaling is essential for osteoblast differentiation and function. Although we detected substantial levels of various chemokine ligands (CCL4, CCL5, CCL9, and CCL11) in osteoblastic cells, these levels were greatly reduced in cells isolated from Ccr1−/− mice (Fig. 3D). This observation implies a chemokine-dependent amplification loop by which a given chemokine signaling sustains or amplifies the expressions of its participating ligands and receptors, which has been previously reported in several contexts. For instance, the activation of CD14+ monocytes form a CCR2-CCL2 axis-dependent amplification loop that ultimately leads to fibrosis (34). Several other studies have reported that macrophage infiltration in injured tissue is mediated by a CCR1-mediated loop (35–37) and a CCR5–CCL5 loop (38). Reports of renal inflammatory signals and abdominal inflammation have described CCR7–CCL19/CCL21 (39) and CCR8–CCL1 loops (17), respectively. Therefore, the CCR1-mediated loop is likely to be involved in osteoblast differentiation, function, and cellular interactions that regulate bone metabolism. Possible roles of the CCR1-mediated loop in osteoblast differentiation and function suggest that changes in the bone marrow microenvironment by a CCR1 deficiency affected the osteoblastic lineage and/or the intercellular regulation of osteoblast differentiation and function. CCR1 conventional knock-down seems to have affected many cell types that express CCR1, affecting the bone marrow microenvironment, which regulates whole process of osteoblast differentiation and function. Our in vitro experiments did not successfully retrieve this point. Nevertheless, the present experiments have confirmed an essential role for CCR1-mediated signaling in osteoblastic cells. The expression and possible roles of CCR1 in osteoclast lineage cells have been reported by several studies (4, 10, 11). We observed the up-regulation of Ccr1 expression and down-regulation of Ccr2 during cultured osteoclastogenesis (Fig. 6A). The bone histomorphometric analyses demonstrated impaired osteoclast differentiation and function in Ccr1−/− mice (Fig. 1F). In addition, we observed impaired bone resorption activity by osteoclasts isolated from CCR1−/− mice (Fig. 4, B and C). A potential reason for the impaired bone resorption is due to defects in osteoclast differentiation. Indeed, the flow cytometric analyses revealed that the component of CD11b+CD115+ myeloid-lineage precursors in Ccr1−/− mice are drastically changed; this population of cells lacked the RANKlo CD11blo subpopulation, which is required for cellular fusion (29) (Fig. 5B). Recent live observation of calvarial bone marrow by two-photon microscopy clarified the roles of chemoattractant S1P1 (sphingosine-1-phosphate 1) and its receptors in the migration of osteoclast precursors to the bone surface (40). Therefore, it is indeed intriguing to speculate that elevated levels of S1P1 expression in Ccr1−/− osteoclasts (Fig. 1F) reduced the supply of osteoclast precursors from peripheral circulation in the bone marrow to the bone surface. Further investigation will reveal whether the CCR1 axis is involved in the chemotactic migration of osteoclast precursors to the bone surface.
One of the possible reasons for osteoclast dysfunction in Ccr1−/− mice may be diminished signaling along the RANK–RANKL axis. The down-regulation of both Rank and Rankl mRNA was observed in the bone tissue of Ccr1−/− mice (Fig. 2D). Cultured osteoblastic cells and osteoclasts isolated from Ccr1−/− mice exhibited remarkable reductions in Rank and Rankl expression levels, respectively (Figs. 5B and 7B). Furthermore, Ccr1-deficient osteoclasts had discouraged the levels of osteoclastic maturation markers such as c-fos, Nfatc1, CathepsinK, and several integrins (Fig. 5A). These results suggest that CCR1-mediated signaling controls the RANK–RANKL axis through the regulation of both osteoblasts and osteoclasts. Our intercross co-cultures of pre-osteoclasts with osteoblastic cells from wild-type and Ccr1−/− mice obviously demonstrated an impaired interaction between these two cell types, resulting in the impaired induction of functional mature osteoclasts (Fig. 7, B and C). These findings, interestingly, support the idea that the chemokines produced by the osteoblasts and osteoclasts that stimulate CCR1-mediated signaling could be categorized as putative “bone-coupling factors” (41), which mediate the cross-talk between osteoclasts and osteoblasts to maintain bone remodeling.
Our data imply that the regulatory mechanism of Rankl expression is associated with osteoblast maturation. Runx2 reportedly induce a low steady-state level of Rankl expression and is also required for the stimulatory effect of vitamin D3 on Rankl transcription possibly by condensing or decondensing the chromatin structure (42). It is possible that the inverse-temporal Runx2 expression in CCR1-deficient mice is causative of the down-regulation of Rankl, due to a reduced cellular response to bone-targeted hormones such as vitamin D3 and parathyroid hormone. However, a more direct role of CCR1-mediated signaling on Rankl transcription remains to be elucidated.
CCR1-mediated signaling pathways on both osteoblasts and osteoclasts raise important questions on how the several members of murine chemokine ligands for CCR1 (in rodents, CCL3, CCL4, CCL5, CCL6, CCL8, CCL9, and CCL11) (43) distinguish the downstream signaling pathways, despite sharing the same CCR1 receptor. Each chemokine may possess specific regulatory control for binding to the receptor and inducing a specific cellular response. For example, the osteoclasts may have a distinct intrinsic signaling adaptor protein for cellular response, as well as the adaptor protein FROUNT for CCR2-mediated signaling (44). It has also been demonstrated that the spatiotemporal expression of chemokine receptors and their ligands may relay chemokine signaling and sequential output that regulate bone metabolism. This is related to several findings in this study, including the distinct temporal expression patterns of different ligands as observed in Fig. 6 (B and C) and supplemental Fig. 1, the chemokine-dependent amplification loop, and the possible chemokine-mediated cellular interaction. Further studies are warranted to investigate the intracellular signaling pathways downstream of each chemokine receptor.
Our current results also support the concept that chemokine receptor antagonists are potentially novel therapeutic candidates for the treatment of patients with certain inflammatory bone diseases. Several reports suggest that CCL3 promotes pathological bone destruction by excessively triggering osteoclast activation (2, 4, 7, 31, 32). However, we were unable to detect increased CCL3 production by cultured osteoclasts (Fig. 6, B and C, and data not shown), suggesting that physiological osteoclastogenesis is primarily maintained by CCL9 rather than CCL3. It is probable that pro-inflammatory CCL3 overcomes the physiological process of osteoclastogenesis by CCL9 expression and signaling, thereby inducing ectopic osteoclastogenesis that causes bone destruction mediated by T-lymphocyte-mediated activation (45). Alternatively, the species differences between rodents and humans must be considered; CCL9 is described only in rodents, and the putative human homologue is predicted to be CCL15 and CCL23 (46), which are potent osteoclastogenesis mediators in humans (47). It is therefore worthwhile to dissect the distinct roles of chemokine signaling in both the pathological and physiological contexts, which would provide novel information that may help researchers identify new therapeutic targets.
In conclusion, the present observations provide the first evidence for the physiological roles of CCR1-mediated chemokines in the bone metabolism. Further studies on chemokine receptors in the bone metabolism will enable the targeted development of new therapeutic strategies for the treatment of patients with bone destruction diseases and osteoporosis.
Supplementary Material
Acknowledgments
We thank Dr. Taeko Dohi, Dr. Harumi Suzuki, Dr. Yasuhiro Natori, and Mikiko Uwano (International Medical Center of Japan (IMCJ)), Dr. Philip M. Murphy (NIH), Dr. Tomoki Nakashima (Tokyo Medical and Dental University), and T. Sakai for valuable advices and supports. The authors are grateful to Dr. Takuro Shimbo and Dr. Tetsuya Mizoue (IMCJ) for statistical support.
This work was supported in part by Grant H19-nano-012 from the Ministry of Health, Labor and Welfare (to K. Y.) and by a research fellowship from the Japan Society for the Promotion of Science for Young Scientists (2007–2009) (to A. H.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- CCR
- C-C chemokine receptor
- M-CSF
- macrophage-colony stimulation factor
- BALP
- bone-specific alkaline phosphatase
- CCL
- C-C chemokine ligand
- MCP-1
- macrophage chemoattractant protein-1
- MIP-1
- macrophage inflammatory protein-1
- CT
- computed tomography
- PTX
- pertussis toxin from Bordetella pertussis
- RANK
- receptor activator of NF-κB
- RANKL
- receptor activator of NF-κB ligand
- RANTES
- regulated upon activation normal T expression and secreted
- TRAP
- tartrate-resistant acid phosphatase
- NTx
- N-telopeptides.
REFERENCES
- 1.Charo I. F., Ransohoff R. M. (2006) N. Engl. J. Med. 354, 610–621 [DOI] [PubMed] [Google Scholar]
- 2.Oba Y., Lee J. W., Ehrlich L. A., Chung H. Y., Jelinek D. F., Callander N. S., Horuk R., Choi S. J., Roodman G. D. (2005) Exp. Hematol. 33, 272–278 [DOI] [PubMed] [Google Scholar]
- 3.Kim M. S., Magno C. L., Day C. J., Morrison N. A. (2006) J. Cell Biochem. 97, 512–518 [DOI] [PubMed] [Google Scholar]
- 4.Menu E., De Leenheer E., De Raeve H., Coulton L., Imanishi T., Miyashita K., Van Valckenborgh E., Van Riet I., Van Camp B., Horuk R., Croucher P., Vanderkerken K. (2006) Clin. Exp. Metastasis 23, 291–300 [DOI] [PubMed] [Google Scholar]
- 5.Haringman J. J., Smeets T. J., Reinders-Blankert P., Tak P. P. (2006) Ann. Rheum. Dis. 65, 294–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi S. J., Cruz J. C., Craig F., Chung H., Devlin R. D., Roodman G. D., Alsina M. (2000) Blood 96, 671–675 [PubMed] [Google Scholar]
- 7.Han J. H., Choi S. J., Kurihara N., Koide M., Oba Y., Roodman G. D. (2001) Blood 97, 3349–3353 [DOI] [PubMed] [Google Scholar]
- 8.Vallet S., Raje N., Ishitsuka K., Hideshima T., Podar K., Chhetri S., Pozzi S., Breitkreutz I., Kiziltepe T., Yasui H., Ocio E. M., Shiraishi N., Jin J., Okawa Y., Ikeda H., Mukherjee S., Vaghela N., Cirstea D., Ladetto M., Boccadoro M., Anderson K. C. (2007) Blood 110, 3744–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang M., Mailhot G., MacKay C. A., Mason-Savas A., Aubin J., Odgren P. R. (2006) Blood 107, 2262–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X., Huang Y., Collin-Osdoby P., Osdoby P. (2004) J. Bone Miner. Res. 19, 2065–2077 [DOI] [PubMed] [Google Scholar]
- 11.Lean J. M., Murphy C., Fuller K., Chambers T. J. (2002) J. Cell Biochem. 87, 386–393 [DOI] [PubMed] [Google Scholar]
- 12.Okamatsu Y., Kim D., Battaglino R., Sasaki H., Spate U., Stashenko P. (2004) J. Immunol. 173, 2084–2090 [DOI] [PubMed] [Google Scholar]
- 13.Kominsky S. L., Abdelmagid S. M., Doucet M., Brady K., Weber K. L. (2008) Cancer Res. 68, 1261–1266 [DOI] [PubMed] [Google Scholar]
- 14.Binder N. B., Niederreiter B., Hoffmann O., Stange R., Pap T., Stulnig T. M., Mack M., Erben R. G., Smolen J. S., Redlich K. (2009) Nat. Med. 15, 417–424 [DOI] [PubMed] [Google Scholar]
- 15.Gao J. L., Wynn T. A., Chang Y., Lee E. J., Broxmeyer H. E., Cooper S., Tiffany H. L., Westphal H., Kwon-Chung J., Murphy P. M. (1997) J. Exp. Med. 185, 1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doi M., Nagano A., Nakamura Y. (2002) Biochem. Biophys. Res. Commun. 290, 381–390 [DOI] [PubMed] [Google Scholar]
- 17.Hoshino A., Kawamura Y. I., Yasuhara M., Toyama-Sorimachi N., Yamamoto K., Matsukawa A., Lira S. A., Dohi T. (2007) J. Immunol. 178, 5296–5304 [DOI] [PubMed] [Google Scholar]
- 18.Ito M., Ikeda K., Nishiguchi M., Shindo H., Uetani M., Hosoi T., Orimo H. (2005) J. Bone Miner. Res. 20, 1828–1836 [DOI] [PubMed] [Google Scholar]
- 19.Parfitt A. M., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R. (1987) J. Bone Miner. Res. 2, 595–610 [DOI] [PubMed] [Google Scholar]
- 20.Liotta L. A., Stetler-Stevenson W. G. (1990) Semin. Cancer Biol. 1, 99–106 [PubMed] [Google Scholar]
- 21.Gogly B., Groult N., Hornebeck W., Godeau G., Pellat B. (1998) Anal. Biochem. 255, 211–216 [DOI] [PubMed] [Google Scholar]
- 22.Wilson M. J., Strasser M., Vogel M. M., Sinha A. A. (1991) Biol. Reprod. 44, 776–785 [DOI] [PubMed] [Google Scholar]
- 23.Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., Shimizu Y., Bronson R. T., Gao Y. H., Inada M., Sato M., Okamoto R., Kitamura Y., Yoshiki S., Kishimoto T. (1997) Cell 89, 755–764 [DOI] [PubMed] [Google Scholar]
- 24.Ducy P., Zhang R., Geoffroy V., Ridall A. L., Karsenty G. (1997) Cell 89, 747–754 [DOI] [PubMed] [Google Scholar]
- 25.Liu W., Toyosawa S., Furuichi T., Kanatani N., Yoshida C., Liu Y., Himeno M., Narai S., Yamaguchi A., Komori T. (2001) J. Cell Biol. 155, 157–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delmas P. D. (1993) J. Bone Miner. Res. 8, Suppl. 2, S549–S555 [DOI] [PubMed] [Google Scholar]
- 27.Takahashi M., Kushida K., Hoshino H., Ohishi T., Inoue T. (1997) J. Endocrinol. Invest 20, 112–117 [DOI] [PubMed] [Google Scholar]
- 28.Schneider D. L., Barrett-Connor E. L. (1997) Arch. Intern. Med. 157, 1241–1245 [PubMed] [Google Scholar]
- 29.Arai F., Miyamoto T., Ohneda O., Inada T., Sudo T., Brasel K., Miyata T., Anderson D. M., Suda T. (1999) J. Exp. Med. 190, 1741–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saitoh Y., Koizumi K., Sakurai H., Minami T., Saiki I. (2007) Biochem. Biophys. Res. Commun. 364, 417–422 [DOI] [PubMed] [Google Scholar]
- 31.Abe M., Hiura K., Wilde J., Moriyama K., Hashimoto T., Ozaki S., Wakatsuki S., Kosaka M., Kido S., Inoue D., Matsumoto T. (2002) Blood 100, 2195–2202 [PubMed] [Google Scholar]
- 32.Chintalacharuvu S. R., Wang J. X., Giaconia J. M., Venkataraman C. (2005) Immunol. Lett. 100, 202–204 [DOI] [PubMed] [Google Scholar]
- 33.Kanatani N., Fujita T., Fukuyama R., Liu W., Yoshida C. A., Moriishi T., Yamana K., Miyazaki T., Toyosawa S., Komori T. (2006) Dev. Biol. 296, 48–61 [DOI] [PubMed] [Google Scholar]
- 34.Sakai N., Wada T., Furuichi K., Shimizu K., Kokubo S., Hara A., Yamahana J., Okumura T., Matsushima K., Yokoyama H., Kaneko S. (2006) J. Leukoc. Biol. 79, 555–563 [DOI] [PubMed] [Google Scholar]
- 35.Furuichi K., Gao J. L., Horuk R., Wada T., Kaneko S., Murphy P. M. (2008) J. Immunol. 181, 8670–8676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma B., Zhu Z., Homer R. J., Gerard C., Strieter R., Elias J. A. (2004) J. Immunol. 172, 1872–1881 [DOI] [PubMed] [Google Scholar]
- 37.Shang X., Qiu B., Frait K. A., Hu J. S., Sonstein J., Curtis J. L., Lu B., Gerard C., Chensue S. W. (2000) Am. J. Pathol. 157, 2055–2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anders H. J., Frink M., Linde Y., Banas B., Wörnle M., Cohen C. D., Vielhauer V., Nelson P. J., Gröne H. J., Schlöndorff D. (2003) J. Immunol. 170, 5658–5666 [DOI] [PubMed] [Google Scholar]
- 39.Coates P. T., Colvin B. L., Ranganathan A., Duncan F. J., Lan Y. Y., Shufesky W. J., Zahorchak A. F., Morelli A. E., Thomson A. W. (2004) Kidney Int. 66, 1907–1917 [DOI] [PubMed] [Google Scholar]
- 40.Ishii M., Egen J. G., Klauschen F., Meier-Schellersheim M., Saeki Y., Vacher J., Proia R. L., Germain R. N. (2009) Nature 458, 524–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuo K., Irie N. (2008) Arch. Biochem. Biophys. 473, 201–209 [DOI] [PubMed] [Google Scholar]
- 42.Kitazawa R., Mori K., Yamaguchi A., Kondo T., Kitazawa S. (2008) J. Cell Biochem. 105, 1289–1297 [DOI] [PubMed] [Google Scholar]
- 43.Murphy P. M., Baggiolini M., Charo I. F., Hébert C. A., Horuk R., Matsushima K., Miller L. H., Oppenheim J. J., Power C. A. (2000) Pharmacol. Rev. 52, 145–176 [PubMed] [Google Scholar]
- 44.Terashima Y., Onai N., Murai M., Enomoto M., Poonpiriya V., Hamada T., Motomura K., Suwa M., Ezaki T., Haga T., Kanegasaki S., Matsushima K. (2005) Nat. Immunol. 6, 827–835 [DOI] [PubMed] [Google Scholar]
- 45.Sato K., Suematsu A., Okamoto K., Yamaguchi A., Morishita Y., Kadono Y., Tanaka S., Kodama T., Akira S., Iwakura Y., Cua D. J., Takayanagi H. (2006) J. Exp. Med. 203, 2673–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Votta B. J., White J. R., Dodds R. A., James I. E., Connor J. R., Lee-Rykaczewski E., Eichman C. F., Kumar S., Lark M. W., Gowen M. (2000) J. Cell Physiol. 183, 196–207 [DOI] [PubMed] [Google Scholar]
- 47.Rioja I., Hughes F. J., Sharp C. H., Warnock L. C., Montgomery D. S., Akil M., Wilson A. G., Binks M. H., Dickson M. C. (2008) Arthritis Rheum 58, 2257–2267 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.