Summary
The brain tumor stem cell (BTSC) hypothesis is based on the premise that there is a subpopulation of cells within tumors with tumorigenic and pluripotent properties. BTSC are believed to be responsible for both the initiation of brain tumors and their resistance to current therapeutic modalities. This new paradigm stresses the need for adequate techniques to culture and characterize this special population of cells. Furthermore, the use of different cell migration assays offers the possibility to evaluate the processes involved in glioma metastasis. In this chapter, we summarize a method to culture, analyze the cellular characteristics, and study the invasion of BTSCs using a neurosphere assay, cryostat sectioning, and human organotypic brain cortex migration assay, respectively.
Keywords: Brain tumor stem cell, Neurospheres, Human organotypic cultures, GBM, Cell migration
1. Introduction
The invasion of tumor cells within normal tissue is thought to be a multifactorial process, requiring the expression of specific proteins, activation of various enzymes, and formation of different types of cell interactions (1). The diffuse infiltration of glioblastoma multiforme (GBM) cells into the healthy brain parenchyma makes complete surgical resection nearly impossible. In fact, there is a recurrence incidence of 99% following gross total resection of these tumors (1–3). Nevertheless, not all the tumor cells have the ability to form a new tumor (4–6). There is increasing evidence that suggests this tumor-initiating ability resides only in a specific subpopulation of cells with characteristics similar to normal neural stem cells (NSC) (5, 7, 8). These cells have been aptly named brain tumor stem cells (BTSC) because they, like NSC, possess self-renewal and multipotential properties, with the added ability to initiate tumor growth (8). Therefore, the development of a BTSC migration model that accurately recapitulates what occurs in the human brain is essential for the study of tumor invasion.
The first findings that showed evidence of glioma-derived BTSC were obtained by Steindler and colleagues (7). With the use of single-cell cultures in a methyl-cellulose (MC) matrix and the addition of epidermal growth factor (EGF) and fibroblast growth factor (FGF), they showed that glioma-derived cells were able to form clones in the MC matrix (7). These clonal cells were also able to express markers specific for glial or neuronal cells (7). Subsequently, several groups have also shown that these cells, like NSCs, have self-renewal and multipotential capabilities (4–6, 9–11). In addition, they had the capability of forming tumors at low cell concentrations (100–1,000 cells). More importantly, they formed tumors that recapitulated the histological characteristics of the parent tumor when implanted into an animal model (8, 11, 12). Interestingly, cells within other tumors, including medulloblastomas (4, 8) and ependymomas (13), also possess these same BTSC characteristics. These findings have led many to believe that brain tumors are initiated and maintained by a small population of BTSC that possess self-renewal, multipotentiality, and tumor-initiating capacity (14).
Advances in research have created the need for experimental techniques to study both NSC and BTSC. Neurosphere assays are currently the standard for identifying these unique stem cell populations (15–17). These assays utilize a selective serum-free culture system that allows NSC and BTSC to proliferate and generate multipotent floating cell clusters called neurospheres (15–17). The neurosphere assay protocols, however, are not uniform and vary significantly between studies. Therefore, the use of specific culture and passaging protocols, as well as different characterization methods, is necessary to correctly identify, maintain, and characterize a true BTSC population (15, 17).
The characterization of BTSC neurospheres using immunocytochemistry (ICC) is difficult due to their floating condition, size, and fragility. As a result, different techniques have been implemented for their staining. This includes the use of a cytospin device (Thermo scientific, USA) to centrifuge the neurospheres against a glass slide (9) or manually adhering neurospheres to a plate (18) for future staining, as well as flotation staining protocols (15). These techniques have significant disadvantages because they deform the neurosphere architecture and prevent clear staining and visualization of the neurospheres. The use of cryostat sectioning of neurospheres, however, gives the best reported resolution without affecting the neurosphere architecture (19). This method also offers the added benefit of obtaining multiple sections from the same neurosphere. We will describe the techniques we use to section BTSC neurospheres with a cryostat, which will allow for effective characterization of these cells using immunocytochemistry.
In addition to the study of BTSC neurospheres, investigating tumor migration and invasion is essential. Understanding how brain tumor-derived cells invade normal tissue is necessary to develop effective strategies for preventing tumor recurrence, which can largely be attributed to their invasive abilities. The most commonly used approaches to study brain tumor cell migration and/or invasion in vitro include the wound healing assay (20), microliter-scale migration assay (21), spot assay (22), and transwell migration assay (23, 24). These methods, however, do not accurately represent the human brain matrix, the natural environment in which the cells migrate. The brain slice invasion assay allows the study of tumor cell invasion using actual brain matrix (25, 26). We will therefore summarize methods used to study BTSC migration using brain slice or organotypic cultures from human intraoperative specimens.
In this chapter, we will describe the techniques we use to identify and maintain GBM-derived BTSCs, as well as some of the methods for characterizing neurospheres and studying BTSC migration.
2. Materials
2.1. Neurosphere Culture from Brain Tumors
Laminar flow culture hood.
Dissecting microscope.
HBSS plus Ca and Mg (Gibco/BRL, Bethesda, MD).
HBSS without Ca and Mg (Gibco/BRL, Bethesda, MD).
Neurosphere culture media. D-MEM/F12 (1:1)(Invitrogen, Carlsbad, Ca) plus 1× B27 supplement (Gibco/BRL, Bethesda, MD), 1× Antibiotic–antimycotic (Invitrogen, Carlsbad, Ca) and 20 ng/ml of Epidermal Growth Factor (EGF) (Prepotech Inc. Rocky Hill, NY) and 20 ng/ml basic Fibroblastic Growth Factor (bFGF) (Prepotech Inc. Rocky Hill, NY). The neurosphere media can be prepared without the growth factors and stored at 4°C. The growth factors are stored in aliquots at −20°C. Complete neurosphere media is made in small volumes (50 ml) and stored for no more than 2 weeks at 4°C.
Trypsin–EDTA (Gibco/BRL, Bethesda, MD).
Microsurgical instruments (forceps, scissors, and scalpel).
Wide- and narrow-tipped fire-polished Pasteur pipettes. Pasteur pipette tips are narrowed by exposing them briefly to the flame of a Bunsen burner while spinning the pipette; check the tip frequently to get the desired caliber.
Hemocytometer or cell counter machine.
2.2. Neurosphere Cutting
4% paraformaldehyde (4% PFA) in 0.1 M Phosphate buffer. 4% PFA is prepared fresh every time and stored at 4°C for no longer than 1 week.
30% sucrose (Sigma-Aldrich St. Louis, MO) in 0.1 M PBS.
OCT compound (Sakura Finetek USA, Torrance, CA, USA).
Peel-A-Way® Embedding Mold (Polysciences Inc. Warrington, PA) for cryosectioning.
Cryostat.
2.3. Human Organotypic Model
Organotypic culture media. Minimal Essential Medium (MEM) (Sigma, St. Louis, MO, USA) containing 25% heat-inactivated horse serum (Gibco/BRL, Bethesda, MD), 25% HBSS (Gibco/BRL, Bethesda, MD) with 25.8 mg/ml of glucose, and 12 mg/ml of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (Sigma, St. Louis, MO, USA) and 1% 0.2 M glutamine (Gibco/BRL, Bethesda, MD) pH 7.2
McIlwain Tissue Chopper (Mickle laboratory engineering Co. Ltd. UK)
12-mm culture plate inserts (Millipore, Billerica, MA)
Angled dissecting microscope or surgical microscope
Stereotactic frame
Glass micropipette needle (Drummond Scientific Co. Broomall, PA). micropipettes are pulled with a needle pipette puller (David Kopf Instrument s Tujunga, CA) and beveled with a 48,000 Micropipette Beveler (World Precision Instruments Sarasota Fl). The final pipette must have a sharp tip of 30–50 µm diameter by 400–500 µm length.
3. Methods
3.1. Neurosphere Culture from Brain Tumors
Tumor sample must be transported in saline solution, PBS, or cell culture media, and kept on ice until cultured. Before starting the culture, the following materials are prepared as follows: warm DMEM/F12 plus 10% heat-inactivated FBS and 1% of 100× antibiotic–antimycotic, neurosphere culture media, and trypsin–EDTA 0.25% to 37°C in a water bath; warm HBSS medium with calcium chloride and magnesium chloride and HBSS medium without calcium chloride and magnesium chloride to room temperature; place the dissecting microscope (previously cleaned with EtOH), the sterile surgical instruments in a beaker with 96% EtOH (see Note 1), and a 10-mm Petri dish with 7 ml HBSS + Ca + Mg into a culture hood.
The tumor sample is then placed in the sterile Petri dish with HBSS + Ca + Mg, and necrotic tissue and blood vessels from the tumor are removed under the dissecting microscope (see Note 2). The clean sample is then divided into three pieces for (a) protein extraction, using an appropriate cryovial and snap freezing the sample in liquid nitrogen; (b) RNA extraction, placing the tumor in a 1.5-ml RNAse-free tube and adding 1 mL of RNA for storage at 4°C; (c) cell culture.
The clean sample that is going to be used for cell culture is dissociated enzymatically by adding 2 ml of Trypsin–EDTA and mechanically by cutting the tumor into small pieces using microdissecting scissors.
The pieces of tissue suspended in 2 ml of Trypsin–EDTA are then placed into a 15-mL conical tube to be homogenized with a sterile wide-tipped fire-polished Pasteur pipette. Pipette up and down gently until the solution becomes blurry (do not let the tissue remain in contact with trypsin for more than 10 min to prevent low cell viability). Trypsin is then inhibited by adding 3 mL of DMEM/F12 + 10% FBS media. The tissue is homogenized further with a sterile narrow-tipped fire-polished Pasteur pipette, and any remaining nonhomogenized pieces of tissue are removed by passing the cell suspension through a 40- µm cell strainer.
Cells are counted with a hemocytometer or a cell counter machine and cell viability is determined with the use of trypan blue.
Cells are centrifuged for 5 min at 180 RCF (Relative Centrifugal Force) at 4°C and serum-containing media is decanted. Pre-warmed neurosphere media is added to the cells pellet to get a final concentration of 4 × 104 cells per ml.
The cell suspension is added to nonadherent cell culture flasks (5 ml per flask) and placed in an incubator at 37°C and 5% CO 2. Culture media is changed twice a week (see Note 3) and neurospheres are passaged every 1 or 2 weeks, depending on the growth rate of each sample (see Note 4).
3.2. Neurosphere Embedding for Cryostat Sectioning
Neurospheres from the culture flasks are taken with a 20- µl pipette set to 2 µl and placed in a PCR tube. They are fixed by adding 50 µl of cold 4% paraformaldehyde for 30 min at room temperature.
Neurospheres are then centrifuged at 150 RCF for 5 min at 4°C and supernatant is removed carefully. 30% sucrose is added and the neurospheres are left in this solution for 30 min. At this moment an empty tissue embedding mold is taken and OCT compound is added to have a flat surface so as to place the neurospheres.
Neurospheres are centrifuged again at 150 RCF for 5 min at 4°C and supernatant is removed carefully.
The neurospheres are then resuspend in 50 µl of OCT compound and placed on the top of the frozen OCT in the mold, giving them enough time to freeze (see Notes 6 and 7).
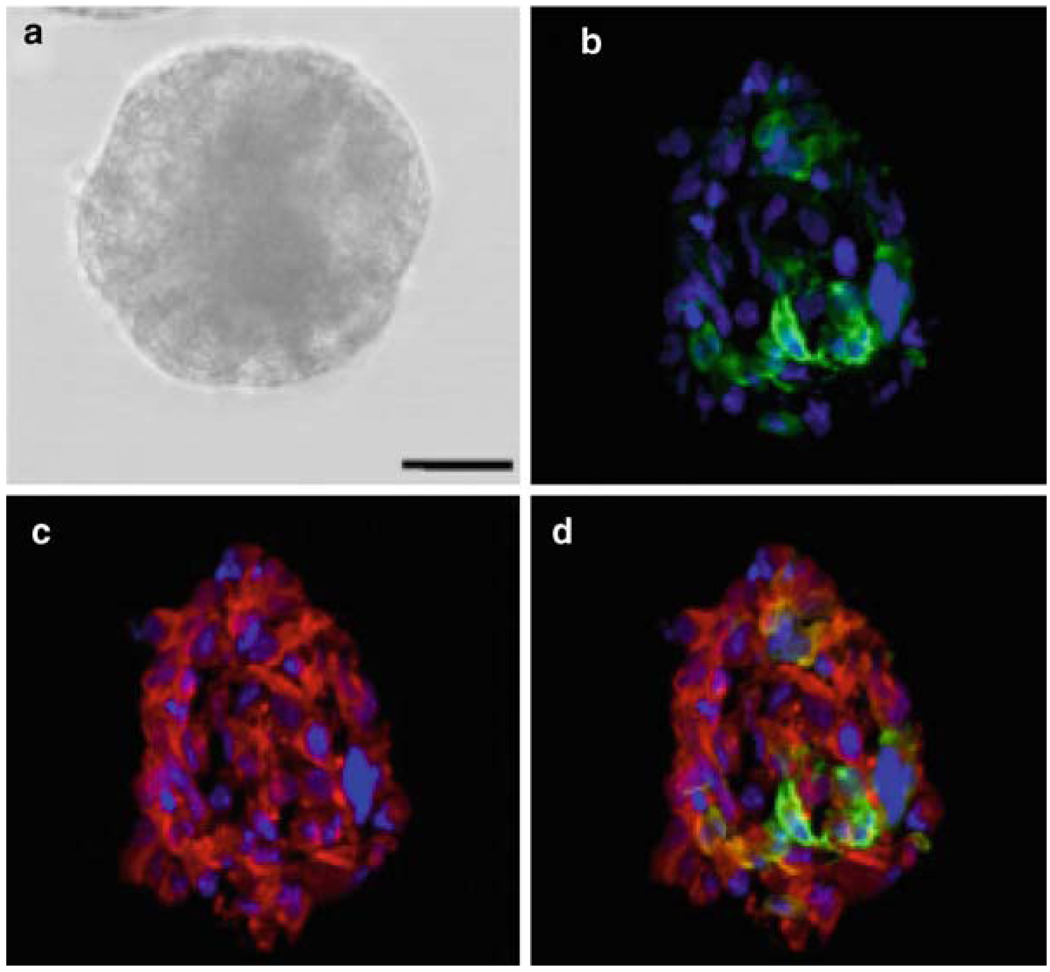
5- µm slices are obtained with a cryostat for further immunostaining (see Note 8) (Fig. 1b–d).
Fig. 1.
GBM-derived neurosphere. (a) In bright field prior to the cryosectioning protocol described; (b–d), immunostained against GFAP (green) and Nestin (red). Dapi was used as a nuclear marker. The finding of more differentiated cells in the core of the neurosphere has been reported previously (9, 15), showing the cell heterogeneity within the neurospheres.
3.3. Human Organotypic Model (27)
A 24-well culture plate is prepared by adding 500 µl of organotypic culture media and placing one 12-mm Millicell insert into each well, taking care to not create bubbles underneath the membrane.
After preparing the plate, a tumor sample (collected and transported as described on section A-1) is placed on a Sylgard plate and cut into rectangular pieces (5–20 mm/1–2 mm). The pieces are then sectioned into 350- µm thick slices using the tissue chopper and placed into a Petri Dish containing high-glucose HBSS (see Notes 9 and 10)
Each slice is put into the 12-mm Millicell inserts with the help of a paint brush (see Note 11). The plate with the organotypic cultures is then placed into an incubator at 37°C and 5% CO2. The organotypic culture media is changed every 2 days taking care to not disturb the explant (see Note 12).
After 2 days of culture, a single-cell suspension of 1 × 105 GFP labeled cells per µl is prepared to be injected into the organotypic explants.
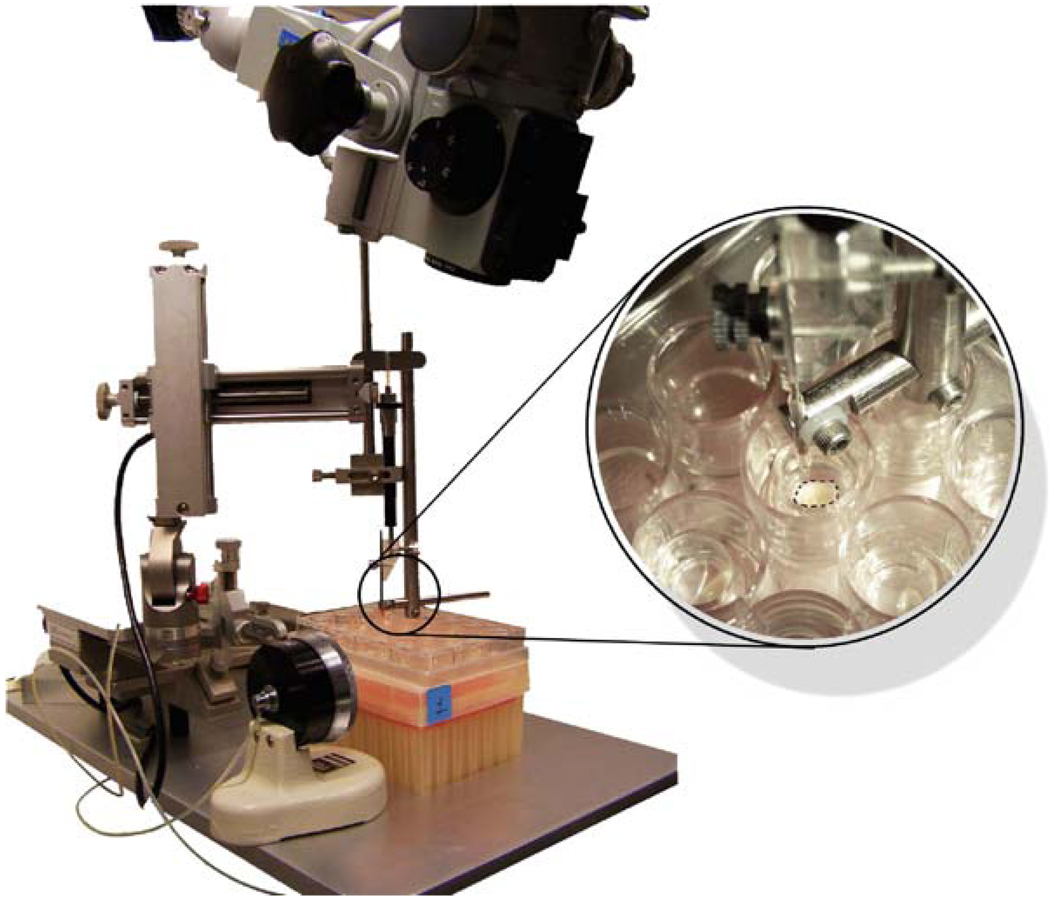
The culture plate with the tissue explants is placed in the hood on a rodent stereotactic frame, under the angled dissecting microscope to obtain the best visualization of the tissue explant inside the millicell insert (Fig. 2).
1 µl of the cell suspension is taken into the glass micropipette needle and then injected into the tissue slice with the help of the stereotactic frame, with particular attention to not penetrate the tissue with the needle.
The cells are injected at a rate of 1 µl per minute while looking under the microscope (see Note 13), and the plate is placed back into the incubator for 2 weeks, changing the culture media every 2 days.
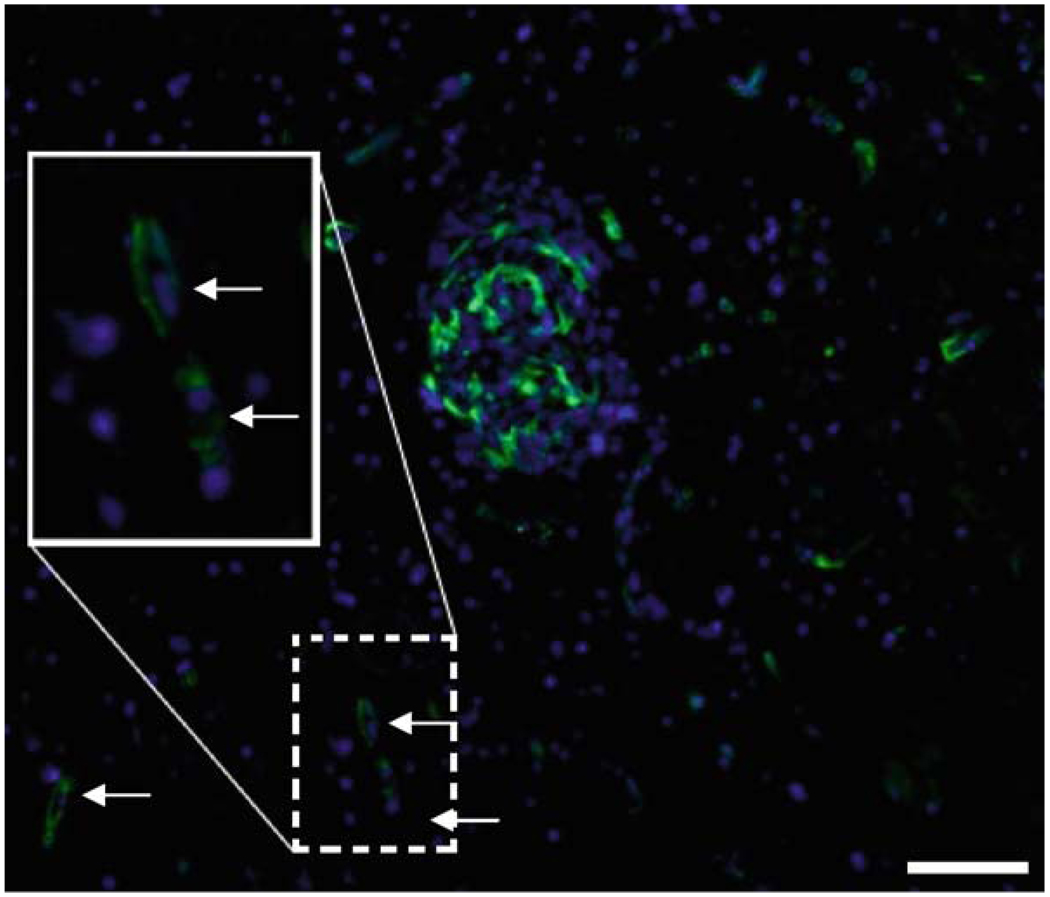
Once the experiment is concluded, the tissue explants are fixed with 4% paraformaldehyde and prepared for cryosectioning and immunostaining (Fig. 3).
Fig. 2.
Setup for the injection of human GBM-derived cells into the human organotypic cultures. The 24-well plate is placed on the stereotactic frame and the surgical microscope is used to visualize both the glass needle and the tissue. Cell injections are performed with a microinjector attached to the glass needle.
Fig. 3.
Migration of human GBM-derived cells in human brain tissue. GFP-labeled GBM cells were injected as described in the methods section, then immunostained after 6 days. DAPI was used as a nuclear marker. Some GFP-labeled GBM cells (arrows) were found to have migrated away from the injection site as shown in the inset. Scale bar = 100 µm.
Acknowledgments
The authors would like to thank Ms. Alyssa Choi for her contributions to the neurosphere-staining technique and Mr. Frank Attenello and Ms. Grettel Zamora-Berridi for their help with the organotypic culture injections. This work was supported by NIH K08NS055851, Children’s Cancer Foundation, and the American Society of Clinical Oncology.
Footnotes
In order to protect the tips of the surgical instruments, it is recommended some cotton be placed in the bottom of a beaker and filled with 96% EtOH.
Necrotic tissue can be identified by its dark color. Blood vessels need to be removed to reduce the presence of contaminant cells such as fibroblasts. Nevertheless, tumor sample are often highly vascularized, which makes it difficult to remove the vessels. In this case, try to avoid culturing the vascularized area if the sample is large enough.
To change the media, avoid the use of centrifuge since this can cause the formation of cell clumps and form structures similar to neurospheres. Preferentially leave the flasks in a vertical position to let the neurospheres precipitate, take out half of the cell culture volume, and replace it with fresh neurosphere media. Some cells can attach to the bottom of the flask and not form neurospheres. When this happens, take out the total volume of the flask and place it in a new one to avoid contact with differentiated cells
The passage of neurospheres with protocols that involve the use of enzymes is widely accepted. Some groups, however, have observed a faster neurosphere growth rate when passaged by the use of mechanical trituration instead of enzymatic digestion. To passage the neurospheres and form a single-cell suspension without the use of enzymes, centri-fuge the neurosphere cell culture for 5 min at 180 × g, discard the supernatant, and resuspend the pellet in 200 µl of neurosphere media. Triturate the pellet by pipetting using a p200- µl pipette tip, where several passes are needed to break the neurospheres. In our experience, this takes on average 200 times.
Freeze the mold with OCT compound by placing it on dry ice with ethanol. Once frozen, mark the surface of the frozen OCT compound with a permanent marker. This will help to identify the place where the neurospheres are when cutting in the cryostat.
Right before adding the OCT-neurosphere solution, take out the mold with frozen OCT from the dry ice. This will allow the OCT-neurosphere suspension to come out from the tip without freezing before getting to the mold.
To resuspend the neurospheres in OCT, add 50 µl of the embedding compound and set the micropipette to 45 µl, and slowly pipette up and down without creating bubbles.
Start cutting until the marks are visible. After the marks are visible, collect the slices and prepare for immunostaining.
When using the tissue chopper, the tissue piece may have a tendency to move as the tissue is being cut. Make sure the cutting surface is dry.
After cutting the tissue, some of the pieces will still be adhered to one another. Use a microsurgical scalpel to cut the adherent portions of the tissue.
When transferring the tissue to the Millicel inserts, try to transfer with a minimal amount of media as excess media will prevent the tissue from adhering to the membrane.
During the media changes, make sure to avoid placing media on top of the Millicel membrane as this may cause the tissue to detach.
Giving the reduce volume that can be injected into the tissue slice, special attention needs to be put on the moment when the cell suspension fills the injection place. At that point take out the needle and aspirate any suspension that could have came out to prevent the deposit of cells on the top of the tissue.
References
- 1.Demuth T, Rennert JL, Hoelzinger DB, Reavie LB, Nakada M, Beaudry C, Nakada S, Anderson EM, Henrichs AN, McDonough WS, Holz D, Joy A, Lin R, Pan KH, Lih CJ, Cohen SN, Berens ME. Glioma cells on the run - the migratory transcriptome of 10 human glioma cell lines. BMC Genomics. 2008;9:54. doi: 10.1186/1471-2164-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Filippini G, Falcone C, Boiardi A, Broggi G, Bruzzone MG, Caldiroli D, Farina R, Farinotti M, Fariselli L, Finocchiaro G, Giombini S, Pollo B, Savoiardo M, Solero CL, Valsecchi MG. Prognostic factors for survival in 676 consecutive patients with newly diagnosed primary glioblastoma. Neuro Oncol. 2008;10:79–87. doi: 10.1215/15228517-2007-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McFerrin MB, Sontheimer H. A role for ion channels in glioma cell invasion. Neuron Glia Biol. 2006;2:39–49. doi: 10.1017/S17440925X06000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemmati HD, Nakano I, Lazareff JA, Masterman-Smith M, Geschwind DH, Bronner-Fraser M, Kornblum HI. Cancerous stem cells can arise from pediatric brain tumors. Proc Natl Acad Sci U S A. 2003;100:15178–15183. doi: 10.1073/pnas.2036535100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh SK, Clarke ID, Hide T, Dirks PB. Cancer stem cells in nervous system tumors. Oncogene. 2004;23:7267–7273. doi: 10.1038/sj.onc.1207946. [DOI] [PubMed] [Google Scholar]
- 6.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 7.Ignatova TN, Kukekov VG, Laywell ED, Suslov ON, Vrionis FD, Steindler DA. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 2002;39:193–206. doi: 10.1002/glia.10094. [DOI] [PubMed] [Google Scholar]
- 8.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 9.Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, Eberhart CG. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F, Vescovi A. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- 11.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 12.Lee LM, Seftor EA, Bonde G, Cornell RA, Hendrix MJ. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dyn. 2005;233:1560–1570. doi: 10.1002/dvdy.20471. [DOI] [PubMed] [Google Scholar]
- 13.Taylor MD, Poppleton H, Fuller C, Su X, Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, Macdonald T, Rutka J, Guha A, Gajjar A, Curran T, Gilbertson RJ. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8:323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 14.Quinones-Hinojosa A, Chaichana K. The human subventricular zone: a source of new cells and a potential source of brain tumors. Exp Neurol. 2007;205:313–324. doi: 10.1016/j.expneurol.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Singec I, Knoth R, Meyer RP, Maciaczyk J, Volk B, Nikkhah G, Frotscher M, Snyder EY. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat Methods. 2006;3:801–806. doi: 10.1038/nmeth926. [DOI] [PubMed] [Google Scholar]
- 16.Chaichana K, Zamora-Berridi G, Camara-Quintana J, Quinones-Hinojosa A. Neurosphere Assays: Growth Factors and Hormone Differences in Tumor and Non-tumor Studies. Stem Cells. 2006 doi: 10.1634/stemcells.2006-0399. [DOI] [PubMed] [Google Scholar]
- 17.Gritti A, Galli R, Vescovi AL. Clonal analyses and cryopreservation of neural stem cell cultures. Methods Mol Biol. 2008;438:173–184. doi: 10.1007/978-1-59745-133-8_14. [DOI] [PubMed] [Google Scholar]
- 18.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 19.Parmar M, Sjoberg A, Bjorklund A, Kokaia Z. Phenotypic and molecular identity of cells in the adult subventricular zone. in vivo and after expansion in vitro. Mol Cell Neurosci. 2003;24:741–752. doi: 10.1016/s1044-7431(03)00239-2. [DOI] [PubMed] [Google Scholar]
- 20.Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonough W, Tran N, Giese A, Norman SA, Berens ME. Altered gene expression in human astrocytoma cells selected for migration: I. Thromboxane synthase. J Neuropathol Exp Neurol. 1998;57:449–455. doi: 10.1097/00005072-199805000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Wichterle H, Alvarez-Dolado M, Erskine L, Alvarez-Buylla A. Permissive corridor and diffusible gradients direct medial ganglionic eminence cell migration to the neocortex. Proc Natl Acad Sci U S A. 2003;100:727–732. doi: 10.1073/pnas.242721899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN. A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res. 1987;47:3239–3245. [PubMed] [Google Scholar]
- 24.Iwasaki K, Rogers LR, Barnett GH, Estes ML, Barna BP. Effect of recombinant tumor necrosis factor-alpha on three-dimensional growth, morphology, and invasiveness of human glioblastoma cells in vitro. J Neurosurg. 1993;78:952–958. doi: 10.3171/jns.1993.78.6.0952. [DOI] [PubMed] [Google Scholar]
- 25.Ohnishi T, Matsumura H, Izumoto S, Hiraga S, Hayakawa T. A novel model of glioma cell invasion using organotypic brain slice culture. Cancer Res. 1998;58:2935–2940. [PubMed] [Google Scholar]
- 26.Valster A, Tran NL, Nakada M, Berens ME, Chan AY, Symons M. Cell migration and invasion assays. Methods. 2005;37:208–215. doi: 10.1016/j.ymeth.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Chaichana KL, Capilla-Gonzalez V, Gonzalez-Perez O, Pradilla G, Han J, Olivi A, Brem H, Garcia-Verdugo JM, Quinones-Hinojosa A. Preservation of glial cytoarchitecture from ex vivo human tumor and non-tumor cerebral cortical explants: A human model to study neurological diseases. J Neurosci Methods. 2007;164:261–270. doi: 10.1016/j.jneumeth.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]