Abstract
The pistil is the specialized plant organ that enables appropriate pollination and ovule fertilization, after which it undergoes growth and differentiation to become a fruit. However, in most species, if ovules are not fertilized around anthesis the pistil irreversibly loses its growth capacity. We used physiological, molecular, and transcriptomic tools to characterize the post-anthesis development of the unfertilized Arabidopsis (Arabidopsis thaliana) pistil. Surprisingly, developmental processes that have been previously described in developing Arabidopsis fruits, such as the collapse of the adaxial epidermis, differentiation of a sclerenchyma layer in the adaxial subepidermis and the dehiscence zone, and valve dehiscence, were also observed in the unfertilized pistil. We determined that senescence is first established in the transmitting tract, stigma, and ovules immediately after anthesis, and that the timing of senescence in the stigma and ovules correlates with the loss of fruit-set responsiveness of the pistil to pollen and the hormone gibberellin (GA), respectively. Moreover, we showed that mutants with altered ovule development have impaired fruit-set response to the GA gibberellic acid, which further indicates that the presence of viable ovules is required for fruit-set responsiveness to GAs in the unfertilized pistil. Our data suggest that a fertilization-independent developmental program controls many of the processes during post-anthesis development, both in unfertilized pistils and seeded fruits, and point to a key role of the ovule in the capacity of pistils to undergo fruit set in response to GA.
The pistil is the female reproductive organ of angiosperms. After receiving the appropriate stimulus at maturity, it undergoes growth and differentiation to form the mature fruit. However, the autonomous or default developmental program of the pistil is not fruit set, but rather senescence (Vercher et al., 1984; van Doorn and Woltering, 2008). In other words, the pistil requires an external signal, generally fertilization of the ovules, to develop into a fruit. Despite the fact that fruit development has been the focus of extensive research (Ferrandiz et al., 1999; Roeder and Yanofsky, 2006), the post-anthesis development of the unfertilized pistil has received little attention. Senescence of floral organs, including the pistil, is finely regulated (van Doorn and Woltering, 2008) and the life span of the pistil, as with other floral organs, is species specific and varies from hours to weeks, usually as an adaptation to the ecological requirements (Primack, 1985; Rogers et al., 2006).
The identification of the physiological and molecular factors regulating pistil senescence is an important goal, because once it is initiated, the capacity of the pistil to develop as a fruit is lost (Garcia-Martinez and Carbonell, 1980). Indeed, increased pistil longevity is a desirable agronomic trait, since it can be a limiting factor for sexual reproduction and fruit production, particularly in self-incompatible species (Yi et al., 2003, 2006; Huang et al., 2004; Hedhly et al., 2005). This is particularly topical given the growing concerns about global warming and changing crop management practices (Yi et al., 2003; Hedhly et al., 2005), which may reduce pistil receptivity and decimate pollinator populations (Kremen et al., 2002, 2007; Hegland et al., 2009).
Another unanswered question is the nature of the mechanisms that terminate the pistil senescence program and induce fruit set. It has been hypothesized that growth regulators produced in fertilized ovules are key factors that determine pistil fate (Gustafson, 1936, 1950) and in this regard, treatment of unpollinated pistils with auxin or gibberellin (GA) can induce parthenocarpic fruit set even in the absence of seeds in many species (Gustafson, 1936; Rappaport, 1956), including Arabidopsis (Arabidopsis thaliana; Vivian-Smith and Koltunow, 1999). More recently, additional evidence has been reported for a link between fertilization and GA biosynthesis in the ovary, mediated by auxins produced in fertilized ovules (Serrani et al., 2008; Dorcey et al., 2009).
Senescence of plant organs is a highly ordered developmental program that eventually leads to death, while enabling nutrient recycling (Reape and McCabe, 2008). Senescence in plants has been extensively studied in vegetative organs, mainly in leaves, and in reproductive organs, especially in petals (Gan, 2007). Microarray-based transcript profiling studies have led to the identification of genes with potential roles in the regulation and execution of senescence (Guo et al., 2004; Buchanan-Wollaston et al., 2005; van der Graaff et al., 2006; Wagstaff et al., 2009), and enhancer trap lines (He et al., 2001) and microarray analyses (Wagstaff et al., 2009) have identified genes induced during leaf senescence that are also expressed in senescent flowers, stems, and siliques, showing that most of the senescence-associated genes (SAGs) are not tissue specific.
Pistil senescence has only been extensively studied in pea (Pisum sativum), where senescence of unpollinated pistils is initiated 2 to 3 d post anthesis (DPA), and by 5 to 6 DPA pistils are fully senescent (Garcia-Martinez and Carbonell, 1980; Vercher and Carbonell, 1991). The onset of senescence correlates with the expression of proteolytic activities (Carrasco and Carbonell, 1988, 1990; Granell et al., 1992) and the loss of capacity to develop into a fruit in response to exogenous gibberellic acid (GA3), which constitutes a good marker for senescence initiation (Carbonell and García-Martínez, 1985). The unpollinated Arabidopsis pistil is also able to develop into a parthenocarpic fruit in response to exogenous application of plant hormones, especially GA, at anthesis but it later loses that capacity (Vivian-Smith and Koltunow, 1999). However, no association of this loss with senescence has been established.
This study is aimed at characterizing the events occurring in the unfertilized Arabidopsis pistil following anthesis, and their relationship with the loss of fruit-set capacity.
RESULTS AND DISCUSSION
Anatomy of the Unfertilized Pistil during Its Post-Anthesis Development
To study unfertilized pistil post-anthesis development in Arabidopsis we used the eceriferum6 (cer6-2) mutant (Preuss et al., 1993; Fiebig et al., 2000), taking advantage of its male conditional sterility to avoid fertilization. This mutant has been successfully used to study fruit set (Vivian-Smith et al., 2001; Dorcey et al., 2009). Under our experimental conditions, at low humidity, Landsberg erecta (Ler) pollen germinated on cer6-2 stigma, while pollen from cer6-2 did not (Supplemental Fig. S1; Preuss et al., 1993). cer6-2 plants did not show any other morphological difference from the parental Ler (data not shown; Preuss et al., 1993).
Unfertilized pistils grew during the first 4 to 5 DPA and remained green until 18 DPA (Fig. 1), after which they yellowed, reflecting advanced senescence. At around 20 to 22 DPA, we observed dehiscence of the valves, a process that has previously been considered a fruit-specific trait for seed dispersal. It has been reported that unfertilized pistils fail to dehisce (Vivian-Smith and Koltunow, 1999; Goetz et al., 2006), but we clearly observed valve dehiscence in unfertilized pistils. We then characterized the morphological changes during post-anthesis unfertilized pistil development and compared them with those of the seeded fruit, focusing on the landmark events of flower/fruit development described previously (Smyth et al., 1990; Ferrandiz et al., 1999). Stages 14 to 20 correspond to fruit development, from pollination to pod shattering and seed dispersal. Overall, we observed a delay in unfertilized pistil development, ranging from several hours (stage 14, long stamens grow above the stigma) to 6 d (stage 19, valves dehisce; Table I).
Figure 1.
Valve dehiscence in unfertilized pistils. Images of cer6-2 pistils at 2, 18, and 21 DPA. Dehiscence of valves is observed around 21 DPA.
Table I. Comparison of the timing of developmental stages of unfertilized pistils and seeded fruits.
| Stage | Age (DPA) |
|
| Pistil | Fruit | |
| 13 - Anthesis | 0 | 0 |
| 14 - Anthers above stigma | 0 | 0 |
| 15 - Stigma above anthers | 1 | 0-1 |
| 16 - Length doubles | 2 | 1 |
| 17a - Petal dehiscence | 2-3 | 1-2 |
| 18 - Valve yellowing | 18–20 | 10–12 |
| 19 - Valve dehiscence | 20–22 | 14–16 |
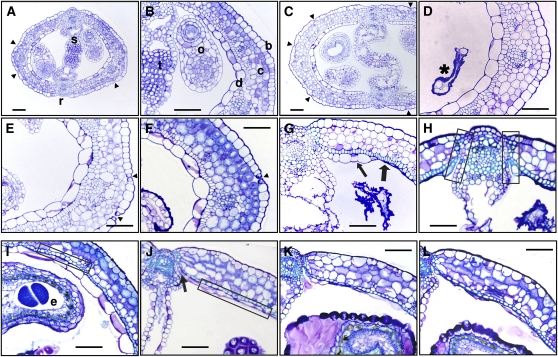
To get a deeper insight into the changes that occur in the unfertilized pistils, we analyzed pistil structure at the tissue level. The cer6-2 pistil showed an ovary anatomy at anthesis identical to that described for wild type (Ferrandiz et al., 1999), with one abaxial epidermal cell layer (that becomes the exocarp in the fruit), three layers of chlorenchyma cells (the mesocarp in the fruit), and adaxial subepidermal and epidermal cell layers (the endocarp b and endocarp a in the fruit, respectively; Fig. 2, A and B). Ovary width increased until 6 DPA, mainly due to cell expansion of chlorenchyma cells (Fig. 2C) as previously reported (Vivian-Smith and Koltunow, 1999). The unfertilized ovary showed the first symptoms of degeneration at 4 DPA, when the transmitting tract cells collapsed (Fig. 2C). Rapid degeneration of the ovules occurred after 4 DPA, and they had disintegrated by 6 DPA (Fig. 2D), while degeneration of the septum was complete at 10 DPA (Fig. 2G). During the first 10 d of post-anthesis development in the unfertilized pistil, the ovary wall did not show any senescence symptoms, but rather resembled that of a developing fruit. However, at 10 DPA, the adaxial epidermal cells of the ovary started to collapse (Fig. 2G), concurrent with the differentiation into sclerenchyma of the adaxial subepidermis, which involved secondary cell wall thickening and lignification. Shortly afterward, all adaxial epidermal cells collapsed, while lignification in the adaxial subepidermal cell layer intensified and extended to cells within the boundaries between the replum and the ovary wall (Fig. 2H). These differentiation processes were identical to those described for the dehiscence zone in the silique (Ferrandiz, 2002). Indeed, lignification of the subepidermal cell layer (endocarp b) and the dehiscence zone occurred similarly in unfertilized pistils and in seeded siliques, both in cer6-2 and in Ler (Supplemental Fig. S2). Next, we compared the timing of those changes between pistils and fruits. A delay in the differentiation events was also observed in the pistils. The endocarp b layer was lignified in fruits by 8 DPA and the endocarp a collapsed between 8 and 9 DPA (Fig. 2, I and J; Supplemental Fig. S2), approximately 2 d earlier than in unfertilized pistils.
Figure 2.
Changes in the structure of pistil and fruit during their post-anthesis development. A to H, Transversal sections of cer6-2 pistil; anthesis (A and B) showing all cellular layers; 4 DPA (C) showing increased size and disorganization of the transmitting tract; 6 DPA (D) with degraded ovules (asterisk); 8 and 9 DPA (E and F); 10 DPA (G) showing septum degradation, sclerenchyma in the adaxial subepidermal layer (thick arrow), and adaxial epidermis collapse (thin arrow); and 11 DPA (H) showing lignification in the dehiscence zone (squared areas). I to L, Ovary transversal sections of cer6-2 seeded fruits; 8 DPA (I) with sclerenchyma in the endocarp a (squared area); 9 DPA (J) with endocarp b fully collapsed (squared area) and dehiscence zone formed (arrow); and 10 and 11 DPA (K and L). b, Abaxial epidermis; c, chlorenchyma; d, adaxial epidermis; e, seed; o, ovule; r, replum; s, septum; t, transmitting tract; arrowhead, stomata. Scale bar is 50 μm.
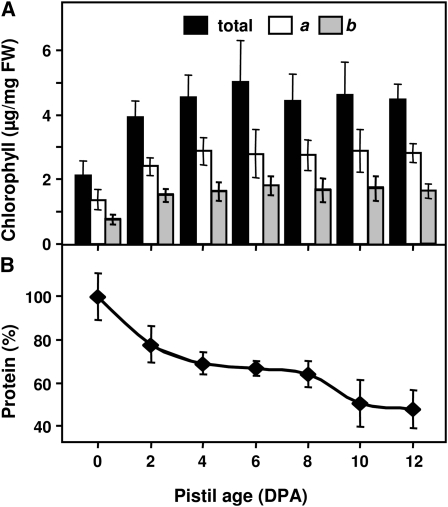
Chlorophyll levels in the unfertilized pistils increased concomitant with pod growth early after anthesis, reaching a plateau at 6 to 8 DPA (Fig. 3A), which is similar to the pattern of chlorophyll accumulation in siliques (Wagstaff et al., 2009). In addition, the number of stomata and stomatal apertures in the abaxial epidermis also increased in unfertilized pistils after anthesis (Table II; Fig. 2, A and C), a trait that has been described in developing fruits after exposure to light at anthesis (Ferrandiz et al., 1999). It is well known that onset of senescence of photosynthetically active tissues is characterized by a progressive decrease in total chlorophyll and protein content (Hensel et al., 1993; Page et al., 2001; Yoshida et al., 2002) and the higher chlorophyll levels and increased stomata number in unfertilized pistils, suggest that both pistils and fruits are photosynthetically active organs at early stages of their post-anthesis development. However, protein levels in the unfertilized pistil showed a continuous decrease, with two major declines, between 0 and 2 DPA and between 8 and 10 DPA (Fig. 3B). In contrast with data from chlorophyll and stomata analysis, the strong decrease of protein levels before 2 DPA may reflect the senescence of nongreen tissues, such as the transmitting tract or the ovules, which were the first degraded structures (Fig. 2).
Figure 3.
Protein and chlorophyll content of unfertilized pistils during post-anthesis development. A, Total, a, and b chlorophyll content in cer6-2 pistils, expressed as μg mg−1 of fresh weight (FW). B, Protein content in cer6-2 pistils, expressed as percentage of content at anthesis. Six biological replicates, each from at least 20 pistils, were used. Data are mean ± sd.
Table II. Stomata number in unfertilized pistils.
Stomata were counted in ovary cross sections.
| Pistil Age (DPA) | Mean | SD |
| 0 | 5.0 | 1.0 |
| 6 | 8.3 | 1.5 |
| 10 | 8.3 | 0.6 |
The morphological changes indicate that fertilization induces fruit set in Arabidopsis, initiating seed development and promoting growth. Strikingly, other differentiation processes associated with fruit development also occur in the unfertilized pistil, concurrent with ovule senescence, suggesting that they are determined independent of pistil fate. This may be a characteristic of the Arabidopsis fruit, whose development does not include the formation of complex anatomical structures, other than differentiation of the dehiscence zone. To summarize, reduced size is the only structural characteristic that differentiates the unfertilized pistil from the fruit. Unfertilized pistil growth is sustained by cell expansion, whereas both cell division and expansion, regulated by auxin and GA, respectively, contribute to silique growth (Vivian-Smith and Koltunow, 1999). Finally, another quantitative difference is a general delay in ovary wall differentiation. The absence of developing seeds could be the cause of the delay, either due to the lack of hormone(s) supplied by them, or the alteration of nutritional competition between ovary and ovules.
Gene Expression Analysis in Unfertilized Pistils
To identify molecular events occurring during post-anthesis pistil development, transcript expression was analyzed using long oligonucleotide microarrays (Supplemental Figs. S3–S9; Supplemental Tables S1–S9; Supplemental File S1). Principal component analysis (PCA; Supplemental Fig. S4) and clustering analysis (Supplemental Figs. S5 and S6) suggested that major changes occurred surprisingly early after anthesis, between 0 and 2 DPA (Supplemental Tables S5 and S6), associated with the apparent up-regulation of genes involved in senescence and degradation (ageing and senescence, autophagy, peroxisomal β-oxidation, and amino acid catabolism), including the senescence-related nucleases RNS1, RNS3, and BFN1 and several peptidases. Other Gene Ontology (GO) categories enriched at 2 DPA were associated with the pod growth, including plastid development, photosynthesis establishment, and increase in stomata activity. The microarray data further suggested that senescence of some tissues within the pistil, probably transmitting tract, stigma, and ovules, initiated shortly after anthesis while the pod grew and became photosynthetically active (Supplemental File S1). This idea is supported by the increase in chlorophyll levels (Fig. 3) together with the absence of structural senescence symptoms in the ovary wall (Fig. 2).
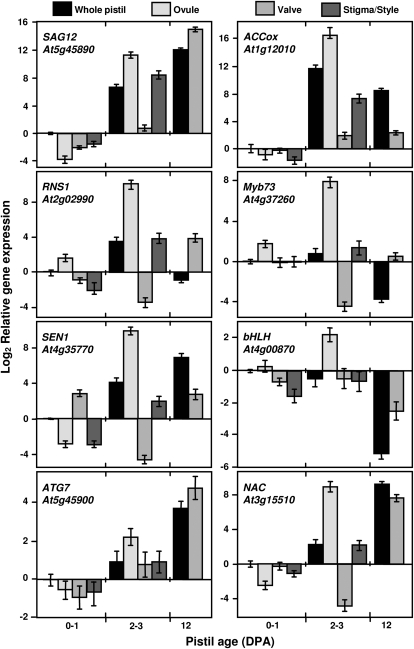
To identify which specific organs in the pistil undergo senescence early after anthesis, a more detailed analysis of gene expression was carried out using quantitative reverse transcription (qRT)-PCR expression analysis of dissected pistils (stigma-style, ovules, and valves) for eight differentially regulated genes during post-anthesis development of the unfertilized pistil (Supplemental Fig. S9). Five of these genes are senescence related and are often used as senescence markers (SAG12 At5g45890, SEN1 At4g35770, RNS1 At2g02990, ATG7 At5g45900, and the 1-aminocyclopropane-1-carboxylic acid oxidase-like encoding gene [ACCox] At1g12010) and three are transcription factors (NAC-domain encoding gene At3g15500, bHLH family gene At4g00870, and MYB73 At4g37260). Expression of all selected genes at 2 to 3 DPA was restricted to the ovule or in some cases to the style-stigma, with little or no expression in the valves (Fig. 4). Expression at anthesis showed a different distribution: Expression of RNS1 and bHLH was detected in the ovule, while SEN1 was expressed mainly in the valve. At 12 DPA, when all ovules are totally degraded, expression of all these genes was high in the valve, with the exception of ACCox and SEN1, whose expression in the valve only contributed partially to the expression in the whole pistil. Therefore, senescence events, including autophagy and ethylene biosynthesis, may be triggered in ovules and style-stigma early after anthesis.
Figure 4.
Real-time qRT-PCR analysis of dissected unfertilized pistils. Relative gene expression is shown for whole pistil, ovule, valve, and stigma-style at 0 to 1, and 2 to 3 DPA; and in whole pistil and valve at 12 DPA in cer6-2. Selected genes were SAG12 At5g45890; ribonuclease RNS1 At2g02990; senescence-related SEN1 At4g35770; ATG7 At5g45900; ACCox At1g12010; MYB73 At4g37260; bHLH At4g00870; and NAC At3g15510. Each experiment was carried out with three technical replicates, and was repeated twice with similar results. Data (log2 expression normalized to ACT8 [At1g49240] and relative to the expression of the whole pistil at 0–1 DPA) are mean ± sd of a single representative experiment.
Senescence Progression in Unfertilized Pistil Monitored by the Expression of PBFN1:GUS
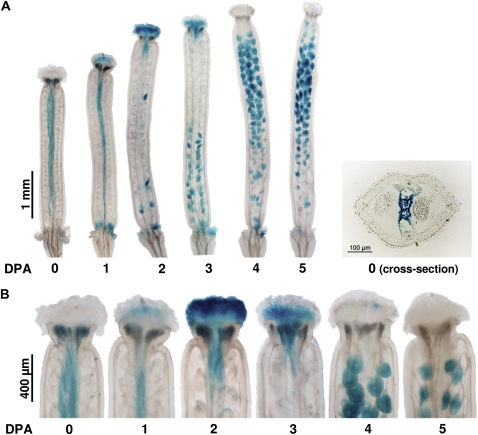
To assess the spatial pattern of senescence in more detail, we used plants expressing the GUS reporter gene under the control of the senescence-activated promoter of the bifunctional nuclease BFN1 gene (Perez-Amador et al., 2000; Farage-Barhom et al., 2009), whose transcript levels increased during unfertilized pistil post-anthesis development (Supplemental Table S2). GUS expression was first observed in the septum (transmitting tract) at anthesis (Fig. 5A) and in the stigma at 1 to 2 DPA (Fig. 5B). At 3 DPA the signal was detected in the ovules located in the base of the pistil, extending later toward the apical ovules. The signal was not detected in senescent valves (data not shown). The activation of the BFN1 promoter was also detected in the septum and stigma of seeded fruits (data not shown). Thus, transmitting tract and stigma senescence is another developmental feature common to unfertilized pistils and fruits.
Figure 5.
BFN1 promoter (PBFN1:GUS) activity as a senescence marker in unfertilized pistil. A, GUS histochemical assay in unfertilized pistils of PBFN1:GUS line. A transversal cross section of a pistil at anthesis is shown. B, Detailed images of the distal part of the unfertilized pistil showing the stigma-style area.
Pistil Loss of Fruit-Set Capacity
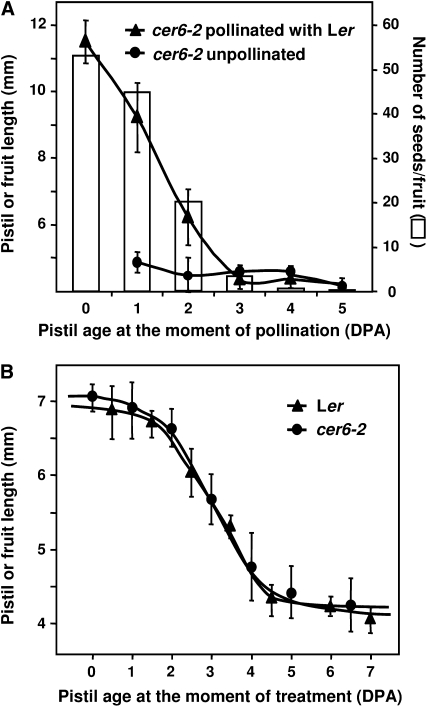
The competence to develop a fruit upon receiving an appropriate stimulus is a distinctive feature of the pistil and the loss of this ability has been associated with pistil senescence in pea (Garcia-Martinez and Carbonell, 1980). Therefore, after defining the structural and molecular events during unfertilized pistil development, we assessed how the loss of pistil capacity to develop a fruit proceeds in Arabidopsis, and how it correlates with the onset of senescence. Response to pollen was found to decrease at 1 DPA, and was completely lost at 3 DPA (Fig. 6A) and the number of seeds per fruit correlated with the final fruit length, as previously described (Cox and Swain, 2006; Dorcey et al., 2009).
Figure 6.
Loss of Ler pistil fruit-set responsiveness to pollen and to GA3. Pistil or fruit length was measured 10 d after pollination or GA3 treatment, and data were plotted against the age of the pistil at the moment of pollination or treatment. A, Response to pollen. Pistils were either hand pollinated with parental Ler pollen (▲) or not pollinated (•). Seed number in pollinated pistils is shown (white bars). B, Response to GA3. Pistils of cer6-2 in Ler (•) or emasculated flowers of Ler (▲) were treated with GA3. Data are mean ± sd of at least 25 pistils per age. The experiment was repeated three times.
Loss of pistil capacity to develop a parthenocarpic fruit was also assayed by treating pistils of different ages with GA3 and measuring their final length (Fig. 6B; Supplemental Fig. S10). While pistils treated with GA3 at anthesis or 1 DPA displayed a full response (i.e. maximum fruit size), 2 DPA pistils were slightly less responsive to the treatment. Thereafter, the responsiveness was progressively reduced and completely absent by 5 DPA. No differences were observed between cer6-2 and unpollinated wild-type pistils, suggesting that neither cer6-2 mutation nor emasculation interfered with the response to GA3. In addition, no differences were observed between Ler and Columbia-0 (Col-0) genetic backgrounds (Fig. 6B; Supplemental Fig. S10).
The fact that fruit-set responsiveness to pollen is lost before responsiveness to exogenous GA3 suggests the involvement of different mechanisms or tissues. Fruit set induced after pollination needs the pollen to be successfully recognized by a functional stigma. Therefore, stigma senescence may be the primary cause for the loss of pistil capacity to set fruits after pollination. In contrast, the specific death of the transmitting tract after pollination does not prevent fertilization in tobacco (Nicotiana tabacum), but rather facilitates proper pollen tube growth and fertilization (Wang et al., 1996). On the other hand, the capacity of the pistil to set a parthenocarpic fruit in response to GA3 is lost later and progressively from 2 to 4 DPA. Strikingly, this closely correlates with the progress of ovule senescence, suggesting that viable ovules may be required for GA-induced fruit set, although not for the subsequent elongation. Therefore, we hypothesize that GA-induced parthenocarpy requires GA perception and/or signaling in ovules; hence, when ovules undergo senescence, GA is not able to induce fruit set. Nonetheless, it cannot be ruled out that the loss of fruit-set response to GA could be due to a loss in the elongation capacity of cells in the ovary wall, independent of ovule fate or hormone perception.
Ovules Are Required for the Establishment of the Parthenocarpic Fruit-Set Response to GA3
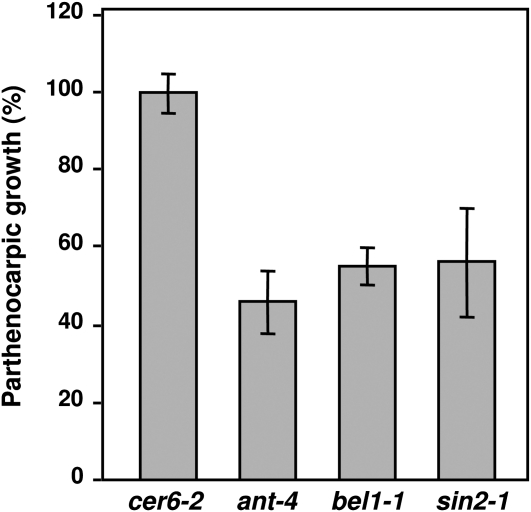
One of the most striking results was that ovule senescence closely correlates with loss of fruit-set responsiveness of the pistil to GA3, suggesting that perception or response to GA3 is localized in the ovule, or that a viable ovule is required for the fruit-set response to GA of the unfertilized pistil. To test this hypothesis, we took advantage of several mutants with different levels of impaired ovule development and evaluated the induction of parthenocarpy following treatment of the pistils with GA3. Three severe ovule-deficient mutants, bel1-1, sin2-1, and ant-4, showed limited fruit-set response to GA3, compared to cer6-2 (Fig. 7) while, as shown before, sterile cer6-2 fully responded to GA3 (Fig. 6B; Supplemental Fig. S10). The ovules from bel1-1 mutant only develop a single integument-like structure that later forms a collar tissue (Robinson-Beers et al., 1992). Embryo sac development takes place, but is not completed and it finally degenerates in bel1-1 ovules; however, pollen tube growth does take place in their transmitting tract (Modrusan et al., 1994; Ray et al., 1994). Although sin2-1 and ant-4 were apparently more severely impaired in ovule development than bel1-1, we found a similar response to GA3. sin2-1 ovules have short or absent integuments, while the embryo sac does not develop (Broadhvest et al., 2000), and ant-4 ovules lack integuments and embryo sac (Elliott et al., 1996; Klucher et al., 1996). While sin2-1 and ant-4 showed slight differences in petal morphology, the latter also showed differences in pistil structure that may account for the GA-response phenotype. As a result of ovule-defective development, these three mutants are completely sterile (Skinner et al., 2004), while cer6-2 has normal ovules and is fertile under nonrestrictive conditions. Furthermore, these three mutants are affected in genes that are predicted to encode functionally diverse proteins (a homeodomain transcription factor, a mitochondrial ribosome assembly factor, and an AP2-family transcription factor). Therefore, lack of response is not correlated with sterility or predicted gene function, but rather to defects in ovule development.
Figure 7.
Reduced responsiveness to GA3 of unfertilized pistils of ovule-deficient mutants. Pistils of cer6-2 (in Ler) and severe ovule-defective mutants bel1-1, sin2-1, and ant-4 were treated at anthesis with GA3. Pistil or fruit length was measured 10 d after GA3 treatment. Data represent the percentage of parthenocarpic growth (final size of GA-treated divided by final size of nontreated pistils), being 100% data from cer6-2. Data are mean ± sd of at least 25 pistils per age. The experiment was repeated three times.
These data support the hypothesis that fully functional ovules are required for parthenocarpic fruit growth in response to GA and that GA perception and/or signaling is localized in the ovule. GA response seems to occur during fruit set in both the ovules and the ovary wall (Dorcey et al., 2009), and it has been reported that GA regulates processes associated with the ovary wall during fruit development (Vivian-Smith and Koltunow, 1999; Dorcey et al., 2009). However, the defective fruit set after GA treatment in ovule development-impaired mutants suggests that GA perception and/or response is first required in the ovules. Further research is necessary to discover the molecular mechanism(s) responsible for the establishment of the GA response from the ovules, and therefore for fruit set.
The results reported here show that the post-anthesis development of the unfertilized pistil in Arabidopsis relies on different developmental pathways that take place simultaneously in different pistil elements. Senescence is established early in the transmitting tract, the stigma, and the ovules, correlating with the loss of the capacity to develop a seeded fruit in response to pollination, or a parthenocarpic fruit in response to GA. Concurrently, the ovary wall, other than showing reduced growth, develops similarly to the silique. Therefore, fertilization in Arabidopsis basically activates seed development and promotes pod growth, since other developmental processes seem to be an inherent part of pistil development. Finally, our data indicate a key role for the ovule in the establishment of the GA response during fruit set, since this is prevented after ovule senescence in the unfertilized pistil.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana) Ler, the cer6-2 mutant in Ler background, and the ovule-deficient mutants bel1-1, sin2-1, and ant-4 were obtained from the Arabidopsis Biological Resource Center (www.biosci.ohio-state.edu). cer6-2 in Col-0 was supplied by Dr. Vera (Universidad Miguel Hernandez, Spain). PBFN1:GUS line was a gift from Dr. Lers (The Volcani Center, Israel). Plants were grown at 22°C under 16-h light/8-h dark regime and 50% relative humidity. To determine the age of each pistil in the primary inflorescence, number and position of flowers at anthesis were recorded every day (Supplemental Fig. S3A). When necessary, emasculation was carried out 1 d before anthesis.
Fruit set was induced by the application of either GA3 or pollen to pistils. GA3 was applied by spraying the bolts with 330 μm GA3 (Duchefa) and 0.01% (v/v) Tween 80, pH 7. Pollen from wild-type Ler flowers was used for pollination. To measure final size, fruit and pistils were harvested 10 d after treatment and scanned, and length was measured using the ImageJ software (Abramoff et al., 2004). For microarray analysis, samples of the different time points on each biological replica were harvested simultaneously. The different biological replicas were harvested at the same time of the day. Each experimental time point consisted of more than 100 pistils, collected from around 40 plants.
Gene Expression Analysis by Microarray and qRT-PCR
Long-oligonucleotide DNA microarrays, from the Operon Arabidopsis genome oligo set version 1.0, provided by Dr. Galbraith (University of Arizona, http://www.ag.arizona.edu/microarray/), were used. Microarray construction, hybridization, and analysis were as described in Bueso et al. (2007) and in Supplemental File S1. Microarray dataset (accession no. GSE13113) is available on the Gene Expression Omnibus.
For qRT-PCR analysis, ovules, valves, and stigma + style of 0 and 2 to 3 DPA, and valves of 12 DPA pistils were harvested. For this, pistils were hand dissected using an acupuncture needle under the stereomicroscope. In addition, whole unfertilized pistils of 0, 2 to 3, and 12 DPA were also harvested. Total RNA was extracted using the RNeasy plant mini kit (Qiagen). Genomic DNA was eliminated with 50 units of DNaseI (Qiagen) for 15 min at room temperature. cDNA was synthesized using the SuperScript first-strand synthesis system for RT-PCR (Invitrogen). qRT-PCR was carried out using the SYBR GREEN PCR master mix (Applied Biosystems) in an ABI PRISM 7000 sequence detection system (Applied Biosystems), essentially as described in Dorcey et al. (2009). Primer sequences are indicated in Supplemental Table S10. In a single experiment, each sample was assayed in triplicate and the experiment was repeated twice, with similar results. ACT8 (At1g49240) was selected as the reference gene (Czechowski et al., 2005).
Total Protein and Chlorophyll Content Quantification
Six biological replicates (25–75 mg each) were harvested. Chlorophyll and protein were extracted using the method described in Mae et al. (1993). For total protein quantification, the method RC DC protein assay (Bio-Rad) was used. Chlorophyll content was calculated as described by Wintermans and De Mots (1965).
Histological Procedures
Pistils and fruits were fixed overnight at 4°C in 4% (w/v) paraformaldehyde (Sigma) in 0.1 M sodium phosphate pH 7.2 with 0.05% (v/v) of Tween 20 (Sigma), dehydrated in ethanol, and embedded in Technovit 7100 resin. Samples were then placed in gelatin capsules for polymerization. Sections (2 μm thickness in a MicromHM330 microtome) were stained with 0.02% (w/v) toluidine blue and images were captured using a microscope Eclipse E600 (Nikon). Staining of callose and lignin are described in Supplemental File S2.
GUS Histochemical Assay
Pistils were fixed 30 min in 90% acetone, washed in staining buffer (50 mm sodium phosphate pH 7.0, 0.1 mm potassium ferricyanide, 0.1 mm potassium hexacyanoferrate, and 0.2% Triton X-100), and incubated overnight at 37°C in staining buffer supplemented with 0.1 mm X-GlcA (5-bromo-4-chloro-3-indolyl-b-d-glucuronide cyclohexylammonium; Duchefa). Samples were dehydrated in ethanol, post fixed 30 min in 50% (v/v) ethanol, 5% (v/v) formaldehyde, 10% (v/v) acetic acid, and further dehydrated in 70% (v/v) ethanol. Finally, samples were cleared for 7 d in chloral hydrate (chloral hydrate:glycerol:water, 8 g:1 mL:2 mL) and observed in an Eclipse 600 microscope.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Germination of pollen in cer6-2.
Supplemental Figure S2. Lignin deposition in unfertilized pistils and fruits.
Supplemental Figure S3. Diagram of experimental design for the microarray analysis.
Supplemental Figure S4. Two component PCA of gene expression during post-anthesis pistil development.
Supplemental Figure S5. Clustering in a 4 × 2 self-organizing map (SOM) analysis of significant genes.
Supplemental Figure S6. Clustering in a 5 × 2 SOM analysis of significant genes identified in the complete dataset.
Supplemental Figure S7. Distribution of genes contributing to the second component in the PCA.
Supplemental Figure S8. Distribution of genes specifically contributing to the second component and not to the first component of the PCA.
Supplemental Figure S9. Microarray expression of genes selected for qRT-PCR analysis.
Supplemental Figure S10. Loss of Col-0 pistil responsiveness to GA3.
Supplemental Table S1. Expression data from microarray analysis in the complete dataset.
Supplemental Table S2. Expression profile of significant differentially expressed genes in the complete dataset.
Supplemental Table S3. Expression profile of significant differentially expressed genes in the dataset reduced to 0 to 1, 2 to 3, and 10 to 14 DPA.
Supplemental Table S4. Genes significantly up-regulated from 0 to 1, to 2 to 3 DPA.
Supplemental Table S5. Significant enriched GO terms within the different SOM clusters.
Supplemental Table S6. Significant enriched GO terms within the genes up-regulated from 0 to 1, to 2 to 3 DPA.
Supplemental Table S7. Genes contributing to the second component but not to the first component in the PCA.
Supplemental Table S8. Significant enriched GO terms for the genes positively contributing to the second component but not to the first component in the PCA using the differentially expressed genes from the complete dataset.
Supplemental Table S9. Genes expressed in seeds that were removed from the lists of significantly regulated genes.
Supplemental Table S10. Primers used for qRT-PCR and genes analyzed.
Supplemental File S1. Microarray analysis (“Materials and Methods”, “Results”, and “Discussion”).
Supplemental File S2. Materials and methods for callose and lignin staining.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Lers for the PBFN1:GUS plants, and Drs. Alabadi, Alonso, Blazquez, and Dorcey for critical reading of the manuscript.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. (2004) Image processing with ImageJ. Biophotonics Int 11: 36–42 [Google Scholar]
- Broadhvest J, Baker SC, Gasser CS. (2000) SHORT INTEGUMENTS 2 promotes growth during Arabidopsis reproductive development. Genetics 155: 895–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan-Wollaston V, Page T, Harrison E, Breeze E, Lim PO, Nam HG, Lin JF, Wu SH, Swidzinsk J, Ishizaki K, et al. (2005) Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J 42: 567–585 [DOI] [PubMed] [Google Scholar]
- Bueso E, Alejandro S, Carbonell P, Perez-Amador MA, Fayos J, Belles JM, Rodriguez PL, Serrano R. (2007) The lithium tolerance of the Arabidopsis cat2 mutant reveals a cross-talk between oxidative stress and ethylene. Plant J 52: 1052–1065 [DOI] [PubMed] [Google Scholar]
- Carbonell J, García-Martínez JL. (1985) Ribulose-1,5-bisphosphate carboxylase and fruit-set or degeneration of unpollinated ovaries of Pisum sativum L. Planta 164: 534–539 [DOI] [PubMed] [Google Scholar]
- Carrasco P, Carbonell J. (1988) Involvement of a neutral proteolytic activity in the senescence of unpollinated ovaries of Pisum sativum. Physiol Plant 72: 610–616 [Google Scholar]
- Carrasco P, Carbonell J. (1990) Changes in the level of peptidase activities in pea ovaries during senescence and fruit set induced by gibberellic acid. Plant Physiol 92: 1070–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CM, Swain SM. (2006) Localized and non-localized promotion of fruit development by seeds in Arabidopsis. Funct Plant Biol 33: 1–8 [DOI] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR. (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139: 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorcey E, Urbez C, Blazquez MA, Carbonell J, Perez-Amador MA. (2009) Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J 58: 318–332 [DOI] [PubMed] [Google Scholar]
- Elliott RC, Betzner AS, Huttner E, Oakes MP, Tucker WQJ, Gerentes D, Perez P, Smyth DR. (1996) AINTEGUMENTA, an APETALA2-like gene of Arabidopsis with pleiotropic roles in ovule development and floral organ growth. Plant Cell 8: 155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farage-Barhom S, Burd S, Sonego L, Perl-Treves R, Lers A. (2009) Expression analysis of the BFN1 nuclease gene promoter during senescence, abscission, and programmed cell death-related processes. J Exp Bot 59: 3247–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrandiz C. (2002) Regulation of fruit dehiscence in Arabidopsis. J Exp Bot 53: 2031–2038 [DOI] [PubMed] [Google Scholar]
- Ferrandiz C, Pelaz S, Yanofsky MF. (1999) Control of carpel and fruit development in Arabidopsis. Annu Rev Biochem 68: 321–354 [DOI] [PubMed] [Google Scholar]
- Fiebig A, Mayfield JA, Miley NL, Chau S, Fischer RL, Preuss D. (2000) Alterations in CER6, a gene identical to CUT1, differentially affect long-chain lipid content on the surface of pollen and stems. Plant Cell 12: 2001–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan S. (2007) Mitotic senescence in plants. Gan S, ed, Senescence Processes in Plants, Annu Plant Rev Vol 26. Blackwell Publishing, Oxford, pp 1–11 [Google Scholar]
- Garcia-Martinez JL, Carbonell J. (1980) Fruit-set of unpollinated ovaries of Pisum sativum L: influence of plat-growth regulators. Planta 147: 451–456 [DOI] [PubMed] [Google Scholar]
- Goetz M, Vivian-Smith A, Johnson SD, Koltunow AM. (2006) AUXIN RESPONSE FACTOR8 is a negative regulator of fruit initiation in Arabidopsis. Plant Cell 18: 1873–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granell A, Harris N, Pisabarro AG, Carbonell J. (1992) Temporal and spatial expression of a thiolprotease gene during pea ovary senescence, and its regulation by gibberellin. Plant J 2: 907–915 [DOI] [PubMed] [Google Scholar]
- Guo Y, Cai Z, Gan S. (2004) Transcriptome of Arabidopsis leaf senescence. Plant Cell Environ 27: 521–549 [Google Scholar]
- Gustafson FG. (1936) Inducement of fruit development by growth-promoting chemicals. Proc Natl Acad Sci USA 22: 628–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson FG. (1950) The role of hormones in fruit development. Am Nat 84: 151–159 [Google Scholar]
- He YH, Tang WN, Swain JD, Green AL, Jack TP, Gan S. (2001) Networking senescence-regulating pathways by using Arabidopsis enhancer trap lines. Plant Physiol 126: 707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedhly A, Hormaza JI, Herrero M. (2005) The effect of temperature on pollen germination, pollen tube growth, and stigmatic receptivity in peach. Plant Biol 7: 476–483 [DOI] [PubMed] [Google Scholar]
- Hegland SJ, Nielsen A, Lázaro A, Bjerknes AL, Totland Ø. (2009) How does climate warming affect plant-pollinator interactions? Ecol Lett 12: 184–195 [DOI] [PubMed] [Google Scholar]
- Hensel LL, Grbic V, Baumgarten DA, Bleecker AB. (1993) Developmental and age-related processes that influence the longevity and senescence of photosynthetic tissues in Arabidopsis. Plant Cell 5: 553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Zhu J, Mu X, Lin J. (2004) Pollen dispersion, pollen viability and pistil receptivity in Leymus chinensis. Ann Bot (Lond) 93: 295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucher KM, Chow H, Reiser L, Fischer RL. (1996) The AINTEGUMENTA gene of Arabidopsis required for ovule and female gametophyte development is related to the floral homeotic gene APETALA2. Plant Cell 8: 137–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen C, Williams NM, Aizen MA, Gemmill-Herren B, LeBuhn G, Minckley R, Packer L, Potts SG, Roulston T, Steffan-Dewenter I, et al. (2007) Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecol Lett 10: 299–314 [DOI] [PubMed] [Google Scholar]
- Kremen C, Williams NM, Thorp RW. (2002) Crop pollination from native bees at risk from agricultural intensification. Proc Natl Acad Sci USA 99: 16812–16816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mae T, Thomas H, Gay AP, Makino A, Hidema J. (1993) Leaf development in Lolium temulentum: photosynthesis and photosynthetic proteins in leaves senescing under different irradiances. Plant Cell Physiol 34: 391–399 [Google Scholar]
- Modrusan Z, Reiser L, Feldmann KA, Fischer RL, Haughn GW. (1994) Homeotic transformation of ovules into carpel-like structures in Arabidopsis. Plant Cell 6: 333–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page T, Griffiths G, Buchanan-Wollaston V. (2001) Molecular and biochemical characterization of postharvest senescence in broccoli. Plant Physiol 125: 718–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Amador MA, Abler ML, De Rocher EJ, Thompson DM, van Hoof A, LeBrasseur ND, Lers A, Green PJ. (2000) Identification of BFN1, a bifunctional nuclease induced during leaf and stem senescence in Arabidopsis. Plant Physiol 122: 169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss D, Lemieux B, Yen G, Davis RW. (1993) A conditional sterile mutation eliminates surface components from Arabidopsis pollen and disrupts cell signalling during fertilization. Genes Dev 7: 974–985 [DOI] [PubMed] [Google Scholar]
- Primack RB. (1985) Longevity of individual flowers. Annu Rev Ecol Syst 16: 15–37 [Google Scholar]
- Rappaport L. (1956) Growth regulating metabolites. Calif Agric 10: 4 [Google Scholar]
- Ray A, Robinson-Beers K, Ray S, Baker SC, Lang JD, Preuss D, Milligan SB, Gasser CS. (1994) Arabidopsis floral homeotic gene BELL (BEL1) controls ovule development through negative regulation of AGAMOUS gene (AG). Proc Natl Acad Sci USA 91: 5761–5765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reape TJ, McCabe PF. (2008) Apoptotic-like programmed cell death in plants. New Phytol 180: 13–26 [DOI] [PubMed] [Google Scholar]
- Robinson-Beers K, Pruitt RE, Gasser CS. (1992) Ovule development in wild-type Arabidopsis two female-sterile mutants. Plant Cell 4: 1237–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder AH, Yanofsky MF. (2006) Fruit development in Arabidopsis. Somerville CR, Meyerowitz EM, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/ http://www.aspb.org/publications/arabidopsis [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CA, Wayne PM, Macklin EA, Muilenberg ML, Wagner CJ, Epstein PR, Bazzaz FA. (2006) Interaction of the onset of spring and elevated atmospheric CO2 on ragweed (Ambrosia artemisiifolia L.) pollen production. Environ Health Perspect 114: 865–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrani JC, Ruiz-Rivero O, Fos M, Garcia-Martinez JL. (2008) Auxin-induced fruit-set in tomato is mediated in part by gibberellins. Plant J 56: 922–934 [DOI] [PubMed] [Google Scholar]
- Skinner DJ, Hill TA, Gasser CS. (2004) Regulation of ovule development. Plant Cell 16: S32–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM. (1990) Early flower development in Arabidopsis. Plant Cell 2: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Graaff E, Schwacke R, Schneider A, Desimone M, Flugge UI, Kunze R. (2006) Transcription analysis of Arabidopsis membrane transporters and hormone pathways during developmental and induced leaf senescence. Plant Physiol 141: 776–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ. (2008) Physiology and molecular biology of petal senescence. J Exp Bot 59: 453–480 [DOI] [PubMed] [Google Scholar]
- Vercher Y, Carbonell J. (1991) Changes in the structure of ovary tissues and in the ultrastructure of mesocarp cells during ovary senescence or fruit-development induced by plant-growth substances in Pisum sativum. Physiol Plant 81: 518–526 [Google Scholar]
- Vercher Y, Molowny A, Lopez C, Garcia-Martinez JL, Carbonell J. (1984) Structural changes in the ovary of Pisum sativum L. induced by pollination and gibberellic acid. Plant Sci Lett 36: 87–91 [Google Scholar]
- Vivian-Smith A, Koltunow A. (1999) Genetic analysis of growth-regulator-induced parthenocarpy in Arabidopsis. Plant Physiol 121: 437–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian-Smith A, Luo M, Chaudhury A, Koltunow A. (2001) Fruit development is actively restricted in the absence of fertilization in Arabidopsis. Development 128: 2321–2331 [DOI] [PubMed] [Google Scholar]
- Wagstaff C, Yang TJ, Stead AD, Buchanan-Wollaston V, Roberts JA. (2009) A molecular and structural characterization of senescing Arabidopsis siliques and comparison of transcriptional profiles with senescing petals and leaves. Plant J 57: 690–705 [DOI] [PubMed] [Google Scholar]
- Wang H, Wu HM, Cheung AY. (1996) Pollination induces mRNA poly(A) tail-shortening and cell deterioration in flower transmitting tissue. Plant J 9: 715–727 [DOI] [PubMed] [Google Scholar]
- Wintermans J, De Mots A. (1965) Spectrophotometric of chlorophyll a and b and their pheophytins in ethanol. Biochim Biophys Acta 109: 448–453 [DOI] [PubMed] [Google Scholar]
- Yi W, Law SE, McCoy D, Wetzstein HY. (2006) Stigma development and receptivity in almond (Prunus dulcis). Ann Bot (Lond) 97: 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi W, Law SE, Wetzstein HY. (2003) Fungicide sprays can injure the stigmatic surface during receptivity in almond flowers. Ann Bot (Lond) 91: 335–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Ito M, Nishida I, Watanabe A. (2002) Identification of a novel gene HYS1/CPR5 that has a repressive role in the induction of leaf senescence and pathogen-defense responses in Arabidopsis thaliana. Plant J 29: 427–437 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.