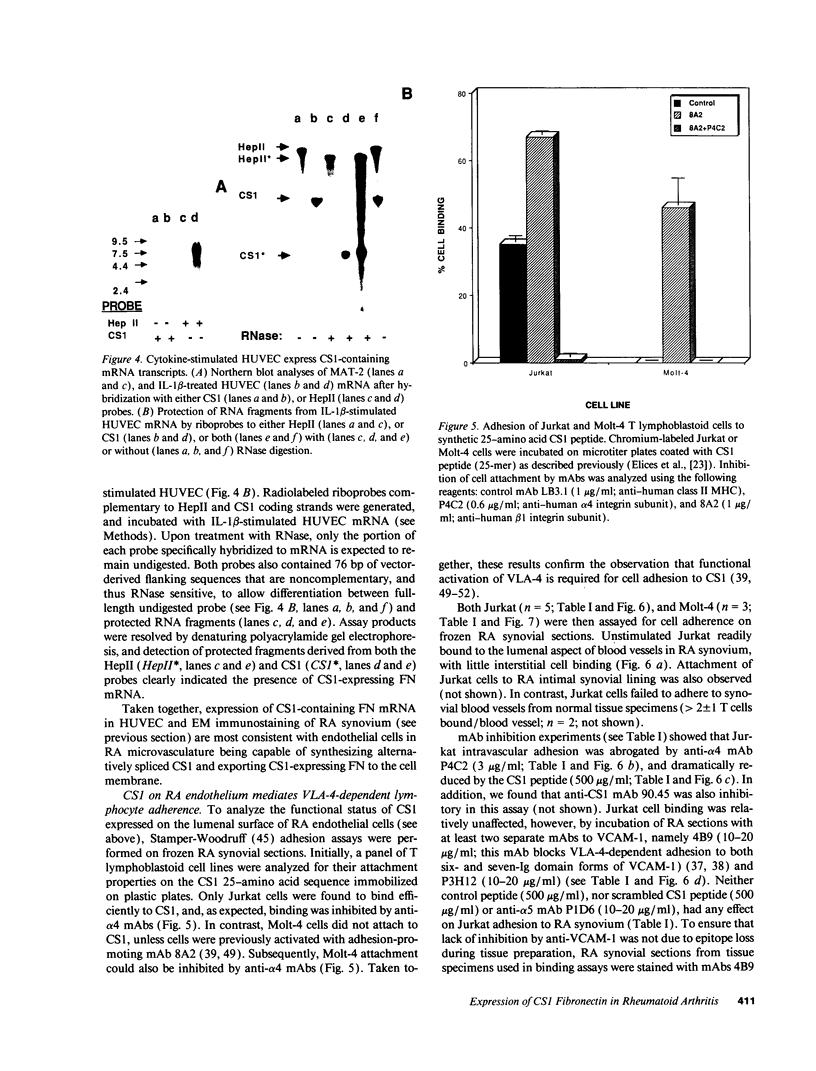
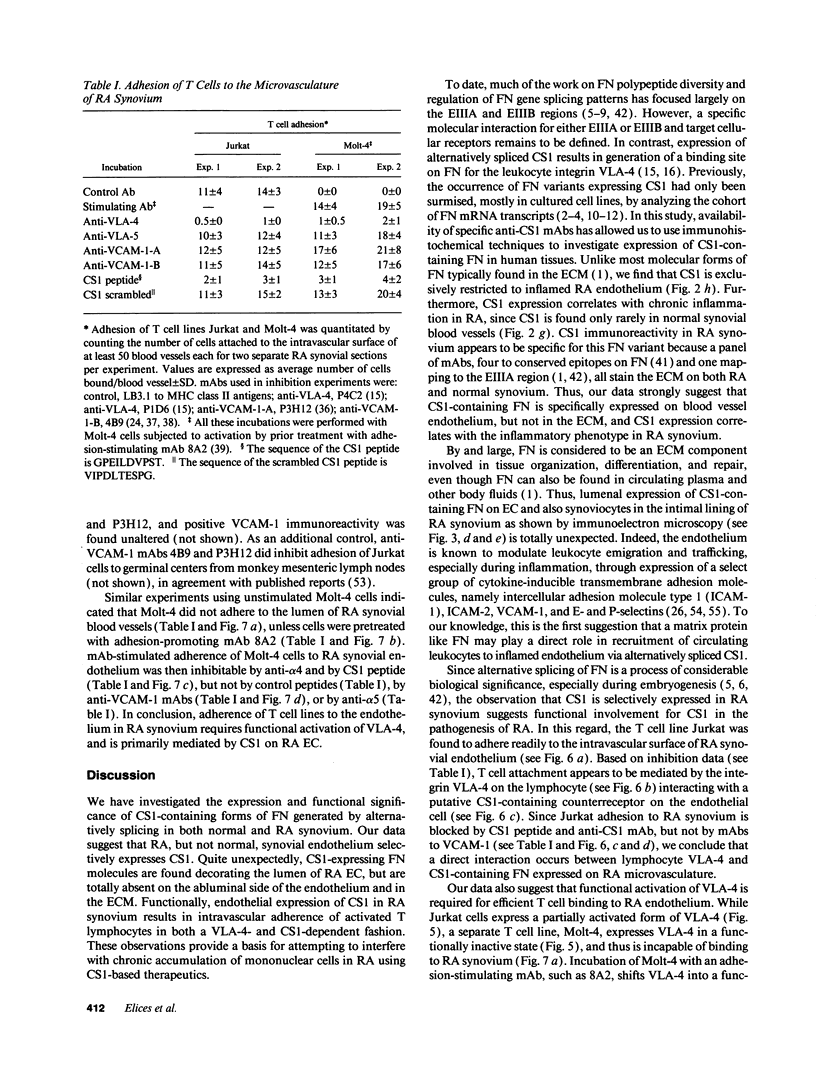
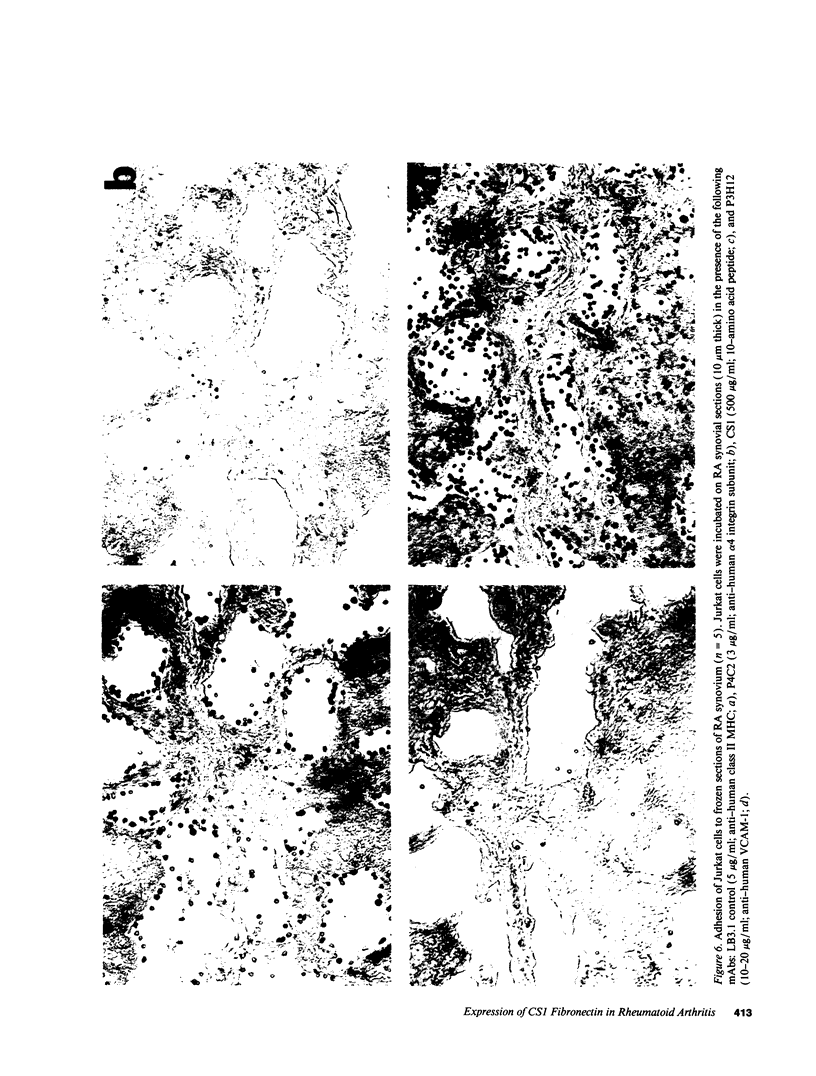
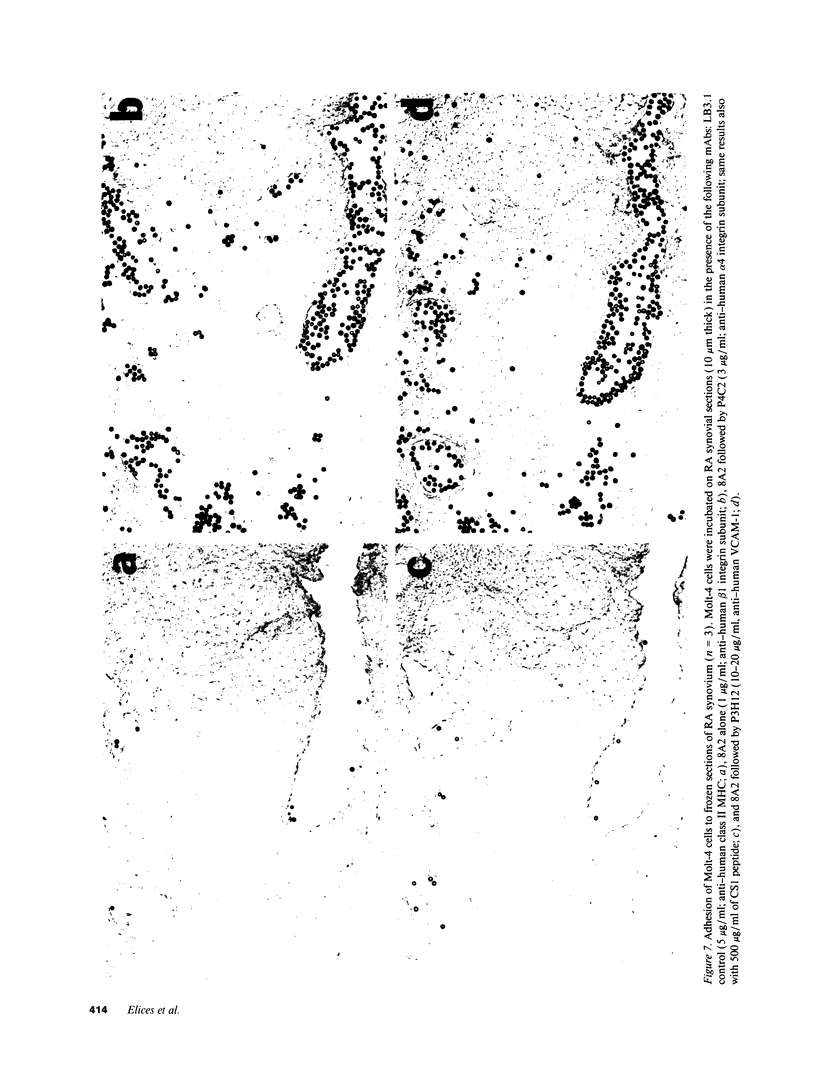
Abstract
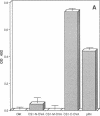
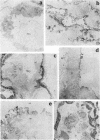
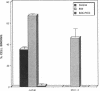
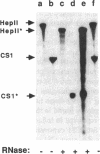
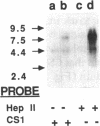
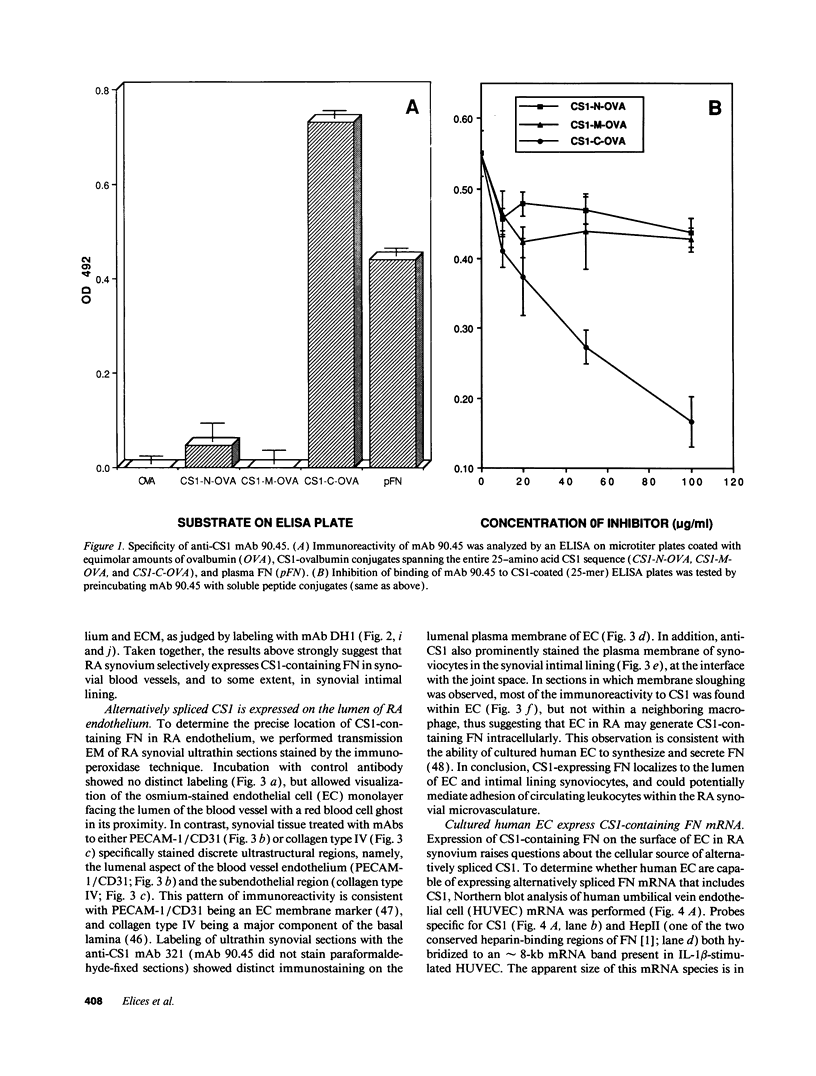
Expression of fibronectin (FN) isoforms containing CS1, a 25-amino acid sequence present within the alternatively spliced IIICS region of FN, has been analyzed in rheumatoid arthritis (RA) synovium. Unexpectedly, CS1-containing FN variants were exclusively found on endothelium but not extracellular matrix (ECM) of RA synovium. Lumenal expression of CS1 on RA endothelial cells, as observed by electron microscopy, correlated with inflammation in RA, since normal synovium expressed little CS1 without appreciable decrease in ECM FN. CS1 expression on human endothelial cells was further shown by FN mRNA analyses. In adhesion assays on frozen RA synovial sections, T lymphoblastoid cells expressing functionally activated alpha 4 beta 1 integrin specifically attached to the intravascular surface of RA endothelium. Binding was abrogated by both anti-alpha 4 integrin and CS1 peptides. Our observations suggest direct involvement of CS1-containing FN in recruitment of alpha 4 beta 1-expressing mononuclear leukocytes in synovitis, and provide basis for therapeutic intervention in RA.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Arroyo A. G., Sánchez-Mateos P., Campanero M. R., Martín-Padura I., Dejana E., Sánchez-Madrid F. Regulation of the VLA integrin-ligand interactions through the beta 1 subunit. J Cell Biol. 1992 May;117(3):659–670. doi: 10.1083/jcb.117.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Dufour S., Duband J. L., Humphries M. J., Obara M., Yamada K. M., Thiery J. P. Attachment, spreading and locomotion of avian neural crest cells are mediated by multiple adhesion sites on fibronectin molecules. EMBO J. 1988 Sep;7(9):2661–2671. doi: 10.1002/j.1460-2075.1988.tb03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Engvall E., Davis G. E., Dickerson K., Ruoslahti E., Varon S., Manthorpe M. Mapping of domains in human laminin using monoclonal antibodies: localization of the neurite-promoting site. J Cell Biol. 1986 Dec;103(6 Pt 1):2457–2465. doi: 10.1083/jcb.103.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson T. A., Mizutani H., Kupper T. S. Two integrin-binding peptides abrogate T cell-mediated immune responses in vivo. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8072–8076. doi: 10.1073/pnas.88.18.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Alternative splicing of fibronectin is temporally and spatially regulated in the chicken embryo. Development. 1989 Jun;106(2):375–388. doi: 10.1242/dev.106.2.375. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Hynes R. O. Patterns of fibronectin gene expression and splicing during cell migration in chicken embryos. Development. 1988 Nov;104(3):369–382. doi: 10.1242/dev.104.3.369. [DOI] [PubMed] [Google Scholar]
- Ffrench-Constant C., Van de Water L., Dvorak H. F., Hynes R. O. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989 Aug;109(2):903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein G. S., Alvaro-Gracia J. M., Maki R., Alvaro-Garcia J. M. Quantitative analysis of cytokine gene expression in rheumatoid arthritis. J Immunol. 1990 May 1;144(9):3347–3353. [PubMed] [Google Scholar]
- Firestein G. S., Tsai V., Zvaifler N. J. Cellular immunity in the joints of patients with rheumatoid arthritis and other forms of chronic synovitis. Rheum Dis Clin North Am. 1987 Aug;13(2):191–213. [PubMed] [Google Scholar]
- Firestein G. S., Xu W. D., Townsend K., Broide D., Alvaro-Gracia J., Glasebrook A., Zvaifler N. J. Cytokines in chronic inflammatory arthritis. I. Failure to detect T cell lymphokines (interleukin 2 and interleukin 3) and presence of macrophage colony-stimulating factor (CSF-1) and a novel mast cell growth factor in rheumatoid synovitis. J Exp Med. 1988 Nov 1;168(5):1573–1586. doi: 10.1084/jem.168.5.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman A. S., Munro J. M., Rice G. E., Bevilacqua M. P., Morimoto C., McIntyre B. W., Rhynhart K., Pober J. S., Nadler L. M. Adhesion of human B cells to germinal centers in vitro involves VLA-4 and INCAM-110. Science. 1990 Aug 31;249(4972):1030–1033. doi: 10.1126/science.1697696. [DOI] [PubMed] [Google Scholar]
- Grober J. S., Bowen B. L., Ebling H., Athey B., Thompson C. B., Fox D. A., Stoolman L. M. Monocyte-endothelial adhesion in chronic rheumatoid arthritis. In situ detection of selectin and integrin-dependent interactions. J Clin Invest. 1993 Jun;91(6):2609–2619. doi: 10.1172/JCI116500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J. L., Hynes R. O. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990 Jan 12;60(1):53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Elices M. J., Parker C., Takada Y. Structure of the integrin VLA-4 and its cell-cell and cell-matrix adhesion functions. Immunol Rev. 1990 Apr;114:45–65. doi: 10.1111/j.1600-065x.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Hemler M. E. VLA proteins in the integrin family: structures, functions, and their role on leukocytes. Annu Rev Immunol. 1990;8:365–400. doi: 10.1146/annurev.iy.08.040190.002053. [DOI] [PubMed] [Google Scholar]
- Hershberger R. P., Culp L. A. Cell-type-specific expression of alternatively spliced human fibronectin IIICS mRNAs. Mol Cell Biol. 1990 Feb;10(2):662–671. doi: 10.1128/mcb.10.2.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J., Akiyama S. K., Komoriya A., Olden K., Yamada K. M. Identification of an alternatively spliced site in human plasma fibronectin that mediates cell type-specific adhesion. J Cell Biol. 1986 Dec;103(6 Pt 2):2637–2647. doi: 10.1083/jcb.103.6.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries M. J., Komoriya A., Akiyama S. K., Olden K., Yamada K. M. Identification of two distinct regions of the type III connecting segment of human plasma fibronectin that promote cell type-specific adhesion. J Biol Chem. 1987 May 15;262(14):6886–6892. [PubMed] [Google Scholar]
- Jaffe E. A., Mosher D. F. Synthesis of fibronectin by cultured human endothelial cells. J Exp Med. 1978 Jun 1;147(6):1779–1791. doi: 10.1084/jem.147.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher O., Kennedy S. P., Madri J. A. Alternative splicing of endothelial cell fibronectin mRNA in the IIICS region. Functional significance. Am J Pathol. 1990 Dec;137(6):1509–1524. [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Vibe-Pedersen K., Baralle F. E. Human fibronectin: cell specific alternative mRNA splicing generates polypeptide chains differing in the number of internal repeats. Nucleic Acids Res. 1984 Jul 25;12(14):5853–5868. doi: 10.1093/nar/12.14.5853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach N. L., Carlos T. M., Yee E., Harlan J. M. A monoclonal antibody to beta 1 integrin (CD29) stimulates VLA-dependent adherence of leukocytes to human umbilical vein endothelial cells and matrix components. J Cell Biol. 1992 Jan;116(2):499–509. doi: 10.1083/jcb.116.2.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffón A., García-Vicuña R., Humbría A., Postigo A. A., Corbí A. L., de Landázuri M. O., Sánchez-Madrid F. Upregulated expression and function of VLA-4 fibronectin receptors on human activated T cells in rheumatoid arthritis. J Clin Invest. 1991 Aug;88(2):546–552. doi: 10.1172/JCI115338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasky H. P., Bauer K., Pope R. M. Increased helper inducer and decreased suppressor inducer phenotypes in the rheumatoid joint. Arthritis Rheum. 1988 Jan;31(1):52–59. doi: 10.1002/art.1780310108. [DOI] [PubMed] [Google Scholar]
- Magnuson V. L., Young M., Schattenberg D. G., Mancini M. A., Chen D. L., Steffensen B., Klebe R. J. The alternative splicing of fibronectin pre-mRNA is altered during aging and in response to growth factors. J Biol Chem. 1991 Aug 5;266(22):14654–14662. [PubMed] [Google Scholar]
- Marlor C. W., Webb D. L., Bombara M. P., Greve J. M., Blue M. L. Expression of vascular cell adhesion molecule-1 in fibroblastlike synoviocytes after stimulation with tumor necrosis factor. Am J Pathol. 1992 May;140(5):1055–1060. [PMC free article] [PubMed] [Google Scholar]
- Martin G. R., Timpl R. Laminin and other basement membrane components. Annu Rev Cell Biol. 1987;3:57–85. doi: 10.1146/annurev.cb.03.110187.000421. [DOI] [PubMed] [Google Scholar]
- Masumoto A., Hemler M. E. Multiple activation states of VLA-4. Mechanistic differences between adhesion to CS1/fibronectin and to vascular cell adhesion molecule-1. J Biol Chem. 1993 Jan 5;268(1):228–234. [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Morales-Ducret J., Wayner E., Elices M. J., Alvaro-Gracia J. M., Zvaifler N. J., Firestein G. S. Alpha 4/beta 1 integrin (VLA-4) ligands in arthritis. Vascular cell adhesion molecule-1 expression in synovium and on fibroblast-like synoviocytes. J Immunol. 1992 Aug 15;149(4):1424–1431. [PubMed] [Google Scholar]
- Muller W. A., Ratti C. M., McDonnell S. L., Cohn Z. A. A human endothelial cell-restricted, externally disposed plasmalemmal protein enriched in intercellular junctions. J Exp Med. 1989 Aug 1;170(2):399–414. doi: 10.1084/jem.170.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton P. A., Hynes R. O. Alternative splicing of chicken fibronectin in embryos and in normal and transformed cells. Mol Cell Biol. 1987 Dec;7(12):4297–4307. doi: 10.1128/mcb.7.12.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Osborn L., Vassallo C., Benjamin C. D. Activated endothelium binds lymphocytes through a novel binding site in the alternately spliced domain of vascular cell adhesion molecule-1. J Exp Med. 1992 Jul 1;176(1):99–107. doi: 10.1084/jem.176.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierschbacher M. D., Hayman E. G., Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981 Oct;26(2 Pt 2):259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Haskard D., Panayi G. The preferential accumulation of helper-inducer T lymphocytes in inflammatory lesions: evidence for regulation by selective endothelial and homotypic adhesion. Eur J Immunol. 1988 Sep;18(9):1397–1404. doi: 10.1002/eji.1830180915. [DOI] [PubMed] [Google Scholar]
- Pitzalis C., Kingsley G., Murphy J., Panayi G. Abnormal distribution of the helper-inducer and suppressor-inducer T-lymphocyte subsets in the rheumatoid joint. Clin Immunol Immunopathol. 1987 Nov;45(2):252–258. doi: 10.1016/0090-1229(87)90040-7. [DOI] [PubMed] [Google Scholar]
- Postigo A. A., Garcia-Vicuña R., Diaz-Gonzalez F., Arroyo A. G., De Landázuri M. O., Chi-Rosso G., Lobb R. R., Laffon A., Sánchez-Madrid F. Increased binding of synovial T lymphocytes from rheumatoid arthritis to endothelial-leukocyte adhesion molecule-1 (ELAM-1) and vascular cell adhesion molecule-1 (VCAM-1). J Clin Invest. 1992 May;89(5):1445–1452. doi: 10.1172/JCI115734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldán E., García-Pardo A., Brieva J. A. VLA-4-fibronectin interaction is required for the terminal differentiation of human bone marrow cells capable of spontaneous and high rate immunoglobulin secretion. J Exp Med. 1992 Jun 1;175(6):1739–1747. doi: 10.1084/jem.175.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B. R., Wayner E. A., Carlos T. M., Ochs H. D., Harlan J. M. Identification of surface proteins mediating adherence of CD11/CD18-deficient lymphoblastoid cells to cultured human endothelium. J Clin Invest. 1990 Jun;85(6):2019–2022. doi: 10.1172/JCI114668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J. E., Tamkun J. W., Lemischka I. R., Hynes R. O. Three different fibronectin mRNAs arise by alternative splicing within the coding region. Cell. 1983 Dec;35(2 Pt 1):421–431. doi: 10.1016/0092-8674(83)90175-7. [DOI] [PubMed] [Google Scholar]
- Sekiguchi K., Klos A. M., Kurachi K., Yoshitake S., Hakomori S. Human liver fibronectin complementary DNAs: identification of two different messenger RNAs possibly encoding the alpha and beta subunits of plasma fibronectin. Biochemistry. 1986 Aug 26;25(17):4936–4941. doi: 10.1021/bi00365a032. [DOI] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Stamper H. B., Jr, Woodruff J. J. Lymphocyte homing into lymph nodes: in vitro demonstration of the selective affinity of recirculating lymphocytes for high-endothelial venules. J Exp Med. 1976 Sep 1;144(3):828–833. doi: 10.1084/jem.144.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamkun J. W., Schwarzbauer J. E., Hynes R. O. A single rat fibronectin gene generates three different mRNAs by alternative splicing of a complex exon. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5140–5144. doi: 10.1073/pnas.81.16.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsumi K., Sawada M., Narumiya S., Nagamine J., Sakata T., Iwagami S., Kita Y., Teraoka H., Hirano H., Ogata M. Adhesion of immature thymocytes to thymic stromal cells through fibronectin molecules and its significance for the induction of thymocyte differentiation. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5685–5689. doi: 10.1073/pnas.88.13.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartio T., Laitinen L., Närvänen O., Cutolo M., Thornell L. E., Zardi L., Virtanen I. Differential expression of the ED sequence-containing form of cellular fibronectin in embryonic and adult human tissues. J Cell Sci. 1987 Nov;88(Pt 4):419–430. doi: 10.1242/jcs.88.4.419. [DOI] [PubMed] [Google Scholar]
- Vibe-Pedersen K., Magnusson S., Baralle F. E. Donor and acceptor splice signals within an exon of the human fibronectin gene: a new type of differential splicing. FEBS Lett. 1986 Oct 27;207(2):287–291. doi: 10.1016/0014-5793(86)81506-x. [DOI] [PubMed] [Google Scholar]
- Vonderheide R. H., Springer T. A. Lymphocyte adhesion through very late antigen 4: evidence for a novel binding site in the alternatively spliced domain of vascular cell adhesion molecule 1 and an additional alpha 4 integrin counter-receptor on stimulated endothelium. J Exp Med. 1992 Jun 1;175(6):1433–1442. doi: 10.1084/jem.175.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Cohen D. S., Palmer E., Sheppard D. Polarized regulation of fibronectin secretion and alternative splicing by transforming growth factor. J Biol Chem. 1991 Aug 25;266(24):15598–15601. [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Kovach N. L. Activation-dependent recognition by hematopoietic cells of the LDV sequence in the V region of fibronectin. J Cell Biol. 1992 Jan;116(2):489–497. doi: 10.1083/jcb.116.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. A., Rios M., Stephens C., Patel V. P. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature. 1991 Aug 1;352(6334):438–441. doi: 10.1038/352438a0. [DOI] [PubMed] [Google Scholar]
- van Dinther-Janssen A. C., Horst E., Koopman G., Newmann W., Scheper R. J., Meijer C. J., Pals S. T. The VLA-4/VCAM-1 pathway is involved in lymphocyte adhesion to endothelium in rheumatoid synovium. J Immunol. 1991 Dec 15;147(12):4207–4210. [PubMed] [Google Scholar]
- van de Wiel-van Kemenade E., van Kooyk Y., de Boer A. J., Huijbens R. J., Weder P., van de Kasteele W., Melief C. J., Figdor C. G. Adhesion of T and B lymphocytes to extracellular matrix and endothelial cells can be regulated through the beta subunit of VLA. J Cell Biol. 1992 Apr;117(2):461–470. doi: 10.1083/jcb.117.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]