Abstract
Growth and carbon (C) fluxes are severely altered in plants exposed to soil water deficit. Correspondingly, it has been suggested that plants under water deficit suffer from C shortage. In this study, we test this hypothesis in Arabidopsis (Arabidopsis thaliana) by providing an overview of the responses of growth, C balance, metabolites, enzymes of the central metabolism, and a set of sugar-responsive genes to a sustained soil water deficit. The results show that under drought, rosette relative expansion rate is decreased more than photosynthesis, leading to a more positive C balance, while root growth is promoted. Several soluble metabolites accumulate in response to soil water deficit, with K+ and organic acids as the main contributors to osmotic adjustment. Osmotic adjustment costs only a small percentage of the daily photosynthetic C fixation. All C metabolites measured (not only starch and sugars but also organic acids and amino acids) show a diurnal turnover that often increased under water deficit, suggesting that these metabolites are readily available for being metabolized in situ or exported to roots. On the basis of 30 enzyme activities, no in-depth reprogramming of C metabolism was observed. Water deficit induces a shift of the expression level of a set of sugar-responsive genes that is indicative of increased, rather than decreased, C availability. These results converge to show that the differential impact of soil water deficit on photosynthesis and rosette expansion results in an increased availability of C for the roots, an increased turnover of C metabolites, and a low-cost C-based osmotic adjustment, and these responses are performed without major reformatting of the primary metabolism machinery.
Water deficit is a common environmental stress experienced by plants and strongly impairs production (Passioura, 1996). It is thought to be one of the main environmental constraints shaping natural variation and evolution of plant growth, development, and physiology (Dudley, 1996). Water deficit leads to large morphological or physiological changes across a range of temporal and spatial scales (Chaves et al., 2002), including reduced expansion of aerial organs (Tardieu et al., 2000), maintenance of root growth (Sharp and Davies, 1979), decrease of transpiration and photosynthesis (Boyer, 1970), accumulation of osmotica (Morgan, 1992), activation of detoxifying processes (Hare et al., 1998), and, in parallel, the transcriptional regulation of a large number of genes (Ozturk et al., 2002; Yu and Setter, 2003, Bray, 2004; Xue et al., 2008).
It has often been proposed that plants exposed to water deficit will experience metabolic limitation (Huber et al., 1984; Cornic et al., 1989; Chaves et al., 2002). Indeed, CO2 diffusion is lowered by stomatal closure, resulting in a lower rate of net carbon (C) assimilation. More C is allocated to nonphotosynthetic organs (e.g. roots) or supporting structures (Sharp and Davies, 1979), while the photosynthetic machinery shows some signs of down-regulation (Farquhar and Sharkey, 1982; Cornic et al., 1989; Xue et al., 2008). In plants exposed to water deficit, soluble sugars usually accumulate, possibly in the vacuole for osmoregulation, and therefore are unavailable to fuel growth (Clifford et al., 1998). Starch levels at the end of the photoperiod are often reduced, indicating that there is less C available for growth in the night (Zrenner and Stitt, 1991; Liu et al., 2004). Moreover, tolerance to water deficit can be improved by high CO2 (Widodo et al., 2003; although high CO2 may also be beneficial by lowering transpiration) or by Suc complementation (McLaughlin and Boyer, 2004).
On the other hand, the response of maize (Zea mays) leaf elongation to soil water deficit is independent of the amount of light intercepted by the plant, suggesting that expansion under water deficit is uncoupled from the C status (Tardieu et al., 1999; Sadok et al., 2007; but see Muller et al., 2001). Furthermore, plants can modify their use of C to face water deficits. In rice (Oryza sativa) grains, starch synthesis rate was correlated with Suc phosphate synthase activity, and both were increased by water deficit, eventually contributing to the acceleration of grain growth (Yang et al., 2003). In maize, a vacuolar invertase induced by water deficit in leaves and root tips contributes to osmoregulation through the accumulation of hexoses in the leaves and to increased root sink strength (Kim et al., 2000). In spinach, under water deficit, posttranslational activation of Suc phosphate synthase directs photosynthate toward Suc rather than starch (Quick et al., 1989; Zrenner and Stitt, 1991).
Thus, no clear conclusion about the impact of water deficit on plant C status can be drawn from the literature. This is not surprising, since it is widely admitted that the consequences of drought depend on the timing, intensity, and mode of imposition of the water deficit (Bray, 2004), the interaction with other environmental variables such as evaporative demand (Tardieu et al., 2000) or CO2 (Huber et al., 1984), and the sensitivity of the plant, which depends on developmental status and intrinsic capacity (Reymond et al., 2003). Reproducible and/or thoroughly documented experiments (with plant or soil water status and climatic conditions) are needed to robustly evaluate responses to various levels of water deficit and ultimately to compare genotypes (Aguirrezabal et al., 2006) and perform genomic (Muller et al., 2007) or genetic (Reymond et al., 2003; Welcker et al., 2007; Tisné et al., 2010) analyses based on multiple experiments. Many protocols for imposing a water deficit are based on cessation of irrigation. These are likely to be biased by differences in transpiration rates due to shoot size, rooting volume, or air humidity, which result in an uncontrolled soil water content and often a rapid fall to very low water availability in the restricted rooting volume. Instead, we chose to impose steady-state water deficits, using the PHENOPSIS platform, which relies on automatic daily measurement of soil water status and controlled readdition of water to maintain a sustained intensity of water deficit (Granier et al., 2006).
Another reason for the conflicting views may be that studies addressing the effect of water deficit on metabolism are usually restricted to particular metabolites such as Pro, Gly betaine, or trehalose (Girousse et al., 1996; Romero et al., 1997; Quan et al., 2004) or particular enzymes such as invertase in sinks (Neumann Andersen et al., 2002) or Suc phosphate synthase in source leaves (Fresneau et al., 2007). Recent research on plant function under water deficit has benefited from unbiased approaches such as proteomics (Plomion et al., 2006) and transcriptomics (Cuming et al., 2007), but integrative approaches that provide a broad overview of how central metabolism responds to water deficit are still missing. The use of multilevel approaches involving a set of high-throughput techniques has recently allowed a reassessment of the molecular, enzymatic, and metabolic responses to moderately low nitrogen (Tschoep et al., 2009), low C (Gibon et al., 2006, 2009; Osuna et al., 2007; Usadel et al., 2008b), low potassium (Armengaud et al., 2009), and small decreases in temperature (Usadel et al., 2008a). Often, metabolism achieved a remarkable homeostasis, with only subtle differences at the level of major pathway enzymes.
In this study, we examine the consequences of water deficit on C metabolism in the model plant Arabidopsis (Arabidopsis thaliana) using the PHENOPSIS platform to impose reproducible levels of water deficit. The changes in C metabolism at various time scales are evaluated through analyses of major metabolites, the activities of 30 enzymes of the central metabolism using a robot-based platform, and a set of sugar-responsive genes. The metabolic and molecular responses are placed in a quantitative perspective by combining them with information on growth, photosynthesis, and osmotic adjustment.
RESULTS
Water Deficit Reduces Biomass Growth and Leaf Expansion While Photosynthesis Is Maintained and Root Growth Is Promoted
In all experiments, an identical water deficit scenario was used, allowing different variables to be collected from independent but comparable experiments (Supplemental Fig. S1). In this scenario, water was withheld from 18 to 22 d after sowing, and plant water status was maintained thereafter by continuously irrigating plants to a target and stable level of soil water content. Mean predawn leaf water potential (Ψw) was maintained at –0.35 MPa in well-watered plants (Fig. 1, A and B). The relationship (Fig. 1A) between Ψw and soil water content was used to impose a moderate (Ψw = –0.6 MPa) or severe (Ψw = –1.1 MPa) water deficit.
Figure 1.
Water deficit management, harvest schedule, and impact of water deficit on plant growth and development. The same color code and acronyms for treatments are used throughout Figures 1 to 7: well watered (black; WW), moderate water deficit (gray; MWD), and severe water deficit (white; SWD). A, Water deficit management was based on daily estimation of soil water content from pot weighing and a preestablished relationship between soil water content and predawn Ψw (on excised leaves [squares] or whole rosettes [circles]). Well-watered plants were maintained in soil at 0.35 to 0.40 g water g−1 dry soil (WW) throughout the experiments. Irrigation for plants exposed to soil water deficit was stopped at day 18 and reestablished after 3 to 5 d once soil water content had reached 0.25 g water g−1 dry soil (MWD) or 0.18 g water g−1 dry soil (SWD). B, Top view of representative plants 14 d after the onset of water deficit. Water potentials shown are averages of those measured at the target soil water content. C, Plant development and harvest schedule. Numbers of visible (circles) or initiated (triangles) leaves, as well as bolting time (BT), are indicated. Arrows refer to harvest dates that were scheduled during the vegetative phase, 7 and 10 d after the onset of water deficit, after the initiation of reproductive organs, or at bolting, 16 and 24 d after the onset of water deficit. D, Relative expansion rate of the whole rosette area at time of harvest based on 12 individuals. The inset shows relative expansion rate (RER) of the whole rosette measured nondestructively 10 d after the onset of stress. Letters refer to ANCOVA [with log(AREAros) as the dependent variable and time as the covariable] followed by lsd grouping at a 0.05 probability threshold. E, Root mass fraction at the four harvests. Means ± sd of 12 plants are shown. Letters refer to lsd grouping following ANOVA. [See online article for color version of this figure.]
While the leaf initiation rate was unaltered (Fig. 1C), water deficit reduced the rate of leaf emergence (from 1.3 leaf d−1 in well-watered plants to 1.0 leaf d−1 in plants under water deficit). As a consequence, total leaf number decreased from 28 to 23. These two effects counterbalanced each other, leading to unchanged bolting time. Further harvests were thus scheduled based on this temporal frame. The relative expansion rate of the rosette was significantly and permanently reduced from day 7 onward following the onset of water deficit in the severe water deficit treatment, whereas it was only reduced 10 d after the onset of the moderate water deficit (Fig. 1D). Rosette expansion was 40% higher during the day than at night in well-watered plants, but day expansion was inhibited by water deficit to a larger extent. The relative growth rate of the rosette determined 10 d after the onset of stress was reduced from 0.182 in well-watered plants to 0.144 and 0.119 under moderate and severe stress, respectively (Table I). As a result, rosette biomass was reduced by about 35% and 45% after 10 d of stress and by about 45% and 75% at bolting under moderate and severe water deficit, respectively (Table I). Water deficit rapidly favored root growth. The root mass fraction increased by about 30% in stressed plants as early as 7 d after stress began (Fig. 1E), and this difference was maintained thereafter.
Table I. Impact of water deficit on a set of physiological, growth, and morphological parameters.
All measurements were performed 10 d after the onset of water deficit (except for dry weight at bolting). Rosette relative growth rate was measured from three consecutive evaluations of rosette dry weight. Photosynthesis and stomatal conductance were measured at growth light intensity on the youngest fully expanded leaves. Photosynthesis at saturating light (Amax) was measured at 1,000 μmol m−2 s−1. Specific leaf area, leaf thickness, water content, and relative water content were measured on the youngest fully expanded leaves on samples harvested at the end of the photoperiod. Protein content was measured on total rosettes. Data are means and (sd) of eight to 12 plants or leaves. Letters refer to lsd grouping following ANOVA for all variables except relative growth rate, where statistics are based on ANCOVA.
| Parameter | Unit | Treatment | ||
| Well Watered | Moderate Water Deficit | Severe Water Deficit | ||
| Relative growth rate | mg dry weight mg−1 dry weight d−1 | 0.182 a | 0.144 b | 0.119 c |
| Rosette dry weight | mg | 14.9 (1.8) a | 10.0 (1.8) b | 8.5 (1.4) b |
| Rosette dry weight at bolting | mg | 204 (17) a | 117 (14) b | 53 (4) c |
| Net photosynthesis | μmol m−2 s−1 | 4.70 (0.61) a | 4.93 (1.17) a | 4.66 (0.81) a |
| Amax | μmol m−2 s−1 | 10.35 (0.85) a | 10.3 (1.15) a | 7.6 (0.53) b |
| Dark respiration | μmol m−2 s−1 | 1.11 (0.2) a | 1.19 (0.12) a | 1.30 (0.18) a |
| Stomatal conductance | mmol m−2 s−1 | 488 (53) a | 347 (27) b | 183 (47) c |
| Protein content | mg g−1 dry weight | 153 (8.0) a | 144 (8.8) ab | 139.3 (20) bc |
| Specific leaf area | mm2 mg−1 dry weight | 45 (5.2) a | 43 (4.5) ab | 36 (3.7) b |
| Leaf thickness | μm | 186 (22) a | 197 (18) b | 208 (16) c |
| Water content | g water g−1 fresh weight | 0.92 (0.01) a | 0.89 (0.01) b | 0.86 (0.02) c |
| Relative water content | g water g−1 water at saturation | 0.92 (0.03) a | 0.84 (0.04) b | 0.71 (0.04) d |
A series of physiological and morphological parameters were evaluated 10 d after the onset of moderate or severe water deficit (Table I). In contrast to growth, neither net photosynthesis at growth irradiance (180 μmol m−2 s−1) nor dark respiration expressed on an area basis was significantly affected by moderate or severe water deficit. Photosynthesis at saturating light was only reduced at the most severe level of water deficit, and stomatal conductance was significantly reduced by 30% and 60% in the two water deficit treatments. Water deficit induced changes of rosette and leaf structure (Table I). The specific leaf area of the youngest fully expanded leaf of stressed plants was reduced under severe deficit by 20%, while its thickness was increased. Both the leaf relative water content and the water content were decreased by water deficit.
Most C Metabolites and K+ Accumulate in Response to Water Deficit at Different Plant Developmental Stages
The concentrations of seven major C metabolites (starch, hexoses, Suc, fumarate, malate, Pro, and total amino acids) and two major ions (nitrate and potassium) were determined at the end of the photoperiod at four dates along plant development. Because flowering was expected to alter plant C metabolism, harvests were scheduled (Fig. 1C) during the vegetative stage (7 and 10 d after onset of water deficit), after the reproductive switch at the shoot apical meristem (16 d after onset of water deficit), and at bolting (24 d after onset of water deficit). Depending on the purpose, metabolite and ion data were expressed either on fresh weight basis after tissue rehydration (saturated fresh weight), in order to account for water loss during stress (Figs. 2 and 3), or on a dry weight basis, in order to estimate the whole plant C balance (Fig. 4).
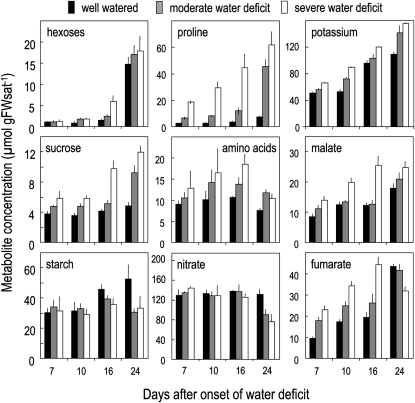
Figure 2.
Impact of water deficit on metabolite concentration at four dates following the onset of water deficit. Means ± sd of four to five biological replicates are shown. Concentrations are expressed per gram of saturated fresh weight (FWsat) in order to account for concentration changes due to water loss imposed by soil water deficit.
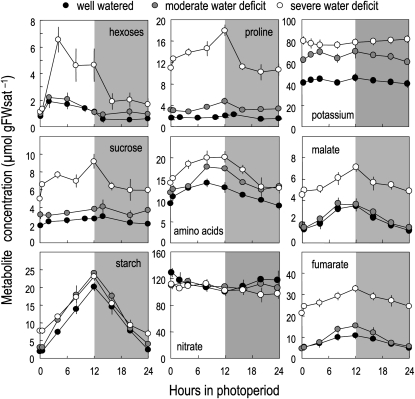
Figure 3.
Impact of water deficit on metabolite concentrations during a 24-h cycle 10 d after the onset of water deficit. Means ± sd of four to five biological replicates are shown. Shaded areas refer to night periods. Concentrations are expressed per gram of saturated fresh weight (FWsat) in order to account for concentration changes due to water loss imposed by soil water deficit.
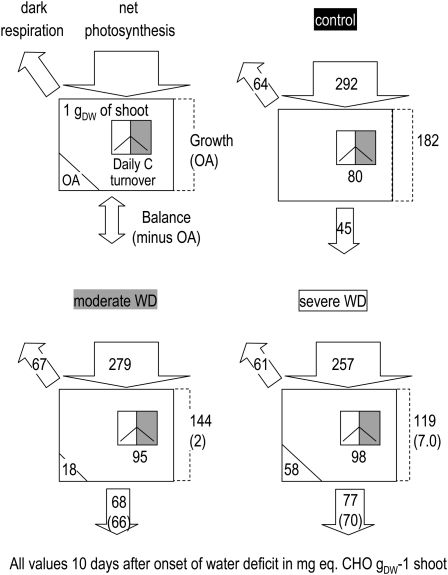
Figure 4.
Impact of water deficit (WD) on C fluxes in the Arabidopsis rosette 10 d after the onset of water deficit. All values are expressed as mg CHO g−1 rosette dry weight d−1 when referred to fluxes. Photosynthesis and respiration measurements on an area basis were transformed on a per dry weight basis using specific leaf area of the whole rosette. Rosette growth demand for CHO was inferred from shoot relative growth rate assuming 40% C in the dry weight. The rosette C balance (potential export to the roots) was evaluated as photosynthesis minus dark respiration minus rosette growth demand for C. Daily C turnover of the sum of the metabolites (starch, Suc, hexoses, fumarate, malate, Pro, other amino acids) was expressed as milligrams of CHO equivalents assuming an average of 4.7 C atoms per molecule of free amino acid. The estimation of the C metabolites immobilized for osmotic adjustment (OA) was based on Table II data reexpressed per gram dry weight. Two values for osmotic adjustment were inferred, either the amount of CHO required for osmotic adjustment in 1 g dry weight (lower left value) or the amount of CHO required to accompany rosette growth (value in parentheses after the flux to growth). The latter was then subtracted from C export (value in parentheses in the bottom arrow).
In well-watered plants, starch and organic acids (malate and fumarate) represented about 50% and 30% of the nonstructural C, respectively, at the end of the photoperiod at the first three harvests (Fig. 2). During development, nitrate as well as some metabolites such as Suc, total amino acids, and Pro remained stable, while starch increased at later stages and organic acids (fumarate and malate) and potassium increased more steadily. Hexose levels were low at the first three harvests but dramatically increased at bolting.
Even when expressed on a saturated fresh weight basis, water deficit induced a global increase in the concentrations of most low-Mr metabolites as well as of K+. A significant decrease of starch level was observed only at the last two harvests and of nitrate only at bolting.
Starch and Several Soluble C Metabolites Accumulate during the Photoperiod in Well-Watered Plants as Well as in Plants Exposed to Water Deficit
Concentrations being very similar between harvests 1 and 2 (Fig. 2), the latter (i.e. 10 d after onset of water deficit) was chosen to perform an in-depth analysis of metabolite and ion contents during a 24-h cycle (Fig. 3).
In well-watered conditions, starch, hexoses, Suc, total amino acids, Pro, and organic acids accumulated during the day and were remobilized at night (Fig. 3). By contrast, potassium, nitrate (Fig. 3), and total protein (Supplemental Fig. S2) remained stable throughout the day/night cycle.
In accordance with the results shown in Figure 2, all soluble metabolites as well as potassium accumulated under water deficit, while nitrate concentration remained constant (Fig. 3). The diel pattern of their concentration depended on the compound. Starch turnover (i.e. the difference between end of day and end of night concentrations) was barely affected under stressing conditions, but there was a slight trend toward lower remobilization at night, which resulted in higher starch content at the end of the night under severe water deficit conditions. In contrast, the concentrations of other major organic metabolites showed increased day/night fluctuations under water deficit and were also not fully remobilized at night. Interestingly, there was a sharp decrease in Pro, soluble sugars, and, to a lesser extent, malate during the first hours of the night, suggesting that the corresponding pools were readily available for catabolism and/or export. Also, the initial rate of accumulation of soluble sugars in the light period was dramatically increased under water deficit. Potassium and nitrate did not show marked diurnal changes under water deficit.
The Daily C Balance Is Increased by Water Deficit
In order to evaluate the C balance of the growing rosette, the rates of photosynthesis, respiration, and rosette expansion measured in plants 10 d after the onset of water deficit were expressed as fluxes of carbohydrate (CHO) equivalents (mg eq. CHO g−1 rosette dry weight 24 h−1; Fig. 4).
Summed daily net photosynthesis was close to 300 mg eq. CHO g−1 dry weight d−1 in nonstressed plants and slightly less under water deficit conditions, due to the decreased specific leaf are (Table I). Dark respiration represented 20% of photosynthesis on a daily basis and was only marginally affected by water deficit. The estimated C demand for growth was strongly reduced by water deficit, from 182 in well-watered plants to 144 and 119 mg eq. CHO g−1 dry weight d−1 in moderately and severely stressed plants, respectively (Fig. 4). The net daily C balance of the shoot (estimated from the difference between C fluxes into photosynthesis, respiration, and rosette growth) indicates a surplus of 15% of the C fixed by photosynthesis in well-watered conditions. This was presumably exported to the roots. The estimated surplus rose to 24% and 30% under moderate and severe water deficit, respectively (Fig. 4). This is in accordance with the higher proportion of total biomass in roots (Fig. 1E).
The daily turnover of all C metabolites (starch + soluble) was 80 mg eq. CHO g−1 dry weight d−1 in well-watered plants and slightly higher under water deficit (95 and 98 mg eq. CHO g−1 dry weight d−1; Fig. 4). The daily metabolite C turnover thus represented a higher proportion of the daily demand for growth under moderate and severe water deficit as compared with well-watered conditions (82% and 66%, respectively, compared with 44%).
K+ and Organic Acids Are the Main Contributors to Osmotic Adjustment
After 10 d of water deficit, the osmotic potential of rosette sap (Ψs[raw]) was close to −1.1 and –1.5 MPa under moderate and severe water deficit, respectively, as compared with –0.9 MPa in well-watered conditions. Moreover, there was a slight tendency toward lower values at the end of the photoperiod under severe water deficit (Table II). About two-thirds of this increase in osmotic potential was due to the decrease in the relative water content. To correct for this and investigate the changes in the contents of osmolytes, the projected osmotic potential was estimated at full turgor (Ψs[sat]), taking the change of relative water content into account. At the end of the night period, Ψs[sat] was −0.84, −0.93, and −1.06 MPa (Table II), leading to a mean osmotic adjustment of 0.086 and 0.213 MPa at moderate and severe water deficit, respectively, with little difference at the end of the photoperiod.
Table II. Impact of water deficit on osmotic potential, osmotic adjustment, and relative contribution of metabolites.
Osmotic potential [mean and (sd) of 10 replicates] of the rosette sap was measured at the end of the night period (EON) and at the end of the photoperiod (EOP) and expressed as raw values (Ψs[raw]) or after tissue rehydration to account for water loss during drought (Ψs[sat]). Osmotic potential difference at saturation between well-watered and water deficit conditions provides an estimate of osmotic adjustment (OA). The concentration differences of solutes between stressed and nonstressed conditions under saturating conditions were summed, giving an estimation of the osmotic adjustment contributed by metabolites (OA[met]). The proportion of the osmotic adjustment accounted for by metabolites (OA[met]) is indicated in parentheses. The relative contribution of each metabolite given is the average of EON and EOP data. All data originate from plants 10 d after the onset of water deficit.
| Parameter | Period | Unit | Treatment | ||
| Well Watered | Moderate Water Deficit | Severe Water Deficit | |||
| Ψs[raw] | EON | MPa | −0.90 (0.09) | −1.07 (0.05) | −1.42 (0.13) |
| Ψs[sat] | EON | MPa | −0.82 (0.08) | −0.91 (0.05) | −1.03 (0.09) |
| Ψs[raw] | EOP | MPa | −0.94 (0.08) | −1.15 (0.13) | −1.61 (0.15) |
| Ψs[sat] | EOP | MPa | −0.87 (0.08) | −0.98 (0.11) | −1.08 (0.10) |
| OA | EON | MPa | 0.086 | 0.213 | |
| OA[met] | EON | MPa | 0.060 (75%) | 0.181 (91%) | |
| OA | EOP | MPa | 0.112 | 0.214 | |
| OA[met] | EOP | MPa | 0.082 (73%) | 0.182 (91%) | |
| Contribution of metabolites to osmotic adjustment (%) | |||||
| Potassium | 53.8 | 35.9 | |||
| Fumarate | 4.4 | 21.7 | |||
| Malate | 0.3 | 3.6 | |||
| Pro | 3.8 | 13.2 | |||
| Amino acids | 8.4 | 6.3 | |||
| Suc | 4.5 | 5.9 | |||
| Hexose | 0.0 | 2.3 | |||
| Unknown | 24.8 | 11.1 | |||
The contributions of the individual metabolites and ions to osmotic adjustment were evaluated from their projected concentrations at full turgor between water deficit and well-watered conditions. This yielded at the end of the night period an average osmotic adjustment of about 0.06 and 0.18 MPa for moderately and severely water-stressed plants, respectively (Table II), with similar estimates at the end of the photoperiod. Our metabolic analysis thus captured 75% (moderate) to 90% (severe) of the contributors to osmotic adjustment. Under moderate water deficit, potassium contributed to 54% of the adjustment, amino acids to 8%, and fumarate, Pro, and Suc to about 4% each. Under severe water deficit, the contribution of potassium was reduced to 36%, fumarate contributed to 22%, and Pro to 13% of the total osmotic adjustment. Nitrate was not involved in the osmotic adjustment, since it remained unaltered in both moderate and severe water deficit (Fig. 3).
An estimation of the C cost of osmotic adjustment based on C metabolites was also performed from these data by scaling the data to a dry weight basis. The background concentrations of C-rich soluble metabolites were 18 and 58 mg eq. CHO g−1 dry weight higher in moderate and severe water deficit conditions, respectively, than in well-watered plants (Fig. 4). This represents both the immobilization of C in existing matter as well as further sequestration of C each day in any new material that is produced. As the analyses were performed after 10 d of water deficit treatment, the average C cost of osmotic adjustment accompanying growth can be estimated to be 2 and 7.0 mg eq. CHO g−1 dry weight d−1, corresponding to 1% and 3% of the daily photosynthesis in moderate and severe water deficit, respectively. These results suggest that osmotic adjustment represents a minor C diversion in growing Arabidopsis exposed to soil water deficit. It should be noted, however, that this estimate does not include any ATP consumed in accumulating the metabolites and ions.
Water Deficit Maintains or Increases Most Enzyme Activities in Central Metabolism
The activities of 30 enzymes from various pathways of central metabolism (Calvin and Benson cycle, starch and Suc metabolism, glycolysis, organic acid metabolism, nitrogen assimilation) were measured under the three watering regimes at the end of the photoperiod and at four time points after the onset of water deficit. Activities were normalized on a protein basis (Fig. 5; Supplemental Table S1; Supplemental Fig. S3A) or a dry weight basis (Supplemental Table S1; Supplemental Fig. S3B), since there was a slight trend toward reduction of protein content per dry weight by water deficit (Table I; Supplemental Fig. S2). In contrast to the metabolites under study, there was no early or dramatic response of enzyme activities to water deficit. Multivariate analyses were carried out to reveal global trends (Fig. 5). A clustering analysis revealed three typical patterns of changes in enzyme activities in response to water deficit throughout plant development. The first cluster (A) contained the majority of enzymes (18 out of 30) and showed a progressive decrease in activity along development that was prevented, or at least delayed, by water deficit. This large cluster included some enzymes from photosynthesis and organic and amino acid processing. A second, much smaller cluster (B) gathered five enzymes that showed an increase in activity at later stages of development in well-watered plants and that tended to occur earlier under water deficit. In particular, this cluster contained enzymes related to the tricarboxylic acid (TCA) cycle (citrate synthase and NAD-isocitrate dehydrogenase) as well as Glu dehydrogenase. Finally, a third cluster (C) contained seven enzymes whose activities showed a progressive decrease along development, which was not influenced by water deficit. This cluster gathered enzymes related to photosynthesis, Suc metabolism, organic acid metabolism, and nitrate assimilation.
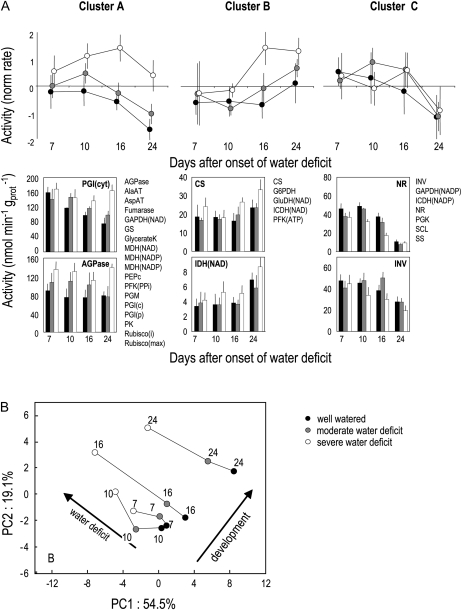
Figure 5.
Multivariate analysis of the impact of water deficit on the activity of 30 enzymes of the central metabolism at four dates following the onset of water deficit, at the end of the photoperiod. Data are based on means ± sd of four to five biological replicates at each date and for each treatment. A, Hierarchical clustering shown is based on the Ward method and Euclidean distance. Results were robust over the different methods. Enzyme activities were centered and scaled to show main trends within clusters. The bottom half of the panels show two examples of enzyme activities within each cluster as well as cluster composition. All other enzyme activities are shown as Supplemental Data. B, PCA showing sample positions in the plane delineated by PC1 and PC2 gathering 74% of the total variance. Projection of variables (activities) on the same plane is shown as Supplemental Data. Lines link the different treatments at the same time after the onset of water deficit (indicated as 7, 10, 16, or 24 d). Arrows show main trends related to development or to water deficit. AGPase, ADP-Glc pyrophosphorylase; AlaAT, Ala aminotransferase; AspAT, Asp aminotransferase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; GS, Gln synthetase; GK, glycerate kinase; MDH, malate dehydrogenase; PEPc, phosphoenolpyruvate carboxylase; PFK, phosphofructokinase; PGM, phosphoglucomutase; PGI, phosphoglucose isomerase; PK, pyruvate kinase; CS, citrate synthase; G6PDH, Glc-6-P dehydrogenase; GluDH, Glu dehydrogenase; ICDH, isocitrate dehydrogenase; INV, acid invertase; NR, nitrate reductase; p, plastidial; PGK, phosphoglycerokinase; SCL, succinyl-CoA ligase; SS, Suc synthase; c, cytosolic activity; i, initial activity; t, total activity.
To further provide insights into enzyme activity changes with water deficit and developmental stages, a principal component analysis (PCA) was performed using samples as individuals and enzyme activities as variables (Fig. 5B). Principal components (PC) 1 and 2 represented 55% and 19% of the total variance, respectively, whereas PC3 accounted for less than 10% and was not further considered. PC1 and PC2 were mainly associated with enzymes from clusters A and B, respectively (see position of enzymes on the first PCA plane in Supplemental Fig. S3C), whereas enzymes from cluster C were located halfway between PC1 and PC2. The PCA clearly discriminated between the effects of water deficit and the effects of the developmental stage, with samples projected in opposite directions on the axis (Fig. 5B). Moreover, shifts in sample position induced by water deficit were parallel at the four dates but much larger at 16 and 24 d than at 10 or 7 d after the onset of water deficit. This suggests that water deficit had a consistent influence on enzyme activities that increased with time.
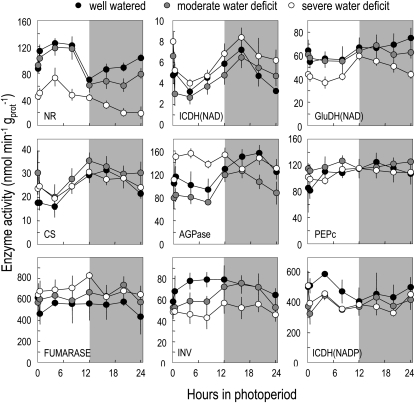
As for metabolites, a detailed time course analysis was then performed 10 d after the onset of water deficit to investigate whether water deficit affects daily fluctuations in a subset of enzymes. Under nonstressing conditions, most enzymes were stable throughout the day/night cycle (Fig. 6). Nitrate reductase and ADP-Glc pyrophosphorylase (AGPase) showed rather large fluctuations, as previously seen. NAD-isocitrate dehydrogenase and citrate synthase also showed significant fluctuations, both of them tending to decrease during the day and recover at night. Under moderate water deficit, most activities had similar patterns to those found in controls, while under severe water deficit, a few enzymes showed a different behavior. Nitrate reductase activity was permanently lower. The diurnal changes in AGPase activity, which are known to increase under C starvation (Gibon et al., 2004a, 2004b), were abolished. Fumarase was slightly increased, suggesting a connection with the accumulation of fumarate under water deficit. Finally, both acidic invertase and Glu dehydrogenase activities, which are known to be induced by C starvation (Gibon et al., 2004b), were decreased by severe water deficit.
Figure 6.
Impact of water deficit on the activities of a set of nine enzymes 10 d after the onset of water deficit along a 24-h cycle. Means ± sd of four to five biological replicates are shown. Shaded areas refer to night periods. Symbol colors are as follows: black, well watered; gray, moderate water deficit; white, severe water deficit. AGPase, ADP-Glc pyrophosphorylase; CS, citrate synthase; GluDH, Glu dehydrogenase; ICDH, isocitrate dehydrogenase; INV, acid invertase; NR, nitrate reductase; PEPc, phosphoenolpyruvate carboxylase.
Water Deficit Influences the Expression of Sugar-Responsive Genes
In order to further evaluate to what extent water deficit would affect the C status of the plant, we studied the expression of 20 sugar-responsive genes, which were selected on the basis of their response to sugar levels (Bläsing et al., 2005; Osuna et al., 2007). The list included three genes encoding Glu dehydrogenase and three genes encoding cell wall, acidic, or neutral invertase, because previous data suggest regulation by C status (Gibon et al., 2004b).
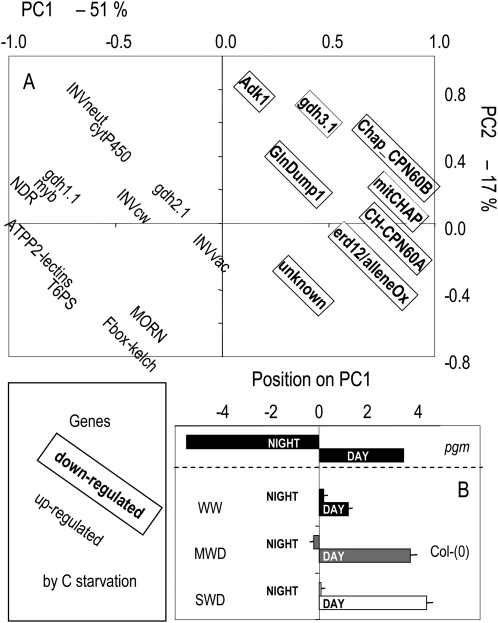
Expression was assayed in samples collected from plants 10 d after the onset of moderate or severe water deficit at the end or at the beginning of the photoperiod. To provide an overview of the results, a PCA was performed (Fig. 7). PC1 accounted for more than 50% of the total variance, while PC2 and PC3 showed much lower contributions (17% and 8%, respectively). Very clearly, all genes located on the left-hand side (respectively right-hand side) of PC1 are those that were chosen because they were induced (respectively repressed) by low sugar levels. This suggests that the sugar content of the cells is the main driving force of expression of these genes in our conditions and that PC1 can be viewed as a C-status axis. This view was further confirmed when samples from the starchless phosphoglucomutase (pgm) mutant that is experiencing severely negative sugar status during the night were projected in the PCA analysis (Fig. 7B). Ecotype Columbia coordinates on PC1 were also higher at the end of the day than at the end of the night, but variation was much less than in the pgm mutant. Interestingly, coordinates at the end of the day were higher in stressed plants than in well-watered plants, while night coordinates remained globally unaltered.
Figure 7.
Impact of water deficit on the expression levels of a set of 20 sugar-responsive genes. Genes were chosen a priori from previous studies (Bläsing et al., 2005; Osuna et al., 2007). GenBank numbers and detailed annotations are given in Supplemental Table S2 together with primer sequences. PCA was performed on expression levels in 18 samples (two time points at three levels of soil water deficit × three biological replicates). A, Position of each variable (expression of each single gene) within the space delineated by the first two axes and gathering 68% of total variance. Genes known to be up- and down-regulated by C starvation are shown without and with frames, respectively. PC1 separates up-regulated from down-regulated genes and thus defines a sugar status axis. B, Mean ± sd position of each sample on PC1 at the end of the photoperiod (day) and the end of the dark period (night) at the three levels of soil water content. Day and night coordinates along PC1 of the starchless mutant pgm are also shown.
DISCUSSION
Several Lines of Evidence Support the View That the C Status Increases under Water Deficit
The initial question addressed in our study was whether drought leads to C shortage due to reduced photosynthesis, the diversion of C for osmotic adjustment, or other alterations in metabolism. Several independent lines of evidence suggest that, at least under our conditions, drought does not lead to acute C shortage but, rather, to an increase of the availability of C.
First, the daily C balance (i.e. the amount of C left available in the rosette after the requirements for growth and respiration are fulfilled) was strongly increased by water deficit. The reason was a consistent maintenance of photosynthesis under water deficit, in accordance with at least part of the literature (Kaiser, 1987; Quick et al., 1992; Bogeat-Triboulot et al., 2007), no drift in respiration (McCree et al., 1984), and a strong reduction in leaf expansion (which is a commonly observed drought syndrome; Chaves et al., 2002; Granier et al., 2006). Such a discrepancy of sensitivities to water deficit between photosynthesis and growth has been known for decades to be the rule for various crop species such as maize, soybean (Glycine max), and sunflower (Helianthus annuus; Boyer 1970) and has been reported in several studies since then (for examples, see Tardieu et al., 1999), including tree species (Bogeat-Triboulot et al., 2007). A different response could occur under conditions of acute water deficit, which result in stomatal closure, a long-term drop in photosynthesis, and ultimately C starvation (McDowell et al., 2008). In our conditions, we did not observe any drop in photosynthesis (on an area basis) even at later stages of water deficit (F. Pantin, unpublished data), at least at the growth light intensity. Since photosynthesis at saturating light was reduced at severe water deficit, a different response might occur under more variable light conditions. Moreover, because specific leaf area was rapidly decreased by water deficit, photosynthesis on a dry weight basis was decreased. Over the long term, this could contribute to rendering the plant C balance less favorable.
Second, C metabolite concentrations and their turnover were higher under water deficit. This is in agreement with several earlier studies (Sharp and Davies, 1979; Kim et al., 2000) and gives the picture of metabolites not being immobilized by osmotic adjustment (see below).
Third, enzymatic and molecular evidence argue for an increased C status under water deficit. The activities of both invertase and Glu dehydrogenase are known to increase under C starvation (Gibon et al., 2006). The latter is thought to be particularly important to fuel the TCA cycle upon C starvation (Miyashita and Good, 2008). Both along development and during a 24-h cycle, our results show opposite tendencies with water deficit, suggesting an improvement of the C status. Leaf AGPase activity fluctuates between day and night (Jeannette and Prioul, 1994), with a sharp drop at the beginning of the day that is more dramatic in the starchless mutant pgm, probably as a result of C starvation occurring at night (Gibon et al., 2004a, 2004b). These fluctuations were abolished under severe water deficit. Finally, an increase in C status during the day, but not at night, was independently suggested by profiling the expression of a subset of sugar-responsive genes. This might be related to the stronger reduction in leaf expansion during the day, eventually resulting in higher sugars, while growth at night was less affected.
No Sign of Massive Reprogramming of C Metabolism in Response to Water Deficit
When assayed under optimal conditions, enzyme activities reflect enzyme capacities and/or protein levels (Piques et al., 2009). Thus, changes of their activities during water deficit give an evaluation of the reprogramming of C metabolism under drought. Our results show (1) that most enzyme activities were maintained or increased under water deficit, when expressed on a protein or a dry weight basis, with only a few exceptions (invertase, nitrate reductase), and (2) that the impact of water deficit became more obvious as the duration of the stress increased. The first result suggests that large changes of metabolite concentrations and diurnal patterns observed as early as 7 to 10 d after the onset of stress do not require an in-depth reprogramming of metabolism. The second result cannot be explained by the progressive establishment of stress, as water deficit was rapidly imposed and maintained at a constant level thereafter. Moreover, 7 d after the onset of water deficit, steady-state drought had already been established for 3 to 4 d, whereas estimated half-times for the synthesis of most enzymes in central metabolism are in this range (Piques et al., 2009). A possible interpretation could thus be that changes in enzyme activities mainly occurred in the tissues and organs that were formed after stress onset, which were proportionally more abundant after 24 d than after 7 d of stress. The composition of tissues produced that is likely to change with water deficit (e.g. increased wall thickness; Bogeat-Triboulot et al., 2007) could be another reason for this temporal drift. Thus, similar to results found in Arabidopsis after transfer to 4°C (Strand et al., 1999), our results suggest that the enzymatic machinery of preexisting tissues would respond only marginally to water deficit, whereas tissues that are produced under water deficit would show larger changes.
Our results strongly differ from a part of the literature that suggests major reprogramming and/or global down-regulation of C metabolism under water deficit (Chaves et al., 2002; Ozturk et al., 2002). However, several of these studies were performed by rapidly imposing drought or desiccation through immersion in osmotica (Kreps et al., 2002), drying out of the plants (Seki et al., 2002), or other drastic protocols (Rabbani et al., 2003; Kilian et al., 2007). It is now well acknowledged that “omics” results from such experiments show little overlap with those obtained by progressive water deficit, suggesting that different processes are engaged in these two contrasting situations (Bray, 2004). Omics studies in which soil water deficit was progressively established and characterized tend to suggest subtle changes rather than massive reprogramming of transcription networks or C metabolism (see Yu and Setter, 2003, in maize; Bogeat-Triboulot et al., 2007, in poplar [Populus spp.]; and Cramer et al., 2007, in grape [Vitis vinifera]). In addition, when some enzymes or functions are focused on, several examples of C metabolism enzymes being up-regulated or maintained under water deficit can be found: Rubisco (Cornic, 2000; Flexas et al., 2006; Bogeat-Triboulot et al., 2007); glycolysis and the TCA cycle (Riccardi et al., 1998); and Suc synthase and acid invertase (Pinheiro et al., 2001). In conclusion, in our study, C metabolism is only marginally altered, and there is a general trend toward increased rather than decreased enzyme activities. This fits with a part of the literature in which drought is imposed progressively, but it differs strongly from studies where osmotic shock or rapid desiccation is imposed.
Our results also show that, independent of water deficit, developmental stage exerted a strong influence on enzyme activities from central metabolism. TCA cycle enzymes were among those most greatly influenced by developmental stage. This could be related to the increase in respiration of the rosette at metabolically active stages such as bolting (onset of rapid elongation of the flower stem) when a boost in hexose concentration is observed. Consistently, reports support the idea that flowering is strongly intertwined with alterations of C metabolism (Corbesier et al., 1998).
One clear-cut change of enzyme activity was a strong decrease in nitrate reductase activity. This confirms earlier studies (Foyer et al., 1998). The decrease in nitrate reductase activity is unlikely to be due to decreased induction by nitrate, because nitrate levels remained constant and high until at least 16 d after the onset of water deficit. Another possible explanation could be that the low internal CO2 associated with the observed decreased stomatal conductance contributed to the posttranslational inactivation of nitrate reductase (Kaiser and Brendle-Behnisch, 1991).
The Dual Role of C Metabolites for Daily C Turnover and Osmotic Adjustment
Our results confirm that starch is not the only C form participating in the daily C turnover in Arabidopsis (Gibon et al., 2009). While soluble sugars made only a small contribution, organic acids and amino acids made a substantial contribution to the transient C stores during a diurnal cycle. The contribution of fumarate was as high as half that of starch, as noted previously in Arabidopsis and other species (Chia et al., 2000; Gibon et al., 2009). Under water deficit, the turnover of soluble C metabolites was increased, suggesting that these are readily used for growth, respiration, or export. A marked increase in the overall level and the daily accumulation of Pro was detected, as already pointed out (Hayashi et al., 2000), indicating that in these conditions Pro can have a role as a temporary C storage pool. Globally, a larger transient accumulation of soluble metabolites during the light period is likely to give more flexibility to the plant to accommodate drought by giving these metabolites a dual role as a transient store of C and as actors in osmotic adjustment.
It has been suggested previously that osmotic adjustment represents a large cost in terms of C metabolites sequestered. Our results quantify this sequestration and show that it represents a minor cost compared with the global plant C budget. The C sequestered in low-Mr metabolites was estimated to represent 1% to 3% of the daily photosynthesis under moderate and severe water deficit, respectively. Under severe water deficit, it represented only 10% of the C exported to the roots. Moreover, the accumulation of low-Mr metabolites was favored by the surplus generated by a maintained rate of photosynthesis and a decreased C demand for growth and was not accompanied by increased C lost in respiration. Finally, it is noteworthy that osmotic adjustment was greatly facilitated by the large decrease of the relative water content, which accounted for two-thirds of the drop in osmotic potential. This drop is presumably due to both a decrease in the vacuolar volume of individual cells and overall changes of the leaf structures (increase in thickness and/or density) and possibly cell wall properties to contribute to turgor maintenance.
CONCLUSION
Together, our results suggest the following in Arabidopsis.
(1) Water deficit does not lead to C depletion. We consistently observed the opposite with a set of metabolites accumulating and turning over at a higher rate and a set of markers for C starvation showing an opposite regulation to that expected in C depletion.
(2) A major reason is that growth decreases more than photosynthesis under water deficit, leading to a more favorable C balance. One consequence is an increased surplus of C for root growth.
(3) Several metabolites may have a dual role as transient stores for C and as contributors to osmotic adjustment, and both roles increase under water deficit.
(4) The organic part of the osmotic adjustment represents a low cost, as compared with the other C fluxes.
(5) In-depth reprogramming of the machinery of central metabolism is not necessary for these adjustments.
MATERIALS AND METHODS
Plant Material, Growth Conditions, and Harvests
Arabidopsis (Arabidopsis thaliana Columbia) seeds were sown in 200-mL cylindrical plastic pots filled with a mixture (1:1, v/v) of sieved loamy soil and organic compost. Each pot was weighed daily and its soil water content kept constant at a control level (0.35–0.40 g water g−1 dry soil; corresponding to a predawn water potential of –0.35 MPa) by watering with one-tenth-strength modified Hoagland solution. Eighteen days after sowing, irrigation was stopped on two-thirds of the plants until soil relative water content reached a target value corresponding to a moderate water deficit (0.25 g water g−1 dry soil; corresponding to a predawn water potential of –0.6 MPa) or to a severe water deficit (0.18 g water g−1 dry soil; corresponding to a predawn water potential of –1.1 MPa). Once the target soil water content was reached, after 3 to 5 d, it was kept constant by daily watering until plant harvest using the automaton PHENOPSIS (Granier et al., 2006). Climatic conditions were identical in all experiments, with a 12-h photoperiod, 180 μmol m−2 s−1 light intensity, a 0.8-kPa leaf-to-air vapor pressure deficit, and a mean air temperature of 20°C days and nights. Five seeds per pot were sown and progressively thinned to two homogenous plants by 2 weeks after sowing and ultimately to one plant after another 1 or 2 weeks, depending on individual plant size. We checked that several morphological (e.g. root mass fraction) and physiological (e.g. photosynthesis) parameters were insensitive to plant number per pot providing that rosettes did not overlap (data not shown).
During experiment 1, harvests were performed successively by day 24, 28, 34, or 42 after sowing (7, 10, 16, or 24 d after onset of water deficit) at the end of the photoperiod. The four successive harvests corresponded to the vegetative stage (first two harvests), to the initiation of reproductive organs on the shoot apical meristem, and to bolting. Four independent samples of five to eight whole rosettes per sample were gathered per treatment and immediately frozen in liquid nitrogen. Inflorescences were discarded when visible. Roots were collected and their dry weights measured separately (root mass fraction was calculated as root dry weight/plant dry weight). Experiments 2 to 5 were devoted to 24-h time-course analyses, which were carried out 10 d after the onset of water deficit. Four independent samples of five to eight whole rosettes per sample were collected every 4 h, with a supplemental harvest 30 min after the beginning of the light period. During experiment 6, whole rosettes were harvested 10 d after the onset of water deficit at the end and at the beginning of the photoperiod, frozen under liquid nitrogen, and later used for RNA extraction. Well-watered plants from the starchless mutant pgm were included in this experiment and harvested together with the wild-type Columbia for gene expression analysis.
Growth Analysis and Gas Exchange
In experiment 1, the number of initiated (after dissection with a binocular microscope) and visible leaves was counted on at least six and 12 plants per treatment, respectively. During experiments 1 and 6, eight plants per treatment were harvested at 7, 10, 16, and 24 d after the onset of water deficit and used for the evaluation of dry weight accumulation and relative growth rate (g dry weight g−1 dry weight d−1) as the slope of the log(dry weight) versus time relationship over three time points. During experiments 1, 3, and 6, a top-view photograph of eight to 12 plants per treatment was taken twice daily with a camera to evaluate day and night expansion rates separately. The projected rosette area (AREAros) was measured using a customized adaptation of ImageJ software (http://www.mri.cnrs.fr). From these measurements, the relative expansion rate (mm2 mm−2 d−1) was calculated as the slope of log(AREAros) over 24 or 12 h.
The net CO2 assimilation rate was measured using either a single leaf or a whole plant cuvette, both designed for Arabidopsis and connected to a gas analyzer (CIRAS-2; PP Systems). Measurements were performed under the environmental conditions of the growth chamber either at growth irradiance (180 μmol m−2 s−1) or, in the case of single leaves, at various light intensities provided by a series of light-emitting diodes located above the cuvette. Dark respiration was measured on whole rosettes only using the same protocol on plants kept in the dark for at least 1 h before measurements. Stomatal conductance was measured using a diffusion porometer (AP4; Delta T Device).
Metabolite Analysis and Enzymatic Assays
Glc, Fru, Suc, starch, total soluble protein, Pro, total amino acids, malate, fumarate, and nitrate contents were measured using spectrophotometric analysis of soluble and residual fractions of ethanol-water extracts as described by Cross et al. (2006). Malate and fumarate contents were determined as described by Nunes- Nesi et al. (2007). Pro content was determined using ninhydrin in acidic reaction (adapted from Troll and Lindsley, 1955). Potassium content was measured using an atomic absorption spectrometer as described by Armengaud et al. (2009). For enzymatic assays, 20 mg of frozen rosette powder were extracted as described by Armengaud et al. (2009) and all enzymes activities were measured according to Gibon et al. (2004b, 2006, 2009) and Sulpice et al. (2007). Enzyme activities were measured under optimized conditions and therefore can be assimilated to maximum catalytic activities (Newsholme and Start, 1973). For simplicity, we refer to them in the text as enzyme activities.
Relative Water Content, Specific Leaf Area, and Leaf Thickness
Relative water content, water content, and specific leaf area were determined on both the youngest fully expanded leaves and the whole rosette on eight leaves or rosettes per treatment. Fresh weight was scored immediately after excision, fresh weight after rehydration after the leaf or the rosette was left floating on deionized water at 4°C in the dark for 24 h, and dry weight after the leaf or the rosette was dried in an oven at 70°C for 3 d. Mean leaf thickness was estimated on the youngest fully expanded leaf using a linear displacement transducer (5-mm full scale; Solartron) incorporating a compression spring. This allowed imposing a moderate force to maintain the 0.5-mm2 plate at the end of the moving cylinder of the linear displacement transducer firmly to the leaf but without damaging it. The cylinder was moved to 20 different points throughout the blade of eight leaves, avoiding the central vein.
C Balance
The C balance of the growing rosette was estimated 10 d after the onset of water deficit by expressing the contents and fluxes as CHO eq. g−1 dry weight. Daily net photosynthesis and dark respiration were expressed on a dry weight basis using specific leaf area of the rosette and upscaled assuming that fluxes remain constant during days and nights (Caspar et al., 1985). The CHO demand associated with rosette growth was estimated from relative growth rate data, assuming that the dry matter contained 40% C in Arabidopsis (Hendrik Poorter, personal communication), which is also the C content in major CHOs (hexoses, Suc, starch). The daily C balance of the rosette (i.e. the C potentially exported to roots) was then estimated from the difference between photosynthesis, respiration, and rosette growth (ExportCHO = PhotosynthesisCHO − Dark respirationCHO − GrowthCHO).
C turnover of metabolites was evaluated by subtracting end-of-night concentrations from end-of-day concentrations and summing them based on their C composition. We assumed an average of 4.7 atoms of C per amino acid molecule, according to the amino acid composition in Arabidopsis rosettes grown under similar conditions (Tschoep et al., 2009).
Expression of Concentration on a Saturated Fresh Weight Basis, Leaf Water Potential, Osmotic Potential, and Estimation of Metabolite Contribution to Osmotic Potential
Metabolite concentrations were expressed on a saturated fresh weight basis from concentration on a fresh weight basis using mean relative water content (RWC) and water content (WC) values. Indeed, let W be the mass of water in a tissue and ΔW be the mass of water it incorporates upon rehydration, then RWC = W/(W + ΔW), which gives after reordering ΔW = W × [(1 − RWC)/RWC]. Then, the relationship between saturated fresh weight (FWsat) and fresh weight (FW) is deduced {FWsat/FW = (FW + ΔW)/FW = 1 + [WC × (1 − RWC)/RWC]} and is used to estimate metabolite concentration per saturated fresh weight from metabolite concentration per fresh weight.
Ψw was measured either on excised single leaves or whole rosettes using a Scholander pressure chamber (Soil Moisture Equipment). For determination of osmotic potential, two rosettes were quickly gathered in the top of filter tips inserted into microtubes and immediately frozen in liquid nitrogen. Sap was extracted by centrifugation (10,000g, 5 min). Ten microliters of the resulting sap was analyzed using a vapor pressure osmometer (Wescor Vapro 5520). Osmotic potential (MPa) was deduced from osmolarity (mOsm) using the Van’t Hoff equation at 20°C. Osmotic potential at full turgor was deduced by multiplying osmotic potential and relative water content.
The contribution of metabolites to osmotic potential was estimated from concentrations. Indeed, if n is the summed mole number of soluble metabolites and Osm is the osmolarity, Osm = n/W = n/(FW − DW) and thus 1/Osm = (FW/n) − (DW/n) = (1/CONCFW) − (1/ CONCDW), where CONCDW is the metabolite concentration on a dry weight basis and CONCFW is the metabolite concentration on a fresh weight basis. It comes that Osm = 1/[(1/CONCFW) − (1/ CONCDW)]. The same reasoning was used for the determination of osmolarity at saturation allowed by metabolites.
RNA Extraction and cDNA Synthesis
Tissue samples were ground in liquid nitrogen. Approximately 100 mg was used for RNA extraction using Trizol reagent (Invitrogen) according to the manufacturer’s instructions followed by DNase treatment (RQ1 RNase-free DNase I; Promega). RNA quality and quantity were checked by electrophoresis and optical density at 260 nm. DNase I-treated RNA was subsequently spiked with 150,000 copies of GeneAmplimer pAW109 RNA per μg of total RNA (GeneAmp RNA PCR Control Kit; Applied Biosystems), which served as a control for the cDNA synthesis efficiency and normalization. The cDNA was synthesized from 2 μg of total RNA using SuperScript III reverse transcriptase (Invitrogen) according to the manufacturer’s instructions using random hexamer primers. The cDNAs were diluted to 400 μL. Absence of genomic DNA contamination in the RNA samples, RNA integrity, and reverse transcription processivity were carried out as described by Piques et al. (2009).
Primer Design and Real-Time PCR Analysis
A set of 14 genes was selected on the basis of overexpression or underexpression under conditions of C starvation (either in the pgm mutant or under extended night; Gibon et al., 2006; Usadel et al., 2008b), and three reference genes (GAPDH3, YLS8, and ACT2) were chosen based on their stability under these conditions (Czechowski et al., 2005). Genes coding for Glu dehydrogenase and invertase (three of each) were included in the set given the high susceptibility of the enzyme activities to C starvation. The primers were designed by Eurofins MWG Biotech, and their quality was tested according to Piques et al. (2009). The primer list and specifications are given in Supplemental Table S2.
Real-time PCR was performed on a 384-well plate with an ABI PRISM 7900 HT sequence detection system (Applied Biosystems) using Power SYBR Green PCR Master Mix (Applied Biosystems) according to Piques et al. (2009). The PCR and data analysis were performed as described by Czechowski et al. (2005). The reference genes were used for normalization. Relative expression ratios of target transcripts were then calculated according to Pfaffl (2001). The reference sample was well-watered plants harvested at the end of the night period. All cycle time values are means of four technical replicates. Three to four biological replicates were processed.
Statistical Analysis of Data
All analyses were performed using Statistica software (version 7.0; StatSoft). Differences described in the text are systematically statistically grounded based on ANOVA followed by lsd grouping for most analyses or analysis of covariance (ANCOVA) for comparing relative expansion rate and relative growth rate [using log(AREAros) or log(dry weight) as dependent variables and time after onset of stress as a covariable]. Hierarchical clustering of enzyme data was performed using the Ward method and Euclidean distance. The clustering method had marginal impact on cluster composition. For gene expression analysis, PCA was performed using Columbia data only, but day and night coordinates of the pgm mutant along PC1 were evaluated in well-watered plants harvested in the same experiment that was performed for collecting Columbia samples.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Experimental design and reproducibility of phenotypes.
Supplemental Figure S2. Mean protein content in rosettes during development and a 24-h cycle.
Supplemental Figure S3. Mean enzyme activities of central metabolism as influenced by developmental stage and water deficit.
Supplemental Table S1. Mean enzyme activities of central metabolism as influenced by developmental stage and water deficit.
Supplemental Table S2. Sequences of primers used for real-time PCR.
Supplementary Material
Acknowledgments
The technical help of Jean-Jacques Thioux and Cris Balsera is gratefully acknowledged. Philippe Hamard designed the leaf thickness probe. Eva Pyl helped in selecting sugar-responsive genes. The continuous contribution of Christine Granier into the development of the PHENOPSIS platform is gratefully acknowledged.
References
- Aguirrezabal L, Bouchier-Combaud S, Radziejwoski A, Dauzat M, Cookson SJ, Granier C. (2006) Plasticity to soil water deficit in Arabidopsis thaliana: dissection of leaf development into underlying growth dynamic and cellular variables reveals invisible phenotypes. Plant Cell Environ 29: 2216–2227 [DOI] [PubMed] [Google Scholar]
- Armengaud P, Sulpice R, Miller AJ, Stitt M, Amtmann A, Gibon Y. (2009) Multilevel analysis of primary metabolism provides new insights into the role of potassium nutrition for glycolysis and nitrogen assimilation in Arabidopsis roots. Plant Physiol 150: 772–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bläsing OE, Gibon Y, Gunther M, Hohne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible WR, Stitt M. (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 17: 3257–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogeat-Triboulot MB, Brosche M, Renaut J, Jouve L, Le Thiec D, Fayyaz P, Vinocur B, Witters E, Laukens K, Teichmann T. (2007) Gradual soil water depletion results in reversible changes of gene expression, protein profiles, ecophysiology, and growth performance in Populus euphratica, a poplar growing in arid regions. Plant Physiol 143: 876–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer JS. (1970) Differing sensitivity of photosynthesis to low leaf water potentials in corn and soybean. Plant Physiol 46: 236–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray EA. (2004) Genes commonly regulated by water-deficit stress in Arabidopsis thaliana. J Exp Bot 55: 2331–2341 [DOI] [PubMed] [Google Scholar]
- Caspar T, Huber SC, Somerville C. (1985) Alterations in growth, photosynthesis, and respiration in a starchless mutant of Arabidopsis thaliana (L.) deficient in chloroplast phosphoglucomutase activity. Plant Physiol 79: 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osorio ML, Carvalho I, Faria T, Pinheiro C. (2002) How plants cope with water stress in the field? Photosynthesis and growth. Ann Bot (Lond) 89: 907–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia DW, Yoder TJ, Reiter WD, Gibson SI. (2000) Fumaric acid: an overlooked form of fixed carbon in Arabidopsis and other plant species. Planta 211: 743–751 [DOI] [PubMed] [Google Scholar]
- Clifford S, Arndt S, Corlett J, Joshi S, Sankhla N, Popp M, Jones H. (1998) The role of solute accumulation, osmotic adjustment and changes in cell wall elasticity in drought tolerance in Ziziphus mauritiana (Lamk.). J Exp Bot 49: 967–977 [Google Scholar]
- Corbesier L, Lejeune P, Bernier G. (1998) The role of carbo-hydrates in the induction of flowering in Arabidopsis thaliana: comparison between the wild type and a starchless mutant. Planta 206: 131–137 [DOI] [PubMed] [Google Scholar]
- Cornic G. (2000) Drought stress inhibits photosynthesis by decreasing stomatal aperture—not by affecting ATP synthesis. Trends Plant Sci 5: 187–188 [Google Scholar]
- Cornic G, le Gouallec JL, Briantais JM, Hodges M. (1989) Effect of a high light treatment during a drought stress on photosynthetic capacities of two C3 plants: Phaseolus vulgaris and Elatostema repens. Planta 177: 84–90 [DOI] [PubMed] [Google Scholar]
- Cramer G, Ergül A, Grimplet J, Tillett R, Tattersall E, Bohlman M, Vincent D, Sonderegger J, Evans J, Osborne C, et al. (2007) Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Funct Integr Genomics 7: 111–134 [DOI] [PubMed] [Google Scholar]
- Cross JM, von Korff M, Altmann T, Bartzetko L, Sulpice R, Gibon Y, Palacios N, Stitt M. (2006) Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions. Plant Physiol 142: 1574–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuming AC, Cho SH, Kamisugi Y, Graham H, Quatrano RS. (2007) Microarray analysis of transcriptional responses to abscisic acid and osmotic, salt, and drought stress in the moss, Physcomitrella patens. New Phytol 176: 275–287 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible W. (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley SA. (1996) Differing selection on plant physiological traits in response to environmental water availability: a test of adaptative hypothesis. Evolution 50: 92–102 [DOI] [PubMed] [Google Scholar]
- Farquhar G, Sharkey T. (1982) Stomatal conductance and photosynthesis. Annu Rev Plant Physiol 33: 317–345 [Google Scholar]
- Flexas J, Ribas-Carbo M, Bota J, Galmes J, Henkle M, Martinez-Canellas S, Medrano H. (2006) Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol 172: 73–82 [DOI] [PubMed] [Google Scholar]
- Foyer CH, Valadier MH, Migge A, Becker TW. (1998) Drought-induced effects on nitrate reductase activity and mRNA and on the coordination of nitrogen and carbon metabolism in maize leaves. Plant Physiol 117: 283–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresneau C, Ghashghaie J, Cornic G. (2007) Drought effect on nitrate reductase and sucrose-phosphate synthase activities in wheat (Triticum durum L.): role of leaf internal CO2. J Exp Bot 58: 2983–2992 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Blaesing O, Palacios-Rojas N, Pankovic D, Hendriks JHM, Fisahn J, Höhne M, Günther M, Stitt M. (2004a) Adjustment of diurnal starch turnover to short days: depletion of sugar during the night leads to a temporary inhibition of carbohydrate utilization, accumulation of sugars, and posttranslational activation of ADP glucose pyrophosphorylase in the following light period. Plant J 39: 847–862 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Blaesing OE, Hannemann J, Carillo P, Hohne M, Hendriks JHM, Palacios N, Cross J, Selbig J, Stitt M. (2004b) A robot-based platform to measure multiple enzyme activities in Arabidopsis using a set of cycling assays: comparison of changes of enzyme activities and transcript levels during diurnal cycles and in prolonged darkness. Plant Cell 16: 3304–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Pyl E, Sulpice R, Lunn JE, Höhne M, Günther M, Stitt M. (2009) Adjustment of growth, starch turnover, protein content and central metabolism to a decrease of the carbon supply when Arabidopsis is grown in very short photoperiods. Plant Cell Environ 32: 859–874 [DOI] [PubMed] [Google Scholar]
- Gibon Y, Usadel B, Blaesing OE, Kamlage B, Hoehne M, Trethewey R, Stitt M. (2006) Integration of metabolite with transcript and enzyme activity profiling during diurnal cycles in Arabidopsis rosettes. Genome Biol 7: R76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girousse C, Bournoville R, Bonnemain JL. (1996) Water deficit-induced changes in concentrations in proline and some other amino acids in the phloem sap of alfalfa. Plant Physiol 111: 109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granier C, Aguirrezabal L, Chenu K, Cookson SJ, Dauzat M, Hamard P, Thioux J, Rolland G, Bouchier-Combaud S, Lebaudy A, et al. (2006) PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit. New Phytol 169: 623–635 [DOI] [PubMed] [Google Scholar]
- Hare PD, Cress WA, Van Staden J. (1998) Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 21: 535–553 [Google Scholar]
- Hayashi F, Ichino T, Osanai M, Wada K. (2000) Oscillation and regulation of proline content by P5CS and ProDH gene expressions in the light/dark cycles in Arabidopsis thaliana L. Plant Cell Physiol 41: 1096–1101 [DOI] [PubMed] [Google Scholar]
- Huber SC, Rogers HH, Mowry FL. (1984) Effects of water stress on photosynthesis and carbon partitioning in soybean (Glycine max [L.] Merr.) plants grown in the field at different CO2 levels. Plant Physiol 76: 244–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannette E, Prioul J. (1994) Variations of ADPglucose pyrophosphorylase activity from maize leaf during day/night cycle. Plant Cell Physiol 35: 869–878 [Google Scholar]
- Kaiser WM. (1987) Effects of water deficit on photosynthetic capacity. Physiol Plant 71: 142–149 [Google Scholar]
- Kaiser WM, Brendle-Behnisch E. (1991) Rapid modulation of spinach leaf nitrate reductase activity by photosynthesis. I. Modulation in vivo by CO2 availability. Plant Physiol 96: 363–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian J, Whitehead D, Horak J, Wanke D, Weinl S, Batistic O, D’Angelo C, Bornberg-Bauer E, Kudla J, Harter K. (2007) The AtGenExpress global stress expression data set: protocols, evaluation and model data analysis of UV-B light, drought and cold stress responses. Plant J 50: 347–363 [DOI] [PubMed] [Google Scholar]
- Kim JY, Mahé A, Brangeon J, Prioul JL. (2000) A maize vacuolar invertase, IVR2, is induced by water stress: organ/tissue specificity and diurnal modulation of expression. Plant Physiol 124: 71–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreps JA, Wu Y, Chang HS, Zhu T, Wang X, Harper JF. (2002) Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol 230: 2129–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Jensen CR, Andersen MN. (2004) Drought stress effect on carbohydrate concentration in soybean leaves and pods during early reproductive development: its implication in altering pod set. Field Crops Res 86: 1–13 [Google Scholar]
- McCree KJ, Kallsen CE, Richardson SG. (1984) Carbon balance of sorghum plants during osmotic adjustment to water stress. Plant Physiol 76: 898–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell N, Pockman WT, Allen CD, Breshears DD, Cobb N, Kolb T, Plaut J, Sperry J, West A, Williams DG. (2008) Mechanisms of plant survival and mortality during drought: why do some plants survive while others succumb to drought? New Phytol 178: 719–739 [DOI] [PubMed] [Google Scholar]
- McLaughlin JE, Boyer JS. (2004) Sugar-responsive gene expression, invertase activity, and senescence in aborting maize ovaries at low water potentials. Ann Bot (Lond) 94: 675–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita Y, Good AG. (2008) NAD(H)-dependent glutamate dehydrogenase is essential for the survival of Arabidopsis thaliana during dark-induced carbon starvation. J Exp Bot 59: 667–680 [DOI] [PubMed] [Google Scholar]
- Morgan J. (1992) Osmotic components and properties associated with genotypic differences in osmoregulation in wheat. Aust J Plant Physiol 19: 67–76 [Google Scholar]
- Muller B, Bourdais G, Reidy B, Bencivenni C, Massonneau A, Condamine P, Rolland G, Conejero G, Rogowsky P, Tardieu F. (2007) Association of specific expansins with growth in maize leaves is maintained under environmental, genetic, and developmental sources of variation. Plant Physiol 143: 278–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller B, Reymond M, Tardieu F. (2001) The elongation rate at the base of a maize leaf shows an invariant pattern during both the steady-state elongation and the establishment of the elongation zone. J Exp Bot 52: 1259–1268 [PubMed] [Google Scholar]
- Neumann Andersen M, Asch F, Wu Y, Jensen CR, Naested H, Mogensen VO, Koch KE. (2002) Soluble invertase expression is an early target of drought stress during the critical, abortion-sensitive phase of young ovary development in maize. Plant Physiol 130: 591–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme EA, Start C. (1973) Regulation in Metabolism. John Wiley & Sons, London [Google Scholar]
- Nunes-Nesi A, Carrari F, Gibon Y, Sulpice R, Lytovchenko A, Fisahn J, Graham J, Ratcliffe RG, Sweetlove LJ, Fernie AR. (2007) Deficiency of mitochondrial fumarase activity in tomato plants impairs photosynthesis via an effect on stomatal function. Plant J 50: 1093–1106 [DOI] [PubMed] [Google Scholar]
- Osuna D, Usadel B, Morcuende R, Gibon Y, Blasing OE, Hohne M, Gunter M, Kamlage B, Trethewey R, Scheible WR. (2007) Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. Plant J 49: 463–491 [DOI] [PubMed] [Google Scholar]
- Ozturk ZN, Talame V, Deyholos M, Michalowski CB, Galbraith DW, Gozukirmizi N, Tuberosa R, Bohnert HJ. (2002) Monitoring large-scale changes in transcript abundance in drought- and salt-stressed barley. Plant Mol Biol 48: 551–573 [DOI] [PubMed] [Google Scholar]
- Passioura JB. (1996) Drought and drought tolerance. Plant Growth Regul 20: 79–83 [Google Scholar]
- Pfaffl MW. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: 2002–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro C, Chaves MM, Ricardo CP. (2001) Alterations in carbon and nitrogen metabolism induced by water deficit in the stems and leaves of Lupinus albus L. J Exp Bot 52: 1063–1070 [DOI] [PubMed] [Google Scholar]
- Piques M, Schulze WX, Hohne M, Usadel B, Gibon Y, Rohwer J, Stitt M. (2009) Ribosome and transcript copy numbers, polysome occupancy and enzyme dynamics in Arabidopsis. Mol Syst Biol 5: 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomion C, Lalanne C, Claverol S, Meddour H, Kohler A, Bogeat-Triboulot M, Barre A, Le Provost G, Dumazet H, Jacob D, et al. (2006) Mapping the proteome of poplar and application to the discovery of drought-stress responsive proteins. Proteomics 6: 6509–6527 [DOI] [PubMed] [Google Scholar]
- Quan R, Shang M, Zhang H, Zhao Y, Zhang J. (2004) Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. Plant Biotechnol J 2: 477–486 [DOI] [PubMed] [Google Scholar]
- Quick WP, Chaves MM, Wendler R, David M, Rodrigues ML, Passaharinho JA, Pereira JS, Adcock MD, Leegood RC, Stitt M. (1992) The effect of water stress on photosynthetic carbon metabolism in four species grown under field conditions. Plant Cell Environ 15: 25–35 [Google Scholar]
- Quick WP, Siegl G, Neuhaus HE, Feil R, Stitt M. (1989) Short term water stress leads to a stimulation of sucrose synthesis by activating sucrose phosphate synthase. Planta 177: 536–546 [DOI] [PubMed] [Google Scholar]
- Rabbani MA, Maruyama K, Abe H, Khan MA, Katsura K, Ito Y, Yoshiwara K, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. (2003) Monitoring expression profiles of rice genes under cold, drought and high salinity stresses and abscisic acid application using cDNA microarray and RNA gel blot analyses. Plant Physiol 133: 1755–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond M, Muller B, Leonardi A, Charcosset A, Tardieu F. (2003) Combining quantitative trait loci analysis and an ecophysiological model to analyze the genetic variability of the responses of maize leaf growth to temperature and water deficit. Plant Physiol 131: 664–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi F, Gazeau P, de Vienne D, Zivy M. (1998) Protein changes in response to progressive water deficit in maize: quantitative variation and polypeptide identification. Plant Physiol 117: 1253–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero C, Bellés JM, Vayá JL, Serrano R, Culiáñez-Macià FA. (1997) Expression of the yeast trehalose-6-phosphate synthase gene in transgenic tobacco plants: pleiotropic phenotypes include drought tolerance. Planta 201: 293–297 [DOI] [PubMed] [Google Scholar]
- Sadok W, Naudin P, Boussuge B, Muller B, Welcker C, Tardieu F. (2007) Leaf growth rate per unit thermal time follows QTL-dependent daily patterns in hundreds of maize lines under naturally fluctuating conditions. Plant Cell Environ 30: 135–146 [DOI] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninci P, Hayashizaki Y, Shinozaki K. (2002) Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J 31: 279–292 [DOI] [PubMed] [Google Scholar]
- Sharp RE, Davies WJ. (1979) Solute regulation and growth by roots and shoots of water-stressed maize plants. Planta 147: 43–49 [DOI] [PubMed] [Google Scholar]
- Strand A, Hurry V, Henkes S, Huner N, Gustafsson P, Gardestrom P, Stitt M. (1999) Acclimation of Arabidopsis leaves developing at low temperatures: increasing cytoplasmic volume accompanies increased activities of enzymes in the Calvin cycle and in the sucrose-biosynthesis pathway. Plant Physiol 119: 1387–1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulpice R, Tschoep H, von Korff M, Büssis D, Usadel B, Höhne M, Witucka-Wall H, Altmann T, Stitt M, Gibon Y. (2007) Description and applications of a rapid and sensitive non-radioactive microplate-based assay for maximum and initial activity of D-ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Cell Environ 30: 1163–1175 [DOI] [PubMed] [Google Scholar]
- Tardieu F, Granier C, Muller B. (1999) Modelling leaf expansion in a fluctuating environment: are changes in specific leaf area a consequence of changes in expansion rate? New Phytol 143: 33–43 [Google Scholar]
- Tardieu F, Reymond M, Hamard P, Granier C, Muller B. (2000) Spatial distributions of expansion rate, cell division rate and cell size in maize leaves: a synthesis of the effects of soil water status, evaporative demand and temperature. J Exp Bot 51: 1505–1514 [DOI] [PubMed] [Google Scholar]
- Tisné S, Schmalenbach I, Reymond M, Dauzat M, Pervent M, Vile D, Granier C. (July 16, 2010) Keep on growing under drought: genetic and developmental bases of the response of rosette area using a recombinant inbred line population. Plant Cell Environ http://dx.doi.org/10.1111/j.1365-3040.2010.02191.x [DOI] [PubMed]
- Troll W, Lindsley J. (1955) A photometric method for the determination of proline. J Biol Chem 215: 655–660 [PubMed] [Google Scholar]
- Tschoep H, Gibon Y, Carillo P, Armengaud P, Szecowka M, Nunes-Nesi A, Fernie AR, Koehl K, Stitt M. (2009) Adjustment of growth and central metabolism to a mild but sustained nitrogen-limitation in Arabidopsis. Plant Cell Environ 32: 300–318 [DOI] [PubMed] [Google Scholar]
- Usadel B, Blasing O, Gibon Y, Poree F, Hohne M, Gunter M, Trethewey R, Kamlage B, Poorter H, Stitt M. (2008a) Multilevel genomic analysis of the response of transcripts, enzyme activities and metabolites in Arabidopsis rosettes to a progressive decrease of temperature in the non-freezing range. Plant Cell Environ 31: 518–547 [DOI] [PubMed] [Google Scholar]
- Usadel B, Blasing OE, Gibon Y, Retzlaff K, Hohne M, Gunther M, Stitt M. (2008b) Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol 146: 1834–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welcker C, Boussuge B, Bencivenni C, Ribaut J-M, Tardieu F. (2007) Are source and sinks strengths genetically linked in maize plants subjected to water deficit? A QTL study of the responses of leaf growth and of anthesis-silking interval to water deficit. J Exp Bot 58: 339–349 [DOI] [PubMed] [Google Scholar]
- Widodo W, Vu JCV, Boote KJ, Baker JT, Allen LH. (2003) Elevated growth CO2 delays drought stress and accelerates recovery of rice leaf photosynthesis. Environ Exp Bot 49: 259–272 [Google Scholar]
- Xue GP, McIntyre CL, Glassop D, Shorter R. (2008) Use of expression analysis to dissect alterations in carbohydrate metabolism in wheat leaves during drought stress. Plant Mol Biol 67: 197–214 [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang J, Wang Z, Zhu Q, Liu L. (2003) Activities of enzymes involved in sucrose-to-starch metabolism in rice grains subjected to water stress during filling. Field Crops Res 81: 69–81 [Google Scholar]
- Yu L, Setter TL. (2003) Comparative transcriptional profiling of placenta and endosperm in developing maize kernels in response to water deficit. Plant Physiol 131: 568–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zrenner R, Stitt M. (1991) Comparison of the effect of rapidly and gradually developing water-stress on carbohydrate metabolism in spinach leaves. Plant Cell Environ 14: 939–946 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.