Abstract
Rab1a is a member of the Rab family of small GTPases with a well characterized function in the regulation of vesicle trafficking from the endoplasmic reticulum to the Golgi apparatus and within Golgi compartments. The integrin family heterodimeric transmembrane proteins serve as major receptors for extracellular matrix proteins, which play essential roles in cell adhesion and migration. Although effects on intracellular trafficking of integrins or other key cargos by Rab1a could influence cell migration, the regulatory mechanisms linking Rab1a to cell migration are not well understood. Here, we report identification of Rab1a as a novel regulator of cell migration using an unbiased RNAi screen targeting GTPases. Inhibition of Rab1a reduced integrin-mediated cell adhesion and spreading on fibronectins, reduced integrin β1 localization to lipid rafts, and decreased recycling of integrin β1 to the plasma membrane. Analysis of Rab1a effector molecules showed that p115 mediated Rab1a regulation of integrin recycling and lipid raft localization in cell migration. Taken together, these results suggest a novel function for Rab1a in the regulation of cell migration through controlling integrin β1 recycling and localization to lipid rafts via a specific downstream effector pathway.
Keywords: Cell Adhesion, Cell Migration, G Proteins, Integrin, Lipid Raft, Small G Protein
Introduction
Cell migration plays critical roles in a variety of biological and disease processes such as embryonic development, wound repair, inflammation, and cancer metastasis (1, 2). The integrin family heterodimeric transmembrane proteins serve as major receptors for extracellular matrix proteins, which play essential roles in cell adhesion and migration. Integrins function to link the extracellular matrix and intracellular actin cytoskeleton and are capable of mediating bidirectional signal transduction across the plasma membrane in migration and other cellular processes (3). Besides these relatively well understood functions of integrins, it is increasingly evident that cell-surface integrins undergo dynamic internalization and recycling processes, which may also play crucial roles in the regulation of cell migration (4, 5). The internalization of integrins is through a clathrin-independent pathway (6–8). The endocytosed integrins are subsequently degraded or recycled back to the plasma membrane through caveolin-1-positive structure or early endosomes, either directly or via a perinuclear recycling complex in a cell type- and stimulus-dependent manner (4).
Recent studies also suggested that cell-surface integrins can localize to lipid rafts on the plasma membrane (9–11). Lipid rafts are cholesterol- and sphingolipid-rich plasma membrane domains that contain a variety of proteins, including glycosylphosphatidylinositol-anchored proteins. They have been proposed to act as signaling platforms by recruiting distinct sets of signaling pathways whose core components display high affinity for the liquid-ordered environment of rafts (12). In migrating cells, lipid rafts are preferentially localized in the leading edges, where new sites of integrin-mediated adhesion to the extracellular matrix are forming (13). Furthermore, integrins have been shown to facilitate recruitment of Rac to lipid rafts, where it couples to the effectors to promote cell migration (11, 14). Nevertheless, the mechanisms controlling the intracellular trafficking of integrins and their localization to lipid rafts are still not well understood (15).
The small GTPases are major intracellular signaling molecules involved in the regulation of diverse cellular functions (1, 16). Members of the Rho subfamily small GTPases such as Rho, Rac, and Cdc42 have been well characterized to regulate cell migration directly through controlling dynamics of the actin cytoskeleton (17). The Rab and Arf subfamilies of small GTPases regulate protein endocytosis, exocytosis, and recycling and thus could also influence cell migration through their effects on intracellular trafficking of proteins, including integrins (18). Furthermore, several members of the Rab and Arf subfamilies have been shown to regulate trafficking of integrins directly in cell migration. Rab11a and Rab25, both members of the Rab11 family of GTPases, have been shown to regulate recycling of internalized integrin α5β1, which is dependent on at least one Rab11 effector molecule (19). Another Rab family member, Rab4, has been shown recently to regulate recycling of integrin αvβ3, but not integrin α5β1, from early endosomes in a “short loop” pathway stimulated by PDGF, without the involvement of Rab11 (20). Recent studies also showed that Arf6 can regulate recycling of integrin β1 together with Rab11 in a sequential manner (8). Despite extensive studies characterizing the role of individual GTPases in various functions, including cell migration (21), much remains to be learned about possible novel roles of other members of the small GTPase family in the regulation of cell migration, given the large size of the family.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
BSA, fibronectin, collagen type I, poly-l-lysine, streptavidin-agarose, protease inhibitor mixture, GSH, and cholera toxin B subunit (CTxB)4 were from Sigma. NHS-SS-biotin was from Pierce. Lipofectamine was from Invitrogen. The antibodies used were rabbit anti-HA, rabbit anti-Rab1a, rabbit anti-Fyn, goat anti-actin, and mouse anti-clathrin heavy chain (Santa Cruz Biotechnology, Santa Cruz, CA); mouse anti-Rac (Millipore); and rabbit anti-caveolin-1 (Cell Signaling Technologies, Danvers, MA). Rabbit anti-integrin β1 antibody was as described previously (42).
Cell Cultures
Drosophila S2 cells were incubated in insect medium (Invitrogen) at 30 °C with 95% humidity. HEK293 cells and MDA-MB-231 cells were cultured in DMEM (Invitrogen) with 10% FBS (Atlanta Biologicals) and 100 units/ml penicillin/streptomycin. NIH 3T3 cells were cultured in DMEM with 10% calf serum (Atlanta Biologicals) and 100 units/ml penicillin/streptomycin. HEK293 and NIH 3T3 cells were incubated in a 5% CO2 incubator with 95% humidity at 37 °C.
RNAi Screening
Drosophila S2 cells were treated with individual dsRNA of a collection of dsRNAs targeting 152 different Drosophila GTPases as described previously (22). Three days after the RNAi treatment, S2 cells were measured for their migration using Boyden chamber assays essentially as described previously (23), except that polycarbonate membranes with 5-μm pores (Neuro Probe, Inc.) were used because of the small size of S2 cells. The target GTPases whose knockdown by RNAi reduced migration of S2 cells at least by 2-fold were then subjected to two additional rounds of validation by RNAi, followed by Boyden chamber assays.
Preparation of Recombinant Lentiviruses and Infection of Mammalian Cells
The psPAX2 and pMD2G vectors and the pGIPZ lentiviral vectors (Open Biosystems) encoding shRNA targeting Rab1a, Rab9b, Arf4, Arl1, GM130, Golga5, or p115 were purchased through the University of Michigan shRNA Core Facility. HEK293 cells were transfected with 10 μg of pGIPZ lentiviral vector encoding each shRNA, 10 μg of psPAX2, and 5 μg of pMD2G by the calcium phosphate method according to the instructions recommended by the manufacturer. Twelve h after transfection, the media were replaced with DMEM containing 5% FBS. The conditioned media were then collected twice at 1-day intervals and combined. After centrifugation and filtration, the supernatant was used to infect HEK293, MDA-MB-231, and NIH 3T3 cells. In some experiments, the infected HEK293 and MDA-MB-231 cells were selected with 1 μg/ml puromycin in DMEM containing 10% FBS to obtain pools that stably expressed shRNA.
Plasmid DNA Construction and Transient Transfection of NIH 3T3 Cells
pEYFPC-Rab1a was kindly provided by Dr. Yanzhuang Wang (University of Michigan). DNA fragments were excised from the pEYFPC vector and cloned into pKH3 (43) to generate HA-tagged Rab1a and mutant S25N. The plasmid DNA was used for transient transfection of HEK293 and NIH 3T3 cells via Lipofectamine (Invitrogen) according to the manufacturer's instructions.
Cell Migration, Adhesion, and Spreading Assays for Mammalian Cells
Boyden chamber assays were performed to measure migration for both transiently transfected HEK293 cells and HEK293 cells with stable expression of various shRNA constructs using 8-μm pore polycarbonate membranes as described previously (23). For transiently transfected NIH 3T3 cells and stably transfected MDA-MB-231 cells, wound closure migration assays were carried out as described previously (44). Cell adhesion assays were performed as described previously (45). Spreading assays for transfected NIH 3T3 cells were performed as described previously (46) with slight modifications. Briefly, coverslips were coated with 10 μg/ml collagen I, 10 μg/ml fibronectin, or 0.1 mg/ml poly-l-lysine overnight at 4 °C. Cells were washed twice with PBS, trypsinized with 0.25% trypsin (Invitrogen), and kept in suspension in DMEM for 1 h. They were then seeded on the coated coverslips and incubated for 1 h in 5% CO2 with 95% humidity at 37 °C. The fraction of spread cells (i.e. phase-dark cells) was determined by viewing 10 random fields under a phase-contrast microscope.
Immunofluorescent Staining and Labeling with CTxB-FITC
Cells were fixed in 4% paraformaldehyde for 15 min and then permeabilized with 0.2% Triton X-100 in PBS for 5 min at room temperature. After blocking with 3% BSA in PBS for 2 h at room temperature, cells were incubated with primary antibodies diluted in 3% BSA overnight at 4 °C or for 2 h at room temperature. After washing twice with PBS for 10 min, the cells were incubated with Texas Red-conjugated secondary antibodies (1:200) for 1 h at room temperature. The coverslips were then mounted with SlowFade® antifade reagent (Invitrogen) and kept at 4 °C. In some experiments, cells were incubated with 1 μg/ml CTxB-FITC in PBS with 0.1% BSA at 4 °C for 20 min to stain lipid rafts.
Sucrose Gradient Fractionation
Detection of components of lipid rafts by sucrose gradient fraction was performed as described previously (47) with minor modification. Cells were washed twice with ice-cold PBS and once with Mes-buffered saline (25 mm Mes and 150 mm NaCl, pH 6.5). They were then resuspended in 2 ml of 1% Triton X-100 in Mes-buffered saline and incubated at 4 °C for 20 min. The solubilized cells were homogenized with 10 strokes of a Dounce homogenizer, and 1.5 ml of the homogenate was added to 2.5 ml of 70% (w/v) sucrose in Mes-buffered saline. The samples were overlaid successively with 5 ml of 30% sucrose and 3 ml of 5% sucrose. They were then centrifuged at 240,000 × g in a Beckman SW 41 Ti rotor for 18 h, and 12 1-ml fractions were collected from the top of the gradient. The pellet was resuspended and designated fraction 13. The fractions were then analyzed by Western blotting with various antibodies as described previously (48). In some experiments, the cells were solubilized in 0.5% Triton X-100 and 0.5% saponin instead of 1% Triton X-100.
Cell-surface Biotinylation, Endocytosis, and Recycling Assay
Cell-surface protein biotinylation and endocytosis assay were performed essentially as described previously (43), except that endocytosis was allowed to proceed for 30 min. To measure recycling of internalized proteins, the cells were further incubated at 37 °C for 15 min, followed by two successive GSH washes. The cells were then lysed, and the lysates were precipitated by streptavidin-agarose beads and analyzed by Western blotting with anti-integrin β1 antibody.
Experimental Metastasis Assay Using Tail Vein Injection
Tail vein injection in nude mice was performed as described previously (49). Briefly, 6 × 105 cells in 200 μl of DMEM were injected into the tail veins of nude mice. Four weeks after injection, the mice were killed, and lungs were removed. They were fixed in Bouin's solution, paraffin-embedded, and sectioned. After staining with hematoxylin and eosin, the samples were examined for metastatic nodules under a light microscope and quantified.
Statistical Analysis
Data are presented as means ± S.E. All statistical analysis was performed using GraphPad Prism Version 4.00 for Windows (GraphPad Software, San Diego, CA).
RESULTS
Identification of Rab1a as a Novel Regulator of Cell Migration
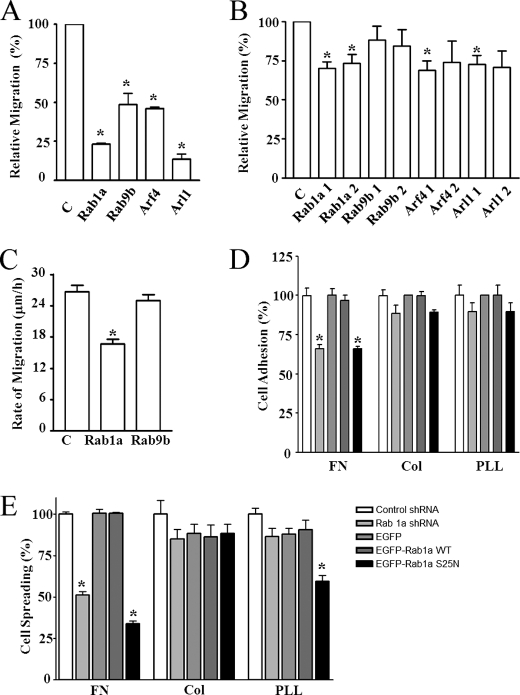
To identify new signaling molecules in the regulation of cell migration, we performed an RNAi screen of a collection of 152 annotated Drosophila GTPases using Drosophila S2 cells, taking advantage of the high RNAi efficiency of the system (22). Three rounds of screening and validation were performed as described under “Experimental Procedures.” Among those GTPases whose mammalian homologs are not known to function in cell migration, four fly GTPases were found to play a role in promoting cell migration, as their RNAi-mediated knockdown significantly reduced migration of Drosophila S2 cells (Fig. 1A). To evaluate a potential role of these genes in mammalian cells, we prepared mammalian expression vectors encoding shRNA targeting two different sequences for each of the mammalian orthologs of these four genes (human rab1a, rab9b, arf4, and arl1). HEK293 cells were transiently transfected with these vectors, and the effects on cell migration were measured using Transwell assays as described previously (23). Fig. 1B shows that Rab1a knockdown inhibited mammalian cell migration, whereas Rab9b knockdown did not. For Arf4 and Arl1, shRNAs targeting two different sequences in the same gene had different results. Similar to HEK293 cells, knockdown of Rab1a by transient transfection of shRNA vectors also significantly inhibited migration of mouse fibroblast NIH 3T3 cells (Fig. 1C). Taken together, these results identify Rab1a as a new regulatory molecule of cell migration in various mammalian cells as well as in Drosophila S2 cells.
FIGURE 1.
Identification of Rab1a as a novel regulatory protein for cell migration. Drosophila S2 cells (A) or HEK293 cells (B) were treated with siRNA targeting fly orthologs of Rab1a, Rab9b, Arf4, or Arl1 (A) or were transiently transfected with lentiviral plasmids encoding shRNA for these human small GTPases (two for each) (B) as indicated. Three days after, the cells were subjected to the Transwell migration assays as described under “Experimental Procedures.” The means ± S.E. of cell migration were determined from three independent experiments and normalized to those of cells treated with control siRNA (C). *, p < 0.05 in comparison with the value from control siRNA/shRNA-treated cells. C, NIH 3T3 cells were transiently transfected with lentiviral plasmids encoding GFP and shRNA for Rab1a, Rab9b, or a control sequence as indicated. Two days after transfection, a wound was generated in the cell monolayer to induce cell migration. The rate of cell migration was measured by quantifying the total distance that the positively transfected cells (GFP+) moved from the edge of the wound toward the center of the wound in 4 h. The mean ± S.E. from three independent experiments is shown. *, p < 0.01 in comparison with the value from cells transfected with control shRNA. D and E, NIH 3T3 cells were transiently transfected with lentiviral plasmids encoding shRNA for Rab1a or a control sequence or by pEGFP plasmids encoding Rab1a, the S25N mutant, or enhanced GFP (EGFP) as a control as indicated. Two days after transfection, the cells were suspended and replated on dishes coated with fibronectins (FN), collagen I (Col), or poly-l-lysine (PLL) for 1 h. The mean ± S.E. of cell adhesion (D) or spreading (E) from at least three experiments is shown. *, p < 0.05 in comparison with the value from cells transfected with control shRNA.
Regulation of Integrin-mediated Cell Adhesion by Rab1a
To investigate potential mechanisms of Rab1a regulation of cell migration, we examined whether knockdown of Rab1a can affect cell adhesion and spreading on extracellular matrix proteins. As shown in Fig. 1D, knockdown of Rab1a significantly inhibited cell adhesion on fibronectin compared with cells treated with the control shRNA. Interestingly, expression of Rab1a shRNA did not reduce adhesion of the cells on either collagen I or poly-l-lysine. We also measured the effect of overexpression of wild-type Rab1a or its mutants on cell adhesion. Overexpression of wild-type Rab1a did not affect cell adhesion on any of the substrates. However, the S25N mutant, which functions as a dominant-negative mutant to inhibit endogenous Rab1a (24), significantly decreased cell adhesion on fibronectin, but not on collagen I or poly-l-lysine. Similar results were obtained when cell spreading was measured, except that the S25N mutant also reduced cell spreading on poly-l-lysine (Fig. 1E). Taken together, these results suggest that Rab1a may regulate cell migration by affecting integrin-mediated cell adhesion and spreading on extracellular matrix protein fibronectin.
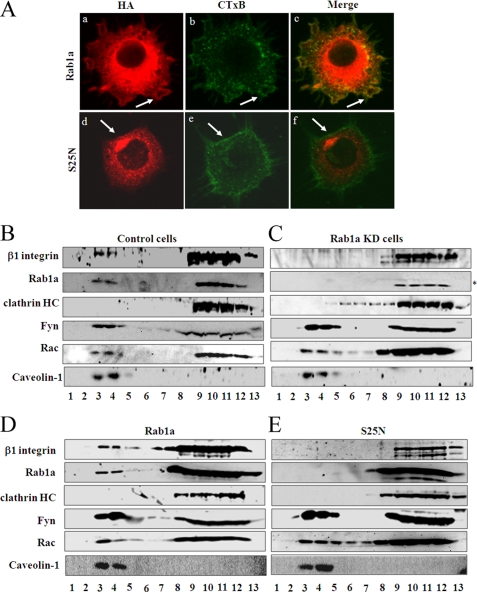
To explore a possible mechanism of regulation of integrins by Rab1a in cell adhesion and migration, we examined the subcellular localization of Rab1a in mammalian cells. Besides its well characterized localization in the endoplasmic reticulum, the Golgi, endoplasmic reticulum-Golgi intermediate components, and intracellular vesicles, we detected the presence of some Rab1a in the plasma membrane (Fig. 2A, panel a, arrow). Furthermore, co-staining of the cells with CTxB, a marker for lipid rafts (25), revealed a fraction of Rab1a localized in the lipid raft subdomain of the plasma membrane in a ruffle-like structure of migrating cells (Fig. 2A, panels a–c, arrows). Interestingly, the S25N mutant was absent in lipid rafts as indicated by CTxB staining (Fig. 2A, panels d–f). As previous studies have shown localization of several integrins, including β1, in lipid rafts of migrating cells (26, 27), the putative localization of Rab1a in lipid rafts as detected by immunofluorescent staining suggests that Rab1a may affect integrin β1 partition in lipid rafts to regulate cell migration.
FIGURE 2.
Localization of Rab1a and its regulation of integrin β1 localization to lipid rafts. A, NIH 3T3 cells were transiently transfected with pKH3 plasmids encoding HA-tagged Rab1a (panels a–c) or mutant S25N (panels d–f). They were then analyzed by immunofluorescent labeling using anti-HA antibody (panels a and d) or by staining with CTxB-FITC (panels b and e). The merged images are shown in panels c and f. B and C, control or Rab1a KD cells, respectively, were solubilized in 1% Triton X-100 and fractionated on a discontinuous sucrose gradient as described under “Experimental Procedures.” Equal volumes from each fraction were resolved by SDS-PAGE and analyzed by Western blotting using antibodies against integrin β1, Rab1a, clathrin, Fyn, Rac, and caveolin-1 as indicated. Fractions 3 and 4 represent lipid raft localization, and fractions 9–12 are non-rafts. The asterisk denotes a nonspecific band recognized by anti-Rab1a antibody. D and E, HEK293 cells were transiently transfected with pKH3 plasmids encoding HA-tagged Rab1a or mutant S25N, respectively, as indicated. They were then solubilized and analyzed as described for B. The data are representative of at least three experiments.
Partitioning of Integrins into Lipid Rafts Is Dependent on Rab1a
To study the potential regulation of integrin partitioning in lipid rafts by Rab1a, stable HEK293 cell pools expressing shRNA for Rab1a (Rab1a knockdown (KD) cells) or a control scrambled sequence (control cells) were established using lentiviral vectors as described under “Experimental Procedures.” The cells were solubilized and subjected to sucrose gradient fractionation, followed by Western blotting to detect the localization of various proteins in each fraction. As shown in Fig. 2B, lipid rafts were recovered in fractions 3 and 4 as indicated by the presence of Fyn, Rac, and caveolin-1 (proteins known to reside in lipid rafts) in these fractions. Integrin β1 was also present in lipid raft fractions in control cells as reported previously (26). Consistent with the immunofluorescent staining results (Fig. 2A), endogenous Rab1a was detected in the lipid raft fractions. In Rab1a KD cells, Rab1a was not detected in either lipid rafts or other fractions, as it was specifically depleted in these cells (Fig. 2C). Interestingly, the lipid raft localization (i.e. fractions 3 and 4) of integrin β1 was also eliminated in Rab1a KD cells, suggesting that partitioning of integrin β1 to lipid rafts is dependent on Rab1a. Knockdown of Rab1a in Rab1a KD cells did not disrupt the lipid rafts per se, as localization of other resident proteins such as Fyn, Rac, and caveolin-1 was not affected in these cells. The localization of clathrin heavy chain (a marker for proteins not localized in lipid rafts) in the Triton X-100-soluble fractions (fractions 9–12) was not affected by Rab1a depletion in Rab1a KD cells. As expected, the lipid raft localization of Rab1a, as well as integrin β1, Fyn, Rac, and caveolin-1, was abolished by treatment of cells with 0.5% saponin, which dissolved cholesterol and shifted all lipid raft components to fractions 9–12 (data not shown).
To complement the studies by Rab1a knockdown, we also examined the effect of the S25N mutant on lipid raft localization of integrin β1. Similar to the results from immunofluorescent staining, whereas ectopically expressed wild-type Rab1a was localized in the lipid raft fractions (Fig. 2D), the S25N mutant was absent in these fractions (Fig. 2E). Furthermore, expression of the S25N mutant also significantly inhibited integrin β1 partitioning into lipid rafts while not affecting localization of Fyn, Rac, and caveolin-1 (Fig. 2E). These results provide further support that lipid raft localization of integrin β1 is dependent on functional Rab1a. Together, the above data suggest that Rab1 may regulate cell migration through its control of integrin β1 localization to lipid rafts (28).
Regulation of Integrin β1 Recycling by Rab1a
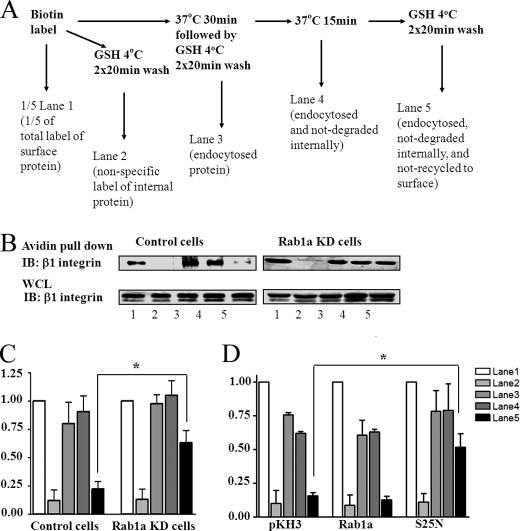
Integrins on the cell surface are dynamically regulated through continuous endocytosis from and recycling to the plasma membrane during cell migration (4, 29, 30). Rab family proteins play key roles in regulating intracellular trafficking of receptors, although a role for Rab1a in controlling integrin dynamics has not been described previously. To investigate the possible mechanisms by which Rab1a may regulate integrin β1 localization to lipid rafts, we examined the effect of Rab1a knockdown on the endocytosis and recycling of integrin β1 by pulse-chase analysis of surface-labeled proteins as outlined in Fig. 3A. As shown in Fig. 3B, a significant fraction of integrin β1 was endocytosed during the 30-min incubation in control cells (upper left panel, lane 3). Furthermore, the majority of endocytosed integrin β1 was recycled back to the cell surface after an additional 15-min incubation, as little remaining labeled integrin β1 was detected internally (upper left panel, lane 5). In Rab1a KD cells, a comparable amount of integrin β1 endocytosis was observed (upper right panel, lane 3), suggesting that endocytosis of integrin β1 is not significantly affected by depletion of Rab1a. In contrast, recycling of integrin β1 was greatly inhibited in Rab1a KD cells compared with control cells, as a significant amount of endocytosed integrin β1 was still protected from GSH wash after the additional 15-min incubation (upper right panel, lane 5). Quantification of data from multiple experiments indicated that ∼70% of endocytosed integrin β1 recycled back to the cell surface in control cells, but only ∼30% did so in Rab1a KD cells (Fig. 3C, compare lanes 3 and 5). There was no appreciable degradation of internalized integrin β1 in either control or Rab1a KD cells (i.e. a similar amount in Fig. 3, B and C, lanes 3 and 4), suggesting that the differential amount of integrin β1 remaining inside control and Rab1a KD cells is not caused by any difference in degradation in these two cells. Analysis of total integrin β1 at various times after inhibition of protein synthesis by cycloheximide showed that it is very stable in both control and Rab1a KD cells (data not shown). These results provide further support that the regulation of recycling of integrin β1 by Rab1a is not due to any alteration of its degradation.
FIGURE 3.
Regulation of integrin β1 recycling by Rab1a. A, shown is the experimental scheme. B, control (left panels) or Rab1a KD (right panels) cells were labeled by surface biotinylation and then processed as outlined in A. The labeled proteins were precipitated by streptavidin (upper panels) and analyzed by Western blotting with antibodies against integrin β1. An aliquot of total lysates from each fraction were also analyzed directly by Western blotting with antibodies against integrin β1 (whole cell lysate (WCL); lower panels). IB, immunoblot. C, the biotinylated integrin β1 bands in B (upper panels) were quantified by densitometry from three independent experiments, and the mean ± S.E. of relative intensity is shown. *, p < 0.01 compared with the value in control cells. D, HEK293 cells were transiently transfected with pKH3 vectors encoding HA-tagged Rab1a, mutant S25N, or vector alone as a control. They were then labeled by surface biotinylation and analyzed as described for C. *, p < 0.01 compared with the value in cells transfected with the pKH3 vector alone.
We also carried out a similar analysis of the effect of ectopic expression of wild-type Rab1a and the S25N mutant on integrin β1 recycling. Fig. 3D shows that whereas expression of wild-type Rab1a did not affect any aspect of integrin β1 dynamics, expression of the S25N mutant inhibited recycling of integrin β1 back to the cell surface without significant perturbation of its endocytosis. Together, these data suggest that Rab1a is required for efficient recycling of integrin β1, which may be necessary for lipid raft localization and promotion of cell spreading and migration.
Regulation of Integrin β1 Partitioning to Lipid Rafts and Recycling to the Cell Surface by the Rab1a Effector p115
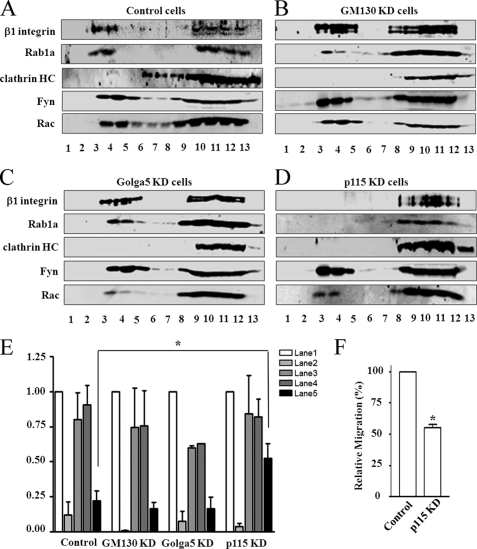
A number of downstream effectors of Rab1a have been described, including GM130, Golga5, and p115 (31–33). To study whether Rab1a regulates cell migration through one or more of these effectors, stable HEK293 cell pools expressing shRNAs for GM130 (GM130 KD cells), Golga5 (Golga5 KD cells), and p115 (p115 KD cells) were established using lentiviral vectors as described under “Experimental Procedures.” The effects on integrin β1 localization to lipid rafts and recycling to the cell surface were then examined in these cells. Similar to control cells (Fig. 4A), integrin β1 was detected in both lipid rafts and non-raft fractions in GM130 KD and Golga5 KD cells (Fig. 4, B and C). In contrast, localization of integrin β1 to lipid rafts was significantly reduced in p115 KD cells (Fig. 4D). Interestingly, Rab1a localization to lipid rafts was also abolished in p115 KD cells, but not in GM130 KD or Golga5 KD cells. Analysis of integrin β1 dynamics in these cells showed that recycling of integrin β1 to the cell surface was inhibited in p115 KD cells, whereas a similar extent of integrin β1 recycling was found in GM130 KD and Golga5 KD cells compared with that in control cells (Fig. 4E). Consistent with its effect on integrin β1 recycling and localization to lipid rafts, knockdown of p115 also reduced cell migration in p115 KD cells compared with control cells (Fig. 4F). Together, these results identify p115 as a key mediator of Rab1a in the regulation of integrin β1 recycling and localization to lipid rafts in cell migration.
FIGURE 4.
Regulation of integrin dynamics and cell migration by Rab1a effectors. Control (A), GM130 KD (B), Golga5 KD (C), or p115 KD (D) cells were solubilized in 1% Triton X-100 and analyzed as described in the legend to Fig. 2. HC, heavy chain. E, control, GM130 KD, Golga5 KD, and p115 KD cells were labeled by surface biotinylation and analyzed as described in the legend to Fig. 3C. *, p < 0.01 compared with the value in control cells. F, control and p115 KD cells were subjected to Transwell migration assays as described under “Experimental Procedures.” The means ± S.E. of migration were determined from three independent experiments and normalized to those of control cells. *, p < 0.01 in comparison with the value from control cells.
Inhibition of Cell Migration by Rab1a Depletion Reduces Cancer Cell Metastasis in Vivo
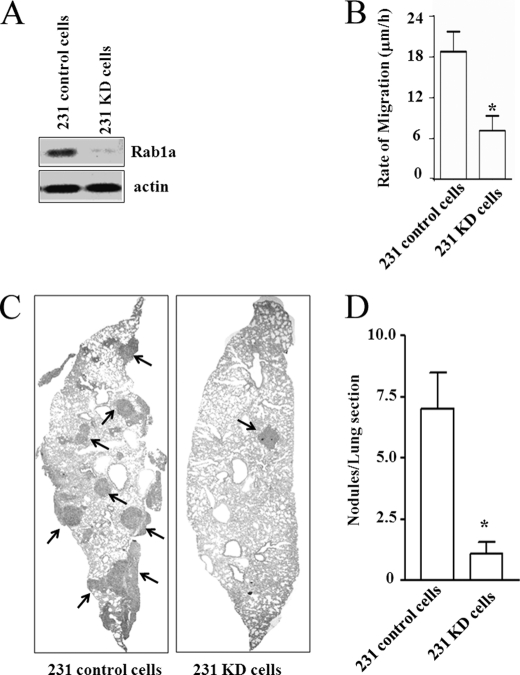
To evaluate a potential role of Rab1a regulation of cell migration in vivo, we also created stable human breast cancer cells (MDA-MB-231) expressing Rab1a or control shRNA (designated 231 KD and 231 control cells, respectively). Similar to the observations obtained with 293 and 3T3 cells (see Fig. 1), knockdown of Rab1a also reduced migration of MDA-MB-231 cells (Fig. 5, A and B). We then injected 231 KD or 231 control cells into the tail veins of recipient nude mice and monitored for metastasis to the lungs, as migration of tumor cells is critical for cancer metastasis. Numerous metastatic nodules were found in the lungs of recipient mice injected with 231 control cells, but only few were detected in mice injected with 231 KD cells (Fig. 5C). Quantification of the metastatic nodules in lung sections indicated an ∼7-fold reduction in the metastatic activity of 231 KD cells compared with 231 control cells (Fig. 5D). These results provide further support for Rab1a regulation of cell migration through controlling integrin dynamics in physiological and disease processes.
FIGURE 5.
Regulation of cell migration and metastasis by Rab1a in human breast cancer cells. A, lysates were prepared from 231 control and 231 KD cells and analyzed by Western blotting using antibodies against Rab1a (upper panel) and actin (lower panel). B, 231 control and 231 KD cells were subjected to wound closure assays as described in the legend to Fig. 1C. *, p < 0.01 compared with the value in 231 control cells. C and D, 231 control and 231 KD cells were injected into the tail veins of nude mice as described under “Experimental Procedures.” Lung sections were prepared 4 weeks after injection and stained with hematoxylin and eosin, and the micrometastatic nodules were quantitated under a microscope. C shows representative images of a lung section, and D shows the mean ± S.E. of nodules/lung section from multiple animals (n ≥ 5). *, p < 0.01 compared with the value in 231 control cells.
DISCUSSION
In this work, we have identified a novel function for Rab1a in cell migration using an unbiased RNAi-mediated gene knockdown screening of GTPases. Although proper control of intracellular protein trafficking is likely crucial in the regulation of cell migration, this is the first report directly demonstrating a specific role for Rab1a in cell migration. We further demonstrated that Rab1a regulated cell migration through controlling integrin β1 recycling and localization to lipid rafts via its downstream effector p115.
Recent studies suggest that partitioning of integrin in lipid rafts is important for its functions in mediating signaling and regulation of cellular events such as migration (10, 12, 27, 28). Interestingly, we demonstrated the localization of a fraction of Rab1a in lipid rafts of the plasma membrane, in addition to its well characterized localization in the Golgi (24). This observation raised the possibility that Rab1a regulates integrin localization and intracellular dynamics directly to control cell migration. In support of this hypothesis, we found that either knockdown of Rab1a or expression of dominant-negative Rab1a mutant S25N abolished the localization of integrin β1 in lipid rafts. Moreover, inhibition of Rab1a did not affect the localization of other resident proteins such as caveolin-1, Rac, and Fyn, suggesting that Rab1a regulates cell migration through its specific effect on integrin β1 localization to lipid rafts rather than by disrupting the structure of lipid rafts per se, which are critical for cell migration also (34). Interestingly, Arf6, another small GTPase involved in endosome recycling (35), has been shown to regulate trafficking of the lipid raft marker GM1 and the recruitment of Rac1 to lipid rafts (36). It is unclear, however, whether Arf6 may also affect integrin localization to lipid rafts to modulate cell migration. Nevertheless, these results suggest that different small GTPases could regulate different components of lipid rafts and possibly affect different cellular processes.
Previous studies have shown that surface integrins undergo dynamic endocytosis and recycling processes during cell migration and that integrin recycling is essential for sustained cell migration (4, 29). Our analysis of each of these steps in cells with either knockdown of Rab1a or expression of dominant-negative mutant Rab1a showed that inhibition of Rab1a significantly reduced recycling of integrin β1 but did not affect endocytosis of integrin β1 or its total level on the cell surface. Consistent with the lack of an effect on its endocytosis, inhibition of Rab1a did not affect the endosome localization of integrin β1 (data not shown). Furthermore, we did not observe significant degradation of internalized integrin β1 either with or without inhibition of Rab1a, consistent with previous observations that the internalized integrins traffic through a recycling endosomal pathway rather than a late endosomal/lysosomal system (37). One possible explanation for the lack of change in the total level of surface integrin β1 is that only a small fraction of the integrin is undergoing endocytosis and recycling. This pool of integrins could be those localized in lipid rafts only that play more critical roles in cell migration. Alternatively, it is possible that internalization of integrin β1 is from the pool of total surface integrin β1 but that the recycling process is specifically targeted to lipid rafts, which subsequently diffuse to the bulk part of the plasma membranes. Our results showing reduction in both recycling of integrin and its localization in lipid rafts, but not other steps in the dynamics, are consistent with the idea of integrin β1 recycling to the lipid rafts on the surface in a Rab1a-dependent manner. Further support for this possibility is provided by observations that disruption of lipid rafts by methyl-β-cyclodextrin reduces integrin endocytosis (28) and inhibited cell migration without a significant effect on the total surface level of integrins (data not shown).
A number of other small GTPases, including Rab4, Rab11, and Arf6, have been shown to regulate recycling of integrins to the cell surface (8, 18, 20, 38). It will be interesting to determine whether or not they also affect integrin localization to lipid rafts on the plasma membrane. If so, this will provide further support for the possibility that integrins are recycled to the surface in lipid rafts. On the other hand, these studies may reveal that some or all of them will not affect integrin localization in lipid rafts. Such a scenario would suggest that recycling and lipid raft localization are separate events (i.e. integrin recycling is targeted to the bulk plasma membrane, and integrin localization to lipid rafts within the plasma membrane is controlled by other mechanisms). This would suggest that Rab1 regulates both recycling to the membrane and lipid raft localization of integrin β1 but through distinct mechanisms.
Like other small GTPases, Rab1a exerts its regulatory functions through multiple effector molecules (32, 33, 39). Our data suggest that Rab1a regulates integrin recycling and localization to lipid rafts through its effector p115, but not GM130 or Golga5. p115 has been shown to regulate other intracellular trafficking as an effector of Rab1a, including the major function of Rab1a in endoplasmic reticulum-to-Golgi trafficking as well as transcytosis. Examination of multiple effectors of Rab1a suggested that p115 is the only known Rab1a effector in the regulation of transport of polymeric IgA receptor from basolateral membranes to the apical plasma membrane (40). p115 has also been shown to regulate the transport of GLUT4 after insulin stimulation from perinuclear recycling complex to the plasma membrane (41). In the best characterized trafficking pathway mediated by Rab1a, p115 was shown to directly tether a set of SNARE (soluble NSF attachment protein receptor) proteins on COPII vesicles, thus facilitating vesicle fusion (33). Although SNARE proteins such as SNAP25 are present in lipid rafts, it remains to be determined whether p115 regulates integrin recycling to lipid rafts through its interaction with SNARE proteins and facilitation of the fusion events there.
Acknowledgments
We are grateful to Dr. Yanzhuang Wang for the generous gifts of plasmids pEYFPC-Rab1a and pEYFPC-S25N. We thank Ming Luo, Huei Jin Ho, Xiaofeng Zhao, Huijun Wei, Fei Liu, Becky Bae, and Shaogang Sun for critical reading of the manuscript and helpful comments.
This work was supported, in whole or in part, by National Institutes of Health grant HL073394 (to J.-L. G.).
- CTxB
- cholera toxin B subunit
- KD
- knockdown.
REFERENCES
- 1.Jaffe A. B., Hall A. (2005) Annu. Rev. Cell Dev. Biol. 21, 247–269 [DOI] [PubMed] [Google Scholar]
- 2.Ridley A. J., Schwartz M. A., Burridge K., Firtel R. A., Ginsberg M. H., Borisy G., Parsons J. T., Horwitz A. R. (2003) Science 302, 1704–1709 [DOI] [PubMed] [Google Scholar]
- 3.Hynes R. O. (2002) Cell 110, 673–687 [DOI] [PubMed] [Google Scholar]
- 4.Caswell P. T., Norman J. C. (2006) Traffic 7, 14–21 [DOI] [PubMed] [Google Scholar]
- 5.Jones M. C., Caswell P. T., Norman J. C. (2006) Curr. Opin. Cell Biol. 18, 549–557 [DOI] [PubMed] [Google Scholar]
- 6.Ng T., Shima D., Squire A., Bastiaens P. I., Gschmeissner S., Humphries M. J., Parker P. J. (1999) EMBO J. 18, 3909–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weigert R., Yeung A. C., Li J., Donaldson J. G. (2004) Mol. Biol. Cell 15, 3758–3770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powelka A. M., Sun J., Li J., Gao M., Shaw L. M., Sonnenberg A., Hsu V. W. (2004) Traffic 5, 20–36 [DOI] [PubMed] [Google Scholar]
- 9.Krauss K., Altevogt P. (1999) J. Biol. Chem. 274, 36921–36927 [DOI] [PubMed] [Google Scholar]
- 10.Leitinger B., Hogg N. (2002) J. Cell Sci. 115, 963–972 [DOI] [PubMed] [Google Scholar]
- 11.Del Pozo M. A. (2004) Cell Cycle 3, 725–728 [PubMed] [Google Scholar]
- 12.Simons K., Toomre D. (2000) Nat. Rev. Mol. Cell Biol. 1, 31–39 [DOI] [PubMed] [Google Scholar]
- 13.Mañes S., Mira E., Gómez-Moutón C., Lacalle R. A., Martínez C. (2000) IUBMB Life 49, 89–96 [DOI] [PubMed] [Google Scholar]
- 14.del Pozo M. A., Alderson N. B., Kiosses W. B., Chiang H. H., Anderson R. G., Schwartz M. A. (2004) Science 303, 839–842 [DOI] [PubMed] [Google Scholar]
- 15.Huttenlocher A. (2005) Nat. Cell Biol. 7, 336–337 [DOI] [PubMed] [Google Scholar]
- 16.Bar-Sagi D., Hall A. (2000) Cell 103, 227–238 [DOI] [PubMed] [Google Scholar]
- 17.Hall A. (1998) Science 279, 509–514 [DOI] [PubMed] [Google Scholar]
- 18.Nielsen E., Cheung A. Y., Ueda T. (2008) Plant Physiol. 147, 1516–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caswell P. T., Spence H. J., Parsons M., White D. P., Clark K., Cheng K. W., Mills G. B., Humphries M. J., Messent A. J., Anderson K. I., McCaffrey M. W., Ozanne B. W., Norman J. C. (2007) Dev. Cell 13, 496–510 [DOI] [PubMed] [Google Scholar]
- 20.Roberts M., Barry S., Woods A., van der Sluijs P., Norman J. (2001) Curr. Biol. 11, 1392–1402 [DOI] [PubMed] [Google Scholar]
- 21.Nobes C. D., Hall A. (1999) J. Cell Biol. 144, 1235–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim E., Goraksha-Hicks P., Li L., Neufeld T. P., Guan K. L. (2008) Nat. Cell Biol. 10, 935–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen T. L., Park A. Y., Alcaraz A., Peng X., Jang I., Koni P., Flavell R. A., Gu H., Guan J. L. (2005) J. Cell Biol. 169, 941–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nuoffer C., Davidson H. W., Matteson J., Meinkoth J., Balch W. E. (1994) J. Cell Biol. 125, 225–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nichols B. J., Kenworthy A. K., Polishchuk R. S., Lodge R., Roberts T. H., Hirschberg K., Phair R. D., Lippincott-Schwartz J. (2001) J. Cell Biol. 153, 529–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holleran B. J., Barbar E., Payet M. D., Dupuis G. (2003) J. Leukocyte Biol. 73, 243–252 [DOI] [PubMed] [Google Scholar]
- 27.Fabbri M., Di Meglio S., Gagliani M. C., Consonni E., Molteni R., Bender J. R., Tacchetti C., Pardi R. (2005) Mol. Biol. Cell 16, 5793–5803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vassilieva E. V., Gerner-Smidt K., Ivanov A. I., Nusrat A. (2008) Am. J. Physiol. Gastrointest. Liver Physiol. 295, G965–G976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellinen T., Ivaska J. (2006) J. Cell Sci. 119, 3723–3731 [DOI] [PubMed] [Google Scholar]
- 30.Caswell P., Norman J. (2008) Trends Cell Biol. 18, 257–263 [DOI] [PubMed] [Google Scholar]
- 31.Nakamura N., Rabouille C., Watson R., Nilsson T., Hui N., Slusarewicz P., Kreis T. E., Warren G. (1995) J. Cell Biol. 131, 1715–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satoh A., Wang Y., Malsam J., Beard M. B., Warren G. (2003) Traffic 4, 153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allan B. B., Moyer B. D., Balch W. E. (2000) Science 289, 444–448 [DOI] [PubMed] [Google Scholar]
- 34.Mañes S., Viola A. (2006) Mol. Membr. Biol. 23, 59–69 [DOI] [PubMed] [Google Scholar]
- 35.D'Souza-Schorey C., van Donselaar E., Hsu V. W., Yang C., Stahl P. D., Peters P. J. (1998) J. Cell Biol. 140, 603–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Balasubramanian N., Scott D. W., Castle J. D., Casanova J. E., Schwartz M. A. (2007) Nat. Cell Biol. 9, 1381–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh R. N., Mallet W. G., Soe T. T., McGraw T. E., Maxfield F. R. (1998) J. Cell Biol. 142, 923–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collins R. N. (2003) Mol. Membr. Biol. 20, 105–115 [DOI] [PubMed] [Google Scholar]
- 39.Moyer B. D., Allan B. B., Balch W. E. (2001) Traffic 2, 268–276 [DOI] [PubMed] [Google Scholar]
- 40.Sztul E., Colombo M., Stahl P., Samanta R. (1993) J. Biol. Chem. 268, 1876–1885 [PubMed] [Google Scholar]
- 41.Hosaka T., Brooks C. C., Presman E., Kim S. K., Zhang Z., Breen M., Gross D. N., Sztul E., Pilch P. F. (2005) Mol. Biol. Cell 16, 2882–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcantonio E. E., Hynes R. O. (1988) J. Cell Biol. 106, 1765–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu X., Gan B., Yoo Y., Guan J. L. (2005) Dev. Cell 9, 185–196 [DOI] [PubMed] [Google Scholar]
- 44.Liang C. C., Park A. Y., Guan J. L. (2007) Nat. Protocols 2, 329–333 [DOI] [PubMed] [Google Scholar]
- 45.Wu C., Chung A. E., McDonald J. A. (1995) J. Cell Sci. 108, 2511–2523 [DOI] [PubMed] [Google Scholar]
- 46.Yoo Y., Wu X., Egile C., Li R., Guan J. L. (2006) J. Biol. Chem. 281, 15352–15360 [DOI] [PubMed] [Google Scholar]
- 47.Chamberlain L. H., Burgoyne R. D., Gould G. W. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 5619–5624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu X., Yoo Y., Okuhama N. N., Tucker P. W., Liu G., Guan J. L. (2006) Nat. Cell Biol. 8, 756–763 [DOI] [PubMed] [Google Scholar]
- 49.Hauck C. R., Hsia D. A., Puente X. S., Cheresh D. A., Schlaepfer D. D. (2002) EMBO J. 21, 6289–6302 [DOI] [PMC free article] [PubMed] [Google Scholar]