Abstract
Tie2 is a receptor tyrosine kinase that is expressed predominantly in the endothelium and that plays key roles in both physiological and pathological angiogenesis. The ligands for Tie2, the angiopoietins (Ang), perform opposing functions in vascular maintenance and angiogenesis; Ang1 regulates vascular quiescence, while Ang2 is thought to promote vascular destabilization and facilitate angiogenesis. However, the mechanisms responsible for these differences are not understood. To begin to elucidate the molecular differences between the angiopoietins, we previously developed a specific RNA aptamer inhibitor of Ang2. Here, we used the same iterative in vitro selection process, termed SELEX (Systematic Evolution of Ligands by EXponential enrichment), to screen a library of 2′-fluoro-modified ribonucleotides for Ang1-binding aptamers. After 9 rounds of selection, we identified a single clone, ANG9-4, that bound with high affinity to human Ang1 (Kd 2.8 nM) but not Ang2 (Kd >1 μM), demonstrating specificity for Ang1. ANG9-4 blocked Ang1-mediated Tie2 phosphorylation and downstream Akt activation. Moreover, ANG9-4 inhibited Ang1-induced endothelial cell survival. Together, these findings demonstrate the feasibility of developing an Ang1-inhibitory aptamer. ANG9-4 and its derivatives may provide useful tools for elucidating the biology of Ang1 and for treating certain angiogenic diseases.
Keywords: Akt, Angiogenesis, Angiopoietin, Apoptosis, Aptamer, Endothelium, Tie2
Introduction
Tie2 is an endothelial receptor tyrosine kinase (RTK) that is required for both embryonic vascular development and pathological angiogenesis [1-4]. Tie2 is unique among RTKs in that its two best-characterized ligands have largely opposing actions, despite sharing approximately 60% amino acid sequence identity and similar binding affinity for Tie2 (Kd ∼3 nM) [5,6]. Based on both genetic and biochemical studies, angiopoietin-1 (Ang1) has been characterized as an agonist for Tie2 [5,7], while angiopoietin-2 (Ang2) is generally considered to be a context-dependent antagonist [6,8]. Mice lacking Tie2 or Ang1 die midway through gestation due to abnormalities of vascular morphogenesis characterized by deficient recruitment of supporting smooth muscle cells and pericytes [7]. Intriguingly, transgenic over-expression of Ang2 in the endothelium results in a similar phenotype, supporting a role for Ang2 as an inhibitor of Ang1/Tie2 signaling [6]. Ang1 promotes endothelial cell survival [9,10] and prevents increases in endothelial cell adhesion molecule expression and vascular permeability induced by vascular endothelial growth factor (VEGF) and inflammatory cytokines [11,12]. In contrast, Ang2 appears to promote vascular regression [6,8], and Ang2 has been shown to sensitize endothelial cells to the effects of tumor necrosis factor (TNF)-α [13]. Taken together, these observations support a model in which Ang1 has anti-inflammatory and vascular stabilizing effects, whereas Ang2 promotes blood vessel destabilization, inflammation, and angiogenesis. However, the molecular mechanisms responsible for the angiopoietins' biological differences remain unclear, particularly in light of their high degree of structural similarity.
The angiopoietins have been implicated in the regulation of numerous physiological and pathological processes, including tumor angiogenesis [14], psoriasis [15], rheumatoid arthritis [16], retinal angiogenesis [17], and hematopoiesis [18]. The need for improved understanding of these ligands' functions is particularly important in the study of tumor angiogenesis, as studies of Ang1 have yielded contradictory results, suggesting that Ang1 may promote [19-21], inhibit [22-24], or have no effect on tumor angiogenesis [25]. In this regard, a specific inhibitor of Ang1 would provide an important tool with which to elucidate the role of Ang1 in the adult vasculature, particularly in the setting of tumor angiogenesis. Moreover, such an inhibitor could be a valuable therapeutic agent in the treatment of cancer or other diseases in which Ang1 is found to play a role.
To develop a specific inhibitor of Ang1, we used an iterative in vitro selection process termed SELEX (Systematic Evolution of Ligands by EXponential enrichment) [26] to generate a nuclease-resistant RNA ligand (aptamer) that binds Ang1. We previously described the utility of this approach in the development of an Ang2 inhibitory aptamer [27]. Here, we describe the use of the SELEX approach to develop an aptamer specific for Ang1, and we demonstrate that it inhibits Ang1 binding to Tie2 and prevents Ang1-induced Tie2 phosphorylation, signaling, and endothelial cell survival in vitro.
Materials and methods
Reagents and cell lines
Recombinant human Ang1 was provided by George Yancopoulos (Regeneron Pharmaceuticals, Tarrytown, NY) or obtained from R&D Systems. Recombinant human Ang2 and TNFα were from R&D Systems. Rabbit polyclonal anti-phospho-Tie2 (Ab1) was from Calbiochem; mouse monoclonal anti-Tie2 (clone 33) has been described previously [28]; rabbit polyclonal anti-Tie2 (C-20) was from Santa Cruz Biotechnology; mouse monoclonal anti-phosphotyrosine (clone 4G10) was purified from hybridoma cells; rabbit polyclonal anti-phospho-Akt (Ser473) and anti-Akt were from Cell Signaling Technology. Human embryonic kidney (HEK)-293 cells stably expressing the full-length human Tie2 cDNA (293-hTie2) were provided by Kevin Peters (Procter & Gamble Health Care Research Center) [27]. Human umbilical vein endothelial cells (HUVECs) were purchased from Clonetics Corp. or were isolated from fresh umbilical cords obtained from healthy donors. Briefly, umbilical veins were flushed with RPMI 1640 medium (Invitrogen) containing 2% penicillin/streptomycin/fungizone (Sigma) to remove all blood and then perfused with 5% collagenase (Sigma) in RPMI 1640 and incubated 15 min at room temperature. Cells were flushed out with RPMI 1640, pelleted by centrifugation at 200×g for 5 minutes, and resuspended and grown in EGM-MV (Clonetics). HUVECs were expanded to passage 2 and characterized by indirect immunofluorescence microscopy for Tie2 (clone 33) [28] and CD31 (clone 9G11, R&D Systems). HUVECs were used for all assays before passage 5.
Systematic evolution of ligands by exponential enrichment (SELEX)
A random pool of RNA oligonucleotides was generated with the sequence 5′-GGGAGAGAGGAAGAGGGAUGGG-N40-CAUAACCCAGAGGUCGAUAGUACUGGAUCCCCCC-3′ (where N40 represents 40 random nucleotides with equimolar 2′-hydroxy ATP, 2′-hydroxy GTP, 2′-fluoro CTP, and 2′-fluoro UTP content), as described previously [26,27]. In vitro selection was performed using recombinant human Ang1 provided by George Yancopoulos, Regeneron Pharmaceuticals. RNA was incubated with Ang1 for 15 min at 37°C in a selection buffer with ionic composition similar to extracellular fluid but with increasing salt concentration over the rounds of selection (20 mM HEPES, pH 7.4, 50 mM [round 1] - 150 mM [round 7] NaCl, 2 mM CaCl2, 0.05% CHAPS). RNAs that bound Ang1 were isolated by vacuum filtration through a 0.45-μm nitrocellulose membrane (Schleicher and Schuell). Bound RNAs were recovered and amplified, and the process was repeated, as described previously [27], with modifications. Successive rounds of selection were performed with decreasing concentrations of Ang1, increasing RNA/protein ratios, and increasing NaCl concentrations. Specifically, initial rounds of selection were performed with 1 nmole of RNA incubated with 200 pmoles of Ang1 at a concentration of 300 nM in 50 mM NaCl buffer, whereas final round selection conditions incubated 200 pmoles of RNA with 8 pmoles of Ang1 at a concentration of 4 nM in 150 mM NaCl. Following round 9, selected RNAs were reverse-transcribed, amplified, cloned, and sequenced, as described previously [27].
Binding affinity assays
RNA-protein equilibrium dissociation constants (Kd) were determined by the double-filter nitrocellulose-filter binding method, as described previously [29], with modifications [27]. Direct binding assays were performed by incubating [32P]-labeled RNA (<0.1 nM) in selection buffer with a range of Ang1 (R&D Systems) or Ang2 protein concentrations at 37°C. The fraction of bound RNA was quantified and corrected for non-specific background binding to the nitrocellulose filter, as described previously [29]. Kds were reported as the mean and 95% confidence interval of three separate experiments. For assays in which saturable competition was not achieved at the highest RNA concentration tested, Kds were estimated and reported as approximate values or as values greater than the highest concentration tested.
Tie2 and Akt activation assays
To evaluate the effects of the Ang1 aptamer, ANG9-4, on Tie2 phosphorylation, Ang1 (14 nM; 1,000 ng/ml) was preincubated with a molar excess of either ANG9-4 or control scramble RNA for 5 minutes at 37°C in selection buffer containing 150 mM NaCl. Confluent 293-hTie2 cells were treated with the Ang1-RNA mixtures for 10 minutes at 37°C. The cells were lysed in Triton lysis buffer (137 mM NaCl, 2 mM EDTA, 10% glycerol, 1% Triton X-100, 20 mM Tris-HCl, pH 8.0) supplemented with protease and phosphatase inhibitors (1 mM sodium orthovanadate, 1 mM PMSF, 1 mM NaF, 1 μg/ml leupeptin, 1 μg/ml pepstatin), and protein complexes were separated by SDS-PAGE, transferred to nitrocellulose, and analyzed by Western blotting with anti-phospho-Tie2. Membranes were then stripped and re-probed with mouse monoclonal anti-Tie2 [28]. Alternatively, cell lysates were immunoprecipitated with monoclonal anti-Tie2 and Protein G-PLUS agarose (Santa Cruz Biotechnology) and probed with mouse monoclonal anti-phosphotyrosine (clone 4G10). Membranes were then stripped and re-probed with polyclonal anti-Tie2 (C-20). To evaluate the effects of ANG9-4 on Tie2-mediated Akt activation, HUVECs were grown in EGM-MV until confluent then serum-starved 3 hours in endothelial basal medium (EBM, Clonetics). Prior to stimulation of HUVECs, Ang1 was preincubated with a molar excess of ANG9-4 or scramble RNA, as described above. The medium was changed to fresh EBM, and cells were either left untreated or were treated 10 min at 37°C with Ang1 (14 nM; 1,000 ng/ml) in the presence or absence of aptamer. Cells were then lysed as described above and proteins were separated by SDS-PAGE and Western blotted with rabbit polyclonal anti-phospho-Akt (Ser473) then stripped and re-probed with rabbit polyclonal anti-Akt.
Endothelial cell apoptosis assay
HUVECs were plated in wells of a 48-well plate and grown until they reached confluence. Cells were then serum-starved in EBM and treated with TNFα (50 ng/ml) for 3 hours in the absence or presence of Ang1 (2 nM; 150 ng/ml) and a molar excess of either ANG9-4 or control scramble RNA. The cells were lysed and DNA fragmentation was measured using the Cell Death Detection ELISA-Plus kit (Roche Molecular Biochemicals). Results are expressed as the mean absorbance and standard error of quadruplicate wells.
Results
ANG9-4 binds Ang1 with high affinity and specificity
To develop a specific inhibitor of Ang1, we used an iterative in vitro selection process termed SELEX (Systematic Evolution of Ligands by EXponential enrichment) [26,27] to identify a nuclease-resistant Ang1-binding aptamer from a library of 2′-fluoro-modified ribonucleotides. Each clone within the library contains a unique 40-nucleotide region with potential protein-binding capabilities. After 9 rounds of selection against Ang1 protein using increasingly stringent conditions, the RNA pool was cloned and sequenced, and a single Ang1-binding clone (termed ANG9-4) was identified with the following random region sequence: 5′-ACUCGAACAUUUCCACUAACCAACCAUACUAAAGCACCGC-3′. Direct binding of ANG9-4 RNA was plotted as a function of Ang1 protein concentration (Figure 1). ANG9-4 bound Ang1 with a calculated Kd of 2.8 nM (95% confidence interval 1.2 – 7.0 nM) and bound the related Ang2 ligand with an estimated Kd of greater than 1 μM. ANG9-4 therefore demonstrates approximately 1,000-fold specificity for Ang1 over Ang2. To serve as a control in subsequent experiments, we generated an RNA aptamer of identical nucleic acid composition in which the random region was rearranged. This scramble RNA binds Ang1 with an estimated Kd of 50 nM (data not shown).
Figure 1.
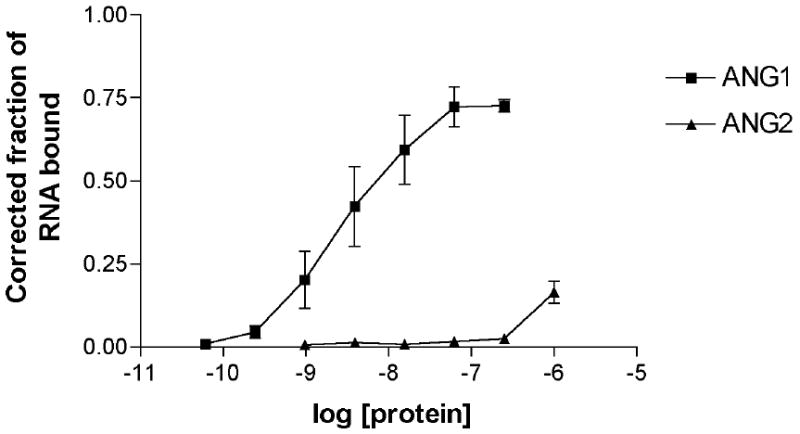
ANG9-4 binds Ang1 with high affinity and specificity. Ligand binding assay demonstrating binding of the ANG9-4 aptamer to recombinant human Ang1 or Ang2.
ANG9-4 inhibits Ang1-mediated activation of Tie2 and Akt in cultured cells
To determine whether binding of ANG9-4 to Ang1 inhibits its ability to bind and activate Tie2, cultured 293 cells stably expressing human Tie2 were incubated with Ang1 (14 nM) in the absence or presence of a 10- to 100-fold molar excess of ANG9-4 or scramble aptamer. ANG9-4 completely abrogated Tie2 autophosphorylation as detected by Western blotting with an antibody specific for phospho-Tie2 (pTie2), whereas the scramble aptamer had no effect on Ang1-mediated Tie2 activation (Figure 2A). We next tested the effects of lower concentrations of aptamer on Tie2 phosphorylation. Whereas treatment with a 5-fold molar excess of ANG9-4 resulted in modest inhibition of Tie2 phosphorylation, a 25-fold molar excess completely abrogated Ang1-induced Tie2 phosphorylation (Figure 2B). ANG9-4 aptamer in the absence of Ang1 had no effect on Tie2 expression, and the scramble aptamer had no effect on Tie2 phosphorylation at lower concentrations (Figure 2B). At an equimolar concentration or a two-fold molar excess of ANG9-4, the inhibition of Ang1-mediated Tie2 phosphorylation was no longer observed (Figure 2C). Together, these findings indicate that a 5-fold molar excess of ANG9-4 is likely required for inhibition of Ang1's effects. Therefore, subsequent studies examined effects of 5- to 10-fold molar excesses of aptamer. Ang2 has been shown to induce Tie2 phosphorylation in non-endothelial cells [6]. Importantly, a 10-fold excess of ANG9-4 did not inhibit Ang2-mediated Tie2 phosphorylation in 293 cells expressing Tie2 (Figure 2C), confirming specificity of ANG9-4 for Ang1.
Figure 2.
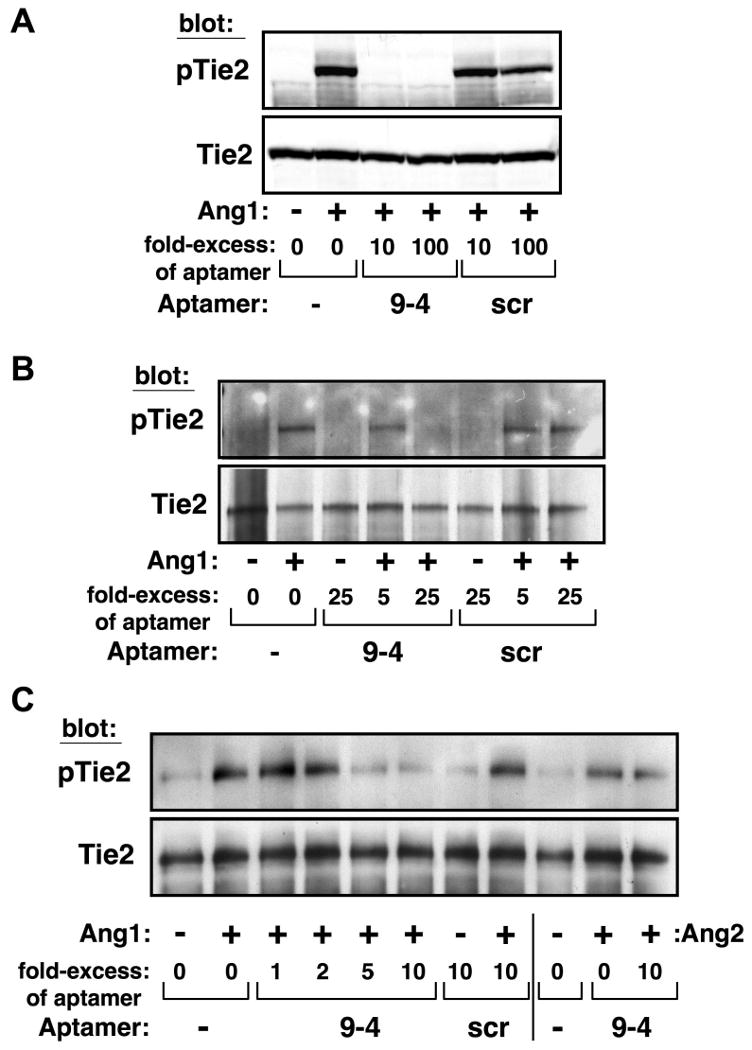
ANG9-4 inhibits Ang1-mediated Tie2 phosphorylation. HEK-293 cells expressing Tie2 were stimulated with Ang1 (14 nM) in the absence or presence of the indicated molar excess of ANG9-4 (9-4) or a scrambled control aptamer (scr), and cell lysates were Western blotted sequentially with anti-phospho-Tie2 (pTie2) and anti-Tie2 as a loading control. (A-C) Progressively lower concentrations of ANG9-4 or control aptamer were tested, as indicated. In (C), cells were also treated with Ang2 (14 nM) to determine effects of ANG9-4 on Tie2 phosphorylation.
Ang1-mediated activation of Tie2 leads to downstream activation of the phosphoinositide 3-kinase (PI3K)/Akt pathway to promote endothelial cell survival [9,10]. To investigate whether the inhibition of Tie2 phosphorylation by ANG9-4 translated into effects on this pathway, HUVECs were treated with Ang1 in the absence or presence of a 10-fold molar excess of ANG9-4 or control aptamer and activation of Akt was assessed. Ang1 alone induced robust activation of Akt in HUVECs, but this effect was completely blocked by ANG9-4 (Figure 3). However, scramble aptamer had no effect on Ang1-mediated Tie2 signaling.
Figure 3.
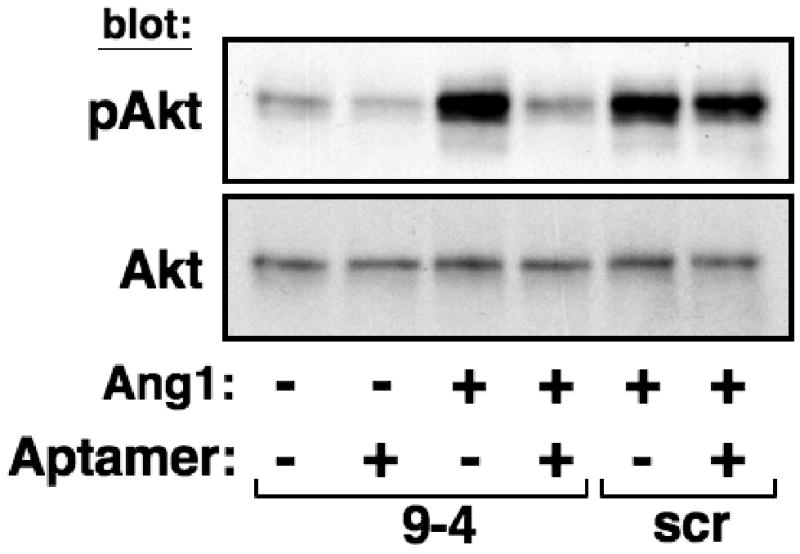
ANG9-4 inhibits Ang1-mediated Tie2 signaling. HUVECs were stimulated with Ang1 (14 nM) in the absence or presence of a 10-fold molar excess of ANG9-4 (9-4) or a scrambled control aptamer (scr), and cell lysates were Western blotted with anti-phospho-Akt (pAkt) or anti-Akt as a loading control.
ANG9-4 inhibits Ang1-mediated survival in HUVECs
To determine effects of ANG9-4 on Ang1-mediated endothelial cell survival, HUVECs were serum starved in the presence of TNFα to induce apoptosis. In the absence of Ang1, serum deprivation plus TNFα resulted in a significant increase in DNA fragmentation, a marker of cellular apoptosis (Figure 4). The addition of ANG9-4 alone did not increase DNA fragmentation, suggesting that the aptamer itself is not directly cytotoxic. Co-treatment with low dose Ang1 (2 nM) protected cells from apoptosis, resulting in DNA fragmentation levels similar to those of serum-treated cells. The addition of a 5- to 10-fold molar excess of ANG9-4, but not scramble RNA, resulted in dose-dependent inhibition of Ang1's ability to protect cells from apoptosis, consistent with its effects on Tie2 phosphorylation. Neither ANG9-4 nor scramble RNA increased DNA fragmentation in unstarved, untreated cells (data not shown). Taken together, these findings demonstrate that ANG9-4 specifically blocks Ang1 and inhibits its ability to activate Tie2 signaling and endothelial cell survival.
Figure 4.
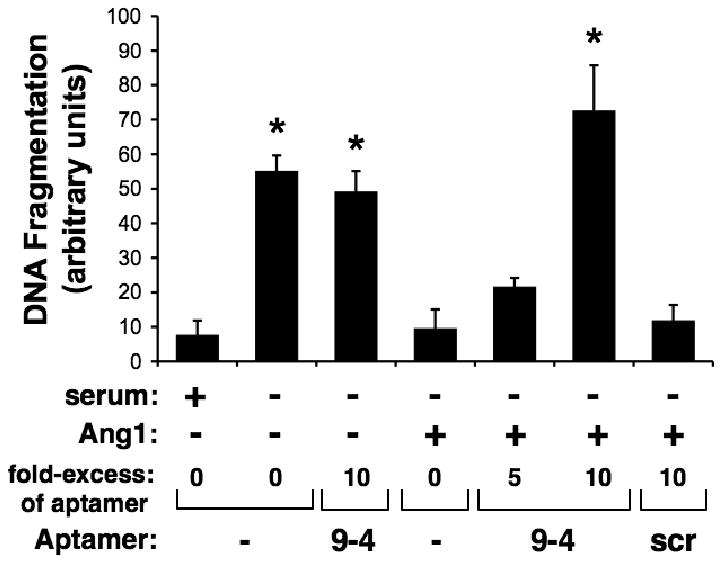
ANG9-4 inhibits Ang1-mediated endothelial cell survival. Apoptosis was induced in HUVECs by serum starvation in the presence of TNFα (50 ng/ml), and cells were treated with or without Ang1 (2 nM) in the presence of the indicated molar excess of ANG9-4 (9-4) or a scrambled control aptamer (scr). Medium containing 5% serum was used as a control for cell survival, and apoptosis was quantified using a DNA fragmentation ELISA. *, P<0.001 vs. Ang1 treatment without aptamer.
Discussion
The angiopoietins and Tie2 play key roles in vascular maintenance and remodeling in a variety of physiological and pathological processes. Substantial evidence indicates that Ang1 and Ang2 play opposing roles in the vasculature, with Ang1 mediating vascular maintenance and stabilization and Ang2 promoting vessel destabilization and angiogenesis [30]. The fact that Tie2 binds both of these ligands with similar affinity positions Tie2 at the interface between quiescent and remodeling blood vessels. To date, however, the mechanistic basis for the differential effects of the angiopoietins remains unclear. Thus, there is a need for molecular tools with which to specifically target the angiopoietins in order to elucidate their respective functions. In this report, we have described the development of a nuclease-resistant RNA aptamer that binds Ang1 with high affinity and specificity and inhibits Ang1-mediated activation of Tie2. We also demonstrated that the ANG9-4 aptamer inhibits Ang1-mediated signaling and cellular responses (i.e., endothelial cell survival) downstream of Tie2.
Previously, we used the SELEX approach to generate a nuclease-resistant RNA aptamer (11-1.41) that binds with high affinity to Ang2, but not Ang1 [27], and inhibits Tie2 binding. Local delivery of this Ang2 aptamer in a rat corneal micropocket assay inhibited angiogenesis. We subsequently showed that systemic delivery of a PEGylated version of this aptamer inhibited tumor growth in a murine model of metastatic colon cancer [31]. Similarly, antibodies and peptide-Fc fusion proteins that specifically inhibit Ang2 have been shown to inhibit tumor growth and angiogenesis in a xenograft model of colon cancer [32]. However, comparable inhibitors of Ang1 have not yet been described or evaluated in physiological or pathological models of angiogenesis.
In addition to its beneficial effects on the endothelium, Ang1 has been shown to activate neutrophils and vascular smooth muscle cells [33,34], suggesting that it may also have detrimental effects in certain contexts. The context-dependence of Ang1's effects may be particularly important in the setting of tumor angiogenesis. Studies have shown that inhibition of the angiopoietins with soluble Tie2 blocks tumor growth, angiogenesis, and metastasis [12, 13]. However, since soluble Tie2 binds both Ang1 and Ang2, it is unclear which ligand is responsible for Tie2's effects on tumor growth. Studies in which the angiopoietins have been either overexpressed or delivered exogenously have yielded conflicting results. In certain tumors Ang1 appears to promote blood vessel and tumor growth [19-21], and in these cases specific inhibition of Ang1 would be desirable. Inhibition of Ang1 expression using antisense RNA has been shown to inhibit tumor growth in xenograft models of cervical and gastric cancer [35,36]. However, this approach is limited by the ability to efficiently deliver nucleic acids into target cells. Our identification of ANG9-4, a nuclease-resistant RNA aptamer that not only binds Ang1 protein with high affinity and specificity but also inhibits Ang1-mediated functions, provides a potential tool with which to dissect the molecular mechanisms of the angiopoietins. Moreover, the use of ANG9-4 or its derivatives to specifically inhibit Ang1, either alone or in combination with inhibition of Ang2, may have therapeutic benefits.
RNA aptamers are promising tools with which to study the angiopoietins in vitro and in vivo. The use of 2′-modified nucleotides confers resistance to nuclease degradation, and 2′-fluoro-modified RNAs have plasma half-lives of greater than 12 hours [37]. The in vivo circulating half-life of these aptamers can be improved further by the addition of PEG or cholesterol moieties to their 5′ ends [38]. The full-length ANG9-4 aptamer (96 nucleotides) can be transcribed in vitro in quantities sufficient for cell culture and some small animal models. However, chemical synthesis, with or without 5′ modification, is inefficient for RNAs greater than 60 nucleotides in length. Work is ongoing to truncate and modify the full-length aptamer for large-scale synthesis and application in animal models.
Acknowledgments
This work was supported in part by a grant from the Duke University Comprehensive Cancer Center to BAS and CDK, NIH grant HL70165 to CDK, and a post-doctoral fellowship award from the Howard Hughes Medical Institute to RRW.
References
- 1.Jones N, Iljin K, Dumont DJ, et al. Tie receptors: new modulators of angiogenic and lymphangiogenic responses. Nat Rev Mol Cell Biol. 2001;2:257–267. doi: 10.1038/35067005. [DOI] [PubMed] [Google Scholar]
- 2.Dumont DJ, Gradwohl G, Fong GH, et al. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes & Development. 1994;8:1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 3.Lin P, Polverini P, Dewhirst M, et al. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie-2 in pathologic vascular growth. J Clin Invest. 1997;100:2072–2078. doi: 10.1172/JCI119740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato TN, Tozawa Y, Deutsch U, et al. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–74. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- 5.Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the Tie-2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 6.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 7.Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the Tie-2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 8.Gale NW, Thurston G, Hackett SF, et al. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–423. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim I, Kim HG, So JN, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 10.Papapetropoulos A, Fulton D, Mahboubi K, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 11.Kim I, Moon SO, Park SK, et al. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ Res. 2001;89:477–479. doi: 10.1161/hh1801.097034. [DOI] [PubMed] [Google Scholar]
- 12.Thurston G, Suri C, Smith K, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 13.Fiedler U, Reiss Y, Scharpfenecker M, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 14.Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 15.Kuroda K, Sapadin A, Shoji T, et al. Altered expression of angiopoietins and Tie2 endothelium receptor in psoriasis. J Invest Dermatol. 2001;116:713–720. doi: 10.1046/j.1523-1747.2001.01316.x. [DOI] [PubMed] [Google Scholar]
- 16.Gravallese EM, Pettit AR, Lee R, et al. Angiopoietin-1 is expressed in the synovium of patients with rheumatoid arthritis and is induced by tumour necrosis factor alpha. Ann Rheum Dis. 2003;62:100–107. doi: 10.1136/ard.62.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hackett SF, Wiegand S, Yancopoulos G, et al. Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol. 2002;192:182–187. doi: 10.1002/jcp.10128. [DOI] [PubMed] [Google Scholar]
- 18.Hattori K, Dias S, Heissig B, et al. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machein MR, Knedla A, Knoth R, et al. Angiopoietin-1 promotes tumor angiogenesis in a rat glioma model. Am J Pathol. 2004;165:1557–1570. doi: 10.1016/S0002-9440(10)63413-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shim WS, Teh M, Bapna A, et al. Angiopoietin 1 promotes tumor angiogenesis and tumor vessel plasticity of human cervical cancer in mice. Exp Cell Res. 2002;279:299–309. doi: 10.1006/excr.2002.5597. [DOI] [PubMed] [Google Scholar]
- 21.Zadeh G, Reti R, Koushan K, et al. Regulation of the pathological vasculature of malignant astrocytomas by angiopoietin-1. Neoplasia. 2005;7:1081–1090. doi: 10.1593/neo.05424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad SA, Liu W, Jung YD, et al. The effects of angiopoietin-1 and -2 on tumor growth and angiogenesis in human colon cancer. Cancer Res. 2001;61:1255–1259. [PubMed] [Google Scholar]
- 23.Hayes AJ, Huang WQ, Yu J, et al. Expression and function of angiopoietin-1 in breast cancer. Br J Cancer. 2000;83:1154–1160. doi: 10.1054/bjoc.2000.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stoeltzing O, Ahmad SA, Liu W, et al. Angiopoietin-1 inhibits vascular permeability, angiogenesis, and growth of hepatic colon cancer tumors. Cancer Res. 2003;63:3370–3377. [PubMed] [Google Scholar]
- 25.Yu Q, Stamenkovic I. Angiopoietin-2 is implicated in the regulation of tumor angiogenesis. Am J Pathol. 2001;158:563–570. doi: 10.1016/S0002-9440(10)63998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fitzwater T, Polisky B. A SELEX primer. Methods Enzymol. 1996;267:275–301. doi: 10.1016/s0076-6879(96)67019-0. [DOI] [PubMed] [Google Scholar]
- 27.White RR, Shan S, Rusconi CP, et al. Inhibition of rat corneal angiogenesis by a nuclease-resistant RNA aptamer specific for angiopoietin-2. Proc Natl Acad Sci U S A. 2003;100:5028–5033. doi: 10.1073/pnas.0831159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters KG, Coogan A, Berry D, et al. Expression of Tie2/TEK in breast tumour vasculature provides a new marker for evaluation of tumour angiogenesis. Br J Cancer. 1998;77:51–56. doi: 10.1038/bjc.1998.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong I, Lohman TM. A double-filter method for nitrocellulose-filter binding: application to protein-nucleic acid interactions. Proc Natl Acad Sci U S A. 1993;90:5428–5432. doi: 10.1073/pnas.90.12.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yancopoulos GD, Davis S, Gale NW, et al. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 31.Sarraf-Yazdi S, Mi J, Moeller BJ, et al. Inhibition of in vivo tumor angiogenesis and growth via systemic delivery of an angiopoietin 2-specific RNA aptamer. J Surg Res. 2008;146:16–23. doi: 10.1016/j.jss.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 32.Oliner J, Min H, Leal J, et al. Suppression of angiogenesis and tumor growth by selective inhibition of angiopoietin-2. Cancer Cell. 2004;6:507–516. doi: 10.1016/j.ccr.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 33.Lemieux C, Maliba R, Favier J, et al. Angiopoietins can directly activate endothelial cells and neutrophils to promote proinflammatory responses. Blood. 2005;105:1523–1530. doi: 10.1182/blood-2004-09-3531. [DOI] [PubMed] [Google Scholar]
- 34.Nykanen AI, Pajusola K, Krebs R, et al. Common protective and diverse smooth muscle cell effects of AAV-mediated angiopoietin-1 and -2 expression in rat cardiac allograft vasculopathy. Circ Res. 2006;98:1373–1380. doi: 10.1161/01.RES.0000225987.52765.13. [DOI] [PubMed] [Google Scholar]
- 35.Shim WS, Teh M, Mack PO, et al. Inhibition of angiopoietin-1 expression in tumor cells by an antisense RNA approach inhibited xenograft tumor growth in immunodeficient mice. Int J Cancer. 2001;94:6–15. doi: 10.1002/ijc.1428. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Wu KC, Zhang DX, et al. Antisense angiopoietin-1 inhibits tumorigenesis and angiogenesis of gastric cancer. World J Gastroenterol. 2006;12:2450–2454. doi: 10.3748/wjg.v12.i15.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green LS, Jellinek D, Bell C, et al. Nuclease-resistant nucleic acid ligands to vascular permeability factor/vascular endothelial growth factor. Chem Biol. 1995;2:683–695. doi: 10.1016/1074-5521(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 38.White RR, Sullenger BA, Rusconi CP. Developing aptamers into therapeutics. J Clin Invest. 2000;106:929–934. doi: 10.1172/JCI11325. [DOI] [PMC free article] [PubMed] [Google Scholar]


