Abstract
The plant response to abiotic stresses involves both abscisic acid (ABA)-dependent and ABA-independent signaling pathways. Here we describe TaCHP, a CHP-rich (for cysteine, histidine, and proline rich) zinc finger protein family gene extracted from bread wheat (Triticum aestivum), is differentially expressed during abiotic stress between the salinity-sensitive cultivar Jinan 177 and its tolerant somatic hybrid introgression cultivar Shanrong No.3. TaCHP expressed in the roots of seedlings at the three-leaf stage, and the transcript localized within the cells of the root tip cortex and meristem. TaCHP transcript abundance was higher in Shanrong No.3 than in Jinan 177, but was reduced by the imposition of salinity or drought stress, as well as by the exogenous supply of ABA. When JN17, a salinity hypersensitive wheat cultivar, was engineered to overexpress TaCHP, its performance in the face of salinity stress was improved, and the ectopic expression of TaCHP in Arabidopsis (Arabidopsis thaliana) also improved the ability of salt tolerance. The expression level of a number of stress reporter genes (AtCBF3, AtDREB2A, AtABI2, and AtABI1) was raised in the transgenic lines in the presence of salinity stress, while that of AtMYB15, AtABA2, and AtAAO3 was reduced in its absence. The presence in the upstream region of the TaCHP open reading frame of the cis-elements ABRE, MYBRS, and MYCRS suggests that it is a component of the ABA-dependent and -independent signaling pathways involved in the plant response to abiotic stress. We suggest that TaCHP enhances stress tolerance via the promotion of CBF3 and DREB2A expression.
Abiotic stresses such as salinity, drought, or cold can adversely affect crop quality and/or yield, so improved stress tolerance is an important breeding trait. Bread wheat (Triticum aestivum) is a moderately salinity-tolerant species (Maas and Hoffman, 1977), but some of its related species have a much higher level of tolerance (Munns and Tester, 2008). One of these is tall wheatgrass (Thinopyrum ponticum), which is among the most salinity tolerant of all monocotyledonous species (Yuan et al., 1999). Somatic hybridization has been successfully employed to transfer blocks of wheatgrass chromatin into bread wheat genome (Xia et al., 2003), and this approach has resulted in the release of an introgression cultivar Shanrong No.3 (SR3), which has inherited enhanced salinity tolerance from tall wheatgrass in a genetic background of the salinity-sensitive wheat cultivar Jinan 177 (JN177; Shan et al., 2008).
The elucidation of the mechanism in stress response is an important area of research in the context of improving stress tolerance. Tolerance to salinity is achieved in plants via one or more of an improved tolerance of osmotic stress, a better ability to selectively exclude toxic ions, and the development of better tissue tolerance to elevated ionic concentrations (Munns and Tester, 2008). The genetic analysis of the stress response of Arabidopsis (Arabidopsis thaliana) and other model plants has suggested that all these processes are dependent on concerted action of many genes. Two specific pathways have been identified, one of which relies on the phytohormone abscisic acid (ABA), and the other is ABA independent (Ergen et al., 2009). The latter is mediated mainly through an IP3-Ca2+-DREB cascade (Zhu, 2001), while the former involves AREB and other modules. Both pathways require participation of many regulatory factors (Chinnusamy et al., 2004), and there is a degree of cross talk between the two pathways (Haake et al., 2002; Narusaka et al., 2003). However, the detailed characteristic of the cross talk is still not well dissected.
The zinc finger proteins belong to a prominent family of regulatory proteins. Some wheat zinc finger protein encoding genes have been identified in silico, and several are responsive to both drought (Kam et al., 2008) and salinity stress (Houde et al., 2006). One of these is TaCHP, which is more highly expressed in SR3 than in its parent cultivar JN177, and is down-regulated in the presence of both salinity and drought stress. The TaCHP product is a CHP-rich (for Cys, His, and Pro rich) zinc finger protein with three divergent C1 (DC1) domains and the capacity to bind two zinc ions. DC1 domain-containing proteins have been implicated in plant response to abiotic stress, as for example the tobacco (Nicotiana tabacum) genes NtDC1A and NtDC1B that responded rapidly and strongly to AaGlucan, an elicitor of some stress-related genes (Shinya et al., 2007). DC1 domain, which is able to bind diacylglycerol (DAG) and phorbol esters (an analog of DAG) in a phospholipid and zinc-dependent fashion, presents in the N-terminal region of protein kinase C (PKC), a family of Ser/Thr protein kinases (Ono et al., 1989). DAG activates PKC (Azzi et al., 1992) and acts as a signal transducer in a Ca2+-dependent manner. However, in plants, there is little evidence for the existence of either PKC or any IP3 receptors (Munnik and Testerink, 2008). As a result, the function of DC1 domain-containing proteins in plants remains unclear.
Here, we show that the expression of TaCHP in SR3 is associated with an improvement in salinity tolerance, achieved via antagonizing an ABA signaling pathway. The ectopic expression of TaCHP was also shown to increase the accumulation of DREB gene products. The indications are that TaCHP may represent an important component of the cross talk between the ABA-dependent and the ABA-independent signaling pathways involved in plant stress response.
RESULTS
The Differential Expression of TaCHP in SR3 and JN177
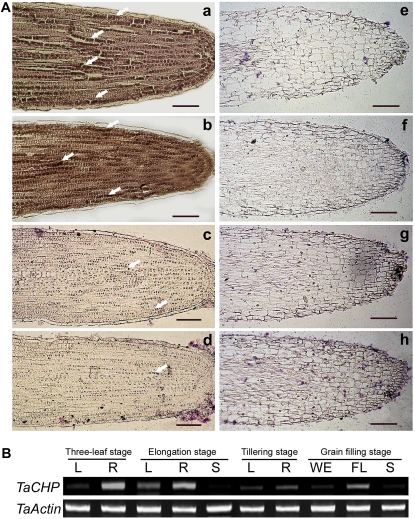
Microarray analysis had indicated that the expression pattern of a CHP family gene, named TaCHP, differed markedly between SR3 and JN177. Consistent with the microarray assay, TaCHP mRNA was present in higher abundance in SR3 roots rather than in JN177 roots, in both stressed and nonstressed conditions, but was hardly detectable in leaves (Fig. 1, A and B). Even during salinity stress, the gene was expressed in SR3 at a higher level than in nonstressed JN177 (Fig. 1, A and B). Both reverse transcription (RT)-PCR and real-time PCR analysis confirmed that this gene was down-regulated in the roots of stressed SR3 and JN177 plants (Fig. 1, A–C). In SR3 roots, TaCHP was down-regulated at the early stages (0.5 h) of imposed salinity stress, and later stayed at a constant level (Fig. 1C). In contrast, in the roots of JN177, TaCHP was expressed at a much lower level than SR3 at every sampling time point, but a decreased transcript level at the 0.5 h is observed as well (Fig. 1C).
Figure 1.
The differential expression of TaCHP in SR3 and JN177. A, Transcript levels in the root compared by RT-PCR. Salt, 12 h exposure to 200 mm NaCl. Control, no imposed stress. B, Real-time PCR analysis of root and leaf expression. TaCHP transcript in root of NaCl-stressed SR3 was used as a standard (100%). Black column indicates control and white column shown as NaCl treatment. C, Real-time PCR analysis of roots at different time points of stress. The vertical coordinate indicates relative transcript levels compared to TaActin. All data represented as means ± sd of three independent experiments.
The Structure and Upstream Sequence of TaCHP and Its Chromosomal Location
RACE PCR was successfully used in obtaining a full-length sequence of TaCHP cDNA from both SR3 and JN177, comprising 955 nucleotides and including a 645-bp open reading frame, a 63-bp 5′ untranslated region (UTR), and a 238-bp 3′ UTR (Supplemental Fig. S1, A and B; Fig. 2A). The deduced TaCHP polypeptide consisted of 214 residues, with a predicted Mr of 23.7 kD and a pI of 6.54. A Blastp search indicated that the peptide is a CHP-rich zinc finger protein-like protein, with three DC1 domains (Fig. 2B). Its first DC1 domain contains five conserved Cys and one conserved His capable of binding zinc (Fig. 2C). A phylogenetic analysis indicated that its most closely related protein is a putative CHP-rich zinc finger protein-like protein from rice (Oryza sativa; GI:14192864; Fig. 2D). The 1.3-kb upstream genomic sequence in SR3 contains several cis-elements, including ABRE, MYC, and MYB, whereas the binding sites in JN177 were relatively diverse. The frequency of CAAT and TATA boxes in the JN177 sequence was lower than in the SR3 one (Supplemental Table S3). A PCR based on SR3 and JN177 genomic DNA template amplified a 790-bp sequence comprising the open reading frame together with a 144-bp intron at its 3′ end (Supplemental Fig. S1, A and C). Analysis of aneuploid stocks cultivar Chinese Spring (CS) showed that this product was not amplified from a template of Dt7DS, a stock that lacks long arm of 7D. Thus, we concluded that TaCHP must map to chromosome arm 7DL (Fig. 2E).
Figure 2.
Structure, phylogeny, and chromosomal location of TaCHP. A, The TaCHP gene. Exons shown as black boxes, and the 5′ UTR, introns, and 3′ UTR as black bars. B, Peptide domain analysis of TaCHP. The DC1 domains are underlined. C, Amino acid sequence alignment of the DC1 domain with other CHP-rich zinc finger proteins. Conserved residues are shaded. D, Phylogeny of TaCHP and related proteins. The tree was derived from the DC1 domain peptide sequence. Branch length numbers are shown. GI15224369, 15224373, 15239894, 15218093, 4836889, 15226210, and 15224871 are from Arabidopsis; GI14192864, 24059846, 125559145, 24059841, 125559137 34394191, 125601034, and 24059843 from rice. E, Chromosomal location of TaCHP. Using the ditelosomic lines of CS, the gene was located to the long arm of chromosome 7D. In the CS ditelosomic lines Dt1AS, Dt1AL, Dt7DS, and Dt7DL, the deleted chromosome arms are 1AL, 1AS, 7DL, and 7DS, respectively.
Expression Pattern of TaCHP
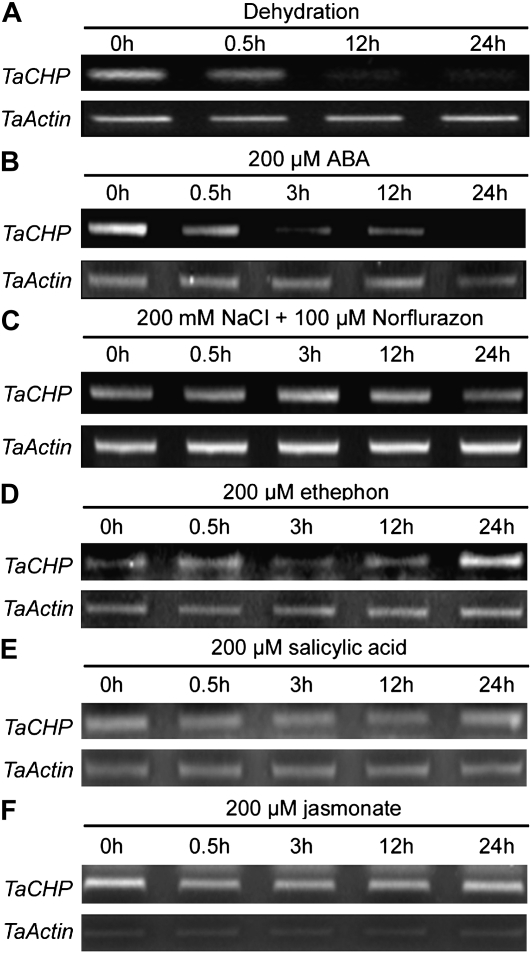
In nonstressed plants, in situ hybridization assay detected the presence of TaCHP mRNA in the cells of the root tip cortex and meristem with more messages present in SR3 than in JN177 (Fig. 3A). There was more signal present in the roots of salinity-stressed SR3 plants than in those of either stressed or nonstressed JN177 plants (Fig. 3A), consistent with the RT-PCR and real-time PCR results discussed above. The RT-PCR analysis showed that TaCHP transcript was present throughout the plant’s life cycle, peaking at the seedling three-leaf stage (Fig. 3B). The reaction to dehydration was a gradual decline in TaCHP abundance over the first 24 h (Fig. 4A). The presence of 200 μm ABA also substantially reduced transcript abundance (Fig. 4B), while the down-regulation of TaCHP expression induced by salinity stress was relieved by the provision of norflurazon, an inhibitor of ABA biosynthesis (Fig. 4C). Ethephon treatment led to an increase in expression after 24 h (Fig. 4D). Neither exogenously provided jasmonic acid (JA) nor salicylic acid (SA) had any noticeable effect on TaCHP transcript level (Fig. 4, E and F).
Figure 3.
The expression analysis of TaCHP. A, In situ localization of TaCHP mRNA in SR3 and JN177 root tip. a to d, Antisense probe. e to h, Sense probe. a and e, Nontreated SR3. b and f, Salinity-stressed (200 mm NaCl for 12 h) SR3. c and g, Nontreated JN177. d and h, Salinity-stressed (200 mm NaCl for 12 h) JN177. Hybridization signals (indicated by arrows) were tracked in the root tip meristem and cortex. Bar: 10 μm. B, Expression of TaCHP at various developmental stages in SR3. R, Root; L, leaf; S, shoot; WE, immature wheat ear; FL, flag leaf.
Figure 4.
TaCHP expression profile (RT-PCR) in roots of SR3 seedlings. A, Plants subjected to dehydration. B, Challenged with 200 μm ABA. C, Challenged with 200 mm NaCl and 100 μm norflurazon. D to F, TaCHP transcript levels (RT-PCR) as affected by exogenously applied ethephon, JA, and SA.
The Stress Response of Wheat and Arabidopsis Lines Expressing TaCHP
Of the 12 independent ubiquitin::TaCHP transgenic wheat lines recovered from a salinity hypersensitive cultivar JN17, two transgenic homozygous T3 selections (TaOE1 and TaOE2) were analyzed for their biochemical and morphological responses to stress. Under nonstressed conditions, both lines accumulated more TaCHP transcript in the root than control plants carrying an empty vector (Fig. 5A). The two OE lines showed larger leaves (Fig. 5B) and 17% to 21% higher relative root growth (Fig. 5C) in the presence of 200 mm NaCl, their leaf malondialdehyde (MDA) content was less under both stressed and nonstressed conditions (Fig. 5D), their leaf peroxidase (POD) activity was 50% higher in the presence of NaCl (Fig. 5E), and their leaf Pro concentration was lower under both stressed and nonstressed conditions (Fig. 5F).
Figure 5.
The overexpression of TaCHP in wheat enhanced its salinity tolerance. A, RT-PCR profiles based on root cDNA showing the expression of TaCHP in the positive homozygous transgenic and control lines. B, Overexpression lines subjected to 200 mm NaCl and absence of NaCl. The white bar indicates 1 cm. C to F, Relative root growth and biochemical response of transgenic and control seedlings treated with 200 mm NaCl. Contents of MDA, POD, and Pro sampled from leaves. VC, Vector control. All data presented as means ± sd. *, Means differ P < 0.05; **, means differ P < 0.01. FW, Fresh weight.
Two homozygous TaCHP-expressing transgenic Arabidopsis lines (BS and A5) along with one empty vector control line (C13) were used similarly to assess the effect of the TaCHP transgene (Fig. 6A). There was no noticeable difference between the phenotype of BS, A5, and C13 either in the presence or absence of 50 mm NaCl (Fig. 6B). However, when the NaCl concentration was raised to 100 mm and 150 mm, BS and A5 were able to grow more freely than C13, and the formers’ roots were longer (Fig. 6, B and C). Similarly, the MDA content in the BS and A5 seedlings was 50% below that in C13 (Fig. 6D), while POD activity was increased by 20% to 30% (Fig. 6E) and Pro content was reduced, particularly in the stressed seedlings of BS (Fig. 6F).
Figure 6.
Stress response of TaCHP-expressing Arabidopsis seedlings. A, RT-PCR showing no TaCHP expression in the control line C13 and its expression in the homozygous transgenic lines A5 and BS in the absence of stress. B, Growth of C13, A5, BS stressed with various concentrations of NaCl (results representative of four independent experiments). C, Quantitative assay of root length in B. d to F, MDA, POD, and Pro contents of transgenic and control seedlings exposed to 100 mm NaCl. Data shown are means ± sd. *, Means differ P < 0.05; **, means differ P < 0.01. Bar: 1 cm. FW, Fresh weight.
Salinity Stress-Induced Gene Expression in Arabidopsis TaCHP Expression Lines
An in vivo analysis of a set of putative genes taking part in the TaCHP signaling pathway was undertaken to study the stress response of the Arabidopsis TaCHP expression lines. A comparison between the transcript abundance in BS and A5 with that in C13 showed that AtHSP101, AtSOS1, AtSOS2, AtSOS3, AtMYB2, AtABF3, and AtP5CS1 shared a similar salt-responsive expression (Fig. 7A), while AtRAB18, AtRD29A, AtCBF3, AtDREB2A, AtABI1, and AtABI2 were all induced by salinity stress (Fig. 7, C–E). The expression of AtCBF1, AtCBF2, AtCBF3, AtDREB2A, and AtDREB2B in BS/A5 and C13 was similar under nonstressed conditions (Fig. 7B). None of AtICE1, AtHOS1, AtHOS2, AtZAT12, AtFRY2, or AtZAT10 was differentially expressed in the absence of salinity stress (Fig. 7B). However, AtMYB15 appeared to be down-regulated in BS and A5 in the absence of salinity stress (Fig. 7B).
Figure 7.
Expression patterns of important marker genes involved in salinity response pathways in Arabidopsis. A, RT-PCR profiles of stress-responsive genes in the roots of Arabidopsis plants expressing TaCHP with (NaCl) or without (CK) salinity stress. B, RT-PCR patterns of stress-responsive genes in nonstress conditions. C to E, Real-time PCR analysis of stress-responsive gene to salinity stress and control (non-NaCl). NaCl, 3 h exposure to 100 mm NaCl for 3 h; CK, no stress imposed.
The Interaction between the Transgenic Expression of TaCHP and the Presence of ABA
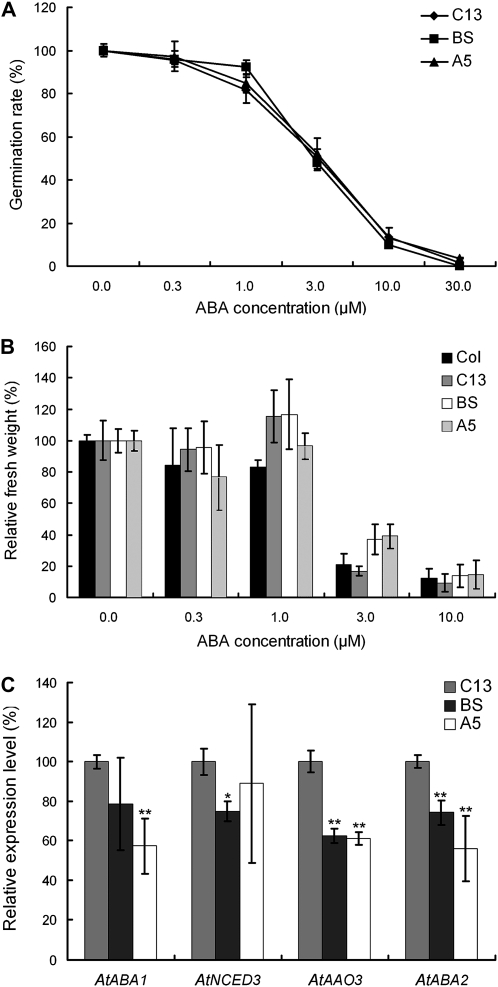
Since TaCHP was down-regulated by ABA treatment, it was of interest to study the effect of this treatment on the Arabidopsis lines expressing TaCHP. Varying the concentration of exogenous ABA had no differential effect on either their germination rate (Fig. 8A), or their growth (Fig. 8B). However, among the genes involved in ABA synthesis, there was some evidence for differential expression in lines A5 and BS under salinity stress—for example, AtAAO3 and AtABA2 were strongly down-regulated, and AtNCED3 and AtABA1 noticeably so (Fig. 8C).
Figure 8.
Phenotypic and molecular analysis of TaCHP transgenic Arabidopsis with respect to ABA. A, Germination rate in the presence of various concentrations of ABA (mean of three replicates). B, Relative fresh weight of seedlings. C, Relative expression of ABA biosynthesis genes in TaCHP overexpression Arabidopsis lines in comparison with the vector control line after 3 h exposure to 100 mm NaCl. Amplicons of RT-PCR were quantified using Image J software (http://rsbweb.nih.gov/ij/, v1.21). Amplicon intensities of genes among were normalized using AtTublin as a standard, then their relative expression levels were calculated as the ratio of the normalized amplicon intensity of A5 and BS to those of C13. Data are means ± sd (n = 4). *, Means differ P < 0.05; **, means differ P < 0.01. A5 and BS, Expressing the TaCHP transgene; C13, negative control (empty vector only).
DISCUSSION
Zinc finger proteins play various roles in plant development and their response to the environment. This large protein family includes many members whose function remains unknown. The major observations here regarding the DC1 domain-containing zinc finger protein gene TaCHP were that its abundance was notably higher in the salinity-resistant introgression line SR3 than in its parent JN177, whether or not the plants were subjected to salinity stress, and that its overexpression as a transgene increased the salinity resistance of both wheat and Arabidopsis, suggesting that Arabidopsis can be used as a heterogonous system for the functional study of this gene.
Gene Family Encoding DC1 Proteins of Arabidopsis in Relation to Stress Resistance
TaCHP transcript level was higher in SR3 than in JN177, but was reduced at the early stage under salinity or drought stress, as well as exogenous ABA (Figs. 1 and 4B). However, it is interesting that when TaCHP was overexpressed in wheat and Arabidopsis, it increased salt tolerance of transgenic plants (Figs. 5B and 6B). We hypothesize that this inconsistency may be the result of different expression pattern of DC1 protein genes. To verify this, we conducted a bioinformatic analysis on this gene family in both wheat and Arabidopsis, but we could not find TaCHP homologs in wheat genome in current public accessible databases. There are 16 DC1 genes in Arabidopsis genome (http://www.arabidopsis.org/). The expression pattern of two DC1 genes in Arabidopsis (At2g42060 and At5g46670) is similar to that of TaCHP, they are down-regulated when plant exposed to abiotic stresses and ABA treatment, whereas other three DC1 genes (At1g61830, At1g55410, and At5g46660) are up-regulated. The different expression pattern of the DC1 genes may indicate that DC1 gene family play complicated roles in plant stress tolerance improvement, and partially explain such inconsistency of TaCHP. These results suggest that the higher expression of TaCHP in SR3 is responsible for its salt tolerance.
TaCHP Enhances Salinity Tolerance by Acting on Antioxidation
Elevated intracellular Na+ concentrations inhibit the activity of many essential enzymes and reduce the cell’s ability to divide or expand. Prolonged exposure to salinity results in membrane disorganization and osmotic imbalance, and the cessation of growth (Tuteja, 2007). These events are typically accompanied by the accumulation of reactive oxygen species (ROS), which interact with a variety of molecules to cause irreversible cell damage, necrosis, and death (Girotti, 2001). Salinity-sensitive barley (Hordeum vulgare) cultivars appear to be particularly susceptible to ROS-induced damage (Chen et al., 2007), and the ability to neutralize ROS has therefore been proposed as an important component of stress tolerance (Xue et al., 2009). It was reported that higher POD activity can alleviate more rapid and severe ROS changes (Laloi et al., 2004). In the presence of salinity stress, the overexpression of TaCHP in wheat promoted the elongation of the root, while simultaneously increasing POD content (Fig. 5, B, C, and E). The parallel fall in MDA content probably reflected this increased POD activity (Fig. 5D). In Arabidopsis, the accumulation of the zinc finger protein ZAT10 promotes the expression of ascorbate POD (APX2; Mittler et al., 2006), while ZAT12 is required for APX1 expression (Rizhsky et al., 2004). APX is one of the most important ROS-scavenging enzymes in plants (Pitzschke et al., 2006). Pro accumulation is a common plant response to a wide range of biotic and abiotic stresses (Verbruggen and Hermans, 2008). The TaCHP expressors tended to accumulate less Pro than the control lines (Fig. 5F). Meanwhile, the expression of TaCHP in transgenic Arabidopsis also increased salt tolerance (Fig. 6, B and C). The indications are therefore that the greater stress tolerance induced by the overexpression of TaCHP is achieved by an enhancement of the antioxidant pathway (Figs. 5, D and E and 6, D and E), rather than by an increased capacity for Pro accumulation (Figs. 5F and 6F).
TaCHP Is Involved in an ABA-Dependent Pathway
A boost in ABA biosynthesis is a frequently observed plant response to abiotic stresses (Ingram and Bartels, 1996), and this has the effect of triggering a number of ABA-dependent genes involved in the stress response. However, the regulation of ABA biosynthesis under stressful conditions is not well researched (Chinnusamy et al., 2004). Here, we have shown that salinity stress reduced the expression of TaCHP (Fig. 1, A and C), as did the exogenous supply of ABA (Fig. 4B), while the ABA biosynthesis inhibitor norflurazon overcame this repression (Fig. 4C). In contrast, the overexpression of TaCHP significantly down-regulated several ABA biosynthesis pathway genes (Fig. 8C). Furthermore, the TaCHP promoter contains the cis-elements ABRE, MYCRS, and MYBRS (Supplemental Table S2). Thus, it appears that TaCHP can act as a negative regulator of ABA biosynthesis, so that its down-regulation in response to salinity stress may partially account for the accumulation of ABA. Some MYB transcription factors are known to be required for ABA biosynthesis (Zhu et al., 2005), and the abundance of AtABA1 transcript was increased when AtMYB15 was overexpressed in nonstressed Arabidopsis seedlings (Ding et al., 2009). Here, the overexpression of TaCHP significantly reduced AtMYB15 transcription (Fig. 7B), indicating that the down-regulation of AAO3 and ABA2 by TaCHP (Fig. 8C) is likely mediated through its action on AtMYB15.
The abundance of AtABI1 and AtABI2 transcript was higher in the Arabidopsis TaCHP expressors than in the control line under stressful conditions (Fig. 7E). ABI1 and ABI2 are both key negative regulators of the ABA signaling pathway (Leung et al., 1994, 1997; Meyer et al., 1994). Thus, in addition to its involvement in the ABA biosynthesis pathway, TaCHP appears also to negatively modulate the ABA signaling pathway, and such modulation is activated by salinity stress. The observation that TaCHP expression did not alter the sensitivity of Arabidopsis to ABA during germination and early seedling growth (Fig. 8, A and B) provides further evidence that the gene plays a specific role in the ABA biosynthesis and signaling pathway during salinity response.
TaCHP Expression Promotes the ABA-Independent Pathway
DREB transcription factors are a well-known component of the ABA-independent response to abiotic stress (Liu et al., 1998; Medina et al., 1999; Haake et al., 2002; Dubouzet et al., 2003; Sakuma et al., 2006). The overexpression in wheat of TaCHP was associated with an increased accumulation of AtDREB2A, AtCBF3, AtRD29A, and AtRAB18 under salinity conditions (Fig. 7, C and D). The overexpression of these genes in Arabidopsis tends to affect plant development (Liu et al., 1998), but there was no such effect noted here, which possibly owes to that their dramatic accumulation in TaCHP overexpression lines is salinity stress dependent (Fig. 6A). A complex regulatory network controls the accumulation of DREB gene products (Gilmour et al., 1998; Zarka et al., 2003; Knight et al., 2004; Fowler et al., 2005). In Arabidopsis, ICE1 and MYB15 bind directly to the CBF promoter region (Zhu et al., 2007), and ICE1 interacts physically with MYB15. ICE1 activates CBF transcription, while MYB15 is negative regulator for the accumulation of CBF transcript (Chinnusamy et al., 2003; Agarwal et al., 2006). Here, AtICE1 was not differentially expressed, although the level of AtMYB15 expression was reduced in the TaCHP-expressing Arabidopsis lines (Fig. 7B). AtCBF3 and AtDREB2A transcript levels were not affected by the presence of the transgene under nonstressed condition (Fig. 7B), so TaCHP appears not to be able to directly activate the transcription of DREB genes. It is therefore possible that the enhanced accumulation of AtCBF3 and AtDREB2A (Fig. 7C) shown by the salinity-stressed transgenic lines may be also mediated by a decrease in the expression of AtMYB15.
TaCHP Is a Key Link between the ABA-Dependent and the ABA-Independent Pathways
Drought and salinity both provoke plants to synthesize ABA (Ingram and Bartels, 1996). In Arabidopsis, both ABA-dependent and ABA-independent response pathways to drought and high salinity have been defined (Tran et al., 2007). The former depend on either MYC/MYB- or bZIP-regulated gene expression (Shinozaki and Yamaguchi-Shinozaki, 1997). Stress-responsive genes, such as AtRD29A, AtRD22, AtCOR15A, AtCOR47, AtP5CS, AtRD19, AtKIN1, and AtADH, are up-regulated via an ABA-dependent pathway (Pitman and Lauchli, 2002; Xiong et al., 2002), and the promoters of these genes commonly contain ABRE, MYCRS, and MYBRS cis-elements (Zhu, 2002), as does the TaCHP promoter. The regulation of DRE elements may be mediated by an ABA-dependent pathway (Haake et al., 2002). The ABA-independent pathways are mediated by DREB genes or other as yet unidentified factors. The ABA-dependent and ABA-independent pathways do converge at several points. Both DRE and AREB cis-elements are present in the AtRD29A promoter, and the two pathways coactivate the expression of AtRD29A (Narusaka et al., 2003).
In Figure 9, we present the model we have elaborated to describe the function of TaCHP. First, salinity and dehydration induce ABA synthesis and the triggering of relevant signaling cascades (Ingram and Bartels, 1996), which serve to inhibit the expression of TaCHP (Fig. 1, A and B). Meanwhile, the expression of ABI1 and ABI2 is also induced, and these have a negative feedback effect on the ABA signaling cascades (Merlot et al., 2001), which alleviates the repression of TaCHP expression as salinity stress continues (Fig. 1C). The overexpression of TaCHP inhibits the expression of both AAO3 and ABA2 in the absence of salinity stress, and promotes the expression of ABI1 and ABI2 under stressed conditions (Figs. 7E and 8C). Thus, TaCHP may also act as a negative modulator or even a cofactor of ABI1/ABAI2 regulation in ABA synthesis and the relevant signaling cascades. Second, the overexpression of TaCHP restricts the expression of MYB15 in the absence of stress (Fig. 7B). MYB15 is known to be a negative transcription factor for CBF3/DREB2A (Chinnusamy et al., 2003; Agarwal et al., 2006). The contribution of TaCHP to salinity tolerance is therefore achieved via the up-regulation of CBF3/DREB2A transcription by inhibiting MYB15 expression (Fig. 7, B and D). It should be noted that some detected marker genes were either not altered by ectopic expression of TaCHP, or displayed a higher steady-state level under salt stress in transgenic Arabidopsis, which might result from the complicated mechanisms of TaCHP in a salinity-dependent manner or even a secondary effect of the stress in the overexpressing plants. TaCHP appears to represent a component of the cross-talk machinery between the two pathways in the stress, the detail of its interaction with other factors remains to be elucidated.
Figure 9.
A model for the regulatory network involving TaCHP in Arabidopsis. Solid lines indicate support from previously published data, dashed ones derived from this study. T-shaped line: repression; arrow: promotion.
MATERIALS AND METHODS
Wheat Material and Treatments
Twenty-five-day-old seedlings of SR3 and JN177 grown in half-strength Hoagland liquid medium under a 16-h light/8-h dark regime at 25°C were subject to following treatments for TaCHP stress-responsive expression analysis: salinity stress by adding 200 mm NaCl to the liquid medium; dehydration by placing seedlings on dry filter paper; a variety of hormonal treatments by supplying one of ABA (200 μm), ethephon (200 μm), JA (200 μm), or SA (200 μm) directly to the medium; inhibition of ABA biosynthesis by the inclusion of 100 μm norflurazon along with 200 mm NaCl. Seven-day-old seedlings of JN17, a salinity-sensitive wheat (Triticum aestivum) cultivar, and a number of its homozygous transgenic derivatives (Ubiquitin::TaCHP) were transferred to the liquid medium and soil with or without NaCl under same condition for phenotypic analysis, here NaCl treatment was achieved by a stepwise twice-daily addition of 25 mm NaCl until a level of 200 mm was reached, at which concentration the seedlings were held until 25 d old. SR3 grown in greenhouse under a 16-h light/8-h dark regime at 25°C were used to trace temporal expression patterns of TaCHP during the whole developmental course. Transformation was achieved using the shoot apical meristem method (Zhao et al., 2006).
Arabidopsis Transgenic Lines and Treatments
A 35S::TaCHP construct in vector pCAMBIA1301 or the empty vector alone were transformed into Arabidopsis (Arabidopsis thaliana) Columbia-0 using the floral-dip method (Clough and Bent, 1998). The surface-sterilized seeds were plated on the surface of one-half Murashige and Skoog agar medium, which was first held at 4°C in the dark for 2 d to break dormancy, and subsequently transferred to a 22°C, 16-h light/8-h dark for 2 or 14 days. Two-day-old plants were transferred to fresh one-half Murashige and Skoog agar medium supplemented with various concentrations of NaCl for 3 weeks to assess the phenotypic response to salinity stress, and to the medium supplemented with 0 to approximately 10 μm ABA for 7 d to determine their hormonal response. Fourteen-day-old plants were transferred into one-half Murashige and Skoog liquid medium containing 100 mm NaCl for 3 h, then RNA was extracted for RT-PCR and real-time PCR assay. Germination assay was conducted using approximately 50 surface-sterilized seeds placed on one-half Murashige and Skoog solid medium containing 0 to approximately 30 μm ABA at 4°C for 2 d followed by at 22°C for 3 d to score germination rate. The emergence of radicle was taken as representing a successfully germinated seed. Germination rates were expressed as a percentage of the number of germinated seeds to that of seeds plated. All measurements were carried out with three repeats.
RT and Real-Time PCR Analysis
RNA was isolated using TRIzol reagent (Invitrogen), and converted to cDNA using the M-MLV reverse transcriptase kit (Invitrogen). RT-PCR was conducted in 20 μL solution containing 100 ng, 1× Easy Taq buffer (Transgene), 1 unit Easy Taq (Transgene), 100 μm deoxyribonucleotide triphosphates, 0.5 μm forward and 0.5 μm reverse primers, and 1 μL diluted (1:10 v/v) cDNA. The PCR regime consisted of a 5 min denaturation at 94°C, followed by 28 cycles of 94°C/30 s, 55°C/30 s, 72°C/30 s, completed by an extension step of 10 min at 72°C. Amplicons were visualized in ethidium bromide-stained 1% agarose gels. Real-time PCR was performed in 20 μL volumes containing 10 μL 2×SYBR Premix Ex Taq mix (Takara), 0.2 μm forward and 0.2 μm reverse primers, 1 μL diluted (1:10 v/v) first-strand cDNA, with a cycling regime comprising an initial denaturation step (95°C/2 min) followed by 45 cycles of 95°C/10 s, 60°C/20 s, 72°C/20 s. A melting curve analysis was performed over the range 80°C to 95°C at 0.5°C intervals. A positive control was provided by a parallel analysis based on the wheat actin gene, and three independent replicates were performed per experiment. The specificity of the real-time PCR was confirmed by agarose gel electrophoresis of the amplicon. Relevant primer sequences are listed in Supplemental Table S1.
Full-Length cDNA Cloning
RNA harvested from nonstressed or salinity-stressed SR3 and JN177 seedlings was subjected to both 5′ and 3′ RACE PCR to isolate the full-length TaCHP cDNA, using the SMART RACE kit (CLONTECH). The gene-specific primer sequences were based on differentially expressed probes in a microarray experiment (C. Li and G. Xia, unpublished data) and are listed in Supplemental Table S1. The RACE reactions employed a touchdown protocol of five cycles of 94°C/30 s, 72°C/2 min, followed by five cycles of 94°C/30 s, 70°C/30 s, 72°C/60 s, and 30 cycles of 94°C/30 s, 65°C/30 s, 72°C/60 s. PCR products were cloned and sequenced using the pMD18-T vector according to the manual (Takara).
Gene Structure Analysis and Phylogenetic Tree Analysis
Gene structure was predicted using SMART software (Schultz et al., 1998), and sequence alignments and phylogenetic analyses were conducted using DNAMAN software (Version 5.2.2, Lynnon Biosoft). Phylogenetic trees were constructed using the neighbor-joining algorithm method.
Chromosomal Assignment
The chromosomal assignment of TaCHP was achieved by an analysis based on cultivar CS cytogenetic stocks. Plant material for DNA extraction included CS nullisomic-tetrasomic lines of all 42 chromosomes (Sears, 1954) and some ditelomeric lines. The ditelomeric lines included Dt1AS, Dt1AL, Dt7DS, and Dt7DL. For Dt7DS the long arm of 7D is missing and the short arm of 7D is present. Genomic DNA extracted from these lines was used as template for 20 μL PCRs containing 1× Easy Taq buffer, 0.2 mm deoxyribonucleotide triphosphates, 0.5 μm TaCHP-specific primers, 0.5 units Easy Taq, and 100 ng DNA, with a cycling regime of 94°C/5 min, followed by 35 cycles of 94°C/60 s, 60°C/60 s, 72°C/30 s.
Promoter Sequence Isolation
The promoter sequence was obtained using the sitefinding-PCR method described by Tan et al. (2005). The used primers were listed in Supplemental Table S2. The cis-elements of the promoter sequence were identified using www.dna.affrc.go.jp/PLACE/index.html (Higo et al., 1999).
In Situ Hybridization
The roots of 25-d-old SR3 and JN177 seedlings were used for in situ hybridization, consisting of the most distal 3 to 5 mm root tip of the first lateral root genesis region. The full-length TaCHP cDNA was labeled with digoxigenin using SP6 or T7 RNA polymerase (Roche Diagnostics GmbH) following Shan et al. (2008), and were purified and hydrolyzed as described by Hejatko et al. (2006). Signal detection was achieved using an alkaline phosphatase-linked immunoassay (DIG nucleic acid detection kit, Roche).
Biochemical Markers of Stress
Wheat seedlings were exposed to salinity stress by the stepwise twice-daily introduction of 25 mm NaCl from 50 mm to 200 mm as described above. Arabidopsis seedlings (21-d-old) were cultured in liquid medium supplemented with 100 mm NaCl for 3 d. A POD assay was conducted on the wheat leaves and the whole Arabidopsis seedlings following Sequeira and Mineo (1966), the content of MDA was measured as described by Heath and Packer (1968), and Pro concentration was determined according to Troll and Lindsley (1955).
Accession number of TaCHP in the National Center for Biotechnology Information is GQ379226.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Isolation of cDNA TaCHP and its corresponding genomic sequence.
Supplemental Table S1. Primers for RACE, real-time PCR, and RT-PCR.
Supplemental Table S2. Primers for SR3 and JN177 TaCHP promoter isolation.
Supplemental Table S3. Binding sites in the upstream genomic sequences of TaCHP of SR3 and JN177.
Supplementary Material
Acknowledgments
We thank Prof. T. Endo (Kyoto University, Japan) for providing the stocks of aneuploid wheat.
References
- Agarwal M, Hao Y, Kapoor A, Dong CH, Fujii H, Zheng X, Zhu JK. (2006) A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J Biol Chem 281: 37636–37645 [DOI] [PubMed] [Google Scholar]
- Azzi A, Boscoboinik D, Hensey C. (1992) The protein kinase C family. Eur J Biochem 208: 547–557 [DOI] [PubMed] [Google Scholar]
- Chen Z, Cuin TA, Zhou M, Twomey A, Naidu BP, Shabala S. (2007) Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. J Exp Bot 58: 4245–4255 [DOI] [PubMed] [Google Scholar]
- Chinnusamy V, Ohta M, Kanrar S, Lee BH, Hong X, Agarwal M, Zhu JK. (2003) ICE1: a regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev 17: 1043–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Schumaker K, Zhu JK. (2004) Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. J Exp Bot 55: 225–236 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Ding Z, Li S, An X, Liu X, Qin H, Wang D. (2009) Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in Arabidopsis thaliana. J Genet Genomics 36: 17–29 [DOI] [PubMed] [Google Scholar]
- Dubouzet JG, Sakuma Y, Ito Y, Kasuga M, Dubouzet EG, Miura S, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. (2003) OsDREB genes in rice, Oryza sativa L., encode transcription activators that function in drought-, high-salt- and cold-responsive gene expression. Plant J 33: 751–763 [DOI] [PubMed] [Google Scholar]
- Ergen NZ, Thimmapuram J, Bohnert HJ, Budak H. (2009) Transcriptome pathways unique to dehydration tolerant relatives of modern wheat. Funct Integr Genomics 9: 377–396 [DOI] [PubMed] [Google Scholar]
- Fowler SG, Cook D, Thomashow MF. (2005) Low temperature induction of Arabidopsis CBF1, 2, and 3 is gated by the circadian clock. Plant Physiol 137: 961–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour SJ, Zarka DG, Stockinger EJ, Salazar MP, Houghton JM, Thomashow MF. (1998) Low temperature regulation of the Arabidopsis CBF family of AP2 transcriptional activators as an early step in cold-induced COR gene expression. Plant J 16: 433–442 [DOI] [PubMed] [Google Scholar]
- Girotti AW. (2001) Photosensitized oxidation of membrane lipids: reaction pathways, cytotoxic effects, and cytoprotective mechanisms. J Photochem Photobiol B 63: 103–113 [DOI] [PubMed] [Google Scholar]
- Haake V, Cook D, Riechmann JL, Pineda O, Thomashow MF, Zhang JZ. (2002) Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis. Plant Physiol 130: 639–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath RL, Packer L. (1968) Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125: 189–198 [DOI] [PubMed] [Google Scholar]
- Hejatko J, Blilou I, Brewer PB, Friml J, Scheres B, Benkova E. (2006) In situ hybridization technique for mRNA detection in whole mount Arabidopsis samples. Nat Protoc 1: 1939–1946 [DOI] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. (1999) Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res 27: 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houde M, Belcaid M, Ouellet F, Danyluk J, Monroy AF, Dryanova A, Gulick P, Bergeron A, Laroche A, Links MG, et al. (2006) Wheat EST resources for functional genomics of abiotic stress. BMC Genomics 7: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram J, Bartels D. (1996) The molecular basis of dehydration tolerance in plants. Annu Rev Plant Physiol Plant Mol Biol 47: 377–403 [DOI] [PubMed] [Google Scholar]
- Kam J, Gresshoff PM, Shorter R, Xue GP. (2008) The Q-type C2H2 zinc finger subfamily of transcription factors in Triticum aestivum is predominantly expressed in roots and enriched with members containing an EAR repressor motif and responsive to drought stress. Plant Mol Biol 67: 305–322 [DOI] [PubMed] [Google Scholar]
- Knight H, Zarka DG, Okamoto H, Thomashow MF, Knight MR. (2004) Abscisic acid induces CBF gene transcription and subsequent induction of cold-regulated genes via the CRT promoter element. Plant Physiol 135: 1710–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laloi C, Apel K, Danon A. (2004) Reactive oxygen signalling: the latest news. Curr Opin Plant Biol 7: 323–328 [DOI] [PubMed] [Google Scholar]
- Leung J, Bouvier-Durand M, Morris PC, Guerrier D, Chefdor F, Giraudat J. (1994) Arabidopsis ABA response gene ABI1: features of a calcium-modulated protein phosphatase. Science 264: 1448–1452 [DOI] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J. (1997) The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9: 759–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Kasuga M, Sakuma Y, Abe H, Miura S, Yamaguchi-Shinozaki K, Shinozaki K. (1998) Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell 10: 1391–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maas EV, Hoffman GJ. (1977) Crop salt tolerance: current assessment. J Irrig Drain Div 103: 115–134 [Google Scholar]
- Medina J, Bargues M, Terol J, Perez-Alonso M, Salinas J. (1999) The Arabidopsis CBF gene family is composed of three genes encoding AP2 domain-containing proteins whose expression is regulated by low temperature but not by abscisic acid or dehydration. Plant Physiol 119: 463–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Gosti F, Guerrier D, Vavasseur A, Giraudat J. (2001) The ABI1 and ABI2 protein phosphatases 2C act in a negative feedback regulatory loop of the abscisic acid signalling pathway. Plant J 25: 295–303 [DOI] [PubMed] [Google Scholar]
- Meyer K, Leube MP, Grill E. (1994) A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science 264: 1452–1455 [DOI] [PubMed] [Google Scholar]
- Mittler R, Kim Y, Song L, Coutu J, Coutu A, Ciftci-Yilmaz S, Lee H, Stevenson B, Zhu JK. (2006) Gain- and loss-of-function mutations in Zat10 enhance the tolerance of plants to abiotic stress. FEBS Lett 580: 6537–6542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnik T, Testerink C. (2008) Plant phospholipid signaling: “in a nutshell”. J Lipid Res (Suppl) 50: S260–S265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R, Tester M. (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59: 651–681 [DOI] [PubMed] [Google Scholar]
- Narusaka Y, Nakashima K, Shinwari ZK, Sakuma Y, Furihata T, Abe H, Narusaka M, Shinozaki K, Yamaguchi-Shinozaki K. (2003) Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J 34: 137–148 [DOI] [PubMed] [Google Scholar]
- Ono Y, Fujii T, Igarashi K, Kuno T, Tanaka C, Kikkawa U, Nishizuka Y. (1989) Phorbol ester binding to protein kinase C requires a cysteine-rich zinc-finger-like sequence. Proc Natl Acad Sci USA 86: 4868–4871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman MG, Lauchli A. (2002) Global impact of salinity and agricultural ecosystems. Lauchli A, Luttge U, , Salinity: Environment-Plants-Molecules. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 3–20 [Google Scholar]
- Pitzschke A, Forzani C, Hirt H. (2006) Reactive oxygen species signaling in plants. Antioxid Redox Signal 8: 1757–1764 [DOI] [PubMed] [Google Scholar]
- Rizhsky L, Davletova S, Liang H, Mittler R. (2004) The zinc finger protein Zat12 is required for cytosolic ascorbate peroxidase 1 expression during oxidative stress in Arabidopsis. J Biol Chem 279: 11736–11743 [DOI] [PubMed] [Google Scholar]
- Sakuma Y, Maruyama K, Osakabe Y, Qin F, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. (2006) Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 18: 1292–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz J, Milpetz F, Bork P, Ponting CP. (1998) SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci USA 95: 5857–5864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears ER. (1954) The aneuploids of common wheat. Mo Agric Exp Stn Res Bull 572: 1–59 [Google Scholar]
- Sequeira L, Mineo L. (1966) Partial purification and kinetics of indoleacetic acid oxidase from tobacco roots. Plant Physiol 41: 1200–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, Li CL, Chen F, Zhao SY, Xia GM. (2008) A Bowman-Birk type protease inhibitor is involved in the tolerance to salt stress in wheat. Plant Cell Environ 31: 1128–1137 [DOI] [PubMed] [Google Scholar]
- Shinozaki K, Yamaguchi-Shinozaki K. (1997) Gene expression and signal transduction in water-stress response. Plant Physiol 115: 327–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinya T, Galis I, Narisawa T, Sasaki M, Fukuda H, Matsuoka H, Saito M, Matsuoka K. (2007) Comprehensive analysis of glucan elicitor-regulated gene expression in tobacco BY-2 cells reveals a novel MYB transcription factor involved in the regulation of phenylpropanoid metabolism. Plant Cell Physiol 48: 1404–1413 [DOI] [PubMed] [Google Scholar]
- Tan G, Gao Y, Shi M, Zhang X, He S, Chen Z, An C. (2005) SiteFinding-PCR: a simple and efficient PCR method for chromosome walking. Nucleic Acids Res 33: e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran LS, Nakashima K, Shinozaki K, Yamaguchi-Shinozaki K. (2007) Plant gene networks in osmotic stress response: from genes to regulatory networks. Methods Enzymol 428: 109–128 [DOI] [PubMed] [Google Scholar]
- Troll W, Lindsley J. (1955) A photometric method for the determination of proline. J Biol Chem 215: 655–660 [PubMed] [Google Scholar]
- Tuteja N. (2007) Mechanisms of high salinity tolerance in plants. Methods Enzymol 428: 419–438 [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C. (2008) Proline accumulation in plants: a review. Amino Acids 35: 753–759 [DOI] [PubMed] [Google Scholar]
- Xia GM, Xiang FN, Zhou AF, Wang H, Chen HM. (2003) Asymmetric somatic hybridization between wheat (Triticum aestivum L.) and Agropyron elongatum (Host) Nevishi. Theor Appl Genet 107: 299–305 [DOI] [PubMed] [Google Scholar]
- Xiong L, Lee H, Ishitani M, Zhu JK. (2002) Regulation of osmotic stress-responsive gene expression by the LOS6/ABA1 locus in Arabidopsis. J Biol Chem 277: 8588–8596 [DOI] [PubMed] [Google Scholar]
- Xue T, Li X, Zhu W, Wu C, Yang G, Zheng C. (2009) Cotton metallothionein GhMT3a, a reactive oxygen species scavenger, increased tolerance against abiotic stress in transgenic tobacco and yeast. J Exp Bot 60: 339–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan WY, Tomita Y, Tanaka H, Yasumuro Y. (1999) Introduction of salt-tolerance chromatin from Agropyron elongatum into the wheat genome. Breeding Research 1: 102 [Google Scholar]
- Zarka DG, Vogel JT, Cook D, Thomashow MF. (2003) Cold induction of Arabidopsis CBF genes involves multiple ICE (inducer of CBF expression) promoter elements and a cold-regulatory circuit that is desensitized by low temperature. Plant Physiol 133: 910–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao TJ, Zhao SY, Chen HM, Zhao QZ, Hu ZM, Hou BK, Xia GM. (2006) Transgenic wheat progeny resistant to powdery mildew generated by Agrobacterium inoculum to the basal portion of wheat seedling. Plant Cell Rep 25: 1199–1204 [DOI] [PubMed] [Google Scholar]
- Zhu J, Dong CH, Zhu JK. (2007) Interplay between cold-responsive gene regulation, metabolism and RNA processing during plant cold acclimation. Curr Opin Plant Biol 10: 290–295 [DOI] [PubMed] [Google Scholar]
- Zhu J, Verslues PE, Zheng X, Lee BH, Zhan X, Manabe Y, Sokolchik I, Zhu Y, Dong CH, Zhu JK, et al. (2005) HOS10 encodes an R2R3-type MYB transcription factor essential for cold acclimation in plants. Proc Natl Acad Sci USA 102: 9966–9971 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhu JK. (2001) Cell signaling under salt, water and cold stresses. Curr Opin Plant Biol 4: 401–406 [DOI] [PubMed] [Google Scholar]
- Zhu JK. (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53: 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.