Abstract
Green light added to blue light has been proposed to shift cryptochromes from their semireduced active form to the reduced, inactive state. Whether the increased proportion of green light observed under leaf canopies compared to open places reduces cryptochrome-mediated effects remained to be elucidated. Here we report that the length of the hypocotyl of Arabidopsis (Arabidopsis thaliana) seedlings grown under controlled conditions decreased linearly with increasing blue/green ratios of the light within the range of ratios found in natural environments. This effect was stronger under higher irradiances. We developed a model, parameterized on the basis of field experiments including photoreceptor mutants, where hypocotyl growth of seedlings exposed to different natural radiation environments was related to the action and interaction of phytochromes and cryptochromes. Adding the blue/green ratio of the light in the term involving cryptochrome activity improved the goodness of fit of the model, thus supporting a role of the blue/green ratio under natural radiation. The blue/green ratio decreased sharply with increasing shade by green grass leaves to one-half of the values observed in open places. The impact of blue/green ratio on cryptochrome-mediated inhibition of hypocotyl growth was at least as large as that of irradiance. We conclude that cryptochrome is a sensor of blue irradiance and blue/green ratio.
The extension growth of the hypocotyl is strongly regulated by light signals (Chen et al., 2004). If a seed germinates beneath the soil, hypocotyl extension growth proceeds in darkness at a maximum rate and this reduces the time needed by the apex and the cotyledons to emerge from the soil. The rate of extension growth decreases when the upper region of the hypocotyl becomes exposed to light. A refined adjustment of hypocotyl length is important because if it is too short the foliage can be easily covered even by weak disturbances of the soil and if the hypocotyl is too long the shoot can be damaged by the excessive exposure to wind impact (Casal et al., 1994).
Hypocotyl growth in Arabidopsis (Arabidopsis thaliana) is controlled mainly by the red and far-red photoreceptors phytochrome A (phyA) and B (phyB; Quail et al., 1995) and the blue UV-A photoreceptors cryptochromes 1 (cry1) and 2 (cry2; Cashmore et al., 1999). The length of the hypocotyl is largely unaffected by light in the phyA phyB cry1 cry2 quadruple mutant of Arabidopsis (Mazzella et al., 2001). Phototropins (Briggs and Christie, 2002) exert a more transient control of hypocotyl growth (Folta and Spalding, 2001). Genetic experiments have revealed a significant dependency of the action of one photoreceptor on the presence of the others. Under suboptimal light conditions, phyB and cry act synergistically (Casal and Mazzella, 1998; Hennig et al., 1999), allowing the inhibition of hypocotyl growth to persist during the night (Sellaro et al., 2009). Under red light, phyA negatively regulates phyB signaling (Cerdán et al., 1999). In the Landsberg erecta background, inhibition of hypocotyl growth by cry2 requires the presence of phyA and phyB and the absence of cry1 (Mazzella et al., 2001). These interactions operate under natural radiation not filtered by leaf canopies or layers of soil (Mazzella and Casal, 2001), where the ratio between red light and far-red light is approximately 1.1. However, the selective absorption of red light compared to far-red light by photosynthetic pigments of green canopies (Holmes and Smith, 1977b) or by soil layers (Mandoli et al., 1990) can also reduce the red/far-red ratio. The quantitative impact of changes in the red/far-red ratio on the action and interactions of phyA and phyB remains largely unexplored.
A number of responses to green light have been reported in the literature (Folta and Maruhnich, 2007). In particular, the flavin chromophore of cry is in the oxidized state in darkness, is driven to the semireduced form by blue light, and this semireduced form is shifted to the reduced state by the absorption of green light, because green light is particularly absorbed by the neutral semiquinone form of FAD (Banerjee et al., 2007; Bouly et al., 2007). Since the biologically active form is that in the semireduced state, green light is able to counteract cry-mediated effect of blue light in vivo (Banerjee et al., 2007; Bouly et al., 2007). The potential role of these green-light effects under natural radiation remains to be elucidated.
The aim of this article is (1) to generate a database of hypocotyl length and light environment for a series of conditions under natural radiation, (2) to use this information to produce a model and analyze the contribution of each photoreceptor and their interactions under shadelight, and (3) to test the role of cry-mediated effects of green light under natural radiation.
RESULTS
Generation of a Database of Hypocotyl Length under Natural Radiation Regimes
To model the contribution of the phyA, phyB, cry1, and cry2 and their interactions to the control of hypocotyl growth in Arabidopsis Landsberg erecta, we generated a database of hypocotyl length under 20 different conditions, including unfiltered sunlight, canopies of divergent structure and composition, litter and soil layers of different texture. We have not included very dense green canopies that reduce the red/far-red ratio below 0.2 to 0.3, because under such conditions phyA is already known to be the dominant photoreceptor (Yanovsky et al., 1995). We measured the light environment at midday (Supplemental Table S1; Supplemental Fig. S1) and the length of the hypocotyl under the different conditions.
Model Stage I: Relationship between Hypocotyl Length and Photoreceptor Input
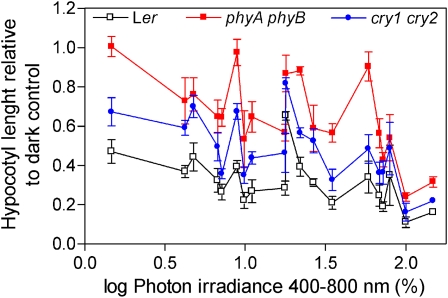
The difference in hypocotyl length between the wild type and either the phyA phyB or the cry1 cry2 double mutants was narrowed down with the exposure to higher irradiances (Fig. 1), indicating that in more open environments the contribution of different photoreceptors becomes increasingly redundant. To reflect this saturation of the hypocotyl-length response to photoreceptor input, we used a hyperbolic relationship, where:
 |
(1) |
Figure 1.
Photoreceptor redundancy increases with the irradiance of natural radiation environments. The length of the hypocotyl of 8-d-old seedlings of Arabidopsis is plotted against the average midday irradiance (400–800 nm) to which they were exposed in field experiments (expressed as a percentage of full sunlight under the experimental conditions). Note that the difference between the wild type and the phyA phyB or the cry1 cry2 double mutants decreases under well-illuminated conditions, indicating that the photoreceptors that remain in each double mutant are almost sufficient to produce wild-type inhibition of hypocotyl growth under strong irradiance. Data are means and se of six replicate boxes. Ler, Landsberg erecta. [See online article for color version of this figure.]
The actions and interactions of phyA, phyB, cry1, and cry2 in the denominator of Equation 1 were disaggregated as follows:
| (2) |
Where k is a constant, a through e are the regression coefficients, PHYA, PHYB, CRY1, and CRY2 equal 1 if the functional PHYA, PHYB, CRY1, and CRY2 alleles are respectively present and equal 0 if the phyA, phyB, cry1, and cry2 mutant (null) alleles are respectively present. R, FR, R + FR, and B are the photon irradiances of red, far-red, red plus far red, and blue light expressed as a percentage of sunlight under our experimental conditions (the use of percentages facilitates the application of the model when the range of integration of the waveband is different from the one used here), and R:FR is the red/far-red ratio (values higher than 1.1 are taken as 1.1).
The hypocotyl of triple-mutant plants containing phyA, phyB, or cry1 is shorter than that of the phyA phyB cry1 cry2 quadruple mutant (Mazzella and Casal, 2001), indicating that phyA, phyB, and cry1 can operate in the absence of the other three photoreceptors. These independent effects are incorporated in the first three terms of the denominator. Based on experiments with restricted wavebands under controlled conditions we expressed phyA activity as a function of red (Franklin et al., 2007) and far-red light (Whitelam et al., 1993; Quail et al., 1995), phyB activity as a function of red light (Reed et al., 1994; Quail et al., 1995), and cry1 activity as a function of blue light (Ahmad and Cashmore, 1993; Cashmore, 1997). Although phyA can operate as a blue light sensor (Whitelam et al., 1993), the effects of blue light on the status of phys is weak in the presence of red and far-red light (Mancinelli, 1986). Based on experiments with different irradiances of sunlight obtained by using spectrally neutral filters (Mazzella and Casal, 2001), percent photon irradiances were log transformed.
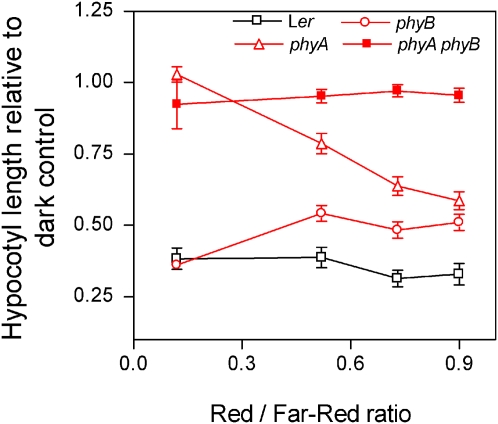
To obtain quantitative information about the effects of red/far-red ratios on phyA and phyB activities, we conducted experiments under natural radiation with selective filters allowing similar transmission of far-red light and different proportions of red light (neither blue nor green light). As expected, these red plus far-red environments failed to inhibit hypocotyl growth in the phyA phyB double mutant (Fig. 2). The contribution of phyB to the inhibition of hypocotyl growth (revealed by the difference between phyA and phyA phyB) decreased with decreasing red/far-red ratio and became negligible at ratios ≤0.3 (Fig. 2). For this reason, the second term contains the coefficient 1.25 [R:FR − 0.3] that decreases the contribution of phyB with reduced red/far-red ratios. This coefficient achieves a maximum value (1) when the red/far-red ratio is ≥1.1 and a minimum value (0) when this ratio is ≤0.3 (Fig. 2). The contribution of phyA (revealed by the difference between the phyB and phyA phyB mutants) was relatively stable at least for red/far-red ratios ≥0.5 (Fig. 2) and therefore the first term does not include a coefficient to correct phyA activity by red/far-red ratio. The contribution of phyA to the inhibition of hypocotyl growth is increased when the red/far-red ratio is reduced below the range used here, i.e. below 0.1 under continuous red plus far-red mixtures in controlled conditions (Smith et al., 1997) or below 0.3 under natural canopies (Yanovsky et al., 1995).
Figure 2.
The contribution of phyA and phyB to the inhibition of hypocotyl growth under different red/far-red ratios. The length of the hypocotyl of 8-d-old seedlings of Arabidopsis is plotted against the red/far-red ratio to which they were exposed in field experiments under sunlight in combination with selective filters. Note that the phyA phyB double mutant is unaffected by light, indicating that no other phy contributes significantly to the inhibition of hypocotyl growth by red and far-red light. The difference between the phyA and phyA phyB mutants reveals the contribution of phyB, which increases with red/far-red ratios above 0.3. The difference between the phyB and phyA phyB mutants reveals the contribution of phyA, which is largely unaffected by red/far-red ratio in the range tested here. Data are means and se of 16 replicate boxes. Ler, Landsberg erecta. [See online article for color version of this figure.]
The fourth term of the denominator reflects that in the Landsberg erecta background, cry2 effects are observed if phyA and phyB are present and cry1 is absent and this contribution of cry2 does not show significant irradiance dependency (Mazzella and Casal, 2001). The fifth term of the denominator incorporates the interaction between phyA and phyB (Cerdán et al., 1999). The sum of the contributions of phyA and phyB in Figure 2 predicts hypocotyls shorter than actually observed in the wild type. For instance, for a red/far-red ratio of 0.9, the reduction of hypocotyl length caused by phyA (difference in length between phyB and phyA phyB mutants) is 0.49 and that caused by phyB (difference in length between phyA and phyA phyB mutants) is 0.41, therefore the predicted hypocotyl length in the wild type (phyA plus phyB activity) would be 0.1 (i.e. 1 − 0.49 − 0.41) but the actual length is 0.33 (Fig. 2). This is caused by the negative regulation of phyB signaling by phyA (Cerdán et al., 1999). This interaction is fluence-rate dependent under continuous red light (Mazzella et al., 1997) and under sunlight combined with neutral filters (Mazzella and Casal, 2001). The sixth term of the denominator incorporates the synergism between phyB and cry1, which does not increase with irradiance (Mazzella and Casal, 2001) and is not reduced by low red/far-red ratios (Casal and Mazzella, 1998).
We used multiple regression to fit hypocotyl-length data to the light inputs according to Equation 2. This analysis yielded a R2 = 0.61 and indicated that each one of the terms of the model made a highly significant contribution (Supplemental Fig. S2A). To determine the model goodness of fit we plotted observed against predicted values (Piñeiro et al., 2008). Linear regression of observed versus predicted values shows an intercept marginally different from 0 (P < 0.1, Supplemental Fig. S2A), suggesting a weak bias of the model (Smith and Rose, 1995). The root mean squared deviation, which estimates the mean deviation of predicted values with respect to the observed values expressed in the units of the model (Kobayashi and Salam, 2000; Piñeiro et al., 2008) was 0.16. To validate the model stage I we used published hypocotyl-length data from wild-type, phyA, phyB, and cry1 seedlings grown under wheat (Triticum aestivum) canopies of different densities (Yanovsky et al., 1995), as these data had not been incorporated in the generation of the model. This analysis demonstrates the good predictive value of the model (Supplemental Fig. S2B).
The Range of Hypocotyl-Length Response to the Blue/Green Ratio
Long-wavelength green light can antagonize the effects of cry activation by blue light by reducing the concentration of the flavosemiquinone signaling state (Banerjee et al., 2007; Bouly et al., 2007). However, short-wavelength green light actually activates cry-mediated inhibition of hypocotyl growth (Ahmad et al., 2002; Bouly et al., 2007). Light reflected or transmitted by green leaves is enriched in both long- and short-wavelength green light (Holmes and Smith, 1977b). We therefore used broad-band light sources to investigate the effect of the blue/green ratio under controlled conditions. Broad-band green light is predicted to have a dual effect on cry activity due to its short- and long-wavelength components.
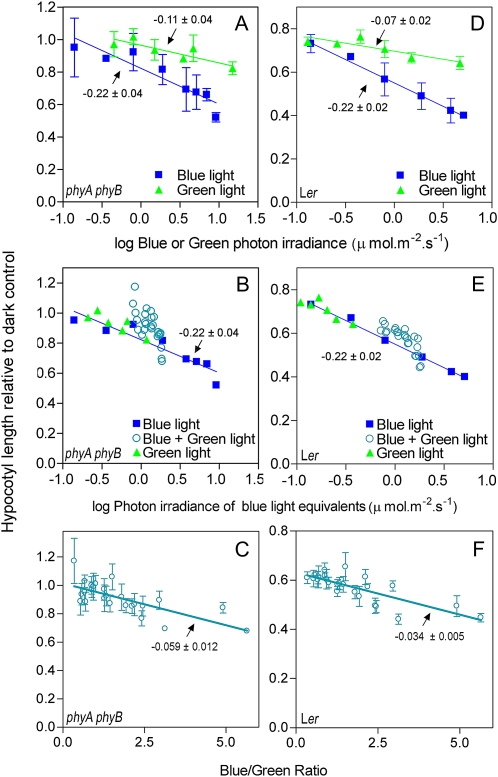
In Landsberg erecta, the length of the phyA phyB cry1 or phyA phyB cry1 cry2 mutants is largely unaffected by light (Mazzella et al., 2001), indicating that the response of the phyA phyB double mutant reflects the activity of cry1. We cultivated phyA phyB seedlings under controlled conditions under different irradiances of continuous broad-band blue light, broad-band green light, or mixtures with different blue/green ratio. We plotted relative hypocotyl length of phyA phyB against the log photon irradiance of blue or green light. As expected, the slope of the responses to blue light was steeper than the slope of response to green light (2.0-fold steeper; Fig. 3A). This means that blue light is 2-fold more effective than green light to inhibit hypocotyl growth via cry. We then transformed green light into blue light equivalents by dividing the log photon irradiance of green light by 2. As expected, when hypocotyl length is plotted against log photon irradiance of blue light equivalents, data from seedlings treated with either blue or green light fall on the same line (Fig. 3B). The blue plus green mixtures were also transformed into blue light equivalents (=photon irradiance of blue light + antilog [log photon irradiance of green light/2.0]). When hypocotyl length of green plus blue mixtures is plotted against log photon irradiance of blue light equivalents, the hypocotyls appear taller than predicted from the data obtained with blue or green light alone, particularly when the mixture has a low blue/green ratio (Fig. 3B). In seedlings exposed to blue plus green mixtures, the length of the hypocotyl decreased linearly with the blue/green ratio over a range including that observed in natural environments (Fig. 3C). A similar pattern was observed for the wild type (Fig. 3, D–F).
Figure 3.
Broad-band green light inhibits hypocotyl growth of phyA phyB mutant (A–C) or wild-type (D–F) seedlings when compared to darkness but blue plus green mixtures are less effective than predicted by the actions of blue and green alone. A, Hypocotyl length of 4-d-old seedlings of phyA phyB (the mutant is used to reveal cry action) against the log photon irradiance of continuous blue light (P < 0.0001) or green light (P < 0.05) under controlled conditions. B, Hypocotyl length against the log photon irradiance of continuous blue light or green light transformed into blue light equivalents (i.e. green light divided by the ratio between the slope for blue and for green light in A). Note that while data from seedlings treated with either green or blue light alone fall on the same line, seedlings exposed to blue plus green mixtures tend to show longer hypocotyls. C, Hypocotyl length of phyA phyB seedlings exposed to blue plus green mixtures plotted against the blue/green ratio of the mixture (P < 0.0001). D, Hypocotyl length of the wild type against the log photon irradiance of continuous blue (P < 0.0001) or green light (P < 0.05). E, Hypocotyl length of the wild type against the log photon irradiance of continuous blue light or green light transformed into blue light equivalents. F, Hypocotyl length of the wild type plotted against the blue/green ratio of the mixture (P < 0.0001). Data are means and se (omitted in B and E for clarity) of three replicate boxes. The slopes and their se are indicated. Ler, Landsberg erecta. [See online article for color version of this figure.]
The Effect of the Blue/Green Ratio Depends on the Irradiance
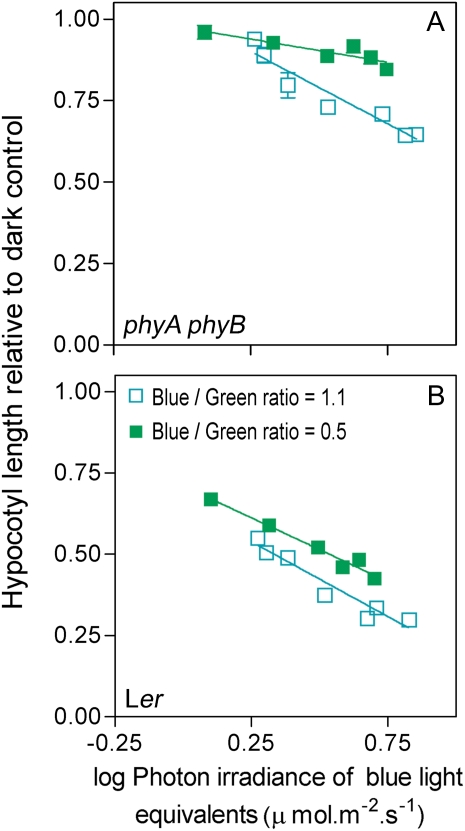
In an independent set of experiments, the seedlings were grown under different irradiances of two blue plus green light mixtures one with a ratio of 1.1 and the other with a ratio of 0.5, because these are values that can be recorded under natural radiation conditions (see below). This reduction of the blue/green ratio significantly promoted hypocotyl growth both in wild-type and phyA phyB seedlings, particularly at higher irradiances (Fig. 4). In phyA phyB (which reflects cry activity), lowering the blue/green ratio from 1.1 to 0.5 at a log photon irradiance (μmol m−2 s−1) of 0.72 increased hypocotyl length from 0.68 to 0.87 (Fig. 4A). A similar increase in hypocotyl length can be achieved if the blue/green ratio remains in 1.1 but the log photon irradiance (μmol m−2 s−1) is reduced to 0.19 (Fig. 4A). In other words, to phenocopy the effect of approximately halving the green/blue ratio (i.e. from 1.1 to 0.5) the log irradiance of blue light has to be reduced to less than one-third (i.e. from 0.72 to 0.19). The effect of the blue/green ratio on cry-mediated inhibition of hypocotyl growth was still evident in the wild type, where phy are also active (Fig. 4B).
Figure 4.
Inhibition of hypocotyl growth by high, compared to low blue/green ratios within the range of natural radiation. Four-day-old seedlings of the phyA phyB mutant (A) or of the wild type (B) grown under different irradiances of continuous light of two contrasting blue/green ratios in controlled conditions. Hypocotyl length of the phyA phyB cry1 triple mutant under log blue light equivalent of 0.8 was 0.99 ± 0.01 and 0.89 ± 0.02 for blue/green ratios of 1.1 and 0.5, respectively. Data are means and se (whenever larger than the symbols) of eight replicate boxes. Ler, Landsberg erecta. [See online article for color version of this figure.]
Model Stage II Incorporates the Blue/Green Light Ratio
The blue/green ratio changed significantly in our experimental conditions and its correlation with blue irradiance was not significant (Fig. 5). Based on the experiments under controlled conditions, where the inhibition of hypocotyl growth is enhanced by the combined action of high irradiances and blue/green ratios (Figs. 3C and 4A), we expressed the contribution of cry1 to the inhibition of hypocotyl growth as a function of the product [log B] × B:G (where B:G is the blue/green ratio) rather than as a function of [log B] alone. This defines the stage II of the model:
| (3) |
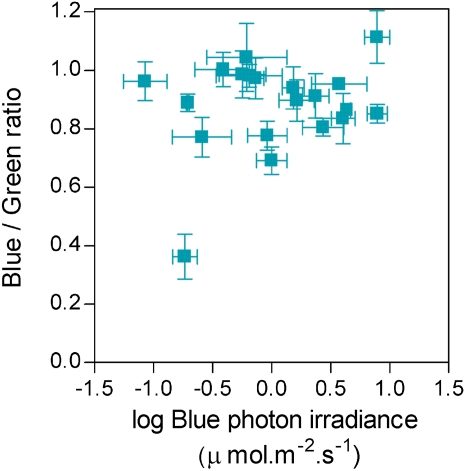
Figure 5.
Blue light irradiance and blue/green ratio are not correlated. R² = 0.05, P > 0.3. Data correspond to the 20 field stations (Supplemental Table S1; Supplemental Fig. S1). Photon irradiances are expressed as a percentage of sunlight values under unshaded conditions. Data are means and se of four measurements. [See online article for color version of this figure.]
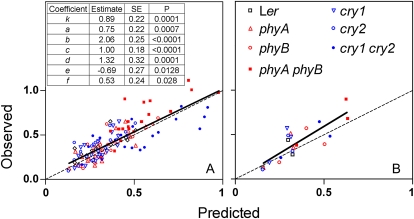
Compared to the basic term, the addition of the B:G component increased the significance of the term involving cry1 activity and the R2 values of the model from 0.61 to 0.64. The plot of observed against predicted values shows no obvious bias as the slope and intercept are not significantly different from 1 and 0, respectively (Fig. 6A). The root mean squared deviation showed a 25% reduction as a result of the inclusion of the blue/green ratio in the model, from 0.16 in stage I to 0.12 in stage II.
Figure 6.
Parametization, analysis of the goodness of fit, and validation of the model stage II. A, Observed versus predicted values of hypocotyl length for the data used to parametize the model. The embedded table shows the estimated coefficients of the model and their significance. B, Validation: observed versus predicted values for an independent set of data. The observed-predicted regression lines and the 1:1 lines (dashed) are included. Ler, Landsberg erecta. [See online article for color version of this figure.]
The product [log B] × B:G gives the same weight to a proportionally similar change in log photon irradiance or in the blue/green ratio. However, halving the blue/green ratio has a stronger effect on cry-mediated activity than halving the log photon irradiance (Fig. 4A), indicating that using the product between both variables would underestimate the weight of changes in the blue/green ratio. The impact of changes in the blue/green ratio can be increased mathematically by using variants of the model where B:G is replaced by ([B:G] − h), where h is a constant (for instance 0.2). When the impact of changes in blue/green ratio is increased in that way, there is some increase in the R2 of the model (data not shown). This confirms that model stage II does involve some underestimation of the impact of changes in blue/green ratio.
We validated model in stage II by conducting an independent field experiment involving five different conditions (Fig. 6B). The root mean squared deviation was 0.13, very close to that obtained with the data used to calibrate the model.
Changes in Blue/Green Ratio in Response to Increasing Canopy Shade
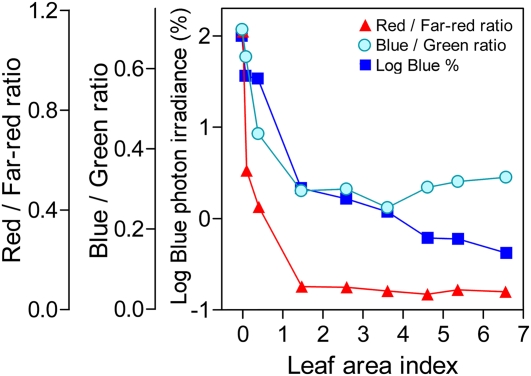
The data shown in Figure 5 illustrate the range of blue/green ratios that result from the combination of different degrees of shade and different shading objects (leaves, soil, dead plant material). To investigate the specific variation of the blue/green ratio in response to different degrees of shading by a green plant canopy, we measured the spectral photon distribution of the light at different positions within a green grass (Paspalum dilatatum) canopy at a clear midday. Then, we harvested the plant material above each measurement position and plotted the blue/green ratio against the leaf area index traversed by the light. The blue/green ratio of unfiltered sunlight (leaf area index = 0) was lower than that indicated in the database used to parametize the model (confront Figs. 5 and 7). This is due to the fact that the measurements in Figure 7 correspond to a clear midday and those shown in Figure 5 were obtained in winter, when most days showed some degree of cloudiness, because clouds increase the blue/green ratio (see spectra by Holmes and Smith, 1977a). The blue/green ratio decreased with increasing shade down to approximately 50% of sunlight values for a leaf area index of 2 and remained relatively stable at these low values under more intense shade (Fig. 7). Above a leaf area index of 2, the log photon irradiance of blue light decreased with a slope comparable to that of the blue/green ratio but, in contrast to the blue/green ratio, under deep shade (leaf area index >4) the log photon irradiance of blue light continued to decrease (Fig. 7). The red/far-red ratio, shown for comparative purposes, decreased more sharply and reached values of approximately 10% those of sunlight for a leaf area index of 2 (Fig. 7).
Figure 7.
Response of the blue/green ratio to increasing degrees of shade by a green canopy of P. dilatatum. The blue/green ratio, the log photon irradiance of blue light expressed as a percentage of the values above the canopy and the red/far-red ratio were calculated from the same scans and are plotted against the leaf area index above the sensor. [See online article for color version of this figure.]
DISCUSSION
Green light has both cry-dependent and transient, cry-independent effects on hypocotyl growth (Folta, 2004; Folta and Maruhnich, 2007). Compared to darkness, maximal cry activity occurs between 380 and 500 nm but there is some activity up to 550 nm (Ahmad et al., 2002), i.e. green light inhibits hypocotyl growth via cry (Lin et al., 1995; Ahmad et al., 2002; Fig. 3). However, long-wavelength green light added to blue light reduces cry-mediated effects, suggesting that the semireduced flavin, which accumulates under blue light, is the biologically active form of cry and that green light absorption by the flavosemiquinone signaling state of cry shifts the photoequilibrium to a higher proportion of the biologically inactive reduced state (Banerjee et al., 2007; Bouly et al., 2007). The results presented here provide three pieces of evidence in favor of a significant regulation of cry activity by the blue/green ratio under natural radiation.
First, the blue/green ratio varies significantly in different seedling environments characterized by the presence of shading by soil layers or green or senescent plant material compared to unfiltered sunlight (Fig. 5). Under different shading materials the blue/green ratio does not correlate with blue light irradiance (Fig. 5), indicating that blue light irradiance and the blue/green ratio can provide independent information. Furthermore, increasing degrees of shade imposed by a green grass canopy reduced the blue/green ratio to about 50% of unfiltered sunlight values at a leaf area index of 2, indicating that the blue/green ratio may provide information about of the degree of shading by neighbors. Blue and green light have different patterns of penetration into green plant tissues (Vogelmann, 1994) and therefore, the ratio could also provide information about deepness within the organs.
Second, under controlled conditions, hypocotyl length decreased linearly with the blue/green ratio over a wide range of ratios (Fig. 3, C and F). Lowering the blue/green ratio from 1.1 to 0.5 more than halved the cry-mediated inhibition of hypocotyl growth (Fig. 4A). Although supplementary green light had already been shown to reduce the effectiveness of blue or white light (Banerjee et al., 2007; Bouly et al., 2007), present data demonstrate that changes in blue/green ratios that are within the natural range of values are effective. We also show that the impact of the blue/green ratio is higher at higher irradiances (Fig. 4). The green light enrichment caused by chlorophyll-rich tissues is broad band and therefore it includes green wavelengths that are effective to activate cry and wavelengths that are more effective to inactivate cry. We present a methodology to translate green light into blue light equivalents based on the comparison of broad-band blue and green light to induce cry-mediated inhibition of hypocotyl growth. This methodology has helped to elucidate the actual quantitative contribution of the blue/green ratio by taking into account the induction of cry activity by green light (Figs. 3 and 4).
Third, we produced and validated a model that quantifies the contribution of phyA, phyB, cry1, and cry2 and their interactions to the control of hypocotyl growth in Arabidopsis (Landsberg erecta) seedlings grown under natural radiation. Adding a factor to reduce cry1-mediated inhibition of hypocotyl growth with decreasing blue/green ratios caused a statistically significant improvement of the model goodness of fit and eliminated a systematic bias toward the underestimation of hypocotyl length under shaded conditions (Fig. 6A).
The irradiance of blue light and the red/far-red ratio are two well-established signals of the degree of shade by green vegetation. The results presented here help to compare the impact and significance of the blue/green ratio with that of these signals. To reduce the cry-mediated effect, lowering the blue/green ratio was at least as effective as lowering the log photon irradiance of blue light in a same proportion (and at least in some cases the blue/green ratio was significantly more effective; Fig. 4A). In relatively sparse canopies (leaf area indexes above 2), the blue/green ratio or the log irradiance of blue light decreased approximately in parallel with increasing shade (Fig. 5), indicating that under these conditions the blue/green ratio would be at least as important as the log blue irradiance. In the range of very dense canopies, the log irradiance of blue light continued to decrease while the blue/green ratio became stable for leaf area indexes above 2. For the control of hypocotyl growth, the red/far-red ratio appears quantitatively more important than the blue/green ratio for two reasons: The red/far-red ratio affects phyB activity, which weights more than cry1 activity, and shows a steeper decrease with increasing leaf are index to values where phyB activity becomes negligible (Fig. 5). Furthermore, as a canopy signal the red/far-red ratio appears more refined than the blue/green ratio because the former is weakly affected by cloudiness, which has a significant impact on the blue/green ratio (Holmes and Smith, 1977a).
The combination of photoreceptor mutants with complex light protocols, often devised without any intention to simulate the natural environment, has helped to characterize the key photobiological features of these photoreceptors, such as their wavelength and fluence-rate dependence and mutual interdependence (interactions). The analysis presented here demonstrates that most of these features have a correlate in the action of phy and cry in the natural radiation environment. The model developed on the basis of this knowledge provides a reasonable prediction of the inhibition of hypocotyl growth in different natural environments by using very simple input variables (the proportion of blue, green, red, and far-red light relative to incoming radiation measured at midday). For a set of data independent from that used for parametization, the estimated mean deviation of predicted values with respect to the observed values was 0.13 in units of hypocotyl length relative to dark controls (in a context where these values range from 1.00 in darkness to approximately 0.1 in open places). We provide a calculator of hypocotyl length (Supplemental Table S2) to facilitate the application of the model. Here, the model has helped to evaluate the function of cry as a sensor of blue/green ratio under natural radiation; beyond this analysis, the comparison of new data with the predictions of the model will provide a tool to identify gaps in the knowledge and generate new hypothesis.
MATERIALS AND METHODS
Plant Material and Measurements of Hypocotyl Length
The wild type, the phyA-201, phyB-5, phyA-201 phyB-5, cry1 (hy4- 2.23n), cry2 (fha), and cry1 (hy4- 2.23n) cry2 (fha) mutants are in the Landsberg erecta background (see Mazzella and Casal [2001] for original sources of these mutants). Seeds (15 per genotype) were sown on 3 mL of 0.8% agar in clear plastic boxes (40-mm long, 33.3-mm wide, 31.5-mm tall). The boxes were incubated in the dark at 7°C for 3 d, given 8 h of red light (to induce seed germination) followed by 16 h darkness (22°C), and transferred to the light conditions either in the field or in a growth room at 22°C. The boxes remained 7 d in the field or 3 d under the light treatments (temperature in the field was lower and hypocotyl growth proceeded more slowly than under controlled conditions). Then, hypocotyl length was measured to the nearest 0.5 mm with a ruler and the length of the 10 tallest seedlings per genotype and per box were averaged (defining one replicate box).
Growth Conditions in Field Experiments
In the field, the boxes were placed beneath different plant canopies, litter or soil layers, or remained exposed to unfiltered sunlight. These conditions defined 20 stands located in the experimental field of the Faculty of Agronomy (University of Buenos Aires) latitude 34° 35′ S, longitude 58° 28′ W (Supplemental Table S1). In each experiment, three boxes were exposed to the light environment of the specific stand and one box was placed under the same conditions but wrapped with black plastic (inner cover) and aluminum foil (outer cover). We did not observe consistent differences in hypocotyl length of dark controls placed under the different stand conditions and therefore, hypocotyl length in each experiment was expressed relative to the average length of the relevant dark control of that experiment and then averaged for the different experiments. The experiment was conducted on two different occasions during the winter (August). Average maximum and minimum temperatures during the experiments were 14.2°C and 7.3°C, respectively.
Additional experiments were conducted to investigate the effect of different red/far-red ratios on hypocotyl length. The seeds were sown as described above but at the time of transfer to the field the boxes were placed in recipients covered with selective plastic filters and placed under unfiltered sunlight. All filter combinations included one red filter (Lee Filters, 106), one orange filter (Lee Filters, 105), and one yellow filter (Lee Filters 101) to allow the seedlings to receive only red and far-red light and a clear diffusing filter. No further filters were included to provide the high red/far-red ratio condition. A green filter (Lee Filters 89) was used to reduce the transmittance of red light without affecting far-red light and small holes in this filter generated intermediate red/far-red ratios.
Description of the Light Environment in Field Experiments
The light environment of the different stands was scanned at 1-nm resolution between 400 and 800 nm with a spectroradiometer (FieldSpec Pro FR; Analytical Spectral Devices [ASD]) at midday. The remote probe of the spectroradiometer was placed at the same position where the seedlings were grown, covered with the lid of a plastic box equal to those used for the seedlings (to record the environment experienced by the seedlings). The photon irradiance values (μmol m−2 s−1 nm−1) were divided by those measured out of the stand to obtain relative values. Since overcast conditions were frequent during the experiments and light measurements, the expression relative to the values out of the stand correct for temporal fluctuations in cloud cover. Ten scans were obtained per stand at each one of four different dates during the experimental period and averaged. Blue light is the integral between 420 and 490 nm, green light is the integral between 500 and 570 nm, red light is the integral between 620 and 680 nm, and far-red light is the integral between 700 and 750 nm. However, to calculate the red/far-red ratio red and far-red light were integrated between 650 and 670 nm and between 720 and 740 nm, respectively, because these bandwidths are more common in the literature to calculate the ratio.
Model Parametization and Evaluation of the Goodness of Fit
To parametize the model we linearized the relationship by using the inverse of Equation 2 or 3 (i.e. the inverse of hypocotyl length relative to dark controls was directly related to the denominator of these equations). Then, we used multiple regression analysis with Infostat software to test and parametize the model. To evaluate the goodness of fit we calculated the root mean squared deviation as described (Kobayashi and Salam, 2000; Piñeiro et al., 2008).
Light Treatments under Controlled Conditions
Blue light or green light were provided by fluorescent tubes (Osram L30W/10) in combination with either a blue filter (Lee Filters, 363, www.leefilters.com) or a green filter (Lee Filters, 89), respectively. The light fields were scanned with the spectroradiometer (Supplemental Fig. S3). For the blue light source, the peak of maximum photon irradiance was at 437 nm and the limits of the band with an irradiance of at least 0.1 of the peak irradiance were 405 and 486 nm. For the green light source, the peak was at 547 nm and the band limits were 492 and 573 nm. The irradiances and the blue/green light ratios were modified by varying the distance between the blue or green light sources and the boxes containing the seedlings or by using neutral filters.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Scans of the light environment at midday in the 20 different stations where hypocotyl length was measured.
Supplemental Figure S2. Parametization, goodness-of-fit analysis, and validation of the model stage I.
Supplemental Figure S3. Spectral irradiance of the blue and green broad-band sources.
Supplemental Table S1. Light environment at midday in the 20 different stations where hypocotyl length was measured.
Supplemental Table S2. Hypocotyl length estimator based on model stage II.
Supplementary Material
Acknowledgments
We thank Emeritus Professor Antonio J. Hall for his encouraging discussions; Dr. Santiago Verón, Martín Durante, and Carlos Mazza for their valuable help with scans of the light treatments and conditions; and Silvia Ibarra for her help with seed stocks.
References
- Ahmad M, Cashmore AR. (1993) HY4 gene of Arabidopsis thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 366: 162–166 [DOI] [PubMed] [Google Scholar]
- Ahmad M, Grancher N, Heil M, Black RC, Giovani B, Galland P, Lardemer D. (2002) Action spectrum for cryptochrome-dependent hypocotyl growth inhibition in Arabidopsis. Plant Physiol 129: 774–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, Schleicher E, Meier S, Viana RM, Pokorny R, Ahmad M, Bittl R, Batschauer A. (2007) The signaling state of Arabidopsis cryptochrome 2 contains flavin semiquinone. J Biol Chem 282: 14916–14922 [DOI] [PubMed] [Google Scholar]
- Bouly JP, Schleicher E, Dionisio-Sese M, Vandenbussche F, Van Der Straeten D, Bakrim N, Meier S, Batschauer A, Galland P, Bittl R, et al. (2007) Cryptochrome blue light photoreceptors are activated through interconversion of flavin redox states. J Biol Chem 282: 9383–9391 [DOI] [PubMed] [Google Scholar]
- Briggs W, Christie J. (2002) Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci 7: 204–210 [DOI] [PubMed] [Google Scholar]
- Casal JJ, Ballaré CL, Tourn M, Sánchez RA. (1994) Anatomy, growth and survival of a long-hypocotyl mutant of Cucumis sativus deficient in phytochrome B. Ann Bot (Lond) 73: 569–575 [Google Scholar]
- Casal JJ, Mazzella MA. (1998) Conditional synergism between cryptochrome 1 and phytochrome B is shown by the analysis of phyA, phyB and hy4 simple, double and triple mutants in Arabidopsis. Plant Physiol 118: 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashmore AR. (1997) The cryptochrome family of photoreceptors. Plant Cell Environ 20: 764–767 [Google Scholar]
- Cashmore AR, Jarillo JA, Wu YJ, Liu D. (1999) Cryptochromes: blue light receptors for plants and animals. Science 284: 760–765 [DOI] [PubMed] [Google Scholar]
- Cerdán PD, Yanovsky MJ, Reymundo FC, Nagatani A, Staneloni RJ, Whitelam GC, Casal JJ. (1999) Regulation of phytochrome B signaling by phytochrome A and FHY1 in Arabidopsis thaliana. Plant J 18: 499–507 [DOI] [PubMed] [Google Scholar]
- Chen M, Chory J, Fankhauser C. (2004) Light signal transduction in higher plants. Annu Rev Genet 38: 87–117 [DOI] [PubMed] [Google Scholar]
- Folta KM. (2004) Green light stimulates early stem elongation, antagonizing light-mediated growth inhibition. Plant Physiol 135: 1407–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folta KM, Maruhnich SA. (2007) Green light: a signal to slow down or stop. J Exp Bot 58: 3099–3111 [DOI] [PubMed] [Google Scholar]
- Folta KM, Spalding EP. (2001) Unexpected roles for cryptochrome 2 and phototropin revealed by high-resolution analysis of blue light-mediated hypocotyl growth inhibition. Plant J 26: 471–478 [DOI] [PubMed] [Google Scholar]
- Franklin KA, Allen T, Whitelam GC. (2007) Phytochrome A is an irradiance-dependent red light sensor. Plant J 50: 108–117 [DOI] [PubMed] [Google Scholar]
- Hennig L, Funk M, Whitelam GC, Schäfer E. (1999) Functional interaction of cryptochrome 1 and phytochrome D. Plant J 20: 289–294 [DOI] [PubMed] [Google Scholar]
- Holmes MG, Smith H. (1977a) The function of phytochrome in the natural environment. I. Characterization of daylight for studies in photomorphogenesis and photoperiodism. Photochem Photobiol 25: 533–538 [Google Scholar]
- Holmes MG, Smith H. (1977b) The function of phytochrome in the natural environment. II. The influence of vegetation canopies on the spectral energy distribution of natural daylight. Photochem Photobiol 25: 539–545 [Google Scholar]
- Kobayashi K, Salam MU. (2000) Comparing simulated and measured values using mean squared deviation and its components. Agron J 92: 345–352 [Google Scholar]
- Lin C, Ahmad M, Gordon D, Cashmore AR. (1995) Expression of an Arabidopsis cryptochrome gene in transgenic tobacco results in hypersensitivity to blue, UV-A and green light. Proc Natl Acad Sci USA 92: 8423–8427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancinelli AL. (1986) Comparison of spectral properties of phytochromes from different preparations. Plant Physiol 82: 956–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandoli DF, Ford GA, Waldron LJ, Nemson JA, Briggs WR. (1990) Some spectral properties of several soil types: implications for photomorphogenesis. Plant Cell Environ 13: 287–294 [Google Scholar]
- Mazzella MA, Alconada Magliano TM, Casal JJ. (1997) Dual effect of phytochrome A on hypocotyl growth under continuous red light. Plant Cell Environ 20: 261–267 [Google Scholar]
- Mazzella MA, Casal JJ. (2001) Interactive signalling by phytochromes and cryptochromes generates de-etiolation homeostasis in Arabidopsis thaliana. Plant Cell Environ 24: 155–162 [Google Scholar]
- Mazzella MA, Cerdán PD, Staneloni R, Casal JJ. (2001) Hierarchical coupling of phytochromes and cryptochromes reconciles stability and light modulation of Arabidopsis development. Development 128: 2291–2299 [DOI] [PubMed] [Google Scholar]
- Piñeiro G, Perelman S, Guerschman JP, Paruelo JM. (2008) How to evaluate models: observed vs. predicted or predicted vs. observed? Ecol Modell 216: 316–322 [Google Scholar]
- Quail PH, Boylan MT, Parks BM, Short TW, Xu Y, Wagner D. (1995) Phytochromes: photosensory perception and signal transduction. Science 268: 675–680 [DOI] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. (1994) Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol 104: 1139–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellaro R, Hoecker U, Yanovsky M, Chory J, Casal JJ. (2009) Synergism of red and blue light in the control of Arabidopsis gene expression and development. Curr Biol 19: 1216–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EP, Rose KA. (1995) Model goodness-of-fit analysis using regression and related techniques. Ecol Modell 77: 49–64 [Google Scholar]
- Smith H, Xu Y, Quail PH. (1997) Antagonistic but complementary actions of phytochromes A and B allow optimum seedling de-etiolation. Plant Physiol 114: 637–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelmann TC. (1994) Light within the plant. Kendrick RE, Kronenberg GHM, , Photomorphogenesis in Plants. Kluwer Academic Press, Dordrecht, The Netherlands, pp 491–535 [Google Scholar]
- Whitelam GC, Johnson E, Peng J, Carol P, Anderson ML, Cowl JS, Harberd NP. (1993) Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell 5: 757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovsky MJ, Casal JJ, Whitelam GC. (1995) Phytochrome A, phytochrome B and HY4 are involved in hypocotyl growth responses to natural radiation in Arabidopsis: weak de-etiolation of the phyA mutant under dense canopies. Plant Cell Environ 18: 788–794 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.