Abstract
A role for histone H2AX, one of the variants of the nucleosome core histone H2A, has been demonstrated in DNA repair, tumor suppression, apoptosis, and cell cycle checkpoint function. However, the physiological function and post-translational modification of histone H2AX during vertebrate development have not been elucidated. Here, we provide evidence showing that Xenopus histone H2AX (XH2AX) has a role in the anterior neural plate for eye field formation during Xenopus embryogenesis. A loss-of-function study clearly demonstrated a critical role of XH2AX in anterior neural specification. Through a differentiation assay with Xenopus animal cap embryonic stem cells, we confirmed that XH2AX is required for the activin-induced anterior neural specification of the ectoderm. Furthermore, we found that Chk1 is an essential kinase to phosphorylate histone XH2AX at Thr16, which is involved in the biological function of this histone. Taken together, our findings reveal that XH2AX has a specific role in anterior neural formation of Xenopus, which is mediated through phosphorylation of XH2AX at Thr16 by Chk1.
Keywords: Development, Histones, Protein Phosphorylation, Threonine/Serine Protein Kinase, Xenopus, Chk1, XH2AX, Anterior Neural Development
Introduction
The basic unit of chromatin is the nucleosome, typically composed of an octamer of the four core histones H2A, H2B, H3, and H4 (1). Histones are highly evolutionarily conserved proteins with a globular domain composed of a nucleosome core and tail motif in both the C- and N-terminal regions, which are targets for post-translational modification such as phosphorylation, acetylation, methylation, and ubiquitination (2–5). Recent studies indicated that covalent modification of some histone variants results in specialized biological functions beyond canonical function, such as the packaging of DNA into nucleosomes (6–9).
In particular, the specialized biological functions of histone H2AX have been studied extensively. Histone H2AX is a variant of the histone H2A family and has an extended C-terminal tail with an evolutionary conserved SQ motif. A functional role of histone H2AX phosphorylation in the SQ motif (γ-H2AX) induced by external genotoxic agents was demonstrated in DNA repair, cell cycle checkpoints, regulated gene recombination events, and tumor suppression (10–14).
During Xenopus laevis embryogenesis, one of the most important processes is gastrulation. Gastrulation converts the embryonic ball into the three layers (ectoderm, mesoderm, and endoderm) and establishes definitive anteroposterior and dorsoventral axes. Neural specification of anteroposterior patterning is generated on the basis of the neural ectoderm and requires inductive interaction between the dorsal mesoderm and presumptive neuroectoderm. The initial basal state of the neural ectoderm comprises the anterior part, and additional factors are required to generate the posterior portions of the nervous system (15–17). In particular, the eye tissue is derived from the anterior neural tissue forebrain. The early eye field is characterized by expression of several marker genes, including the homeobox transcription factors Rx1 and Otx2 and the paired box transcription factor Pax6 (15–18).
The involvement of histone variants in the X. laevis development process has been reported previously. For example, histone variants of the H1 linker histone were reported to control embryonic gene expression patterns in X. laevis. The depletion of histone H2AZ, a variant of histone H2A, perturbs gastrulation, leading to embryos with malformed, shortened trunks (19–22). Recently, a role for histone modification in X. laevis development was reported (23, 24). Perturbation of histone H3 lysine 4 methylation in X. laevis resulted in a mesodermal and endodermal patterning defect (23). Histone H2A deubiquitination mediates embryonic anteroposterior patterning through the regulation of hox gene expression (24). These results thus suggest that general chromatin components are involved in pattern formation processes during early X. laevis development.
However, to date, the physiological function of histone H2AX in vertebrate development has not yet been elucidated. Moreover, the role of histone H2AX post-translational modification in vertebrate development is unexplored. In this study, we report the function of histone H2AX in anterior neural development of X. laevis as a developmental model of vertebrates. Notably, we identified Chk1 (checkpoint kinase 1) as a functional kinase of histone H2AX Thr16 and this phosphorylation might be important for the function of histone H2AX in anterior neural development. Taken together, our results suggest a novel embryonic function of histone proteins, as well as Chk1 as a novel histone H2AX-modifying kinase in X. laevis.
EXPERIMENTAL PROCEDURES
Embryonic Manipulation, Microinjection, and RNA Analysis
X. laevis embryos were obtained by artificial fertilization. Vitelline membranes were removed by immersing embryos in a 2% cysteine solution (pH 8). Embryos at the one- or two-cell stage were injected in the animal pole with mRNA as described in the figure legends. Animal caps, the area around the animal (pigmented) pole of the blastula embryos, were dissected from the injected embryos at stages 8–9 and cultured to various stages in 67% Leibovitz's L-15 medium (Invitrogen, Carlsbad, CA) containing 1 mg/ml BSA, 7 mm Tris-HCl (pH 7.5), and 50 μg/ml gentamycin for 1 or 2 days. Activin was added to l-15 medium (25–27). For RT-PCR, total RNA was extracted from whole embryos or cultured explants using TRIzol reagent (Tel-Test, Inc., Friendswood, TX) following the manufacturer's instructions. RT-PCR primers and cycling conditions are described at the Xenopus Molecular Marker Resource (XMMR, University of Texas). The morpholino antisense oligonucleotide (Gene Tools, LLC, Philomath, OR) directed against Xenopus histone H2AX (referred to as XH2AX-MO)2 was TGACGGCTTTTCCTCTGCCCGACAT, and its mismatching control (Control-MO) was TcACGGgTTTTCgTCTcCCCGAgAT. The morpholino antisense oligonucleotide of Xenopus Chk1 was 5′-TTCAACAAACGGAACTGCCATTTTG-3′. In situ hybridization was performed with digoxigenin-labeled probes as described previously (20).
Plasmid Constructs
For expression in Xenopus embryos, an XH2AX cDNA (Swiss-Prot accession number Q6GM86) was screened by RT-PCR using stage 11 embryos and cloned into the pCS2-FLAG vector at the EcoRI/XbaI restriction sites. To examine XH2AX-MO specificity, the XH2AX cDNA was cloned into the pCS2-HA vector at the BamHI/EcoRV sites. For rescue experiments, the XH2AX region (amino acid residues 1–8) targeted by morpholino was deleted by PCR and cloned into the pCS2-FLAG vector at the EcoRI/XbaI sites (referred to as FLAG-XH2AX-8aa). The Xenopus Chk1 cDNA (cDNA clone MGC 79140) was cloned into pCS2-FLAG or the HA vector at the EcoRI/XbaI or BamHI/EcoRV sites. The T16A mutant of histone XH2AX was generated using the QuikChange II site-directed mutagenesis kit (Stratagene). To make the GST fusion protein, full-length XH2AX, an N-terminal fragment of XH2AX (amino acids 1–39), the same N-terminal fragment with the T16A mutant, or a C-terminal fragment of XH2AX (amino acids 102–138) was cloned into the pGEX vector at the BamHI/XhoI sites.
Immunoprecipitation and Western Blot Analysis
The embryonic lysates were homogenized in 50 mm Tris (pH 7.4), 150 mm NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 0.1% SDS, 50 mm NaF, and 1 mm Na3VO4 containing 1 mm PMSF, 15 mm β-glycerophosphate, and 1× proteinase inhibitor mixture (Calbiochem) and then used for Western blotting and immunoprecipitation. The protein bands were visualized using ECL reagent (Amersham Biosciences).
Antibodies
For Western blotting, peroxidase-conjugated monoclonal anti-FLAG M2 (Sigma), peroxidase-conjugated anti-HA (Roche), anti-phosphothreonine (Cell Signaling, Danvers, MA), and monoclonal anti-actin (Sigma) antibodies were used. Immunoprecipitation experiments were performed by incubating extracts overnight with monoclonal anti-HA and anti-Myc antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). The anti-phospho-Thr16 XH2AX antibody was obtained by immunizing rabbits against peptide RGKAVSKTRAKAK(pT)RSSRAG, followed by purification using a column linked to the non-phosphorylated peptide and a final purification using a column linked to the phosphorylated peptide (Bio-Synthesis Inc., Lewisville, TX).
In Vitro Kinase Assays
The indicated GST-XH2AX proteins (25 ng) were mixed together with the active Chk1 or Chk2 protein (Upstate Biotechnology), 0.2 mm ATP, and 1× kinase buffer (Invitrogen) and incubated at 30 °C for 30 min. Equal amounts of reactive products were divided into two portions for Western blotting and Coomassie Brilliant Blue staining.
Embryos were injected with the mRNA of FLAG-Chk1. Each of 30 embryos expressing FLAG-Chk1 was homogenized on ice in lysis buffer (50 mm Tris (pH 7.5), 100 mm NaCl, 2 mm DTT, 10% glycerol, 0.1% Nonidet P-40, and protease inhibitor including phosphatase inhibitors (Calbiochem)) and cleared by centrifugation at 14,000 rpm. Cell extracts were immunoprecipitated with anti-FLAG antibody (Sigma) at 4 °C. After two or three washes with lysis buffer and one wash with kinase buffer (10 mm Tris (pH 7.5), 10 mm MgCl2, 1 mm DTT, 1 mm Na3VO4, and 1 mm NaF), the kinase reaction was performed as described above.
RESULTS
Temporal and Spatial Expression Pattern of XH2AX
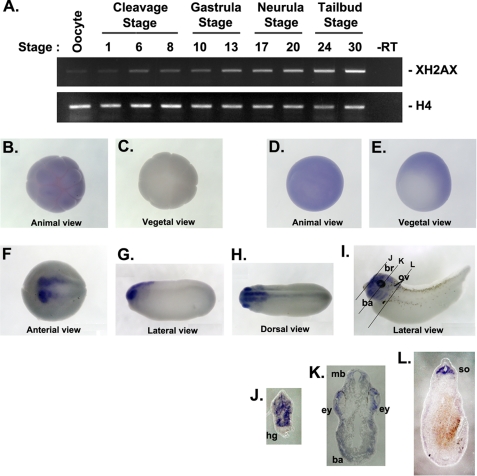
We first determined the spatiotemporal expression pattern of XH2AX during X. laevis development to investigate a potential role for this histone. Using RT-PCR, we analyzed the temporal expression of XH2AX mRNA throughout embryogenesis. Temporally, XH2AX is expressed both maternally and zygotically throughout early development. XH2AX expression is markedly increased from the early neurula stage to the tail bud stage (Fig. 1A). To examine the spatial expression pattern of XH2AX, we performed whole-mount in situ hybridization during the embryonic stages. The unfertilized Xenopus egg is polarized along the animal to vegetal axis before fertilization, as shown by the pigmentation of the animal hemisphere and the presence of larger yolk platelets in the vegetal hemisphere. Spatially, XH2AX is detected in the animal hemisphere (Fig. 1B) and not in the vegetal hemisphere of the embryo during cleavage (Fig. 1C). During gastrulation, XH2AX is broadly detected in the animal hemisphere (Fig. 1D) throughout the marginal zone except the blastopore region (Fig. 1E). At the neurula and tail bud stages, XH2AX shows a restricted expression pattern in the anterior region containing the anterior central nervous system (Fig. 1, F and G). At later stages, XH2AX is localized in the brain, eye, otic vesicle, and branchial arches (Fig. 1, H and I). Transverse sections of embryos stained for H2AX show restricted XH2AX mRNA expression into the edge of the anterior region except for the hatching gland (Fig. 1J); anterior neural tissue, including eye, brain, and branchial arches (Fig. 1K); and the somite region (Fig. 1L). This result suggests that XH2AX might have a potential role in anterior neural development.
FIGURE 1.
Temporal and spatial expression profile of XH2AX during embryogenesis. A, RT-PCR analysis of temporal mRNA expression of XH2AX. Developmental stages are indicated above each lane. Histone H4 served as a loading control. −RT, control reaction without reverse transcriptase. B–I, whole-mount in situ hybridization showing the spatial expression of XH2AX during early Xenopus development. B and C, blastula stage: animal hemisphere view (B) and vegetal hemisphere view (C); D and E, gastrula stage: animal hemisphere view (D) and vegetal hemisphere view (E); F, neurula stage: anterior view with posterior right; G and H, tail bud stage: lateral view with anterior left (G) and dorsal view with anterior left (H); I, stages 33–34. The black lines represent the angle of sectioning for J–L. J–L, transverse section through a stage 33–34 embryo stained by whole-mount in situ hybridization for XH2AX mRNA. ba, branchial arches; br, brain; ey, eye; hg, hatching gland; mb, midbrain; ov, otic vesicle; so, somite.
Depletion of XH2AX Causes Defective Anterior Neural Development
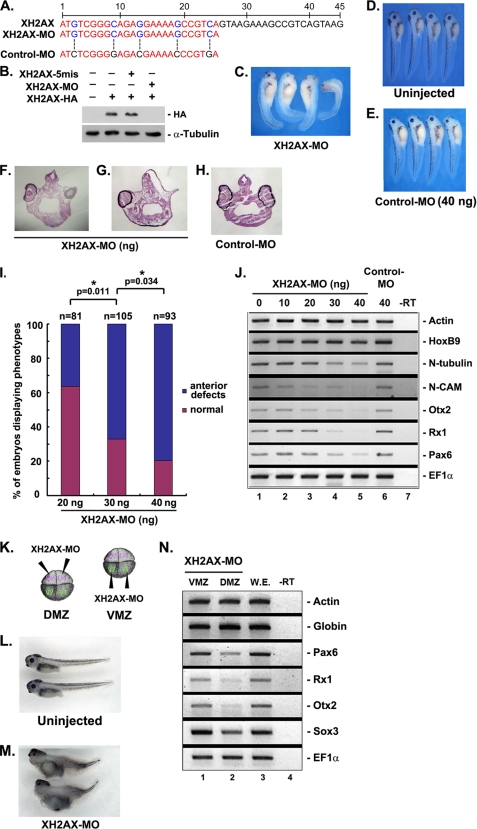
To investigate the physiological role of XH2AX in X. laevis development, we performed a loss-of-function study using a morpholino antisense oligonucleotide directed against XH2AX (XH2AX-MO). We generated an XH2AX-MO that targets the first 25 nucleotides of the XH2AX mRNA coding sequence (Fig. 2A). As a specificity control, the same 5-mismatched morpholino oligonucleotide (Control-MO) was used (Fig. 2A). XH2AX-MO was effective in specifically reducing the level of the 3′-terminal end HA-tagged H2AX protein (XH2AX-HA), whereas Control-MO had no effect on the translation of XH2AX mRNA (Fig. 2B).
FIGURE 2.
Depletion of XH2AX causes defective anterior neural formation. Control-MO or XH2AX-MO was injected at the one- or two-cell stage in the animal pole region, and embryos were cultured until the tadpole stage. A, the MO sequence targeting XH2AX. B, XH2AX-MO (30 ng) specifically knocked down the translation of the overexpressed C-terminal HA-tagged histone H2AX protein. α-Tubulin served as a specificity control. C, phenotypes of XH2AX-depleted embryos. D, β-gal mRNA (200 pg)-injected embryos were used as a control phenotype. E, injection of Control-MO did not result in severe defects. F–H, hematoxylin-stained transverse section of the head region of tadpole embryos from Type A (F and G) or Control-MO (H). I, quantitative results of relative defects in whole embryos. *, p < 0.05. J, RT-PCR analysis of whole embryos expressing XH2AX-MO. XH2AX-MO caused repression of anterior neural markers (Otx2, Rx1, and Pax6), the pan-neural marker (N-CAM), and the neural differentiation marker (N-tubulin) without changing the mesoderm marker actin and the posterior marker HoxB9. Control-MO did not change the anterior neural markers tested above. K, XH2AX-MO (20 ng) was injected into the DMZ or VMZ of four-cell stage embryos. L, ventrally XH2AX-MO-injected embryos showed a normal phenotype. M, dorsally injected XH2AX-MO caused a weak head defect with small eyes and shortened axis. N, RT-PCR analysis of whole embryos dorsally or ventrally expressing XH2AX-MO. Dorsally (but not ventrally) expressed XH2AX-MO repressed anterior neural markers, including neural markers, but not the actin and globin mesoderm markers, compared with the whole embryo (W.E.) that was not injected. −RT, control reaction without reverse transcriptase.
Injection of XH2AX-MO into the animal region at the one- or two-cell stage caused anterior neural defects, including microcephaly and eye defects (Fig. 2C), compared with uninjected normal embryos (Fig. 2D). The Control-MO-injected embryos showed a normal phenotype, indicating the specificity of XH2AX-MO (Fig. 2E). A hematoxylin-stained transverse section of the head region showed small eyes or eyes with defects (Fig. 2, F and G) compared with normal embryos that were not injected (Fig. 2H). Anterior neural defects were quantified and represented by histogram as the percent of embryos displaying developmental defects (Fig. 2I). The results indicate that the severity of the phenotypic defect induced by XH2AX-MO was dose-dependent.
To characterize the phenotypic defects induced by depletion of XH2AX at the molecular level, we used RT-PCR to examine the expression of several neural markers in XH2AX-MO-injected embryos. Otx2, Rx1, and Pax6 are general anterior neural markers. N-CAM, a general pan-neural marker, and N-tubulin, a general neural differentiation marker, were used for RT-PCR. Consistent with the morphological defects, injection of XH2AX-MO suppressed the expression of general anterior neural markers (Otx2, Rx1, and Pax6), the pan-neural marker (N-CAM), and the neural differentiation marker (N-tubulin). The Control-MO-injected embryos had no effect on the expression of these markers (Fig. 2J, lane 6). Additionally, we examined the expression of the mesoderm marker actin and the posterior marker HoxB9. XH2AX-MO could not suppress the expression of actin or HoxB9 (Fig. 2J). These results provide evidence for a specific role of XH2AX in anterior neural development, but not in mesoderm and posterior formation at the molecular level.
To further investigate the effect of XH2AX-MO in neural formation, including anterior neural tissue, XH2AX-MO was injected into the marginal zone of two dorsal (DMZ) or ventral (VMZ) blastomeres of four-cell stage embryos (Fig. 2K). The DMZ of amphibian embryos is a key embryonic region involved in body axis organization and neural induction (28, 29). Compared with the phenotype of control embryos that were not injected (Fig. 2L), the embryos injected with XH2AX-MO dorsally showed severe defects in neural formation, including the anterior neural tissue (Fig. 2M), which is similar to the phenotypes caused by XH2AX depletion in the animal region of one-cell stage embryos. In contrast, embryos injected ventrally with XH2AX-MO showed no significant changes in phenotype (data not shown). Consistent with these phenotypes, RT-PCR analysis showed that the dorsally XH2AX-depleted embryos repressed neural markers, including specific anterior neural markers (Fig. 2N, lane 2), compared with either control embryos (lane 3) or ventrally XH2AX-depleted embryos (lane 1) without affecting the mesoderm markers actin and globin. These RT-PCR results are also paralleled with those changes caused by XH2AX-MO injection into the animal region. Taken together, these results indicate that depletion of XH2AX disrupts normal anterior neural development without affecting mesoderm induction.
XH2AX Is Required for Anterior Neural Development
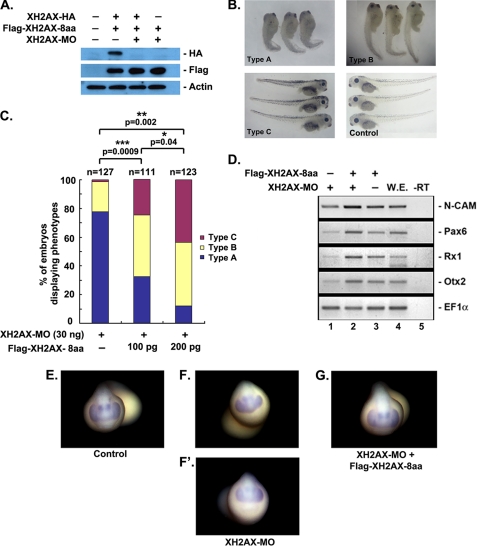
We then examined whether overexpression of XH2AX in XH2AX-MO-injected embryos could rescue the normal phenotype and reinstate the expression of neural molecular markers. For the rescue experiment, we used wild-type XH2AX-8aa mRNA, which lacks the MO-binding sites and is resistant to its translational inhibition as confirmed by Western blotting (Fig. 3A). The anterior neural defects observed in XH2AX-MO-injected embryos were effectively rescued by co-injection of wild-type XH2AX-8aa (Fig. 3B). XH2AX-MO-injected embryos showed a severe anterior neural defective phenotype (Fig. 3B, Type A panel). However, by coexpression with XH2AX-8aa, the normal phenotype was rescued (Fig. 3B, Type B and C panels) in a dose-dependent manner. The data are represented in the histogram as the percent of embryos displaying the particular phenotype (Fig. 3C).
FIGURE 3.
XH2AX is required for anterior neural development. A–D, one- or two-cell stage embryos were injected with XH2AX-MO (30 ng) alone or in combination with the indicated dose of FLAG-XH2AX-8aa mRNA for rescue experiments. A, XH2AX-MO (30 ng) specifically knocked down the translation of the overexpressed C-terminal HA-tagged histone H2AX protein, but not the FLAG-H2AX-8aa protein, which lacks the MO target site. α-Tubulin served as a control for specificity. B, rescued phenotypes were classified into three types: Type A, severely defective embryo; Type B, moderately defective embryo; and Type C, fully rescued embryo. The control was an uninjected embryo. C, quantitative results of relative rescue in whole embryos are shown. *, p < 0.05; **, p < 0.01; ***, p < 0.001. D, RT-PCR analysis of sibling embryos: FLAG-XH2AX-8aa mRNA (150 pg) rescue of anterior neural markers (Otx2, Rx1, and Pax6) and the pan-neural marker (N-CAM) that were repressed by XH2AX-MO. W.E., whole embryo as a positive control for PCR; −RT, control reaction without reverse transcriptase. E–G, whole-mount in situ hybridizations on embryos injected as described for A with a Pax6 probe, an anterior neural marker. FLAG-XH2AX-8aa mRNA (G) rescued Pax6 expression previously repressed by XH2AX-MO (F and F′). E, control-MO-injected embryo.
At the molecular level, the expression of anterior neural markers (Pax6, Rx1, and Otx2) and the pan-neural marker (N-CAM) (Fig. 3C, lane 1) in XH2AX-MO-injected embryos was also effectively rescued by coexpression of wild-type XH2AX-8aa (Fig. 3D, lane 2). Additionally, we performed in situ hybridization using whole embryos to analyze the expression of Pax6. Consistent with other results, the in situ hybridization analysis revealed that wild-type XH2AX-8aa could rescue Pax6 (Fig. 3G) previously suppressed by XH2AX-MO (Fig. 3, F and F′). Taken together, these results demonstrate that XH2AX is critical for anterior neural development in X. laevis.
Depletion of XH2AX Inhibits Activin-induced Anterior Neurogenesis
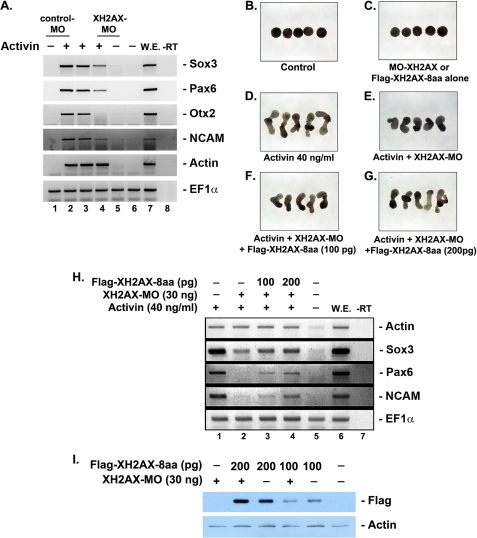
To further verify the role of XH2AX in anterior neural development, we performed animal cap assays with activin, a well known morphogen of dorsoventral and anteroposterior neural axis determination. The so-called animal cap assay (isolation and differentiation of animal cap cells) is a useful method for investigating the mechanism of cell differentiation at the molecular level. The cells of the animal caps retain pluripotentiality, and upon exposure to specific inducers, the animal cap can differentiate into neural, mesodermal, or endodermal tissues, equivalent to mammalian embryonic stem cells (25, 27). A high dose of activin can induce more anterior neural markers (30–33) in the animal cap assay. To investigate whether depletion of XH2AX can inhibit activin-induced anterior neural marker expression, animal caps isolated from XH2AX-MO-injected embryos were incubated with or without activin. XH2AX-MO, but not Control-MO, effectively inhibited the activin-induced expression of anterior neural markers, including pan-neural markers (Fig. 4A, compare lanes 2–4). Consistent with these results, depletion of XH2AX inhibited the elongation of animal cap explants induced by activin (Fig. 4, D and E). Animal cap explants cultured in the absence of activin (Fig. 4B) or derived from embryos injected with wild-type XH2AX alone or XH2AX-MO alone (Fig. 4C) did not show any detectable morphological changes or expression of anterior neural specific markers (supplemental Fig. S1).
FIGURE 4.
XH2AX is required for the induction of anterior neural markers by activin. A–I, animal caps, explanted from embryos injected with Control-MO or XH2AX-MO (30 ng) either alone or in combination with the indicated dose of FLAG-XH2AX-8aa mRNA, were incubated with or without the activin protein (40 ng/ml) until stage 24, anterior neural markers (Otx2, Rx1, and Pax6), pan-neural markers (N-CAM and Sox3), a mesoderm marker (actin), and EF1α as a loading control. W.E., whole embryo as a positive control for PCR; −RT, control reaction without reverse transcriptase. A, RT-PCR analysis of animal cap explants. XH2AX-MO selectively blocked the activin-induced expression of Sox3, N-CAM, Pax6, and Otx2, but not actin (lane 4). Control-MO did not change activin induction of any of the genes (lane 2). B–G, phenotype of animal caps explanted from embryos injected as described for A–I. XH2AX-MO blocked activin-induced elongation of animal caps, and this phenotype was rescued by XH2AX-8aa mRNA in a dose-dependent manner. H, RT-PCR analysis of samples shown in B–G. XH2AX-8aa mRNA injection rescued activin induction of Sox3, Pax6, N-CAM in XH2AX-MO-injected animal caps. I, Western blot of embryonic extracts probed with anti-FLAG antibody, showing that all injected constructs were translated equally when injected into Xenopus embryos. Actin served as a loading control.
Additionally, the suppressed expression of neural markers, as well as the defective phenotype of animal caps induced by XH2AX-MO, was effectively rescued by co-overexpression of wild-type XH2AX-8aa mRNA (Fig. 4, E–H, lanes 2–4). Overexpression of XH2AX was confirmed by Western blotting (Fig. 4I). Interestingly, consistent with the data from whole embryos (Fig. 2, G and K), the expression of the mesoderm marker actin, induced by activin, was not changed by XH2AX-MO (Fig. 4, A and H).
To determine whether overexpression of XH2AX can be incorporated into chromatin in embryos, histone proteins were isolated by acidic extraction (34) from the nuclei of embryos. The incorporation of overexpressed FLAG-XH2AX into chromatin was confirmed by Western blotting with an anti-FLAG antibody (supplemental Fig. S2). Histone H2B was detected as a control to verify histone extraction. Taken together, these results provide strong evidence that XH2AX is required for anterior neural formation without affecting mesoderm formation.
Depletion of Chk1 Disrupts Normal Neurogenesis, Including Anterior Neural Tissue
Covalent modification of histone variants is closely linked to transcriptional regulation and is required for many biological processes, including the differentiation of pluripotent stem cells into specific tissue lineage (6, 35, 36). These studies provide evidence supporting the possibility that histone covalent modifications play crucial roles throughout development.
A recent study showed that histone H3 phosphorylation at Thr11 by Chk1 has an important role in transcriptional regulation in unperturbed cells (37). Additionally, it was suggested that Chk1-mediated phosphorylation of additional sites on chromatin might prove to be functionally important. Chk1 is an evolutionarily conserved serine/threonine-specific kinase classified as a checkpoint kinase because of its critical role in regulating cell cycle checkpoint responses to DNA damage (38–41). Recent studies have extended the role of Chk1 into genome maintenance, as a chromatin-modifying transcription factor in unperturbed proliferating cells, and as a target for anticancer therapy (37, 42, 43). Furthermore, Chk1-deficient mice are embryonic lethal, indicating that Chk1 is essential for cell growth and differentiation at the early stages of development (44).
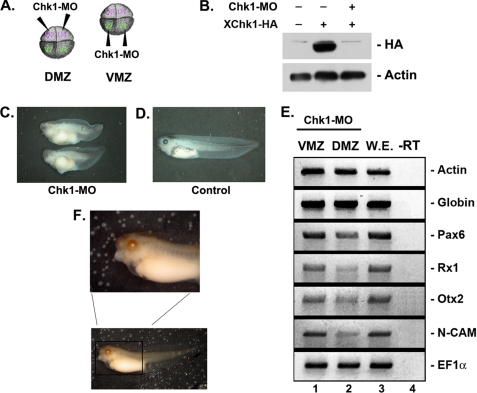
To understand the function of Chk1 in X. laevis normal development, we observed the phenotypes of Chk1-depleted embryos by injecting Chk1-MO into the dorsal or ventral regions at the four-cell stage (Fig. 5A). Chk1-MO was effective in specifically reducing the level of the C-terminal end HA-tagged Chk1 protein (Fig. 5B). Dorsally Chk1-depleted embryos showed a severe defect in the anterior neural tissue (Fig. 5C) compared with control embryos (Fig. 5D). Consistent with this result, the dorsally Chk1-depleted embryos repressed the expression of anterior neural markers without affecting the mesoderm markers (Fig. 5E). In contrast, ventrally Chk1-depleted embryos showed no significant changes in phenotype (data not shown). These results agree well with the phenotype observed in dorsally XH2AX-depleted embryos (Fig. 2, M and N). Moreover, expression of the Chk1 protein was restricted to the anterior region, including the eye (Fig. 5F), in stage 40 embryos, and the distribution of Chk1 is consistent with the XH2AX expression pattern (Fig. 1I).
FIGURE 5.
Depletion of Chk1 causes defective anterior neural formation. A, Chk1-MO (20 ng) was injected into the DMZ or VMZ of four-cell stage embryos. B, Chk1-MO specifically knocked down the translation of an overexpressed C-terminal HA-tagged Chk1 protein. Actin served as a control for specificity. C and D, dorsally injected Chk1-MO caused severe head and eye defects with a shortened axis (C) compared with the normal embryo that was not injected (D). E, RT-PCR analysis of whole embryos dorsally or ventrally expressing Chk1-MO. Dorsally (but not ventrally) expressed Chk1-MO repressed anterior neural markers (Pax6, Rx1, and Otx2) and the neural marker (N-CAM), but not the actin and globin mesoderm markers, compared with whole embryos (W.E.) not injected. EF1α served as a loading control. −RT, control reaction without reverse transcriptase. F, whole-mount in situ hybridization showing the spatial expression of Chk1 at the tadpole stage. The upper panel shows a magnified anterior region of an embryo.
Thr16 of XH2AX Is Phosphorylated by Xenopus Chk1
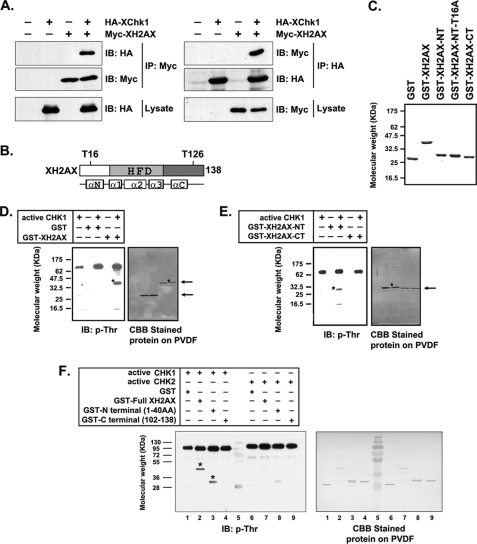
To further investigate the relationship between Chk1 and XH2AX, we examined the localization of injected Chk1 and XH2AX proteins by immunostaining. As expected, the injected Chk1 and XH2AX proteins were localized in the nucleus (supplemental Fig. S3). Furthermore, the direct association of these proteins was examined by immunoprecipitation assay in vivo. HA-Chk1 is co-immunoprecipitated with Myc-XH2AX by anti-Myc antibody. A reverse immunoprecipitation experiment confirmed this result (Fig. 6A). This physical interaction between Chk1 and XH2AX in vivo suggested that Chk1 might have a role as an XH2AX kinase.
FIGURE 6.
XH2AX is phosphorylated by Chk1 at Thr16 of the N terminus. A, in vivo interaction between XH2AX and Chk1. Lysates from embryos injected with mRNAs for HA-tagged Xenopus Chk1 and Myc-tagged XH2AX expression were used to immunoprecipitate (IP) Myc-XH2AX (left panels) or HA-Xenopus Chk1 (right panels). IB, immunoblot. B, schematic representation of the structure of XH2AX. The histone fold domain (HFD) is a globular domain comprising the nucleosome core. αN, α-helix of the N-terminal tail; αC, α-helix of the C-terminal tail. C, Coomassie Brilliant Blue (CBB) staining of a bacterially expressed GST-XH2AX deletion mutant. D–G, Western blot using an anti-phosphothreonine antibody after an in vitro kinase reaction. D, Chk1 phosphorylated a threonine residue of XH2AX. E, Chk1 phosphorylated a threonine residue in the N terminus (but not the C terminus) of XH2AX. F, Chk1 phosphorylated a threonine residue in the N terminus of XH2AX (*), whereas Chk2 had little effect. G, phosphorylation of a threonine residue in the N terminus of XH2AX (*) was absent when Thr16 was mutated to alanine. H–J, Western blot using anti-phospho-Thr16 XH2AX antibody after an in vitro kinase reaction. H, anti-phospho-Thr16 XH2AX antibody was used for detecting phosphorylation of XH2AX. The upper arrow indicates full-length GST-XH2AX, and the lower arrow indicates the N terminus of GST-XH2AX. Asterisks indicate phosphorylation of full-length XH2AX and its N terminus. I, phosphorylation of XH2AX at Thr16 by Chk1 is shown by an in vitro kinase assay using immunoprecipitated FLAG-tagged Chk1. Extracts from embryos injected or uninjected (control) with FLAG-Chk1 mRNA were immunoprecipitated using an anti-FLAG antibody, and the kinase reaction was performed with the indicated GST-recombinant protein as a substrate. Phosphorylation of the threonine residue was analyzed by Western blotting using anti-phospho-Thr16 XH2AX antibody. Western blotting with anti-FLAG antibody showed that equal amounts of Chk1 immunoprecipitates were loaded. The upper arrow indicates the N terminus of GST-XH2AX, and the lower arrow indicates the IgG light chain. The asterisk indicates phosphorylated XH2AX. J, immunoprecipitated Chk1 from DMZ-expressed FLAG-Chk1 mRNA phosphorylated Thr16 of XH2AX. Extracts from embryos dorsally or ventrally injected with FLAG-Chk1 mRNA were immunoprecipitated using an anti-FLAG antibody and subjected to a kinase reaction with the indicated GST-recombinant protein as a substrate. Western blotting using anti-FLAG antibody showed that equal amounts of Chk1 were immunoprecipitated. The upper arrow indicates the N terminus of GST-XH2AX, and the lower arrow indicates the IgG light chain. The asterisk indicates phosphorylated XH2AX.
To investigate the possibility that XH2AX is a substrate of Chk1, we performed an in vitro kinase assay with anti-phosphothreonine antibody to determine whether XH2AX could be phosphorylated by Chk1. A schematic representation of the structure of XH2AX (Fig. 6B) and expression of GST-recombinant XH2AX constructs are shown (Fig. 6C). We purified recombinant full-length XH2AX (GST-XH2AX), an N-terminal fragment (GST-XH2AX-NT, amino acids 1–40), a C-terminal fragment (GST-XH2AX-CT, amino acids 102–138), and an N-terminal mutant of XH2AX in which Thr16 was replaced with alanine (GST-XH2AX-NT-T16A) (Fig. 6C). At least one threonine residue of XH2AX was phosphorylated by Chk1 (Fig. 6D). To determine which portion of XH2AX is phosphorylated by Chk1 at threonine residues, we tested the phosphorylation of XH2AX-NT and XH2AX-CT by Chk1. Using this approach, we demonstrated that the N terminus of XH2AX is responsible for the phosphorylation by Chk1 (Fig. 6E).
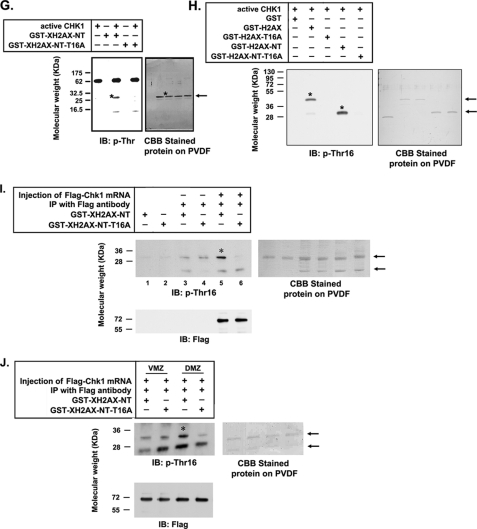
Additionally, we showed that Chk2 could not phosphorylate threonine residues of XH2AX (Fig. 6F, lanes 7–9). This result suggests the specificity of Chk1 for the N-terminal phosphorylation of XH2AX at threonine residues. We recently discovered that Ser16 of human H2AX (analogous to Thr16 of XH2AX) might be a crucial phosphorylation site.3 To determine whether Chk1 can specifically phosphorylate Thr16 of XH2AX, we tested the phosphorylation of wild-type XH2AX-NT and XH2AX-NT-T16A by Chk1. In vitro kinase assay revealed that Chk1 phosphorylated wild-type XH2AX-NT, but not XH2AX-NT-T16A (Fig. 6G), indicating that Thr16 is a primary site of phosphorylation by Chk1.
To determine the specificity of XH2AX phosphorylation at Thr16 in more detail, we developed an anti-phospho-Thr16 XH2AX antibody as described under “Experimental Procedures.” Consistent with the results obtained with the anti-phosphothreonine antibody (Fig. 6, D–G), our data confirmed that Chk1 could specifically phosphorylate Thr16 of XH2AX (Fig. 6H). To confirm that Chk1 phosphorylates Thr16 of XH2AX, we immunoprecipitated FLAG-tagged Chk1 from embryos expressing FLAG-tagged Chk1 and performed an in vitro kinase assay using wild-type XH2AX-NT and XH2AX-NT-T16A as substrates. Wild-type XH2AX-NT, but not the T16A mutant, was remarkably phosphorylated by the anti-FLAG-Chk1 immunoprecipitates (Fig. 6I, lanes 5 and 6) compared with the control (lanes 3 and 4). Chk1-MO knockdown assay results (Fig. 5) indicated that the function of Chk1 in DMZ, but not VMZ, of embryos is important in neurogenesis. To evaluate the ability of Chk1 to phosphorylate XH2AX Thr16 in the DMZ and VMZ of embryos, recombinant wild-type XH2AX-NT and mutant XH2AX-NT-T16A proteins were used as substrates of anti-FLAG-Chk1 immunoprecipitates from embryos dorsally or ventrally expressing FLAG-tagged Chk1. Consistent with the biological effect of Chk1 in DMZ and VMZ, Chk1 immunoprecipitated from DMZ, not from VMZ, obviously phosphorylated Thr16 of XH2AX (Fig. 6J). These results demonstrate that Chk1 specifically phosphorylates Thr16 of XH2AX.
Thr16 of XH2AX Has a Crucial Role in Anterior Neurogenesis
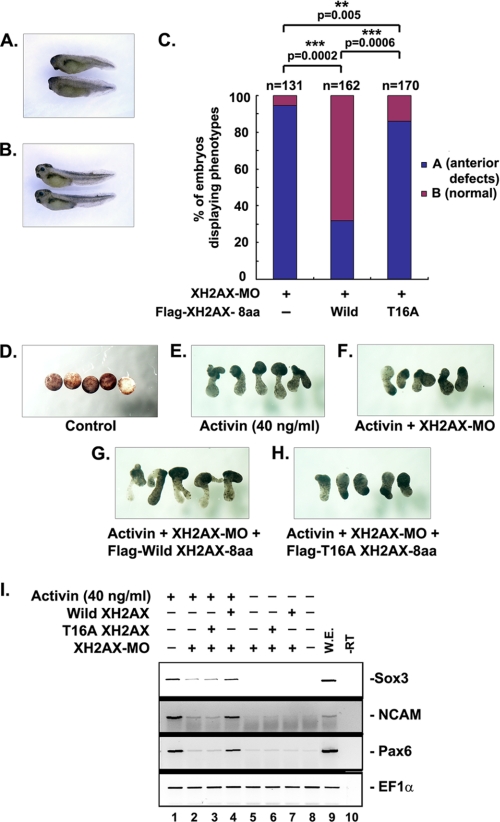
To address the biological role of Thr16 phosphorylation in anterior neurogenesis of the X. laevis embryo, we determined whether the expression of the XH2AX-T16A mutant could rescue the anterior neural defective phenotype induced by XH2AX-MO. XH2AX-MO-injected embryos showed a severe anterior neural defective phenotype (Fig. 7A), and the normal phenotype could be rescued by wild-type XH2AX or XH2AX-T16A (Fig. 7B). The recovery ratio is represented by histogram as a percent of embryos displaying a particular phenotype (Fig. 7C). The XH2AX-T16A mutant was less able to rescue the normal phenotype (10% rescue) compared with wild-type XH2AX (60% rescue) (Fig. 7C). Compared with wild-type XH2AX, the XH2AX-T16A mutant could not rescue the activin-induced inhibited elongation of animal cap explants exhibited by XH2AX-MO embryos (Fig. 7, D–H) or the activin-induced expression of anterior neural markers (Fig. 7I, lanes 2–4). Taken together, these results indicate that XH2AX Thr16 is phosphorylated by Chk1 and is required for anterior neural formation of X. laevis.
FIGURE 7.
Thr16 of XH2AX has a critical role in neurogenesis. A and B, one or two-cell stage embryos injected with XH2AX-MO (30 ng) alone or in combination with 150 pg of FLAG-XH2AX-8aa or FLAG-XH2AX-T16A mRNA and cultured until the tadpole stage. A, severely defective embryo. B, fully rescued embryo. C, quantitative results of relative rescue in whole embryos shown in A and B. **, p < 0.01; ***, p < 0.001. D–I, animal caps explanted from embryos injected with XH2AX-MO (30 ng) alone or in combination with FLAG-XH2AX-8aa or FLAG-XH2AX-T16A mRNA were incubated with or without the activin protein (40 ng/ml) until stage 24. D–H, phenotype of animal cap explans. I, RT-PCR analysis of samples shown in E–H. The anterior neural marker Pax6 and the pan-neural markers N-CAM and Sox3 were used; EF1α served as a loading control. W.E., whole embryo as a positive control of PCR; −RT, control reaction without reverse transcriptase.
DISCUSSION
Several lines of evidence have firmly established a canonical role for phosphorylated XH2AX (γ-XH2AX). Distinct from other H2A variants, H2AX contains a C-terminal tail with a serine residue that is phosphorylated quickly by ATM (ataxia-telangiectasia mutated gene) in response to DNA damage. γ-H2AX recruits and retains DNA damage factors, thus playing a crucial role in both checkpoint activation and DNA repair (6, 10–14, 45, 46).
Here, we have demonstrated, for the first time, a novel non-canonical function of XH2AX in the anterior neural development of X. laevis as a developmental model of vertebrates. We discovered that phosphorylation of XH2AX at Thr16 has a critical role in anterior neural formation. Although the role of other histone variants has been reported in X. laevis development, the physiological function of H2AX had not yet been elucidated in vertebrate development. Moreover, very little is known about the role of histone post-translational modification in vertebrate development. Therefore, our findings represent a novel function of histones that expands the knowledge of the role of histones beyond DNA damage and into a new biological function in development.
According to our results, knockdown of XH2AX resulted in defects of anterior and neural formation without affecting mesoderm and posterior tissue formation in whole embryos or animal caps treated with activin. Although we did not clarify how XH2AX regulates the expression of anterior neural markers, this result provides interesting new evidence suggesting that anterior neural defects might be the primary effect of the depletion of XH2AX.
Chk1 has been known to restrain mitotic entry in response to DNA damage and replication blocks, in part by inhibiting Cdk activity (39–41). In our study, we elucidated an additional role for Chk1 in X. laevis development. Consistent with XH2AX, knockdown of Chk1 in DMZ, but not VMZ, resulted in a severe defect in neural formation, including anterior tissue, without affecting mesoderm formation (Fig. 5E). Furthermore, we found that Chk1 phosphorylation of XH2AX was detectable in DMZ (Fig. 6J). As indicated, the DMZ of amphibian embryos is a key embryonic region involved in body axis organization and neural induction. The DMZ houses the Spemann-Mangold organizer induced by TGF-β and Vg1-like signals, and grafts of DMZ to the ventral side induce secondary neural axes from the ventral tissue of the host (29). Thus, our findings suggest that the kinase activity of Chk1 toward XH2AX might be regulated by signals originating from DMZ, which indicates that Chk1, as well as XH2AX, has a critical role in neurogenesis.
C-terminal Ser139 phosphorylation of XH2AX (γ-XH2AX) by ATM/ATR and JNK is a well known response to DNA damage and leads to initiation of DNA repair or apoptosis (11, 45, 46). Recently, Tyr142 was reported as a new phosphorylation site on H2AX with a role in regulating the DNA damage response (47). Here, we found that Thr16 is a novel phosphorylation site on XH2AX that is associated with neural specification rather than the DNA damage response. We also discovered that Chk1 is a novel kinase responsible for phosphorylating XH2AX at Thr16. To demonstrate the specificity of Chk1 for XH2AX, we showed that Chk2, another Chk family member, could not phosphorylate XH2AX at any threonine residue (Fig. 6F). We also observed that Chk1, as well as Chk2, could phosphorylate serine residues in the XH2AX N terminus (data not shown), and the function of the phosphorylation of these sites is the subject of future studies.
In conclusion, we propose a novel role for XH2AX and Chk1 in anterior neural formation during X. laevis development. Phosphorylation of XH2AX (Thr16) by Chk1 is important for the biological role of XH2AX in neural specification during X. laevis development. These findings suggest that XH2AX phosphorylation is important not only in the DNA damage response but also in X. laevis development.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants CA077646, CA111536, CA120388, R37 CA081064, and ES016548. This work was also supported by The Hormel Foundation.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Experimental Procedures” and Figs. S1–S3.
F. Zhu, T. A. Zykova, C. Peng, J. Zheng, Y. Y. Cho, D. Zheng, K. Yao, W.-Y. Ma, A. T. Y. Lau, A. M. Bode, and Z. Dong, unpublished data.
- MO
- morpholino oligonucleotide
- DMZ
- dorsal marginal zone
- VMZ
- ventral marginal zone.
REFERENCES
- 1.Isenberg I. (1979) Annu. Rev. Biochem. 48, 159–191 [DOI] [PubMed] [Google Scholar]
- 2.Cosgrove M. S., Wolberger C. (2005) Biochem. Cell Biol. 83, 468–476 [DOI] [PubMed] [Google Scholar]
- 3.Ehrenhofer-Murray A. E. (2004) Eur. J. Biochem. 271, 2335–2349 [DOI] [PubMed] [Google Scholar]
- 4.Kouzarides T. (2007) Cell 128, 693–705 [DOI] [PubMed] [Google Scholar]
- 5.Shilatifard A. (2006) Annu. Rev. Biochem. 75, 243–269 [DOI] [PubMed] [Google Scholar]
- 6.Bhaumik S. R., Smith E., Shilatifard A. (2007) Nat. Struct. Mol. Biol. 14, 1008–1016 [DOI] [PubMed] [Google Scholar]
- 7.Grant P. A. (2001) Genome Biol. 2, REVIEWS0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadnagy A., Beaulieu R., Balicki D. (2008) Mol. Cancer Ther. 7, 740–748 [DOI] [PubMed] [Google Scholar]
- 9.Ausió J. (2006) Brief Funct. Genomic Proteomic 5, 228–243 [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Capetillo O., Lee A., Nussenzweig M., Nussenzweig A. (2004) DNA Repair 3, 959–967 [DOI] [PubMed] [Google Scholar]
- 11.Lu C., Zhu F., Cho Y. Y., Tang F., Zykova T., Ma W. Y., Bode A. M., Dong Z. (2006) Mol. Cell 23, 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redon C., Pilch D., Rogakou E., Sedelnikova O., Newrock K., Bonner W. (2002) Curr. Opin. Genet. Dev. 12, 162–169 [DOI] [PubMed] [Google Scholar]
- 13.Wuebbles R. D., Jones P. L. (2004) Cell. Mol. Life Sci. 61, 2148–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaid O., Downs J. A. (2005) Cell. Mol. Life Sci. 62, 1653–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eyal-Giladi H. (1954) Arch. Biol. 65, 179–259 [PubMed] [Google Scholar]
- 16.Heasman J. (2006) Development 133, 1205–1217 [DOI] [PubMed] [Google Scholar]
- 17.Zuber M. E., Gestri G., Viczian A. S., Barsacchi G., Harris W. A. (2003) Development 130, 5155–5167 [DOI] [PubMed] [Google Scholar]
- 18.Lupo G., Andreazzoli M., Gestri G., Liu Y., He R. Q., Barsacchi G. (2000) Int. J. Dev. Biol. 44, 627–636 [PubMed] [Google Scholar]
- 19.Iouzalen N., Moreau J., Méchali M. (1996) Nucleic Acids Res. 24, 3947–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ridgway P., Brown K. D., Rangasamy D., Svensson U., Tremethick D. J. (2004) J. Biol. Chem. 279, 43815–43820 [DOI] [PubMed] [Google Scholar]
- 21.Steinbach O. C., Wolffe A. P., Rupp R. A. (1997) Nature 389, 395–399 [DOI] [PubMed] [Google Scholar]
- 22.Vermaak D., Steinbach O. C., Dimitrov S., Rupp R. A., Wolffe A. P. (1998) Curr. Biol. 8, 533–536 [DOI] [PubMed] [Google Scholar]
- 23.Wysocka J., Swigut T., Milne T. A., Dou Y., Zhang X., Burlingame A. L., Roeder R. G., Brivanlou A. H., Allis C. D. (2005) Cell 121, 859–872 [DOI] [PubMed] [Google Scholar]
- 24.Joo H. Y., Zhai L., Yang C., Nie S., Erdjument-Bromage H., Tempst P., Chang C., Wang H. (2007) Nature 449, 1068–1072 [DOI] [PubMed] [Google Scholar]
- 25.Ariizumi T., Takahashi S., Chan T. C., Ito Y., Michiue T., Asashima M. (2009) Curr. Protocols Stem Cell Biol., Chapter 1, Unit 1D.5 [DOI] [PubMed] [Google Scholar]
- 26.Xu R. H., Dong Z., Maeno M., Kim J., Suzuki A., Ueno N., Sredni D., Colburn N. H., Kung H. F. (1996) Proc. Natl. Acad. Sci. U.S.A. 93, 834–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okabayashi K., Asashima M. (2003) Curr. Opin. Genet. Dev. 13, 502–507 [DOI] [PubMed] [Google Scholar]
- 28.Papan C., Boulat B., Velan S. S., Fraser S. E., Jacobs R. E. (2007) Dev. Biol. 305, 161–171 [DOI] [PubMed] [Google Scholar]
- 29.Shih J., Keller R. (1992) Development 116, 887–899 [DOI] [PubMed] [Google Scholar]
- 30.Ruiz i Altaba A., Melton D. A. (1989) Nature 341, 33–38 [DOI] [PubMed] [Google Scholar]
- 31.Green J. B., Smith J. C. (1990) Nature 347, 391–394 [DOI] [PubMed] [Google Scholar]
- 32.Green J. B., Cook T. L., Smith J. C., Grainger R. M. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 8596–8601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomsen G., Woolf T., Whitman M., Sokol S., Vaughan J., Vale W., Melton D. A. (1990) Cell 63, 485–493 [DOI] [PubMed] [Google Scholar]
- 34.Shechter D., Dormann H. L., Allis C. D., Hake S. B. (2007) Nat. Protocols 2, 1445–1457 [DOI] [PubMed] [Google Scholar]
- 35.Margueron R., Trojer P., Reinberg D. (2005) Curr. Opin. Genet. Dev. 15, 163–176 [DOI] [PubMed] [Google Scholar]
- 36.Torres-Padilla M. E., Parfitt D. E., Kouzarides T., Zernicka-Goetz M. (2007) Nature 445, 214–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimada M., Niida H., Zineldeen D. H., Tagami H., Tanaka M., Saito H., Nakanishi M. (2008) Cell 132, 221–232 [DOI] [PubMed] [Google Scholar]
- 38.Bartek J., Lukas J. (2003) Cancer Cell 3, 421–429 [DOI] [PubMed] [Google Scholar]
- 39.Boddy M. N., Furnari B., Mondesert O., Russell P. (1998) Science 280, 909–912 [DOI] [PubMed] [Google Scholar]
- 40.Furnari B., Rhind N., Russell P. (1997) Science 277, 1495–1497 [DOI] [PubMed] [Google Scholar]
- 41.Sanchez Y., Wong C., Thoma R. S., Richman R., Wu Z., Piwnica-Worms H., Elledge S. J. (1997) Science 277, 1497–1501 [DOI] [PubMed] [Google Scholar]
- 42.Enders G. H. (2008) J. Biol. Chem. 283, 17749–17752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaugg K., Su Y. W., Reilly P. T., Moolani Y., Cheung C. C., Hakem R., Hirao A., Liu Q., Elledge S. J., Mak T. W. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 3805–3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takai H., Tominaga K., Motoyama N., Minamishima Y. A., Nagahama H., Tsukiyama T., Ikeda K., Nakayama K., Nakanishi M., Nakayama K. (2000) Genes Dev. 14, 1439–1447 [PMC free article] [PubMed] [Google Scholar]
- 45.Ward I. M., Chen J. (2001) J. Biol. Chem. 276, 47759–47762 [DOI] [PubMed] [Google Scholar]
- 46.Chowdhury D., Keogh M. C., Ishii H., Peterson C. L., Buratowski S., Lieberman J. (2005) Mol. Cell 20, 801–809 [DOI] [PubMed] [Google Scholar]
- 47.Xiao A., Li H., Shechter D., Ahn S. H., Fabrizio L. A., Erdjument-Bromage H., Ishibe-Murakami S., Wang B., Tempst P., Hofmann K., Patel D. J., Elledge S. J., Allis C. D. (2009) Nature 457, 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.