Abstract
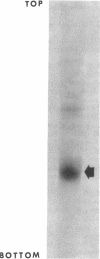
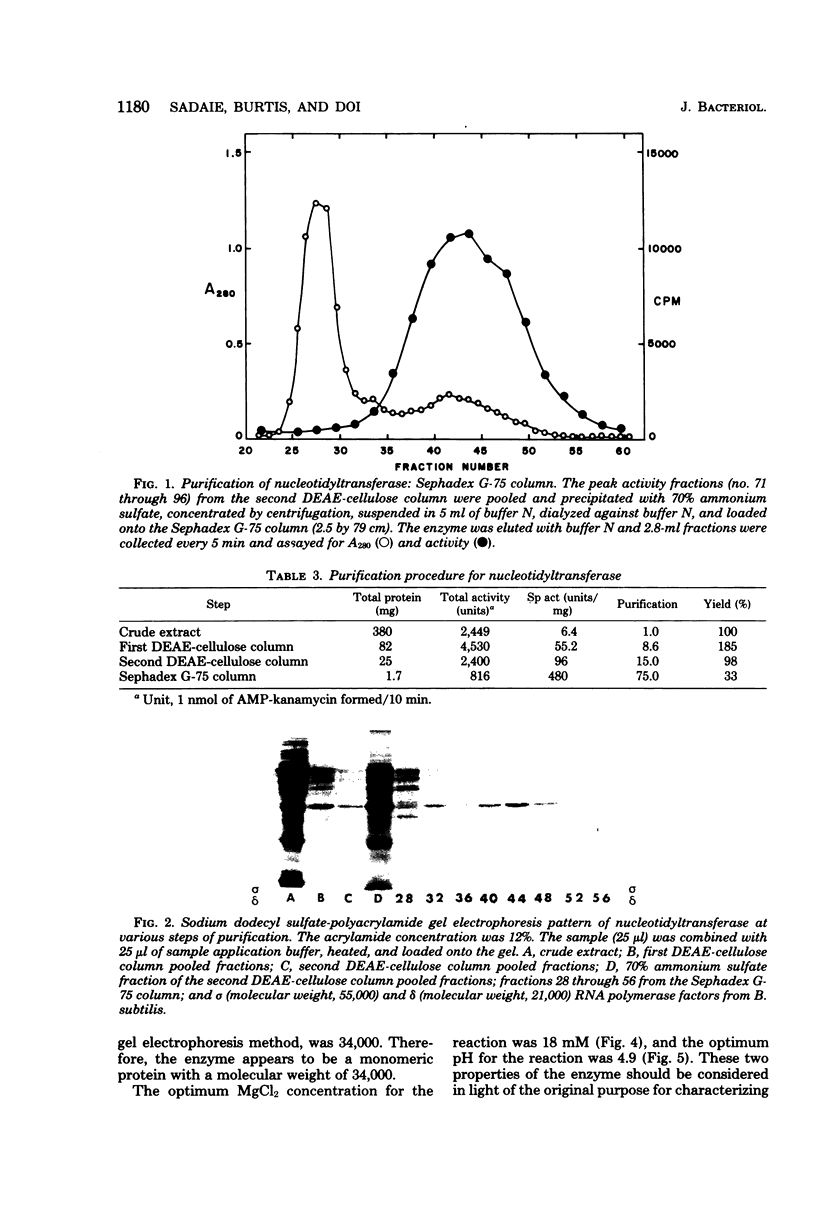
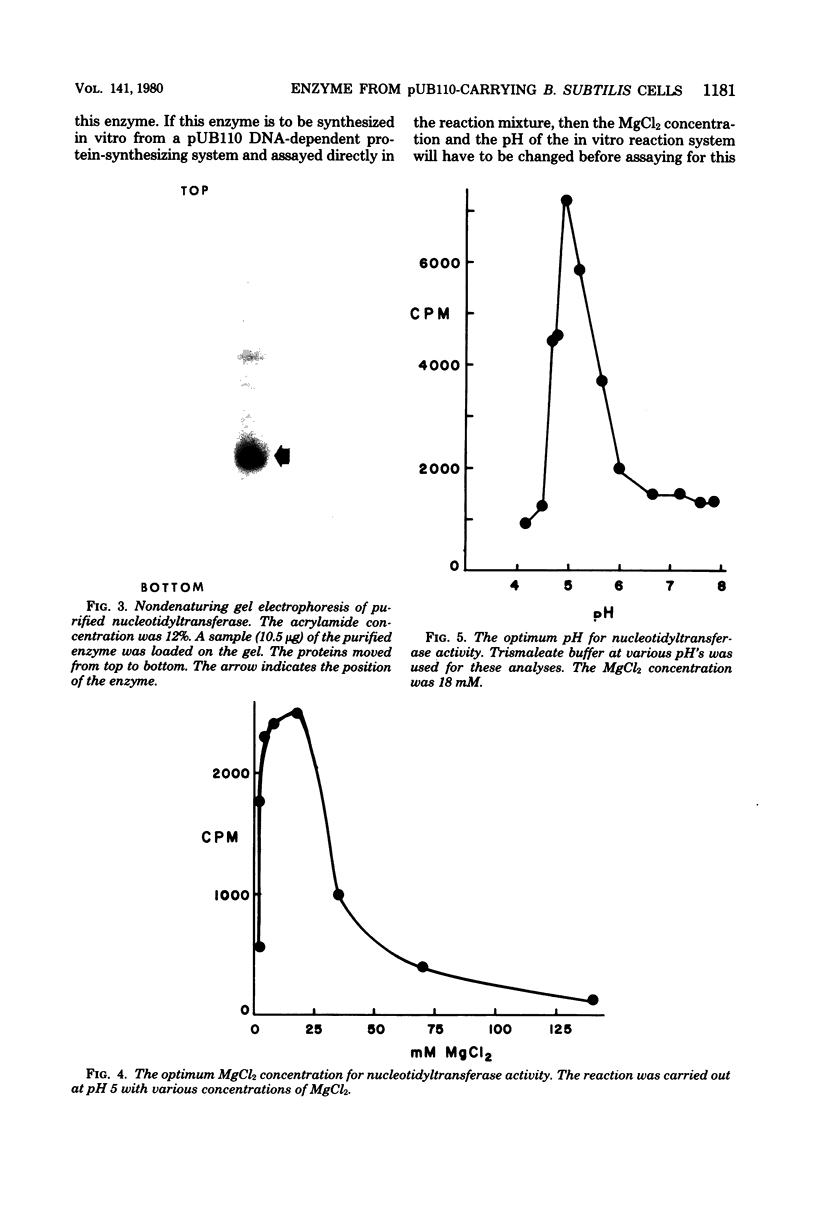
The nucleotidyltransferase encoded by plasmid pUB110 was purified to greater than 95% purity with a 33% yield. The enzyme is a monomeric protein with a molecular weight of 34,000. The optimum pH for activity is 5, and the optimum MgCl2 concentration for activity is 18 mM. The enzyme, which is synthesized constitutively, is stable for several weeks at 4 degrees C. This enzyme would appear to be a good model gene product for the development of a pUB110 deoxyribonucleic acid-dependent in vitro protein-synthesizing system from Bacillus subtilis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste R., Davies J. Enzymatic acetylation of aminoglycoside antibiotics by Escherichia coli carrying an R factor. Biochemistry. 1971 May 11;10(10):1787–1796. doi: 10.1021/bi00786a009. [DOI] [PubMed] [Google Scholar]
- Davies J., Smith D. I. Plasmid-determined resistance to antimicrobial agents. Annu Rev Microbiol. 1978;32:469–518. doi: 10.1146/annurev.mi.32.100178.002345. [DOI] [PubMed] [Google Scholar]
- Doi R. H. Genetic control of sporulation. Annu Rev Genet. 1977;11:29–48. doi: 10.1146/annurev.ge.11.120177.000333. [DOI] [PubMed] [Google Scholar]
- Doi R. H. Role of ribonucleic acid polymerase in gene selection in procaryotes. Bacteriol Rev. 1977 Sep;41(3):568–594. doi: 10.1128/br.41.3.568-594.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich S. D. Replication and expression of plasmids from Staphylococcus aureus in Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1680–1682. doi: 10.1073/pnas.74.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Contente S., Dubnau D. Characterization of Staphylococcus aureus plasmids introduced by transformation into Bacillus subtilis. J Bacteriol. 1978 Apr;134(1):318–329. doi: 10.1128/jb.134.1.318-329.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryczan T. J., Dubnau D. Construction and properties of chimeric plasmids in Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1428–1432. doi: 10.1073/pnas.75.3.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halling S. M., Burtis K. C., Doi R. H. Reconstitution studies show that rifampicin resistance is determined by the largest polypeptide of Bacillus subtilis RNA polymerase. J Biol Chem. 1977 Dec 25;252(24):9024–9031. [PubMed] [Google Scholar]
- Halling S. M., Burtis K. C., Doi R. H. beta' subunit of bacterial RNA polymerase is responsible for streptolydigin resistance in Bacillus subtilis. Nature. 1978 Apr 27;272(5656):837–839. doi: 10.1038/272837a0. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- Keggins K. M., Lovett P. S., Duvall E. J. Molecular cloning of genetically active fragments of Bacillus DNA in Bacillus subtilis and properties of the vector plasmid pUB110. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1423–1427. doi: 10.1073/pnas.75.3.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Goffic F., Martel A., Capmau M. L., Baca B., Goebel P., Chardon H., Soussy C. J., Duval J., Bouanchaud D. H. New plasmid-mediated nucleotidylation of aminoglycoside antibiotics in Staphlococcus aureus. Antimicrob Agents Chemother. 1976 Aug;10(2):258–264. doi: 10.1128/aac.10.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne B., Benveniste R., Tipper D., Davies J. Aminoglycoside antibiotics: inactivation by phosphorylation in Escherichia coli carrying R factors. J Bacteriol. 1969 Nov;100(2):1144–1146. doi: 10.1128/jb.100.2.1144-1146.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadaie Y., Narui K. Repair deficiency, mutator activity, and thermal prophage inducibility in dna-8132 strains of Bacillus subtilis. J Bacteriol. 1976 Jun;126(3):1037–1041. doi: 10.1128/jb.126.3.1037-1041.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Shivakumar A. G., Dubnau D. Differential effect of hydroxyurea on the replication of plasmid and chromosomal DNA in Bacillus subtilis. J Bacteriol. 1978 Dec;136(3):1205–1207. doi: 10.1128/jb.136.3.1205-1207.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivakumar A. G., Dubnau D. Plasmid replication in DNA Ts mutants of Bacillus subtilis. Plasmid. 1978 Jun;1(3):405–416. doi: 10.1016/0147-619x(78)90055-0. [DOI] [PubMed] [Google Scholar]