Abstract
Focal adhesions (FAs) are large clusters of transmembrane receptors of the integrin family and a multitude of associated cytoplasmic “plaque” proteins, which connect the extracellular matrix-bound receptors with the actin cytoskeleton. The formation of nearly stationary focal adhesions defines a boundary between dense and highly dynamic actin network in lamellipodium and the sparser and more diverse cytoskeletal organization in the lamella proper, creating a template for the organization of entire actin network. The major “mechanical” and “sensory” functions of FAs, namely, the nucleation and regulation of the contractile, myosin-II-containing, stress fibers and the mechanosensing of external surfaces depend, to a major extent, on the dynamics of molecular components within FAs. A central element in FA regulation concerns the positive feedback loop, based on the most intriguing feature of FAs, namely, their dependence on mechanical tension developing by the growing stress fibers. FAs grow in response to such tension, and rapidly disassemble upon its relaxation. In this article we address the mechanistic relationships between the process of FA development, maturation and dissociation and the dynamic molecular events, which take place in different regions of the FA, primarily in the distal end of this structure (the “toe”) and the proximal “heel”, and discuss the central role of local mechanical forces in orchestrating the complex interplay between FAs and the actin system.
Introduction
Focal adhesions (FA, also known as “focal contacts”) were first visualized in live cells in culture, using interference reflection microscopy (Curtis 1964), by Abercrombie and Dunn [1975]; Izzard and Lochner [1976]. These discrete areas, located at the cell’s ventral surface and measuring several square micrometers, lie within 10–15 nm of the substratum. When first discovered, these apparent adhesion sites attracted immediate attention, since they appeared to be located at the termini of “stress fibers,” prominent bundles of actin filaments [Heath and Dunn, 1978; Wehland et al., 1979], suggesting mechanical continuity between the cell’s contractile machinery and the extracellular matrix (ECM). The notion of such mechanical continuity was also suggested by earlier, electron microscopy studies of vertical sections of fibroblasts, which revealed electron-dense plaques, associated with bundles of microfilaments and apparently attached to the substrate [Abercrombie et al., 1971].
The “molecular era” of FAs began in the late 70’s and early 80’s, with the discovery of their first molecular component; namely, vinculin [Burridge and Feramisco. 1980; Geiger,1979] and pp60src [Rohrschneider,1980], work that was highlighted in a mini-review in Nature, entitled, “Hot Foot” [Lloyd, 1980]. At that time, it was already clear that FAs play a major role in the dynamic crosstalk between the actin cytoskeleton and the ECM in the course of cell movement, cell adhesion and matrix remodeling. The complex molecular composition of FAs, their intricate structure, and their dynamic organization were, however, obviously underestimated in those early years. Three decades later, we can state that though remarkable progress has been made in identifying novel FA components and dynamic characteristics, our knowledge and understanding of FAs remain far from saturation, keeping the “foot” still pretty “hot”.
Recent bioinformatic approaches revealed that FAs contain more than 80 types of proteins (commonly referred to as “plaque proteins”), located at the interface between the transmembrane adhesion receptors (mainly α5β1 and αvβ3 integrins, and probably also syndecans [Morgan et al., 2007] and the actin cytoskeleton [Zaidel-Bar et al., 2007]. Among these proteins, there are a few molecules that directly connect integrin with actin (mainly talin, along with tensin, filamin and alpha-actinin), as well as multiple indirect linkers and numerous bona fide signaling proteins, including different kinases and phosphatases, their many targets, exchange factors for small G-proteins, and the like [Geiger et al., 2009; Zaidel-Bar et al., 2007]. Bioinformatic analyses of existing experimental data on the interactions between FA components enables identification of functional circuits within this complex molecular network [Zaidel-Bar et al., 2007]. In fact, the molecular machinery located at FAs generates a variety of signals that not only affect the fates of the adhesion itself and the associated actin cytoskeleton, but also cell growth, differentiation, and survival [Assoian and Klein, 2008; LaFlamme et al., 2008; Legate et al., 2009; Reddig and Juliano, 2005]. Thus, individual FAs appear to act as an “intelligent system” capable of responding to multiple sensory cues, integrating them in time and space, and responding to the input signals, both locally and globally.
However, unlike man-made ”intelligent machines”, the most immediate and apparent FA-mediated response involves the modulation of FA size, location and, most likely, composition. Indeed, time-lapse movies of FAs indicated that these structures are dynamic and versatile, and can grow, shrink, and sometimes translocate along the substratum [Ballestrem et al., 2001; Holt et al., 2008; Rid et al., 2005; Zamir et al., 2000; see also Zaidel-Bar et al., 2005] (Fig. 1). These continuous changes in FA structure are apparently necessary, if FAs are to accommodate themselves to the ever-changing mechanical stress under which they operate, and to changes in the underlying matrix, particularly prominent during cell migration. Local changes in FAs also have long-range effects on the remodeling and mechanical activities of the actin cytoskeleton. For example, they locally promote actin polymerization [Gupton et al., 2007; Hirata et al. 2008; Turnacioglu et al. 1998], a process which is required for the assembly of straight actomyosin bundles (stress fibers) and their end-on anchorage in the FA plaque [Endlich et al., 2007; Hotulainen and Lappalainen, 2006]. In fact, stress fibers have never been observed in cells lacking mature FAs, suggesting that FAs are an integral part of these cytoskeletal structures, analogous to the relationship between the soles of our feet, and the musculature of the leg.
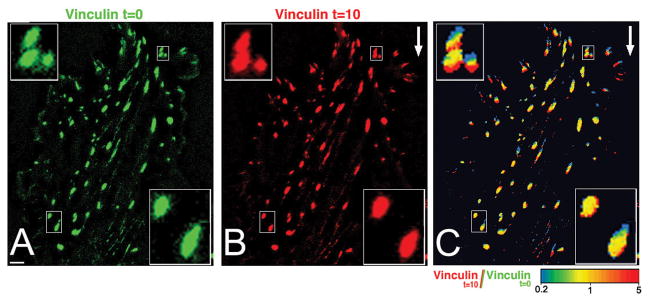
Figure 1.
Shear stress-induced growth of “upstream focal adhesions” and reduction in the size of “downstream focal adhesions”. Cultured endothelial cells expressing CFP-vinculin were monitored at 2-minute intervals before and after application of flow (20 dyn/cm2, direction is indicated by white arrow). Frames from such a movie, taken 10 minutes appart (A and B), and an intensity ratio image of the two time points (C) are shown. Scale bar (in A): 5 μm. Selected groups of focal adhesions (in small rectangles) are shown at high magnification in the insets. In the ratio image (C), the colors represent the intensity ratio values in a spectral scale. Extension of focal adhesions appears red whereas loss of focal adhesion area appears blue. Note that focal adhesions grow centripetally (toe-to-heel), and shrink in opposite direction [Adapted from Zaidel-Bar et al., 2005].
The input cues affecting FAs are numerous, and can be both external or internal. Notable among them are mechanical signals – typically, forces generated by the actomyosin machinery within the cell, as well as mechanical cues transmitted via the extracellular environment (for example, the stretching or rigidity of the ECM). How the FA mechanosensory machinery interprets these input cues and transforms them into output events is not yet clear, but general behavioral characteristics of these structures have begun to emerge.
The birth, maturation, and death of focal adhesions
The commonly held view that FAs evolve from initial “punctate”, “dot-like”, “nascent” “focal complexes” appearing continuously at the cell’s leading edge, into mature structures is supported by many studies [Alexandrova et al., 2008; Bershadsky et al., 1985; Choi et al., 2008; Clark et al., 1998; Rottner et al., 1999]; see also Fig. 2. The so-called “leading edge” of moving or spreading cells can be subdivided into peripheral lamellipodium (a 2–4 micrometer-wide ribbon immediately adjacent to the cell border and filled with a very dense, branched network of actin filaments, and the more internal “lamella proper,” containing a sparser network composed of circumferential and radial actin filament bundles and individual, unbranched actin filaments, along with intermediate filaments and microtubules [Danuser, 2005; Ponti et al., 2004; Small et al., 2002b; Svitkina and Borisy, 1999; Svitkina et al., 1984]. In both the lamellipodium and the lamella proper, the bulk of the cellular material is continuously moving backward, but the velocity and, perhaps, driving forces underlying this centripetal flow differ noticeably [Alexandrova et al., 2008; Ponti et al., 2004]. In lamellipodia, the flow is always 2–5 fold faster than in the lamella, and is generated by Arp2/3 complex-dependent actin polymerization [Alexandrova et al., 2008; Ponti et al., 2004, 2005; Vallotton et al., 2004]. (In fact, some observations would suggest that the centripetal movement of the upper layer of the lamellipodial network is also myosin-II-dependent [Giannone et al., 2007]). The slow flow in the lamella proper depends entirely on contractile forces, primarily generated by myosin-IIA [Alexandrova et al., 2008; Ponti et al., 2004]. In many cell types, the boundary between the lamellipodium and the lamella is a site where FA development apparently takes place [Alexandrova et al., 2008; Choi et al., 2008; Hu et al., 2007].
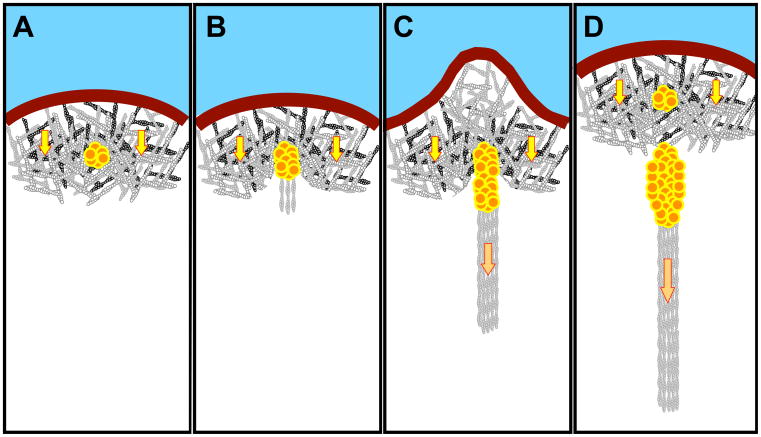
Figure 2.
A cartoon that summarizes the main stages of FA formation and maturation, and the simultaneous advancement of the boundary between the lamellipodium and the lamella domains. The substrate is marked blue, the plasma membrane at the cell edge shown as a brown thick line. The cell is viewed from its “ventral” substrate-attached aspect. Nascent and mature FAs of different sizes are shown as clusters of red-yellow circular “subunits”. The lamellipodium is filled with dense branched network of actin filaments; yellow arrows symbolize the centripetal actin flow characteristic to that area. The stress fibers are shown as actin filament bundles; cross-linking proteins and myosin-II are not shown. The forces generated by the actomyisin contraction in the stress fibers are indicated by the orange arrows. (A) Formation of nascent adhesion underneath the branched actin network in the lamellipodium. (B) Early stage of FA maturation, formation of the precursor of stress fiber (by filament nucleation and crosslinking) and appearance of a new border between the lamellipodium and the lamella. (C) Formation of contractile stress fiber and force-dependent growth of FAs. Simultaneously, bulging of lamellipodial protrusion just opposite the growing FA occurs. (D) The lamellipodial network moves forward due to FA-triggered disassembly and the assembly at the tip. The mature FA and the associated stress fiber continue to grow in the lamella, while new nascent adhesion appears in the lamellipodium. The sequence of events is based on refs Alexandrova et al. [2008]; Choi et al. [2008]; Vicente-Manzanares et al. [2009].
Initial, or “nascent” FAs mainly form underneath the lamellipodium (Fig. 2). They appear as submicron-sized vinculin- or paxillin-positive dots, sometimes traveling for short distances with the actin flow, but usually, they remain immobile [Alexandrova et al., 2008; Choi et al., 2008]. The fast centripetal flow in the lamellipodium plays a critical role in the initiation of FA formation, since mild treatment with cytochalasin D, which halts the flow, leads to the rapid disappearance of these structures [Alexandrova et al., 2008; Choi et al., 2008]. The mechanism underlying the association of the lamellipodial actin meshwork with these early adhesions is not clear, though some data suggest that a direct link may be formed between the Arp2/3 complex, and either vinculin [DeMali et al., 2002] or focal adhesion kinase (FAK) [Serrels et al., 2007]. Both in lamellipodia and filopodia (finger-like cell projections capable of establishing small, transient integrin-mediated contacts with the ECM), the location of integrin molecules in an active conformation [Banno and Ginsberg, 2008; Calderwood, 2004] strongly correlates with sites of actin polymerization [Galbraith et al., 2007].
Nascent FAs (or focal complexes) are short-lived structures (on the order of seconds) that either disappear or rapidly grow centripetally, undergoing transition into elongated, mature FAs. Several important features typically underlie such growth and maturation (Fig. 2). First, the entire process is strictly myosin-II-dependent; the myosin-IIA isoform seems to play a major role [Alexandrova et al., 2008; Choi et al., 2008; Riveline et al., 2001]. Secondly, the FA always grows in a centripetal direction, from the initial adhesion toward the cell center, corresponding with the centripetal direction of the actin flow [Alexandrova et al., 2008]. Thirdly, FA maturation is accompanied by formation of an actomyosin bundle associated with the growing proximal end of the FA, and also oriented centripetally [Endlich et al., 2007; Hotulainen and Lappalainen, 2006].
Thus, transition from a nascent to a mature FA creates a symmetry break in the FA structure: instead of a seemingly isotropic dot-like focal complex, an elongated polar structure with a distal tip (“toe”), and proximal end (“heel”) associated with the growing actin bundle, emerge. The changes in protein composition or phosphorylation underlying this reorganization remain to be elucidated, but one interesting feature is well documented: the plaque protein zyxin is never recruited to nascent adhesions, but rather binds to FAs upon their maturation [Zaidel-Bar et al., 2003].
The F-actin cross-linker α-actinin, along with myosin-II, are among the first proteins to appear in the actin filament bundle growing from the “heel” portion of the maturing FA [Choi et al., 2008; Hotulainen and Lappalainen, 2006]. Notably, these two proteins occupy distinct, even mutually exclusive, domains along the actin bundle in skeletal muscle, smooth muscle and striated stress fibers [Draeger et al., 1990; Naumanen et al., 2008; Sanger et al., 2002]. At early stages of maturation, these proteins are thought to crosslink the actin filaments into a coherent bundle [Choi et al., 2008; Vicente-Manzanares et al., 2009]. Later on, myosin-IIA produces contractile forces that become major regulators of FA dynamics and growth (see following section). The directionality of such growth coincides with the direction of retrograde actomyosin flow also driven essentially by myosin IIA [Cai et al. 2006]. Moreover, several bona fide components of the FAs are carried along the centripetal flow, continuously moving within the FAs in the direction of the flow [Guo and Wang, 2007; Hu et al., 2007].
As it matures, the growing FA defines a new boundary between the lamellipodium and the lamella [Alexandrova et al., 2008]. This boundary apparently connects neighboring young FAs by arcs, concave in the centripetal direction (Fig. 2); thus, the formation of new FAs shifts the boundary forward. Older, apparently more mature adhesions remain, and sometimes continue to grow, within the lamella.
Once their maturation is essentially complete, FAs within the lamella domain stop growing, remain stationary for up to tens of minutes, and gradually disappear. In migrating cells, stationary FAs usually undergo disassembly when, due to the forward movement of the entire cell, they find themselves at the rear part of the lamella [Rid et al., 2005]. The high density of microtubules and microtubule tips found in this region is believed to be a major factor promoting FA disassembly [Broussard et al., 2008; Kaverina et al., 1999]. Indeed, in cells with disrupted microtubules or inhibited kinesin motor activity, FAs and stress fibers are larger and more stable [Bershadsky et al., 1996; Krylyshkina et al., 2002; Liu et al., 1998].
Though the physiological mechanism underlying FA disassembly is not completely understood, it is well established that FA disassembly can be triggered by any treatment interfering with myosin-IIA-driven contractility both in the presence and in the absence of microtubules [Bershadsky et al., 1996; Helfman et al., 1999]. A plausible hypothesis is that FA disassembly might involve local down-regulation of the myosin-II-driven pulling forces. If this is the case, microtubules may serve as (local) regulators of myosin-II contractility, consistent with numerous previous observations [Elbaum et al., 1999; Small et al., 2002a] and with more recent findings of their inhibitory role in the regulation of the RhoA exchange factor, RhoGEF-H1 [Chang et al., 2008; Krendel et al., 2002]. However, other mechanisms, such as local proteolysis by the protease calpain resident in FAs [Bhatt et al., 2002] or enhanced, microtubule-dependent endocytosis activated by the FAK-dynamin pathway [Burridge, 2005; Ezratty et al., 2005] may also participate in this process. How all of these factors work in concert is still unknown.
It is interesting that in well-controlled situations such as FA disassembly following myosin-II inhibition, the disassembly proceeds in a vectorial manner – from heel to toe. Thus, FAs grow from toe to heel and shrink from heel to toe, so that the heel (proximal part) of the FA should be more dynamic than the distal, toe part. This is consistent with recent, direct measurements of FA dynamics [Wolfenson et al., 2009] (and see below). However, the possibility that, in addition to the disassembly from heel to toe, disassembly at the distal toe end could also occur, cannot be excluded. In particular, it has often been observed that some FA translocate, as a whole, in the centripetal direction [Ballestrem et al., 2001; Zamir et al., 2000]. Such translocations can occur due to expansion of FA at the heel area, and disassembly at the toe (a “treadmilling” mechanism). On the other hand, true centripetal “sliding” of large FAs (namely translocation of the FA, as a coherent unit, relative to the substrate) often occurring at the rear of the cell, is most probably the result of partial or complete detachment of such adhesions from the substrate, or the centripetal “dragging” of loosely bound components of the ECM [Broussard et al., 2008].
Adhesion-dependent mechanosensitivity: phenomenology and elementary mechanisms
A variety of myosin-II inhibitors efficiently interfere with the maturation, growth and maintenance of mature FAs, but not with the formation of nascent adhesions (focal complexes). These include agents inhibiting myosin-II light chain phosphorylation by suppressing Rho-, Rho kinase (ROCK) and myosin light chain kinase [Alexandrova et al., 2008; Chrzanowska-Wodnicka and Burridge, 1996; Geiger and Bershadsky, 2001; Riveline et al., 2001; Volberg et al., 1994], as well as the low molecular-weight inhibitor blebbistatin [Even-Ram et al., 2007; Gupton and Waterman-Storer, 2006] and the regulatory protein caldesmon [Grosheva et al., 2006; Helfman et al., 1999], which block the actin-activated myosin-II ATPase by inhibiting productive binding of myosin-II to actin [Alahyan et al., 2006; Kovacs et al., 2004; Zhao et al., 2008]. Experiments with RNAi-mediated knockdowns of myosin-II isoforms revealed that myosin-IIA plays a major role in FA growth and maintenance, at least in cultured fibroblasts and epithelial cells [Even-Ram et al., 2007; Sandquist et al., 2006; Vicente-Manzanares et al., 2007]. Myosin-IIB in these cells is normally responsible for the formation of FAs at the rear of the cell, and therefore in the establishment of cell polarity in 2D cultures [Lo et al., 2004; Vicente-Manzanares et al., 2008]; in 3D conditions, the distribution of functions between myosins IIA and IIB may differ [Ahmed et al., 2007; Meshel et al., 2005]. Localization and possibly functions of myosin IIA and IIB isoforms depend on their short C-terminal tail region [Sandquist and Means, 2008]. The role of the third myosin-II isoform, myosin-IIC, remains to be elucidated. Surprisingly, another related myosin, Myosin 18A (MYO18A) was recently shown to function in concert with the myosin IIA and to be required for the proper assembly of lamellar actomyosin bundles [Tan et al., 2008].
Besides myosin-driven contractility, actin polymerization can also create mechanical forces [Footer et al., 2007; Marcy et al., 2004; Prass et al., 2006], which may play a role in the formation of nascent adhesions underneath lamellipodia and filopodia [Alexandrova et al., 2008; Choi et al., 2008; Galbraith et al., 2007].
The notion of FA mechanosensitivity gained support from experiments involving the more or less direct application of mechanical stimuli to these structures. Local interventions involving the stretching of the cell edge by micromanipulation [Riveline et al., 2001], or by application of local stretching forces to either the elastic substrate adjoining the cell edge [Kaverina et al., 2002], or to the PDMS micro-pillars to which the cell is attached [Sniadecki et al., 2007], all seem to result in the apparent growth of FAs experiencing the pulling force. The incorporation of new FA components from solution into the FAs can be triggered even by the stretching of Triton X100-treated demembranated cytoskeletons [Sawada and Sheetz, 2002]. The effect of stretching on FA subunit incorporation was also evident in cells with inhibited actomyosin contractility [Riveline et al., 2001]. Altogether, these and other findings suggest that FAs are, by nature, mechanosensory units responding to stretching forces by growth, and to relaxation by disassembly. On average, the force required to maintain the FA is about 5 nN per square micrometer [Balaban et al., 2001; Bershadsky et al., 2003], though some adhesions at the leading edge can experience stronger forces [Beningo et al., 2001].
Several novel pathways that could, in part, be responsible for the reinforcement of FAs were recently discovered. Among FA components, talin seems to form the most important link between the cytoplasmic portion of β-integrin and the actin filament [Brown et al., 2002; Jiang et al., 2003; Ziegler et al., 2008]; it is also required for integrin activation [Calderwood, 2004; Wegener et al., 2007] and FA formation [Zhang et al., 2008]. Besides actin and β-integrin, talin binds another FA component, vinculin. There are several sites on the talin rod that bind the vinculin head [Ziegler et al., 2008]. The majority of these sites are cryptic (buried inside the rod), but can be opened by unfolding the talin molecule, as was proposed in structural studies [Fillingham et al., 2005; Gingras et al., 2006; Papagrigoriou et al., 2004], verified by steered molecular dynamic simulations [Hytonen and Vogel, 2008 ; Lee et al., 2007, 2008], and finally directly demonstrated in experiments involving the stretching of talin rods in vitro [del Rio et al., 2009]. Notably, binding of the vinculin head to talin promotes binding of the vinculin tail to the actin filament [Bois et al., 2006]. Thus, one scenario for the force-dependent reinforcement of a talin-mediated integrin-actin link could be based on the force-mediated binding of the extra vinculin molecules to the talin rod and, consequently, formation of additional, vinculin-mediated talin-actin bonds. It is not yet clear whether this reinforcement mechanism is only involved at the initial stages of FA formation, or if it is operative throughout the FA life cycle.
Other components of FAs were also shown to demonstrate force-dependent conformational changes (unfolding), thereby serving as individual mechanosensory components in the FA molecular network [Schwartz, 2009; Vogel, 2006]. Among such components is the signaling protein p130Cas which, upon stretching, exposes the Src-phosphorylation site [Sawada et al., 2006]. Molecular dynamic simulation shows that stretching forces applied to an integrin molecule could facilitate its transition from an inactive to an active [Puklin-Faucher et al., 2006]. Indeed, in a recent experimental study [Friedland et al., 2009], myosin-II-dependent conformational transition in α5β1 integrin, enhancing its interaction with the ECM protein fibronectin, was detected. Finally, stretch-induced unfolding of fibronectin itself may also play a role in adhesion mechanosensitivity [Smith et al., 2007; Vogel, 2006]. Theoretical considerations imply the involvement of other mechanisms (e.g, a “thermodynamic” model, not requiring stretch-induced unfolding [Shemesh et al., 2005]), possibly underlying the force-induced assembly processes occurring in FAs (summarized in Bershadsky et al. [2006]). An in-depth examination of these issues is beyond the scope of this review.
Asymmetric molecular dynamics of focal adhesions
Recent studies involving fluorescence correlation spectroscopy (FCS), fluorescent speckle microscopy (FSM), and fluorescence recovery after photobleaching (FRAP) revealed intriguing facets of FA dynamics. Direct measurements (mostly using FRAP) indicated that FAs are molecularly dynamic sites in which molecules from within the structure exchange with molecules from the cytoplasm. These measurements also indicated significant variations in the exchange rates among the different FA plaque proteins, suggesting differences in the repertoire of binding sites specific to each protein [Ballestrem et al., 2001; Chandrasekar et al., 2005; Edlund et al., 2001; Hamadi et al., 2005]. One study [Lele et al., 2006], for example, has demonstrated that the molecular binding kinetics of zyxin are sensitive to mechanical forces. The authors used several methods, including treatment with cytochalasin D, selective ROCK inhibition, or laser incision of stress fibers, to reduce or dissipate cell tension, to show that the rate at which zyxin unbound from FAs increases, under such conditions. These results are in line with zyxin’s role as a typical constituent of mature FAs [Zaidel-Bar et al., 2003] and a component of mechanosensing machinery [Colombelli et al., 2009; Hirata et al., 2008], since, as mentioned above, the maturation of focal complexes into FAs depends upon the tension exerted on the adhesion proper.
Fluorescent speckle microscopy was also used to analyze the dynamic interactions of several resident adhesion proteins within FAs [Gardel et al., 2008; Hu et al., 2007; Ji et al., 2008]. Studies by Hu and coworkers [Hu et al., 2007] revealed that motions of different FA components correlate, to different degrees with motions of F-actin speckles, suggesting a hierarchical system of transmission of actin-driven forces through FAs. Assuming that the temporal variations of the F-actin flow gradients depends on the friction forces between F-actin and FAs/ECM, Ji and coworkers [Ji et al., 2008] were able to calculate these adhesion forces and correlate them with the degrees of coupling between the motions of F-actin and vinculin speckles. It appeared that the predicted adhesion forces were higher when the motion of vinculin speckles was incompletely coupled to the F-actin flow, than when the motions of vinculin and F-actin speckles were fully coupled [Ji et al., 2008]. This suggests that vinculin is, indeed, a component of a slippage clutch that transmits forces from F-actin to the FAs/ECM.
The study by [Gardel et al., 2008] represents the first attempt to correlate actin dynamics with the directly measured mechanical forces, transduced from the actin cytoskeleton to the ECM via FAs. The authors employed quantitative fluorescent speckle microscopy to assess actin dynamics and high-resolution traction force microscopy (TFM) to monitor cell-generated traction forces. They then superimposed the map of actin flow velocities onto the map of cell-generated traction forces, as well as onto FA distribution, and plotted the traction forces against the velocity of actin flow through the FAs. Consistent with previous observations, they noticed that the flow velocity drops when FAs are formed. Furthermore, they discovered a biphasic (“upside-down V-shaped”) dependence of the cell-mediated traction force on the actin flow velocity. Both low and high velocities corresponded to low traction force, while at intermediate velocities, the observed traction force was maximal. Analysis of such plots specifically for FAs revealed that the F-actin flow speed is inversely related to stress during FA assembly and growth, but is directly related to force during FA weakening. The authors conclude that FA-transduced traction force on the ECM responds in varying ways to F-actin motion, depending on the state of FA assembly and maturation. It is interesting that such behavior was observed in cells even under conditions of myosin-II inhibition, or upon expression of constitutively active Rac and Rho. Thus, the authors claim that a biphasic response to actin flow velocity is an intrinsic feature of FA complexes [Gardel et al., 2008].
In a series of FCS microscopy papers, Digman and colleagues [Digman et al. 2008, 2009a, b) explored the dependence of the molecular dynamics of several FA resident proteins, on the assembly/disassembly stage of the adhesion. In these papers, the authors employed several novel correlation microscopy-based methods, including temporal image correlation spectroscopy (TICS) and raster image correlation spectroscopy (RICS), along with particle number and brightness (N&B) analyses, and photon-counting histograms. These techniques enabled the detection of molecular complexes within FAs, and measurement of their dynamics at different regions of the adhesion, as well as comparison of adhesions at different stages of their life cycle. In these studies, the authors used several FA proteins to demonstrate that the binding equilibrium that occurs during adhesion assembly is characterized by monomers binding to immobile structures, whereas in disassembling adhesions or regions of adhesions, the equilibrium is characterized by the release of large protein aggregates. In addition, the treadmilling mechanism by which FAs perform their sliding movement involves the addition of monomers at one end, and removal of relatively large protein aggregates/complexes from the other. Once released from the FA, these complexes disassemble rapidly in the cytoplasm.
In a recent study, we combined FRAP studies with in silico simulations and mathematical modeling to characterize the dynamics of FA proteins in FAs proper, in non-adherent regions, and in the cytoplasm [Wolfenson et al., 2009]. Our findings demonstrated differences in the exchange rates of FA plaque proteins (paxillin and vinculin) between the two ends (heel and toe) not only in sliding adhesions, but also in FAs at steady state. At the heel, or proximal end, closest to the attached actin bundle, the majority of the paxillin and vinculin populations (~80%) underwent exchange at relatively high rates, while at the toe, or distal end, further from the actin attachment, the exchange rate was very slow; most of the plaque protein population remained immobile, and did not exchange (Fig. 3). β3-integrin, a transmembrane protein, was laterally mobile outside FAs, but exhibited very low mobility, accompanied by a large immobile fraction, within FAs, indicating that the exchange of plaque proteins does not occur through movement of molecular complexes connected to integrin, but rather through the actual exchange of proteins between the FA and the cytoplasm.
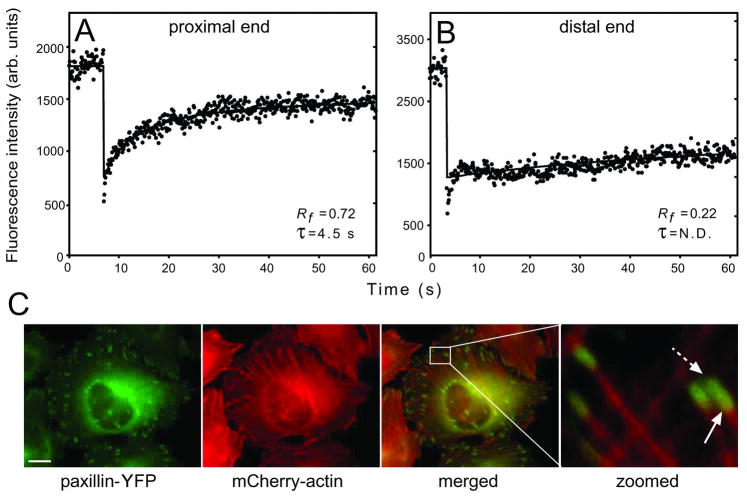
Figure 3.
Paxillin and vinculin display different dynamics at the FA proximal and distal ends. FRAP experiments were carried out, focusing the beam on the two FA ends. (A) A typical FRAP curve of paxillin-YFP at the proximal (heel) FA end (60 s timescale). (B) A typical curve at the distal (toe) FA end (60 s timescale). Fast recovery by diffusion exists, but the ensuing exchange is very slow. (C) Paxillin co-localizes with actin at the FA proximal end. HeLa-JW cells co-expressing paxillin-YFP and mCherry-actin were visualized by fluorescence microscopy. Co-localization was visible at the proximal edge (solid arrow), but not at the distal edge (dashed arrow). Scale bar: 10 μm. [Adapted from Wolfenson et al. 2009]
Together with earlier observations suggesting a polarized distribution of actin within FAs, with the actin bundles mainly contacting the proximal end (heel) and, to a far lesser extent, the distal end (toe) [Zamir et al. 2000] (and Fig. 3). These findings imply that the forces exerted by the actin stress fibers may differ at the two ends, resulting in more rapid exchange in the region with higher actin density. This suggestion is in line with models designed to explain the regulation of FA dynamics by the actomyosin machinery [Besser and Safran, 2006; Shemesh et al., 2005].
Another novel observation in the Wolfenson et al. [2009] study was the identification of a juxtamembrane region surrounding FAs, which displays a gradient of FA proteins with respect to both concentration and dynamics (the greater the distance from the adhesion, the more rapid the dynamics). This region may act as an intermediate layer between the cytoplasm and the membrane-associated FAs, dynamically trapping FA components to create a high local density of plaque subunits. This, in turn, would enable the rapid recruitment of plaque proteins into the FA, as may be required for FA growth in response to local, rapidly applied stress.
Conclusions, and more open questions
At a nearby gym, the cycling trainer Kobi Appelbaum made the following “motivational statement”: “Muscle understands only one language – force! But it is not the force per se that matters; rather, the cell’s response to it.” This review addresses this precise issue of adhesion mechanosensitivity, focusing on multiple features of the response to force, ranging from the effects of mechanosensitive components on integrin adhesions, force-responsive multi-protein complexes, intact FAs, and integrated networks of FAs and the associated contractile cytoskeleton. The challenge lies in understanding the dynamic inter-relationships between all of these hierarchical states. Understanding such relationships requires a detailed characterization of the inner molecular architecture of FAs, which has only now begun to emerge, based on photo-activated light microscopy (PALM) [Betzig et al., 2006; Shroff et al., 2007, 2008a, b] and cryo-electron tomography (Patla, I., Volberg, T., Elad, N., Hirschfeld-Warneken, V.C., Grashoff, C., Fässler, R., Spatz, J., Geiger, B., and Medalia, O., unpublished data). These findings indicate that FAs contain sub-structure(s) characterized by arrays of “plaque” protein complexes.
Of particular interest is a unique feature of FAs; namely, their tendency to develop a toe-to-heel polarity. This polarity manifests itself in the tendency of FAs to grow, under tension, centripetally (“toe-to-heel”) and to fade, upon mechanical relaxation, in the opposite direction (“heel-to-toe”). It would appear that FA organization is primarily driven by mechanical forces acting somewhere along the interface between the cytoskeleton and the ECM-attached membrane.
Some of these force-dependent features are presented in a highly schematic and hypothetical manner in Fig. 4. We suggest that the mechanical forces applied by the actomyosin system to the FA plaque are higher in the heel region and lower in the toe region, thereby differentially distorting the plaque components to which it is anchored. We further propose that the higher tension at the heel region enables an active exchange process to take place. While the lower tension at the toe region is sufficient to keep the plaque components in place, it is insufficient to drive their active exchanges. Accordingly, an increase in the force (and stretching of the plaque) beyond steady-state levels can induce FA growth, whereas relaxation below that of the “toe levels” might be insufficient for maintaining the basic FA structure, thus leading to its dissociation (Fig. 4). The subunit topology hypothesized in this Figure is just one example of many possible architectures (compare with models [Bershadsky et al., 2006; Besser and Safran, 2006; Shemesh et al., 2005]), and should be considered in that light.
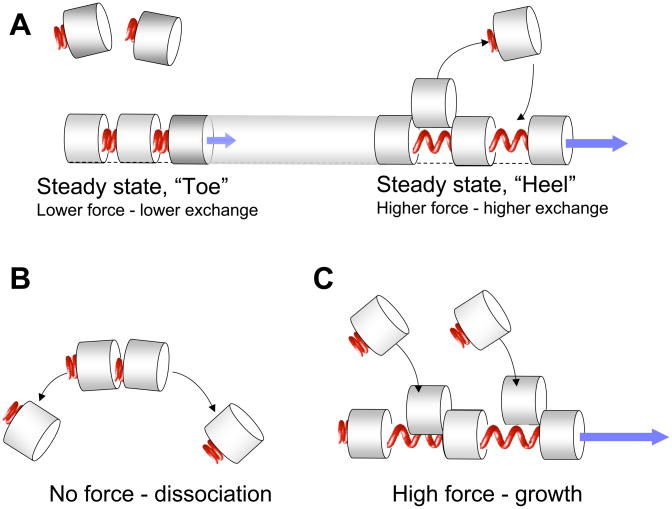
Figure 4.
(A) A diagram illustrating a hypothetical mechanism of asymmetric dynamics of FAs. Proteins or protein complexes corresponding to building blocks (subunits) of FA (grey cylinders) bind to each other forming “elastic” bonds (red springs), which expand upon stretching and permit incorporation of new subunits in between them (this topology of subunit organization, similar to that proposed by (Shemesh et al. 2005) is obviously an over-simplification). We hypothesize that the pulling forces experienced by the subunits (blue arrows) are higher at the “heel” region (immediately adjacent to the stress fiber), and lower at the distal “toe” region. Accordingly, the incorporation of the new subunits and thus the subunit exchange rate at steady state are higher at the “heel”, than at the “toe”, in agreement with the experimental data [Wolfenson et al., 2009]. (B) Under conditions of complete relaxation (no force), the incorporation of new subunits is completely blocked, and the FA undergoes gradual disassembly. (C) Application of strong pulling force (i.e. higher that the steady-state force, marked by a blue arrow) promotes the incorporation of new FA subunits and hence stimulates FA growth.
The paradigm of differential force-dependent regulation of FA dynamics fits local measurements of the dynamic reorganization of plaque components [Wolfenson et al., 2009]. It could also account for such supra-molecular processes as FA growth, shrinking, and centripetal “migration,” most likely due to a treadmilling process, whereby the “heel” is extending and the “toe” is dissociating. At this stage, however, evidence for the suggested mechanism is rather circumstantial, and the underlying mechanisms are still poorly understood. Furthermore, the identity of presumably multiple mechanosensitive components of FAs remains obscure. Are these molecules directly involved in diverse adhesion-driven processes, such as the regulation of the cytoskeletal assembly, differential force transduction, and even signaling activities? What is the relationship between the topological and spatial organization of FA subunits and their mechanosensing activities? How is the complex information collected at FAs about the chemical and physical properties of the cell’s environment integrated and interpreted, thereby triggering long-range responses? The growing interest in the structure and function of FAs, coupled with the development of advanced molecular perturbation techniques as well as sensitive tools for measuring cellular forces at high spatial and temporal resolution, and the persistent attempts to model the physical properties of FAs will likely result in a new, more comprehensive view of the mechanisms underlying FA-mediated sensing.
Acknowledgments
The authors are grateful to Barbara Morgenstern for editorial assistance. Y.H. holds the Zalman Weinberg Chair in Cell Biology. A.D.B. holds the Joseph Moss Professorial Chair in Biomedical Research. His work was partially supported by the Israel Science Foundation, the Minerva Foundation, and the Maurice Janin Fund. B.G. holds the Erwin Neter Professorial Chair in Cell and Tumor Biology and acknowledges support from the Cell Migration Consortium (NIH Grant U54GM64346). and the NanoMedicine Consortium (GM-54508).
References
- Abercrombie M, Dunn GA. Adhesions of fibroblasts to substratum during contact inhibition observed by interference reflection microscopy. Exp Cell Res. 1975;92(1):57–62. doi: 10.1016/0014-4827(75)90636-9. [DOI] [PubMed] [Google Scholar]
- Abercrombie M, Heaysman JE, Pegrum SM. The locomotion of fibroblasts in culture. IV. Electron microscopy of the leading lamella. Exp Cell Res. 1971;67(2):359–67. doi: 10.1016/0014-4827(71)90420-4. [DOI] [PubMed] [Google Scholar]
- Ahmed I, Ponery AS, Nur EKA, Kamal J, Meshel AS, Sheetz MP, Schindler M, Meiners S. Morphology, cytoskeletal organization, and myosin dynamics of mouse embryonic fibroblasts cultured on nanofibrillar surfaces. Mol Cell Biochem. 2007;301(1–2):241–9. doi: 10.1007/s11010-007-9417-6. [DOI] [PubMed] [Google Scholar]
- Alahyan M, Webb MR, Marston SB, El-Mezgueldi M. The mechanism of smooth muscle caldesmon-tropomyosin inhibition of the elementary steps of the actomyosin ATPase. J Biol Chem. 2006;281(28):19433–48. doi: 10.1074/jbc.M507602200. [DOI] [PubMed] [Google Scholar]
- Alexandrova AY, Arnold K, Schaub S, Vasiliev JM, Meister JJ, Bershadsky AD, Verkhovsky AB. Comparative dynamics of retrograde actin flow and focal adhesions: formation of nascent adhesions triggers transition from fast to slow flow. PLoS ONE. 2008;3(9):e3234. doi: 10.1371/journal.pone.0003234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian RK, Klein EA. Growth control by intracellular tension and extracellular stiffness. Trends Cell Biol. 2008;18(7):347–52. doi: 10.1016/j.tcb.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban NQ, Schwarz US, Riveline D, Goichberg P, Tzur G, Sabanay I, Mahalu D, Safran S, Bershadsky A, Addadi L, et al. Force and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat Cell Biol. 2001;3(5):466–72. doi: 10.1038/35074532. [DOI] [PubMed] [Google Scholar]
- Ballestrem C, Hinz B, Imhof BA, Wehrle-Haller B. Marching at the front and dragging behind: differential alphaVbeta3-integrin turnover regulates focal adhesion behavior. J Cell Biol. 2001;155(7):1319–32. doi: 10.1083/jcb.200107107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno A, Ginsberg MH. Integrin activation. Biochem Soc Trans. 2008;36(Pt 2):229–34. doi: 10.1042/BST0360229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol. 2001;153(4):881–8. doi: 10.1083/jcb.153.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky A, Chausovsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr Biol. 1996;6(10):1279–89. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- Bershadsky A, Kozlov M, Geiger B. Adhesion-mediated mechanosensitivity: a time to experiment, and a time to theorize. Curr Opin Cell Biol. 2006;18(5):472–81. doi: 10.1016/j.ceb.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–95. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- Bershadsky AD, Tint IS, Neyfakh AA, Jr, Vasiliev JM. Focal contacts of normal and RSV-transformed quail cells. Hypothesis of the transformation-induced deficient maturation of focal contacts. Exp Cell Res. 1985;158(2):433–44. doi: 10.1016/0014-4827(85)90467-7. [DOI] [PubMed] [Google Scholar]
- Besser A, Safran SA. Force-induced adsorption and anisotropic growth of focal adhesions. Biophys J. 2006;90(10):3469–84. doi: 10.1529/biophysj.105.074377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzig E, Patterson GH, Sougrat R, Lindwasser OW, Olenych S, Bonifacino JS, Davidson MW, Lippincott-Schwartz J, Hess HF. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313(5793):1642–5. doi: 10.1126/science.1127344. [DOI] [PubMed] [Google Scholar]
- Bhatt A, Kaverina I, Otey C, Huttenlocher A. Regulation of focal complex composition and disassembly by the calcium-dependent protease calpain. J Cell Sci. 2002;115(Pt 17):3415–25. doi: 10.1242/jcs.115.17.3415. [DOI] [PubMed] [Google Scholar]
- Bois PR, O'Hara BP, Nietlispach D, Kirkpatrick J, Izard T. The vinculin binding sites of talin and alpha-actinin are sufficient to activate vinculin. J Biol Chem. 2006;281(11):7228–36. doi: 10.1074/jbc.M510397200. [DOI] [PubMed] [Google Scholar]
- Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol. 2008;20(1):85–90. doi: 10.1016/j.ceb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Brown NH, Gregory SL, Rickoll WL, Fessler LI, Prout M, White RA, Fristrom JW. Talin is essential for integrin function in Drosophila. Dev Cell. 2002;3(4):569–79. doi: 10.1016/s1534-5807(02)00290-3. [DOI] [PubMed] [Google Scholar]
- Burridge K. Foot in mouth: do focal adhesions disassemble by endocytosis? Nat Cell Biol. 2005;7(6):545–7. doi: 10.1038/ncb0505-545. [DOI] [PubMed] [Google Scholar]
- Burridge K, Feramisco JR. Microinjection and localization of a 130K protein in living fibroblasts: a relationship to actin and fibronectin. Cell. 1980;19(3):587–95. doi: 10.1016/s0092-8674(80)80035-3. [DOI] [PubMed] [Google Scholar]
- Cai Y, Biais N, Giannone G, Tanase M, Jiang G, Hofman JM, Wiggins CH, Silberzan P, Buguin A, Ladoux B, et al. Nonmuscle myosin IIA-dependent force inhibits cell spreading and drives F-actin flow. Biophys J. 2006;91(10):3907–20. doi: 10.1529/biophysj.106.084806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood DA. Integrin activation. J Cell Sci. 2004;117(Pt 5):657–66. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- Chandrasekar I, Stradal TE, Holt MR, Entschladen F, Jockusch BM, Ziegler WH. Vinculin acts as a sensor in lipid regulation of adhesion-site turnover. J Cell Sci. 2005;118(Pt 7):1461–72. doi: 10.1242/jcs.01734. [DOI] [PubMed] [Google Scholar]
- Chang YC, Nalbant P, Birkenfeld J, Chang ZF, Bokoch GM. GEF-H1 couples nocodazole-induced microtubule disassembly to cell contractility via RhoA. Mol Biol Cell. 2008;19(5):2147–53. doi: 10.1091/mbc.E07-12-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CK, Vicente-Manzanares M, Zareno J, Whitmore LA, Mogilner A, Horwitz AR. Actin and alpha-actinin orchestrate the assembly and maturation of nascent adhesions in a myosin II motor-independent manner. Nat Cell Biol. 2008;10(9):1039–50. doi: 10.1038/ncb1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133(6):1403–15. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142(2):573–86. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombelli J, Besser A, Kress H, Reynaud EG, Girard P, Caussinus E, Haselmann U, Small JV, Schwarz US, Stelzer EH. Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J Cell Sci. 2009;122(Pt 10):1665–79. doi: 10.1242/jcs.042986. [DOI] [PubMed] [Google Scholar]
- Curtis AS. The Mechanism of Adhesion of Cells to Glass. a Study by Interference Reflection Microscopy. J Cell Biol. 1964;20:199–215. doi: 10.1083/jcb.20.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danuser G. Coupling the dynamics of two actin networks--new views on the mechanics of cell protrusion. Biochem Soc Trans. 2005;33(Pt 6):1250–3. doi: 10.1042/BST0331250. [DOI] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323(5914):638–41. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMali KA, Barlow CA, Burridge K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J Cell Biol. 2002;159(5):881–91. doi: 10.1083/jcb.200206043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digman MA, Brown CM, Horwitz AR, Mantulin WW, Gratton E. Paxillin dynamics measured during adhesion assembly and disassembly by correlation spectroscopy. Biophys J. 2008;94(7):2819–31. doi: 10.1529/biophysj.107.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digman MA, Wiseman PW, Choi C, Horwitz AR, Gratton E. Stoichiometry of molecular complexes at adhesions in living cells. Proc Natl Acad Sci U S A. 2009a;106(7):2170–5. doi: 10.1073/pnas.0806036106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digman MA, Wiseman PW, Horwitz AR, Gratton E. Detecting protein complexes in living cells from laser scanning confocal image sequences by the cross correlation raster image spectroscopy method. Biophys J. 2009b;96(2):707–16. doi: 10.1016/j.bpj.2008.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draeger A, Amos WB, Ikebe M, Small JV. The cytoskeletal and contractile apparatus of smooth muscle: contraction bands and segmentation of the contractile elements. J Cell Biol. 1990;111(6 Pt 1):2463–73. doi: 10.1083/jcb.111.6.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund M, Lotano MA, Otey CA. Dynamics of alpha-actinin in focal adhesions and stress fibers visualized with alpha-actinin-green fluorescent protein. Cell Motil Cytoskeleton. 2001;48(3):190–200. doi: 10.1002/1097-0169(200103)48:3<190::AID-CM1008>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Elbaum M, Chausovsky A, Levy ET, Shtutman M, Bershadsky AD. Microtubule involvement in regulating cell contractility and adhesion-dependent signalling: a possible mechanism for polarization of cell motility. Biochem Soc Symp. 1999;65:147–72. [PubMed] [Google Scholar]
- Endlich N, Otey CA, Kriz W, Endlich K. Movement of stress fibers away from focal adhesions identifies focal adhesions as sites of stress fiber assembly in stationary cells. Cell Motil Cytoskeleton. 2007;64(12):966–76. doi: 10.1002/cm.20237. [DOI] [PubMed] [Google Scholar]
- Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol. 2007;9(3):299–309. doi: 10.1038/ncb1540. [DOI] [PubMed] [Google Scholar]
- Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7(6):581–90. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- Fillingham I, Gingras AR, Papagrigoriou E, Patel B, Emsley J, Critchley DR, Roberts GC, Barsukov IL. A vinculin binding domain from the talin rod unfolds to form a complex with the vinculin head. Structure. 2005;13(1):65–74. doi: 10.1016/j.str.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Footer MJ, Kerssemakers JW, Theriot JA, Dogterom M. Direct measurement of force generation by actin filament polymerization using an optical trap. Proc Natl Acad Sci U S A. 2007;104(7):2181–6. doi: 10.1073/pnas.0607052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedland JC, Lee MH, Boettiger D. Mechanically activated integrin switch controls alpha5beta1 function. Science. 2009;323(5914):642–4. doi: 10.1126/science.1168441. [DOI] [PubMed] [Google Scholar]
- Galbraith CG, Yamada KM, Galbraith JA. Polymerizing actin fibers position integrins primed to probe for adhesion sites. Science. 2007;315(5814):992–5. doi: 10.1126/science.1137904. [DOI] [PubMed] [Google Scholar]
- Gardel ML, Sabass B, Ji L, Danuser G, Schwarz US, Waterman CM. Traction stress in focal adhesions correlates biphasically with actin retrograde flow speed. J Cell Biol. 2008;183(6):999–1005. doi: 10.1083/jcb.200810060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979;18(1):193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol. 2001;13(5):584–92. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10(1):21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- Giannone G, Dubin-Thaler BJ, Rossier O, Cai Y, Chaga O, Jiang G, Beaver W, Dobereiner HG, Freund Y, Borisy G, et al. Lamellipodial actin mechanically links myosin activity with adhesion-site formation. Cell. 2007;128(3):561–75. doi: 10.1016/j.cell.2006.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AR, Vogel KP, Steinhoff HJ, Ziegler WH, Patel B, Emsley J, Critchley DR, Roberts GC, Barsukov IL. Structural and dynamic characterization of a vinculin binding site in the talin rod. Biochemistry. 2006;45(6):1805–17. doi: 10.1021/bi052136l. [DOI] [PubMed] [Google Scholar]
- Grosheva I, Vittitow JL, Goichberg P, Gabelt BT, Kaufman PL, Borras T, Geiger B, Bershadsky AD. Caldesmon effects on the actin cytoskeleton and cell adhesion in cultured HTM cells. Exp Eye Res. 2006;82(6):945–58. doi: 10.1016/j.exer.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Guo WH, Wang YL. Retrograde fluxes of focal adhesion proteins in response to cell migration and mechanical signals. Mol Biol Cell. 2007;18(11):4519–27. doi: 10.1091/mbc.E07-06-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupton SL, Eisenmann K, Alberts AS, Waterman-Storer CM. mDia2 regulates actin and focal adhesion dynamics and organization in the lamella for efficient epithelial cell migration. J Cell Sci. 2007;120(Pt 19):3475–87. doi: 10.1242/jcs.006049. [DOI] [PubMed] [Google Scholar]
- Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125(7):1361–74. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Hamadi A, Bouali M, Dontenwill M, Stoeckel H, Takeda K, Ronde P. Regulation of focal adhesion dynamics and disassembly by phosphorylation of FAK at tyrosine 397. J Cell Sci. 2005;118(Pt 19):4415–25. doi: 10.1242/jcs.02565. [DOI] [PubMed] [Google Scholar]
- Heath JP, Dunn GA. Cell to substratum contacts of chick fibroblasts and their relation to the microfilament system. A correlated interference-reflexion and high-voltage electron-microscope study. J Cell Sci. 1978;29:197–212. doi: 10.1242/jcs.29.1.197. [DOI] [PubMed] [Google Scholar]
- Helfman DM, Levy ET, Berthier C, Shtutman M, Riveline D, Grosheva I, Lachish-Zalait A, Elbaum M, Bershadsky AD. Caldesmon inhibits nonmuscle cell contractility and interferes with the formation of focal adhesions. Mol Biol Cell. 1999;10(10):3097–112. doi: 10.1091/mbc.10.10.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Tatsumi H, Sokabe M. Mechanical forces facilitate actin polymerization at focal adhesions in a zyxin-dependent manner. J Cell Sci. 2008;121(Pt 17):2795–804. doi: 10.1242/jcs.030320. [DOI] [PubMed] [Google Scholar]
- Holt MR, Calle Y, Sutton DH, Critchley DR, Jones GE, Dunn GA. Quantifying cell-matrix adhesion dynamics in living cells using interference reflection microscopy. J Microsc. 2008;232(1):73–81. doi: 10.1111/j.1365-2818.2008.02069.x. [DOI] [PubMed] [Google Scholar]
- Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol. 2006;173(3):383–94. doi: 10.1083/jcb.200511093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Ji L, Applegate KT, Danuser G, Waterman-Storer CM. Differential transmission of actin motion within focal adhesions. Science. 2007;315(5808):111–5. doi: 10.1126/science.1135085. [DOI] [PubMed] [Google Scholar]
- Hytonen VP, Vogel V. How force might activate talin's vinculin binding sites: SMD reveals a structural mechanism. PLoS Comput Biol. 2008;4(2):e24. doi: 10.1371/journal.pcbi.0040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzard CS, Lochner LR. Cell-to-substrate contacts in living fibroblasts: an interference reflexion study with an evaluation of the technique. J Cell Sci. 1976;21(1):129–59. doi: 10.1242/jcs.21.1.129. [DOI] [PubMed] [Google Scholar]
- Ji L, Lim J, Danuser G. Fluctuations of intracellular forces during cell protrusion. Nat Cell Biol. 2008;10(12):1393–400. doi: 10.1038/ncb1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang G, Giannone G, Critchley DR, Fukumoto E, Sheetz MP. Two-piconewton slip bond between fibronectin and the cytoskeleton depends on talin. Nature. 2003;424(6946):334–7. doi: 10.1038/nature01805. [DOI] [PubMed] [Google Scholar]
- Kaverina I, Krylyshkina O, Beningo K, Anderson K, Wang YL, Small JV. Tensile stress stimulates microtubule outgrowth in living cells. J Cell Sci. 2002;115(Pt 11):2283–91. doi: 10.1242/jcs.115.11.2283. [DOI] [PubMed] [Google Scholar]
- Kaverina I, Krylyshkina O, Small JV. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 1999;146(5):1033–44. doi: 10.1083/jcb.146.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. J Biol Chem. 2004;279(34):35557–63. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- Krendel M, Zenke FT, Bokoch GM. Nucleotide exchange factor GEF-H1 mediates cross-talk between microtubules and the actin cytoskeleton. Nat Cell Biol. 2002;4(4):294–301. doi: 10.1038/ncb773. [DOI] [PubMed] [Google Scholar]
- Krylyshkina O, Kaverina I, Kranewitter W, Steffen W, Alonso MC, Cross RA, Small JV. Modulation of substrate adhesion dynamics via microtubule targeting requires kinesin-1. J Cell Biol. 2002;156(2):349–59. doi: 10.1083/jcb.200105051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme SE, Nieves B, Colello D, Reverte CG. Integrins as regulators of the mitotic machinery. Curr Opin Cell Biol. 2008;20(5):576–82. doi: 10.1016/j.ceb.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Chunsrivirot S, Kamm RD, Mofrad MR. Molecular dynamics study of talin-vinculin binding. Biophys J. 2008;95(4):2027–36. doi: 10.1529/biophysj.107.124487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SE, Kamm RD, Mofrad MR. Force-induced activation of talin and its possible role in focal adhesion mechanotransduction. J Biomech. 2007;40(9):2096–106. doi: 10.1016/j.jbiomech.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23(4):397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- Lele TP, Pendse J, Kumar S, Salanga M, Karavitis J, Ingber DE. Mechanical forces alter zyxin unbinding kinetics within focal adhesions of living cells. J Cell Physiol. 2006;207(1):187–94. doi: 10.1002/jcp.20550. [DOI] [PubMed] [Google Scholar]
- Liu BP, Chrzanowska-Wodnicka M, Burridge K. Microtubule depolymerization induces stress fibers, focal adhesions, and DNA synthesis via the GTP-binding protein Rho. Cell Adhes Commun. 1998;5(4):249–55. doi: 10.3109/15419069809040295. [DOI] [PubMed] [Google Scholar]
- Lloyd C. Hot foot. Nature. 1980;288(5786):13–4. doi: 10.1038/288013a0. [DOI] [PubMed] [Google Scholar]
- Lo CM, Buxton DB, Chua GC, Dembo M, Adelstein RS, Wang YL. Nonmuscle myosin IIb is involved in the guidance of fibroblast migration. Mol Biol Cell. 2004;15(3):982–9. doi: 10.1091/mbc.E03-06-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcy Y, Prost J, Carlier MF, Sykes C. Forces generated during actin-based propulsion: a direct measurement by micromanipulation. Proc Natl Acad Sci U S A. 2004;101(16):5992–7. doi: 10.1073/pnas.0307704101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshel AS, Wei Q, Adelstein RS, Sheetz MP. Basic mechanism of three-dimensional collagen fibre transport by fibroblasts. Nat Cell Biol. 2005;7(2):157–64. doi: 10.1038/ncb1216. [DOI] [PubMed] [Google Scholar]
- Morgan MR, Humphries MJ, Bass MD. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8(12):957–69. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumanen P, Lappalainen P, Hotulainen P. Mechanisms of actin stress fibre assembly. J Microsc. 2008;231(3):446–54. doi: 10.1111/j.1365-2818.2008.02057.x. [DOI] [PubMed] [Google Scholar]
- Papagrigoriou E, Gingras AR, Barsukov IL, Bate N, Fillingham IJ, Patel B, Frank R, Ziegler WH, Roberts GC, Critchley DR, et al. Activation of a vinculin-binding site in the talin rod involves rearrangement of a five-helix bundle. Embo J. 2004;23(15):2942–51. doi: 10.1038/sj.emboj.7600285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti A, Machacek M, Gupton SL, Waterman-Storer CM, Danuser G. Two distinct actin networks drive the protrusion of migrating cells. Science. 2004;305(5691):1782–6. doi: 10.1126/science.1100533. [DOI] [PubMed] [Google Scholar]
- Ponti A, Matov A, Adams M, Gupton S, Waterman-Storer CM, Danuser G. Periodic patterns of actin turnover in lamellipodia and lamellae of migrating epithelial cells analyzed by quantitative Fluorescent Speckle Microscopy. Biophys J. 2005;89(5):3456–69. doi: 10.1529/biophysj.104.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prass M, Jacobson K, Mogilner A, Radmacher M. Direct measurement of the lamellipodial protrusive force in a migrating cell. J Cell Biol. 2006;174(6):767–72. doi: 10.1083/jcb.200601159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puklin-Faucher E, Gao M, Schulten K, Vogel V. How the headpiece hinge angle is opened: New insights into the dynamics of integrin activation. J Cell Biol. 2006;175(2):349–60. doi: 10.1083/jcb.200602071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddig PJ, Juliano RL. Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 2005;24(3):425–39. doi: 10.1007/s10555-005-5134-3. [DOI] [PubMed] [Google Scholar]
- Rid R, Schiefermeier N, Grigoriev I, Small JV, Kaverina I. The last but not the least: the origin and significance of trailing adhesions in fibroblastic cells. Cell Motil Cytoskeleton. 2005;61(3):161–71. doi: 10.1002/cm.20076. [DOI] [PubMed] [Google Scholar]
- Riveline D, Zamir E, Balaban NQ, Schwarz US, Ishizaki T, Narumiya S, Kam Z, Geiger B, Bershadsky AD. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J Cell Biol. 2001;153(6):1175–86. doi: 10.1083/jcb.153.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider LR. Adhesion plaques of Rous sarcoma virus-transformed cells contain the src gene product. Proc Natl Acad Sci U S A. 1980;77(6):3514–8. doi: 10.1073/pnas.77.6.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9(12):640–8. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Sandquist JC, Means AR. The C-terminal tail region of nonmuscle myosin II directs isoform-specific distribution in migrating cells. Mol Biol Cell. 2008;19(12):5156–67. doi: 10.1091/mbc.E08-05-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandquist JC, Swenson KI, Demali KA, Burridge K, Means AR. Rho kinase differentially regulates phosphorylation of nonmuscle myosin II isoforms A and B during cell rounding and migration. J Biol Chem. 2006;281(47):35873–83. doi: 10.1074/jbc.M605343200. [DOI] [PubMed] [Google Scholar]
- Sanger JW, Chowrashi P, Shaner NC, Spalthoff S, Wang J, Freeman NL, Sanger JM. Myofibrillogenesis in skeletal muscle cells. Clin Orthop Relat Res. 2002;(403 Suppl):S153–62. doi: 10.1097/00003086-200210001-00018. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Sheetz MP. Force transduction by Triton cytoskeletons. J Cell Biol. 2002;156(4):609–15. doi: 10.1083/jcb.200110068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, Tamada M, Dubin-Thaler BJ, Cherniavskaya O, Sakai R, Tanaka S, Sheetz MP. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell. 2006;127(5):1015–26. doi: 10.1016/j.cell.2006.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA. The force is with us. Science. 2009;323(5914):588–9. doi: 10.1126/science.1169414. [DOI] [PubMed] [Google Scholar]
- Serrels B, Serrels A, Brunton VG, Holt M, McLean GW, Gray CH, Jones GE, Frame MC. Focal adhesion kinase controls actin assembly via a FERM-mediated interaction with the Arp2/3 complex. Nat Cell Biol. 2007;9(9):1046–56. doi: 10.1038/ncb1626. [DOI] [PubMed] [Google Scholar]
- Shemesh T, Geiger B, Bershadsky AD, Kozlov MM. Focal adhesions as mechanosensors: a physical mechanism. Proc Natl Acad Sci U S A. 2005;102(35):12383–8. doi: 10.1073/pnas.0500254102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff H, Galbraith CG, Galbraith JA, Betzig E. Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nat Methods. 2008a;5(5):417–23. doi: 10.1038/nmeth.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff H, Galbraith CG, Galbraith JA, White H, Gillette J, Olenych S, Davidson MW, Betzig E. Dual-color superresolution imaging of genetically expressed probes within individual adhesion complexes. Proc Natl Acad Sci U S A. 2007;104(51):20308–13. doi: 10.1073/pnas.0710517105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff H, White H, Betzig E. Photoactivated localization microscopy (PALM) of adhesion complexes. Curr Protoc Cell Biol. 2008b;Chapter 4(Unit 4):21. doi: 10.1002/0471143030.cb0421s41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small JV, Geiger B, Kaverina I, Bershadsky A. How do microtubules guide migrating cells? Nat Rev Mol Cell Biol. 2002a;3(12):957–64. doi: 10.1038/nrm971. [DOI] [PubMed] [Google Scholar]
- Small JV, Stradal T, Vignal E, Rottner K. The lamellipodium: where motility begins. Trends Cell Biol. 2002b;12(3):112–20. doi: 10.1016/s0962-8924(01)02237-1. [DOI] [PubMed] [Google Scholar]
- Smith ML, Gourdon D, Little WC, Kubow KE, Eguiluz RA, Luna-Morris S, Vogel V. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 2007;5(10):e268. doi: 10.1371/journal.pbio.0050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniadecki NJ, Anguelouch A, Yang MT, Lamb CM, Liu Z, Kirschner SB, Liu Y, Reich DH, Chen CS. Magnetic microposts as an approach to apply forces to living cells. Proc Natl Acad Sci U S A. 2007;104(37):14553–8. doi: 10.1073/pnas.0611613104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Borisy GG. Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol. 1999;145(5):1009–26. doi: 10.1083/jcb.145.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina TM, Shevelev AA, Bershadsky AD, Gelfand VI. Cytoskeleton of mouse embryo fibroblasts. Electron microscopy of platinum replicas. Eur J Cell Biol. 1984;34(1):64–74. [PubMed] [Google Scholar]
- Tan I, Yong J, Dong JM, Lim L, Leung T. A tripartite complex containing MRCK modulates lamellar actomyosin retrograde flow. Cell. 2008;135(1):123–36. doi: 10.1016/j.cell.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Turnacioglu KK, Sanger JW, Sanger JM. Sites of monomeric actin incorporation in living PtK2 and REF-52 cells. Cell Motil Cytoskeleton. 1998;40(1):59–70. doi: 10.1002/(SICI)1097-0169(1998)40:1<59::AID-CM6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Vallotton P, Gupton SL, Waterman-Storer CM, Danuser G. Simultaneous mapping of filamentous actin flow and turnover in migrating cells by quantitative fluorescent speckle microscopy. Proc Natl Acad Sci U S A. 2004;101(26):9660–5. doi: 10.1073/pnas.0300552101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Choi CK, Horwitz AR. Integrins in cell migration--the actin connection. J Cell Sci. 2009;122(Pt 2):199–206. doi: 10.1242/jcs.018564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Koach MA, Whitmore L, Lamers ML, Horwitz AF. Segregation and activation of myosin IIB creates a rear in migrating cells. J Cell Biol. 2008;183(3):543–54. doi: 10.1083/jcb.200806030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Zareno J, Whitmore L, Choi CK, Horwitz AF. Regulation of protrusion, adhesion dynamics, and polarity by myosins IIA and IIB in migrating cells. J Cell Biol. 2007;176(5):573–80. doi: 10.1083/jcb.200612043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct. 2006;35:459–88. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- Volberg T, Geiger B, Citi S, Bershadsky AD. Effect of protein kinase inhibitor H-7 on the contractility, integrity, and membrane anchorage of the microfilament system. Cell Motil Cytoskeleton. 1994;29(4):321–38. doi: 10.1002/cm.970290405. [DOI] [PubMed] [Google Scholar]
- Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128(1):171–82. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- Wehland J, Osborn M, Weber K. Cell-to-substratum contacts in living cells: a direct correlation between interference-reflexion and indirect-immunofluorescence microscopy using antibodies against actin and alpha-actinin. J Cell Sci. 1979;37:257–73. doi: 10.1242/jcs.37.1.257. [DOI] [PubMed] [Google Scholar]
- Wolfenson H, Lubelski A, Regev T, Klafter J, Henis YI, Geiger B. A role for the juxtamembrane cytoplasm in the molecular dynamics of focal adhesions. PLoS ONE. 2009;4(1):e4304. doi: 10.1371/journal.pone.0004304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J Cell Sci. 2003;116(Pt 22):4605–13. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- Zaidel-Bar R, Itzkovitz S, Ma'ayan A, Iyengar R, Geiger B. Functional atlas of the integrin adhesome. Nat Cell Biol. 2007;9(8):858–67. doi: 10.1038/ncb0807-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidel-Bar R, Kam Z, Geiger B. Polarized downregulation of the paxillin-p130CAS-Rac1 pathway induced by shear flow. J Cell Sci. 2005;118(Pt 17):3997–4007. doi: 10.1242/jcs.02523. [DOI] [PubMed] [Google Scholar]
- Zamir E, Katz M, Posen Y, Erez N, Yamada KM, Katz BZ, Lin S, Lin DC, Bershadsky A, Kam Z, et al. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nat Cell Biol. 2000;2(4):191–6. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jiang G, Cai Y, Monkley SJ, Critchley DR, Sheetz MP. Talin depletion reveals independence of initial cell spreading from integrin activation and traction. Nat Cell Biol. 2008;10(9):1062–8. doi: 10.1038/ncb1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao FQ, Padron R, Craig R. Blebbistatin stabilizes the helical order of myosin filaments by promoting the switch 2 closed state. Biophys J. 2008;95(7):3322–9. doi: 10.1529/biophysj.108.137067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler WH, Gingras AR, Critchley DR, Emsley J. Integrin connections to the cytoskeleton through talin and vinculin. Biochem Soc Trans. 2008;36(Pt 2):235–9. doi: 10.1042/BST0360235. [DOI] [PubMed] [Google Scholar]