Abstract
Developmental responses associated with end-of-day far-red light (EOD-FR) signaling were investigated in maize (Zea mays subspecies mays) seedlings. A survey of genetically diverse inbreds of temperate and tropical/semitropical origins, together with teosinte (Zea mays subspecies parviglumis) and a modern hybrid, revealed distinct elongation responses. A mesocotyl elongation response to the EOD-FR treatment was largely absent in the tropical/semitropical lines, but both hybrid and temperate inbred responses were of the same magnitude as in teosinte, suggesting that EOD-FR-mediated mesocotyl responses were not lost during the domestication or breeding process. The genetic architecture underlying seedling responses to EOD-FR was investigated using the intermated B73 × Mo17 mapping population. Among the different quantitative trait loci identified, two were consistently detected for elongation and responsiveness under EOD-FR, but none were associated with known light signaling loci. The central role of phytochromes in mediating EOD-FR responses was shown using a phytochromeB1 phytochromeB2 (phyB1 phyB2) mutant series. Unlike the coleoptile and first leaf sheath, EOD-FR-mediated elongation of the mesocotyl appears predominantly controlled by gibberellin. EOD-FR also reduced abscisic acid (ABA) levels in the mesocotyl for both the wild type and phyB1 phyB2 double mutants, suggesting a FR-mediated but PHYB-independent control of ABA accumulation. EOD-FR elongation responses were attenuated in both the wild type and phyB1 phyB2 double mutants when a chilling stress was applied during the dark period, concomitant with an increase in ABA levels. We present a model for the EOD-FR response that integrates light and hormonal control of seedling elongation.
Plants utilize a complex network of photoreceptors to monitor the spectral quality, fluence, direction, and duration of light (Smith, 1995). These photosensory pigments include phytochromes that sense red light (R; 580–690 nm) and far-red light (FR; 690–800 nm) and the cryptochromes, phototropins, and zeitlupes for blue light (380–495 nm) and UV-A light (320–380 nm). The light reflected and transmitted by the vegetation creates a canopy characterized by reductions in both the R-to-FR ratio (R:FR) and the photosynthetically active radiation (400–700 nm). This light environment induces adaptive biochemical and morphological responses known as the shade avoidance syndrome (Smith and Whitelam, 1997). These responses can be induced early in development, before canopy closure, through FR reflected from adjacent neighbor plants (Ballare et al., 1990) or from low-lying weeds (Rajcan and Swanton, 2001), which can negatively impact yields in maize (Zea mays subspecies mays; Rajcan et al., 2004), even if only present early in the growing season (Liu et al., 2009).
R:FR signals are transduced by the phytochrome family of photoreceptors (Franklin and Whitelam, 2007b). In rice (Oryza sativa) and sorghum (Sorghum bicolor), three genes constitute the phytochrome family: PhytochromeA (PhyA), PhytochromeB (PhyB), and PhytochromeC (PhyC). In maize, an ancient alloploidization has doubled the family size to six: PhyA1, PhyA2, PhyB1, PhyB2, PhyC1, and PhyC2 (Sheehan et al., 2004). Although many similarities are apparent between maize and Arabidopsis (Arabidopsis thaliana) light response, there are significant differences between members of the phytochrome gene family in copy number and selection pressures that have resulted in the divergence of phytochrome signaling networks (Sawers et al., 2005; Sheehan et al., 2007). Thus far, only three phytochrome mutants have been characterized in maize: elongated mesocotyl1 (elm1), phyB1, and phyB2. The elm1 mutant carries a mutation in phytochromobilin synthase, necessary for the biosynthesis of the chromophore common to all phytochromes (Sawers et al., 2004). The mutation severely reduces the total phytochrome pool, but the weak nature of the allele enables a partial responsiveness to R and FR (Markelz et al., 2003). At maturity, elm1 mutants have elongated internodes, pale green leaves, and flower early (Sawers et al., 2002). Mutations at phyB1 and phyB2 also impair light signal transduction. At maturity, both PHYB1 and PHYB2 contribute to plant height, stem diameter, and sheath-internode length, but PHYB2 predominates in the control of flowering (Sheehan et al., 2007). Like the sorghum and rice phyB mutants (Childs et al., 1997; Takano et al., 2005; Kebrom et al., 2010), both elm1 and phyB1 phyB2 double mutants constitutively display several traits associated with low R:FR response (Sawers et al., 2002; Markelz et al., 2003; Sheehan et al., 2007).
In Arabidopsis, R/FR-mediated responses are developmentally complex and involve the PIF proteins (Duek and Fankhauser, 2005) and many hormones including auxins (Tao et al., 2008), ethylene (Khanna et al., 2007), jasmonate (Moreno et al., 2009), and GA (Djakovic-Petrovic et al., 2007). In particular, there is a direct interaction between PIF and DELLA proteins that govern phytochrome-mediated elongation (de Lucas et al., 2008; Feng et al., 2008; Lorrain et al., 2008). DELLA proteins also regulate FR inhibition of germination by abscisic acid (ABA; Piskurewicz et al., 2009), suggesting an interaction between the PIFs and ABA signaling. Complex cross talk between light and temperature has also been reported (Franklin, 2009). In Arabidopsis, colder temperatures can repress the early-flowering phenotype of the phyB mutant (Halliday et al., 2003). These studies suggest a complex interplay between light, hormone, and temperature to fine-tune the elongation response.
The end-of-day far-red light (EOD-FR) treatment consists of a pulse of FR given at subjective dusk (Kasperbauer, 1971) and triggers a circadian clock-gated response (Salter et al., 2003). EOD-FR treatments result in a minimal pool of active Pfr during the dark period (Fankhauser and Casal, 2004), and plants submitted to daily treatments display similar developmental responses to those elicited by a continuous photoperiod with low R:FR (Smith, 1994). One of the key features that contributed to the discovery of the phytochromes is the photoreversibility of the response (Borthwick et al., 1952). These low-fluence responses (LFRs) are induced or repressed by alternating R and FR treatments (Mancinelli, 1994). The LFR nature of EOD-FR in maize was previously demonstrated in 5-d-old mesocotyl and coleoptile tissues (Gorton and Briggs, 1980). The EOD-FR treatment offers several advantages over growing plants in continuous low R:FR, including exposing plants to relatively brief treatment periods, thus potentially reducing genotype × environment effects. It also facilitates kinetic assays of phytochrome response, as treatments are limited to a single point in the diurnal cycle and can be delivered at any stage in plant development. Finally, as relatively low fluences of light are needed to saturate EOD-FR responses, large populations of seedlings can be screened without the need for large numbers of FR light-emitting diodes (LEDs) or sophisticated light chambers.
Here, we have examined EOD-FR-mediated responses in maize and its closest relative, teosinte (Zea mays subspecies parviglumis). A survey of genetically diverse maize and teosinte accessions revealed extensive tissue-specific variations in mesocotyl, coleoptile, and first leaf sheath elongation. EOD-FR responses were greatly attenuated in tropical/semitropical (TS) accessions but present in teosinte, temperate inbreds, and a modern commercial hybrid, suggesting that the EOD-FR response is plastic in Z. mays. To investigate the genetic regulation underlying seedling responses to EOD-FR, we performed a quantitative trait locus (QTL) analysis using the intermated B73 × Mo17 (IBM) recombinant inbred population. We identified several QTLs that regulate mesocotyl and first leaf sheath response to EOD-FR and show that these QTLs mediate tissue-specific responses. The phyB1 phyB2 mutant series was also evaluated, indicating that the two PhyB paralogs are largely redundant in mediating the EOD-FR response. Pharmacological assays revealed a major role for GA in promoting mesocotyl, but not coleoptile or first leaf sheath, elongation in response to EOD-FR treatments. In contrast, EOD-FR reduced mesocotyl ABA levels. A chill treatment (10°C) applied during dark breaks attenuated EOD-FR elongation responses. Based on these observations, we discuss a model that integrates temperature, light, and hormonal inputs in the regulation of mesocotyl elongation.
RESULTS
An EOD-FR Treatment Induces a Phytochrome-Mediated Low-Fluence Response in Seedlings
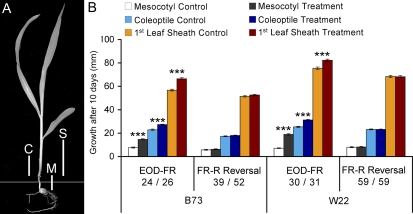
We developed an EOD-FR assay to screen large numbers of maize seedlings in a conventional growth chamber (see “Materials and Methods”). One section was equipped with white (W) fluorescent lights, plus lateral R and FR LED modules, while a similar section was used for the W control treatments. Spectral irradiances were measured for all light treatments (Supplemental Fig. S1). Seedlings grown under W only in both sections showed no significant differences in elongation responses between the two chamber sections, confirming equivalent growth conditions (data not shown). The robustness of a daily 15-min EOD-FR treatment was initially confirmed using two maize inbreds belonging to two heterotic groups: B73 (stiff stalk) and W22 (nonstiff stalk). After a 10-d growth period, mesocotyl, coleoptile, and first leaf sheath lengths were measured (Fig. 1A). For both inbred lines, the EOD-FR treatment caused a significant increase in the length of all three seedling tissues measured. Proportionally, the emerged mesocotyl was the most responsive tissue, followed by the coleoptile and the first leaf sheath (Fig. 1B). EOD-FR-treated plants were also significantly thinner and paler than controls, both features of elm1 and phyB1 phyB2 double mutant plants (Sawers et al., 2002; Sheehan et al., 2007) and reminiscent of seedling phenotypes observed at high planting densities (Dubois and Brutnell, 2009).
Figure 1.
Seedling responses to EOD-FR and FR-R reversal treatments. A, Seedling traits measured 10 d after planting. The horizontal gray line represents the soil surface. C, Coleoptile; M, mesocotyl; S, first leaf sheath. B, Responses to daily EOD-FR treatments on maize seedlings and photoreversibility by subsequent R treatments. Seedlings of B73 and W22 inbreds were subjected to either a daily pulse of 15 min of FR at the end of a 10-h photoperiod (EOD-FR) or the EOD-FR treatment immediately followed by an additional 15-min pulse of R (FR-R). For both EOD-FR and FR-R treatments, control seedlings received 10 h of W only. The values are representative means ± se of seedlings used for each light treatment. The number of seedlings measured for W control/EOD-FR (left side) or W control/FR-R (right side) are shown below each graph. Asterisks indicate significant differences between the two light treatments using Student’s t test for each line (*** P < 0.001).
To examine the photoreversibility of the EOD-FR treatment on mesocotyl, coleoptile, and first leaf sheath elongation, a pulse of 15 min of R (47.4 μmol m−2 s−1) was applied immediately after FR (51.1 μmol m−2 s−1). Seedlings of both inbreds were not significantly different from the W control for all three traits measured (Fig. 1B). In vitro measurements on Pr/Pfr reversibility (Kelly and Lagarias, 1985) indicated that 15 min of FR is sufficient to photoconvert approximately 97% of Pfr to Pr. The subsequent R treatment converts approximately 85% the phytochrome pools to Pfr before developmental responses are irreversibly transduced. Thus, the EOD-FR response induced by this treatment regime is likely mediated by phytochromes acting in the LFR mode.
The impact of EOD-FR on aboveground biomass was examined using the B73 inbred and a commercial hybrid (34P88; Pioneer Hi-Bred). Dry weights were measured after 10 d of growth. As in all the experiments, identical planting densities were used for both light treatments to reduce the confounding effect of crowding on seedling development. For both inbred and hybrid seedlings, no significant differences in dry weights (B73, Student’s t = 0.58, P = 0.59; 34P88, Student’s t = 1.4, P = 0.21) were observed between the two light treatments. These results suggest that at this developmental stage, the EOD-FR treatment causes a repartitioning of photosynthate between tissues rather than a change in biomass.
Responsiveness to EOD-FR Varies among a Genetically Diverse Germplasm Panel
To examine the range of variation in the EOD-FR response in maize, a panel of genetically diverse maize lines was screened. The panel consisted of the 26 founders of the Nested Association Mapping (NAM) population (McMullen et al., 2009), Mo17, and W22. These 28 lines were screened for their response to EOD-FR (Table I) and include stiff stalk, nonstiff stalk, popcorn, sweet corn, TS, and mixed (likely TS admixtures) inbred accessions (Liu et al., 2003). Seedlings displayed significant variation for tissue elongation under control and EOD-FR treatments (Supplemental Fig. S2). As a group, TS accessions were generally significantly shorter than non-TS (mainly temperates) for all three seedling traits and under both light treatments (Student’s t test, all P values < 0.001 except for first leaf sheath under EOD-FR, where P = 0.03; Supplemental Fig. S2). When comparing the three most common inbred lines used in genetic research (B73, Mo17, and W22), B73 and W22 had the two longest mesocotyl lengths under both light treatments among the panel, while Mo17 was much shorter but displayed the strongest EOD-FR elongation response of the three. This developmental pattern was also observed for coleoptile and first leaf sheath. Thus, significant variation was observed both between TS and non-TS lines and also within non-TS lines.
Table I. Seedling responses to EOD-FR treatments in a panel of genetically diverse maize inbreds.
Maize inbreds are grouped based on population structure. Limited seed availability for Tzi8, combined with poor germination, contributed to reduced statistical power in analyzing this line. C, Control; INC, significant elongation increase over control treatment; NS, nonsignificant difference between the two treatments based on Student’s t test (* P < 0.05, ** P < 0.01, *** P < 0.001); NSS, temperate nonstiff stalk; SS, temperate stiff stalk.
| Inbreds | Classification | Individuals C/EOD-FR | Mesocotyl |
Coleoptile |
First Leaf Sheath |
|||
| EOD-FR Response Ratio | Percentage | EOD-FR Response Ratio | Percentage | EOD-FR Response Ratio | Percentage | |||
| B73 | SS | 57/58 | INC*** | 56.6 | INC*** | 13.3 | INC*** | 11.5 |
| B97 | NSS | 29/30 | INC*** | 55.9 | INC** | 14.5 | NS | – |
| Ky21 | NSS | 16/23 | INC** | 63.9 | NS | – | NS | – |
| M162W | NSS | 20/19 | NS | – | INC*** | 19.0 | INC** | 14.3 |
| Mo17 | NSS | 29/25 | INC*** | 134.2 | INC*** | 22.2 | INC*** | 23.0 |
| MS71 | NSS | 31/31 | INC* | 41.8 | INC*** | 9.0 | INC*** | 11.0 |
| Oh43 | NSS | 30/30 | INC*** | 105.0 | INC*** | 18.5 | INC*** | 20.9 |
| W22 | NSS | 32/31 | INC*** | 77.3 | INC*** | 16.4 | INC** | 5.6 |
| HP301 | Popcorn | 18/26 | INC*** | 87.8 | NS | – | INC* | 11.6 |
| Il14H | Sweet | 28/28 | INC*** | 43.2 | NS | – | INC*** | 15.8 |
| P39 | Sweet | 24/30 | NS | – | INC* | 17.4 | INC** | 17.0 |
| M37W | Mixed | 27/30 | INC* | 47.7 | INC* | 5.6 | NS | – |
| Mo18W | Mixed | 17/17 | INC*** | 109.8 | NS | – | NS | – |
| Oh7B | Mixed | 31/31 | INC*** | 117.8 | INC*** | 15.7 | INC*** | 25.7 |
| Tx303 | Mixed | 29/31 | INC** | 68.1 | NS | – | INC** | 8.8 |
| CML103 | TS | 24/28 | INC* | 162.3 | INC** | 13.1 | NS | – |
| CML228 | TS | 30/32 | INC* | 160.9 | INC*** | 14.1 | INC*** | 20.2 |
| CML247 | TS | 31/28 | NS | – | INC*** | 21.7 | INC*** | 19.5 |
| CML277 | TS | 30/26 | NS | – | INC*** | 14.8 | INC*** | 18.3 |
| CML322 | TS | 26/19 | NS | – | INC* | 17.4 | INC*** | 20.2 |
| CML333 | TS | 30/27 | NS | – | INC*** | 18.1 | NS | – |
| CML52 | TS | 32/32 | NS | – | INC*** | 19.1 | INC*** | 18.3 |
| CML69 | TS | 23/20 | NS | – | INC*** | 15.8 | INC** | 15.6 |
| Ki11 | TS | 31/32 | NS | – | NS | – | INC*** | 11.1 |
| Ki3 | TS | 27/23 | NS | – | INC*** | 33.3 | INC*** | 21.7 |
| NC350 | TS | 31/32 | INC* | 87.1 | INC*** | 18.3 | INC*** | 22.4 |
| NC358 | TS | 32/28 | INC*** | 64.4 | INC*** | 18.3 | INC* | 7.2 |
| Tzi8 | TS | 12/17 | NS | – | NS | – | NS | – |
To compare seedling responsiveness to EOD-FR, a response ratio was defined as the percentage increase in tissue length induced by the EOD-FR treatment relative to the W control (EOD-FR response ratio; Table I). Most of the TS lines did not display a significant response for mesocotyl elongation, such that the TS lines as a group were significantly different from all others in mesocotyl behavior (χ2 = 9.69, P = 0.002). The non-TS lines have on average 29% and 30% Southern Dent and Northern Flint landrace backgrounds, respectively, versus 13% and 6% for TS lines (Liu et al., 2003). Contrary to the general tendency, mesocotyl tissues of only four TS lines (CML103, CML228, NC350, and NC358) responded significantly (P < 0.05) to EOD-FR. Interestingly, these four inbreds have a higher proportion of Southern Dent (averaging 23%) relative to the other TS lines of the panel (averaging 0.9%; Student’s t = 3.48, P = 0.02). No such distinction within the TS lines exists for the contribution of the three other historic landrace groupings considered: Tropical Lowland, Tropical Highland, and Northern Flint (Liu et al., 2003). It is worth noting that the photoperiodic regulation of flowering associated with several tropical accessions was attenuated by introgression with temperate germplasm to facilitate their study under long-day seasons (Holland and Goodman, 1995). Thus, selection for early flowering may have accompanied selection for mesocotyl sensitivity to low R:FR. However, no such distinction based on origin could be made for coleoptile or first leaf sheath EOD-FR response ratios, suggesting that only the mesocotyl tissues of TS accessions are less sensitive to low R:FR signals, while coleoptile and first leaf sheath tissues respond in TS as well as non-TS lines. Among the three seedling traits, only coleoptile and first leaf sheath EOD-FR response ratios were weakly correlated (r = 0.53), indicating that a tissue-specific control of EOD-FR-mediated responses occurs in the seedling.
To further assess the consequences of domestication and breeding selection on FR-mediated responses, the maize ancestor teosinte (Doebley et al., 2006) and a commercial hybrid (34P88) were also grown under EOD-FR. In both cases, the mesocotyl, coleoptile, and first leaf sheath were all significantly responsive to EOD-FR (Supplemental Fig. S3). Teosinte EOD-FR elongation ratios were 65%, 17%, and 14% for the mesocotyl, coleoptile, and first leaf sheath, respectively, while the hybrid ratios were 128%, 16%, and 16%, respectively. In both cases, these results are within the range observed in the inbred diversity panel. Interestingly, teosinte and 34P88 mesocotyl responsiveness is similar to temperate, but not TS, inbred lines.
The Genetic Control of EOD-FR-Mediated Elongation Responses
The survey of a diverse panel of inbred accessions identified a range of EOD-FR-mediated elongation responses. It also revealed significant variation between B73 and Mo17, the two parents of the IBM mapping population (Lee et al., 2002). In order to map the genetic components regulating EOD-FR responses, the IBM population was screened under W control and EOD-FR treatments. Seedling traits analyzed were mesocotyl and first leaf sheath length under W control and EOD-FR treatments as well as EOD-FR response ratios. The distribution of mesocotyl and first leaf sheath lengths and corresponding EOD-FR response ratios were all symmetrical and unimodal (Supplemental Fig. S4); thus, no transformation of the data was made prior to QTL analysis. Transgressive segregation in the IBM population was observed for all traits measured (Supplemental Fig. S4). Broad-sense heritability (H2) of the mesocotyl length was 0.80 for W control and 0.87 for EOD-FR, while first leaf sheath length heritability was 0.89 for W control and 0.90 for EOD-FR. EOD-FR response ratio H2 was lower, 0.52 for the mesocotyl and 0.63 for the first leaf sheath.
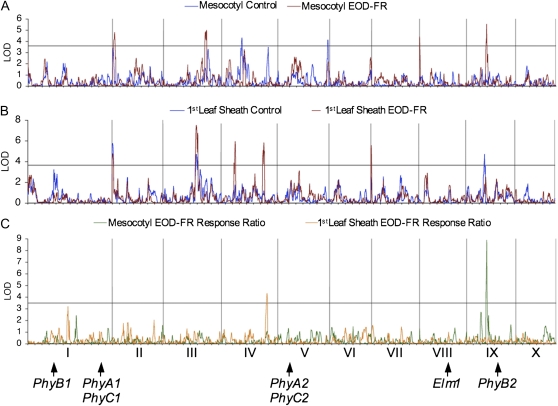
A total of 16 QTLs were identified among all traits analyzed. Plots of the composite interval mapping are presented in Figure 2 and summarized in Table II. Only two independent QTLs were identified for mesocotyl and first leaf sheath EOD-FR response ratios. Interestingly, these two QTLs were also detected as EOD-FR elongation QTLs for the corresponding seedling trait (Fig. 2). The mesocotyl QTL specific to both EOD-FR elongation and EOD-FR response ratio is located on chromosome 9 (bin 9.03), while the first leaf sheath QTL is located on chromosome 4 (bin 4.09). For both QTLs, the Mo17 allele is responsible for the enhanced response to EOD-FR. These results are consistent with the diversity inbred panel survey (Table I), which also identified Mo17 as having a greater responsiveness to EOD-FR than B73. Furthermore, when all QTLs were considered together, a majority of the alleles conferring enhanced EOD-FR responsiveness were from Mo17 (11 out of 16; Table II).
Figure 2.
QTL analysis of EOD-FR-mediated seedling elongation in the IBM mapping population. Logarithm of the odds (LOD) plots for seedling lengths and EOD-FR response ratios are shown. A, Mesocotyl length for W control and EOD-FR treatments. B, First leaf sheath length for W control and EOD-FR treatments. C, EOD-FR response ratios of mesocotyl and first leaf sheath. The horizontal line across each plot indicates the 95% confidence threshold (mesocotyl, 3.58; first leaf sheath, 3.63; EOD-FR response ratios, 3.48). Vertical lines across each plot delimit each maize chromosome and are numbered along the chromosomal position in C. Arrows indicate the positions of the phytochrome gene family members and Elm1. Increments on the x axis represent approximately 80 centimorgans.
Table II. Significant QTLs identified by composite interval analysis for each trait.
For each QTL detected, the following are listed: the logarithm of the odds (LOD), the parent donor allele, the IBM version 1 (centimorgan [cM]) interval distance at threshold α = 0.05, the location of the maximum LOD score (peak), the marker closest to the peak location, the chromosomal bin location, the r2 value at the peak, and an ANOVA model summary including the adjusted r2 for each trait, the F test score, and its associated P value. r2 represents the proportion of phenotypic variation explained by a QTL, and adjusted r2 represents the percentage of the phenotypic variation explained by all the significant QTLs detected for each trait.
| Trait | LOD | Donor Allele | Interval | Peak Position | Marker at Peak | Bin | r2 at Peak | Model Summary |
|||
| r2 Adjusted | F | P | |||||||||
| cM | % | % | |||||||||
| Mesocotyl | Control | 4.29 | B73 | 244–253 | 250.8 | umc191 | 4.04 | 8.0 | 13.0 | 19.38 | <0.001 |
| 4.12 | Mo17 | 582–589 | 584.3 | mmp175 | 5.08 | 7.8 | |||||
| EOD-FR | 4.78 | B73 | 12–34 | 28.0 | php20568b | 2.01 | 7.2 | 26.7 | 16.09 | <0.001 | |
| 4.84 | Mo17 | 473–496 | 485.4 | umc1404 | 3.07 | 6.0 | |||||
| 4.39 | Mo17 | 0–5 | 0.0 | npi220a | 8.01 | 6.1 | |||||
| 5.52 | Mo17 | 226–236 | 233.5 | umc1921 | 9.03 | 6.8 | |||||
| EOD-FR response ratio | 8.87 | Mo17 | 222–236 | 233.1 | umc20 | 9.03 | 14.0 | 5.1 | 12.94 | <0.001 | |
| First leaf sheath | Control | 5.74 | B73 | 0–23 | 6.0 | isu53a | 2.01 | 15.9 | 23.9 | 20.99 | <0.001 |
| 4.68 | Mo17 | 369–402 | 381.4 | umc60 | 3.06 | 7.5 | |||||
| 4.69 | Mo17 | 214–222 | 219.8 | mmp2 | 9.03 | 6.4 | |||||
| EOD-FR | 4.70 | B73 | 0–19 | 12.0 | isu144a | 2.01 | 15.2 | 30.2 | 16.94 | <0.001 | |
| 7.47 | Mo17 | 367–402 | 379.4 | umc60 | 3.06 | 13.2 | |||||
| 5.92 | Mo17 | 161–185 | 179.4 | umc1902 | 4.03 | 8.7 | |||||
| 5.80 | Mo17 | 498–523 | 511.6 | umc2135 | 4.09 | 6.9 | |||||
| 5.55 | B73 | 0–7 | 2.7 | csu582 | 7.00 | 6.7 | |||||
| EOD-FR response ratio | 4.31 | Mo17 | 534–552 | 545.3 | umc1999 | 4.09 | 11.0 | 4.9 | 13.30 | <0.001 | |
To examine in more detail the contribution of QTLs in bins 4.09 and 9.03, a series of teosinte-maize near-isogenic lines (NILs) was used to evaluate their EOD-FR responsiveness. These lines carry small introgressed regions of the teosinte genome in the B73 inbred background. A NIL containing a teosinte introgression corresponding to bin 4.09 did not show enhanced EOD-FR responsiveness relative to the B73 parent and was not characterized further (data not shown). Two additional NILs were also characterized. The NIL Z033E0026 carries a teosinte introgression of approximately 100 Mb of chromosome 9 (18.6–118.5 Mb, between markers PZA00860 to PZA01819), which spans the QTL interval within bin 9.03 (between 86 and 96 Mb). This NIL also carries two small introgressions at the end of chromosomes 1 (2 Mb, PHM9807 to PZA00243) and 4 (6 Mb, PHM5599 to PZA00282). The NIL Z31E0512 contains a single teosinte introgression on the short arm of chromosome 9 (12.2 Mb, PHM3925 to PZA00466), and this interval does not overlap with the bin 9.03 QTL interval. Thus, Z31E0512 was selected as a control for the evaluation of Z033E0026. Neither of the introgressions found in the NILs used contain the candidate gene PhyB2, which is found at 130.6 Mb on chromosome 9 (Fig. 2). These two lines and the B73 parent were grown under EOD-FR, and EOD-FR response ratios were measured (Supplemental Fig. S5). Line Z033E0026 displayed significantly higher EOD-FR response ratios than the control line for all three of the measured traits. This was surprising, as the maize QTL at 9.03 regulated mesocotyl and not first leaf sheath elongation. The mesocotyl and coleoptile lengths of Z033E0026 were also longer than B73 in both W control and EOD-FR treatments. These results suggest that the QTL that displays a tissue-specific response in the IBM population may be broader in its effect on seedling development. Line Z033E0026 has been crossed with B73 to dissociate the two nontarget introgressions on chromosomes 1 and 4 and to initiate fine-mapping of the 9.03 EOD-FR-responsive QTL.
PhyB1 and PhyB2 Are Largely Redundant in Mediating EOD-FR Responses
In maize, only three light-signaling mutants have been described: elm1 is a weak allele encoding phytochromobilin synthase (Sawers et al., 2004), phyB1 is a Mutator (Mu) insertion loss-of-function allele, and phyB2 is the naturally occurring deletion allele found in the Northern Flint inbred France 2 (Sheehan et al., 2007). A second loss-of-function allele of phyB2, carrying a Mu insertion (May et al., 2003), was identified during the course of these studies. The elm1, phyB1-Mu, and phyB2-Mu alleles were backcrossed multiple generations into the B73 and W22 inbreds to generate a single and double mutant series. Introgressions into an inbred line facilitate detailed phenotypic comparisons between NILs. The use of more than one background allows the evaluation of trans-acting genetic modifiers on a mutant phenotype (Neuffer et al., 1997). Seedling phenotypes of the phyB series along with its recurrent parent are presented for both B73 and W22 (Supplemental Fig. S6). Ten days after sowing, the third leaf blade had fully emerged in the B73 seedlings. However, in the W22 background, this leaf blade was only visible in the wild type under both light treatments and for the two single phyB mutants under W control treatments. The development of the phyB1 phyB2 double mutant in W22 also was delayed relative to B73.
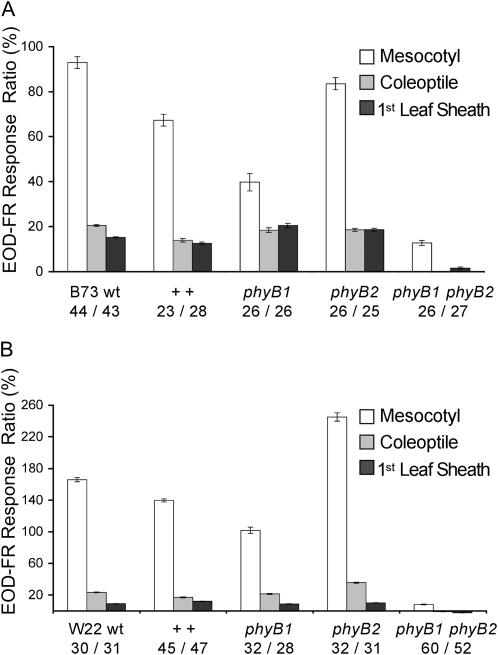
EOD-FR response ratios for mesocotyl, coleoptile, and first leaf sheath are presented for B73 (Fig. 3A) and W22 (Fig. 3B) introgressions. These data show that the responsiveness to EOD-FR is almost completely attenuated in the phyB1 phyB2 double mutant. However, a small response can still be detected in the mesocotyl (Supplemental Fig. S7). Each of the single mutants and wild-type lines was responsive to EOD-FR. However, significant differences in EOD-FR response ratios exist between phyB1 and phyB2 single mutants, especially for the mesocotyl. These results suggest that PhyB1 and PhyB2 are redundant in mediating EOD-FR responses in the seedling, but with a predominant role for PhyB1 over PhyB2. This is consistent with previous studies of phyB1 and phyB2 mutants, which show that PhyB1 has a greater influence on mesocotyl elongation under W and R than PhyB2 (Sheehan et al., 2007). The mesocotyl response to EOD-FR of the phyB1 phyB2 double mutant demonstrates that other photoreceptors also control, to a lesser degree, FR-mediated elongation.
Figure 3.
EOD-FR response ratios of the phyB mutant series. Responses are shown for mutants introgressed into B73 (A) and W22 (B). The number of seedlings measured at 10 d after planting is shown below each genotype as W/EOD-FR treatments. wt, Wild type; ++, PhyB1 PhyB2 nonmutant segregant.
The response to EOD-FR was also examined in the phytochromobilin-deficient elm1 mutant (Supplemental Fig. S7). The elm1 mutant is homologous to the Arabidopsis hy2 mutant, and both condition a pale-green, elongated-seedling phenotype (Kohchi et al., 2001; Sawers et al., 2002). In the B73 introgressions, the mesocotyl of elm1 seedlings was longer than in the phyB1 phyB2 double mutant but nonresponsive to EOD-FR. The length of the coleoptile was not significantly different from the phyB1 phyB2 double mutant under W but significantly longer under EOD-FR. The first leaf sheath of elm1 was longer than in the phyB1 phyB2 double mutant under both light treatments and was also responsive to EOD-FR. In the W22 introgressions, the elm1 mutant mesocotyl was responsive to the EOD-FR, the coleoptile was nonresponsive, while the first leaf sheath was shorter following EOD-FR treatments than when grown under W only. The reduction in the apparent length of the first leaf sheath under EOD-FR may be a consequence of the slightly greater extension of the mesocotyl, resulting in fewer resources committed to sheath tissues. In both inbred backgrounds, reduced but detectable EOD-FR responses may be attributed to the presence of low levels of PHYB1 and PHYB2, as the elm1 allele is not a complete loss-of-function mutation (Sawers et al., 2004).
Tissue-Specific Regulation of EOD-FR Responses by GA
The role of GA in the downstream transduction of FR signaling was investigated by pharmacological treatments using synthetic GA3 and the GA biosynthesis inhibitor paclobutrazol (PBZ). The effectiveness of a 50 μg mL−1 PBZ seed imbibition treatment (Pinhero et al., 1997) was confirmed in wild-type W22 and elm1 mutant seedlings and through comparisons with a mock-treated dwarf1 (d1) mutant (Emerson, 1912). The d1 mutation has not been defined molecularly, but the homozygous mutant is impaired in the conversion of GA20 to GA1, GA20 to GA5, and GA5 to GA3 (Spray et al., 1996). The d1 mutant can be restored to a stature similar to the wild type by exogenous application of GA3. PBZ-treated seedlings had thicker and greener tissues, characteristic features of dwarf mutants (Neuffer et al., 1997). Both wild-type W22 and elm1 PBZ-treated seedlings were phenotypically similar to d1 (Supplemental Fig. S8). To establish whether exogenous GA3 would enhance seedling EOD-FR responses, seedlings were treated with 50 μm GA3 (Ogawa et al., 1999) by daily soil drench. Morphological responses to PBZ and GA3 treatments are presented in Supplemental Figure S8. GA3 caused exaggerated elongation of the mesocotyl, coleoptile, and first leaf sheath and a delay in leaf blade emergence. Leaf blades were paler, narrower, and hyponastic, responses similar to the ones caused by EOD-FR.
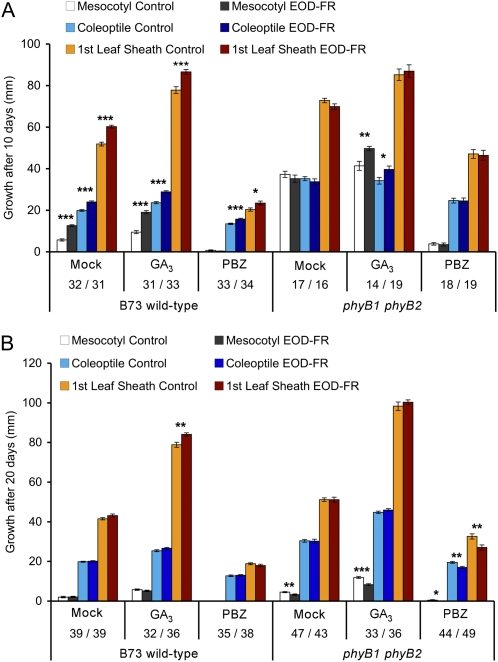
To further investigate the role of GA in EOD-FR responses, we examined the effects of EOD-FR when combined with GA3 and PBZ treatments in the B73 wild type and the phyB1 phyB2 double mutant (Fig. 4A). Wild-type mesocotyl, coleoptile, and first leaf sheath tissues were all significantly longer in GA3-treated seedlings relative to a mock treatment, and all responded to EOD-FR despite significant elongation responses induced by GA3. Thus, at this concentration of GA3, EOD-FR-mediated responses are not saturated by GA3 treatments. In the wild type, PBZ treatment repressed mesocotyl elongation under both light treatments, indicating that GA is required for the elongation of mesocotyl tissues. The coleoptile and first leaf sheath responded to EOD-FR following PBZ treatment. As seedling tissues were capable of responding to EOD-FR following PBZ treatments, other growth-stimulating factors (such as auxins) likely contribute to EOD-FR responses. These results also suggest that GA plays a predominant role in mediating FR-induced mesocotyl elongation relative to coleoptile and sheath tissues.
Figure 4.
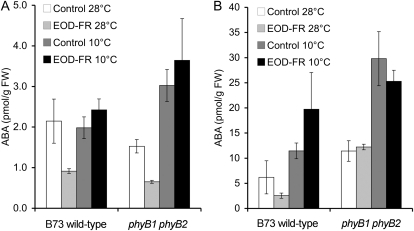
Effects of GA and chilling temperatures on EOD-FR-mediated elongation response. Mock-, GA3-, and PBZ-treated B73 wild-type and phyB1 phyB2 double mutant seedlings were used. A, Seedlings were grown at a constant temperature of 28°C, and measurements were taken 10 d after planting. B, Seedlings were subjected to a nightly chilling temperature of 10°C and grown at 28°C during the photoperiod. Measurements were taken 20 d after planting. The values are representative means ± se. The number of seedlings measured for W/EOD-FR are shown below each treatment. Asterisks indicate Student’s t test significant differences between the two light treatments for each line (* P < 0.05, ** P < 0.01, *** P < 0.001).
The phyB1 phyB2 double mutant was also evaluated for its response to GA3 and PBZ (Fig. 4A). While double mutants failed to respond to EOD-FR, they responded to EOD-FR following GA3 applications. This response was observed for mesocotyl and coleoptile tissues but not first leaf sheath tissue. This suggests that in addition to PHYB-mediated EOD-FR responses, PHYA and/or PHYC may contribute to the EOD-FR response when GA3 is not limiting. PBZ treatments inhibited responsiveness of the double mutants to EOD-FR, but all tissues were significantly taller than wild-type PBZ-treated seedlings. These results suggest that the double mutants are either more responsive to endogenous GA or produce more active GA than the wild type. Furthermore, GA regulation is tissue specific, with the greatest effect in mesocotyl tissues.
Chilling Temperatures Applied during Dark Breaks Modulate EOD-FR Responses
To investigate the effect of temperature on EOD-FR response, growth temperature was alternated between 28°C during the photoperiod and a chilling temperature of 10°C during dark breaks. This chill treatment was made in combination with EOD-FR, GA3, and PBZ treatments. Growth chamber temperature was reduced only during dark breaks to simulate the broad daily fluctuations that can take place in early field season under temperate climates. To ensure robust germination, maize seeds were imbibed in soil at a constant temperature of 28°C for 2 d prior to the first chill treatment. Coleoptiles had not emerged from the soil at this time. The duration of the experiment under chill treatments was extended to 20 d to allow sufficient development of the seedlings prior to measurements.
The chill treatment attenuated the EOD-FR response in wild-type B73 for all three traits measured (mock treatment; Fig. 4B). Coleoptiles and first leaf sheaths in the phyB1 phyB2 double mutant were nonresponsive to EOD-FR. However, mesocotyl tissues were significantly shorter following EOD-FR treatments in the phyB1 phyB2 double mutants. In addition, seedlings were more similar in appearance to the wild type (Supplemental Fig. S9) than when grown at constant 28°C. Surprisingly, both GA and PBZ treatments enhanced the effect of EOD-FR in phyB1 phyB2 seedlings, resulting in less elongation following EOD-FR treatments. These results suggest a complex interplay between temperature and GA-mediated elongation. They also suggest a role for PhyA or PhyC in the regulation of EOD-FR response under low temperatures in the absence of PhyB.
FR-Mediated Regulation of ABA Levels in the Mesocotyl
Transient exposure to cold is associated with increased levels of ABA (Penfield, 2008), and in Arabidopsis, PhyB was identified as the primary light receptor for the activation of cold-dependent light signaling (Kim et al., 2002). To evaluate the role of ABA in modulating maize seedling development in response to transient chilling temperatures and EOD-FR, mesocotyl and leaf blade tissues of the B73 wild type and phyB1 phyB2 double mutants were harvested at subjective dawn and ABA content was measured. All tissues were harvested within 30 min from the beginning of the photoperiod. ABA content was assayed by indirect ELISA (see “Materials and Methods”). At constant 28°C, EOD-FR reduced ABA levels approximately 50% (P = 0.09) in wild-type B73 seedlings (Fig. 5A). When a chill treatment was applied during dark breaks, ABA levels in the wild type remained at similar levels to seedlings grown in W at 28°C (Fig. 5A). The EOD-FR treatment of phyB1 phyB2 double mutants caused a 50% reduction in mesocotyl levels of ABA (P = 0.02) at 28°C but had little effect with chill treatments, suggesting a role for PhyA or PhyC in regulating ABA levels in the mesocotyl at 28°C in addition to contributing to elongation under chill treatments, as discussed above. In contrast to mesocotyl tissues, ABA levels in the leaf blades (Fig. 5B) of both the wild type and phyB1 phyB2 double mutants appeared to increase in response to chilling but failed to respond to EOD-FR. In summary, these results reveal a temperature-dependent and PHYB-independent pathway that regulates ABA levels in response to EOD-FR treatments.
Figure 5.
ABA concentration in mesocotyl (A) and leaf blade (B) of the B73 wild type and phyB1 phyB2 double mutants grown under two different temperature regimes. All seedling tissues were harvested at the beginning of the photoperiod (dawn) at 10 d (28°C constant) or 15 d (10°C dark chill) after planting. Each measurement consists of the mean ± se derived from three biological replicates (each from a pool of eight seedlings). FW, Fresh weight.
DISCUSSION
Early detection of FR reflected by surrounding vegetation is an important factor influencing plant development (Ballare et al., 1990; Rajcan et al., 2004). To examine this process in maize, a crop grown at high planting density, we developed an EOD-FR assay that mimics several developmental responses caused by low R:FR as described previously (Fankhauser and Casal, 2004). The light treatment offers the benefit of using a uniform controlled environment to simultaneously conduct screens on large numbers of seedlings and minimizes environmental differences between control and treated plants, as only a 15-min pulse of daily FR differentiates the treatments. Previous studies have shown that coleoptile and mesocotyl tissues are responsive to EOD-FR through a LFR mode of PHY action (Gorton and Briggs, 1980). Here, we show that elongation responses, triggered by the EOD-FR treatment, are completely reversible through a PHYB-mediated LFR and validated on two different inbred lines. The two maize paralogs PHYB1 and PHYB2 are largely redundant in mediating the elongation responses of all three seedling tissues measured, likely as a result of similar structural characteristics and expression profiles (Sheehan et al., 2004).
Natural variation has been used to define components of the light signal transduction pathway in Arabidopsis and demonstrated the role of variation at phytochromes and cryptochromes in mediating light response (Maloof et al., 2001; Wolyn et al., 2004). Surveys of Arabidopsis accessions exposed to low R:FR revealed wide variations in hypocotyl elongation and flowering time, but no correlations were observed between the two traits (Botto and Smith, 2002). To explore the variation in responses to EOD-FR in maize, we evaluated a genetically diverse panel of inbred lines largely composed of the founders of the NAM population (McMullen et al., 2009). A wide range of responses to EOD-FR was observed for all three tissues measured, suggesting that genetic variation is present in the panel. For mesocotyl, an association between EOD-FR responsiveness and population structure was established, as TS lines were the least responsive to EOD-FR. A previous study demonstrated that TS seedlings were more photomorphogenic and displayed less mesocotyl elongation upon light exposure (Markelz et al., 2003). Thus, it appears that mesocotyl tissues are highly responsive to R and W in TS, but EOD-FR responses have been partially attenuated. Robust EOD-FR responses of mesocotyl tissues were observed for teosinte and a commercial hybrid, suggesting that EOD-FR responses may have fitness benefits under both natural and artificial selection.
To gain a better understanding of the genetic architecture of EOD-FR and the loci underlying its control, the IBM mapping population (Lee et al., 2002) was screened for responses to EOD-FR. This population has been used to map QTLs for cell wall composition, flowering time, and resistance to southern leaf blight (Hazen et al., 2003; Balint-Kurti et al., 2007). In our analysis, none of the 16 QTLs identified mapped to phytochrome gene family or Elm1 loci. This was somewhat unexpected, as photoreceptors have been identified as QTLs for light response and flowering time in Arabidopsis (Wolyn et al., 2004). Furthermore, association analysis in Arabidopsis identified phyB as a target of natural selection in regulating R responses (Filiault et al., 2008) and PhyC as a source of flowering time variation (Balasubramanian et al., 2006). In maize, variation in photoreceptors may modulate FR responses, but allelic variation is likely not present or was undetected in our study. Only two significant QTLs for EOD-FR elongation ratio were identified in the analysis, where the peaks coincided with two QTLs specific to EOD-FR but not to elongation in W. Unfortunately, the low resolution of the mapping population precludes speculation on candidate genes in these intervals. However, the heritability of the mesocotyl and first leaf sheath traits mapped for EOD-FR are both high (0.87 and 0.90, respectively), which should greatly facilitate the fine-mapping effort that is currently under way. The inability to capture the majority of the QTLs that were found for elongation in EOD-FR as response ratio QTLs suggests that many of these QTLs mediate elongation independent of FR signaling. Alternatively, the lower heritability inherent to response ratios between two light treatments results in less power in detection and precludes the mapping of small-effect QTLs.
A teosinte × maize NIL was used to further investigate the QTL located within bin 9.03. This QTL was first identified in the IBM population as specific to mesocotyl elongation, but the teosinte introgression also revealed that the same or linked region may also affect coleoptile and first leaf sheath. This result suggests that the tissue-specific nature of the QTL may also be influenced by genetic variation segregating in mapping populations. At present, it is unclear if QTLs that affect elongation responses to EOD-FR in seedlings also exert control of leaf growth and canopy morphology in later stages of plant growth. A study using a B73 × Mo17 recombinant inbred line (RIL) planted at two different densities identified several QTLs associated with traits affecting light penetration in the canopy (Mickelson et al., 2002). Two of these QTLs, one for tassel branch number and the other for leaf angle, are located in close proximity to the bin 4.09 QTL that controls first leaf sheath EOD-FR elongation and response ratio. A similar study, also using a B73 × Mo17 RIL population, identified QTLs associated with mature plant height in regions overlapping the 4.09 and 9.03 seedling QTLs. As in our study, it was the Mo17 parent that contributed the responsive allele (Gonzalo et al., 2010). In a separate study, the density response of several Tx303 × B73 segmental introgression lines was evaluated (Gonzalo et al., 2006) and several density-dependent QTLs were identified, one of which mapped to 4.03, a location where we identified a QTL for first leaf sheath elongation under EOD-FR. Further investigations should help reveal if EOD-FR responses in the seedling can be used as a proxy for high-density responses. If so, the colocation of QTLs across these studies suggests some pleiotropic regulation of the R/FR signaling throughout development.
Characterization of phyB single and double mutants revealed the primary role of PhyB in regulating responses to EOD-FR. However, as was observed for seedling responses to R and W (Sheehan et al., 2007), PhyB1 plays a more prominent role in regulating the response. Furthermore, introgressions into two different inbreds revealed distinct genetic modifiers in B73 and W22. The elm1 mutant, which is a weak allele (Sawers et al., 2004), is still responsive to EOD-FR, likely because of the presence of low levels of PhyB1 and PhyB2. It is also interesting that roles for PhyA or PhyC in EOD-FR response were revealed only in the double mutants. These included an EOD-FR response to chill treatments and the suppression of ABA accumulation following EOD-FR treatments. It is likely that in wild-type plants, these roles for PHYA and PHYC are largely masked by PhyB. However, under conditions where PHYB is inactivated (e.g. FR light treatments) or in accessions with nonfunctional alleles of PhyB (e.g. France 2), PhyA or PhyC may play a more important role in regulating responses to FR signals.
The role of GA in mediating the elongation response to EOD-FR was investigated using GA3 and PBZ. Previous studies demonstrated that GA3 combined with EOD-FR had a synergistic effect on elongation in Phaseolus vulgaris (Downs et al., 1957) and on the induction of flowering in sorghum (Williams and Morgan, 1979). The accumulation profile of bioactive GAs is disrupted in the sorghum phyB mutant, and biosynthesis inhibitors can reduce shoot elongation in both the wild type and the phyB mutant (Lee et al., 1998). The responses observed in this report were consistent with the possibility that there are additive signaling pathways for EOD-FR response, one with GA involvement and the other independent of GA. Additional hormones such as ABA, auxin, and ethylene are also likely to be involved in these responses (Vandenbussche et al., 2005). GA-stimulated elongation was greater in the mesocotyl than in either the coleoptile or first leaf sheath, as elongation responses were completely suppressed in PBZ-treated wild-type mesocotyl. It is possible that the endogenous GA profiles are also altered in the phyB1 phyB2 double mutant. The tissue-specific control of EOD-FR response by different hormones is likely to complicate the analysis of mutant phenotypes, particularly when whole seedlings are used for molecular studies. Our results suggest that the responses in mesocotyl may be distinct from leaf blade or sheath tissues and warrant a fine-grain (e.g. tissue-specific) analysis of the molecular signaling networks.
The effect of a chilling temperature during dark breaks on EOD-FR-induced responses was also investigated. A previous study identified European Flint and Highland Tropical lines as having a greater chilling tolerance than Corn Belt Dent material (Leipner and Stamp, 2009), and chilling-tolerant maize genotypes accumulate higher amounts of ABA during cold weather periods (Janowiak et al., 2003). Chilling temperatures are also associated with prolonged cell cycle progression and reduced cell production in maize leaves (Rymen et al., 2007). Thus, there is evidence for both genetic variation and a molecular mechanism for cold tolerance in maize. In Arabidopsis, there is also good evidence supporting a role for phytochromes in regulating responses to cold stress that involve ABA signaling (Franklin and Whitelam, 2007a). Previous studies in maize and Arabidopsis have shown an antagonism of ABA and GA signaling in the regulation of seed germination (White and Rivin, 2000; Seo et al., 2006; Oh et al., 2007; Sawada et al., 2008). In Arabidopsis, phytochromes can modulate endogenous levels of both GA and ABA (Seo et al., 2009), and we have shown here that ABA levels in mesocotyl tissues are negatively regulated by EOD-FR. R promotes Arabidopsis germination through the activation of genes that encode GA biosynthetic enzymes and the repression of genes involved in GA catabolism. R also induces the expression of genes required for the repression of ABA biosynthesis, whereas FR promotes ABA accumulation (Seo et al., 2006). Here, our data suggest a similar antagonism between ABA and GA signaling, but the control of ABA and GA levels is likely mediated through an alternative mode of phytochrome control. That is, R is likely to repress GA accumulation in the mesocotyl and result in increased levels of ABA. In the absence of PHYB, seedlings displayed an increased sensitivity to chilling, resulting in less elongation following FR treatments. This response is likely mediated by PHYA acting to increase ABA levels. Further studies, however, will be necessary to characterize these interactions in detail that are likely to be mediated in part through transcriptional changes (Seo et al., 2009).
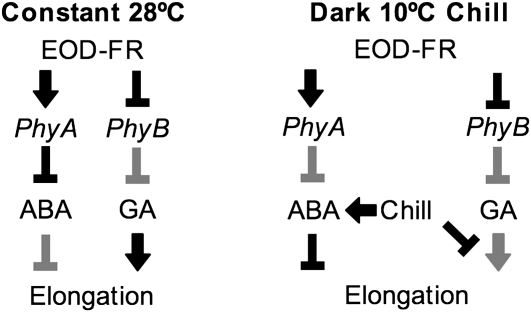
A model integrating these data is presented in Figure 6. In this model, both ABA and GA contribute to mesocotyl elongation, but the contribution of each is modulated by temperature. Under low temperatures, ABA signaling predominates, and under constant temperatures, GA signaling through PHYB predominates. This fine-tuning of mesocotyl elongation by temperature through hormone regulation is likely an important component of seedling emergence in temperate environments.
Figure 6.
Interaction between light, temperature, and hormonal pathways in the control of maize mesocotyl elongation. At constant 28°C (left), FR photoconverts PhyB-Pfr to PhyB-Pr, resulting in a derepression of elongation mediated by GA. In addition, ABA levels are reduced following EOD-FR exposure during night breaks in a PHYB-independent but FR-dependent manner, which strongly suggests a PHYA-mediated response. When a chilling treatment is applied during night breaks (right), ABA levels remain high and result in the repression of mesocotyl elongation. It is unclear if chilling reduces the GA response directly or through an independent pathway (e.g. PHYA or ABA). As mesocotyl tissues are longer in the phyB1 phyB2 double mutants than in the wild type following chilling treatments, some GA signaling likely persists following chill treatments. Gray arrows indicate a minor pathway and black arrows indicate the predominant pathway.
MATERIALS AND METHODS
Plant Materials
Inbred maize (Zea mays subspecies mays) lines used in these experiments include B73, B97, CML52, CML69, CML103, CML288, CML247, CML277, CML322, CML333, HP301, Il14H, Ki3, Ki11, Ky21, M162W, M37W, Mo17, Mo18W, MS71, NC350, NC358, Oh43, Oh7B, P39, Tx303, Tzi8, W22, and the hybrid 34P88 (Pioneer Hi-Bred). The phyB1 (phyB1-563::Mu) allele carries a Mu insertion and was identified from the Pioneer Trait Utility System for Corn collection (Bensen et al., 1995; Sheehan et al., 2007). The phyB2 (phyB2-12058::Mu) allele also carries a Mu insertion and was identified from the Cold Spring Harbor Maize Targeted Mutagenesis (MTM) collection (May et al., 2003). The phyB1 and phyB2 loss-of-function alleles were introgressed into B73 (backcrossed four times for the EOD-FR phyB mutant series analysis and three times for the remaining experiments, except for the GA3 and PBZ experiment at constant temperature, where, due to limited availability, the seeds used for the analysis were backcrossed only two times) and into W22 (backcrossed four times) inbred backgrounds. The elm1 mutant was initially identified in the W22 background (Sawers et al., 2002) and was also introgressed into the B73 background by backcrossing five times. The d1 mutant was introgressed into the W22 background by backcrossing five times. A subset of 272 of the 302 IBM lines (Lee et al., 2002) was used in this analysis and corresponds to the lines capable of flowering in upstate New York. Teosinte (Zea mays subspecies parviglumis)-maize NILs were derived from crosses between teosinte and B73, followed by backcrossing to B73 four times. The BC4 plants were self-pollinated twice (BC4S2) and genotyped using 768 single-nucleotide polymorphism markers (www.panzea.org).
PCR-Based Genotyping
The phyB1 and phyB2 alleles refer to phyB1-563::Mu and phyB2-12058::Mu loss-of-function alleles, respectively. All introgressions of the phyB1-563::Mu and phyB2-12058::Mu alleles and their corresponding wild-type alleles were confirmed using PCR-based genotyping. A 950-bp fragment specific to the phyB1-563::Mu allele was amplified using the Mu-specific primer MuMTM (5′-GCCTCCATTTCGTCGAATC-3′) located in the transposon inverted terminal repeat and the PhyB1-specific primer 563Mu-R1 (5′-CAATTCTAGCTCCCGAAGCTGAAC-3′). A 979-bp PhyB1 wild-type allele fragment was amplified using the PhyB1-specific primer 563Mu-NegF1 (5′-GAACCGCGTGCGAATGATTGCCGAT-3′) and 563Mu-R1. Both phyB1-563::Mu and PhyB1 alleles were amplified using these PCR conditions: 95°C for 3 min, followed by 35 cycles of 95°C for 30 s, 57°C for 30 s, and 72°C for 3 min. The presence of the phyB2-12058::Mu allele was assayed using MuMTM and the PhyB2-specific primer 12058-R3 (5′-AGTAGCTTTTCGACTATATCATCAC-3′). Amplification conditions for the 1,450-bp fragment were 95°C for 3 min, followed by 35 cycles of 95°C for 30 s, 57°C for 30 s, and 72°C for 3 min. The presence of the PhyB2 wild-type allele was assayed using the PhyB2-specific primer 12058Mu-F1 (5′-CCGTTCCCTCGCTCGACTCCGTG-3′) and the PhyB (non-homolog-specific) primer 12058Mu-NegR2 (5′-CATGCTCCACGACTGTGTCGC-3′). Amplification conditions for the 357-bp fragment were 95°C for 3 min, followed by seven cycles of 95°C for 30 s, 67°C for 30 s (decreasing by 1°C at each subsequent cycle), and 72°C for 1.5 min, followed by 28 cycles of 95°C for 30 s, 61°C for 30 s, and 72°C for 1.5 min. Introgression of the elm1 allele were confirmed as described previously (Sawers et al., 2004). Genomic DNA was isolated from leaf tissue (Ahern et al., 2009), and DNA fragments were amplified using GoTaq DNA polymerase according to the manufacturer’s recommendations (Promega).
Growth Conditions and Light Treatments
All seeds were uniformly sown at a depth of 2 cm in germination trays with internal plastic cell dividers (6 cm × 6 cm) filled to the top with a soil mixture composed of 35% peat moss, 10% vermiculite, 35% baked clay, 10% sand, and 10% topsoil. The same planting density of four kernels per cell divider was used for all experiments. All kernel pedicels were oriented toward the bottom of the tray to improve emergence uniformity. All experiments were conducted using a complete random block design in a Conviron TC30 growth chamber. For each experiment, seeds were planted at the same time, and the randomization pattern was identical between the upper (treatment) and lower (control) sections of the growth chamber to minimize possible positional effects inside the chamber when comparing light treatments. Phenotypic data were collected using an electronic caliper (Fowler) with direct data entry to a computer. Seedling trays awaiting measurements were kept at 4°C to halt growth. Four replicates were used for density experiments composed of 488 seedlings for W and 481 seedlings for EOD-FR (B73) or six replicates and 324 seedlings (hybrid). Mesocotyl length was scored as the aboveground portion of the tissue, except in the IBM screen, where total mesocotyl length was scored. Coleoptile and sheath length was measured from the point of insertion at the base of the tissue to the tip.
Plants were grown under a 10-h photoperiod of W, 28°C, and 40% relative humidity. Each section was fitted with eight fluorescent bulbs (cool-white F72T8-CW; General Electric) emitting a photosynthetically active radiation photon flux of 133.2 μmol m−2 s−1 in the lower section (W control) and 130.0 μmol m−2 s−1 in the upper section (treatment). The R and FR treatments were performed using LED module banks (2:1 R:FR; Quantum Devices) positioned at the left and right edges of the upper section. Since LED modules and cables were found to absorb a significant amount of W, a similar W photon flux for the control section was obtained with the addition of mock equipment similar to that used in the treatment section. Each light module (15 cm × 15 cm) was positioned approximately 15 cm above soil level. Spectral irradiances of each light treatment were measured using a spectroradiometer (Apogee Instruments), and results are presented in Supplemental Figure S1. The FR-R reversal experiment was performed using two R LED modules (47.4 μmol m−2 s−1) and four FR LED modules (51.1 μmol m−2 s−1). All EOD-FR experiments were conducted using six FR modules (76.7 μmol m−2 s−1), with the exception of the IBM screen, where four FR LED modules were used. The EOD-FR treatment consisted of a 15-min pulse of FR immediately following each W photoperiod (including an overlap of 1 min where both W and FR were on). The FR-R reversal treatment consisted of the aforementioned EOD-FR treatment immediately followed by a 15-min pulse of R. Synchronization of all the light treatments was made by connecting the digital controls of the growth chamber to a remote power supply controlling the LED system. The growth conditions for the chill temperature treatment were similar to those previously stated with the exception of imposing, beginning at the end of day 2 of growth, a temperature of 10°C during the 14-h dark period until seedlings were measured. The temperature was maintained at 28°C for the first 2 d so as not to interfere with germination. The transition between 28°C and 10°C was made over a 1-h period at the beginning and end of each photoperiod.
Pharmacological Treatments and ABA Assay
PBZ (Phytotechnology Laboratories) was dissolved in 100% dimethyl sulfoxide to a concentration of 50 mg mL−1 (170 mm), and seeds were imbibed for 18 h in a solution of 50 μg mL−1 PBZ (Pinhero et al., 1997) prior to planting. Seeds not treated with PBZ were imbibed with a mock solution of 0.1% (v/v) dimethyl sulfoxide. GA3 (Phytotechnology Laboratories) was dissolved in 100% ethanol, and a dilution of 50 μm (0.1% [v/v]; Ogawa et al., 1999) was applied daily by soil drench using 50 mL per cell divider. Non-GA3-treated seedlings received mock soil drench treatment using water supplemented with 0.1% (v/v) ethanol. ABA content was assayed by indirect ELISA. Mesocotyl and leaf blade tissue samples were harvested within 0.5 h from the beginning of the photoperiod (dawn) and immediately flash frozen in liquid nitrogen and then ground with a mortar and pestle. Three biological replicates were harvested, each consisting of pooled seedling tissues from eight seedlings. A powder aliquot was weighed and extracted three times with 80% (v/v) methanol; the extracts were pooled. Chromatographic separation and ABA determination purification were performed according to the procedure of Setter and Parra (2010). Briefly, compounds were separated with reverse-phase flash chromatography on columns packed with C18-silica material, and ABA was quantified by indirect ELISA with monoclonal antibody specific to (+)ABA (Agdia, Inc.).
Statistical Analysis
The EOD-FR screen of the IBM population was repeated four times, each time using four kernels per line and per light treatment. Since germination rate and seed quality were highly variable, a single median value was derived for each RIL from all four replicates to attenuate the effect of outliers. The traits analyzed included the four primary measurements: total mesocotyl length and first leaf sheath length for both control and EOD-FR treatments. Median length of mesocotyl of B73 in W was 17.14 mm and that in EOD-FR was 23.97 mm. Median length of mesocotyl of Mo17 in W was 12.94 mm and that in EOD-FR was 18.35 mm. Median values for first leaf sheath lengths for W control and EOD-FR were 49.76 and 58.72 mm (B73 inbred) and 49.31 and 62.09 mm (Mo17 inbred), respectively. Two additional traits were derived from these initial measurements, representing the elongation ratios of mesocotyl and first leaf sheath caused by the EOD-FR treatment relative to the W control. Defined as the EOD-FR response ratio, it was calculated for each line using the equation (EOD-FR − control)/control and expressed as a percentage. EOD-FR response ratios for the mesocotyl were 39.8% (B73 inbred) and 41.8% (Mo17 inbred), and those for the first leaf sheath were 18.0% (B73 inbred) and 25.9% (Mo17 inbred). H2 was estimated using a one-way ANOVA for each trait measured or derived. For mesocotyl and first leaf sheath elongation, all data were used for estimates of heritability. Heritability of the EOD-FR response ratios was estimated using the median values of each repetition. For estimates of primary trait heritability, effects included in the model were lines, repetitions, and the line × repetition interaction (the interaction was excluded for the EOD-FR response ratio estimates, as a median value was used for each repetition). The mean square value of the model (between-line variability) over the mean square values of both model and error (between-line and within-line variability) was used to estimate the heritability. QTL analysis was conducted using the composite interval-mapping module in the QTL Cartographer software version 2.5. The genotypic data used for the analysis consisted of 1,339 publicly available markers (www.maizegdb.org). Marker distances were based on the IBM version 1 map. Logarithm of the odds threshold significance level was set at α = 0.05 and calculated from 1,000 permutations.
Statistical analyses were performed using JMP software version 7.0 (SAS Institute). With the exception of the IBM screen, analyses were based on averages and their corresponding se values. EOD-FR response ratios were also calculated using the mean value of each light treatment. The se for each ratio was estimated using a bootstrap permutation analysis, where each of the two data sets used to calculate a EOD-FR response ratio were resampled 10,000 times to sizes of n1 and n2, and for each of the 10,000 iterations, a ratio was calculated. The distribution of this bootstrap sample of ratios was used to estimate sd. The se was then calculated by dividing the sd by the square root of the smallest of the two sample sizes, n1 or n2. Significant differences between lengths or ratios were calculated using Student’s t test.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Growth chamber experimental setup and spectral measurements of the different light treatments.
Supplemental Figure S2. Growth responses in a genetically diverse maize inbred panel.
Supplemental Figure S3. Seedling responses to EOD-FR treatments in teosinte and maize hybrid 34P88.
Supplemental Figure S4. Distribution of seedling lengths and EOD-FR response ratios for the IBM mapping population.
Supplemental Figure S5. EOD-FR response ratios of B73 NILs containing a teosinte introgression.
Supplemental Figure S6. Phenotypes of phyB single and double mutants in B73 and W22 inbreds.
Supplemental Figure S7. EOD-FR response in phyB1, phyB2, phyB1 phyB2, and elm1 mutants.
Supplemental Figure S8. Effects of pharmacological treatments on maize seedling development.
Supplemental Figure S9. Pharmacological and chilling temperature treatments of maize seedlings.
Supplementary Material
Acknowledgments
We thank Dr. Marty Sachs for providing the d1 mutant, Dr. Edward Buckler for providing the NAM founder inbred lines, Dr. John Doebley for the teosinte seeds, Mr. Donald Slocum for the customization of the growth chamber, Mr. Matthias Kormaksson for assistance with statistical analysis, Mr. Karl Kremling for technical assistance, and Dr. Ruairidh Sawers for critical reading of the manuscript.
References
- Ahern KR, Deewatthanawong P, Schares J, Muszynski M, Weeks R, Vollbrecht E, Duvick J, Brendel VP, Brutnell TP. (2009) Regional mutagenesis using Dissociation in maize. Methods 49: 248–254 [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Sureshkumar S, Agrawal M, Michael TP, Wessinger C, Maloof JN, Clark R, Warthmann N, Chory J, Weigel D. (2006) The PHYTOCHROME C photoreceptor gene mediates natural variation in flowering and growth responses of Arabidopsis thaliana. Nat Genet 38: 711–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balint-Kurti PJ, Zwonitzer JC, Wisser RJ, Carson ML, Oropeza-Rosas MA, Holland JB, Szalma SJ. (2007) Precise mapping of quantitative trait loci for resistance to southern leaf blight, caused by Cochliobolus heterostrophus race O, and flowering time using advanced intercross maize lines. Genetics 176: 645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballare CL, Scopel AL, Sanchez RA. (1990) Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science 247: 329–332 [DOI] [PubMed] [Google Scholar]
- Bensen RJ, Johal GS, Crane VC, Tossberg JT, Schnable PS, Meeley RB, Briggs SP. (1995) Cloning and characterization of the maize An1 gene. Plant Cell 7: 75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick HA, Hendricks SB, Parker MW, Toole EH, Toole VK. (1952) A reversible photoreaction controlling seed germination. Proc Natl Acad Sci USA 38: 662–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botto JF, Smith H. (2002) Differential genetic variation in adaptive strategies to a common environmental signal in Arabidopsis accessions: phytochrome-mediated shade avoidance. Plant Cell Environ 25: 53–63 [Google Scholar]
- Childs KL, Miller FR, Cordonnier-Pratt MM, Pratt LH, Morgan PW, Mullet JE. (1997) The sorghum photoperiod sensitivity gene, Ma3, encodes a phytochrome B. Plant Physiol 113: 611–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lucas M, Daviere JM, Rodriguez-Falcon M, Pontin M, Iglesias-Pedraz JM, Lorrain S, Fankhauser C, Blazquez MA, Titarenko E, Prat S. (2008) A molecular framework for light and gibberellin control of cell elongation. Nature 451: 480–484 [DOI] [PubMed] [Google Scholar]
- Djakovic-Petrovic T, de Wit M, Voesenek LA, Pierik R. (2007) DELLA protein function in growth responses to canopy signals. Plant J 51: 117–126 [DOI] [PubMed] [Google Scholar]
- Doebley JF, Gaut BS, Smith BD. (2006) The molecular genetics of crop domestication. Cell 127: 1309–1321 [DOI] [PubMed] [Google Scholar]
- Downs RJ, Hendricks SB, Borthwick HA. (1957) Photoreversible control of elongation of pinto beans and other plants under normal conditions of growth. Bot Gaz 118: 199–208 [Google Scholar]
- Dubois PG, Brutnell TP. (2009) Light signal transduction networks in maize. Hake S, Bennetzen JL, , Handbook of Maize: Its Biology, Vol 1 Springer, New York, pp 205–228 [Google Scholar]
- Duek PD, Fankhauser C. (2005) bHLH class transcription factors take centre stage in phytochrome signalling. Trends Plant Sci 10: 51–54 [DOI] [PubMed] [Google Scholar]
- Emerson RW. (1912) The inheritance of certain “abnormalities” in maize. Am Breeder’s Assoc Annu Rep 8: 385–399 [Google Scholar]
- Fankhauser C, Casal JJ. (2004) Phenotypic characterization of a photomorphogenic mutant. Plant J 39: 747–760 [DOI] [PubMed] [Google Scholar]
- Feng S, Martinez C, Gusmaroli G, Wang Y, Zhou J, Wang F, Chen L, Yu L, Iglesias-Pedraz JM, Kircher S, et al. (2008) Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 451: 475–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiault DL, Wessinger CA, Dinnery JR, Lutes J, Borevitz JO, Weigel D, Chory J, Maloof JN. (2008) Amino acid polymorphisms in Arabidopsis phytochrome B cause differential responses to light. Proc Natl Acad Sci USA 105: 3157–3162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KA. (2009) Light and temperature signal crosstalk in plant development. Curr Opin Plant Biol 12: 63–68 [DOI] [PubMed] [Google Scholar]
- Franklin KA, Whitelam GC. (2007a) Light-quality regulation of freezing tolerance in Arabidopsis thaliana. Nat Genet 39: 1410–1413 [DOI] [PubMed] [Google Scholar]
- Franklin KA, Whitelam GC. (2007b) Red:far-red ratio perception and shade avoidance. Whitelam GC, Halliday KJ, , Light and Plant Development. Blackwell Publishing, Oxford, UK, pp, 211–234 [Google Scholar]
- Gonzalo M, Holland JB, Vyn TJ, McIntyre LM. (2010) Direct mapping of density response in a population of B73 × Mo17 recombinant inbred lines of maize (Zea mays L.). Heredity 104: 583–599 [DOI] [PubMed] [Google Scholar]
- Gonzalo M, Vyn TJ, Holland JB, McIntyre LM. (2006) Mapping density response in maize: a direct approach for testing genotype and treatment interactions. Genetics 173: 331–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorton HL, Briggs WR. (1980) Phytochrome responses to end-of-day irradiations in light-grown corn grown in the presence and absence of Sandoz 9789. Plant Physiol 66: 1024–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday KJ, Salter MG, Thingnaes E, Whitelam GC. (2003) Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT. Plant J 33: 875–885 [DOI] [PubMed] [Google Scholar]
- Hazen SP, Hawley RM, Davis GL, Henrissat B, Walton JD. (2003) Quantitative trait loci and comparative genomics of cereal cell wall composition. Plant Physiol 132: 263–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland JB, Goodman MM. (1995) Combining ability of tropical maize accessions with U.S. germplasm. Crop Sci 35: 767–773 [Google Scholar]
- Janowiak F, Luck E, Dorffling K. (2003) Chilling tolerance of maize seedlings in the field during cold periods in spring is related to chilling-induced increase in abscisic acid level. J Agron Crop Sci 189: 156–161 [Google Scholar]
- Kasperbauer MJ. (1971) Spectral distribution of light in a tobacco canopy and effects of end-of-day light quality on growth and development. Plant Physiol 47: 775–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebrom TH, Brutnell TP, Finlayson SA. (2010) Suppression of sorghum axillary bud outgrowth by shade, phyB and defoliation signaling pathways. Plant Cell Environ 33: 48–58 [DOI] [PubMed] [Google Scholar]
- Kelly JM, Lagarias JC. (1985) Photochemistry of 124-kilodalton Avena phytochrome under constant illumination in vitro. Biochemistry 24: 6003–6010 [Google Scholar]
- Khanna R, Shen Y, Marion CM, Tsuchisaka A, Theologis A, Schafer E, Quail PH. (2007) The basic helix-loop-helix transcription factor PIF5 acts on ethylene biosynthesis and phytochrome signaling by distinct mechanisms. Plant Cell 19: 3915–3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kim YK, Park JY, Kim J. (2002) Light signalling mediated by phytochrome plays an important role in cold-induced gene expression through the C-repeat/dehydration responsive element (C/DRE) in Arabidopsis thaliana. Plant J 29: 693–704 [DOI] [PubMed] [Google Scholar]
- Kohchi T, Mukougawa K, Frankenberg N, Masuda M, Yokota A, Lagarias JC. (2001) The Arabidopsis HY2 gene encodes phytochromobilin synthase, a ferredoxin-dependent biliverdin reductase. Plant Cell 13: 425–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IJ, Foster KR, Morgan PW. (1998) Photoperiod control of gibberellin levels and flowering in sorghum. Plant Physiol 116: 1003–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Sharopova N, Beavis WD, Grant D, Katt M, Blair D, Hallauer A. (2002) Expanding the genetic map of maize with the intermated B73 × Mo17 (IBM) population. Plant Mol Biol 48: 453–461 [DOI] [PubMed] [Google Scholar]
- Leipner J, Stamp P. (2009) Chilling stress in maize seedlings. Hake S, Bennetzen JL, , Handbook of Maize: Its Biology, Vol 1 Springer, New York, pp 291–310 [Google Scholar]
- Liu JB, Mahoney KJ, Sikkema PH, Swanton CJ. (2009) The importance of light quality in crop-weed competition. Weed Sci 49: 217–224 [Google Scholar]
- Liu K, Goodman M, Muse S, Smith JS, Buckler E, Doebley J. (2003) Genetic structure and diversity among maize inbred lines as inferred from DNA microsatellites. Genetics 165: 2117–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorrain S, Allen T, Duek PD, Whitelam GC, Fankhauser C. (2008) Phytochrome-mediated inhibition of shade avoidance involves degradation of growth-promoting bHLH transcription factors. Plant J 53: 312–323 [DOI] [PubMed] [Google Scholar]
- Maloof JN, Borevitz JO, Dabi T, Lutes J, Nehring RB, Redfern JL, Trainer GT, Wilson JM, Asami T, Berry CC, et al. (2001) Natural variation in light sensitivity of Arabidopsis. Nat Genet 29: 441–446 [DOI] [PubMed] [Google Scholar]
- Mancinelli A. (1994) The physiology of phytochrome action. Kendrick RE, Kronenberg GHM, , Photomorphogenesis in Plants, Ed 2 Kluwer, Dordrecht, The Netherlands, pp 211–269 [Google Scholar]
- Markelz NH, Costich DE, Brutnell TP. (2003) Photomorphogenic responses in maize seedling development. Plant Physiol 133: 1578–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May BP, Liu H, Vollbrecht E, Senior L, Rabinowicz PD, Roh D, Pan X, Stein L, Freeling M, Alexander D, et al. (2003) Maize-targeted mutagenesis: a knockout resource for maize. Proc Natl Acad Sci USA 100: 11541–11546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen MD, Kresovich S, Villeda HS, Bradbury P, Li H, Sun Q, Flint-Garcia S, Thornsberry J, Acharya C, Bottoms C, et al. (2009) Genetic properties of the maize nested association mapping population. Science 325: 737–740 [DOI] [PubMed] [Google Scholar]
- Mickelson SM, Stuber CS, Senior L, Kaeppler SM. (2002) Quantitative trait loci controlling leaf and tassel traits in a B73 × Mo17 population of maize. Crop Sci 42: 1902 [Google Scholar]
- Moreno JE, Tao Y, Chory J, Ballare CL. (2009) Ecological modulation of plant defense via phytochrome control of jasmonate sensitivity. Proc Natl Acad Sci USA 106: 4935–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuffer MG, Coe EH, Wessler SR. (1997) Mutants of Maize. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- Ogawa M, Kusano T, Koizumi N, Katsumi M, Sano H. (1999) Gibberellin-responsive genes: high level of transcript accumulation in leaf sheath meristematic tissue from Zea mays L. Plant Mol Biol 40: 645–657 [DOI] [PubMed] [Google Scholar]
- Oh E, Yamaguchi S, Hu J, Yusuke J, Jung B, Paik I, Lee HS, Sun TP, Kamiya Y, Choi G. (2007) PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19: 1192–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penfield S. (2008) Temperature perception and signal transduction in plants. New Phytol 179: 615–628 [DOI] [PubMed] [Google Scholar]
- Pinhero RG, Rao MV, Paliyath G, Murr DP, Fletcher RA. (1997) Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings. Plant Physiol 114: 695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piskurewicz U, Tureckova V, Lacombe E, Lopez-Molina L. (2009) Far-red light inhibits germination through DELLA-dependent stimulation of ABA synthesis and ABI3 activity. EMBO J 28: 2259–2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajcan I, Chandler KJ, Swanton CJ. (2004) Red-far-red ratio of reflected light: a hypothesis of why early season weed control is important in corn. Weed Sci 52: 774–778 [Google Scholar]
- Rajcan I, Swanton CJ. (2001) Understanding maize-weed competition: resource competition, light quality and the whole plant. Field Crops Res 71: 139–150 [Google Scholar]
- Rymen B, Fiorani F, Kartal F, Vandepoele K, Inze D, Beemster GT. (2007) Cold nights impair leaf growth and cell cycle progression in maize through transcriptional changes of cell cycle genes. Plant Physiol 143: 1429–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter MG, Franklin KA, Whitelam GC. (2003) Gating of the rapid shade-avoidance response by the circadian clock in plants. Nature 426: 680–683 [DOI] [PubMed] [Google Scholar]
- Sawada Y, Aoki M, Nakaminami K, Mitsuhashi W, Tatematsu K, Kushiro T, Koshiba T, Kamiya Y, Inoue Y, Nambara E, et al. (2008) Phytochrome- and gibberellin-mediated regulation of abscisic acid metabolism during germination of photoblastic lettuce seeds. Plant Physiol 146: 1386–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers RJ, Linley PJ, Farmer PR, Hanley NP, Costich DE, Terry MJ, Brutnell TP. (2002) elongated mesocotyl1, a phytochrome-deficient mutant of maize. Plant Physiol 130: 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers RJ, Linley PJ, Gutierrez-Marcos JF, Delli-Bovi T, Farmer PR, Kohchi T, Terry MJ, Brutnell TP. (2004) The Elm1 (ZmHy2) gene of maize encodes a phytochromobilin synthase. Plant Physiol 136: 2771–2781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawers RJ, Sheehan MJ, Brutnell TP. (2005) Cereal phytochromes: targets of selection, targets for manipulation? Trends Plant Sci 10: 138–143 [DOI] [PubMed] [Google Scholar]
- Seo M, Hanada A, Kuwahara A, Endo A, Okamoto M, Yamauchi Y, North H, Marion-Poll A, Sun TP, Koshiba T, et al. (2006) Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J 48: 354–366 [DOI] [PubMed] [Google Scholar]
- Seo M, Nambara E, Choi G, Yamaguchi S. (2009) Interaction of light and hormone signals in germinating seeds. Plant Mol Biol 69: 463–472 [DOI] [PubMed] [Google Scholar]
- Setter TL, Parra R. (2010) Relationship of carbohydrate and abscisic acid levels to kernel set in maize under postpollination water deficit. Crop Sci 50: 980–988 [Google Scholar]
- Sheehan MJ, Farmer PR, Brutnell TP. (2004) Structure and expression of maize phytochrome family homeologs. Genetics 167: 1395–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan MJ, Kennedy LM, Costich DE, Brutnell TP. (2007) Subfunctionalization of PhyB1 and PhyB2 in the control of seedling and mature plant traits in maize. Plant J 49: 338–353 [DOI] [PubMed] [Google Scholar]
- Smith H. (1994) Sensing the light environment: the functions of the phytochrome family. Kendrick RE, Kronenberg GMH, , Photomorphogenesis in Plants, Ed 2 Kluwer, Dordrecht, The Netherlands, pp 377–416 [Google Scholar]
- Smith H. (1995) Physiological and ecological function within the phytochrome family. Annu Rev Plant Physiol Plant Mol Biol 46: 289–315 [Google Scholar]
- Smith H, Whitelam GC. (1997) The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant Cell Environ 20: 840–844 [Google Scholar]
- Spray CR, Kobayashi M, Suzuki Y, Phinney BO, Gaskin P, MacMillan J. (1996) The dwarf-1 (dt) mutant of Zea mays blocks three steps in the gibberellin-biosynthetic pathway. Proc Natl Acad Sci USA 93: 10515–10518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano M, Inagaki N, Xie X, Yuzurihara N, Hihara F, Ishizuka T, Yano M, Nishimura M, Miyao A, Hirochika H, et al. (2005) Distinct and cooperative functions of phytochromes A, B, and C in the control of deetiolation and flowering in rice. Plant Cell 17: 3311–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, et al. (2008) Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell 133: 164–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche F, Pierik R, Millenaar FF, Voesenek LA, Van Der Straeten D. (2005) Reaching out of the shade. Curr Opin Plant Biol 8: 462–468 [DOI] [PubMed] [Google Scholar]
- White CN, Rivin CJ. (2000) Gibberellins and seed development in maize. II. Gibberellin synthesis inhibition enhances abscisic acid signaling in cultured embryos. Plant Physiol 122: 1089–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EA, Morgan PW. (1979) Floral initiation in sorghum hastened by gibberellic acid and far-red light. Planta 145: 269–272 [DOI] [PubMed] [Google Scholar]
- Wolyn DJ, Borevitz JO, Loudet O, Schwartz C, Maloof J, Ecker JR, Berry CC, Chory J. (2004) Light-response quantitative trait loci identified with composite interval and eXtreme array mapping in Arabidopsis thaliana. Genetics 167: 907–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.