Abstract
The trichomes of the wild tomato species Solanum habrochaites subsp. glabratum synthesize and store high levels of methylketones, primarily 2-tridecanone and 2-undecanone, that protect the plants against various herbivorous insects. Previously, we identified cDNAs encoding two proteins necessary for methylketone biosynthesis, designated methylketone synthase 1 (ShMKS1) and ShMKS2. Here, we report the isolation of genomic sequences encoding ShMKS1 and ShMKS2 as well as the homologous genes from the cultivated tomato, Solanum lycopersicum. We show that a full-length transcript of ShMKS2 encodes a protein that is localized in the plastids. By expressing ShMKS1 and ShMKS2 in Escherichia coli and analyzing the products formed, as well as by performing in vitro assays with both ShMKS1and ShMKS2, we conclude that ShMKS2 acts as a thioesterase hydrolyzing 3-ketoacyl-acyl carrier proteins (plastid-localized intermediates of fatty acid biosynthesis) to release 3-ketoacids and that ShMKS1 subsequently catalyzes the decarboxylation of these liberated 3-ketoacids, forming the methylketone products. Genes encoding proteins with high similarity to ShMKS2, a member of the “hot-dog fold” protein family that is known to include other thioesterases in nonplant organisms, are present in plant species outside the genus Solanum. We show that a related enzyme from Arabidopsis (Arabidopsis thaliana) also produces 3-ketoacids when recombinantly expressed in E. coli. Thus, the thioesterase activity of proteins in this family appears to be ancient. In contrast, the 3-ketoacid decarboxylase activity of ShMKS1, which belongs to the α/β-hydrolase fold superfamily, appears to have emerged more recently, possibly within the genus Solanum.
Many plants develop glandular trichomes, or appendages, on their aerial parts that synthesize and store specialized (secondary) metabolites involved in plant defense (Schilmiller et al., 2008). Plants in the Solanaceae family exhibit a particularly wide range of different types of glandular trichomes (Luckwill, 1943), each with its own repertoire of specialized compounds that also varies across species. This chemodiversity is particularly pronounced in the genus Solanum (Schilmiller et al., 2010). For example, type VI glands in Solanum lycopersicum (cultivated tomato) produce mostly terpenes (Schilmiller et al., 2009), while the type VI glands of Solanum habrochaites subsp. glabratum produce high levels of methylketones (up to 8 mg g−1 leaf fresh weight) consisting mostly of 2-tridecanone and 2-undecanone (Williams et al., 1980; Antonious, 2001; Fridman et al., 2005).
The biosynthetic pathway to methylketones has only recently begun to be investigated (Fridman et al., 2005; Ben-Israel et al., 2009). It is well established that 3-ketoacids are somewhat unstable and can readily undergo decarboxylation when subjected to high temperature and/or nonphysiological pH values; a low-level spontaneous decarboxylation occurs under milder conditions (Kornberg et al., 1948). Decarboxylation of 3-keto fatty acids could thus give rise to straight-chain methylketones such as those found in the S. habrochaites glands (Fig. 1). In plants, 3-keto fatty acids could themselves be derived from the hydrolysis of either 3-ketoacyl-acyl carrier proteins (ACPs), which are intermediates in the fatty acid biosynthetic pathway of chloroplasts, or could be derived from 3-ketoacyl-CoAs, which are intermediates in the degradation of fatty acids in the peroxisomes (Buchanan et al., 2000; Fig. 1).
Figure 1.
A schematic reaction sequence for the synthesis of straight-chain methylketones. 3-Ketoacyl-ACP or 3-ketoacyl-CoA intermediates of fatty acid synthesis and degradation, respectively, are first hydrolyzed, and the resulting 3-ketoacids are then decarboxylated to give the corresponding 2-methylketone.
Initial analysis of a type VI-specific EST database from a methylketone-producing line of S. habrochaites glabratum (accession no. PI126449) for highly expressed genes, followed by comparative gene expression analysis (using S. habrochaites accessions with varying amounts of methylketones), identified the gene methylketone synthase 1 (ShMKS1), whose expression level positively correlated with high levels of methylketone formation (Fridman et al., 2005). The 265-residue-long protein encoded by ShMKS1 belongs to the α/β-hydrolase superfamily of proteins (Hotelier et al., 2004; Forouhar et al., 2005; Yang et al., 2008). Although ShMKS1 does not have a cleavable N-terminal transit peptide, chloroplast import experiments indicated that it could be transported into this organelle (Fridman et al., 2005). Initially, using in vitro biochemical assays, recombinant ShMKS1 appeared to catalyze he conversion of 3-ketomyristoyl-ACP, an intermediate in fatty acid biosynthesis in the chloroplasts, to 2-tridecanone, suggesting that ShMKS1 possesses both thioesterase and decarboxylase activities that sequentially remove the ACP moiety and decarboxylate the 3-ketomyristic acid intermediate (Fridman et al., 2005). However, it was noted that the in vitro rate of production of 2-tridecanone from 3-ketomyristoyl-ACP using ShMKS1 was extremely slow (Fridman et al., 2005).
More recently, extensive genetic and genomic analyses have identified additional genes associated with the high-level production of methylketones in S. habrochaites (Ben-Israel et al., 2009). These results validated earlier genetic analysis that concluded that methylketone production had a polygenic basis, which explains why it has proven difficult to breed cultivated tomato lines that produce high levels of methylketones in their trichomes (Zamir et al., 1984). Some of the loci identified encode fatty acid biosynthetic enzymes, a result that is consistent with the need to increase the flux in fatty acid anabolism that provides, directly or indirectly, the substrates for methylketone biosynthesis. Another locus, designated mks2, identified in this second study encodes a protein with homology (but less than 15% identity) to a 4-hydroxybenzoyl-CoA thioesterase (4HBT), a protein belonging to the “hot-dog fold” family, from a Pseudomonas bacterium (Benning et al., 1998). Our analysis indicated that high-level expression of the S. habrochaites glabratum gene, ShMKS2, in the glandular trichomes was required for high-level production of methylketones (Ben-Israel et al., 2009). Evolutionarily related proteins are encoded in the genomes of various plants, but no functions have yet been assigned to any such plant proteins (Ben-Israel et al., 2009).
Genetic analysis of an interspecific F2 population between the cultivated and wild species identified significant epistatic interaction between the mks1 and mks2 loci. Plants lacking the ShMKS2 allele failed to accumulate any methylketones regardless of the allelic state of the mks1 locus, while absence of the ShMKS1 allele resulted in significantly reduced levels of methylketones. This raised the possibility that the MKS2 protein acts upstream of MKS1 in the pathway for methylketone biosynthesis (Ben-Israel et al., 2009). Furthermore, expression of ShMKS2 cDNA in Escherichia coli cells resulted in the production of 2-tridecanone, 2-undecanone, and several other methylketones (Ben-Israel et al., 2009). These genetic and biochemical observations raised the questions of what specific catalytic role ShMKS2 plays in the biosynthesis of methylketones in wild tomato trichomes and whether it works in parallel or in tandem with ShMKS1. Here, we show that ShMKS2 catalyzes the hydrolysis of the 3-ketoacyl-ACP thioester bond, and ShMKS1 catalyzes the subsequent decarboxylation of the released 3-keto fatty acid, during methylketone biosynthesis.
RESULTS
Genes Encoding MKS1 in S. lycopersicum and S. habrochaites glabratum
Data mining of the genomic “scaffolds” of S. lycopersicum (http://solgenomics.net/) indicated that its genome includes at least four genes on two scaffolds, 05390 and 05477, encoding proteins of 264 to 283 amino acids in length that are more than 75% identical to ShMKS1. We designated these genes SlMKS1a, SlMKS1b, SlMKS1d, and SlMKS1e (Supplemental Figs. S1–S4); a gene designated as SlMKS1c on scaffold 05477 appears to be a nonfunctional gene because it contains a premature stop codon (Supplemental Fig. S5). SlMKS1a is the most similar gene to ShMKS1, encoding a protein with 95% identity to ShMKS1 (Fig. 2). Proteins with similar size that are approximately 54% identical to ShMKS1 have recently been found in the genomes of poplar (Populus trichocarpa) and grape (Vitis vinifera; Fig. 2), although their functions are unknown at present. However, the most similar protein encoded by a gene in the Arabidopsis (Arabidopsis thaliana) genome, AtMES3 (a protein capable of hydrolyzing methyl indole-3-acetic acid and methyl jasmonate [Yang et al., 2008]), is only 40% identical to ShMKS1 (Fig. 2). As reported previously for ShMKS1 (Fridman et al., 2005), the N-terminal region of all these newly identified S. lycopersicum MKS1 proteins as well as the analogous region within the closely related homologs from other species do not appear to constitute N-terminal extensions that could function as cleavable transit peptides.
Figure 2.
Comparison of the protein sequence of S. habrochaites glabratum ShMKS1 with homologous (MKS1-Like, or MKS1L) sequences from S. lycopersicum, grape (Vv), poplar (Pt), and Arabidopsis (At). Accession numbers are as follows: ShMKS1, GU987105; SlMKS1a, GU987107; SlMKS1b, GU987108; SlMKS1d, GU987110; SlMKS1e, GU987111; PtMKS1L, XM_002313048; VvMKS1L, XM_002284871; AtMES3, At2g23610.
Interestingly, while SlMKS1a is the most similar gene to ShMKS1, only one cDNA for it was found in the National Center for Biotechnology Information database, consistent with its low expression level (Fridman et al., 2005; Ben-Israel et al., 2009). Moreover, no cDNAs/ESTs were found for SlMKS1b, while a small number of ESTs for SlMKS1d and SlMKS1e were observed, the majority of which were obtained from trichomes.
We used oligonucleotide primers encoding the beginning and end of the coding region of ShMKS1 in PCR experiments with genomic DNA to isolate the DNA fragment containing all exons and introns of this gene (Supplemental Fig. S6). The number and positions of introns in ShMKS1 were found to be the same as those found in the S. lycopersicum MKS1 genes.
Genes Encoding MKS2 in S. lycopersicum and S. habrochaites glabratum
We previously reported that the longest available ShMKS2 cDNA contained an open reading frame, starting with a Met codon (ATG), of 149 codons. In addition, we showed that the protein encoded by this cDNA was highly similar (more than 70% identity across the equivalent region) to putative, functionally uncharacterized proteins from numerous plant species, including four from Arabidopsis, as well as showing limited similarity (less than 15% identity) to 4HBT from Pseudomonas species (Ben-Israel et al., 2009). Based on this ShMKS2 cDNA sequence and analysis of homologous ESTs from S. lycopersicum available at the time, the orthologous S. lycopersicum gene was deemed to encode a protein similar in size to that of ShMKS2; consequently, a cDNA was isolated by RT-PCR from S. lycopersicum and named SlMKS2 (Ben-Israel et al., 2009). Protein sequence comparisons indicated that the homologous proteins from all other plant species, with the exception of the Solanaceae proteins, have an N-terminal extension that was predicted to function as a transit sequence to direct the protein into the plastids (Ben-Israel et al., 2009).
Mining the S. lycopersicum genome resulted in the identification of three genes on the same scaffold (scaffold 04161) that encode proteins with more than 90% identity (within the equivalent region) to previously reported ShMKS2 sequences; we named these genes SlMKS2a, SlMKS2b, and SlMKS2c (Fig. 3; Supplemental Figs. S7–S9). The EST databases contain ESTs for SlMKS2a and SlMKS2b but not for SlMKS2c. Consistent with this observation, our previously reported SlMKS2 cDNA is derived from SlMKS2a, although SlMKS2c encodes a protein with a higher identity to ShMKS2 (95%). All three of these S. lycoperiscum MKS2 genes have five exons and four introns (whose positions are conserved in comparison with the intron positions in the homologous Arabidopsis genes). By comparing the sequence of the previously reported SlMKS2 cDNA with the genomic sequence of SlMKS2a, from which it is derived, and the sequence of ShMKS2 cDNA with the genomic sequence of SlMKS2c, to which it is most similar, we noted that the first ATG codon of the open reading frame in each of these cDNAs was equivalent to the ATG codon that occurs in positions 2 to 4 of exon 2 in the SlMKS2a and SlMKS2c genes (see underlined codons in Supplemental Figs. S7 and S9). This suggested that these previously reported MKS2 cDNAs from both species were incomplete. Indeed, although no SlMKS2a EST that contains the entire coding region of exon 1 is available, the sequence of one SlMKS2b EST that includes the entire coding region of exon 1 is now in the EST database of the National Center for Biotechnology Information (accession no. DB688740).
Figure 3.
Comparison of the protein sequence of S. habrochaites glabratum ShMKS2 with homologous sequences from S. lycopersicum, Arabidopsis, and Pseudomonas species (Ps). Accession numbers are as follows: ShMKS2, GU987106; SlMKS2a, GU987112; SlMKS2b, GU9877113; SlMKS2c, GU987114; Ps4HB, EF569604. The initiating Met codon used to produce ShMKS2 protein without the transit peptide is underlined.
To determine the beginning of the transcript of ShMKS2, two independent 5′ RACE experiments were performed using two specific primers complementary to the 3′ end and middle of the coding region, respectively (Supplemental Fig. S10). Analysis of the DNA fragments produced in these experiments by agarose gel electrophoresis gave a single sharp band in both cases. The sequences of the resulting fragments from both experiments were determined, and in both cases the sequences obtained indicated that ShMKS2 transcripts are considerably longer at their 5′ ends than was previously seen in the cDNA. This newly uncovered 5′ end sequence, identical in both 5′ RACE experiments, included the region homologous to exon 1 in the SlMKS2 genes, which encodes a putative transit peptide, as well as 63 nucleotides of the 5′ untranslated region (Supplemental Fig. S10). To determine the complete genomic structure of the ShMKS2 gene, we used a forward oligonucleotide primer based on the sequence at the beginning of the coding region in exon 1 of ShMKS2 (as determined by the 5′ RACE experiment) and a reverse primer based on the sequence at the end of the coding region in a PCR experiment with S. habrochaites glabratum genomic DNA and isolated and characterized the genomic fragment containing ShMKS2 (Supplemental Fig. S10). Using a homology-based PCR approach, we also isolated a 1.5-kb fragment upstream of exon 1 of ShMKS2, with a forward oligonucleotide primer whose sequence was based on the sequence of the promoter of SlMKS2c (Supplemental Fig. S9) and a reverse primer derived from the beginning of the ShMKS2 coding region. Analysis of the complete sequence of the ShMKS2 gene (Supplemental Fig. S10) indicates that its structure, with five exons and four introns and encoding a protein of 208 amino acid residues with a predicted plastidic transit peptide, is very similar to that of the S. lycopersicum MKS2 genes (Fig. 3).
Subcellular Localization of ShMKS2
To determine the subcellular localization of ShMKS2 proteins, we injected tobacco (Nicotiana benthamiana) leaves with a solution of Agrobacterium tumefaciens cells carrying various constructs in which the ShMKS2 had been fused to the enhanced GFP (eGFP) under the control of the cauliflower mosaic virus 35S promoter and visualized the targeting by confocal microscopy. No green fluorescence was detected in tobacco leaf cells transformed with an empty binary vector (Fig. 4, A–C). In the tobacco leaf cells transformed with the full-length ShMKS2-eGFP construct, GFP-labeled signals, seen as a punctate pattern, were observed from the same area from which red fluorescence was observed and therefore were identified as the chloroplasts (Fig. 4, D–F). In tobacco leaf cells transformed with a ShMKS2-eGFP construct that lacked the putative ShMKS2 transit peptide, the green fluorescence dots no longer coincided with chloroplast red fluorescence (Fig. 4, G–I).
Figure 4.
Subcellular localization of ShMKS2-eGFP fusion proteins in N. benthamiana leaf cells. The panels shown on the left exhibit green fluorescence from eGFP, the panels in the middle show red fluorescence from plastidic chlorophyll, and each panel in the right column exhibits an overlay of the two panels to its left. A to C, Tobacco cells infiltrated with an empty binary vector. D to F, Tobacco cells infiltrated with a binary vector carrying the complete opening reading frame of ShMKS2 fused to eGFP. G to I, Tobacco cells infiltrated with a binary vector carrying the ShMKS2 gene lacking the putative transit peptide and fused to eGFP. Bars = 10 μm.
Expression of ShMKS1 and ShMKS2 in E. coli and Production of Methylketones
We previously reported that analysis of the spent medium of E. coli cells expressing ShMKS2 demonstrated the presence of several methylketones, with 2-tridecanone, 2-tridecenone, and 2-undecanone predominating (Ben-Israel et al., 2009). However, in those studies, no attempt was made to measure the production of 3-ketoacids, which are the putative intermediates in the synthesis of the final methylketone products (Fig. 1). Direct measurement of 3-ketoacids is difficult, since these compounds are unstable. However, a chemical approach employing sulfuric acid and heat treatment was developed, leading to greatly enhanced decarboxylation and conversion of the water-soluble 3-ketoacids into easily extractable methylketones, which can then be directly measured by gas chromatography-mass spectrometry (GC-MS; Matiasek et al., 2001).
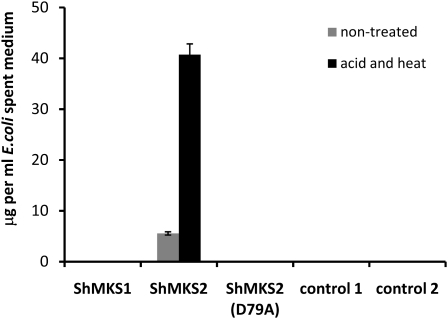
To test if expression of either ShMKS1 or ShMKS2 (without its transit peptide) in E. coli results in the formation of 3-ketoacids, we collected spent medium of bacterial cells expressing each of them (by centrifuging out the cells at the end of the incubation period), heated the spent medium at 75°C for 30 min in the presence of 1 m sulfuric acid, extracted with hexane, then injected the hexane fraction in a GC-MS device. Spent medium of cells expressing either ShMKS1 or a plant gene unrelated to the methylketone biosynthetic pathway, as well as of cells carrying the same vector (pEXP5-CT/TOPO) without an introduced gene, contained no methylketones with or without the acid and heat treatment (Fig. 5). On the other hand, the spent medium of E. coli cells expressing ShMKS2 contained 5.6 ± 0.32 μg μL−1 total methylketones, and the amount of methylketones increased 8-fold, to 40.7 ± 2.1 μg μL−1, after the spent medium was treated with acid and heat (Fig. 5).
Figure 5.
Total amount of methylketones found in spent medium of E. coli cells expressing ShMKS1, ShMKS2, and ShMKS2(D79A) (all missing the transit peptide-coding region) from the pEXP-TOPO-CT bacterial expression vector. Cells were grown and spent medium was collected and treated as described in “Materials and Methods.” Control 1 cells expressed Clarkia breweri Isoeugenol synthase1 on pEXP5-CT/TOPO (Koeduka et al., 2008). Control 2 cells contained a pEXP5-CT/TOPO vector with no insert. Values are averages ± se calculated from three experiments.
In the bacterial thioesterase 4HBT, the Asp residue at position 17 was identified as the catalytic residue required for thioester bond cleavage (Benning et al., 1998). We mutated the equivalent Asp codon in ShMKS2 (the Asp encoded by codon 79 of the complete open reading frame) to an Ala codon and expressed the mutated gene (without the transit peptide-encoding region) in E. coli. The spent medium of cells expressing this mutant ShMKS2 protein did not contain any methylketones, with or without acid and heat treatment (Fig. 5).
A more detailed analysis of the spent medium of E. coli cells expressing ShMKS2 showed that the major compounds in the untreated spent medium were 2-undecanone (0.51 μg μL−1), 2-tridecanone (2.6 μg μL−1), 2-tridecenone (1.1 μg μL−1), and 2-pentadecenone (1.3 μg μL−1; Fig. 6). Lower amounts of 2-nonanone (0.05 μg μL−1) were also detected (Fig. 6). When the spent medium was heated at 75°C for 30 min in the absence of sulfuric acid, the yield of methylketones increased up to 0.60 ± 0.05 μg μL−1 2-nonanone (11.9-fold over the nontreated control), 6.22 ± 0.34 μg μL−1 2-undecanone (12.2-fold), 9.61 ± 0.27 μg μL−1 2-tridecanone (3.7-fold), 9.94 ± 0.83 μg μL−1 2-tridecenone (9-fold), and 3.94 ± 0.54 μg μL−1 2-pentadecenone (3.0-fold). The yield was increased even further in the combined heat and acid treatment, reaching maximum levels of 0.95 ± 0.04 μg μL−1 2-nonanone (19.1-fold over the nontreated control), 9.33 ± 0.61 μg μL−1 2-undecanone (18.3-fold), 11.99 ± 0.51 μg μL−1 2-tridecanone (4.6-fold), 13.04 ± 0.63 μg μL−1 2-tridecenone (11.8-fold), and 5.69 ± 0.21 μg μL−1 2-pentadecenone (4.4-fold).
Figure 6.
Methylketone production by E. coli cells expressing ShMKS2. Treated and nontreated spent medium of E. coli cells expressing ShMKS2 (without the transit peptide-coding region) was extracted with hexane, and the methylketone content was measured by GC-MS. Treatments included heat, acid and heat, purified ShMKS1 protein in phosphate buffer, and phosphate buffer alone. Values are averages ± se calculated from three experiments. See text for details.
When purified ShMKS1 (75 μg mL−1 in 12.5 mm Na+-phosphate buffer, pH 6.8) was added to the spent medium (3 μg mL−1 final concentration) and incubated for 2 h prior to hexane extraction, levels of extractable methylketones were significantly higher than in spent medium treated with phosphate buffer alone, although not as high as the levels of methylketones observed after acid and heat treatment. Moreover, the treatment with purified ShMKS1 seemed to favor an increase in 2-tridecanone over other methylketones (Fig. 6).
In Vitro Decarboxylase Activity Assays for ShMKS1 and ShMKS2
To examine the possible decarboxylase activity of ShMKS1 as well as ShMKS2 in vitro, we tested homogenous recombinant ShMKS1 and partially purified recombinant ShMKS2 proteins (without their transit peptides) for their ability to convert 3-ketomyristic acid into its 2-tridecanone decarboxylated product. Notably, ShMKS1 produced 2.6 nm 2-tridecanone μg−1 protein min−1, while ShMKS2 showed no decarboxylase activity (Fig. 7). In steady-state kinetic assays, ShMKS1 was determined to have a Km of 18.4 ± 5.6 μm for 3-ketomyristic acid, with an apparent kcat of 227.9 ± 24.1 min−1.
Figure 7.
Decarboxylase activity assays for ShMKS1 and ShMKS2 using 3-ketomyristic acid as the substrate. Purified recombinant proteins were assayed as described in “Materials and Methods,” and the mean and sd values were calculated from three replicates and given as shown.
In Vitro Thioesterase Activity Assays for ShMKS1 and ShMKS2
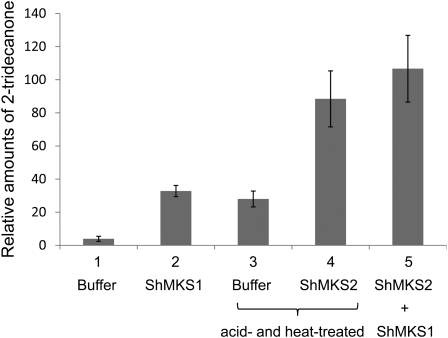
To examine the potential thioesterase activity of ShMKS1 and ShMKS2 in vitro, we added 2.5 μg of each protein to a 500-μL solution of freshly prepared 3-ketomyristoyl-ACP. This protein-linked substrate was prepared using a sequential in vitro enzymatic system involving the addition of multiple starting materials and enzymes, and the reaction was allowed to proceed for 5 h (see “Materials and Methods”). Due to the highly unstable nature of 3-ketomyristoyl-ACP, this compound was not further purified; instead, the solution in which it was synthesized was used as the “substrate solution” for in vitro thioesterase activity assays.
Aliquots of this substrate solution were incubated with either buffer, ShMKS1, ShMKS2, or both ShMKS1 and ShMKS2 for 30 min at 23°C. Extraction of buffer-incubated substrate solution with hexane, followed by GC-MS analysis, resulted in detection of almost no 2-tridecanone (Fig. 8). However, when the buffer-incubated substrate solution was treated with acid, heated at 75°C, cooled, and then extracted with hexane, 2-tridecanone was detected (Fig. 8), indicating that the substrate solution contained free 3-ketomyristic acid in addition to 3-ketomyristoyl-ACP. When the substrate solution was incubated with ShMKS1 and then directly extracted with hexane (i.e. without first treating the sample with acid and heat), the amount of 2-tridecanone obtained was slightly higher, but not significantly so (t test, P = 0.062, α = 0.05), than the amount found in the buffer-incubated sample treated with acid and heat (Fig. 8). However, when the substrate solution was incubated with ShMKS2 and then further treated with acid and heat, the amount of 2-tridecanone formed was approximately 3-fold higher than levels found in buffer-incubated substrate solution treated with acid and heat. Finally, when the substrate solution was coincubated with both ShMKS1 and ShMKS2 for 30 min and then directly extracted with hexane, the amount of 2-tridecanone was slightly higher than that found in ShMKS2-incubated substrate solution treated with acid and heat, but again not significantly so (t test, P = 0.102, α = 0.05; Fig. 8).
Figure 8.
Thioesterase activity assays for ShMKS1 and ShMKS2. To a 500-μL solution of enzymatically prepared 3-ketomyristoyl-ACP (see “Materials and Methods”), a 20-μL solution of the following was added: lane 1, enzyme buffer; lane 2, 2.5 μg of ShMKS1 in buffer; lane 3, enzyme buffer; lane 4, 2.5 μg of ShMKS2; lane 5, 2.5 μg of ShMKS1 and 2.5 μg of ShMKS2. Each reaction was incubated for 30 min at 23°C, after which the reaction solution was either extracted directly with hexane (lanes 1, 2, and 5) or first treated with acid and heated at 75°C for 30 min (lanes 3 and 4), then cooled down to room temperature and extracted with hexane. Hexane extracts were analyzed by GC-MS. Mean and sd values were calculated from three replicates.
DISCUSSION
Enzymatic Activities of ShMKS1 and ShMKS2
Although we previously reported that incubation of crude preparations of 3-ketomyristoyl-ACP derived from a complex mixture of fatty acid biosynthetic components with purified ShMKS1 resulted in the appearance of 2-tridecanone, we also noted that the yield of the in vitro reaction was exceedingly low (Fridman et al., 2005). Here, we report that the expression of ShMKS1 in E. coli does not result in the production of methylketones. On the other hand, we confirm and expand on a subsequent finding (Ben-Israel et al., 2009) that methylketones are present in the growth medium of E. coli expressing ShMKS2 (Fig. 6). Furthermore, treatments of this spent medium with acid and heat, or with purified ShMKS1 protein, greatly elevate the levels of methylketones extracted and detected by GC-MS analysis. Since it is well established that treatment with acid and heat, or even heat alone, greatly accelerates decarboxylation of 3-ketoacids to form methylketones (Matiasek et al., 2001), the increase in levels of methylketones extracted from acid- and heat-treated spent medium of E. coli cells expressing ShMKS2 indicates that substantial amounts of 3-ketoacids were present in this spent medium prior to this treatment and further suggests that ShMKS2 acted as a thioesterase, producing 3-ketoacids from either 3-ketoacyl-CoA or 3-ketoacyl-ACP precursors.
Because treatment of the spent medium of ShMKS2-expressing E. coli cells with purified ShMKS1 also increased the extractable levels of methylketones by several fold without the use of heat or acid, it appears that ShMKS1 possesses decarboxylase activity. This latter activity was later confirmed quantitatively. However, ShMKS1 did not seem to possess a thioesterase activity when expressed in E. coli, since no methylketones could be detected in the spent medium of these cells even after acid and heat treatment, indicating a lack of 3-ketoacids in the spent medium (Fig. 5).
To directly test the enzymatic activities of ShMKS1 and ShMKS2, we carried out in vitro assays. ShMKS1 exhibited decarboxylase activity on 3-ketomyristic acid, while ShMKS2 did not (Fig. 7). When ShMKS1 was added to a solution containing enzymatically synthesized 3-ketomyristoyl-ACP but also some free 3-ketomyristic acid (due to the instability of 3-ketomyristoyl-ACP, it could not be further purified), the amount of 2-tridecanone obtained was slightly higher than the amount of 2-tridecanone observed after incubation of the same volume of substrate solution with buffer, followed by acid and heat treatment. However, the difference was not significant, indicating that ShMKS1 possesses little or no in vitro thioesterase activity, a result also consistent with the lack of 3-ketoacids in the spent medium of ShMKS1-expressing E. coli. On the other hand, incubation of the substrate solution with ShMKS2 resulted in a 3-fold higher amount of 2-tridecanone than what would be expected simply from the acid- and heat-induced decarboxylation of the 3-ketomyristic acid present in the solution (Fig. 8). The additional amount of 2-tridecanone most likely resulted from the intrinsic thioesterase activity of ShMKS2 on 3-ketomyristoyl-ACP, leading to the liberation of 3-ketomyristic acid, which then underwent chemically mediated decarboxylation in the acid and heat treatment. Furthermore, coincubation of substrate solution with both ShMKS1 and ShMKS2 resulted in similar amounts to those observed during the incubation with only ShMKS2 followed by acid and heat treatment, indicating that MKS1 was able to decarboxylate the 3-ketomyristic acid that the thioesterase activity of ShMKS2 on 3-ketomyristoyl-ACP produced.
Since the in vitro assays indicate that ShMKS2 lacks decarboxylase activity, the small amount of methylketones (relative to the corresponding 3-ketoacids) found in the spent medium of ShMKS2-expressing E. coli cells (Fig. 6) was thus most likely due to slow nonenzymatic decarboxylation of the 3-ketoacids released by ShMKS2 during the overnight incubation period.
Taken together, these data suggest that in tomato trichomes, ShMKS2 and ShMKS1 work sequentially, ShMKS2 first liberating 3-ketoacids and ShMKS1 catalyzing their decarboxylation to produce the final methylketone products. This is consistent with the observations that severalfold more methylketones are found in the tomato trichomes when ShMKS2 is highly expressed and ShMKS1 is expressed at low levels than in the opposite case, when ShMKS1 is expressed at high levels but ShMKS2 is expressed at low levels (Ben-Israel et al., 2009). This is easily rationalized, since ShMKS2 activity is required for the production of 3-ketoacids while ShMKS1 activity is limited to decarboxylation, and some decarboxylation may occur spontaneously both in planta and in E. coli given sufficient time (although ShMKS1 activity substantially increases methylketone production in both cases). Our earlier observation that small amounts of 2-tridecanone were produced in vitro when a solution containing enzymatically prepared 3-ketomyristoyl-ACP was incubated with ShMKS1 can now be explained to be the result of the decarboxylating activity of ShMKS1 on the free 3-ketomyristic acid present in the substrate solution, as shown in this study (Fig. 8).
Mature ShMKS2 Localizes to the Plastid and Catalyzes 3-Ketoacyl-ACP Hydrolysis
The results of transient expression of the ShMKS2-GFP fusion protein in tobacco leaves indicated that the ShMKS2 protein localized to the plastids, as has been previously shown for ShMKS1 by in vitro chloroplast import studies (Fridman et al., 2005). A plastidic localization is consistent with the hypothesis that ShMKS2 works by competing for 3-ketoacyl-ACP intermediates formed iteratively during fatty acid elongation in the plastids, rather than from 3-ketoacyl-CoA intermediates formed catabolically during fatty acid degradation in peroxisomes. This conclusion is also consistent with the demonstrated in vitro thioesterase activity of ShMKS2 with 3-ketomyristoyl-ACP, although 3-ketomyristoyl-CoA could not be obtained for comparison purposes.
The range of methylketones produced by expressing ShMKS2 in E. coli was very similar to that seen in S. habrochaites glabratum trichomes, with 2-tridecanone and 2-undecanone being the most abundant, suggesting that ShMKS2 displays a preference for similar chain-length intermediates in both plants and E. coli. Intriguingly, substantial amounts of 2-tridecenone (with one double bond present between C3 and C4) and some 2-pentadecenone (also with one double bond present between C3 and C4) were also observed in ShMKS2-expressing E. coli cultures (Fig. 6), whereas these compounds were not detectable in S. habrochaites glabratum trichomes (Fridman et al., 2005). The position of the double bond indicates that ShMKS2 is able to act on 2-oxo-4-en acyl-ACPs that are at least 14 carbons long. Such intermediates could have resulted from the elongation of unreduced 2-en acyl-ACPs. Whether ShMKS2 acts on such intermediates in S. habrochaites glabratum trichomes or whether this activity is simply a peculiarity of its heterologous expression in E. coli is not known at present.
Evolution of ShMKS1 and ShMKS2
Low levels of methylketones have occasionally been found in plant species from diverse taxa outside the genus Solanum (Jasperson and Jones, 1947; Henricsson et al., 1996), but their mode of synthesis has not yet been determined. In Solanum, only S. habrochaites glabratum has been reported to synthesize and store high levels of methylketones (up to 8 mg leaf−1 fresh weight) in their type VI glandular trichomes, while the trichomes of the cultivated tomato (S. lycopersicum) contain methylketones at levels that are about 1,000-fold lower (Ben-Israel et al., 2009). We also previously showed that the expression of both SlMKS1 and SlMKS2 genes in S. lycoperiscum trichomes is considerably lower than in their related wild species (Ben-Israel et al., 2009). The presence of proteins in species outside Solanum with homology to MKS1 and MKS2 raises the question of whether such proteins are involved in methylketone biosynthesis, albeit at very low rates, and if not, how the Solanum MKS1 and MKS2 proteins cooperatively acquired the catalytic ability to biosynthesize considerable amounts of methylketones.
It is possible that regardless of the original function of MKS2-like genes, simply increasing the expression of such a gene possessing a low level of an alternative activity (with a concomitant increase in fatty acid biosynthetic flux) will lead to the production of some methylketones (for example, high-level expression in E. coli of a ShMKS2 homolog from Arabidopsis also leads to substantial methylketone production; Supplemental Fig. S11). The ability to produce methylketones, with their insecticidal properties, would then be positively selected. However, overexpression of a ShMKS2-type protein without the presence of a dedicated decarboxylase will also lead to accumulation of the 3-ketoacid intermediates, which could interfere with fatty acid biosynthesis, and perhaps an ancestral MKS1 possessing low-level 3-ketoacid decarboxylation activity was selected because it conferred an advantage to the plant by decomposing such acids and increasing the production of methylketones. It is interesting that, unlike MKS2-like proteins from Arabidopsis, which catalyze a similar reaction to ShMKS2 in E. coli, ShMKS1 and its ortholog SlMKS1a are fundamentally different from the other SlMKS1 and MKS1-like proteins in other species, in that the first two are missing what at first glance appears to be a catalytically essential Ser (at position 87 in ShMKS1), part of the catalytic triad necessary for the α/β-hydrolase activity of many proteins in the α/β-hydrolase superfamily (Hotelier et al., 2004; Forouhar et al., 2005). Although ShMKS1 and SlMKS1a clearly belong to this family, they have an Ala substitution at this position and therefore are unlikely to possess hydrolase activity. It thus appears that a bona fide MKS1 evolved recently in the Solanum lineage by acquiring decarboxylase activity and attenuating its more ancient hydrolase activity.
MATERIALS AND METHODS
Bioinformatics
Homologs of Solanum habrochaites subsp. glabratum ShMKS1 and ShMKS2 were identified by BLAST search of the Tomato WGS Scaffolds Prelease data set (http://solgenomics.net/). The genomic sequences identified in this search were checked (by BLAST) with the EST database (http://bioinfo.bch.msu.edu/trichome_est) from the trichomes of Solanum lycopersicum. The positions of exons were determined by comparisons with ESTs directly derived from these genes or, in the absence of ESTs, from a comparison with ShMKS1 and ShMKS2 cDNAs, respectively. Protein sequence comparisons were performed with the ClustalX protocol (Thompson et al., 1997).
Gene Isolation
A full-length cDNA of ShMKS2 was isolated by RT-PCR using the oligonucleotides 5′-ATGTCTCATTCGTTCAGCA-3′ and 5′-GAGATGATGTTGTACACCGCAACT-3′ (oligonucleotides 1 and 4; Supplemental Fig. S10) with total RNA from S. habrochaites. The genomic sequence of ShMKS2 was obtained using total DNA as the template. The promoter sequence of ShMKS2 was isolated by PCR using the oligonucleotides 5′-CTGTGGCAATTGTTAATTGGTGGGAGT-3′ (oligonucleotide 1; Supplemental Fig. S9) and 5′-GAGCGGGAGTTGCCGGTGAG-3′ (oligonucleotide 2; Supplemental Fig. S10). The genomic sequence of ShMKS1 was obtained by PCR with nucleotides 5′-ATGGAGAAAAGCATGTCGCCA-3′ and 5′-TTTATACTTGTTAGCGATGCTTAGAAGAGT-3′ (oligonucleotides 1 and 2; Supplemental Fig. S6). All PCRs employed KOD hot-start polymerase (Novagen). Products were spliced into the pGEM-T easy vector (Promega) and sequenced.
5′ RACE
The 5′ RACE procedure used the SMART RACE cDNA amplification kit (Clontech Laboratories) with SuperScript II reverse transcriptase and anchored oligo(dT)20 (Invitrogen). Two independent experiments with different primers were performed for each gene, with RACE-ready cDNA synthesized from total RNA from the leaves. Products were spliced into the pGEM-T easy vector (Promega) and sequenced.
Genome Walking
Isolation of the promoter region of ShMKS1 was done with the GenomeWalker Universal Kit (Clontech) according to the manufacturer’s instructions.
Constructs for Subcellular Localization
Full-length ShMKS2 cDNA and ShMKS2 without the coding region of exon 1 (starting with the first ATG codon in exon 2) were amplified by KOD polymerase to add BglII and SalI restriction sites and spliced into pSAT6A-EGFP-N1 (Tzfira et al., 2005). The expression cassettes were digested by PspI, ligated to pPZP-RCS2 binary vector, and transferred into Agrobacterium tumefaciens strain EHA105 (Tzfira et al., 2005).
Transient Expression in Nicotiana benthamiana and Confocal Microscopy
A. tumefaciens cells were grown in a shaker-incubator at 30°C at 200 rpm in LB medium supplemented with 200 μg mL−1 spectinomycin and 200 μg mL−1 streptomycin until the optical density of the culture at 600 nm reached 0.7 to 0.9. Bacteria were pelleted by centrifugation at 5,000 rpm for 10 min at room temperature and resuspended to optical density of 0.4 in fresh infiltration buffer containing 10 mm MgCl2 and 0.1 μm acetosyringone. The resulting mix was diluted with infiltration buffer to optical density of 0.1 and infiltrated into the abaxial air spaces of 4- to 6-week-old N. benthamiana plants by a syringe, as described previously (Yang et al., 2000). The plants were then returned to the growth chamber for 48 to 72 h for an optimal expression of the gene.
To test for the localization of the ShMKS2 protein, the infiltrated tobacco leaves were dissected and mounted on a microscope slide with distilled water and examined using a Leica SP5 confocal system and a 63× (1.3 numerical aperture) glycerin immersion lens. eGFP was visualized using an argon gas 488-nm laser, an RP500 dichroic mirror, and photomultiplier detection from 500 to 530 nm. Chloroplast fluorescence was visualized using the same argon gas 488-nm laser, an RP500 dichroic mirror, and photomultiplier detection from 650 nm long pass.
Expression of ShMKS1 and ShMKS2 in Escherichia coli
The coding regions of ShMKS1 and ShMKS2 (minus the transit peptide-encoding region) were each amplified by PCR and inserted into the E. coli expression vector pEXP5-CT/TOPO (Invitrogen). The expression vectors were introduced into E. coli BL21 Star (DE3) cells, and gene expression was induced by the addition of 0.5 mm isopropylthio-β-galactoside after the culture optical density at 600 nm had reached 0.65. After induction with isopropylthio-β-galactoside and growth at 18°C overnight, the cells expressing ShMKS1 or ShMKS2 were centrifuged at 5,000 rpm for 15 min, and 1-mL aliquots of the spent medium were placed in individual vials for further analysis.
GC-MS Analysis of Spent Medium of E. coli Cells Expressing ShMKS2
Aliquots (1 mL) of the spent medium of E. coli expressing ShMKS2, obtained by centrifuging the culture solution after the incubation time at 5,000 rpm for 15 min and collecting the solution without the cells, were treated in the following ways: (1) incubated with 40 μL (3 μg) of purified MKS1 in phosphate buffer (12.5 mm NaH2PO4, 125 mm NaCl, and 2 mm dithiothreitol [DTT], pH 6.8) for 2 h at 30°C; (2) incubated at 75°C for 30 min followed by 30 min at 30°C; (3) incubated with 1 mL of 2 m H2SO4 at 75°C for 30 min followed by 30 min at 30°C. After the various treatments, 1 mL of hexane containing 5 ng μL−1 linalool as an internal standard was added, and the resulting mixture was vortexed and centrifuged at 5,000 rpm for 10 min. Two microliters of the resulting extract was injected into the GC-MS device for determination of methylketones. GC-MS and product analysis were performed as described previously (Ben-Israel et al., 2009).
Affinity Purification of ShMKS1 and ShMKS2
His-tagged ShMKS1 and ShMKS2 (Ben-Israel et al., 2009) were affinity purified by nickel-agarose chromatography using the protocol described by Fridman et al. (2005). After elution from the nickel-agarose column, the proteins were analyzed by SDS-PAGE and ShMKS2 was dialyzed against 50 mm phosphate buffer, pH 6.8, 500 mm NaCl, 1 m (NH4)2SO4, and 2 mm DTT and ShMKS1 was dialyzed against 12.5 mm phosphate buffer, pH 6.8, 50 mm NaCl, and 2 mm DTT. ShMKS1 purity was estimated at 99%, and ShMKS2 purity was estimated at 6%.
Decarboxylase Activity Assays
A typical decarboxylase assay consisted of a 500-μL reaction solution containing ShMKS1 or ShMKS2 (2.5 μg), 3-ketomyristic acid (0.1 mm), and 1,3-bis(tris[hydroxymethyl]methylamino)propane-Na+ (20 mm, pH 7.0). For measuring kinetic parameters, substrate concentrations ranged from 5 to 75 μm. Assays were performed at 23°C for 10 min after addition of protein. Reactions were quenched by the addition of 25 μL of 3 m NaOH to ensure that any remaining 3-ketoacid was anionic and unlikely to be extracted by hexane. Omission of the base neutralization step resulted in the extraction of free 3-ketoacids and spontaneous decarboxylation upon heating in the GC-MS device inlet. Methylketone products were extracted with 500 μL of hexane. For reaction normalization, a standard concentration of 2-undecanone was added prior to extraction to a final concentration of 4 μm. Reaction products (5 μL) were analyzed by a modified procedure described by O’Maille et al. (2004) using a Hewlett-Packard 6890 gas chromatograph coupled to a 5973 mass selective detector equipped with an HP-5MS capillary column (0.25 mm i.d., 30 m length, 0.25 μm film thickness; Agilent Technologies). Product quantification was performed using total ion monitoring mode, where all ions in the mass spectrum contribute to the measured response. The gas chromatograph was operated at a helium flow rate of 1.5 mL min−1, and the mass selective detector was operated at 70 eV. Splitless injections (5 μL) were performed with an inlet temp of 280°C. The gas chromatograph was programmed with an initial oven temperature of 60°C (2-min hold), which was then increased 5°C min−1 up to 200°C, followed by a 50°C min−1 ramp to 280°C (5-min hold). A solvent delay of 8.5 min was included prior to the acquisition of the MS data. 2-Tridecanone was quantified by integration of peak areas using Enhanced Chemstation (version B.01.00; Agilent Technologies). The GC-MS instrument was calibrated with an authentic 2-undecanone standard included in the quenched reactions prior to hexane extraction.
3-Ketomyristic acid was prepared from methyl 3-oxotetradecanoate (1 mmol) by the addition of 6 mL of 3.0 m aqueous NaOH in addition to several drops of tetrahydrofuran to aid in the dissolution of the esterified starting material. The mixture was stirred at 23°C for 12 h. The mixture was then diluted with 10 mL of water and acidified to pH 2 to 3 by adding 3 m HCl dropwise while monitoring pH. The acidified mixture was next extracted five times with 30 mL of methylene chloride. The organic phases were pooled, washed with saturated NaCl, and then dried using anhydrous sodium sulfate. The methylene chloride solvent was removed under reduced pressure, yielding an opaque yellowish powder. This powder was purified using a normal phase silica gel column after dissolution in a minimal amount of column solvent (methylene chloride:methanol, 3:1) to afford 3-ketomyristic acid.
Thioesterase Activity Assays
3-Ketomyristoyl-ACP was synthesized in a 500-μL reaction volume containing 1,3-bis(tris[hydroxymethyl]methylamino)propane (20 mm, pH 7.0), malonyl-CoA (0.2 mm), lauroyl-CoA (0.2 mm), ShACP (0.1 mm), EcFabD (10 μg), and MtFabH (10 μg). After 5 h at 37°C, the in vitro reaction was used as the substrate solution for subsequent treatments. A 20-μL solution containing 2.5 μg of MKS1 or MKS2 (or buffer only) was added, and the reaction was incubated for an additional 30 min at 23°C. Hexane was used for extraction either directly or after being treated with acid and heat. Hexane extracts were analyzed by GC-MS as described above. The values for heat-treated samples shown in Figure 8 were corrected for loss of methylketones during the heating step, as determined by comparisons with standards.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers GU987105 to GU987114.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Sequence of SlMKS1a
Supplemental Figure S2. Sequence of SlMKS1b
Supplemental Figure S3. Sequence of SlMKS1d
Supplemental Figure S4. Sequence of SlMKS1e
Supplemental Figure S5. Sequence of SlMKS1c
Supplemental Figure S6. Sequence of ShMKS1
Supplemental Figure S7. Sequence of SlMKS2a
Supplemental Figure S8. Sequence of SlMKS2b
Supplemental Figure S9. Sequence of SlMKS2c
Supplemental Figure S10. Sequence of ShMKS2
Supplemental Figure S11. Methylketones produced in E. coli by the expression of At1g68260.
Supplementary Material
Acknowledgments
We thank Drs. Tzvi Tzfira and Laura Olsen for the generous gift of plasmids for the subcellular localization studies, Dr. Michael Austin for critical reading and suggestions for improvement, and Drs. Basil Nikolau and Tom Bobik for useful discussion of the fabA system in E. coli.
References
- Antonious GF. (2001) Production and quantification of methyl ketones in wild tomato accessions. J Environ Sci Health B 36: 835–848 [DOI] [PubMed] [Google Scholar]
- Ben-Israel I, Yu G, Austin MB, Bhuiyan N, Auldridge M, Nguyen T, Schauvinhold I, Noel JP, Pichersky E, Fridman E. (2009) Multiple biochemical and morphological factors underlie the production of methylketones in tomato trichomes. Plant Physiol 151: 1952–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benning MM, Wesenberg G, Liu RQ, Taylor KL, Dunaway-Mariano D, Holden HM. (1998) The three-dimensional structure of 4-hydroxybenzoyl-CoA thioesterase from Pseudomonas sp. strain CBS-3. J Biol Chem 50: 33572–33579 [DOI] [PubMed] [Google Scholar]
- Buchanan BB, Gruissem W, Jones RL. (2000) Biochemistry and Molecular Biology of Plants. American Society of Plant Biology, Rockville, MD [Google Scholar]
- Forouhar F, Yang Y, Kumar D, Chen Y, Fridman E, Park SW, Chiang Y, Acton TB, Montelione GT, Pichersky E, et al. (2005) Identification of methyl salicylate as a substrate for salicylic acid binding protein 2 (SABP2) and implications for plant host defense. Proc Natl Acad Sci USA 102: 1773–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman E, Wang J, Iijima Y, Froehlich JE, Gang DR, Ohlrogge J, Pichersky E. (2005) Metabolic, genomic, and biochemical analyses of glandular trichomes from the wild tomato species Lycopersicon hirsutum identify a key enzyme in the biosynthesis of methylketones. Plant Cell 17: 1252–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricsson S, Westerholm R, Nilsson S, Berggren B. (1996) Chemical characterisation of extractable compounds found in the coating of birch (Betula) pollen. Grana 35: 179–184 [Google Scholar]
- Hotelier T, Renault L, Cousin X, Negre V, Marchot P, Chatonnet A. (2004) ESTHER, the database of the alpha/beta-hydrolase fold superfamily of proteins. Nucleic Acids Res 32: D145–D147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasperson H, Jones R. (1947) Some unsaponifiable constituents of deodorization distillate of vegetable oils. J Soc Chem Ind 66: 13–17 [Google Scholar]
- Koeduka T, Louie GV, Orlova I, Kish CM, Wilkerson CG, Bowman ME, Baiga TJ, Noel JP, Dudareva N, Pichersky E. (2008) The multiple phenylpropene synthases in both Clarkia breweri and Petunia hybrida represent two distinct protein lineages. Plant J 54: 362–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A, Ochoa S, Mehler AH. (1948) Spectrophotometric studies on the decarboxylation of beta-ketoacids. J Biol Chem 174: 159–172 [PubMed] [Google Scholar]
- Luckwill L. (1943) The Genus Lycopersicon: A Historical, Biological, and Taxonomic Survey of the Wild and Cultivated Tomatoes. Aberdeen University Press, Aberdeen, Scotland [Google Scholar]
- Matiasek MG, Choudhury K, Nemecek-Marshall M, Fall R. (2001) Volatile ketone formation in bacteria: release of 3-oxopentanoate by soil pseudomonads during growth on heptanoate. Curr Microbiol 42: 276–281 [DOI] [PubMed] [Google Scholar]
- O’Maille P, Chappell J, Noel JP. (2004) A single-vial analytical and quantitative gas chromatography-mass spectrometry assay for terpene synthases. Anal Biochem 335: 210–217 [DOI] [PubMed] [Google Scholar]
- Schilmiller A, Shi F, Kim J, Charbonneau AL, Holmes D, Jones AD, Last RL. (2010) Mass spectrometry screening reveals widespread diversity in trichome specialized metabolites of tomato chromosomal substitution lines. Plant J 62: 391–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilmiller AL, Last RL, Pichersky E. (2008) Harnessing plant trichome biochemistry for the production of useful compounds. Plant J 54: 702–711 [DOI] [PubMed] [Google Scholar]
- Schilmiller AL, Schauvinhold I, Larson M, Xu R, Charbonneau AL, Schmidt A, Wilkerson C, Last RA, Pichersky E. (2009) Monoterpenes in the glandular trichomes of tomato are synthesized from a neryl diphosphate precursor rather than geranyl diphosphate. Proc Natl Acad Sci USA 106: 10865–10870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. (1997) The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzfira T, Tian GW, Lacroix B, Vyas S, Li J, Leitner-Dagan Y, Krichevsky A, Taylor T, Vainstein A, Citovsky V. (2005) pSAT vectors: a modular series of plasmids for autofluorescent protein tagging and expression of multiple genes in plants. Plant Mol Biol 57: 503–516 [DOI] [PubMed] [Google Scholar]
- Williams WG, Kennedy GG, Yamamoto RT, Thacker JD, Bordner J. (1980) 2-Tridecanone: naturally-occurring insecticide from the wild tomato Lycopersicon hirsutum f glabratum. Science 207: 888–889 [DOI] [PubMed] [Google Scholar]
- Yang Y, Xu R, Ma CJ, Vlot AC, Klessig DF, Pichersky E. (2008) Inactive methyl indole-3-acetic acid ester can be hydrolyzed and activated by several esterases belonging to the AtMES esterase family of Arabidopsis thaliana. Plant Physiol 147: 1034–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YN, Li RG, Qui M. (2000) In vivo analysis of plant promoters and transcription factors by agroinfiltration of tobacco leaves. Plant J 22: 543–551 [DOI] [PubMed] [Google Scholar]
- Zamir D, Selilaben-David T, Rudich J, Juvik JA. (1984) Frequency-distributions and linkage relationships of 2-tridecadone in interspecific segregating generations of tomato. Euphytica 33: 481–488 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.