Abstract
Steatotic donors are routinely rejected for transplantation because of their increased rate of primary nonfunction. These grafts are more sensitive to ischemia/reperfusion (I/R) during transplantation. Removal of endotoxin before reperfusion improves liver performance post-I/R. We hypothesize that the main modality of injury in steatotic livers is toll-like receptor 4 (TLR4) signaling. We fed 4-week-old control and TLR4-deficient (TLR4KO) mice a normal diet (ND) or a 60% high-fat diet (HFD) for 4 weeks to induce steatosis. Mice were subjected to total hepatic ischemia (35 minutes) and reperfusion (1 or 24 hours). Survival improved and liver pathology decreased at 24 hours in TLR4KO HFD animals compared to control HFD animals. An investigation of infiltrates showed that neutrophils and CD4+ cells were increased at 24 hours in control HFD animals, whereas TLR4KO HFD animals were similar to ND controls. Messenger RNA levels of interleukin 6 (IL-6), IL-12, and interferon gamma were elevated at 1 hour in control HFD animals, whereas TLR4KO HFD animals were similar to ND controls. IL-10 levels at 1 hour of reperfusion in control HFD and TLR4KO animals were decreased versus control ND animals. In conclusion, these improvements in liver function in TLR4KO HFD animals implicate TLR4 as a mediator of steatotic graft failure after I/R.
Exacerbating the already short supply of donor livers for transplantation is the usability of steatotic donor organs. Livers, whose parenchyma contains more than 30% of fat, have a dramatically increased chance of primary nonfunction, 1 and the vast majority of these organs are discarded. Furthermore, it has been shown that the degree of steatosis correlates with higher postoperative liver enzymes and increased long-term mortality.2 These livers are more sensitive to the stresses of ischemia/reperfusion (I/R) injury, especially the stresses of endotoxin exposure, than normal, lean livers.3,4
Endotoxin from intestinal microflora is translocated across the intestinal barrier during periods of gut hypoperfusion and during periods of mesenteric congestion associated with the anhepatic phase of liver transplantation.5 Research has shown that steatotic animals are much more sensitive to the hepatic effects of endotoxin than their lean counterparts.6 In addition, previous research in our laboratory has shown that neutralization of translocated endotoxin with a monoclonal antibody, when administered intravenously before I/R, dramatically improves the survival and liver function of overtly steatotic animals after a period of warm I/R.7 The main signaling receptor for endotoxin is toll-like receptor 4 (TLR4).
So far, 13 TLRs have been identified in rodents, and each of these recognizes a specific pathogen-associated molecular pattern.8 In addition to several endogenous ligands, TLR4 specifically recognizes bacterial cell wall lipopolysaccharide (LPS) and is an essential signaling component in the endotoxin signaling pathway. Circulating LPS binds to circulating LPS binding protein, and this complex is recognized by membrane-bound or circulating CD14. The complex is then transferred to the TLR4/MD-2 complex, which signals intracellularly via 2 pathways.9 The myeloid differentiation protein 88 (MyD88)–independent pathway signals through TIR-domain-containing adapter-inducing interferon-β/translocation associated membrane protein, stimulates interferon regulatory factor 3 phosphorylation, and ultimately results in the stimulation of interferon (IFN)-inducible genes and the production of type I IFNs, including IFN-β.10 The MyD88-dependent pathway signals interleukin 1 receptor–associated kinase 1/4, goes on to phosphorylate tumor necrosis factor receptor–associated factor 6, and ultimately stimulates nuclear factor kappa B and activator protein 1, which initiate the production of proinflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and IFN-γ.11
The cytokines produced in response to endotoxin are altered in steatotic organs. Upon endotoxin stimulation, previous research has shown a polarization toward a T-helper 1 cytokine profile in a genetic obesity model3; specifically, there is an overproduction of IL-12 and IFN-γ. Accompanying this overproduction, there is a concomitant underproduction or down-regulation of T-helper 2 cytokines, such as IL-4 and IL-10. Overproduction of IL-12 and IFN-γ not only is observed in steatotic organs but also occurs in animals that are otherwise presensitized to TLR signaling (specifically TLR2 and TLR9), such as those injected with Propionibacterium acnes.12
Because endotoxin injury has been implicated in steatotic warm I/R injury, we sought to investigate the specific role of its receptor, TLR4, in steatotic I/R injury. We hypothesized that a deficiency of TLR4 would be hepatoprotective in our model of steatotic warm liver I/R and that because of the deficiency of the receptor, a decreased concentration of proinflammatory signaling molecules would be seen.
MATERIALS AND METHODS
Animals
Male, 4- to 8-week-old inbred C57BL/10J (control; Jackson Laboratory, Bar Harbor, ME) and C57BL/10ScN [TLR4-deficient (TLR4KO); National Cancer Institute, Frederick, MD] mice were used in all experiments. Three to four mice were housed per cage in a temperature-controlled room (22°C–25°C) with a 12-hour light-dark cycle, and they were provided with water and food ad libitum. All experiments were performed under clean conditions, were approved by the Medical University of South Carolina Institutional Animal Care and Use Committee, and were in accordance with the National Institutes of Health guidelines for laboratory animal usage. When it was appropriate, starting at 4 weeks of age, the animals were fed a high-fat diet (HFD; 60% of kilocalories from fat; D12492, Research Diets) ad libitum. This was continued for 4 weeks until the animals were 8 weeks old. Control animals were fed the normal chow diet. Preliminary experiments indicated a decreased survival rate in HFD animals. On the basis of careful power and statistical analysis, we increased these group sizes accordingly. Therefore, depending on the survival rate, there were 5 to 8 animals per group for final analysis.
Total Hepatic Warm I/R
I/R was performed as previously described.13 Blood was collected by sterile cardiac puncture, and whole blood was placed into serum separator tubes and allowed to coagulate for 30 minutes. It was then centrifuged for 5 minutes at 3200 rpm at room temperature to separate the serum, which was then aliquoted into pyrogen-free glass vials. Portions of the liver were placed in 10% neutral buffered formalin for histological analysis, and the remaining liver was snap-frozen in liquid nitrogen and stored at −80°C for later analysis.
Serum Alanine Aminotransferase (ALT)
ALT was evaluated with an ALT reagent (Pointe Scientific, Canton, MI) according to the manufacturer’s instructions. Briefly, 1 volume of serum was incubated with 10 volumes of the ALT reagent, and the reaction was read kinetically at 37°C for approximately 5 minutes.
Serum Endotoxin
Serum samples were diluted 1:10 in limulus amebocyte lysate reagent-grade water and boiled for 5 minutes to irreversibly denature serine proteases. Endotoxin analysis was performed according to the manufacturer’s instructions with a kinetic, turbidimetric assay (Lonza, Switzerland).
Hematoxylin and Eosin (H&E) and Oil Red O (ORO) Staining and Centrilobular Necrotic Index Grading
Upon the sacrifice of an animal, a portion of its liver tissue was placed in 10% neutral-buffered formalin for fixation, embedded in paraffin, cut, placed on a glass slide, and heat-fixed. Frozen sections were placed in liquid nitrogen immediately at sacrifice and later embedded in an optimal cutting temperature compound. Sections were cut and placed on glass slides, at which point they were stained for ORO as previously described.13 H&E staining was performed. Necrosis grading was quantified from H&E-stained slides as previously described on a scale from 0 to 3 with reference to the central vein.14 Briefly, a grade of 0 indicated absent necrosis, 1 indicated individual hepatocyte dropout, 2 indicated small foci of missing hepatocytes up to 2 to 3 cells across, and 3 indicated confluent foci of hepatocyte dropout greater than 3 cells across. Ten high-powered fields per section were analyzed with respect to the central vein. Necrotic indexing was performed in a blinded fashion.
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections were incubated with a primary antibody (GR-1, BD Biosciences, or CD3∊, Santa Cruz Biotechnology) for 1 hour after antigen retrieval with a heat-induced epitope retrieval method. Frozen, acetone-fixed sections were also incubated with a primary antibody (CD4 or CD8, BD Biosciences) without antigen retrieval. Samples were then washed and incubated with a biotinylated secondary antibody. After they were washed, sections were incubated with a Vectastain ABC kit (Vector Laboratories) for an additional hour. Immunoperoxidase staining was performed with a diaminobenzidine substrate kit (Vector Laboratories). When it was appropriate, positive cells were counted as a ratio of total cells in 10 high-powered fields per section.
Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction
Total RNA was extracted from liver samples by RNA-Bee reagent (Tel-Test, Inc., Friendswood, TX). The messenger RNA (mRNA) coding for TLR4, IL-4, IL-6, IL-10, IL-12p40, IFN-γ, TNF, and glyceraldehyde 3-phosphate dehydrogenase was quantified by SYBR Green 2-step reverse-transcription polymerase chain reaction. Briefly, 1 mg of total RNA from each sample was used for reverse transcription with the iScript complementary DNA synthesis kit (Bio-Rad, Hercules, CA) to generate first-strand complementary DNA. A polymerase chain reaction mixture was prepared with the use of SYBR Green polymerase chain reaction master mix (Perfecta SYBR Green Fastmix for iQ, Quanta Biosciences, Gaithersburg, MD).Table 1 lists the sequences of the primers. Thermal cycling conditions were 10 minutes at 95°C followed by 50 cycles of 95°C for 15 seconds and 60°C for 30 seconds on an MyiQ single-color real-time polymerase chain reaction detection system (Bio-Rad). The expression of each gene was normalized to GAPDH mRNA and calculated with respect to the baseline control with the comparative cycle threshold method.
TABLE 1.
Sequences of the Primers for SYBR Green Real-Time Reverse-Transcription Polymerase Chain Reaction
| Target | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Reference |
|---|---|---|---|
| TLR4 | GGACTCTGATCATGGCACTG | CTGATCCATGCATTGGTAGGT | 37 |
| IL-4 | ACAGGAGAAGGGACGCCAT | GAAGCCCTACAGACGAGCTCA | 38 |
| IL-6 | ACAACCACGGCCTTCCCTACTT | CACGATTTCCCAGAGAACATGTG | 39 |
| IL-10 | ACAGGAGAAGGGACGCCAT | GAAGCCCTACAGACGAGCTCA | 40 |
| IL-12p40 | GGAAGCACGGCAGCAGAATA | AACTTGAGGGAGAAGTAGAA | 40 |
| IFN-γ | TCAAGTGGCATAGATGTGGAAGAA | TGGCTCTGCAGGATTTTCATG | 38 |
| TNF | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC | 38 |
| GAPDH | TTCACCACCATGGAGAAGGC | GGCATGGACTGTGGTCATGA | 41 |
NOTE: TLR4 and cytokine levels were measured through the assessment of the levels of messenger RNA with quantitative reverse-transcription polymerase chain reaction and the primers by the methods in the accompanying references.
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IFN-γ, interferon gamma; IL, interleukin; TLR4, toll-like receptor 4; TNF, tumor necrosis factor.
Statistical Analysis
All values are expressed as the mean ± the standard error of the mean. An alpha value of 0.05 was established prior to experimentation as the limit for statistical significance. For a single pairwise comparison, a 2-tailed t test was used when samples were normally distributed. For histological analysis and samples that were not normally distributed, a Mann-Whitney U test was used. For multiple independent groups, a Kruskal-Wallis nonparametric comparison was used, with Tamhane’s test used for post hoc analysis with SPSS statistical software.
RESULTS
Establishment of a Model of Steatosis in TLR4KO Animals
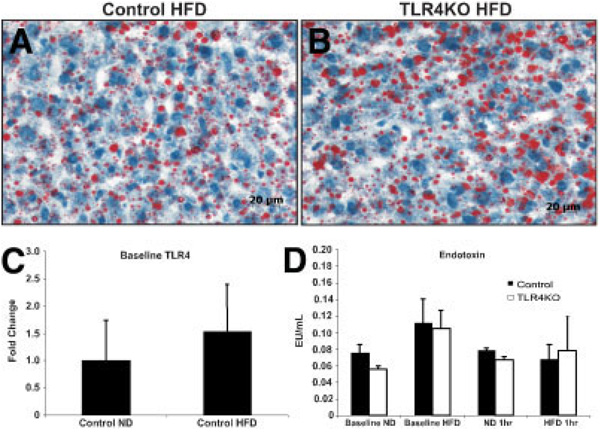
Animal weight and liver weight were similar in both normal diet (ND) control and ND TLR4KO animals. As expected, there was an overall increase in the body weight of the animals fed the HFD, but there was no difference in weight observed between control and TLR4KO animals on this diet (data not shown). Additionally, after 4 weeks of feeding, the HFD produced comparable steatosis in control and TLR4KO groups, as determined by ORO staining, which stains fat (Fig. 1).15 Further measurement of TLR4 expression levels revealed no difference between the groups (Fig. 1).
Figure 1.
Assessment of TLR4 deficiency with respect to the liver phenotype. (A) Control HFD and (B) TLR4KO animals had similar levels of steatosis at baseline, as determined by Oil Red O staining. (C) TLR4 levels were compared via quantitative reverse-transcription polymerase chain reaction. (D) Serum endotoxin levels were measured at baseline and at 1 hour of reperfusion to assess the quantity of liberated gut endotoxin (n = 5–8/group, depending on survival). Abbreviations: HFD, high-fat diet; ND, normal diet; TLR4, toll-like receptor 4; TLR4KO, toll-like receptor 4–deficient.
No Differences in Endotoxin Levels
At baseline, there was no difference in circulating endotoxin levels. To ensure that animals fed an HFD did not release a greater level of endotoxin into the portal circulation after bowel congestion from total hepatic ischemia, serum levels of endotoxin were measured. The levels of endotoxin released did not appreciably vary between any of the groups on either of the diets (Fig. 1).
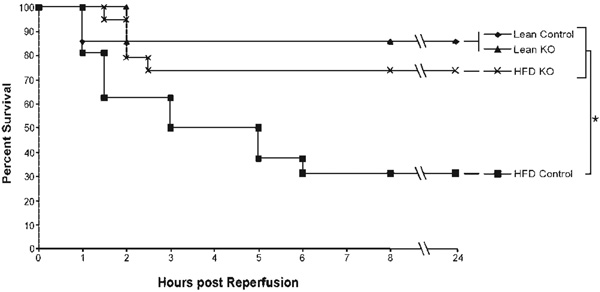
TLR4 Knockout in the Presence of Steatosis Improves Survival
It has been previously shown that ob/ob mice are significantly more sensitive to total hepatic I/R than their lean littermates.16 To investigate the role of TLR4 in steatotic hepatic injury after I/R, mice were fed an HFD for 4 weeks. When the mice were 8 weeks old, they were subjected to 35 minutes of total hepatic ischemia followed by 1 or 24 hours of reperfusion. Animal survival was monitored over the 24-hour period, at which time animal fate was determined. In our model, a lean animal that survives the initial 24-hour period typically does not die from I/R. At 24 hours, 85% of the ND and ND TLR4KO mice were alive (Fig. 2). In the HFD control group, survival rates decreased to 31%. However, the HFD TLR4KO animals had a survival rate of 73%, which was not different from that of the ND groups.
Figure 2.
TLR4 deficiency increases animal survival in steatotic animals following ischemia/reperfusion. TLR4 deficiency significantly increased the 24-hour animal survival of HFD mice following 35 minutes of total hepatic ischemia (*P < 0.05, n = 5–8/group). Abbreviations: HFD, high-fat diet; KO, knockout; TLR4, toll-like receptor 4.
TLR4 Knockout Decreases Liver Damage and Cell Death
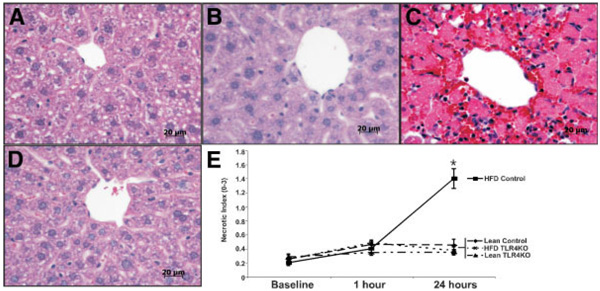
Hepatocyte injury was assessed by the measurement of serum ALT levels. At baseline, there were no differences in ALT levels between ND and HFD mice or between control and TLR4KO mice (Fig. 3). At 1 h of reperfusion, we observed approximately a 4-fold increase in ALT values in all groups. However, at 24 hours of reperfusion, all groups except the control HFD group returned to baseline levels. To gain insight into the type and scope of hepatocyte injury, histological sections were assessed with H&E staining, and injury was graded on a scale of 0 to 3 as described previously.14 Large areas of necrosis were noted around zone 3 (pericentral areas) in the HFD control animals at 24 hours (Fig. 4). These areas were almost completely decreased in the HFD TLR4KO animals at 24 hours and were not observed in either of the ND groups at 24 hours. Scoring of the necrosis revealed no difference in necrosis in the baseline or 1-hour groups. In addition, there was no difference in necrosis in either of the lean groups or the ND TLR4KO group at 24 hours. However, there was a 7-fold increase in the necrotic index in the HFD control animals at 24 hours. These results reveal that HFD control and HFD TLR4KO mice exhibited similar degrees of hepatic injury in terms of ALT release without necrotic cell death 1 hour after reperfusion but exhibited marked differences at 24 hours, with TLR4KO mice exhibiting little ALT release and necrotic cell death.
Figure 3.
TLR4 deficiency decreases hepatic injury in steatotic animals following ischemia/reperfusion. At 24 hours, there was a significant decrease in hepatic injury in TLR4KO HFD animals as assessed by circulating levels of ALT. Animals were sacrificed, and serum was collected prior to surgery (baseline) or 1 or 24 hours post-reperfusion. Values for each group (IU/L) are expressed as the mean ± the standard error of the mean (*P < 0.05 versus TLR4KO HFD, n = 5–8/group). Abbreviations: ALT, alanine aminotransferase; HFD, high-fat diet; ND, normal diet; TLR4, toll-like receptor 4; TLR4KO, toll-like receptor 4–deficient.
Figure 4.
TLR4 deficiency reduces hepatocellular necrosis following ischemia/reperfusion. Prior to surgery and 1 and 24 hours after reperfusion, H&E slides were made from tissue harvested from the left hepatic lobe of each mouse. At 24 hours following reperfusion, no appreciable necrosis was observed in (A) control ND or (B) TLR4KO ND groups, as illustrated by the representative photomicrographs. (C) However, necrosis was marked in the control HFD group, in which large necrotic foci were present around central veins, whereas (D) TLR4 HFD animals showed little appreciable necrosis. (E) Grading of centrilobular necrosis for each animal was conducted on a 0 to 3 scale, scored per high-powered field, and represented graphically. At 24 hours, necrosis was significantly greater in the control HFD livers compared to the TLR4KO HFD livers, whereas it was greater for both groups in comparison with their ND controls. Values are expressed as the mean ± the standard error of the mean (*P < 0.05 versus HFD TLR4KO, n = 5–8/group). Abbreviations: HFD, high-fat diet; ND, normal diet; TLR4, toll-like receptor 4; TLR4KO, toll-like receptor 4–deficient.
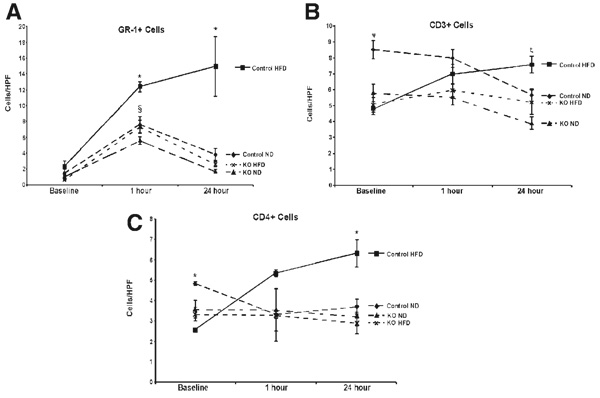
Neutrophil Infiltration Is Decreased in TLR4KO HFDAnimals
TLR4 signaling can result in the production of chemo-tactic and stimulatory factors. Thus, immunostaining for the neutrophil granulocyte marker GR-1 enabled an assessment of the extent of neutrophil infiltration. Ten high-powered fields were counted, and positive cells were quantified. An increase in liver GR-1+ cells was observed in all groups, with a higher number in the control HFD group at 1 hour (Fig. 5). At 24 hours, the control HFD group remained elevated, and the neutrophils were concentrated in areas of necrosis. In contrast, the number of neutrophils in the HFD TLR4KO group returned to baseline levels, as did the ND and TLR4KO ND groups.
Figure 5.
TLR4 deficiency decreases cellular infiltration following ischemia/reperfusion. (A) Sections were stained for GR-1, a granulocyte marker for neutrophils. All groups had increased infiltration at baseline (§P < 0.05 versus baseline). Additionally, there was a significant increase in the neutrophil infiltration in control HFD animals versus TLR4KO HFD animals at 1 hour of reperfusion(*P < 0.05, n = 5–8/group). As the ND groups and the TLR4KO HFD groups returned to baseline levels at 24 hours, the control HFD animals retained a significantly elevated level of neutrophil infiltration (*P < 0.05, n = 5–8/group). (B) At baseline, there were fewer CD3+ cells in the control HFD animals versus the control ND animals (ψP < 0.05). This trend continued when the control ND animals were compared with the TLR4KO ND animals at 1 hour (ψP < 0.05). At 24 hours, the control HFD group was significantly increased over both knockout groups (ζP < 0.05). (C) There was a similar trend at baseline in the CD4+ cells, in that the control HFD animals had significantly fewer cells than the control ND animals (*P < 0.05). There were no differences at 1 hour of reperfusion, but by 24 hours, there were more infiltrating cells in the control HFD animals than in the TLR4KO HFD animals (*P < 0.05). Values are represented as the mean number of cells per HPF ± the standard error of the mean. Abbreviations: HFD, high-fat diet; HPF, high-powered field; KO, knockout; ND, normal diet; TLR4, toll-like receptor 4; TLR4KO, toll-like receptor 4–deficient.
T-Cell Infiltration Is Altered by TLR4 Phenotype
To better understand the immunological processes that were occurring after I/R, liver sections were immunostained for CD3+ cells. Interestingly, a higher number of CD3+ cells were present in the control ND mice in comparison with the control HFD mice at baseline. All of the TLR4KO mice, regardless of diet, remained at baseline levels at all time points (Fig. 5). CD3+ positive cells increased at 1 and 24 hours in control HFD mice after reperfusion (Fig. 5). In contrast, control HFD, control ND, and ND TLR4KO mice did not exhibit an increased number of liver CD3+ cells at 24 hours.
To better characterize these cells, we immunostained for CD4 and CD8. CD4+ T-cells have been shown to be increased in other models of TLR4-induced inflammation and cell death in transplantation.17 At 1 hour of reperfusion, CD4+ levels in livers of control HFD increased and remained elevated at 24 hours (Fig. 5). TLR4KO HFD mice did not exhibit this increase in CD4+ cells at 24 hours. There were only occasional CD8+ cells noted in any of the animals, both at baseline and either of the reperfusion time points (data not shown).
TLR4 Knockout Reduces Transcription of Proinflammatory Cytokines
To assess TLR4 signaling under these conditions, we looked at the transcription levels of key proinflammatory cytokines via real-time quantitative reverse-transcription polymerase chain reaction. One of the hallmark indicators of TLR4 signaling and liver injury is the expression of IL-6. Liver IL-6 mRNA levels were elevated 2- to 4-fold at 1 and 24 hours after reperfusion in ND control and TLR4KO mice and at 24 hours in HFD control and TLR4KO mice. In contrast, liver IL-6 mRNA levels were elevated 14-fold in HFD control mice at 1 hour but only 6-fold in HFD TLR4KO mice, a value not different from that of the other groups. Similar to the IL-6 results, the same trends were observed in the levels of IL-12p40 mRNA, in that there was a dramatic 60-fold increase in the levels observed in the control HFD animals at 1 hour of reperfusion, which was reduced to a 10-fold increase in TLR4KO HFD animals; this was similar to all other groups at 1 or 24 hours (Fig. 6). Finally, we saw this pattern again in the levels of IFN-γ mRNA, with a 25-fold up-regulation in the control HFD animals that was reduced to a 4-fold increase in the TLR4KO HFD animals; this was similar to all other groups at 1 or 24 hours (Fig. 6). In summary, mRNA levels of IL-6, IL-12, and IFN-γ increased 1 hour after reperfusion and remained increased at 24 hours in ND controls. The levels of these cytokines in control HFD mice increased markedly at 1 hour and returned to the levels of the other groups at 24 hours. The absence of TLR4 blocked the marked increases in these cytokines in HFD mice 1 hour after reperfusion.
Figure 6.
Cytokine mRNA levels are altered because of TLR4 deficiency. Real-time reverse-transcriptase polymerase chain reaction was used to compare cytokine mRNA levels between groups, and the results are expressed as the fold change over the baseline. In the proinflammatory T-helper 1 cytokines, there were significant increases in IL-12, IFN-γ, and IL-6 mRNA levels at 1 hour of reperfusion in the control HFD animals versus their TLR4KO counterparts (*P < 0.05, n = 5–8/group). There was also a blunting of anti-inflammatory T-helper 2 cytokine production, as evidenced by the increased levels of IL-10 present in the ND control animals, which were dramatically decreased in the HFD control animals (*P < 0.05, n = 5–8/group). Abbreviations: HFD, high-fat diet; IFN-γ, interferon gamma; IL, interleukin; mRNA, messenger RNA; ND, normal diet; TLR4, toll-like receptor 4; TLR4KO, toll-like receptor 4–deficient.
The pattern for IL-10, our representative T-helper 2 anti-inflammatory cytokine and a negative regulator of proinflammatory responses, was markedly different. In the ND control animals at 1 hour of reperfusion, we observed a 24-fold up-regulation of liver IL-10 mRNA (Fig. 6) in comparison with the ND TLR4KO animals, which had only a 10-fold up-regulation of IL-10. In the HFD control mice, liver IL-10 levels increased only 6-fold; this value was not different from that for HFD or ND mice lacking TLR4. The levels of liver IL-10 in all groups almost completely returned to baseline levels at 24 hours of reperfusion. The levels of IL-4, a second T-helper 2 cytokine, were also examined, and these levels did not show differences between ND and HFD animals at any of the time points (data not shown). Finally, levels of TNF, a common cytokine in endotoxin injury, were examined but failed to show a difference among the groups (data not shown). Overall, we saw marked decreases in proinflammatory cytokines in the TLR4KO HFD animals at 1 hour of reperfusion versus the control HFD animals. There were no differences between any of the other groups, and all levels had returned nearly to baseline levels at 24 hours. Similarly, there was an underproduction of IL-10 at 1 hour in the control HFD group in comparison with the TLR4KO HFD group. This indicates that TLR4KO HFD animals were largely protected from the inflammation present in control HFD animals.
DISCUSSION
The series of experiments presented here addressed the question of what role TLR4 plays in steatotic liver injury and primary nonfunction after I/R. It is known that obese animals are more sensitive to the effects of endotoxin6 and that removing the endotoxin stimulus from the ischemic event improves animal survival and prevents liver injury.7 Therefore, TLR4 was a logical target to investigate in the paradox associated with steatotic graft dysfunction. Numerous studies have previously shown the importance of TLR4 in lean liver damage after ischemia and reperfusion.10,17,18 Here, we attempt to extend that observation into the setting of steatosis, where the response to endotoxin seems to be of greater consequence.6,7 The loss of TLR4 in steatotic grafts led to improved animal survival, a reduction in liver injury as measured by ALT and indices of necrosis, reductions in neutrophil and T-cell infiltration, and decreases in the mRNA levels of proinflammatory cytokines.
These data corroborate previous observations that revealed improvements in survival and reduced hepatic injury when endotoxin was removed via a monoclonal antibody prior to reperfusion.7 In the work presented here, survival was improved from approximately 30% in steatotic animals to approximately 85% when either endotoxin or TLR4 was removed. These 2 independent observations argue that in our model of total hepatic warm ischemia, endotoxin is the major TLR4 signal mitigating injury to the liver after ischemia. However, we do not see complete protection in TLR4KO animals, as their survival rate does not return to 100%. This indicates that there are still alternate pathways that are likely resulting in injury.
Endotoxin is known to be taken up by scavenger receptors, and these receptors may be playing a minor role in this capacity.19 There are also numerous exogenous and endogenous ligands that could be binding to other members of the TLR family and signaling inflammation. Other work from our laboratory has shown that steatotic hepatocyte death is dependent on energy status and occurs through oncosis. Uncoupling protein-2 (UCP2) is highly expressed in steatotic hepatocytes, causes mitochondrial uncoupling, and contributes to the loss of adenosine triphosphate and steatotic hepatocyte oncosis after the insult of I/R.13 Numerous studies have shown connections between signaling mediated by endotoxin through TLR4 and the regulation of energy homeostasis through UCP2.20–23 Additionally, apoptotic signaling secondary to TLR4 activation may be exacerbated by UCP2 expression.24 Thus, the lack of complete protection through the removal of TLR4 may be the result of mitochondrial uncoupling as well as stimulation through other TLR ligands.
One of the key aspects of the response of HFD livers to TLR4 signaling in this model is exemplified by the ALT response seen in these animals. For each of the groups, increased ALT levels were observed at 1 hour. However, at 24 hours, only the control HFD group remained elevated. This indicates sustained liver damage in these animals, which correlates with the increased necrosis observed at the later time point in the control HFD group. Likely offenders for this continued damage include endogenous ligands (both TLR4 and otherwise) that are present because of necrotic cell debris and continued TLR4 downstream signaling.
Significant changes were seen in the levels of cellular necrosis between the HFD control animals and the rest of the groups, giving further insight into the cell fate and probable cause of liver failure. In the TLR4KO HFD animals, necrosis did not increase after I/R, whereas control HFD animals had multiple large necrotic foci and large amounts of hepatocyte dropout around the central veins. We have not in the past seen much appreciable necrosis in lean animals, and we do not now.7,13,16 The key observation from the current study is that TLR4 deficiency returns steatotic animals to a functional phenotype similar to lean animals, and this indicates its major role in steatotic I/R. There is likely an increased sensitivity to TLR4 ligands in steatotic animals, increasing their necrosis via direct inflammation and that induced by cytokines.
Marked changes were observed in the proinflammatory cytokine mRNA levels 1 hour after reperfusion. Specifically, IL-6 levels were markedly elevated in the control HFD animals. Levels of this cytokine have been shown to correlate well with levels of TLR4 activation and to correlate closely with the severity of liver-specific injury in several studies.25–27 Removal of TLR4 greatly diminished the generation of IL-6 in HFD animals. In addition, marked increases in the levels of IL-12 and IFN-γ in the control HFD animals were seen. In addition to being considered inflammatory markers, these cytokines have been shown to play important immunostimulatory roles. IFN-γ stimulates macrophages, can be directly cytotoxic to hepatocytes, 28 and sensitizes cells to TNF-induced killing.29 Although we did not detect differences in our TNF levels, the increased levels of IFN-γ could have hypersensitized cells to this toxic cytokine, making its effect more pronounced in the control HFD animals. Neutrophils can also be primed by IFN-γ and can increase the CD4+ cell infiltration.30 This is consistent with our results, in that we see an increase in necrotic cell death and CD3+ and CD4+ cell infiltration in our control HFD animals that correlates with the increased level of IFN-γ. Again, this increase is abrogated in the TLR4KO HFD animals, in which we see less necrosis, fewer neutrophils infiltrating, fewer CD3+ and CD4+ cells infiltrating, and a reduction in the production of IFN-γ. IL-12 is also known to stimulate IFN-γ release and has been tied to TLR4 stimulation through the MyD88-dependent pathway and the subsequent immune response.31–33 However, because the main type of cell death is oncotic in steatotic animals,34 the interplay of the nonparenchymal mediators, likely the major cytokine producers, and mediators of energy homeostasis remains unclear. What is likely, though, is that the increased TLR4 signaling which we see in the HFD animals is causing an increase in cell death and cytokine production, which is accompanied by an increase in chemotaxis. Such increases in the levels of these cytokines are directly cytotoxic (IFN-γ), are sensitizing cells to LPS-induced damage, or are sensitizing cells to damage induced by other cytotoxic cytokines (TNF). This is likely causing the higher numbers of neutrophils and CD4+ cells, which can contribute to hepatocyte death.
There have been numerous studies that have shown the involvement of oxidative injury to livers after I/R.35–37 These studies have also shown the importance of heme oxygenase 1 to liver I/R, especially with respect to TLR4.17,38,39 We performed experiments to investigate oxidative damage and found slightly decreased damage in our knockout animals, as shown by 8-hydroxydeoxyguanisine staining and by diaminobenzidine staining. However, this difference was far from dramatic, and interpretation of these data is difficult because these animals have less overall damage. Additionally, we found that heme oxygenase 1 levels were not different among any of our groups.
The polarization of fatty livers toward an increased inflammatory profile has been hypothesized to be caused by a decrease in the levels of the hepatic resident CD4+ natural killer T-cell population40 or decreases in the population of CD4+CD25+ T-cells.41 A phenomenon similar to what we see in our warm I/R model has been observed in both ob/ob steatotic mice and models of diet-induced steatosis, in that there is an overproduction of proinflammatory molecules. Therefore, from our study, we can infer that the increases in these cytokines are mediated in part by TLR4. Interesting to note is the consistency with previous reports, in which a decrease in the concentration of T-cells at baseline was observed in the control HFD animals versus the control ND animals, 40,41 whereas the TLR4KO animals showed a similar decrease in CD3+ cells. The data from previous studies implicated this deficiency as a potential cause of the differential response to an inflammatory stimulus of steatotic animals versus lean animals. This difference manifests in a similar manner here (ie, massive necrosis, cytokine production, and cellular infiltration in these animals). However, because the TLR4KO animals lack the key TLR4 inflammatory pathway, we did not see these results in this group of animals. Also, in the control HFD animals at 24 hours, we saw increases in the infiltration of CD3+ and CD4+ cells in comparison with the TLR4KO animals, and this was consistent with reports showing that TLR4 deficiency affects T-cell infiltration, 17 which is likely coupled to damage.
Overall, we continue to observe that animals with steatotic livers have an increased inflammatory phenotype and susceptibility to warm I/R. In this study, it has been demonstrated for the first time that TLR4 plays an important role in steatotic liver warm I/R. We hypothesize that gut-derived LPS is the major TLR4 ligand in this paradigm and that removal of endotoxin or TLR4 reduces hepatic injury after total warm I/R. Taken together, these data support a role for TLR4 in steatotic liver I/R injury and further suggest that therapeutic strategies directed against TLR4 signaling amplification may potentially reduce the damage mediated by I/R in livers and enable the use of steatotic organs, thus expanding the usable donor pool. Although no TLR4 antagonists are commercially available or work well in animals, we anticipate that these molecules will greatly improve the size of the donor pool by allowing the inclusion of more steatotic livers.
ACKNOWLEDGMENT
The authors thank Kathy Haines for her effort and support in keeping the laboratory running.
This work was supported by grants from the National Institutes of Health (1R01DK069369 and 5P20RR017677-05).
Abbreviations
- ALT
alanine aminotransferase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HFD
high-fat diet
- HPF
high-powered field
- I/R
ischemia/reperfusion
- IFN
interferon
- IL
interleukin
- KO
knockout
- LPS
lipopolysaccharide
- mRNA
messenger RNA
- MyD88
myeloid differentiation primary response gene (88)
- ND
normal diet
- TLR
toll-like receptor
- TLR4KO
toll-like receptor 4–deficient
- TNF
tumor necrosis factor
- UCP2
uncoupling protein-2
REFERENCES
- 1.Marsman WA, Wiesner RH, Rodriguez L, Batts KP, Porayko MK, Hay JE, et al. Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation. 1996;62:1246–1251. doi: 10.1097/00007890-199611150-00011. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Daga JA, Santoyo J, Suarez MA, Fernandez-Aguilar JA, Ramirez C, Rodriguez-Canete A, et al. Influence of degree of hepatic steatosis on graft function and postoperative complications of liver transplantation. Transplant Proc. 2006;38:2468–2470. doi: 10.1016/j.transproceed.2006.08.077. [DOI] [PubMed] [Google Scholar]
- 3.Li Z, Lin H, Yang S, Diehl AM. Murine leptin deficiency alters Kupffer cell production of cytokines that regulate the innate immune system. Gastroenterology. 2002;123:1304–1310. doi: 10.1053/gast.2002.35997. [DOI] [PubMed] [Google Scholar]
- 4.Zamboni F, Franchello A, David E, Rocca G, Ricchiuti A, Lavezzo B, et al. Effect of macrovesicular steatosis and other donor and recipient characteristics on the outcome of liver transplantation. Clin Transplant. 2001;15:53–57. doi: 10.1034/j.1399-0012.2001.150109.x. [DOI] [PubMed] [Google Scholar]
- 5.Filos KS, Kirkilesis I, Spiliopoulou I, Scopa CD, Nikolopoulou V, Kouraklis G, et al. Bacterial translocation, endotoxaemia and apoptosis following Pringle manoeuvre in rats. Injury. 2004;35:35–43. doi: 10.1016/s0020-1383(03)00288-2. [DOI] [PubMed] [Google Scholar]
- 6.Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fiorini RN, Shafizadeh SF, Polito C, Rodwell DW, Cheng G, Evans Z, et al. Anti-endotoxin monoclonal antibodies are protective against hepatic ischemia/reperfusion injury in steatotic mice. Am J Transplant. 2004;4:1567–1573. doi: 10.1111/j.1600-6143.2004.00549.x. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Kaisho T, Akira S. Toll-like receptors. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald KA, Rowe DC, Golenbock DT. Endotoxin recognition and signal transduction by the TLR4/MD2-complex. Microbes Infect. 2004;6:1361–1367. doi: 10.1016/j.micinf.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Zhai Y, Shen XD, O’Connell R, Gao F, Lassman C, Busuttil RW, et al. Cutting edge: TLR4 activation mediates liver ischemia/reperfusion inflammatory response via IFN regulatory factor 3-dependent MyD88-independent pathway. J Immunol. 2004;173:7115–7119. doi: 10.4049/jimmunol.173.12.7115. [DOI] [PubMed] [Google Scholar]
- 11.Tazi KA, Quioc JJ, Saada V, Bezeaud A, Lebrec D, Moreau R. Upregulation of TNF-alpha production signaling pathways in monocytes from patients with advanced cirrhosis: possible role of Akt and IRAK-M. J Hepatol. 2006;45:280–289. doi: 10.1016/j.jhep.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu Y, Margenthaler JA, Landeros K, Otomo N, Doherty G, Flye MW. The resistance of P. acnes–primed interferon gamma-deficient mice to low-dose lipopolysaccharide-induced acute liver injury. Hepatology. 2002;35:805–814. doi: 10.1053/jhep.2002.32484. [DOI] [PubMed] [Google Scholar]
- 13.Evans ZP, Ellett JD, Schmidt MG, Schnellmann RG, Chavin KD. Mitochondrial uncoupling protein-2 mediates steatotic liver injury following ischemia/reperfusion. J Biol Chem. 2008;283:8573–8579. doi: 10.1074/jbc.M706784200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neil DA, Hubscher SG. Are parenchymal changes in early post-transplant biopsies related to preservation-reperfusion injury or rejection? Transplantation. 2001;71:1566–1572. doi: 10.1097/00007890-200106150-00014. [DOI] [PubMed] [Google Scholar]
- 15.Fiorini RN, Kirtz J, Periyasamy B, Evans Z, Haines JK, Cheng G, et al. Development of an unbiased method for the estimation of liver steatosis. Clin Transplant. 2004;18:700–706. doi: 10.1111/j.1399-0012.2004.00282.x. [DOI] [PubMed] [Google Scholar]
- 16.Chavin KD, Yang S, Lin HZ, Chatham J, Chacko VP, Hoek JB, et al. Obesity induces expression of uncoupling protein-2 in hepatocytes and promotes liver ATP depletion. J Biol Chem. 1999;274:5692–5700. doi: 10.1074/jbc.274.9.5692. [DOI] [PubMed] [Google Scholar]
- 17.Shen XD, Ke B, Zhai Y, Gao F, Tsuchihashi S, Lassman CR, et al. Absence of toll-like receptor 4 (TLR4) signaling in the donor organ reduces ischemia and reperfusion injury in a murine liver transplantation model. Liver Transpl. 2007;13:1435–1443. doi: 10.1002/lt.21251. [DOI] [PubMed] [Google Scholar]
- 18.Tsung A, Hoffman RA, Izuishi K, Critchlow ND, Nakao A, Chan MH, et al. Hepatic ischemia/reperfusion injury involves functional TLR4 signaling in nonparenchymal cells. J Immunol. 2005;175:7661–7668. doi: 10.4049/jimmunol.175.11.7661. [DOI] [PubMed] [Google Scholar]
- 19.van Oosten M, van Amersfoort ES, van Berkel TJ, Kuiper J. Scavenger receptor-like receptors for the binding of lipopolysaccharide and lipoteichoic acid to liver endothelial and Kupffer cells. J Endotoxin Res. 2001;7:381–384. doi: 10.1177/09680519010070050601. [DOI] [PubMed] [Google Scholar]
- 20.Busquets S, Alvarez B, Van Royen M, Figueras MT, Lopez-Soriano FJ, Argiles JM. Increased uncoupling protein-2 gene expression in brain of lipopolysaccharide-injected mice: role of tumour necrosis factor-alpha? Biochim Bio-phys Acta. 2001;1499:249–256. doi: 10.1016/s0167-4889(00)00126-9. [DOI] [PubMed] [Google Scholar]
- 21.Emre Y, Hurtaud C, Nubel T, Criscuolo F, Ricquier D, Cassard-Doulcier AM. Mitochondria contribute to LPS-induced MAPK activation via uncoupling protein UCP2 in macrophages. Biochem J. 2007;402:271–278. doi: 10.1042/BJ20061430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faggioni R, Shigenaga J, Moser A, Feingold KR, Grunfeld C. Induction of UCP2 gene expression by LPS: a potential mechanism for increased thermogenesis during infection. Biochem Biophys Res Commun. 1998;244:75–78. doi: 10.1006/bbrc.1998.8219. [DOI] [PubMed] [Google Scholar]
- 23.Ruzicka M, Skobisova E, Dlaskova A, Santorova J, Smolkova K, Spacek T, et al. Recruitment of mitochondrial uncoupling protein UCP2 after lipopolysaccharide induction. Int J Biochem Cell Biol. 2005;37:809–821. doi: 10.1016/j.biocel.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Fulop P, Derdak Z, Sheets A, Sabo E, Berthiaume EP, Resnick MB, et al. Lack of UCP2 reduces Fas-mediated liver injury in ob/ob mice and reveals importance of cell-specific UCP2 expression. Hepatology. 2006;44:592–601. doi: 10.1002/hep.21310. [DOI] [PubMed] [Google Scholar]
- 25.Hensler T, Sauerland S, Bouillon B, Raum M, Rixen D, Helling HJ, et al. Association between injury pattern of patients with multiple injuries and circulating levels of soluble tumor necrosis factor receptors, interleukin-6 and interleukin-10, and polymorphonuclear neutrophil elastase. J Trauma. 2002;52:962–970. doi: 10.1097/00005373-200205000-00023. [DOI] [PubMed] [Google Scholar]
- 26.Martin C, Boisson C, Haccoun M, Thomachot L, Mege JL. Patterns of cytokine evolution (tumor necrosis factor-alpha and interleukin-6) after septic shock, hemorrhagic shock, and severe trauma. Crit Care Med. 1997;25:1813–1819. doi: 10.1097/00003246-199711000-00018. [DOI] [PubMed] [Google Scholar]
- 27.Cornell RP. Acute phase responses after acute liver injury by partial hepatectomy in rats as indicators of cytokine release. Hepatology. 1990;11:923–931. doi: 10.1002/hep.1840110604. [DOI] [PubMed] [Google Scholar]
- 28.Greenfield RS, Kiener PA, Chin DP, Curry RC, Spitalny GL. Biochemical analysis and biological activities of immunoaffinity purified natural murine gamma interferon (MuIFN-gamma) J Leukoc Biol. 1986;40:665–676. doi: 10.1002/jlb.40.6.665. [DOI] [PubMed] [Google Scholar]
- 29.Shigeno M, Nakao K, Ichikawa T, Suzuki K, Kawakami A, Abiru S, et al. Interferon-alpha sensitizes human hepatoma cells to TRAIL-induced apoptosis through DR5 up-regulation and NF-kappa B inactivation. Oncogene. 2003;22:1653–1662. doi: 10.1038/sj.onc.1206139. [DOI] [PubMed] [Google Scholar]
- 30.Meyer CN, Nielsen H. Priming of neutrophil and monocyte activation in human immunodeficiency virus infection. Comparison of granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor and interferon-gamma. APMIS. 1996;104:640–646. doi: 10.1111/j.1699-0463.1996.tb04924.x. [DOI] [PubMed] [Google Scholar]
- 31.Pearl-Yafe M, Fabian I, Halperin D, Flatau E, Werber S, Shalit I. Interferon-gamma and bacterial lipopolysaccharide act synergistically on human neutrophils enhancing interleukin-8, interleukin-1beta, tumor necrosis factor-alpha, and interleukin-12 p70 secretion and phagocytosis via upregulation of toll-like receptor 4. Shock. 2007;27:226–231. doi: 10.1097/01.shk.0000239765.80033.37. [DOI] [PubMed] [Google Scholar]
- 32.Sawaki J, Tsutsui H, Hayashi N, Yasuda K, Akira S, Tanizawa T, et al. Type 1 cytokine/chemokine production by mouse NK cells following activation of their TLR/MyD88-mediated pathways. Int Immunol. 2007;19:311–320. doi: 10.1093/intimm/dxl148. [DOI] [PubMed] [Google Scholar]
- 33.Velayudham A, Hritz I, Dolganiuc A, Mandrekar P, Kurt-Jones E, Szabo G. Critical role of toll-like receptors and the common TLR adaptor, MyD88, in induction of granulomas and liver injury. J Hepatol. 2006;45:813–824. doi: 10.1016/j.jhep.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Selzner M, Rudiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology. 2000;32:1280–1288. doi: 10.1053/jhep.2000.20528. [DOI] [PubMed] [Google Scholar]
- 35.Avellini C, Baccarani U, Trevisan G, Cesaratto L, Vascotto C, D’Aurizio F, et al. Redox proteomics and immunohistology to study molecular events during ischemia-reperfusion in human liver. Transplant Proc. 2007;39:1755–1760. doi: 10.1016/j.transproceed.2007.05.082. [DOI] [PubMed] [Google Scholar]
- 36.Tsung A, Klune JR, Zhang X, Jeyabalan G, Cao Z, Peng X, et al. HMGB1 release induced by liver ischemia involves toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–2923. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vairetti M, Ferrigno A, Rizzo V, Richelmi P, Cillo U, Imberti R. Liver damage during ischemia/reperfusion and glutathione: implications for potential organ donors. Transplant Proc. 2007;39:1768–1770. doi: 10.1016/j.transproceed.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Shen XD, Ke B, Zhai Y, Gao F, Busuttil RW, Cheng G, et al. Toll-like receptor and heme oxygenase-1 signaling in hepatic ischemia/reperfusion injury. Am J Transplant. 2005;5:1793–1800. doi: 10.1111/j.1600-6143.2005.00932.x. [DOI] [PubMed] [Google Scholar]
- 39.Tsuchihashi S, Zhai Y, Bo Q, Busuttil RW, Kupiec-Weglinski JW. Heme oxygenase-1 mediated cytoprotection against liver ischemia and reperfusion injury: inhibition of type-1 interferon signaling. Transplantation. 2007;83:1628–1634. doi: 10.1097/01.tp.0000266917.39958.47. [DOI] [PubMed] [Google Scholar]
- 40.Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 41.Ma X, Hua J, Mohamood AR, Hamad AR, Ravi R, Li Z. A high-fat diet and regulatory T cells influence susceptibility to endotoxin-induced liver injury. Hepatology. 2007;46:1519–1529. doi: 10.1002/hep.21823. [DOI] [PubMed] [Google Scholar]