Abstract
In a hospital-based case-control study of 805 non-Hispanic whites with cutaneous melanoma and 841cancer-free age-, sex- and ethnicity-matched control subjects, three VDR polymorphisms (i.e., TaqI, BsmI, and FokI) were genotyped using blood samples collected between 1994 and 2006. We tested the hypothesis that the haplotypes and combined genotypes of these polymorphisms were associated with melanoma risk by interacting with known risk factors. Haplotypes t-B-F (adjusted odds ratio [OR], 0.52; 95 percent confidence interval [CI], 0.34–0.80) and t-B-f (adjusted OR, 0.51; CI, 0.27–0.94) were associated with a reduced risk when compared with T-b-f. The combined genotypes Tt+tt/Bb+BB/Ff+ff (adjusted OR, 0.69; CI, 0.52, 0.90) and Tt+tt/Bb+BB/FF (adjusted OR, 0.58; CI, 0.43, 0.78) were also associated with reduced risk, whereas the combined genotype TT/Bb+BB/Ff+ff genotype (adjusted OR, 2.35; CI, 1.13, 4.98) was associated with increased risk when compared with TT/bb/Ff+ff genotypes. On multivariate analysis, only the TaqI polymorphism was an independent risk factor, while the FokI polymorphism interacted with skin color (p = 0.029), moles (p = 0.017), and first-degree relatives with any cancer (p = 0.013) in modifying risk. Together, these findings suggest that VDR polymorphisms may directly effect or modify the risk associated with known melanoma risk factors. Larger, population-based studies are needed to replicate our findings.
Keywords: case-control studies, vitamin D receptor, genetic polymorphisms, genotypes, melanoma
Interaction
Vitamin D is involved in a variety of biological processes including bone metabolism, immunomodulation, and regulation of cell proliferation and differentiation 1–4. Vitamin D is also known to have a potential protective effect against cancers, including cutaneous melanoma 5, 6, a lethal skin cancer that has an increasing incidence in the United States over the last 30 years 7. Vitamin D exerts its tumor-suppressive effects by binding to the vitamin D receptor (VDR). A ubiquitously expressed intracellular polypeptide that belongs to the steroid/retinoid receptor superfamily of nuclear receptors, VDR specifically binds to 1,252D3 and interacts with target cell nuclei 8. The VDR protein is overexpressed in malignant melanocytes responsive to vitamin D’s antiproliferative effects 2. Several studies have suggested that VDR polymorphisms may alter the functions of genes involved in cell division and adhesion 2, 9, thus implicating such polymorphisms in melanoma development 10.
Located on chromosome 12q12-q14, VDR contains at least five promoter regions 11, eight protein-coding exons, and six untranslated exons, all of these regions bejng alternatively spliced 12. VDR at least has 196 single nucleotide polymorphisms (SNPs) (http://egp.gs.washington.edu/data/vdr/vdrxx.csnps.txt), of which 64 lie in the promoter region, 32 in the 3′ and 5′ untranslated regions, and 2 synonymous and 2 nonsynonymous SNPs in the coding region. FokI (exon 2, rs10735810), BsmI (intron 8, rs1544410), and TaqI (exon 9, rs731236) are the three most frequently investigated SNPs for their associations with various cancers 13–15. Some studies also addressed gene-environment interactions since environmental factors (e.g., sunlight) can influence vitamin D production 16.
Although ultraviolet light plays an important role in melanoma development 17, 18, only three studies to date have assessed the associations between this factor, VDR polymorphisms, and melanoma risk. Moreover, no studies have examined whether VDR polymorphisms modulate the risk associated with other established melanoma risk factors 19–21. Therefore, we conducted a relatively large case-control study of non-Hispanic whites (i.e., 805 patients with melanoma and 841 cancer-free controls) to determine whether the haplotypes and combined genotypes of VDR polymorphisms TaqI, BsmI, and FokI are associated with melanoma risk and whether these polymorphisms can modify the risk associated with known melanoma risk factors.
MATERIALS AND METHODS
Study subjects
The study protocol was approved by The University of Texas M. D. Anderson Cancer Center institutional review board, and written informed consent was obtained from all subjects. The subject recruitment has been described previously 20, 22, 23. In brief, all patients with newly diagnosed, histologically confirmed 24 and untreated cutaneous melanoma, who were referred to M. D. Anderson Cancer Center between May 1994 and February 2006, were recruited as case subjects. Because most of the patients (~99 percent) were non-Hispanic whites, the few minority subjects who were recruited were excluded from analysis. Although there were no restrictions on patient age or tumor stage, only those patients who were free of metastases or other cancers and agreed to donate a blood sample were included in the present study. Approximately 85 percent of eligible patients recruited for this study agreed to participate. Cancer-free control subjects were recruited during the same period from among cancer-free visitors to M. D. Anderson Cancer Center who were accompanying patients to our outpatient clinics, were not seeking medical care, and were not related by blood to the patients. Approximately 90 percent of eligible control subjects recruited for this study agreed to participate. The control subjects were matched by frequency to case subjects by age (±5 years), sex, and ethnicity.
After giving informed consent, all subjects completed a self-administered questionnaire that elicited information on demographic factors (e.g., age, sex, education, and income), ethnicity, medical history, family history, and sunlight exposure history (i.e., tanning ability, lifetime number of severe sunburns, and freckling in the sun as a child) 25. Then, each subject was interviewed in person to assess his or her host characteristics (e.g., natural hair, eye, and skin color) as well as self-reported skin conditions (e.g., color, moles, and pigmented nevi). After each interview, a sample of blood (30 mL) was drawn from the subject and collected in a heparinized tube.
Genotyping
Genotyping was performed as follows. First, 1 mL of each whole blood sample was centrifuged to isolate a leukocyte cell pellet from the buffy coat fraction. Genomic DNA was extracted from the pellet, purified using a DNA blood mini kit (Qiagen, Valencia, CA), and assayed for purity and concentration by spectrophotometry (i.e., absorbance at 260 nm and 280 nm). Next, DNA fragments of VDR containing the TaqI 26, BsmI 27 and FokI 10, 19 polymorphisms were amplified by polymerase chain reaction, subjected to restriction fragment length polymorphism analysis, and sequenced (Figure I). Approximately 10 percent of samples were genotyped a second time; the repeat genotyping results agreed completely with the initial results.
Figure I.
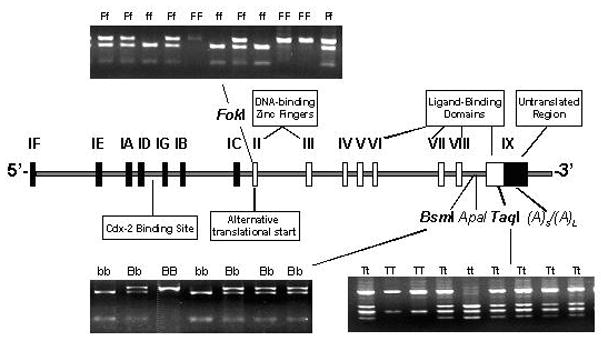
VDR gene structure and locations and genotypes of selected polymorphisms.
Statistical methods
The χ2 test was used to evaluate case-control differences in the frequency distributions of selected demographic variables, known risk factors, and each allele and genotype of the VDR polymorphisms. Because skin color was self-assessed on the screening questionnaire on a scale of 1 (light) to 10 (dark), skin colors were categorized as fair (1 or 2), brown (3 or 4), , or dark (5–10); the aim was to obtain similar numbers of observations in each stratum to facilitate further stratification analysis. Some subjects did not provide information about some variables (e.g., hair color, eye color, skin color, tanning ability, number of sunburns, freckling, pigmented nevi, and family history of skin cancer); the missing variables for those subjects were treated as missing data on multivariate analysis. The linkage disequilibrium for each SNP of interest (i.e., TaqI, BsmI, and FokI) was calculated, and the polymorphism haplotypes for each subject were reconstructed on the basis of the known TaqI, BsmI, and FokI genotypes. Because of the potential effect of locus-locus interactions of the polymorphisms on melanoma risk, associations between risk and the haplotypes and combined genotypes of the three polymorphisms were also evaluated.
Crude and adjusted odds ratios [ORs] and associated 95 percent confidence intervals (CIs) were determined by univariate and multivariate unconditional logistic regression analyses. Multivariate adjustments were made, where appropriate, for age, sex, and other known risk factors. Odds ratios, CIs, and p values for interactions and trend tests were obtained from multivariate logistic regression models.
The null hypotheses of multiplicative gene-gene interactions were tested, and departures from multiplicative interaction models were assessed empirically. A more-than-multiplicative interaction was suggested when OR11 > OR10 * OR01 28. To assess potential departures from a multiplicative model, interaction terms between variables were modeled according to standard unconditional logistic regression techniques. Finally, to determine whether the main effect of the VDR polymorphisms was independent of other known risk factors, selected variables were included in the multivariate logistic regression analyses of data from only those subjects who completely answered their questionnaires 29.
Two models were fitted. The first model included age, sex, and the three polymorphisms of interest, the aim being to control for any potential effects due to associations among the polymorphisms. The second model was to exclude the polymorphism that showed no statistically significant association with risk in the first model and then include all other known risk factors, the aim being to assess further the independent effects of the polymorphisms. A p value of ≤ 0.05 was considered statistically significant. All tests were two-sided and were performed using SAS software (version 9.13; SAS Institute, Cary, NC).
RESULTS
Population characteristics and risk factors
The initial analysis included all cases (n=805) and controls (n=841). The two groups had similar age (p = 0.37), sex (p = 0.16), education (p = 0.99), and household income (p = 0.35) (Table I). The similar age and sex distributions implied adequate frequency matching. Because some subjects did not completely answer their questionnaires, the numbers of subjects in some risk factor strata were less than the total number of subjects in the study. Nevertheless, our results were consistent with previous findings by others 30–32. Except for skin color (p = 0.16), the frequencies of known melanoma risk factors were significantly higher among cases than among controls and were associated with 1.55- to 7.78-fold increased melanoma risk (Table II). Subjects with these risk factors were placed into dichotomized groupings for further stratification and assessment of interactions in multivariate logistic regression analyses.
Table I.
Demographic distributions of non-Hispanic whites in a hospital-based case-control study of cutaneous melanoma, Texas, 1994–2006
| Variables* | Cases (n = 805) |
Controls (n = 841) |
p† | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Age (years) | 0.36 | ||||
| <39 | 123 | 15.3 | 121 | 14.4 | |
| 39–50 | 195 | 24.2 | 189 | 22.5 | |
| 51–60 | 258 | 32.0 | 258 | 30.7 | |
| >60 | 229 | 28.5 | 273 | 32.4 | |
| Sex | 0.16 | ||||
| Male | 514 | 63.8 | 565 | 67.2 | |
| Female | 291 | 36.2 | 276 | 32.8 | |
| Education (years) | 0.99 | ||||
| ≤14 | 239 | 32.7 | 235 | 32.7 | |
| >14 | 493 | 67.4 | 484 | 67.3 | |
| Household income (yearly) | 0.35 | ||||
| <$35,000 | 144 | 20.3 | 127 | 18.4 | |
| ≥$35,000 | 564 | 79.7 | 564 | 81.6 | |
The numbers of subjects in some of the strata were less than the total number of subjects included in this study because some subjects did not provide complete information in their screening questionnaires.
Two-sided χ2 test.
Table II.
Age and sex adjusted odds ratios for selected cutaneous melanoma risk factors, Texas, 1994–2006
| Variable | Cases* (n = 805) |
Controls* (n = 841) |
p† | Odds ratio and 95% confidence interval |
|||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | Crude odds ratio | 95 percent confidence interval | Adjusted odds ratio‡ | 95 percent confidence interval | ||
| Hair color | <0.01 | ||||||||
| Black or brown | 483 | 66.0 | 579 | 80.9 | 1.0 | Referent | 1.0 | Referent | |
| Blond(e) or red | 249 | 34.0 | 137 | 19.1 | 2.18 | 1.71, 2.77 | 2.15 | 1.69, 2.74 | |
| Eye color | <0.01 | ||||||||
| Other | 424 | 58.0 | 534 | 74.4 | 1.0 | Referent | 1.0 | Referent | |
| Blue | 307 | 42.0 | 184 | 25.6 | 2.10 | 1.68, 2.63 | 2.09 | 1.68, 2.62 | |
| Skin color | 0.16 | ||||||||
| Black or brown | 328 | 44.8 | 347 | 48.1 | 1.0 | Referent | 1.0 | Referent | |
| Fair | 404 | 55.2 | 374 | 51.9 | 1.14 | 0.93, 1.41 | 1.13 | 0.92, 1.40 | |
| Tanning ability | <0.01 | ||||||||
| Good | 173 | 23.6 | 267 | 37.1 | 1.0 | Referent | 1.0 | Referent | |
| Moderate or poor | 559 | 76.4 | 453 | 62.9 | 1.91 | 1.52, 2.40 | 1.90 | 1.51, 2.39 | |
| Lifetime sunburn with blistering | <0.01 | ||||||||
| None | 191 | 26.4 | 296 | 45.5 | 1.0 | Referent | 1.0 | Referent | |
| ≥1 time | 533 | 73.6 | 421 | 54.5 | 1.96 | 1.57, 2.45 | 1.95 | 1.56, 2.45 | |
| Freckling in the sun as a child | <0.01 | ||||||||
| No | 343 | 47.1 | 421 | 58.6 | 1.0 | Referent | 1.0 | Referent | |
| Yes | 385 | 52.9 | 297 | 41.4 | 1.59 | 1.29, 1.96 | 1.55 | 1.25, 1.91 | |
| Moles | <0.01 | ||||||||
| No | 175 | 21.7 | 289 | 34.4 | 1.0 | Referent | 1.0 | Referent | |
| Yes | 630 | 78.3 | 552 | 65.6 | 1.89 | 1.51, 2.35 | 1.88 | 1.51, 2.34 | |
| Atypical nevi | <0.01 | ||||||||
| No | 740 | 91.9 | 832 | 98.9 | 1.0 | referent | 1.0 | referent | |
| Yes | 65 | 8.1 | 9 | 1.1 | 8.12 | 4.02, 16.4 | 7.78 | 3.84, 15.8 | |
| First–degree relatives with any cancer | <0.01 | ||||||||
| No | 343 | 42.6 | 431 | 51.3 | 1.0 | Referent | 1.0 | Referent | |
| Yes | 461 | 57.4 | 410 | 48.7 | 1.42 | 1.17, 1.72 | 1.45 | 1.19, 1.76 | |
The numbers of subjects in some of the strata were less than the total number of subjects included in this study because some subjects did not provide complete information in their screening questionnaires.
Two-sided χ2 test.
Adjusted by age and sex.
VDR allele and genotype distributions and association with melanoma risk
Allele and genotype frequencies of the polymorphisms of interest are presented in Table III. Genotype distributions among controls were consistent with the Hardy-Weinberg equilibrium (p = 0.49 for TaqI, p = 0.31 for BsmI, and p = 0.64 for FokI). TaqI alleles t and BsmI alleles B were significantly less frequent among cases than among controls (0.370 vs. 0.429 [p < 0.01] and 0.394 vs. 0.431 [p = 0.03], respectively), whereas the FokI allele f was more frequent, though not significantly so (0.378 vs. 0.356 [p = 0.20]). This suggested that t, B, and F might protect carriers against melanoma or T, b, and f might put them at risk. Moreover, the t and B genotypes (i.e., Tt+tt and Bb+BB) were consistently less frequent among cases than among controls (p < 0.05 for both) and were associated with a significantly lower melanoma risk (i.e., a protective effect) for Tt+tt vs. TT genotypes (adjusted OR [CI], 0.72 [0.58, 0.90] and 0.68, [0.56, 0.83] and Bb+BB vs. bb genotypes, respectively (Table III). In contrast, the f genotypes (i.e., ff+Ff) were significantly more frequent among cases than among controls and were associated with a significantly greater melanoma risk than was the FF genotype (adjusted OR [CI], 1.25 [1.03, 1.53]) (Table III).
Table III.
Genotype and allele frequencies of the VDR polymorphisms among non-Hispanic whites in a case-control study of cutaneous melanoma, Texas, 1994–2006
| VDR genotype | Case (n = 805) |
Control (n = 841) |
p† | Odds ratio and 95% confidence interval |
|||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | Crude odds ratio | 95 percent confidence interval | Adjusted odds ratio‡ | 95 percent confidence interval | ||
| TaqI | <0.01§ | ||||||||
| TT | 330 | 41.0 | 269 | 32.0 | 1 | Referent | 1 | Referent | |
| Tt | 355 | 44.1 | 422 | 50.2 | 0.69 | 0.55, 0.86 | 0.69 | 0.56, 0.86 | |
| tt | 120 | 14.9 | 150 | 17.8 | 0.65 | 0.49, 0.87 | 0.66 | 0.49, 0.87 | |
| Tt +tt | 475 | 59.0 | 572 | 68.0 | <0.01§§ | 0.68 | 0.55, 0.83 | 0.68 | 0.56, 0.83 |
| t allele frequency | 0.370 | 0.429 | <0.01§§§ | ||||||
| BsmI | 0.02§ | ||||||||
| bb | 305 | 37.9 | 265 | 31.5 | 1 | Referent | 1 | Referent | |
| Bb | 366 | 45.5 | 427 | 50.8 | 0.75 | 0.60, 0.92 | 0.75 | 0.60, 0.93 | |
| BB | 134 | 16.6 | 149 | 17.7 | 0.78 | 0.59, 1.04 | 0.78 | 0.59, 1.05 | |
| Bb+BB | 500 | 62.1 | 576 | 68.5 | <0.01§§ | 0.75 | 0.58, 0.92 | 0.72 | 0.58, 0.90 |
| B allele frequency | 0.394 | 0.431 | 0.03§§§ | ||||||
| FokI | 0.05§ | ||||||||
| FF | 287 | 35.7 | 344 | 40.9 | 1 | Referent | 1 | Referent | |
| Ff | 427 | 53.0 | 396 | 47.1 | 1.29 | 1.05, 1.59 | 1.30 | 1.05, 1.60 | |
| ff | 91 | 11.3 | 101 | 12.0 | 1.08 | 0.78, 1.49 | 1.08 | 0.78, 1.49 | |
| Ff +ff | 518 | 64.4 | 497 | 59.1 | 0.03§§ | 1.25 | 1.02, 1.53 | 1.25 | 1.03, 1.53 |
| f allele frequency | 0.378 | 0.356 | 0.20§§§ | ||||||
The observed distribution of genotype frequency among the control subjects appeared to be in Hardy Weinberg equilibrium (χ2 = 0.49, p = 0.49 for TaqI; χ2 = 1.04, p = 0.31 for BsmI; and χ2 = 0.64, p = 0.43 for FokI).
Two-sided χ2 test for distributions of either genotype or allele frequency.
distribution of three genotypes;
distribution of combined genotypes;
allele distribution.
Odds ratios were adjusted for age and sex in a logistic regression model.
Association between VDR haplotypes or combined genotypes and melanoma risk
TaqI, BsmI, and FokI polymorphisms were in linkage disequilibrium (t and B alleles: D′ = 0.918, R2 = 0.855, p < 0.001; t and F alleles: D′ = 0.039, R2 = 0.001, p < 0.001; B and F alleles: D′ = 0.027, R2 = 0.001, p < 0.001), suggesting a potentially joint effect of the haplotypes of the three VDR polymorphisms on melanoma risk. Eight hypothetical haplotypes were estimated based on the observed genotypes (Table IV). However, the overall distributions of these haplotypes did not significantly differ between cases and controls (p = 0.381). When the Tbf haplotype was used as the referent (the T, b, and f alleles being putatively associated with increased melanoma risk), the haplotypes tBF and tBf were both associated with a significantly reduced melanoma risk (adjusted OR [CI], 0.52 [0.33, 0.79]) and (0.51 [0.27, 0.94], respectively) (Table IV). This suggested that the tB haplotype was protective, regardless of the f allele’s presence or absence.
Table IV.
Age- and sex-adjusted odds ratios for association between cutaneous melanoma risk and presence of haplotypes and combined genotypes of VDR TaqI, BsmI, and FokI in non-Hispanic whites, Texas, 1994–2006
| Haplotype | Cases (n = 1610 alleles) | Controls (n = 1682 alleles) | Odds ratio and 95% confidence interval | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TaqI | BsmI | FokI | No. | % | No. | % | Crude odds ratio | 95 percent confidence interval | Adjusted odds ratio † | 95 percent confidence interval |
| T | b | f | 329 | 20.4 | 314 | 18.7 | 1.0 | Referent | 1.0 | Referent |
| T | b | F | 586 | 36.4 | 609 | 36.2 | 0.69 | 0.42,1.12 | 0.69 | 0.42, 1.11 |
| T | B | f | 16 | 1.0 | 8 | 0.5 | 3.81 | 0.51, 28.5 | 4.02 | 0.53, 30.4 |
| T | B | F | 36 | 2.2 | 29 | 1.7 | 1.47 | 0.50, 4.31 | 1.48 | 0.50, 4.36 |
| t | b | F | 19 | 1.2 | 22 | 1.3 | 0.39 | 0.09, 1.73 | 0.39 | 0.09, 1.72 |
| t | b | f | 13 | 0.8 | 12 | 0.7 | 1.06 | 0.14, 7.88 | 1.09 | 0.14, 8.07 |
| t | B | F | 378 | 23.5 | 424 | 25.2 | 0.52 | 0.33, 0.79 | 0.52 | 0.34, 0.80 |
| t | B | f | 233 | 14.5 | 264 | 15.7 | 0.51 | 0.27, 0.94 | 0.51 | 0.27, 0.94 |
| Combined genotype | Cases (n =805) | Controls (n = 841 | Odds ratio and 95% confidence interval | |||||||
| TaqI | BsmI | FokI | No. | % | No. | % | Crude odds ratio | 95 percent confidence interval | Adjusted odds ratio † | 95 percent confidence interval |
| TT | bb | Ff+ff | 181 | 22.5 | 142 | 16.9 | 1.0 | Referent | 1.0 | Referent |
| TT | bb | FF | 99 | 12.3 | 105 | 12.5 | 0.74 | 0.52, 1.05 | 0.74 | 0.52, 1.05 |
| TT | Bb+BB | Ff+ff | 30 | 3.7 | 10 | 1.2 | 2.35 | 1.11, 4.98 | 2.35 | 1.13, 4.98 |
| TT | Bb+BB | FF | 20 | 2.5 | 12 | 1.4 | 1.31 | 0.62, 2.76 | 1.31 | 0.62, 2.77 |
| Tt+tt | bb | Ff+ff | 18 | 2.2 | 12 | 1.4 | 1.18 | 0.55, 2.52 | 1.18 | 0.55, 2.54 |
| Tt+tt | bb | FF | 7 | 0.9 | 6 | 0.7 | 0.92 | 0.30, 2.78 | 0.94 | 0.31, 2.85 |
| Tt+tt | Bb+BB | Ff+ff | 289 | 35.9 | 333 | 39.6 | 0.68 | 0.52, 0.89 | 0.69 | 0.52, 0.90 |
| Tt+tt | Bb+BB | FF | 161 | 20.0 | 221 | 26.3 | 0.57 | 0.42, 0.77 | 0.58 | 0.43, 0.78 |
Odds ratios were adjusted for age and sex in a logistic regression model.
p values for trend were obtained in a logistic regression model after adjustment for age and sex.
p values for interaction were obtained from logistic regression models after adjustment for age, sex, and the main effects of the interactive variables.
When the putative risk genotypes (i.e., TT, bb, and ff+Ff) were combined and used as the referent (TT/bb/ff+Ff), only the Tt+tt/Bb+BB/Ff+ff and Tt+tt/Bb+BB/FF genotypes were associated with a significantly reduced melanoma risk (adjusted OR [CI], 0.69 [0.52, 0.90] and 0.58 [0.43, 0.78], respectively), whereas the TT/Bb+BB/Ff+ff genotype was associated with a significantly increased risk (adjusted OR [CI], 2.35 [1.13, 4.98]). Together, these findings suggested that the Tt+tt/Bb+BB genotypes were protective, consistent with the effect of the tB haplotype, and that the Bb+BB genotypes were not protective in the presence of the TT genotype (Table IV).
Association between melanoma risk and polymorphism genotypes stratified by known risk factors
Because the FokI variants were associated with increased melanoma risk and the TaqI and BsmI variants with reduced risk, subjects bearing the protective TaqI and BsmI variant genotypes were further stratified by the Ff+ff and FF genotypes and all known melanoma risk factors (Table V). In the Ff+ff subgroup, the Tt+tt genotypes were associated with a significantly lower risk of melanoma than was the TT genotype, provided the carriers of the Tt+tt genotypes were old, male, and blue-eyed; had not freckled in the sun as a child; or had no pigmented nevi. In contrast, the protective Bb+BB genotypes were associated with a significantly lower risk than was the bb genotype only if the carriers were old (Table V).
Table V.
Association between cutaneous melanoma risk and VDR TaqI, BsmI, and VDR FokI genotypes, stratified by risk factors, in non-Hispanic whites, Texas, 1994–2006
| Variable | FokI Ff+ff (no. cases/no. controls) | FokI FF (no. cases/no. controls) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TaqI | BsmI | TaqI | BsmI | |||||||||||||||||
| TT | Tt+tt | Odds ratio‡ | CI | p§ | bb | Bb+BB | Odds ratio‡ | CI | p§ | TT | Tt+tt | Odds ratio‡ | CI | p§ | bb | Bb+BB | Odds ratio‡ | CI | p§ | |
| Age (years) | 0.03 | 0.01 | 0.80 | 0.71 | ||||||||||||||||
| ≤50 | 39/55 | 75/78 | 1.35 | 0.80, 2.27 | 35/51 | 79/82 | 1.40 | 0.82–2.38 | 87/56 | 117/121 | 0.62 | 0.41, 0.95 | 85/58 | 119/119 | 0.68 | 0.45, 1.04 | ||||
| >50 | 80/62 | 93/149 | 0.50 | 0.33, 0.76 | 71/60 | 102/151 | 0.58 | 0.38–0.89 | 124/96 | 190/224 | 0.66 | 0.48, 1.92 | 114/96 | 200/224 | 0.76 | 0.54, 1.05 | ||||
| Sex | 0.18 | 0.17 | 0.19 | 0.05 | ||||||||||||||||
| Male | 78/73 | 104/157 | 0.62 | 0.41, 0.93 | 69/68 | 113/162 | 0.69 | 0.46–1.04 | 132/108 | 200/227 | 0.74 | 0.54, 1.02 | 121/111 | 211/224 | 0.89 | 0.64, 1.22 | ||||
| Female | 41/44 | 64/70 | 1.00 | 0.58, 1.72 | 37/43 | 68/71 | 1.11 | 0.64–1.93 | 79/44 | 107/118 | 0.50 | 0.32, 0.79 | 78/43 | 108/119 | 0.50 | 0.32, 0.78 | ||||
| Skin color | 0.07 | 0.65 | 0.70 | |||||||||||||||||
| Black or brown | 55/43 | 72/86 | 0.66 | 0.39, 1.09 | 54/38 | 68/71 | 0.55 | 0.32–0.92 | 82/61 | 119/157 | 0.55 | 0.36, 0.83 | 74/64 | 127/154 | 0.70 | 0.47, 1.06 | ||||
| Fair | 55/53 | 81/109 | 0.74 | 0.45, 1.19 | 44/54 | 73–91 | 1.08 | 0.66–1.77 | 104/62 | 164/150 | 0.65 | 0.44, 0.96 | 104/61 | 164/151 | 0.65 | 0.44, 0.96 | ||||
| Hair color | 0.45 | 0.81 | 0.05 | 0.03 | ||||||||||||||||
| Black or brown | 70/77 | 105/55 | 0.76 | 0.51, 1.15 | 64/73 | 111/159 | 0.81 | 0.53, 1.22 | 124/92 | 184/255 | 0.54 | 0.39, 0.75 | 119/93 | 189/254 | 0.58 | 0.42, 0.81 | ||||
| Blond(e) or red | 40/18 | 48/39 | 0.57 | 0.28, 1.14 | 34/18 | 54/39 | 0.73 | 0.36, 1.50 | 62/31 | 99/49 | 1.04 | 0.60, 1.82 | 59/32 | 102/48 | 1.20 | 0.69, 2.09 | ||||
| Eye color | 0.12 | 0.26 | 0.38 | 0.44 | ||||||||||||||||
| Other | 63/77 | 93/144 | 0.79 | 0.52, 1.21 | 57/73 | 99/148 | 0.86 | 0.56, 1.33 | 63/46 | 93/60 | 0.55 | 0.39, 0.79 | 106/90 | 162/233 | 0.62 | 0.44, 0.87 | ||||
| Blue | 46/17 | 60/51 | 0.42 | 0.21, 0.82 | 40/17 | 66/51 | 0.53 | 0.27, 1.04 | 77/17 | 144/51 | 0.73 | 0.45, 1.20 | 72/35 | 129/81 | 0.79 | 0.48, 1.29 | ||||
| Tanning ability | 0.44 | 0.05 | 0.74 | 0.83 | ||||||||||||||||
| Good (high) | 30/31 | 41/75 | 0.55 | 0.29, 1.05 | 31/28 | 40/78 | 0.43 | 0.22, 0.83 | 40/48 | 62/113 | 0.67 | 0.40, 1.14 | 40/50 | 62/111 | 0.71 | 0.42, 1.20 | ||||
| Poor (low) | 80/65 | 112/119 | 0.77 | 0.51, 1.17 | 67/64 | 125/120 | 1.00 | 0.65, 1.53 | 146/75 | 221/193 | 0.60 | 0.42, 0.84 | 138/75 | 229/193 | 0.66 | 0.47, 0.92 | ||||
| Lifetime sunburn with blistering | 0.83 | 0.92 | 0.42 | 0.93 | ||||||||||||||||
| None | 34/38 | 49/84 | 0.63 | 0.35, 1.14 | 31/38 | 52/84 | 0.73 | 0.40, 1.32 | 38/50 | 70/124 | 0.75 | 0.45, 1.25 | 39/48 | 69/126 | 0.68 | 0.40, 1.14 | ||||
| ≥1 | 74/156 | 105/111 | 0.71 | 0.45, 1.10 | 65/52 | 114/115 | 0.79 | 0.50, 1.23 | 146/173 | 208/181 | 0.58 | 0.41, 0.82 | 137/77 | 217/177 | 0.70 | 0.49, 0.98 | ||||
| Freckling in the sun as a child | 0.45 | 0.71 | 0.10 | 0.23 | ||||||||||||||||
| No | 62/56 | 71/109 | 0.57 | 0.35, 0.91 | 54/54 | 79/111 | 0.69 | 0.42, 1.11 | 83/83 | 127/173 | 0.74 | 0.50, 1.08 | 80/82 | 130/174 | 0.77 | 0.53, 1.13 | ||||
| Yes | 48/39 | 81/85 | 0.77 | 0.45, 1.30 | 44/37 | 85/87 | 0.81 | 0.47, 1.38 | 102/40 | 154/133 | 0.46 | 0.30, 0.71 | 98/43 | 158/130 | 0.55 | 0.36, 0.84 | ||||
| Moles | 0.94 | 0.67 | 0.35 | 0.65 | ||||||||||||||||
| No | 25/30 | 43/71 | 0.73 | 0.38, 1.41 | 21/30 | 47/71 | 0.98 | 0.50, 1.92 | 40/47 | 67/141 | 0.57 | 0.34, 0.95 | 39/53 | 68/135 | 0.70 | 0.42, 1.16 | ||||
| Yes | 94/87 | 125/156 | 0.76 | 0.52, 1.11 | 85/81 | 134/162 | 0.81 | 0.55, 1.19 | 171/105 | 240/204 | 0.74 | 0.54, 1.01 | 160/101 | 251/208 | 0.79 | 0.57, 1.07 | ||||
| Dysplastic nevi | 0.98 | 0.51 | 0.28 | 0.24 | ||||||||||||||||
| No | 113/115 | 155/227 | 0.70 | 0.50, 0.97 | 100/110 | 168/232 | 0.80 | 0.57, 1.12 | 190/151 | 282/339 | 0.66 | 0.51, 0.86 | 178/153 | 294/337 | 0.75 | 0.57, 0.98 | ||||
| Yes | 6/2 | 13/0 | NC | 6/1 | 13/1 | 0.48 | 0.01, 40.2 | 21/1 | 25/6 | 0.07 | 0.01, 0.99 | 21/1 | 25/6 | 0.07 | 0.01, 0.98 | |||||
| First-degree relatives with any cancer | 0.36 | 0.94 | 0.49 | 0.63 | ||||||||||||||||
| No | 43/67 | 67/122 | 0.86 | 0.53, 1.40 | 42/62 | 68/127 | 0.79 | 0.48, 1.29 | 104/77 | 129/165 | 0.58 | 0.40, 0.85 | 96/77 | 137/165 | 0.68 | 0.46, 0.99 | ||||
| Yes | 76/50 | 101/105 | 0.64 | 0.41, 1.00 | 64/49 | 113/106 | 0.83 | 0.52, 1.31 | 107/75 | 178/180 | 0.70 | 0.49, 1.00 | 103/77 | 182/178 | 0.77 | 0.53, 1.10 | ||||
The numbers of subjects in some of the strata were less than the total number of subjects included in this study because some subjects did not provide the information.
CI, confidence interval.
Odds ratios were adjusted for age and sex in a logistic regression model.
p values for interaction were obtained from logistic regression models after adjustment for age, sex, and the main effects of the interactive variables.
In the FF subgroup, the Tt+tt genotypes were more likely to be associated with reduced risk in carriers who were young, female, black- or brown-skinned, black- or brown-haired, or non-blue-eyed; had poor tanning ability, had ≥ 1 lifetime sunburn with blistering, had a childhood history of freckling in the sun, had no moles or pigmented nevi, or had no family history of cancer. The same was generally true of the Bb+BB genotypes, except that being young was not a risk factor. Further tests for interaction were significant for age (p = 0.03 for TaqI and p = 0.01 for BsmI) among subjects carrying the Ff+ff genotype and for sex (p = 0.05 for BsmI) and hair color (p = 0.05 for TaqI and p = 0.03 for BsmI) among subjects carrying the FF genotype. However, these findings may have been due to chance since multiple tests were performed.
Multivariate analysis of association between polymorphisms and melanoma risk
All variables used in the initial analyses were fitted to two multivariate unconditional logistic models after simultaneous adjustment (Table VI). In the first model, which included the age, sex, and polymorphism genotypes for all subjects, the t genotypes (Tt+tt vs. TT) and f genotypes (Ff+ff vs. FF), but not the B genotypes (Bb+BB vs. bb), were associated with a significantly reduced melanoma risk (OR [CI], 0.57 [0.39, 0.84] for TaqI and 1.27 [1.04, 1.55] for FokI). This suggested that the BsmI polymorphism was not an independent melanoma risk factor, consistent with the high linkage disequilibrium between the t and B alleles. Consequently, BsmI was excluded from the second model, and all other selected risk factors were added to the multivariate logistic regression model. The second model included only data from subjects who provided complete questionnaire data (i.e., 712 case subjects and 707 control subjects). Most of the known risk factors were consequently found to be significant independent predictors of melanoma risk, the exceptions being age, sex, skin color, and childhood freckling. Because skin color may be represented by hair or eye color and freckling by the number of sunburns in the same model, and because there was a high correlation between these variables in our study (data not shown), variance in the model was reduced by excluding skin color and freckling from the final model (Table VI). As a result, the VDR TaqI t variant genotypes assessed in the final model were associated with a significantly reduced melanoma risk (OR [CI], 0.68 [0.54, 0.86]), whereas the FokI f variant genotypes were not (1.14 [0.91–1.44]) (Table VI). Further tests for interaction revealed significant associations between the FokI f genotypes and skin color (p = 0.029), moles (p = 0.017), and a family history of cancer (p = 0.013) but not between the TaqI t genotypes and the same variables (data not shown). Since multiple tests were performed, these interactions are only suggestive and require validation in larger future studies.
Table VI.
Multivariate logistic regression analysis of associations between VDR TaqI, BsmI, and FokI genotype frequencies and cutaneous melanoma risk in non-Hispanic whites, Texas, 1994–2006
| Variable | β | Wald χ2− | p | Odds ratio | 95 percent confidence interval |
|---|---|---|---|---|---|
| Model 1 (805 cases and 841 controls) | |||||
| Age (years) | −0.007 | 2.59 | 0.11 | 0.99 | 0.99, 1.00 |
| Sex (male vs. female) | 0.13 | 1.51 | 0.22 | 1.14 | 0.93, 1.40 |
| VDR BsmI (Bb+BB vs. bb) | 0.21 | 1.11 | 0.29 | 1.23 | 0.84, 1.82 |
| VDR FokI (Ff+ff vs. FF) | 0.24 | 5.39 | 0.02 | 1.27 | 1.04, 1.55 |
| VDR TaqI (Tt+tt vs. TT) | −0.57 | 8.20 | 0.004 | 0.57 | 0.39, 0.84 |
| Model 2 (712 cases and 707 controls) * | |||||
| Age (years) | −0.01 | 0.11 | 0.74 | 1.00 | 0.99, 1.01 |
| Sex (male vs. female) | 0.08 | 0.42 | 0.52 | 1.08 | 0.88, 1.37 |
| Eye color (other vs. blue) | 0.58 | 21.7 | <0.001 | 1.79 | 1.40, 2.29 |
| Hair color (blond[e] or red vs. black or brown) | 0.46 | 11.3 | <0.001 | 1.58 | 1.21, 2.07 |
| Lifetime sunburns with blistering (≥1 vs. 0) | 0.45 | 12.9 | <0.001 | 1.56 | 1.23, 1.99 |
| Tanning ability after prolonged sun exposure (moderate or poor vs. good) | 0.37 | 8.55 | 0.003 | 1.45 | 1.13, 1.86 |
| Moles (yes vs. no) | 0.65 | 27.9 | <0.001 | 1.92 | 1.51, 2.44 |
| Pigmented nevi (yes vs. no) | 1.70 | 20.9 | <0.001 | 5.46 | 2.64, 11.3 |
| Family history of skin cancer (yes vs. no) | 0.27 | 5.25 | 0.02 | 1.31 | 1.04, 1.65 |
| VDR FokI (Ff+ff vs. FF) | 0.15 | 1.55 | 0.21 | 1.16 | 0.92, 1.46 |
| VDR TaqI (Tt+tt vs. TT) | −0.38 | 10.3 | 0.001 | 0.68 | 0.54, 0.86 |
The numbers of subjects included in this model were less than the total number of subjects included in this study because this model only included subjects who provided complete information in their screening questionnaires.
DISCUSSION
In this hospital-based case-control study of cutaneous melanoma, we found that TaqI t and BsmI B variant genotypes of the VDR gene (Tt+tt and Bb+BB, respectively) were associated with a reduced risk of melanoma and FokI f variant genotypes (Ff and Ff+ff) with an increased risk when compared with the TT, bb, and FF genotypes, respectively. The tBF and tBf haplotypes were associated with a significantly lower melanoma risk than was the Tbf haplotype. The VDR FokI polymorphisms appeared to interact with other known risk factors to modulate the melanoma risk associated with those factors, while the VDR TaqI polymorphism appeared to exert its protective (i.e., risk-reducing) effect independently of other risk factors.
The VDR protein is expressed in both melanocytes and melanoma cells, and 1,25-[OH]2D3 can apparently inhibit the growth of both normal and malignant melanocytes in vitro 2, 5, 33. However, malignant transformation may inhibit the anticancer actions of 1,252D3 for reasons that include genetic polymorphisms of the VDR gene 34. The VDR gene comprises nine exons harboring several polymorphisms, including a poly-A microsatellite in the 3′ flanking region 35, changes in intron 8 that generate BsmI 36 and ApaI restriction enzyme sites 37, a synonymous change at codon 352 in exon 9 that generates a TaqI restriction enzyme site 38, and a 5′ FokI site in exon 2 8. No apparent association has been found between the TaqI or Bsm1 polymorphisms and altered functional activities. Nevertheless, both of TaqI and BsmI polymorphisms located near to the 3′ end of the gene, thus are thought to affect mRNA stability and VDR gene transcription regulation 39. Among the VDR polymorphisms, the FokI singlenucleotide polymorphism of the translation start site is the only one that results in a VDR protein with a different structure40. This polymorphism is characterized by the presence of either two ATG start codons separated by six nucleotides in the long f-VDR or only one start codon due to a T-to-C substitution in the most 50 ATG codon, resulting in a 3-aa shorter F-VDR protein (424 aa in stead of 427 aa)41.
To date, two other groups have reported their studies on TaqI, BsmI, or FokI polymorphisms in evaluating their association with melanoma risk 19–21 but generated mixed results. In the earliest study of the TaqI polymorphism in 316 melanoma patients and 108 control subjects, neither the Tt nor the combined Tt+tt genotype was associated with altered melanoma risk when compared with the TT genotype 19. However, in the present large study, we found that the t genotypes were in fact associated with a lower melanoma risk. In the Nurses’ Health Study, investigators examined the association between melanoma risk and BsmI polymorphism in 219 melanoma patients and 873 controls and found no association between the two21. However, in our present study, we found an association between Bb+BB genotypes and reduced melanoma risk only in women who carried the FF genotype and not in men (Table V). We found the Ff and Ff+ff genotypes to be associated with increased melanoma risk. Interestingly, even though this finding was consistent with published data from other group 19, it was not consistent with the finding in the Nurses’ Health Study of an association (though not significant) between only the ff genotype and higher melanoma risk21.
There are several possible reasons for the apparent discrepancy between our results and those reported by others. One is the relatively larger size of our control population, and another is the potential for selection bias in our control population. Our present study, with its 805 melanoma cases and 841 control subjects, is the largest study so far to have addressed the possible association between VDR polymorphism and melanoma risk; in addition, the VDR t, B, and f allele frequencies we report here are similar to those reported previously in a large meta-analysis of studies in whites 14. Therefore, we believe it unlikely that the association between the VDR polymorphisms and melanoma risk demonstrated in the present study was biased by our selection of controls. A third possible reason for the discrepancy between our findings and those previously reported is variations in the serum vitamin D levels of study subjects between studies. Indeed, the serum vitamin D level may have affected our results. Unfortunately, none of the studies published so far gathered data on serum vitamin D levels in their subjects. A fourth possible reason is recall biases in exposure data, to which a retrospective study might be prone. Thus, larger, population-based studies are needed to verify our findings.
The functional significance of the VDR TaqI polymorphisms is unknown. As a synonymous change in exon 9, the TaqI polymorphism does not cause the amio acid substitution (http://egp.gs.washington.edu/data/vdr/vdrxx.csnps.txt; http://snp500cancer.nci.nih.gov/snplist.cfm). In addition, no apparent association has been found between the BsmI polymorphism and altered functional activities42. Nevertheless, these polymorphisms might be functional themselves or in linkage disequilibrium with other functional SNPs and associated with melanoma risk. Indeed, previous in vitro functional studies have revealed the baT haplotype (haplotype of Bsm1/ApaI/TaqI) inserted in transfection constructs resulted in lower reporter gene activity compared with BAt 43 and associated with low VDR mRNA expression 44, which is in agreement with our findings that both of the BsmI B allele and TaqI t allele are protective against melanoma in the present study. VDR FokI is the only polymorphism that is not linked to any of the other VDR polymorphisms41. A study recently provided evidence that the VDR FokI polymorphism affects immune cell behavior, with a more active immune system for the short F-VDR45. Consistent with this functional study, we found Ff+ff genotype associated with significantly increased melanoma risk, which might due to the f allele-related reduced anti-tumor immune activity. However, since some in vitro data may not accurately reflect the biologic environment, in which a marker may be acting in humans, our consideration regarding the putative function of VDR polymorphisms should be adequately validated in further functional studies.
As some epidemiologic studies have suggested, adequate vitamin D levels (including sunlight induced) may provide very important protection against colon, breast, and prostate cancers 46–48. However, its protection against skin cancer is a more complex issue. One potential complication is that ultraviolet light exposure not only promotes vitamin D-3 (cholecalciferol) synthesis in the skin but also increases the risk of skin cancer by inducing DNA damage. Therefore, it is very important to consider gene-environment interactions as well as locus-locus interactions when studying associations between VDR polymorphisms and melanoma risk. For example, one recent case-only analysis study revealed an association between the TaqI tt genotype and reduced prostate cancer risk, but only in association with high sun exposure 16. However, in the present study, we found that the TaqI t variant genotypes exerted their protective effects independently of other genotypes and known risk factors. Meanwhile, the effect of FokI genotypes on melanoma risk appeared to be independent of the TaqI polymorphism but dependent on other known risk factors, suggesting that some environmental modification of the VDR gene may have occurred. Indeed, we found that the FokI polymorphism interacted with the known melanoma risk factors of skin color, moles, and family history of cancer. However, because of our study’s limited size and current uncertainty about the biological mechanisms underlying such interactions, these findings are only suggestive. Again, larger, population-based, and functional studies are necessary to validate these interactions.
The present study has several limitations. First, it was a hospital-based case-control study in which selection of the unrepresentive population and retrospective collection of exposure data may have led to uncontrolled biases. Second, despite being the largest study of its kind ever published, the present study was still too underpowered to detect gene-gene or gene-environment interactions. Third, the self-reporting of skin conditions by both case and control subjects created an additional source of potential bias. Finally, like most previous studies on the subject, ours could not account for serum vitamin D and thus did not allow for genotype-phenotype correlation analysis. These limitations can only be overcome in large, well-designed prospective studies that gather data on both genotypes and phenotypes of vitamin D metabolism.
In summary, the VDR TaqI, BsmI, and FokI polymorphisms and their combined variant genotypes do affect melanoma risk. The VDR TaqI polymorphism alters risk independently of BsmI, FokI, and other known melanoma risk factors, while the VDR FokI polymorphism may modify it through interaction with sun exposure-related melanoma risk factors. Larger, population-based studies are needed to confirm these findings.
Acknowledgments
We thank Margaret Lung, Cesar A. Maldonado, and Amanda Francofor assistance in recruiting subjects; Zhaozheng Guo, Yawei Qiao, Jianzhong He, and Kejing Xu for laboratory assistance; Monica Domingue for assistance in preparing the manuscript; and Jude Richard, ELS, for editing the manuscript.
Grant sponsors: National Cancer Institute grants R01 CA 100264 (QW) and P50 CA 093459 (EAG) and National Institute of Environmental Health Sciences grants R01 ES11740 (QW) and P30 CA16672 (M. D. Anderson Cancer Center).
Abbreviations
- CI
95 percent confidence interval
- OR
odds ratio
- SNP
single nucleotide polymorphism
- VDR
vitamin D receptor
References
- 1.Zhu KJ, Zhou WF, Zheng M. 1 alpha, 25-dihydroxyvitamin D3 and its analogues modulate the phagocytosis of human monocyte-derived dendritic cells. Yao Xue Xue Bao. 2002;37:94–7. [PubMed] [Google Scholar]
- 2.Seifert M, Rech M, Meineke V, Tilgen W, Reichrath J. Differential biological effects of 1,25-dihydroxyVitamin D3 on melanoma cell lines in vitro. J Steroid Biochem Mol Biol. 2004;89–90:375–9. doi: 10.1016/j.jsbmb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Dang ST, Lu XH, Zhou J, Bai L. Effects of 1alpha, 25-dihydroxyvitamin D3 on the acute immune rejection and corneal neovascularization in high-risk penetrating keratoplasty in rats. Di Yi Jun Yi Da Xue Xue Bao. 2004;24:892–6. 903. [PubMed] [Google Scholar]
- 4.Vieth R. The role of vitamin D in the prevention of osteoporosis. Ann Med. 2005;37:278–85. doi: 10.1080/07853890510007313. [DOI] [PubMed] [Google Scholar]
- 5.Osborne JE, Hutchinson PE. Vitamin D and systemic cancer: is this relevant to malignant melanoma? Br J Dermatol. 2002;147:197–213. doi: 10.1046/j.1365-2133.2002.04960.x. [DOI] [PubMed] [Google Scholar]
- 6.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, Holick MF. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96:252–61. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geller AC, Miller DR, Annas GD, Demierre MF, Gilchrest BA, Koh HK. Melanoma incidence and mortality among US whites, 1969–1999. Jama. 2002;288:1719–20. doi: 10.1001/jama.288.14.1719. [DOI] [PubMed] [Google Scholar]
- 8.Baker AR, McDonnell DP, Hughes M, Crisp TM, Mangelsdorf DJ, Haussler MR, Pike JW, Shine J, O’Malley BW. Cloning and expression of full-length cDNA encoding human vitamin D receptor. Proc Natl Acad Sci U S A. 1988;85:3294–8. doi: 10.1073/pnas.85.10.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593–8. doi: 10.1111/1523-1747.ep12477126. [DOI] [PubMed] [Google Scholar]
- 10.Li C, Liu Z, Zhang Z, Strom SS, Gershenwald JE, Prieto VG, Lee JE, Ross MI, Mansfield PF, Cormier JN, Duvic M, Grimm EA, et al. Genetic variants of the vitamin D receptor gene alter risk of cutaneous melanoma. J Invest Dermatol. 2007;127:276–80. doi: 10.1038/sj.jid.5700544. [DOI] [PubMed] [Google Scholar]
- 11.Fang Y, van Meurs JB, d’Alesio A, Jhamai M, Zhao H, Rivadeneira F, Hofman A, van Leeuwen JP, Jehan F, Pols HA, Uitterlinden AG. Promoter and 3′-untranslated-region haplotypes in the vitamin d receptor gene predispose to osteoporotic fracture: the rotterdam study. Am J Hum Genet. 2005;77:807–23. doi: 10.1086/497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crofts LA, Hancock MS, Morrison NA, Eisman JA. Multiple promoters direct the tissue-specific expression of novel N-terminal variant human vitamin D receptor gene transcripts. Proc Natl Acad Sci U S A. 1998;95:10529–34. doi: 10.1073/pnas.95.18.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen WY, Bertone-Johnson ER, Hunter DJ, Willett WC, Hankinson SE. Associations between polymorphisms in the vitamin D receptor and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:2335–9. doi: 10.1158/1055-9965.EPI-05-0283. [DOI] [PubMed] [Google Scholar]
- 14.Ntais C, Polycarpou A, Ioannidis JP. Vitamin D receptor gene polymorphisms and risk of prostate cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2003;12:1395–402. [PubMed] [Google Scholar]
- 15.Liu Z, Calderon JI, Zhang Z, Sturgis EM, Spitz MR, Wei Q. Polymorphisms of vitamin D receptor gene protect against the risk of head and neck cancer. Pharmacogenet Genomics. 2005;15:159–65. doi: 10.1097/01213011-200503000-00004. [DOI] [PubMed] [Google Scholar]
- 16.John EM, Schwartz GG, Koo J, Van Den Berg D, Ingles SA. Sun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancer. Cancer Res. 2005;65:5470–9. doi: 10.1158/0008-5472.CAN-04-3134. [DOI] [PubMed] [Google Scholar]
- 17.Boscoe FP, Schymura MJ. Solar ultraviolet-B exposure and cancer incidence and mortality in the United States, 1993–2002. BMC Cancer. 2006;6:264. doi: 10.1186/1471-2407-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wei Q, Lee JE, Gershenwald JE, Ross MI, Mansfield PF, Strom SS, Wang LE, Guo Z, Qiao Y, Amos CI, Spitz MR, Duvic M. Repair of UV light-induced DNA damage and risk of cutaneous malignant melanoma. J Natl Cancer Inst. 2003;95:308–15. doi: 10.1093/jnci/95.4.308. [DOI] [PubMed] [Google Scholar]
- 19.Hutchinson PE, Osborne JE, Lear JT, Smith AG, Bowers PW, Morris PN, Jones PW, York C, Strange RC, Fryer AA. Vitamin D receptor polymorphisms are associated with altered prognosis in patients with malignant melanoma. Clin Cancer Res. 2000;6:498–504. [PubMed] [Google Scholar]
- 20.Li C, Larson D, Zhang Z, Liu Z, Strom SS, Gershenwald JE, Prieto VG, Lee JE, Ross MI, Mansfield PF, Cormier JN, Duvic M, et al. Polymorphisms of the FAS and FAS ligand genes associated with risk of cutaneous malignant melanoma. Pharmacogenet Genomics. 2006;16:253–63. doi: 10.1097/01.fpc.0000199501.54466.de. [DOI] [PubMed] [Google Scholar]
- 21.Han J, Colditz GA, Hunter DJ. Polymorphisms in the MTHFR and VDR genes and skin cancer risk. Carcinogenesis. 2007;28:390–7. doi: 10.1093/carcin/bgl156. [DOI] [PubMed] [Google Scholar]
- 22.Li C, Hu Z, Liu Z, Wang LE, Strom SS, Gershenwald JE, Lee JE, Ross MI, Mansfield PF, Cormier JN, Prieto VG, Duvic M, et al. Polymorphisms in the DNA repair genes XPC, XPD, and XPG and risk of cutaneous melanoma: a case-control analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:2526–32. doi: 10.1158/1055-9965.EPI-06-0672. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Liu Z, Wang LE, Strom SS, Lee JE, Gershenwald JE, Ross MI, Mansfield PF, Cormier JN, Prieto VG, Duvic M, Grimm EA, et al. Genetic variants of the ADPRT, XRCC1 and APE1 genes and risk of cutaneous melanoma. Carcinogenesis. 2006;27:1894–901. doi: 10.1093/carcin/bgl042. [DOI] [PubMed] [Google Scholar]
- 24.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Jr, Kirkwood JM, McMasters KM, Mihm MF, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–48. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 25.Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124:869–71. doi: 10.1001/archderm.124.6.869. [DOI] [PubMed] [Google Scholar]
- 26.Taylor JA, Hirvonen A, Watson M, Pittman G, Mohler JL, Bell DA. Association of prostate cancer with vitamin D receptor gene polymorphism. Cancer Res. 1996;56:4108–10. [PubMed] [Google Scholar]
- 27.Hou MF, Tien YC, Lin GT, Chen CJ, Liu CS, Lin SY, Huang TJ. Association of vitamin D receptor gene polymorphism with sporadic breast cancer in Taiwanese patients. Breast Cancer Res Treat. 2002;74:1–7. doi: 10.1023/a:1016048900049. [DOI] [PubMed] [Google Scholar]
- 28.Brennan P, Lewis S, Hashibe M, Bell DA, Boffetta P, Bouchardy C, Caporaso N, Chen C, Coutelle C, Diehl SR, Hayes RB, Olshan AF, et al. Pooled analysis of alcohol dehydrogenase genotypes and head and neck cancer: a HuGE review. Am J Epidemiol. 2004;159:1–16. doi: 10.1093/aje/kwh003. [DOI] [PubMed] [Google Scholar]
- 29.Wang LE, Hsu TC, Xiong P, Strom SS, Duvic M, Clayman GL, Weber RS, Lippman SM, Goldberg LH, Wei Q. 4-Nitroquinoline-1-oxide-induced mutagen sensitivity and risk of nonmelanoma skin cancer: a case-control analysis. J Invest Dermatol. 2007;127:196–205. doi: 10.1038/sj.jid.5700481. [DOI] [PubMed] [Google Scholar]
- 30.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Abeni D, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: I. Common and atypical naevi. Eur J Cancer. 2005;41:28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 31.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer. 2005;41:45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Zanetti R, Masini C, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer. 2005;41:2040–59. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 33.Reichrath J, Tilgen W, Diedrich K, Friedrich M. Vitamin D analogs in cancer prevention and therapy. Anticancer Res. 2006;26:2511–4. [PubMed] [Google Scholar]
- 34.Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta. 2006;371:1–12. doi: 10.1016/j.cca.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Ingles SA, Ross RK, Yu MC, Irvine RA, La Pera G, Haile RW, Coetzee GA. Association of prostate cancer risk with genetic polymorphisms in vitamin D receptor and androgen receptor. J Natl Cancer Inst. 1997;89:166–70. doi: 10.1093/jnci/89.2.166. [DOI] [PubMed] [Google Scholar]
- 36.Morrison NA, Yeoman R, Kelly PJ, Eisman JA. Contribution of trans-acting factor alleles to normal physiological variability: vitamin D receptor gene polymorphism and circulating osteocalcin. Proc Natl Acad Sci U S A. 1992;89:6665–9. doi: 10.1073/pnas.89.15.6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Faraco JH, Morrison NA, Baker A, Shine J, Frossard PM. ApaI dimorphism at the human vitamin D receptor gene locus. Nucleic Acids Res. 1989;17:2150. doi: 10.1093/nar/17.5.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hustmyer FG, DeLuca HF, Peacock M. ApaI, BsmI, EcoRV and TaqI polymorphisms at the human vitamin D receptor gene locus in Caucasians, blacks and Asians. Hum Mol Genet. 1993;2:487. doi: 10.1093/hmg/2.4.487. [DOI] [PubMed] [Google Scholar]
- 39.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–56. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 40.Arai H, Miyamoto K, Taketani Y, Yamamoto H, Iemori Y, Morita K, Tonai T, Nishisho T, Mori S, Takeda E. A vitamin D receptor gene polymorphism in the translation initiation codon: effect on protein activity and relation to bone mineral density in Japanese women. J Bone Miner Res. 1997;12:915–21. doi: 10.1359/jbmr.1997.12.6.915. [DOI] [PubMed] [Google Scholar]
- 41.Nejentsev S, Godfrey L, Snook H, Rance H, Nutland S, Walker NM, Lam AC, Guja C, Ionescu-Tirgoviste C, Undlien DE, Ronningen KS, Tuomilehto-Wolf E, et al. Comparative high-resolution analysis of linkage disequilibrium and tag single nucleotide polymorphisms between populations in the vitamin D receptor gene. Hum Mol Genet. 2004;13:1633–9. doi: 10.1093/hmg/ddh169. [DOI] [PubMed] [Google Scholar]
- 42.Zmuda JM, Cauley JA, Ferrell RE. Molecular epidemiology of vitamin D receptor gene variants. Epidemiol Rev. 2000;22:203–17. doi: 10.1093/oxfordjournals.epirev.a018033. [DOI] [PubMed] [Google Scholar]
- 43.Morrison NA, Qi JC, Tokita A, Kelly PJ, Crofts L, Nguyen TV, Sambrook PN, Eisman JA. Prediction of bone density from vitamin D receptor alleles. Nature. 1994;367:284–7. doi: 10.1038/367284a0. [DOI] [PubMed] [Google Scholar]
- 44.Carling T, Rastad J, Akerstrom G, Westin G. Vitamin D receptor (VDR) and parathyroid hormone messenger ribonucleic acid levels correspond to polymorphic VDR alleles in human parathyroid tumors. J Clin Endocrinol Metab. 1998;83:2255–9. doi: 10.1210/jcem.83.7.4862. [DOI] [PubMed] [Google Scholar]
- 45.van Etten E, Verlinden L, Giulietti A, Ramos-Lopez E, Branisteanu DD, Ferreira GB, Overbergh L, Verstuyf A, Bouillon R, Roep BO, Badenhoop K, Mathieu C. The vitamin D receptor gene FokI polymorphism: functional impact on the immune system. Eur J Immunol. 2007;37:395–405. doi: 10.1002/eji.200636043. [DOI] [PubMed] [Google Scholar]
- 46.Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1985;1:307–9. doi: 10.1016/s0140-6736(85)91082-7. [DOI] [PubMed] [Google Scholar]
- 47.Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–9. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 48.Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women’s Health Study Am J Epidemiol. 1993;137:1302–17. doi: 10.1093/oxfordjournals.aje.a116640. [DOI] [PubMed] [Google Scholar]


