Abstract
Pregnancy coordinately alters the contractile properties of both vascular and uterine smooth muscles reducing systemic blood pressure and maintaining uterine relaxation. The precise molecular mechanisms underlying these pregnancy-induced adaptations have yet to be fully defined but are likely to involve changes in the expression of proteins regulating myosin phosphorylation. Here we show that smoothelin like protein 1 (SMTNL1) is a key factor governing sexual development and pregnancy induced adaptations in smooth and striated muscle. A primary target gene of SMTNL1 in these muscles is myosin phosphatase-targeting subunit 1 (MYPT1). Deletion of SMTNL1 increases expression of MYPT1 30–40-fold in neonates and during development expression of both SMTNL1 and MYPT1 increases over 20-fold. Pregnancy also regulates SMTNL1 and MYPT1 expression, and deletion SMTNL1 greatly exaggerates expression of MYPT1 in vascular smooth muscle, producing a profound reduction in force development in response to phenylephrine as well as sensitizing the muscle to acetylcholine. We also show that MYPT1 is expressed in Type2a muscle fibers in mice and humans and its expression is regulated during pregnancy, suggesting unrecognized roles in mediating skeletal muscle plasticity in both species. Our findings define a new conserved pathway in which sexual development and pregnancy mediate smooth and striated muscle adaptations through SMTNL1 and MYPT1.
Keywords: Contractile Protein, Myosin, Phosphatase, Signal Transduction, Skeletal Muscle, Smooth Muscle, MYPT1, Pregnancy, Progesterone, SMTNL1
Introduction
Smooth and striated muscles show phenotypic plasticity in response to physiological stresses such as exercise and pregnancy (1–5). Each phenotypic state is characterized by the expression of a unique set of structural, contractile, and receptor proteins that correlate with differing patterns of gene expression (2, 6–10). Exercise-induced changes in muscle phenotype have been most extensively studied at the molecular level in striated muscle, but is less well defined in smooth muscles (2, 3, 11). Pregnancy promotes major changes in smooth muscles throughout the body through the actions of the steroid hormones 17β-estradiol and progesterone (4, 12, 13). Both hormones act by binding to their respective nuclear hormone receptors (PR and ER), coordinately altering gene expression patterns within their respective target tissues (14). For example in uterine muscle during pregnancy, progesterone maintains myometrial relaxation by directly inhibiting the expression of estrogen-sensitive genes e.g. the oxytocin receptor (OXTR), PGF2, C-43, and PGDH. Additionally, mesenteric and uterine arteries regulating uterine blood flow, exhibit less vascular tone and contractility (15). At parturition contractility of uterine smooth muscle is restored by promoting expression of estrogen-regulated genes such as OXTR (16). Although less well studied at the molecular level, vascular smooth muscle undergoes substantial changes during pregnancy that are also thought to be associated with remodeling. These adaptive responses underlie reduced systemic blood pressure (BP) thereby accommodating the increased cardiac out put and blood volume associated with normal pregnancy (16–18). In general vascular smooth muscle plasticity is beneficial, however, under pathological conditions it can also be detrimental. Hypertension can cause blood vessels to become hypertrophic causing blood flow resistance resulting in cardiac hypertrophy (19). Preeclampsia and eclampsia are complications of pregnancy that are associated with persistent hypertension in women.
The major mechanism governing the contractile activity of all smooth muscles is the phosphorylation of the regulatory light chain (MLC20) of myosin by calcium/calmodulin-dependent myosin light chain kinase (MLCK), while relaxation is effected by the dephosphorylation of MLC20 by myosin phosphatase (PP1M) (20, 21). Regulatory factors governing the phosphorylation state of myosin are thought to be primary determinants for promoting adaptive responses in smooth muscles. The role phosphorylation and PP1M in the regulation of striated muscle myosin is less well defined. Both smooth and striated PP1M consist of a heterotrimer composed of the 37 kDa catalytic subunit of protein phosphatase 1 (PP1cδ), a 110–130-kDa myosin-targeting subunit (MYPT)3 and a 20-kDa subunit of unknown function (20, 21). MYPT has three main isoforms, MYPT1, -2, and -3, which function to target PP1C to myosin and regulate its phosphatase activity. MYPT1 is expressed in smooth muscles, whereas MYPT2 and 3 are expressed preferentially in heart, skeletal muscle (SKM), and brain (22). Although the MYPT promoter lacks recognizable transcription factor binding sites suggesting its expression may not be regulated, isoform switching from MYPT1 to MYPT2 was observed in the differentiation of C2C12 cells from non-muscle to skeletal muscle cells (23, 24). Additionally, developmental switch of MYPT1 isoforms caused a significant increase in the rate of relaxation in the transition from the fetal to the adult circulation in rat vascular smooth muscle (25). Fischer et al. (15) recently reported expression of MYPT1 was increased in uterine artery during normal pregnancy, and in a rat model of hypertension in pregnancy, expression of C-terminal leucine zipper splice variants of MYPT1 (LZ+ and LZ−) was regulated. MYPT1 is also a target of several signaling pathways, including G-protein-coupled receptors acting through Rho kinase and NO/cGMP acting through PKG that positively and negatively regulate PP1M to acutely control smooth muscle tone respectively (20, 21).
A potential protein effector of PP1M is smoothelin-like protein 1 (SMTNL1) (26–28). SMTNL1 contains a calponin homology domain (CH2) at its C terminus while the remaining two-thirds of the primary sequence is entirely unique within the mammalian databases (26, 29). Gene deletion studies in mice demonstrated that SMTNL1 plays a role in cGMP/cAMP-mediated adaptations to exercise involving direct modulation of contractile activity in vascular smooth muscle. Inhibition of PP1M activity by SMTNL1 toward myosin and isolated light chains in vitro was also shown (27, 28). Additionally, in striated muscle, SMTNL1 was found to be specifically expressed in Type 2a adaptive fibers and gene deletion promoted increased numbers of these fibers in males, suggesting a role in mediating both striated muscle as well as vascular plasticity in response to physiological stresses such as exercise (28).
From studies with smtnl1−/− mice we noted several sex-related differences involving the SMTNL1 role in mediating adaptive responses in vascular and striated muscle (28). Vascular smooth muscle from sedentary and exercise-trained female smtnl1−/− mice exhibited more significant changes in responsiveness to β and α adrenergic agonists than their male counterparts. Exercise also induced more significant reduction of SMTNL1 expression in females compared with males. Striated muscle from male smtnl1−/− mice showed a greater tendency to switch to an oxidative phenotype. Collectively these findings suggested a role for SMTNL1 in mediating both vascular and striated muscle plasticity in response to the sex-related hormones. To investigate this hypothesis here we examined the function of SMTNL1 during sexual development and pregnancy in smtnl1−/− mice. Our findings suggest that a major mechanism by which development and pregnancy affect smooth muscle contractility is via regulation of SMTNL1, which in turn directly regulates MYPT1 expression, desensitizing smooth muscle containing tissues to Ca2+-mediated signals. Additionally we show a similar development and pregnancy-induced role for SMTNL1 in the regulation of MYPT1 expression in in Type2a oxidative fibers of striated muscle from mice and humans.
EXPERIMENTAL PROCEDURES
Mouse Colony Maintenance, Pregnancy, and Pseudopregnancy Studies
Congenic 129 SvEv smtnl1−/− mouse were created as described (28). All procedures involving mice were approved by Duke University Animal Care and Use Committee. For pregnancy studies 6–8 weeks matured female mice in estrus were selected by the appearance of their vagina (30) and also with lavarge method (31). Female littermates caged together presented synchronized estrus in 98% of cases. Females in estrus were mated with adult males overnight and then examined the following morning(s) for the presence of a vaginal plug. This was designated as day 0 of pregnancy. For pseudopregnancy studies adult, 7–8-week-old matured female in estrus were mated by vasectomized males obtained from Taconic. Males were vasectomized by ligation of the vas deferens and produced no sperm. Vasectomized mice then were mixed with females at a ratio of 1:2; females were observed for vaginal plug formation, which was designated as day 0 of pseudopregnancy.
Phosphatase Assays
Myosin phosphatase activity was assayed using smooth muscle whole myosin from pig bladder as the substrate as described (32).
Immunohistochemistry
Fiber typing of isopenthane-frozen mouse frozen plantarus (PL) sections (8 μm) by immunofluorescent staining using anti-MHC2a and 2b (Developmental Studies Hybridoma Bank, developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology, Iowa City) was performed as described (28, 33). Anti-MYPT1 (provided by D. J. Hartshorne, University of Arizona) and anti-SMTNL1 antibodies (Proteinrech Inc.) were applied simultaneously. Human rectus abdominis (RA) samples of patients of hysterectomy (non-pregnant) or C-sectioning (pregnant) and sections of aged matched healthy woman (35–40 years) were treated similarly to mice. Procedures involving humans were approved by the University and Medical Center Institutional Review Board at East Carolina University. Formalin-fixed mouse tissues were sectioned at 4 μm. Antigen unmasking with heat-induced epitope retrieval was applied using citrate retrieval buffer and Background buster (Innovex Biosc.) buffer were used to reduce background. The same method was applied on formalin-fixed human thoracic aorta sections. A representative set of images from n = 4–7 experiments are shown in figures. The inset shows the images of secondary antibody control.
Protein Analysis, Quantitative Western, and Quantitative Western Blot Analysis and Far Western Blot Analysis
Tissue samples were frozen in liquid N2 at the time of harvest, stored at −80 °C, and homogenized and applied for Western blot analysis as described (28) using the indicated antibodies. For the study of MLC20 phosphorylation MLC20 and MLC20 phospho-Ser-19 (Upstate) antibodies were applied. Protein concentration was determined by Bradford method. Bio-Rad gel doc system was used for data analysis (34). Densitometry of the blots was performed, and scans were analyzed by the Volume Analyze feature of the Molecular Analyst Software (Bio-Rad) and Image J. The density of the protein of interest was normalized to the density of GAPDH (Thermo Scien.) and tubulin (Sigma) internal controls (run on the same gel) and plotted as relative numbers. Far Western analysis was conducted as described before (34) using 0.1 mg of each recombinant proteins (PKA, AMPK, NT-SMTNL1, and GST-MYPT1) and for overlay 10 nm of NF-SMTNL1 and GST-MYPT1. For control experiments no proteins were applied but anti-Flag and anti-GST (Sigma) antibodies were used for Western blotting.
Cell Culture, Immunocytochemistry, and Confocal Microscopy
HeLa and ASMC cells were cultured and maintained in a humidified 37 °C incubator with 5% CO2. HeLa cells were transfected by 1 μg of pcDNA/SMTNL1 (NP_077192) and pcDNA/SMTNL1T301A. Cells were treated with 10 μm 8BrcAMP (cA) for 30 min and with 10 μm isoproterenol (ISO) for 10 min. Subcellular fractionation of control and treated HeLa cells were conducted as described (34). HeLa, T47D, and ASMC cells were cultured on coverslips and were serum-starved for 12 h. After blocking primary antibodies specific for SMTNL1, SMTNLS301, and MYPT1 were applied. Alexa Fluor 488 and 564 were used as secondary antibodies and DAPI (Molecular Probes) staining was applied for nuclear staining. Immunocytochemical and -histochemical images were imaged on Zeiss LSM 510 confocal laser scanning microscope (Carl Zeiss, Jena, Germany) using 85–100 nm pinholes. Individual random fields were collected using Apochromat 40 or 63 × 1.4 oil DIC immersion objective lenses. Final images represents z serial sections with an individual slice thickness of 0.8–1.2 mm. Images were processed using LSM 5 Examiner software programs (Carl Zeiss Microscope Systems) and reproduced using image-editing software (Photoshop, Adobe Systems, CA).
RNA Isolation and Real-time RT-PCR
RNeasy® Lipid Tissue Mini kit (Qiagen) was used to isolate total RNA from the uterus (n = 3) of wild type and/or smtnl1−/− of pregnant and/or non-pregnant mice according to the manufacturer's protocol. 1 μg of RNA was reverse transcribed to cDNA using the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer's protocol. PCR amplification of MYPT-1 was performed (in triplicates) in a total volume of 13 μl and included 5 μl of cDNA, 6.5 μl of iQ SYBR Green Supermix (Bio-Rad), 0.2 mm of forward primer (5′-AAAGCGACGGTCTACTGGAG-3′) and 0.2 mm of reverse primer (5′-AACAGAATCCGTCTGCGTCT-3′). 36B4 gene was used for the normalization of amplification. PCR was performed on an iCycler (Bio-Rad) according to the following cycling conditions: an initial cycle of 15 min at 95 °C, 45 cycles of 45 s at 95 °C, 15 s at 55 °C, and 15 s at 72 °C, followed by a melt-curve analysis cycle with steps of 10 s each at 0.5 °C increments from 60 to 95 °C. Amplification rates were visualized and analyzed on ICYCLER IQ optical system software Version 3.0 (Bio-Rad).
Isometric Force Measurements
Descending thoracic aortas were dissected, and aortic rings were dissected free of adjacent tissues. Segments were cut for mounting in a wire myograph and the passive stretch was set to 15 mNs to simulate the wall tension generated by 100 mmHg of blood pressure. Dose-response curves to phenylephrine (PE) and acetylcholine (Ach) were determined as described (28).
Statistical Analysis
To make conclusions robust to individual variation in the studied variables among experimental animals, data were normalized by taking values obtained for WT males as 1 and by calculating a corresponding proportional value for WT females and both sexes of KO mice. Normalized data were analyzed by Student's t-tests (for two groups) or by general linear models (GLM, for >2 groups). Groups not connected by the same letter are significantly different (p < 0.05). Parametric statistical tests were used if the assumptions of such tests were met. In other cases, we log-transformed data for analyses. In GLMs, we tested all possible interaction terms and report here the final models obtained by excluding non-significant (p > 0.05) interactions. When any covariate or factor was significant in GLMs, we applied Tukey's HSD procedure to test for pair wise differences in group means. GLMs and Tukey tests were conducted using the R statistical environment (R Development Core Team 2008: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (ISBN 3-900051-07-0, version 2.8.1).
RESULTS
SMTNL1 Regulates the Expression of MYPT1 in Striated and Smooth Muscle during Sexual Development
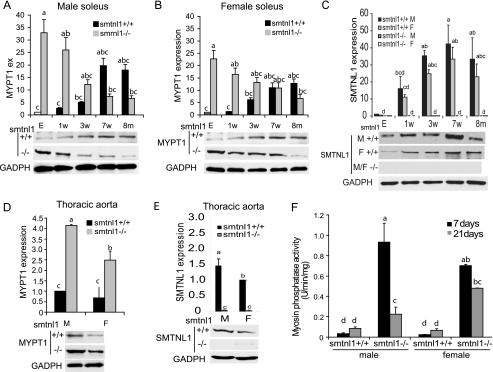
To understand the role of SMTNL1 and its relationship with MYPT1 between the sexes we first examined the expression of this phosphatase subunit during early development in WT and smntl1−/− mice (Fig. 1, A and B). In WT striated and smooth muscle, MYPT1 is expressed at low levels in neonates, but as the animals mature sexually, expression increases 10–20-fold and the levels are 20–30% higher in developed males than females. Remarkably, however, in both male and female smtnl1−/− mice, MYPT1 expression shows a reciprocal age-dependent pattern of expression. MYPT1 expression in neonates is now 30–40-fold higher than the levels found in WT littermates and exhibits a steady decline as the animals become sexually mature (Fig. 1, A and B). In contrast, expression levels of the MYPT2 isoform was unaffected by SMTNL1 deletion, suggesting that SMTNL1 specifically regulates MYPT1 expression. To investigate the relationship between MYPT1 and SMTNL1, we examined the expression of SMTNL1 throughout sexual development (Fig. 1C). Like MYPT1, SMTNL1 expression, increases in striated muscle and also the difference in the level of expression between males and females persists regardless of age. Similar correlations between MYPT1 and SMTNL1 were observed throughout development in thoracic aorta (Fig. 1, D and E).
FIGURE 1.
Development regulates the expression of SMTNL1 and MYPT1 in striated and vascular smooth muscle. A and B, MYPT1 expression increases in both male (A) and female (B) WT mice in striated muscle from the embryonic stage (E) and week 1 to 8 months (1w-8m), p < 0.05. C, SMTNL1 expression from embryonic stage (E) and ages 1 week to 8 months (1w-8m) from male (M) and female soleus (SOL) muscle (D–E), relative MYPT1 (D) and SMTNL1 (E) expression in vascular smooth muscle of males (M) and females (F) at age 5 weeks. F, increased expression of MYPT1 correlates with increased myosin phosphatase activity in muscle extracts of WT and smtnl1−/− animals at 7 (black) and 21 (gray) days. Groups not connected by the same letter are significantly different, n = 4 ± S.E., GLM with Tukey-test, p < 0.05.
To determine if increased expression of MYPT1 in response to SMTNL1 deletion increases myosin phosphatase activity in these tissues we measured PP1M activity following IP with antibody to MYPT1 (Fig. 1F). Fig. 1F shows that the observed age-dependent changes in the expression of MYTP1 correlates with increased total myosin phosphatase activity. This finding suggests that increased expression of MYTP1 itself induces the expression of the catalytic subunit of protein phosphatase 1 (PP1C). Our findings are consistent with recent work by Frohman et al. (35) showing that MYPT1 and PP1cδ expression is interdependent and knocking down of either subunit decreases the expression level of the other subunit. Concomitant increased expression of PP1C has also been observed following forced overexpression of MYPT2 using adenoviral vectors in the heart (22).
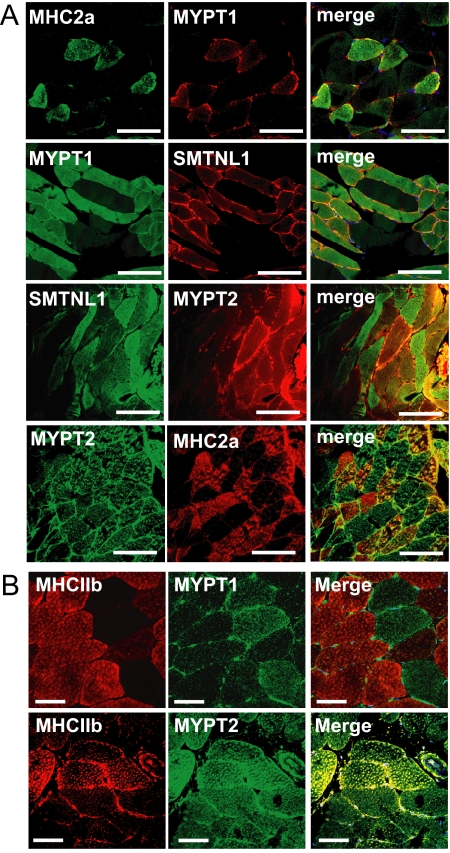
MYPT1 Is Selectively Expressed in Mouse and Human Type2a Striated Fibers
The finding that development, as well as SMTNL1 deletion induce expression of MYPT1 in striated muscle is somewhat surprising since prior to our studies MYPT2 has largely been reported as the major form of the phosphatase subunit in this tissue (36). Previously we had reported that in striated muscle SMTNL1 expression is highly localized to Type2a fibers (28). Therefore given the finding herein that SMTNL1 deletion promotes extraordinary expression of MYPT1 in this muscle, we examined fiber specific expression of the phosphatase subunit. Fig. 2 shows remarkable discrete expression of MYPT1 in Type2a fibers only, indicated by co-staining of SMTNL1 and MHC2a (Fig. 2A). Importantly, a similar staining pattern of MYPT1 and SMTNL1 was also observed in Type 2a human abdominus rectus striated muscle. As discussed, previous work showed that SMTNL1 expression is also confined to Type2a fibers in humans, suggesting that the relationship between this protein and MYPT1 is conserved across species (Fig. 2B). In contrast, MYPT2 expression does not show muscle specific fiber staining. To our knowledge, this is the first report showing fiber-specific staining of MYPT1 in striated muscle and suggests specific roles for the phosphatase in regulating Type 2a fiber function.
FIGURE 2.
Localization of MYPT1 in mouse and human striated muscle. A, discrete expression of MYPT1 to Type2a fibers compared with MYPT2 in plantarus (PL) muscle as determined by co-staining with MHC2a and SMTNL1. B, localization of MYPT1 and MYPT2 in Type2a and Type2b fibers in human skeletal muscle by confocal microscopy. Scale bars represent 20 μm.
Pregnancy Regulates the Expression of MYPT1 in Striated and Smooth Muscle in Mice and Humans
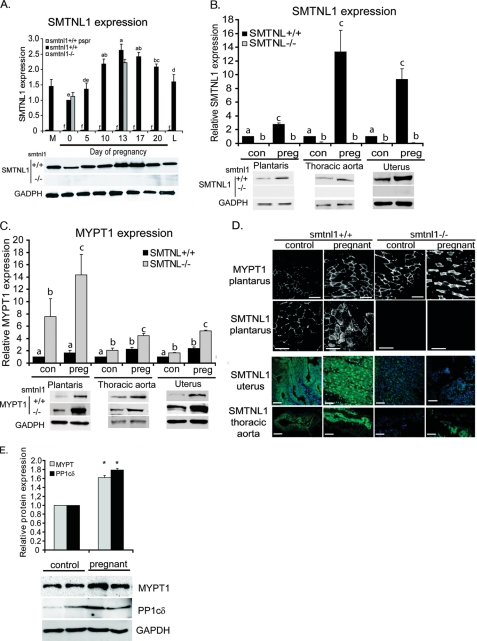
Our observation of gender related differences in both SMTNL1 and MYPT1 expression suggest unrecognized roles for these proteins in mediating the actions of the sex related hormones. To explore this hypothesis further we examined expression of both proteins during pregnancy. Fig. 3A shows that during pregnancy SMTNL1 expression in striated muscle increases 2.5-fold by day 13 compared with non-pregnant mice, and by ∼2-fold over levels expressed in males. Expression steadily declines through parturition and the onset of lactation. Interestingly, expression of SMTNL1 within all tissues examined mirrors that of serum progesterone levels, which also peak at day 13–17 then declines close to parturition. Pregnancy was also found to greatly induce expression of SMTNL1 in vascular and uterine smooth muscle by up to 10–12-fold (Fig. 3B).
FIGURE 3.
Pregnancy regulates the expression of SMTNL1 and MYPT1 in striated and smooth muscle. A, pregnancy and pseudopregnancy induce expression of SMTNL1 severalfold in striated muscle n = 5 ± S.E. p < 0.05. The inset below shows representative example of Western blot data. M, male expression levels. B, pregnancy induces significant expression of SMTNL1 relative to plantarus muscle in vascular (aorta) and uterine smooth muscle. Data shown are from day 0 and day 14 of pregnancy. Groups not connected by the same letter are significantly different n = 4 ± S.E., GLM with Tukey-test, p < 0.01. The inset below shows representative Western blots. C, pregnancy and SMTNL1 deletion induces MYPT1 expression in striated and smooth muscles n = 4 ± S.E., p < 0.05. The insets below show representative Western blots. D, upper panels show black and white images of the effects of pregnancy on MYPT1 and SMTNL1 expression in Type 2a. Lower panels show immunofluorescent images of SMTNL1 localization in uterus and aorta from control and pregnant WT and smtnl1−/− mice by SMTNL1 (green). Images are a representative set of n = 4 experiments. Scale bars represent 20 μm. E, MYPT1 and PP1C expression are induced in striated muscle from pregnant women under going C-section compared non-pregnant women undergoing hysterectomy. Biopsy samples were taken from the abdominus rectus muscles and processed for quantitative Western blot analysis with the indicated antibodies, n = 5 ± S.E. p < 0.05. Representative Western blots are shown below.
Because SMTNL1 expression is regulated by exercise in striated muscle, two possible explanations may account for alterations in the level of the protein in pregnant mice, either the induction is related to the physical effects of increased weight gain of the pregnant mother or SMTNL1 expression is hormonally regulated. To discriminate between these two possibilities we examined SMTNL1 expression in pseudo-pregnant mice. Pseudo-pregnant mice proceed with the normal hormonal cycle observed in fully pregnant animals but do not experience weight gain from the developing fetal mice in utero. The data in Fig. 3A show that SMTNL1 expression in pseudo-pregnant mice mirrors that observed in fully pregnant animals, suggesting that the expression changes are more likely to be related to changes in primary sex hormones present during normal pregnancy, rather than as a result of physical stress arising from the weight gain of the developing fetal mice.
Expression of MYPT1 was also found to closely mirror SMTNL1 levels during pregnancy in all tissues examined (Fig. 3C). Fig. 3C shows that pregnancy promotes a 2–3-fold increase in MYPT1 expression in striated, vascular, and uterine muscle. By contrast, in smtnl1−/− mice by day 13, MYPT1 expression increased dramatically to 6–14 times over the levels observed in pregnant WT littermates. Histological examination of SMTNL1 and MYPT1 expression in plantarus muscle by confocal microscopy shows that pregnancy induces the expression of both proteins within Type 2a fibers only, further confirming a relationship between both proteins and this particular muscle fiber type (Fig. 3D). Deletion of SMTNL1 also greatly enhances expression of MYPT1 within these fibers only. Immunohistochemistry of non-pregnant uterus, showed SMTNL1 is expressed within myometrial cells of the uterus as well as in the endometrial layer, and by day 13 of pregnancy expression is increased dramatically in both cell types. Uterus contrasts with aorta in which SMTNL1 expression is confined to smooth muscle cells, with no significant staining in endothelial cells (Fig. 3D). As indicated in Western analysis, histological studies also demonstrate that expression of SMTNL1 is also greatly induced by pregnancy in aortal smooth muscle. The finding that SMTNL1 is also discretely expressed within cells selectively targeted by the primary sex hormones supports links between the protein, MYPT1 and steroid hormone action. Quantitative Western blotting of abdominus rectus muscles isolated from age matched women undergoing either caesarean section (C-section) or hysterectomy showed that both MYPT1 and PP1 C expression are likely to be similarly regulated during pregnancy in humans. Fig. 3E shows that expression of MYPT1 is induced ∼2-fold by pregnancy with a similar concomitant fold increase in PP1C expression. These findings indicate that the mechanisms of regulation and functions of MYPT1 in striated muscle are likely to be conserved across species.
To determine if the observed changes in MYPT1 at the protein level are due to transcriptional or post-transcriptional regulation we examined mRNA levels by RT-PCR in response to pregnancy and SMTNl1 deletion. Supplemental Fig. S1 shows that where as pregnancy promotes a 2 fold increase in MYPT1 mRNA suggesting hormone-mediated translational control, SMTNL1 deletion alone has no effect on MYPT1 message levels. MYPT1 message was increased to ∼2.5-fold in pregnant smtnl1−/− mice supporting the hypothesis that any effect SMTNL1 has on MYPT1 mRNA synthesis requires additional hormonal factors.
Collectively, at first glance our finding that both development and pregnancy concomitantly increase SMTNL1 and MYPT1 expression, as well increasing total endogenous PP1M activity simultaneously, presents a paradox, since previous work demonstrated that SMTNL1 inhibits PP1M activity in vitro (28). In the discussion we suggest that this paradox can be explained through regulation of SMTNl1 activity by phosphorylation at Ser-301.
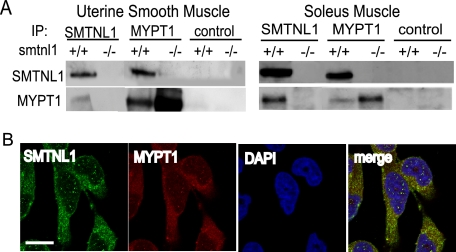
SMTNL1 Binds and Co-localizes with MYPT1 in Vivo
To investigate whether SMTNL1 mediates its cellular effects through direct interactions with MYPT1 we carried out co-IP experiments from uterine and striated muscle isolated from pregnant WT and smtnl1−/− mice (Fig. 4A). Fig. 4A shows that IP with anti-SMTNL1 co-IPs MYPT1 and similarly SMTNL1 is co-IP with anti-MYPT1 antibody. The interactions are specific, because in control IP experiments with extracts from smtnl1−/− mice no MYPT1 is recovered with the SMTNL1 antibody (Fig. 4A). As observed in Western experiments, IP of MYPT1 from striated and uterine muscle extracts prepared from smtnl1−/− mice resulted in recovery of a larger amount of the protein than from WT mice. Co localization of MYPT1 and SMTNL1 was also observed by immunofluorescent staining in arterial smooth muscle cells showing diffuse cytosolic and discrete nuclear localization for both proteins (Fig. 4B). Interactions between MYPT1 and SMTNL1 were also confirmed by far Western analysis in which the purified recombinant forms of each protein were found to recognize each other in overlay experiments (supplemental Fig. S2). Although these findings suggest direct interactions between the two proteins, other proteins may also participate to facilitate binding in vivo.
FIGURE 4.
SMTNL1 binds MYPT1 in striated and uterine smooth muscle. A, SMTNL1 and MYPT1 were co-immunoprecipitated (IP) from uterus or soleus extracts using non-immune serum as a control. B, cytosolic and nuclear co-localization (merge, white) of endogenous SMTNL1 (green) and MYPT1 (red) in aortic smooth muscle cells. Scale bars, 10 μm.
Ser-301 Phosphorylation Regulates Translocation of SMTNL1 to the Nucleus
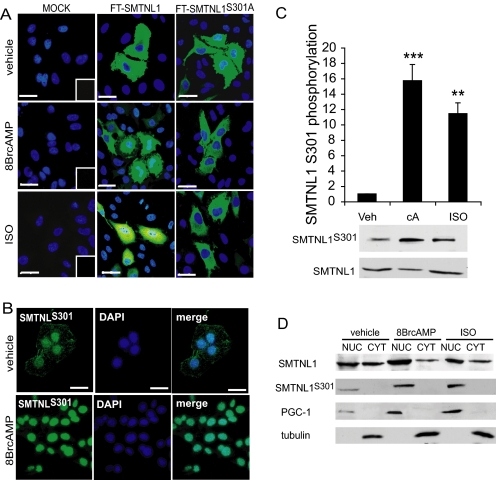
SMNTL1 is phosphorylated at Ser-301 in vivo in response to agonists that elevate intracellular cGMP or cAMP (27, 28). Our finding that SMTNL1 deletion induces expression of MYPT1 in both smooth and striated muscle tissues, and that this effect is greatly exaggerated during pregnancy, suggests a direct role for SMTNL1 in the regulation of MYPT1 expression. Further, as shown in Western blot experiments in Fig. 3, expression of both SMTNL1 and MYPT1 is highly reminiscent of the progesterone cycle during normal pregnancy, which also peaks between day 13 and 17 in mice. To exert its cellular effects progesterone binds to its receptor (PR) in the cytosol and translocates to the nucleus to alter the transcriptional profile of its target tissues. To determine if SMTNL1 has the capacity to translocate to the nucleus and impact MYPT1 expression directly we examined it subcellular location. Fig. 5 shows that in vivo SMTNL1 localization is highly regulated and phosphorylation at Ser-301 promotes translocation of the protein in both non-muscle as well as intact muscle cells transfected with SMTNL1. In its dephosphorylated state, transfected WT SMTNL1 resides in the cytoplasm, but becomes highly localized within the nucleus in response to 8-Br-cAMP or isoproterenol (ISO) (Fig. 5A). In contrast, mutation of Ser-301 to alanine blocks agonist dependant nuclear localization of transfected FLAG-SMTNL1S301A and is unresponsive to cAMP (Fig. 5A). Similar results were observed following measurement of Ser-301 phosphorylation using a phospho-Ser-301 specific antibody (Fig. 5B). To evaluate whether SMTNL1 exhibits similar nuclear localization in intact striated muscle, isolated soleus muscles were treated with either ISO or 8BrcAMP (Fig. 5, C and D). Fig. 5, C and D shows that both agonists elicit robust phosphorylation of Ser-301 with concomitant recovery of SMTNL1 within the nuclear fraction. The Ser-301 phosphorylation site resides C-terminal to a putative nuclear localization sequence (NLS) 290KKDR293 suggesting a potential mechanism of regulation for SMTNL1 nuclear localization.
FIGURE 5.
SMTNL1 Ser-301 phosphorylation is required for translocation to the nucleus. A, FT-SMTNL1 translocates to the nucleus followed by phosphorylation at Ser-301. HeLa cells were transfected with FT-SMTNL1 and FT-SMTNL1S301A, treated with 10 μm 8BrcAMP (cA) and 10 μm ISO, then immunostained by antibody against Flag-tag (green scale bars, 10 μm). B, endogenous SMTNL1 is cytosolic but phosphor-Ser-301 SMTNL1 shows dominantly nuclear subcellular localization in T47D cells. Scale bars, 10 μm. C, SMTNL1 is phosphorylated at Ser-301 in response to acute treatment of 10 μm 8BrcAMP and ISO in different mammalian cell lines (HeLa cells are presented). n = 4 ± S.E. ***, p < 0.001; **, p < 0.01. D, subcellular distribution of SMTNL1 and SMTNL1PS301. The subcellular fractions of HeLa cells as NUC (nucleus) and CYT (cytosol, cytoskeleton) fractions were analyzed. PGC-1 and tubulin were used as nuclear and cytosolic markers, respectively. Representative images are shown, but similar results were obtained on separate occasions.
Increased Expression of MYPT1 in Pregnant smtnl1−/− Mice Results in Profound Reduction in Isometric Force in Vascular Smooth Muscle
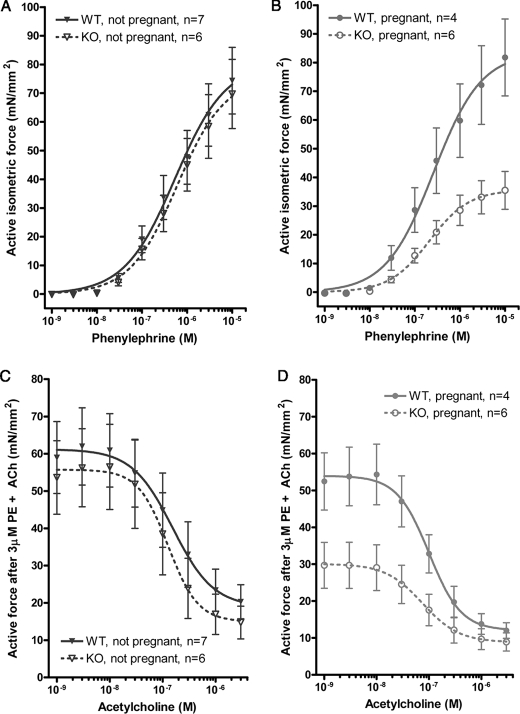
To evaluate the effects of increased MYPT1 on smooth muscle contractile activity during pregnancy thoracic aorta was isolated from WT and smtnl1−/− mice and responsiveness to the contractile agonist Phe and relaxant Ach was measured. Both agonists are known to affect myosin phosphorylation either through increasing intracellular Ca2+ release (Phe) or by elevating intracellular cGMP through generation of endothelial nitric oxide (Ach). Fig. 6A shows that in non-pregnant animals deletion of SMTNL1 promotes modest desensitization to phenylephrine (WT Phe50% = 1.2 ± 0.05 μm; smtnl1−/− Phe50% = 1.6 ± 0.03 μm n = 5 S.E.). In both WT type and smtnl1−/− mice, although pregnancy (day 13) had little observable effect on sensitivity to phenylephrine (WT preg Phe50% = 1.5 ± 0.04 μm; smtnl1−/− preg Phe50% = 1.6 ± 0.06 μm n = 5 S.E.), deletion of the protein had a profound effect on maximal force (Fmax) development (WT preg Fmax = 86 ± 15 mN; smtnl1−/− preg Fmax = 35 ± 9 mN n = 5 S.E.) (Fig. 6B). Measurement of the effects of acetylcholine to promote relaxation following maximal phenylephrine-induced contraction showed that both pregnancy and SMTNL1 deletion had significant effects on responsiveness to the relaxant (Fig. 6, C and D). Fig. 6C shows that SMTNL1 deletion has a sensitizing effect on responsiveness to acetylcholine, increasing potency (WT Ach50% = 0.25 ± 0.006 μm n = 7 S.E.; smtnl1−/− Ach50% = 0.095 ± 0.002 μm n = 6 S.E.) to the agonist as well as producing greater extent of relaxation (Rmax) (WT Rmax = 28 ± 11 mN, n = 7; smtnl1−/− Rmax = 15 ± 8 mN, n = 6 S.E.). Fig. 6D shows that pregnancy increases the relaxant effects of acetylcholine in WT mice and that this effect is greatly exaggerated in smtnl1−/− mice (WT preg Ach50% = 0.2 ± 0.006 μm, Rmax = 15 ± 4 mN, n = 4 S.E.; smtnl1−/− preg Ach50% = 0.06 ± 0.001 μm, Rmax 9.5 ± 3 mN, n = 6 S.E.). The finding that both pregnancy and SMTNL1 deletion reduce force development in response to phenylephrine and promote both the rate and extent of relaxation in response to acetylcholine are consistent with Western blotting, immunohistochemical and biochemical studies showing increased expression of MYPT1 in vascular smooth muscle. Because acetylcholine is able to relax aorta from smtnl1−/− mice these findings suggest that SMTNL1 is not essential for directly mediating all the actions of PKG in vascular smooth muscle and that other targets of the protein kinase remain intact. We and others have shown that several proteins in addition to SMTNL1 participate in the regulation of vascular smooth muscle relaxation including phosphorylation of MYPT1 at Ser-695, the intracellular Ca2+ channel IRAG and the PP1M inhibitor protein CP17 (21). However, examination of basal phosphorylation levels of MLC20 using a phosphospecific antibodies to Ser-19 does show both pregnancy and SMTNL1 deletion promote endogenous myosin dephosphorylation (supplemental Fig. S3A). Furthermore, myosin phosphatase assays of extracts prepared from WT and pregnant tissues showed that both pregnancy and SMTNL1 deletion correlated with an increase in total myosin phosphatase activity (supplemental Fig. S3B). These results are consistent with the induction of MYPT1 expression of observed in Western blot and IHC experiments of aorta. Collectively our data suggest that one of the major mechanisms by which pregnancy promotes reduced systemic BP is by controlling MYPT1 expression levels in vascular smooth muscle to promote Ca2+ desensitization and myosin dephosphorylation. A key regulatory factor in mediating these processes is SMTNL1.
FIGURE 6.
Increased expression of MYPT1 in pregnant smtnl1−/− mice promotes reduction in isometric force in response to phenylephrine and sensitization to acetylcholine in isolated thoracic aorta. Measurements of contractile activity were carried using the wire micrograph as described (36). A, shows effects of SMTNL1 deletion on responsiveness to phenylephrine. B, effects of pregnancy on the contractile response to phenylephrine in thoracic aorta isolated from pregnant WT and smtnl1+/+ mice. In B, differences were significant at 10−8 and 10−5 m Phe n = 4–6, ± S.E. Student's t test, p = 0.0011. C, effects of SMTNL1 deletion on sensitivity to acetylcholine in non-pregnant animals following treatment with 10 μm phenylephrine. D, effects of pregnancy on relaxation in response to acetylcholine in WT and smtnl1−/− mice following precontraction with 10 μm phenylephrine. All points between 1 nm and 0.5 μm were significant at p < 0.001 ± S.E.
DISCUSSION
In this report, we show that SMTNL1 plays a major role in mediating adaptive responses in both smooth and striated muscle during development and pregnancy supporting a role for the protein in mediating the actions of the major sex-related hormones. Consistent with this hypothesis SMTNL1 deletion appears to exaggerate the pregnant state in terms of the contractile responses of isolated vascular smooth muscle and effects on MYPT1 expression. The finding that pregnancy and SMTNL1 deletion greatly increase the expression of MYPT1 in vascular and uterine smooth muscle now provide a molecular mechanism to explain pregnancy-induced relaxation in these tissues i.e. pregnancy promotes MYPT1 expression increasing myosin dephosphorylation, desensitizing uterine and vascular smooth muscle to Ca2+ signals, thereby promoting uterine relaxation and reduced systemic BP. The finding that increased MYPT1 expression correlates with decreased basal levels of LC20 phosphorylation and increased endogenous myosin phosphatase activity support this conclusion. Our data highlight the capacity of MYPT1 expression to be regulated. During normal sexual development MYPT1 expression increases ∼20-fold, in both males and females. Deletion of SMTNL1 produces an extraordinary effect on basal expression in neonates and sexually immature mice, increasing MYPT1 over 40-fold compared with WT litter mates. Prior to this study MYPT1 has not been thought to have much capacity to be regulated at the protein level, largely due to an absence of recognized transcription factor binding sites in its promoter region (23). However, our studies examining MYPT1 mRNA levels by real time PCR in response to pregnancy support recent findings of Fischer et al. (15) in uterine artery showing both an increase in MYPT1 expression at both the protein and message level, suggesting that the protein is transcriptional regulated. In contrast, although SMTNL1 deletion alone induces large changes in the expression of MYPT1 at the protein level in non-pregnant animals, we observed no direct effect on MYPT1 mRNA levels (supplemental Fig. S1). These findings suggest that at the protein level MYPT1 is likely to be regulated both transcriptionally and post-transcriptionally and that SMTNL1 may play a role at both levels. The finding that the expression of MYPT1 in greatly exaggerated in pregnant smntl1−/− suggests that its ability to regulate MYPT1 at the transcriptional level is likely to be hormone dependent and indeed may act in a repressor capacity, perhaps through interactions with the progesterone or estrogen receptor. The finding that MYPT1 mRNA levels were ∼25% higher in pregnant smtnl1−/− animals compared with pregnant WT litter mates supports this mechanism. Non-hormone-dependent post-translational mechanisms of regulation mediated through SMTNL1 could include roles in RNA stability, translational regulation, protein stability or degradation. Sikorska and co-workers (37) recently demonstrated that MYPT1 degradation has the capacity to be regulated in that the Seven in absencia homolog 2 (SIAH2)-ubiquitin-proteosomal pathways is responsible for the turnover of MYPT1. We are currently investigating the effects of SMTNL1 deletion on MYPT1 mRNA and protein turnover to investigate its role in post-translation regulation of the phosphatase subunit. Importantly, alterations of expression of MYPT1 mediated by pregnancy also occurs in humans, with the finding that both MYPT1 and PP1C are induced ∼2-fold in Type2a muscle fibers of abdominus rectus muscle taken from pregnant women undergoing C-section compared with aged matched non-pregnant women undergoing hysterectomy. The finding that MYPT1 is also expressed selectively in Type 2a fibers in humans also suggests that its functions are likely to be conserved from mouse to humans.
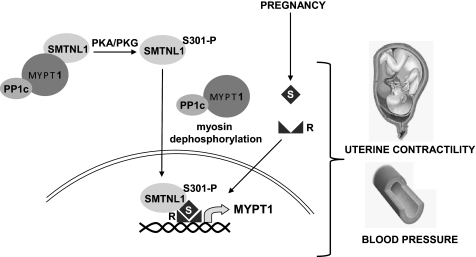
Data showing increased expression of MYPT1 in response to SMTNL1 deletion and pregnancy present a paradox, because pregnancy also induces expression of SMTNL1. This is further complicated by previous findings showing that in vitro recombinant SMTNL1 inhibits PP1M activity toward myosin and MLC20 (26, 28). Consequently one might expect that expression of SMTNL1 would be decreased, not increased by pregnancy if the protein directly regulates PP1M activity in vivo. The finding that SMTNL1 activity and intracellular localization are regulated by Ser-301 phosphorylation offers a mechanism to explain this paradox. In previous work we had shown that agonists that elevate intracellular cAMP or cGMP promote phosphorylation of Ser-301 in vivo, and phosphorylation of this amino acid inhibits the ability of SMTNL1 to inhibit PP1M in vitro (27, 28). Herein we now show that phosphorylation Ser-301 also promotes translocation of SMTNL1 to the nucleus. We therefore hypothesize that SMTNL1 is a signal transduction molecule acting as part of a feedback mechanism to enable cellular signals to regulate both the activity and expression of MYPT1 and consequently PP1M activity (Fig. 7). In non-pregnant tissue SMTNL1 remains in the cytosol and suppresses PP1M activity through interactions with MYPT1. In response to adrenergic signaling Ser-301 becomes phosphorylated and SMTNL1 translocates to the nucleus where it may interact with factors governing the expression MYPT1. As discussed, Ser-301 resides N-terminal to a NLS sequence, therefore, could be transported to the nucleus through interactions with importins. Alternatively the protein could interact with the nuclear hormone receptors targeted by progesterone or estrogen, which in the absence of these hormones, also reside the cytoplasm, but translocate to the nucleus when bound to these steroids. Because progesterone is recognized as the major hormone governing pregnancy, a likely candidate for interaction with SMTNL1 is the progesterone receptor (PR). If SMTNL1 interacts with PR this would provide a mechanism for the protein to not only alter MYPT1 expression at the gene level, but also be involved coordinating wider gene responses related to promoting smooth and striated muscle adaptations in response to pregnancy. Such a mechanism of action may imply that in females the default function for SMTNL1 in non-pregnant animals is to repress sex hormone function from promoting pregnancy-like adaptive responses in both vascular smooth muscle and other tissues. Under this scenario pregnancy would therefore relieve SMTNL1 inhibition of PR for example to promote appropriate adaptive responses such as the increased expression of MYPT1 in uterine and vascular smooth muscles as well as adaptations in striated muscles. Intriguingly, PKA-mediated signaling pathways have long been known to greatly effect progesterone signaling in vivo, synergizing with the hormone (38–41). In summary, loss of SMTNL1 function deregulates pathways that are normally suppressed in non-pregnant animals, therefore explaining the smtnl1−/− phenotype in female mice.
FIGURE 7.
SMTNL1 regulates MYPT1 expression in pregnancy. In non-pregnant tissue SMTNL1 remains in the cytosol and suppresses PP1M activity through interactions with MYPT1. In response to phosphorylation by PKA/PKC at Ser-301 SMTNL1 releases MYPT1 and enters the nucleus where it may interact with factors governing the expression MYPT1. During pregnancy SMTNL1 may also enter the nucleus where it may interact with factors that also regulate MYPT1 expression such as the nuclear hormone receptors targeted by estrogen or progesterone. This coordinated response adapts the mother's physiological state to support the developing fetus. Increased expression of MYPT1 in uterine and vascular smooth muscle promotes myosin dephosphorylation and muscle relaxation. (R: receptor, S: steroid hormone).
The finding that MYPT1 is exclusively expressed in Type2a fibers from both WT and smtnl1−/− mice as well as human striated muscle was unexpected. Currently, the precise function of MYPT1 expression in Type2a fibers can only be inferred from studies in vascular smooth muscle showing that increased expression of the protein promotes profound Ca2+ desensitization and muscle relaxation. However, the fiber specific localization of MYPT1 in Type2a fibers suggests a re-evaluation of myosin phosphatase function in regulating contractile activity in striated muscle is warranted. Prior to data presented herein, MYPT2 has been defined as the major isoform of myosin phosphatase subunit expressed in striated muscle (22, 36). In early in vitro studies MYPT2 was shown to exhibit a preference for striated muscle myosin over smooth muscle isoforms. However, reports reporting the specificity of both MYPT1 and MYPT2 isoforms for striated muscle myosin were performed on mixed myosins isolated from whole skeletal muscle (36). The isolation of purified forms of native MHC2a from MHC1 and MHC2b from the relevant striated muscle types in sufficient quantities for biochemical studies is technically challenging. Based on our finding of selective localization of MYPT1 in Type2a fibers only, we now suggest that when complexed with PP1C, the subunit is likely to show selectivity toward MHC2a over the other forms of striated muscle myosins. Sequence alignments of the 3 major isoforms of striated muscle MHC shows MHC2a shares 91% identity with MHC2b and 94% identity with MHC I, whereas MHC2b and MHC1 share 94% identity, suggesting that MHC2a is more divergent from the other isoforms. Indeed, the majority of the sequence divergence occurs in the motor and hinge regions of MHC2a (42). Additionally, there is considerably more sequence divergence between the types of myosin light chain isoforms expressed in striated fibers. The MLC20s, or slow twitch myosin regulatory light chain, shares ∼52% sequence similarity with the fast twitch MLC20f forms (42, 43). Both types are phosphorylated in vivo and are thought to sensitize striated muscle to Ca2+ to increase shortening velocity. Furthermore, recent evidence suggests that striated muscle remodeling can promote switching of individual light chains between MHC forms (43).
To summarize, we have shown that SMTNL1 functions to regulate MYPT1 expression in both smooth as well as striated muscles. Further, both proteins are regulated during sexual development and pregnancy and form a module that adapts these tissues to alter their contractile properties. In smooth muscle these pregnancy-induced adaptations most likely contribute to reduced systemic blood pressure and uterine relaxation that occurs during normal pregnancy. In striated muscle the role of both proteins has yet to be fully defined, but their co-expression in Type2a adaptive fibers only strongly suggest a previously unrecognized role in mediating pregnancy induced alterations in striated muscle function.
Supplementary Material
Acknowledgment
We thank Dr. Ferenc Erdodi (University of Debrecen, Hungary) for help in the preparation of this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant R01DK065954-05 (to T. A. J. H.) and a Bolyai Fellowship (Hungarian Academy of Science) (to B. L.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- MYPT
- myosin phosphatase-targeting subunit
- SMTNL
- smoothelin-like protein
- Phe
- phenylephrine
- Ach
- acetylcholine.
REFERENCES
- 1.Halayko A. J., Solway J. (2001) J. Appl. Physiol. 90, 358–368 [DOI] [PubMed] [Google Scholar]
- 2.Flück M., Hoppeler H. (2003) Rev. Physiol Biochem. Pharmacol. 146, 159–216 [DOI] [PubMed] [Google Scholar]
- 3.Hoppeler H., Klossner S., Flück M. (2007) Adv. Exp. Med. Biol. 618, 245–254 [DOI] [PubMed] [Google Scholar]
- 4.Wu X., Morgan K. G., Jones C. J., Tribe R. M., Taggart M. J. (2008) J. Cell. Mol. Med. 12, 1360–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiao D., Huang X., Longo L. D., Pearce W. J., Zhang L. (2006) Am. J. Physiol. Heart Circ. Physiol. 291, H413–H420 [DOI] [PubMed] [Google Scholar]
- 6.Flück M., Dapp C., Schmutz S., Wit E., Hoppeler H. (2005) J. Appl. Physiol. 99, 397–413 [DOI] [PubMed] [Google Scholar]
- 7.Harridge S. D. (2007) Exp Physiol. 92, 783–797 [DOI] [PubMed] [Google Scholar]
- 8.Owens G. K. (1995) Physiol. Rev. 75, 487–517 [DOI] [PubMed] [Google Scholar]
- 9.Owens G. K., Kumar M. S., Wamhoff B. R. (2004) Physiol. Rev. 84, 767–801 [DOI] [PubMed] [Google Scholar]
- 10.Reggiani C., Kronnie G. T. (2004) J. Muscle Res. Cell. Motil. 25, 231–234 [DOI] [PubMed] [Google Scholar]
- 11.D'Antona G., Lanfranconi F., Pellegrino M. A., Brocca L., Adami R., Rossi R., Moro G., Miotti D., Canepari M., Bottinelli R. (2006) J. Physiol. 570, 611–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao D., Buchholz J. N., Zhang L. (2006) Am. J. Physiol. Heart Circ. Physiol. 290, H2337–H2343 [DOI] [PubMed] [Google Scholar]
- 13.Minorics R., Gáspár R., Gál A., Klukovits A., Falkay G. (2009) Reproduction 138, 383–390 [DOI] [PubMed] [Google Scholar]
- 14.Edwards D. P. (2005) Annu. Rev. Physiol. 67, 335–376 [DOI] [PubMed] [Google Scholar]
- 15.Lu Y., Zhang H., Gokina N., Mandala M., Sato O., Ikebe M., Osol G., Fisher S. A. (2008) Am. J. Physiol. Cell. Physiol. 294, C564–C571 [DOI] [PubMed] [Google Scholar]
- 16.McDonnell D. P., Shahbaz M. M., Vegeto E., Goldman M. E. (1994) J. Steroid Biochem. Mol. Biol. 48, 425–432 [DOI] [PubMed] [Google Scholar]
- 17.Thornburg K. L., Jacobson S. L., Giraud G. D., Morton M. J. (2000) Semin. Perinatol. 24, 11–14 [DOI] [PubMed] [Google Scholar]
- 18.Veerareddy S., Cooke C. L., Baker P. N., Davidge S. T. (2002) Am. J. Physiol. Heart Circ Physiol. 283, H2226–H2233 [DOI] [PubMed] [Google Scholar]
- 19.Owens G. K. (2007) Novartis Found Symp. 283, 174–191 [DOI] [PubMed] [Google Scholar]
- 20.Matsumura F., Hartshorne D. J. (2008) Biochem. Biophys. Res. Commun. 369, 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Somlyo A. V. (2007) Circ. Res. 101, 645–647 [DOI] [PubMed] [Google Scholar]
- 22.Okamoto R., Kato T., Mizoguchi A., Takahashi N., Nakakuki T., Mizutani H., Isaka N., Imanaka-Yoshida K., Kaibuchi K., Lu Z., Mabuchi K., Tao T., Hartshorne D. J., Nakano T., Ito M. (2006) Cell. Signal. 18, 1408–1416 [DOI] [PubMed] [Google Scholar]
- 23.Machida H., Ito M., Okamoto R., Shiraki K., Isaka N., Hartshorne D. J., Nakano T. (2001) Biochim Biophys Acta. 1517, 424–429 [DOI] [PubMed] [Google Scholar]
- 24.Wu Y., Erdodi F., Murányi A., Nullmeyer K. D., Lynch R. M., Hartshorne D. J. (2003) J. Muscle Res Cell. Motil. 24, 499–511 [DOI] [PubMed] [Google Scholar]
- 25.Dirksen W. P., Vladic F., Fisher S. A. (2000) Am. J. Physiol. Cell. Physiol. 278, C589–C600 [DOI] [PubMed] [Google Scholar]
- 26.Borman M. A., Freed T. A., Haystead T. A., Macdonald J. A. (2009) Mol. Cell. Biochem. 327, 93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borman M. A., MacDonald J. A., Haystead T. A. (2004) FEBS Lett. 573, 207–213 [DOI] [PubMed] [Google Scholar]
- 28.Wooldridge A. A., Fortner C. N., Lontay B., Akimoto T., Neppl R. L., Facemire C., Datto M. B., Kwon A., McCook E., Li P., Wang S., Thresher R. J., Miller S. E., Perriard J. C., Gavin T. P., Hickner R. C., Coffman T. M., Somlyo A. V., Yan Z., Haystead T. A. (2008) J. Biol. Chem. 283, 11850–11859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishida H., Borman M. A., Ostrander J., Vogel H. J., MacDonald J. A. (2008) J. Biol. Chem. 283, 20569–20578 [DOI] [PubMed] [Google Scholar]
- 30.Champlin A. K., Dorr D. L., Gates A. H. (1973) Biol Reprod. 8, 491–494 [DOI] [PubMed] [Google Scholar]
- 31.Grüneberg H., Burnett J. B., Snell G. D. (1941) Proc. Natl. Acad. Sci. U.S.A. 27, 562–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirazi A., Iizuka K., Fadden P., Mosse C., Somlyo A. P., Somlyo A. V., Haystead T. A. (1994) J. Biol. Chem. 269, 31598–31606 [PubMed] [Google Scholar]
- 33.Akimoto T., Ribar T. J., Williams R. S., Yan Z. (2004) Am. J. Physiol. Cell. Physiol. 287, C1311–C1319 [DOI] [PubMed] [Google Scholar]
- 34.Lontay B., Kiss A., Gergely P., Hartshorne D. J., Erdodi F. (2005) Cell. Signal. 17, 1265–1275 [DOI] [PubMed] [Google Scholar]
- 35.Scotto-Lavino E., Garcia-Diaz M., Du G., Frohman M. A. (2009) J. Biol. Chem. 285, 6419–6424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moorhead G., Johnson D., Morrice N., Cohen P. (1998) FEBS Lett. 438, 141–144 [DOI] [PubMed] [Google Scholar]
- 37.Twomey E., Li Y., Lei J., Sodja C., Ribecco-Lutkiewicz M., Smith B., Fang H., Bani-Yaghoub M., McKinnell I., Sikorska M. (2009) Exp. Cell. Res. 316, 68–77 [DOI] [PubMed] [Google Scholar]
- 38.Weigel N. L., Bai W., Zhang Y., Beck C. A., Edwards D. P., Poletti A. (1995) J. Steroid Biochem. Mol. Biol. 53, 509–514 [DOI] [PubMed] [Google Scholar]
- 39.Rowan B. G., Garrison N., Weigel N. L., O'Malley B. W. (2000) Mol. Cell. Biol. 20, 8720–8730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coleman K. M., Dutertre M., El-Gharbawy A., Rowan B. G., Weigel N. L., Smith C. L. (2003) J. Biol. Chem. 278, 12834–12845 [DOI] [PubMed] [Google Scholar]
- 41.Aronica S. M., Katzenellenbogen B. S. (1993) Mol. Endocrinol. 7, 743–752 [DOI] [PubMed] [Google Scholar]
- 42.Bottinelli R., Reggiani C. (2000) Prog Biophys Mol. Biol. 73, 195–262 [DOI] [PubMed] [Google Scholar]
- 43.González B., Negredo P., Hernando R., Manso R. (2002) Pflugers Arch. 443, 377–386 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.