Abstract
Objectives
To evaluate cost-effectiveness of the Tailored Activity Program (TAP) for individuals with dementia and family caregivers.
Design
Cost-effectiveness study of a two-group randomized controlled trial involving 60 patients-caregiver dyads randomized to intervention or wait-list control.
Setting
Participants’ homes in Philadelphia region.
Participants
Caregivers were ≥21 years, lived with patients and provided ≥4 hours of daily care. Patients had mild to moderate dementia and behavioral symptoms
Intervention
8 sessions of occupational therapy over 4-months to identify patients’ preserved capabilities, previous roles, habits and interests, develop customized activities, and train families in their use.
Measurements
Incremental cost-effectiveness ratios (ICER), expressed as the cost to bring about one additional unit of benefit measured by caregiver hours per day “doing things” and hours per day “being on duty”. Decision tree and Monte Carlo analyses tested robustness of the economic models.
Results
Average intervention cost was $941.63 per dyad. ICER showed that intervention caregivers saved one extra hour per day “doing things” at a cost of $2.37 per day; and one extra hour per day “being on duty” at a cost of $1.10 per day. Monte Carlo showed that TAP was cost-effective 79.2% of the time for “doing things” and 79.6% of the time for “being on duty.” Varying the cost assumptions did not change cost-effectiveness.
Conclusions
Findings suggest that investment in TAP is cost-effective and afforded families an important, limited and highly valued resource, needed time off from caregiving. This nonpharmacologic approach should be considered part of the clinical management of dementia.
Keywords: Quality of life, Caregivers, Behavioral symptoms, Dementia care, Willingness-to-pay
OBJECTIVE
Dementia is a major public health concern with considerable costs to society and family caregivers. In the United States, care costs for individuals requiring daily oversight has been estimated to be as high as $152 billion, a considerable proportion of which ($97 billion), is associated with informal or family caregiving costs.1,2,3 One of the most costly aspects of providing care to individuals with dementia is managing behavioral symptoms.4 Behaviors such as agitation, aggressiveness, or resistance to care, are customary, occur throughout disease stages, cause intense family upset, heighten risk of nursing home placement, and increase the need for caregiver time in oversight and management.5–8 Behavioral symptoms also significantly increase health utilization and direct care costs such that even a one-point worsening on the Neuropsychiatric Inventory, a commonly used behavioral symptom scale, results in an estimated $400 increase in total direct care costs.9
Best practices for managing behavioral symptoms and reducing caregiver burden, and hence informal care costs, remain unclear.10 Pharmacologic approaches, specifically the off label use of atypical antipsychotic drugs, yields only modest benefits and at considerable risk.11,12 Some nonpharmacologic approaches have been shown to be safe and to reduce behavioral symptoms.13 However, with few exceptions, nonpharmacologic interventions for at-home patients have not been systematically tested using trial methodology.14,15 Furthermore, there have been only a few economic evaluations of these interventions.16,17 An economic evaluation of a randomized trial of cognitive-stimulation therapy with 91 dementia patients in residential facilities, showed cost effectiveness compared to usual care using cognition and quality of life outcome measures.16 A cost-effectiveness analysis of a 10 session occupational therapy program involving cognitive and behavioral training of 135 community-based patients and caregivers in the Netherlands, similarly demonstrated cost effectiveness using a multi-component quality improvement measure combining improvements in dementia patients’ daily functioning and caregiver subjective burden.17 Cost-effectiveness studies of caregiver interventions similarly report cost savings, but these interventions have not shown improvements in patient outcomes including behavioral symptoms.18,19 Thus, developing and testing nonpharmacologic interventions and evaluating cost-effectiveness remains an important public health priority in dementia care.20,21 Economic evaluations are critical for translating proven nonpharmacologic programs into deliverable services that can become part of the standard of care.22,23
This study evaluates the cost-effectiveness of an innovative home intervention, the Tailored Activity Program (TAP), an 8-session, 4-month structured occupational therapy intervention that provides dementia patients with activities tailored to their capabilities, and trains family caregivers in their use. We previously reported statistically significant (p <= 0.023) and large effect sizes at 4-months, such that compared to a wait-list control group, the TAP group showed reductions in the frequency of behavioral occurrences overall (Cohen’s d=.72), and particularly for shadowing (Cohen’s d=3.10) and repetitive questioning (Cohen’s d=1.22) which were the most frequently occurring behaviors for this sample. We also showed that TAP reduced caregiver time providing instrumental care (Cohen’s d=.88), and in daily oversight (Cohen’s d=1.00).24 To our knowledge, this is the first study to evaluate the cost-effectiveness of a nonpharmacologic approach that shows both behavioral improvements in dementia patients and reduced burden for family caregivers.
METHODS
Study Design and Sample
The cost-effectiveness analysis was consistent with the original TAP trial design as previously reported.24 Briefly, using a two-group parallel design, 60 dyads (dementia patients/caregivers) were recruited between 2005 and 2006 and randomly assigned to treatment or wait-list control. Treatment group participants received TAP whereas the wait-list control group did not receive any study-related contact. At 4-months from baseline, all dyads were reassessed on study outcomes. Four individuals were lost to follow-up due to patient death (3=intervention group; 1=control group).
As reported elsewhere,24,25 dementia patients were English-speaking, had a physician diagnosis or Mini-mental State Examination (MMSE) score < 24,26 able to self feed and participate in at least two self-care activities (bathing, dressing, grooming, toileting, or transferring from bed-to-chair) and had one or more behavioral symptoms as reported by caregivers. Caregivers were English-speaking, ≥21 years of age, living with patients, and provided ≥4 hours of daily care. The study enrolled dementia patients at the moderate stage when behavioral symptoms are most troublesome to families and require heightened vigilance.
Tailored Activity Program
The 4-month, 8-session (6 home/2 telephone contacts) intervention delivered by occupational therapists involved three phases. In phase one, interventionists used standardized neuropsychological and occupational therapy-based observational tools to evaluate the dementia patients’ abilities, deficits, previous roles, habits and interests. Also evaluated was caregiver communication (e.g., negative and positive communication forms), and the home environment for its supportive features and potential barriers to performance. In phase two, based on assessment results, interventionists developed three activities tailored to patient capabilities. “Activity prescriptions” were developed that detailed capabilities of the person, target activity (e.g., sorting beads), and activity goal (e.g., use bead sorting when preparing meals), and set up and supervision needs. The first activity prescription is reviewed and introduced through role-play with caregivers, and then through direct involvement with the person with dementia. Interventionists practiced with dyads, modeling strategies while narrating what was being done and why, and offering feedback as caregivers and individuals with dementia engaged in the activity. Caregivers were instructed in five specific techniques: cueing, relaxing the rules, not rushing, environmental set-up, and simplifying communication. Additionally, caregivers were provided education as to the role of the environment and how to integrate activities in daily care routines. Finally, caregivers were instructed in simple stress reduction techniques to establish a calm tone prior to initiating and during activities. After one tailored activity prescription was mastered, another was introduced. Sessions were spaced to allow opportunities for caregivers and individuals with dementia to practice using the activities. In subsequent home sessions, activity prescriptions were reviewed and modified if necessary. In phase three, as caregivers mastered activity use, interventionists helped to generalize techniques to other care challenges (e.g., resistance to bathing or dressing), and provided instruction on how to simplify prescribed activities to prepare for future declines. Most sessions included both caregivers and individuals with dementia.25
Measures
Time Spent Caregiving
For the economic evaluation, we used two items from the 4-item Caregiver Vigilance Scale of the NIH REACH I multi-site study; hours “doing things” and hours “on duty”.29 At baseline and 4-month interviews, caregivers were asked the number of hours per day “you are actually doing things for” and the number of hours per day “you feel the need to be there or on duty to care for” the dementia patient out of a 24-hour day. Hours “doing things” refers to task performance such as managing self-care; whereas hours “on duty” refers to perceived oversight demands including providing cueing, guidance, and assuring safety and well-being. While the scale has validity and reliability, each item also has content validity and can be used independently.29 Further evidence of construct validity of independent items is supported by Nichols and colleagues19 study in which a treatment effect was found for hours “doing things,” and by Gitlin et al., study of a caregiver intervention involving home instruction in communication and environmental simplification strategies in which a treatment effect was found for hours “on duty.”30
Intervention and Control Group Costs
Costs were estimated from the perspective of the individual caregiver. Because the target population for TAP is family caregivers of community-dwelling patients with dementia we reasoned this perspective would provide a justification for individuals to participate in the program if it is proven to be cost-effective.
The control group incurred no costs. Table 1 presents costs related to all aspects of the intervention and how each was calculated. Cost categories included: interventionist training and supervision, caregiver time in intervention sessions, assessment materials, intervention supplies, interventionist time including travel time, and mileage (Table 1).
Table 1.
Total Per-Person Cost (N=30) of TAP Intervention by Cost Category
| Cost category | Minimum1 | Maximum1 | Sum2 | Mean3 | Cost Calculation Assumptions |
|---|---|---|---|---|---|
| Total TAP Cost | $242.51 | $1,339.95 | $25,380.38 | $941.63 | |
| Training and supervision of interventionists | 10.08 | 10.08 | 302.40 | 10.08 | Interventionist and trainer, supervisor time spent in 3 days face-to- face training and total of 16 hours supervision over 4 months. Cost of training adjusted by dividing cost by typical full home care agency case load (n=333 clients per therapist for 4 months). |
| Caregiver time in intervention | 18.68 | 90.13 | 1659.92 | 55.33 | Caregiver time receiving training multiplied by regional home health aid hourly rate ($10.14).37 |
| Assessment materials | 76.73 | 76.73 | 2301.90 | 76.73 | Actual 2006 costs for assessment forms and manual/workbooks. |
| Intervention Supplies | .00 | 129.09 | 1794.64 | 59.82 | Cost for activity-related materials (e.g., crafts, beading kits, puzzles, videos, sensory objects). |
| Interventionist time in sessions, preparation, documentation | 117.00 | 465.00 | 9089.00 | 302.97 | Interventionist wages calculated using actual regional pay figure of$28.41 per hour, with an additional 25% for fringe benefits for total per hour pay =$35.51.32 Interventionists’ time averaged 90 minutes over 6 home visits, and two 15 minutes telephone calls per participant. Interventionists spent average of 20 minutes preparation for each in-home visit and 10 minutes for documentation of each home and telephone session. |
| Interventionist Travel time | .00 | 426.12 | 9914.68 | 330.48 | Included interventionist time to drive to and from each participant’s homes per in-home session. Average time for travel was 9.86 hours. |
| Mileage | 20.00 | 142.80 | 3186.48 | 106.21 | Estimated based on 20 mile distance to participants’ homes per session. 2008 government rate of .44 per mile was applied. |
Note: Represents the minimum and maximum costs for participants in TAP intervention group for each cost category;
Represents sum of costs across participants receiving TAP intervention for each cost category;
Represents average cost per participant in TAP intervention group for each cost category.
Incremental Cost-Effectiveness Ratio
Cost-effectiveness was examined using the incremental cost-effectiveness ratio (ICER) following the Panel of Cost-Effectiveness in Health and Medicine recommendations,31 for each of two outcomes, hours “doing things” and “on duty.” For this study, ICER represented the additional cost incurred to bring about one additional unit of benefit per day per caregiver (for each measure). It was computed separately for each measure as follows.
Where the numerator, ΣCostIntervention CG and ΣCostControl CG represent the sum of costs per intervention caregiver and per control caregiver, respectively, up to the 120-day endpoint of the study; and for denominator CHsInterventionCG and CHsControl CG represent the difference in change in caregiving hours (“doing for” and “on duty”) per participating intervention and control group caregiver per day at the study end point. The ICER for each outcome therefore represents the cost of an additional hour of caregiving time that can be “purchased” by TAP.
Data Analyses
As reported previously,24 descriptive data included socio-demographic characteristics (age, gender, race, education, relationship), financial difficulty level, cognitive status, number of instrumental and basic activities of living for which assistance is provided. Chi-square and Wilcoxon rank-sum test (Mann-Whitney test) were used to compare experimental and control dyads on characteristics at baseline. Main treatment effects for caregiver time (hours “doing things” and “on duty”) at 4 months were examined using analysis of covariance (ANCOVA) to examine between group differences. To increase precision of treatment comparisons, baseline values, cognitive status and number of functional dependencies of patient, caregiver age, gender, education and relationship to patient were selected a priori as covariates based on previous research showing significant associations between these factors and outcomes. Cohen’s d was determined as a measure of effect size using the following formula: Adjusted mean between-group difference/pooled SD.
The distribution of residuals from the ANCOVAs was examined and found to be somewhat skewed for both outcome variables (hours “doing things” and “on duty”). Log transformations improved distributions.
A decision tree and probabilistic sensitivity analysis (PSA) using Monte Carlo simulation was completed using TreeAge Pro 2008 to estimate the cost effectiveness of TAP on the two caregiver time measures.32, 33 PSA was conducted to test the results of the ICER for each outcome measure over a computer-generated sample of 1,000 patients. We used a willingness-to-pay threshold of $3,893 per person. That is, we assumed an individual would be willing to pay up to this amount to achieve a positive outcome over a 4-month time frame of the intervention. This amount represents the financial savings obtained if a caregiver partakes in the intervention and forgoes hiring a home health aide to perform similar activities as the caregiver during the course of the intervention ($9.83 hourly wage for an aide × 3.3 hours saved doing things for relative × 120 days in the study).34
For each outcome measure, we also conducted univariate sensitivity analyses to evaluate effects of changing the two largest cost categories (Table 1) in the model and determine which cost variable the model was most sensitive to per outcome measure. Cost variables were tested between their minimum and maximum TAP value. When the cost variable was a constant, a 10% interval was used to test robustness of the economic model.
RESULTS
The study sample of dementia patients were primarily male (57%) and White (77%), with a mean age of 79 years. On average, they had a MMSE score of 11.6 (SD=8.1), were dependent in 8 (SD=.90) instrumental and 5 (SD=2.2) basic activities of living and manifested an average of 7.8 (SD=4.1) behaviors (e.g., repetitive vocalization, shadowing, agitation).27, 28
Caregivers were primarily female (88%), white (77%), high school graduates (56%), and spouses (62%) with a mean age of 65 (SD=11.1). Caregivers had high exposure to behavioral symptoms and functional dependencies resulting in an average of 6.25 (SD=3.81) hours of their time spent doing things for patients and 16.85 (SD=7.54) hours in direct oversight responsibilities. At baseline, there were no large or statistically significant differences between the two groups on any demographic or outcome variables in the main trial or for this cost analysis except for caregiver age. Caregivers in the intervention group were younger by five years than those in the control group (Table 2).
Table 2.
Baseline Characteristics of Persons with Dementia and Family Caregivers (N=60)
| Characteristics | Wait-list (n = 30) | Experiment (n = 30) | Total (n = 60) | Range | χ2 | Z | p value |
|---|---|---|---|---|---|---|---|
| Person with Dementia | |||||||
| Mean Age(SD)a | 80.8 (9.5) | 78.0 (9.2) | 79.4 (9.4) | 56.0 -- 96.2 | −1.30 | .192 | |
| Gender (%) | 1.07d | .297 | |||||
| Male | 63.3 | 50.0 | 56.7 | ||||
| Female | 36.7 | 50.0 | 43.3 | ||||
| Race (%) | 0.37e | .542 | |||||
| White | 80.0 | 73.3 | 76.7 | ||||
| Non-white | 20.0 | 26.7 | 23.3 | ||||
| Education (%)b | 0.34e | .559 | |||||
| ≤ HS | 23.3 | 30.0 | 26.7 | ||||
| > HS | 76.7 | 70.0 | 73.3 | ||||
| Mean MMSE (SD)c | 12.2 (8.8) | 11.0 (7.3) | 11.6 (8.1) | 0.0 -- 27.0 | −0.72 | .473 | |
| Mean ADL (SD) | 4.37 (2.1) | 4.6 (2.3) | 4.5 (2.2) | 0.0 -- 7.0 | −0.63 | .529 | |
| Mean IADL (SD) | 7.4 (1.2) | 7.8 (.5) | 7.6 (.9) | 3.0 -- 8.0 | −0.84 | .401 | |
| Mean # of Behavioral Symptoms | 7.5 (4.5) | 8.0 (3.8) | 7.8 (4.1) | 1.0 -- 18.0 | −0.92 | .357 | |
| Caregiver | |||||||
| Mean Age (SD) | 67.9 (10.6) | 62.8 (11.3) | 65.4 (11.1) | 47.2 -- 89.7 | −1.99 | .047 | |
| Gender (%) | 1.46d | .228 | |||||
| Male | 6.7 | 16.7 | 11.7 | ||||
| Female | 93.3 | 83.3 | 88.3 | ||||
| Race (%) | 0.37e | .542 | |||||
| White | 80.0 | 73.3 | 76.7 | ||||
| Non-white | 20.0 | 26.7 | 23.3 | ||||
| Education (%)a | 0.60e | .438 | |||||
| ≤HS | 56.7 | 46.7 | 51.7 | ||||
| > HS | 43.3 | 53.3 | 48.3 | ||||
| Relationship to dementia patient (%) | 1.76d | .184 | |||||
| Spouse | 70.0 | 53.3 | 61.7 | ||||
| Non-spouse | 30.0 | 46.7 | 38.3 | ||||
| Mean Financial Difficulty Level (SD) | 2.0 (1.0) | 1.7 (.8) | 1.9 (.9) | 1.0 -- 4.0 | −1.30 | .195 | |
| Mean Hours doing things (SD) | 6.2 (3.3) | 6.3 (4.3) | 6.3 (3.8) | 1.0 -- 22.0 | −0.26 | .793 | |
| Mean Hours on duty (SD) | 15.5 (7.7) | 18.2 (7.3) | 16.9 (7.5) | 0.0 -- 24.0 | −1.45 | .146 | |
Note: MMSE = Mini Mental Status Examination; ADL = Activities of Daily Living; IADL = Instrumental ADL. Financial difficulty coded as: 1 – “not at all difficult”, 2 = “not very difficult”, 3 = “somewhat difficult”, 4 = “very difficult”. χ2 = Chi Square; Z = Wilcoxon rank-sum test (Mann-Whitney test).
N = 59;
N = 57;
N = 58;
df = 1;
df = 1.
Intervention Effect on Time Spent Caregiving
As reported previously,24 at baseline, TAP caregivers reported an average of 6.3 hours (SD=4.3) “doing things” for their relatives; similarly, control group caregivers reported 6.2 hours (SD=3.3). However, by 4 months, TAP caregivers reported an average of 5.4 hours (SD=2.5) “doing things,” a reduction in time of 1 hour; this is compared to control group caregivers who reported 8.6 hours (SD=5.7) “doing things,” representing a 3 hour increase in time spent caregiving (Adjusted mean effect =−.22; F=8.79 (df=1, 42); p=.005, 95% CI −.36, −.07; Cohen’s d=.88). Differences were also found for hours “on duty.” At baseline, TAP caregivers reported an average of 18.2 hours (SD=7.3) and control group caregivers reported an average of 15.5 hours (SD=7.7). However, by 4 months, TAP caregivers reported 5 hours less caregiving (Mean=13.4, SD=7.6) compared to control group caregivers who reported spending 3 hours more (Mean=17.6; SD=7.1) on duty (Adjusted mean effect=−.25; F=15.78 (df=1, 42); p=.001, 95% CI −.37, −.12; Cohen’s d=1.00).
Intervention Costs
Table 1 shows intervention costs per individual and overall costs across seven cost categories. The lowest average cost was intervention supplies such as activity-related materials ($60), whereas the highest average costs were associated with interventionist time in sessions including preparation and documentation ($303 per participant), and in travel ($330).35 Caregiver time spent in home and telephone sessions with interventionists were valued as being equal to an alternative use of that time. Thus, shadow-price method for costs involved for caregiving time that could be performed by a professional domiciliary caregiver was calculated for comparison.36, 37 Caregiver time receiving training was multiplied by the regional home health aid hourly rate ($10.14)34 resulting in an average cost of $55 for participation in TAP.
Incremental Cost-Effectiveness Analysis
Numerator - TAP Time and Cost
Total average cost for TAP was $942 per intervention dyad whereas cost for the control group was $0. Using average costs for intervention and control groups and the two outcomes of caregiving hours, the ICER was constructed as the net expenditure as the numerator and the net improvement as the denominator.
Denominator #1: Hours “Doing things”
To determine average caregiver hours “doing things” at the 4-month endpoint, control group caregiving hours “doing things” (8.6) were subtracted from intervention group hours “doing things” (5.4) for a difference of 3.2 hours “doing things” per day. However, because the intervention and control groups differed by 0.1 hours at baseline, this amount was added to the difference at 4 months resulting in a net effect of 3.3 hours for the intervention group.
Denominator #2: Hours “On Duty”
To determine average hours “on duty” at the 4-month endpoint, control group caregiving hours “on duty” (17.6) were subtracted from intervention group hours “on duty” (13.4) for a difference of 4.2 hours “on duty” per day. Because intervention and control groups differed by 2.7 hours at baseline, this amount was added to the difference at 4 months resulting in a net effect of 6.9 hours for the intervention group.
Incremental Cost-Effectiveness Ratio
Using total costs for intervention and control groups and the outcome of caregiving hours, the ICER was computed for:
1 hour “doing things” as:
And 1 hour “on duty” as:
Results show that this 4-month intervention is highly cost-effective if one is willing to spend $2.37 per day to save 1 hour of caregiving time “doing things” for a relative with dementia. Moreover, the intervention is cost-effective if one is willing to spend $1.10 per day to save 1 hour of caregiving time being “on duty” per day.
With regard to hours “doing things,” the intervention can be interpreted as being financially positive as it results in $32.44 ($9.83 for a housekeeper hourly wage × 3.3 hours) of time gained for caregivers. As to being “on duty,” the intervention is also financially positive. It results in $67.83 ($9.83 of a housekeeper hourly wage × 6.9 hours) of time gained for caregivers.34 Greater cost savings would occur if the hourly wage of more skilled personnel were considered such as home health aides.
Sensitivity Analyses
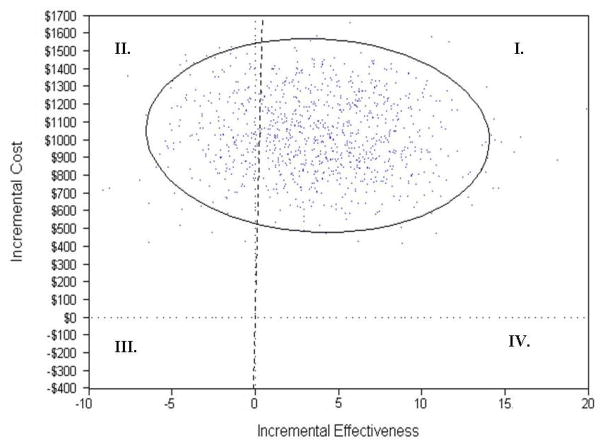
Results of the Monte Carlo PSA suggest that TAP is cost effective 79.2% of the time for the outcome measure “doing things,” and 79.6% of the time for “on duty” based on an individuals’ willingness-to-pay of $3,893 ($9.83 hourly rate for housekeeping × 3.3 hours saved × 120 days). As Figure 1 shows for “doing things,” all points in the upper right quadrant to the right of the diagonal dotted line (I) are cost-effective occurring 79.2% of the time. The remaining dots in the upper left quadrant (II) indicate that the model is more costly and less efficient or not cost-effective compared to the standard of care 20.8% of the time. The plot was similar for hours “on duty” and thus is not shown.
Figure 1.
Probability Sensitivity Analysis (PSA) for Caregiver Hours “Doing Things” for Dementia Patient
Univariate Sensitivity Analysis
To test variability of the models for both outcome measures, the two largest cost inputs interventionist time and travel costs, were varied between their minimum and maximum observation (Table 1). Changing interventionist time and travel costs independently did not change the conclusion of the cost-effectiveness analysis.
DISCUSSION
The vast majority of individuals with dementia are cared for at home by family members. With disease progression, families must devote more time providing hands-on assistance and oversight such that time becomes a precious commodity. Programs that offer respite or time away from both tangible (task performance) and intangible (vigilance) caregiving can help alleviate caregiver burden.
This study examined the cost-effectiveness of a home-based intervention that provides activities customized to patient capabilities and trains caregivers to effectively use those activities in daily care routines. TAP is one of the first home-based intervention studies to systematically identify patient capabilities from which to select and tailor activities, and instruct caregivers in their use as an approach to manage behavioral symptoms and alleviate caregiver burdens. Moreover, to our knowledge, the current study is the first cost-effectiveness analysis of an activity-based nonpharmacologic approach to reduce behavioral symptoms in patients and objective burden of caregivers.
By 4 months, there was a large and statistically significant difference between intervention and control group caregivers in their perceptions of time spent in direct care “doing things” and “being on duty” for their relatives. TAP caregivers had statistically significant more time to allocate to noncaregiving activities. Whereas at baseline, TAP and control group caregivers reported no large or statistically significant differences in time spent doing things or being on duty for their relatives, by 4 months, TAP caregivers had gained 1 hour of noncaregiving time whereas control caregivers had lost 3 hours of noncaregiving time doing things, and had gained 5 hours of noncaregiving time compared to control caregivers who had lost 3 hours of noncaregiving time for being on duty.
Average total cost of TAP was $941. The ICER showed that to reduce caregiving time “doing things” and “being on duty” by 1 hour per day, intervention costs were $2.37 per caregiver ($264 total over 4 months), and $1.10 per caregiver ($206.40 total over 4 months) respectively. Our sensitivity analyses show that TAP was cost-effective 79.2% of the time for reducing time spent “doing things,” and 79.6% of the time for reducing time “on duty.”
TAP compares favorably to the few other economic evaluations of patient and caregiver-based interventions. The Netherland study by Graff and colleagues found that the occupational therapy intervention costs were on average $1,738 (USD) representing $797 more than TAP intervention costs. For cost-effectiveness, they used a combined outcome measure (patient improvement in daily functioning and caregiver improved sense of competence), thus, it is not possible to compare their cost effectiveness outcomes or the cost savings to those of TAP.17 Nichols et al., in their cost-effectiveness study reported a slightly higher intervention cost ($1,214) than TAP ($941) and using the same outcome measure “doing things,”19 found an ICER ($4.96) more than double that of TAP ($2.37).
A major study limitation was the lack of follow-up data after the intervention ended. We do not know if caregivers continued to use activities beyond the 4-month study period and whether a similar time and hence cost-savings persisted. One might argue that using activities in daily care routines requires caregivers to devote more time in order to set-up, initiate or oversee activity participation. However, as we show here, the opposite occurred; caregivers saved time by using activities suggesting that such savings might likely continue over time with consistent activity use. Another limitation is that TAP was a small randomized controlled trial involving 60 patient-dyads who were mostly white. Further study to determine duration of intervention effects and benefits for a larger more diverse sample would be important to substantiate efficacy and cost-effectiveness. Yet another study limitation was the unavailability of objective healthcare utilization data such that we were unable to determine cost-effectiveness from a societal perspective that accounts for such costs. Finally, although time savings resulting from TAP was large (5 hours), it is unclear how caregivers spent noncaregiving time and whether less time in caregiving is related to better health outcomes. We do know that of the 60 caregivers, 37% were employed full (n=13) or part-time (n=9). However, we did not capture lost employment hours nor whether caregivers cut back on employment for caregiving. Nevertheless, by saving time caregiving, TAP may have enabled caregivers to sustain employment. A related point is that we do not know the hourly rate of employment for employed caregivers. We assigned the regional hourly rate of a home health aid ($10.14)34 which may be considerably lower than the true wages of these caregivers, suggesting that the cost savings of TAP may be higher than what we report.
It should be noted that we employed a novel denominator, caregiver time in hours. While we do not view this as a limitation, it is an interesting approach since some may consider time spent caregiving as a cost variable that should be monetized and included in the numerator of the cost-effectiveness ratio. Since time spent caregiving for dementia patients is an indicator of objective burden, we believe that caregiver time is the most relevant outcome measure from TAP, is consistent with the Nichols et al economic analysis,19 has cost implications, and resonates with what caregivers themselves express as important to them.
A methodological consideration is the validity of interview-obtained caregiver estimated time in caregiving compared to direct observation by independent observers. Research suggests however high concordance between interview-based estimates and observation such that asking caregivers directly appears to be a valid and reliable approach.38, 39
Despite limitations in cost-effectiveness analyses, these analyses are at the forefront to quantify benefits of proven programs from which to make judgments for their translation into real-world services. This study contributes to a growing body of evidence demonstrating the cost-effectiveness of occupational therapy in caring for well elderly,40 functionally vulnerable elderly,41 and dementia caregivers.17 Although wages for occupational therapists may be higher than other human service providers, we show that their specialized knowledge plus training in TAP uniquely contributes to dementia care and is highly cost-effective. As such, TAP should be evaluated further with larger and more diverse populations and be considered part of the clinical management of dementia patients and their families contending with behavioral symptoms.
Acknowledgments
Research reported in this paper was supported in part by funds from the National Institute of Mental Health (Grant # R21 MH069425). We wish to acknowledge the significant contributions of our research staff and study participants for their time and effort in this study. A version of this paper was presented at the International Conference on Alzheimer’s Disease, July 12, 2009, Vienna, Austria.
Footnotes
No disclosures to report
Funded by the National Institute of Mental Health (Grant #R21 MH069425).
Contributor Information
Nancy Hodgson, Email: nancy.hodgson@jefferson.edu.
Eric Jutkowitz, Email: eric.jutkowitz@jefferson.edu.
Laura Pizzi, Email: laura.pizzi@jefferson.edu.
References
- 1.Wimo A, Winblad B, Jönsson L. An estimate of the total worldwide societal costs of dementia in 2005. Alzheimers’s & Dementia. 2007;3:81–91. doi: 10.1016/j.jalz.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Moore MJ, Zhu CW, Clipp EC. Informal costs of dementia care: Estimates from the National Longitudinal Caregiver Study. J Gerontol B Psychol Sci Soc Sci. 2001;56B:S219–S228. doi: 10.1093/geronb/56.4.s219. [DOI] [PubMed] [Google Scholar]
- 3.Zhu CW, Leibman C, McLaughlin T, et al. The effects of patient function and dependence on costs of care in Alzheimer’s disease. J Am Geriatr Soc. 2008;56:1497–1503. doi: 10.1111/j.1532-5415.2008.01798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beeri MS, Werner P, Davidson M, et al. The cost of Behavioral and Psychological Symptoms of Dementia (BPSD) in community dwelling Alzheimer’s disease patients. Int J Geriatr Psychiatry. 2002;17:403–408. doi: 10.1002/gps.490. [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulos GS, Jeste DV, Chung H, et al. Treatment of dementia and its behavioral disturbances [Special Report] Post Graduate Medicine. 2005 [PubMed] [Google Scholar]
- 6.Ballard C, Lowery K, Powell I, et al. Impact of behavioral and psychological symptoms of dementia on caregivers. Int Psychogeriatr. 2000;12(1):93–105. [Google Scholar]
- 7.Faiths EB, Zarit SH, Femia EE, et al. Behavioral and psychological symptoms of dementia and caregivers’ stress appraisals: Intra-individual stability and change over short-term observations. Aging Ment Health. 2006;10(6):563–573. doi: 10.1080/13607860600638107. [DOI] [PubMed] [Google Scholar]
- 8.Gilley DW, Bienias JL, Wilson RS, et al. Influence of behavioral symptoms on rates on institutionalization for person’s with Alzheimer’s disease. Psychol Med. 2004;34:1129–1135. doi: 10.1017/s0033291703001831. [DOI] [PubMed] [Google Scholar]
- 9.Murman DL, Chen Q, Powell MC, et al. The incremental direct costs associated with behavioral symptoms in AD. Neurology. 2002;59:1721–1729. doi: 10.1212/01.wnl.0000036904.73393.e4. [DOI] [PubMed] [Google Scholar]
- 10.Clarfield MA. Review: pharmacological and non-pharmacological interventions improve outcomes in patients with dementia and in their caregivers. Evid Based Ment Health. 2001;4(4):109. [Google Scholar]
- 11.Salzman C, Jeste DV, Meyer RE, et al. Elderly patients with Dementia-related symptoms of severe agitation and aggression: Consensus statement on treatment options, clinical trials methodology, and policy [Electronic Version] J Clin Psychiatry. 2008:e1–e10. doi: 10.4088/jcp.v69n0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider LS, Tariot PN, Dagerman KS, et al. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;35(15):1525–1538. doi: 10.1056/NEJMoa061240. [DOI] [PubMed] [Google Scholar]
- 13.Cohen-Mansfield J. Nonpharmacological interventions for inappropriate behaviors in dementia. Alzheimer’s Care Quarterly. 2005;6(2):129–145. [Google Scholar]
- 14.Teri L, Gibbons LE, McCurry SM, et al. Exercise plus behavioral management in patients with Alzheimer disease: a randomized controlled trial. J Am Med Assoc. 2003;290(15):2015–2022. doi: 10.1001/jama.290.15.2015. [DOI] [PubMed] [Google Scholar]
- 15.Gitlin LN, Hauck WW, Dennis MP, et al. Maintenance of effects of the home environmental skill-building program for family caregivers and individuals with Alzheimer’s disease and related disorders. J Geron: Med Sc. 2005;60A(3):368–374. doi: 10.1093/gerona/60.3.368. [DOI] [PubMed] [Google Scholar]
- 16.Knapp M, Thorgrimsen L, Patel A, et al. Cognitive stimulation therapy for people with dementia: Cost-effectiveness analysis. Br J Psychiatry. 2006;188:574–580. doi: 10.1192/bjp.bp.105.010561. [DOI] [PubMed] [Google Scholar]
- 17.Graff MJL, Adang EMM, Vernooij-Dassen MJM, et al. Community occupational therapy for older patients with dementia and their care givers: Cost effectiveness study. [Accessed March 11, 2008];Br Med J [serial online] 2008 January;336:134–138. doi: 10.1136/bmj.39408.481898.BE. Available from http://www.bmj.com/cgi/content/full/336/7636/134. [DOI] [PMC free article] [PubMed]
- 18.Brodaty H, Peters KE. Cost effectiveness of a training program for dementia carers. Int Psychogeriatr. 1991;3:11–22. doi: 10.1017/s1041610291000479. [DOI] [PubMed] [Google Scholar]
- 19.Nichols LO, Chang C, Lummus A, et al. The cost-effectiveness of a behavior intervention for caregivers of patients with Alzheimer’s disease. J Am Geriatr Soc. 2007;56:413–420. doi: 10.1111/j.1532-5415.2007.01569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyketsos CG, Colenda CC, Beck C, et al. Position statement of the American Association for Geriatric Psychiatry regarding principles of care for patients with dementia resulting from Alzheimer disease. Am J Geriatr Psychiatry. 2006;14:561–573. doi: 10.1097/01.JGP.0000221334.65330.55. [DOI] [PubMed] [Google Scholar]
- 21.American Psychiatric Association Work Group on Alzheimer’s Disease and other Dementias. American Psychiatric Association practice guideline for the treatment of patients with Alzheimer’s disease and other dementias. Second edition. Am J Psychiatry. 2007;164(12 Suppl):5–56. [PubMed] [Google Scholar]
- 22.Russell LB, Gold MR, Siegel JE, et al. The role of cost-effectiveness analysis in health and medicine. Panel on cost-effectiveness in health and medicine. JAMA. 1996;276(14):1172–1177. [PubMed] [Google Scholar]
- 23.Detsky AS, Laupacis A. Relevance of cost-effectiveness analysis to clinicians and policy makers. JAMA. 2007;298(2):221–224. doi: 10.1001/jama.298.2.221. [DOI] [PubMed] [Google Scholar]
- 24.Gitlin LN, Winter L, Burke J, et al. Tailored activities to manage neuropsychiatry behavior in persons with dementia and reduce caregiver burden: A randomized pilot study. Am J Geriatr Psychiatry. 2008;16:229–239. doi: 10.1097/JGP.0b013e318160da72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gitlin LN, Winter L, Earland TV, et al. The Tailored Activity Program (TAP): A nonpharmacologic approach to improving quality of life at home for person with dementia and their family caregivers. The Gerontologist: Practice Concepts. 2009;49:428–439. [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Teri L, Truax P, Logsdon R, et al. Assessment of behavioral problems in dementia: The Revised Memory and Behavior Problems Checklist (RMBPC) Psychol Aging. 1992;7:622–631. doi: 10.1037//0882-7974.7.4.622. [DOI] [PubMed] [Google Scholar]
- 28.Logsdon RG, Teri L, Weiner MF, et al. Assessment of agitation in Alzheimer’s disease: The agitated behavior in dementia scale. J Am Geriatr Soc. 1999;47:1354–1358. doi: 10.1111/j.1532-5415.1999.tb07439.x. [DOI] [PubMed] [Google Scholar]
- 29.Mahoney DF, Jones RN, Coon DW, et al. The Caregiver Vigilance Scale: Application and validation in the Resources for Enhancing Alzheimer’s Caregiver Health (REACH) project. Am J Alzheimers Dis Other Demen. 2003;18:39–48. doi: 10.1177/153331750301800110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gitlin LN, Winter L, Corcoran M, et al. Effects of the Home Environmental Skill-building Program on the Caregiver-Care Recipient Dyad: Six-month Outcomes from the Philadelphia REACH Initiative. Gerontologist. 2003;43:532–546. doi: 10.1093/geront/43.4.532. [DOI] [PubMed] [Google Scholar]
- 31.Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the panel of cost-effectiveness in health and medicine. JAMA. 1996;276(15):1253–1258. [PubMed] [Google Scholar]
- 32.TreeAge Software. TreeAge pro 2009 suite. Williamstown, MA: Release 1.0.2. [Google Scholar]
- 33.Mooney CZ. Sage University Paper Series on Quantitative Applications in the Social Sciences. Newbury Park, CA: Sage; 1997. Monte Carlo simulation. Series NO. 07–116. [Google Scholar]
- 34.US Department of Labor. [Accessed November 15, 2008];Chart book: Occupational Employment and Wages. 2008 May; 2006 (Bulletin 2703). Available at http://www.bls.gov/oes/
- 35.Payscale.com: Salary survey report for job: Occupational Therapist. [Accessed May, 2008]; Available at: http://www.payscale.com/research/US/Job=Occupational_Therapist/Salary.
- 36.Dowie J. Why cost-effectiveness should trump (clinical) effectiveness. Health Econ. 2004;13:453–459. doi: 10.1002/hec.861. [DOI] [PubMed] [Google Scholar]
- 37.Posnett J, Jan S. Indirect cost in economical evaluation: the opportunity cost of unpaid inputs. Health Econ. 1996;5:13–23. doi: 10.1002/(SICI)1099-1050(199601)5:1<13::AID-HEC182>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 38.Cotter EM, Burgio LD, Stevens AB, Roth DL, Gitlin LN. Correspondence of the functional independence measure (FIM) self-care subscale with real-tine observations of dementia patients’ ADL performance in the home. Clinical Rehabilitation. 2002;16:38–47. doi: 10.1191/0269215502cr465oa. [DOI] [PubMed] [Google Scholar]
- 39.Wimo A, Nordberg G. Validity and reliability of assessments of time: Comparisons of direct observations and estimates of time by the use of the resource utilization in dementia (RUD)-instrument. Arch Gerontol Geriatr. 2007;44(1):71–81. doi: 10.1016/j.archger.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Hay J, LaBree L, Luo R, et al. Cost- effectiveness of preventative occupational therapy for independent-living older adults. J Am Geriatr Soc. 2002;50(8):1381–1388. doi: 10.1046/j.1532-5415.2002.50359.x. [DOI] [PubMed] [Google Scholar]
- 41.Jutkowitz E, Gitlin LN, Pizzi L, et al. The cost-effectiveness of a home-based program that reduces functional disability in community-dwelling older adults. Poster presented at: Gerontological Society of America Meetings; November 24, 2008; National Harbor, MD. [Google Scholar]