Abstract
Reactive oxygen species (ROS) are potent signal molecules rapidly generated in response to stress. Detection of pathogen-associated molecular patterns induces a transient apoplastic ROS through the function of the NADPH respiratory burst oxidase homologs D (RbohD). However, little is known about the regulation of pathogen-associated molecular pattern-elicited ROS or its role in plant immunity. We investigated ROS production triggered by bacterial flagellin (flg22) in Arabidopsis (Arabidopsis thaliana). The oxidative burst was diminished in ethylene-insensitive mutants. Flagellin Sensitive2 (FLS2) accumulation was reduced in etr1 and ein2, indicating a requirement of ethylene signaling for FLS2 expression. Multiplication of virulent bacteria was enhanced in Arabidopsis lines displaying altered ROS production at early but not late stages of infection, suggesting an impairment of preinvasive immunity. Stomatal closure, a mechanism used to reduce bacterial entry into plant tissues, was abolished in etr1, ein2, and rbohD mutants. These results point to the importance of flg22-triggered ROS at an early stage of the plant immune response.
A rapid and transient increase in reactive oxygen species (ROS), termed an “oxidative burst,” is often associated with responses to abiotic and biotic stresses and could trigger changes in stomatal aperture or programmed cell death in defense against pathogens (Kwak et al., 2003; Torres and Dangl, 2005). ROS production can occur extracellularly through activities of plasma membrane-resident NADPH oxidases (Kangasjärvi et al., 2005; Torres and Dangl, 2005). In plants, Rboh proteins, which are homologs of mammalian NADPH oxidase 2, were shown to be the predominant mediators of apoplastic ROS production (Torres et al., 1998; Galletti et al., 2008). Respiratory burst oxidase homologs D and F (RbohD and RbohF) were identified by mutation to be the responsible oxidases in Arabidopsis (Arabidopsis thaliana) defense responses (Torres et al., 2002). While most ROS generated in response to avirulent Pseudomonas syringae bacteria and Hyaloperonospora oomycete pathogens depend on RbohD function, the induced cell death response by these pathogens appears to be mostly regulated by RbohF. Cell death provoked upon infection with the necrotizing fungus Alternaria, however, is under the control of RbohD (Pogány et al., 2009). The contribution of NADPH oxidases to plant immunity was also described in barley (Hordeum vulgare) and tobacco (Nicotiana benthamiana), where resistance to powdery mildew fungi and the oomycete Phytophthora infestans, respectively, was dependent on Rboh functions (Yoshioka et al., 2003; Trujillo et al., 2006).
An early layer of active plant defense is mediated by pattern recognition receptors, which sense microbes according to conserved constituents, so-called pathogen-associated molecular patterns (PAMPs). These initiate a plethora of defense responses referred to as PAMP-triggered immunity (Boller and Felix, 2009). The Arabidopsis receptor kinase Flagellin Sensitive2 (FLS2) recognizes and physically interacts with flg22, the elicitor-active epitope of bacterial flagellin (Felix et al., 1999; Gomez-Gomez and Boller, 2000; Chinchilla et al., 2006). FLS2 is plasma membrane localized and expressed throughout the plant (Robatzek et al., 2006). FLS2 requires the receptor kinase BRI1-Associated Kinase1 (BAK1), which forms a heteromeric complex upon flg22 binding (Chinchilla et al., 2007). Subsequently, a rapid and transient flg22-stimulated oxidative burst occurs that is dependent on RbohD (Zhang et al., 2007). In addition, flg22 triggers early responses, such as ethylene biosynthesis, activation of mitogen-activated protein (MAP) kinase cascades, and changes in gene expression (Felix et al., 1999; Asai et al., 2002; Zipfel et al., 2004). Late flg22 responses include the accumulation of salicylic acid (SA), callose deposition, and an arrest of seedling growth (Gomez-Gomez et al., 1999; Mischina and Zeier, 2007). This collectively contributes to plant immunity (Zipfel et al., 2004; Melotto et al., 2006).
Little is known about the regulatory components of FLS2-activated early flg22 responses and their relevance in plant resistance to pathogens. Here, we investigated flg22-triggered ROS production in Arabidopsis seedlings and have identified ethylene signaling as a critical component of the oxidative burst in response to flg22, partly through promoting the accumulation of FLS2. We further provide evidence that the flg22-triggered oxidative burst is required for resistance to bacterial infection at the point of pathogen entry through stomata.
RESULTS
ROS Production in Response to Flg22 Is Diminished in Ethylene-Insensitive Mutants
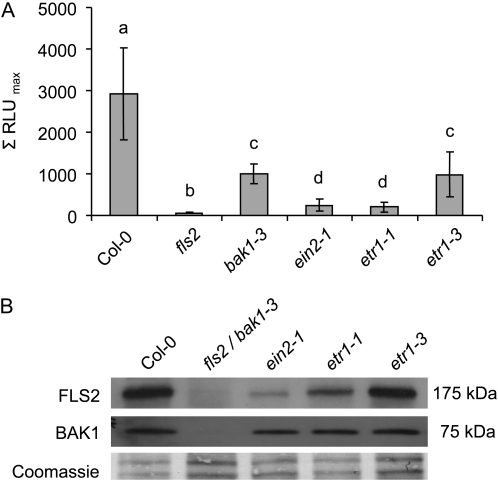
Although flg22-induced resistance to bacterial infection was reported to be independent of single hormones known to play key roles in plant defense (Zipfel et al., 2004), recent studies support a role for each of the SA, ethylene, and jasmonic acid pathways in PAMP-triggered immunity (Tsuda et al., 2009; Wang et al., 2009). However, PAMP-elicited bacterial resistance corresponds to a late flg22 response. To investigate the involvement of different hormone, stress, and kinase signaling pathways in early PAMP-triggered responses, we monitored the flg22-induced oxidative burst in intact seedlings of a collection of known mutants (Supplemental Fig. S1). Nearly all mutants tested were able to mount a wild-type-like oxidative burst. A slight increase in ROS production could be detected in mutants impaired in SA signaling, while some mutants involved in abscisic acid responses, and rcd1, a regulator of ROS-responsive cell death and stress-induced ethylene biosynthesis (Overmyer et al., 2000), were slightly reduced. A significant reduction in the flg22-elicited oxidative burst was detected in dnd1 mutants (Supplemental Fig. S1), which are lowered in cell death (Clough et al., 2000). Most strikingly, ethylene-insensitive mutants displayed a severe decrease in flg22-triggered ROS levels (Fig. 1A; Supplemental Fig. S2). A strong dominant allele of the ethylene receptor mutant, etr1-1, was almost unresponsive, whereas a partially ethylene-insensitive allele, etr1-3, displayed a partially compromised flg22-elicited oxidative burst. Also, flg22-elicited ROS production was nearly abolished in the ethylene-insensitive mutant ein2-1.
Figure 1.
Flg22-stimulated oxidative burst and FLS2 abundance in ethylene-insensitive mutants. A, Flg22-triggered ROS production was monitored in liquid-grown intact seedlings of the indicated genotypes over time. Depicted are average values (n = 18); error bars represent ±sd. Similar results were obtained in multiple independent experiments. Letters indicate significant differences at P < 0.05. B, Immunoblot analysis of the indicated genotypes using specific anti-FLS2 and anti-BAK1 antibodies. Coomassie Brilliant Blue staining is shown for equal loading. Several independent experiments revealed similar results.
Ethylene is a known component of plant immunity and accumulates upon flg22 treatment (Gomez-Gomez et al., 1999). Its recognition is mediated by a family of membrane receptors including ETR1, which acts in concert with the Raf-like kinase CTR1 to negatively regulate ethylene responses (Wang et al., 2002). Upon ethylene perception, the downstream component EIN2 activates the transcription factor EIN3, which drives transcriptional changes in ethylene-responsive genes. Mutants in CTR1 causing constitutive ethylene responses (Kieber et al., 1993), or ETO1, leading to overproduction of ethylene (Wang et al., 2004), were dispensable for flg22-triggered ROS (Supplemental Fig. S1). These findings suggest that an active ethylene signaling pathway is required for the flg22-induced ROS production. By contrast, ein3-1 mutants produced nearly wild-type-like ROS levels upon flg22 elicitation (Supplemental Fig. S1). This might be explained by the less pronounced ethylene insensitivity in ein3 loss-of-function alleles due to functional redundancy within the EIN3-like gene family (Chao et al., 1997; Bleecker and Kende, 2000).
Ethylene Signaling Contributes to FLS2 Expression
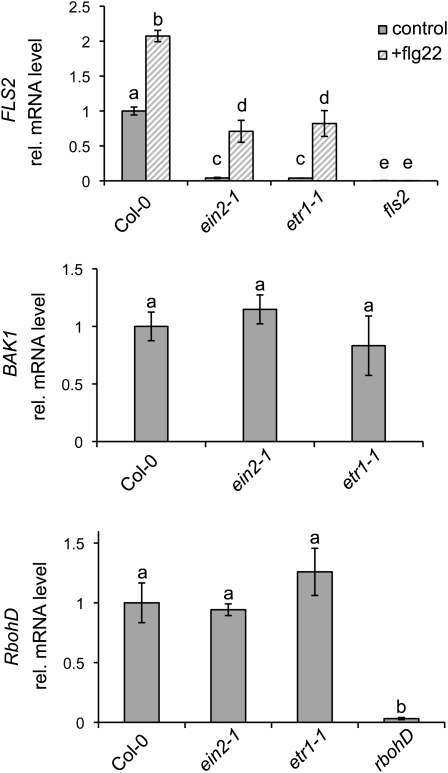
Reduced ROS generation in response to flg22 might be a result of lowered FLS2 or BAK1 abundance. Indeed, we detected reduced FLS2 steady-state levels in the strong ein2-1 and etr1-1 mutants (Fig. 1B) that could explain the compromised flg22-induced oxidative burst in these lines. However, the partial ethylene-insensitive allele, etr1-3, which exhibited a reduced flg22-induced oxidative burst, accumulated FLS2 to levels observed in the wild type. This suggests that additional regulatory mechanisms underlie flg22-triggered ROS production. No differences in BAK1 abundance were observed in all three mutant alleles when compared with Columbia (Col-0) wild-type seedlings (Fig. 1B). Differences in FLS2 steady-state levels between mutants correlated with differences in FLS2 transcript accumulation (Fig. 2). Similarly, induced FLS2 transcript accumulation upon flg22 treatment was lower in the ethylene-insensitive mutants when compared with the Col-0 wild type. This was confirmed by public database analysis (Supplemental Table S1). The BAK1 steady-state transcript levels remained unaltered in etr1-1, etr1-3, and ein2-1 seedlings. Since flg22-induced ROS is dependent on the NADPH oxidase RbohD (Zhang et al., 2007), we also analyzed RbohD transcript levels in the ethylene-insensitive mutants, which were unaltered (Fig. 2).
Figure 2.
FLS2 expression levels in ethylene-insensitive mutants. Quantitative real-time PCR monitoring of FLS2, BAK1, and RbohD transcript levels in the indicated genotypes is shown. Tubulin was used as a control. Depicted are average values of two independent experiments (n = 6); error bars represent ±sd. Letters indicate significant differences at P < 0.05.
Our data revealed that ETR1 and EIN2 could regulate the expression of FLS2 at the level of transcription or translation. A possible transcriptional regulation of FLS2 expression is supported by database searches, which revealed that FLS2 transcript levels increase upon ethylene/1-aminocyclopropane-1-carboxylic acid treatment and decrease in the presence of aminoethoxyvinylglycine, an inhibitor of ethylene biosynthesis (Supplemental Table S2). Several ethylene response elements, which are cis-regulatory elements responsible for ethylene-induced transcriptional changes (Supplemental Table S3; Kosugi and Ohashi, 2000; Hao et al., 2003), can be found within the FLS2 promoter region, which was previously shown to confer mutant complementation (Zipfel et al., 2004). Thus, the expression of FLS2 appears to be under the control of basal ethylene signaling, which influences FLS2 steady-state levels.
Differential Behavior of Flg22 Responses in Ethylene-Insensitive Mutants
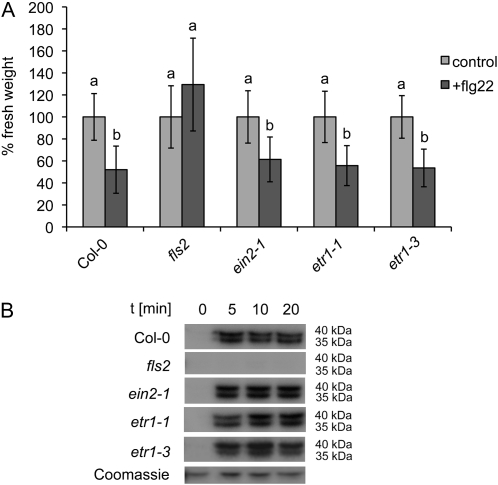
Flg22-triggered callose deposition, a late PAMP response, was shown to depend on EIN2 (Clay et al., 2009). By contrast, when tested for flg22-elicited seedling growth arrest, sensitivity to flg22 in the ethylene-insensitive mutants occurred as in the wild type (Fig. 3A). Also, flg22 activation of signaling MAP kinases, representing another early FLS2-mediated response and involved in ethylene biosynthesis (Liu and Zhang, 2004; Boller and Felix, 2009), remained unaltered in etr1-1, etr1-3, and ein2-1 seedlings (Fig. 3B). The impaired oxidative burst, but wild-type-like activation of MAP kinases and seedling growth arrest in flg22-stimulated ethylene-insensitive mutants, suggest different genetic requirements for individual flg22 responses.
Figure 3.
Flg22-stimulated early and late responses in ethylene-insensitive mutants. A, Seedling growth of the indicated genotypes was measured in the absence or presence of 100 nm flg22. Error bars represent ±sd. Letters indicate significant differences at P < 0.05. Similar results were obtained in three independent experiments. B, Flg22-induced MPK6 and MPK3 activation was determined by in-gel MAP kinase assays at the indicated time points and seedling genotypes. Coomassie Brilliant Blue staining is shown for equal loading.
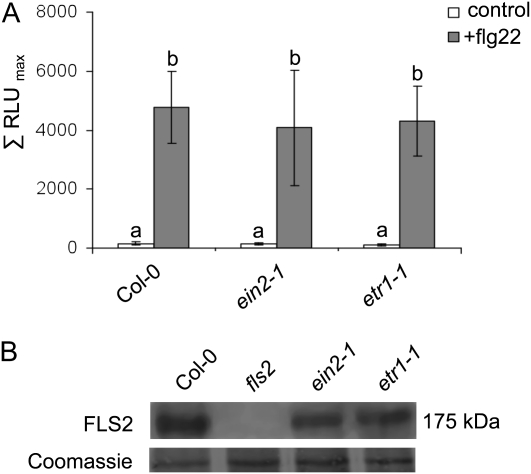
Ethylene is an important regulator of plant development and growth (Ecker, 1995), and ethylene signaling mutants exhibit enhanced steady-state levels of ethylene compared with wild-type plants (Kende, 1993; Supplemental Fig. S3). Therefore, we monitored flg22-triggered ROS production in fully expanded leaves of adult plants and found that the oxidative burst was impaired in the ethylene-insensitive mutants (Supplemental Fig. S4). Remarkably, when we used excised leaf material as described by Felix et al. (1999), the strong ethylene-insensitive etr1-1 and ein2-1 mutants produced almost wild-type-like levels of flg22-triggered ROS (Fig. 4A). Wounding alone did not induce ROS production in this assay. To further exclude conditional developmental differences, we examined leaf discs from seedlings. All tested mutants displayed wild-type-like ROS levels upon flg22 treatment (Supplemental Fig. S5), and FLS2 protein was present at similar levels in wounded etr1-1, ein2-1, and Col-0 wild type (Fig. 4B). Notably, FLS2 expression is enhanced upon wounding (Supplemental Table S2). This result suggests that wounding reverts the compromised flg22-induced oxidative burst in the ethylene-insensitive mutants and points to a stimulus-dependent regulation of flg22-triggered defense responses.
Figure 4.
Flg22-induced oxidative burst in leaf discs. A, Mature leaves of the indicated genotypes were excised into discs of approximately equal sizes and monitored for flg22-mediated ROS production. Depicted are average values (n = 8); error bars represent ±sd. RLU, Relative light unit. Letters indicate significant differences at P < 0.05. B, FLS2 immunoblot analysis. Coomassie Brilliant Blue staining is shown for equal loading.
Ethylene Signaling Contributes to Plant Immunity
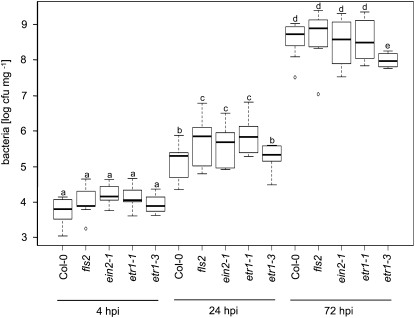
Ethylene has diverse functions in plant-microbe interactions (Van Loon et al., 2006). It is important for defense against necrotrophic fungi (Chagué et al., 2006), but its contribution to bacterial resistance remains unclear. Unaltered, reduced, or enhanced bacterial numbers have been reported depending on infection conditions (Bent et al., 1992; Pieterse et al., 1998). Our data demonstrated that among the flg22 responses tested, the ethylene-insensitive mutants were most impaired in ROS production. Due to the rapid and transient nature of the oxidative burst, we reasoned that differences in pathogen proliferation might be most apparent at early infection time points after exposure to a weak bacterial pathogen. FLS2-mediated immunity is suppressed by the effectors AvrPto and AvrPtoB (Göhre et al., 2008; Shan et al., 2008); therefore, we used the invasive but disarmed Pseudomonas syringae pv tomato DC3000 (PtoDC3000) ΔAvrPto/ΔAvrPtoB strain (Rosebrock et al., 2007). To avoid interference with wounding, we surface inoculated the set of ethylene-insensitive mutants and found that ein2-1 and etr1-1 allowed elevated bacterial multiplication compared with wild-type plants at 1 d post infection (Fig. 5). This is in agreement with the recently reported increased susceptibility of ein2-1 mutants to PtoDC3000 infection (Clay et al., 2009). Bacterial growth rates at later time points were similar or even lower than wild-type levels in ethylene-insensitive mutants (Fig. 5). Moreover, the etr1-3 mutant allele, which confers partial insensitivity to ethylene and still allowed a partial flg22-induced oxidative burst, facilitated bacterial multiplication similar to wild-type plants early during infection. This suggests that ethylene contributes to preinvasive immunity, possibly through regulation of PAMP-triggered ROS production, but has a different role after disease establishment. At 3 d post infection, etr1-3 mutants displayed enhanced bacterial resistance compared with Col-0, unraveling differential effects of altered ethylene signaling in plant immunity.
Figure 5.
Bacterial growth in ethylene-insensitive mutants. Plants of the indicated genotypes were surface inoculated with PtoDC3000 ΔAvrPto/ΔAvrPtoB, and bacterial multiplication was monitored at 4, 24, and 72 h post infection (hpi). Shown are average values of three independent experiments (n = 16); error bars represent ±sd. Letters indicate significant differences at P < 0.05.
Ethylene-Insensitive Mutants Are Impaired in PAMP-Triggered Stomatal Closure
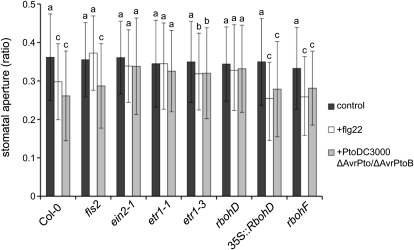
Plants respond to bacterial invasion with closure of stomata in order to prevent pathogens from entering into leaf tissues. It has been shown that stomatal closure is induced upon perception of PAMPs and that successful pathogens need to overcome this level of preinvasive immunity (Melotto et al., 2006). Plant immunity in the ethylene-insensitive mutants was compromised at early but not late stages of bacterial infection; therefore, we analyzed stomatal behavior in the ethylene-insensitive mutants. As a measure of stomatal closure, we monitored the aperture of the inner sites of guard cells in etr1-1 and ein2-1 plant leaves in the absence or presence of flg22 (Lemichez et al., 2001; Desikan et al., 2006; Supplemental Fig. S6A). Elicited leaves of Col-0 exhibited reduced stomatal aperture, which was not observed in fls2 mutants (Fig. 6). The ethylene-insensitive mutants etr1-1 and ein2-1 were not able to reduce stomatal aperture in response to flg22, and the partial ethylene-insensitive allele etr1-3 displayed weak flg22-induced stomatal closure (Fig. 6). A defect in stomatal closure upon bacterial detection is likely the cause of enhanced susceptibility early in the infection process in ethylene-insensitive mutants. Like flg22 treatment, incubation with PtoDC3000 ΔAvrPto/ΔAvrPtoB bacteria reduced stomatal aperture in wild-type plants but not in etr1-1 and ein2-1 mutants and only weakly in etr1-3 mutants. By contrast, stomatal closure occurred in PtoDC3000 ΔAvrPto/ΔAvrPtoB-infected fls2 mutants, likely due to the presence of other PAMPs than bacterial flagellin. Stomatal closure plays an important role in the FLS2-mediated immunity against PtoDC3000 infection (Zeng and He, 2010). FLS2 expression in the epidermis and in guard cells was described previously (Robatzek et al., 2006; Supplemental Fig. S6B). Database analysis in addition revealed that BAK1, RbohD, and ETR1 are expressed in guard cells, and BAK1, ETR1, and EIN2 are expressed in the epidermis, consistent with a role in PAMP-triggered stomatal closure (Supplemental Table S1).
Figure 6.
Flg22-triggered stomatal closure. Leaves of the indicated seedlings were untreated, stimulated with flg22, or infected with PtoDC3000 ΔAvrPto/ΔAvrPtoB, and stomatal aperture was measured microscopically as width to length ratio. Depicted are average values; error bars represent ±sd (n = 140 stomata of three independent experiments). Letters indicate significant differences at P < 0.1 (b) or P < 0.05 (c).
rbohD Mutants Are Compromised in Flg22-Triggered Stomatal Closure and Immunity
Since etr1-1 and ein2-1 were strongly diminished in flg22-induced ROS accumulation but not MAP kinase activation, we reasoned that flg22-induced stomatal closure requires an oxidative burst. Although both RbohD and RbohF were reported to regulate plant defense responses (Torres et al., 2002), RbohD alone was sufficient for the PAMP-triggered oxidative burst (Zhang et al., 2007). Our results confirmed that rbohD, but not rbohF mutants, are impaired in flg22-elicited ROS (Supplemental Fig. S7A). Moreover, Arabidopsis seedlings overexpressing RbohD accumulated higher ROS levels (Supplemental Fig. S7A). Other flg22 responses, such as seedling growth arrest and MAP kinase activation, were not affected by interference with RbohD function, suggesting that these responses occur independent of the flg22-triggered oxidative burst (Supplemental Fig. S6, B and C). But unlike etr1-1 and ein2-1 mutants, FLS2 steady-state levels remained unaltered in rbohD and 35S::RbohD lines (Supplemental Fig. S8A), indicating that RbohD is an essential and rate-limiting component of the flg22-induced ROS production. Stomatal aperture was not affected by flg22 treatment in rbohD mutants (Fig. 6). Also, no stomatal closure was detected in rbohD mutants when incubated with PtoDC3000 ΔAvrPto/ΔAvrPtoB bacteria. By contrast, rbohF mutants and 35S::RbohD lines showed reduced stomatal aperture upon flg22 stimulation and bacterial infection. This demonstrates that stomatal closure provoked by flg22 and PtoDC3000 ΔAvrPto/ΔAvrPtoB infection specifically depends on the RbohD-mediated oxidative burst.
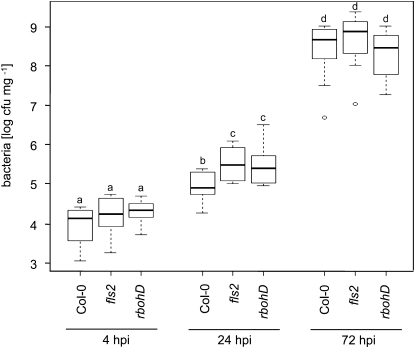
The strong etr1-1 and ein2-1 and the rbohD mutants were all impaired in flg22-triggered ROS production and stomatal closure. However, in contrast to the ethylene-insensitive lines, rbohD mutants were unaffected in FLS2 accumulation. To test whether the rbohD mutants were also compromised in immunity, we similarly investigated bacterial growth at early time points by spray infection of disarmed PtoDC3000 ΔAvrPto/ΔAvrPtoB. We detected significantly increased bacterial proliferation in the rbohD loss-of-function mutant (Fig. 7). Enhanced susceptibility was not observed at later time points. The precise role of PAMP-induced ROS in plant immunity has remained unclear, although it is a closely associated reaction of PAMP perception. Besides being antimicrobial, ROS might serve as signaling molecules promoting rapid plant defense responses in a cell autonomous or nonautonomous manner (D’Autréaux and Toledano, 2007; Miller et al., 2009). Our results provide evidence that PAMP-triggered ROS production through RbohD is important for plant defense at early stages of bacterial infection.
Figure 7.
Bacterial growth in plants with altered ROS accumulation. Plants of the indicated genotypes were surface infected with PtoDC3000 ΔAvrPto/ΔAvrPtoB, and bacterial multiplication was determined at 4, 24, and 72 h post infection (hpi). Shown are average values of three independent experiments (n = 16); error bars represent ±sd. Letters indicate significant differences at P < 0.05.
DISCUSSION
Among the mutant collection surveyed in this study, mutants impaired in ethylene sensing were identified as strongly diminished in the flg22-triggered oxidative burst. This is in accordance with findings that ethylene-insensitive mutants did not accumulate callose in response to flg22, whereas other hormone signaling mutants reacted like the wild type (Clay et al., 2009; Millet et al., 2010). Altogether, ethylene sensing is required for flg22-induced ROS production and callose deposition but not for flg22-triggered MAP kinase activation, seedling growth arrest, and induced resistance (Figs. 1 and 3; Zipfel et al., 2004; Adams-Phillips et al., 2008; Clay et al., 2009). It is evident from these findings that there are different genetic requirements for individual PAMP responses. However, different response assays likely differ in sensitivity, and the possibility that genetic redundancy obscures the importance of other than ethylene signaling pathways cannot be excluded. Indeed, the dde2/ein2/pad4/sid2 quadruple mutant defective in SA, jasmonic acid, and ethylene signaling displayed reduced responsiveness in flg22-induced resistance and seedling growth arrest (Tsuda et al., 2009). Noteworthy, etr1-1 represents a dominant ethylene-insensitive mutant allele, while ein2-1 and ein3-1 are loss-of-function alleles conferring complete and reduced ethylene insensitivity, respectively (Kieber et al., 1993; Chao et al., 1997; McCourt, 1999).
FLS2, but not BAK1, steady-state levels were significantly lower in plants carrying strong etr1-1 and ein2-1 alleles. Moreover, etr1-1 and ein2-1 mutants accumulated reduced amounts of FLS2 transcripts. Ethylene signaling, therefore, is necessary for basal expression of FLS2. Recently, ein3-4, a semidominant ethylene-insensitive mutant allele, was shown to accumulate less callose upon flg22 treatment and concomitantly allow enhanced bacterial multiplication compared with wild-type plants (Chen et al., 2009). This is in accordance with data obtained in our study and provides evidence that FLS2 abundance may be downstream of EIN3. However, plants carrying the weak etr1-3 allele, which were reduced in flg22-induced ROS production, accumulated wild-type-like FLS2 levels. This suggests that ethylene signaling is not only required for proper FLS2 expression but also for the oxidative burst response.
Remarkably, wounding appears to counteract defects in etr1-1 and ein2-1 mutants, because both wild-type-like FLS2 levels and the flg22-triggered oxidative burst were recovered in ethylene-insensitive mutants when leaf discs instead of whole seedlings were used. EIN3-like transcription factors are transcriptionally altered in response to wounding and involved in the regulation of wound-induced genes in rice (Oryza sativa; Hiraga et al., 2009). In addition, wound signaling is mediated by MAP kinase activation via MPK6 and involves ethylene (Schweighofer et al., 2007). Ethylene signaling is required for flg22-triggered dissociation of MPK6 and the ethylene response factor ERF104 (Nühse et al., 2000; Bethke et al., 2009). Genetic interference with ERF104 function altered the growth arrest in seedlings treated with flg22. It is likely, therefore, that wounding primes ethylene-insensitive mutants competent to flg22-induced ROS production by at least partly utilizing the same signaling components.
In this study, we observed that ethylene signaling through ETR1 and EIN2 contributes to flg22-dependent stomatal closure. The etr1-1 and ein2-1 mutants were also impaired in stomatal closure when incubated with PtoDC3000 ΔAvrPto/ΔAvrPtoB bacteria and accordingly facilitated enhanced bacterial growth compared with the wild type at early stages of infection. Increased susceptibility of ethylene-insensitive mutants was described before for nonhost pathogens and when bacteria were surface inoculated (Knoester et al., 1998; Pieterse et al., 1998; Clay et al., 2009). Previously reported unaltered immunity of ethylene-insensitive mutants in flg22-induced resistance might be due to hand inoculation (Zipfel et al., 2004). Bacterial growth rates were comparable between mutants and the wild type later during infection, indicating a different requirement of ethylene signaling at this stage. Notably, the bacterial strain PtoDC3000 ΔAvrPto/ΔAvrPtoB is disarmed in virulence but still able to produce coronatine and therefore is able to reopen stomata and colonize leaf tissues (Melotto et al., 2006; Zhang et al., 2008).
We revealed that RbohD contributes to restricting bacterial numbers at an early stage of infection. PAMP perception is particularly relevant at the level of preinvasive immunity. It can induce stomatal closure and thereby hinder the entry of pathogens into plant tissues (Melotto et al., 2006; Zeng and He, 2010). We identified RbohD as specifically required for flg22- and bacteria-induced stomatal closure. This defect in PAMP-triggered stomatal closure, which would largely compromise preinvasive immunity, is likely to be the cause of elevated bacterial numbers at early but not late stages of infection. An interaction between ROS and stomatal aperture in response to flg22 is consistent with a reduced oxidative burst in mutants devoid of OST1, another mediator of PAMP-induced stomatal closure and indicated to phosphorylate RbohF, which might also be postulated for RbohD (Melotto et al., 2006; Nühse et al., 2007; Sirichandra et al., 2009).
Regulation of stomatal closure in plant immunity also involves nitric oxide and GPA1, the G-subunit of heterotrimeric G protein (Melotto et al., 2006; Zhang et al., 2008; Zeng and He, 2010). GPA1-dependent ROS production through RbohD/F is necessary for stomatal closure triggered by treatment with exogenous calmodulin (Li et al., 2009). Thus, ROS production is an intrinsic component regulating stomatal aperture in various stress responses. It is notable that the functions of Coronatine Insensitive1, previously identified as a crucial component of PAMP-triggered stomatal closure (Melotto et al., 2006), or Abscisic acid Insensitive1 (ABI1) and ABI2, key regulators of stomatal closure upon abiotic stress (Assmann et al., 2001), appear to be dispensable for flg22-induced oxidative burst.
The ETR1 receptor was previously proposed as a point of convergence for ethylene and ROS signaling in stomatal function (Desikan et al., 2005, 2006), and a role for EIN2 in ROS signaling was demonstrated in ozone-dependent accumulation of ROS (Overmyer et al., 2000). Altogether, our data revealed an interaction between flg22-induced ROS production and signaling through ethylene, a hormone that itself accumulates in response to flg22. We propose that ethylene signaling contributes to basal expression of FLS2 and provide evidence for RbohD-dependent PAMP-triggered stomatal closure, which is required for proper plant immunity at early stages of infection.
MATERIALS AND METHODS
Plants and Growth Conditions
Unless stated otherwise, Arabidopsis (Arabidopsis thaliana) genotypes (Col-0, fls2, bak1-3, etr1-1, etr1-3, ein2-1, rbohD, and mutant plants listed in Supplemental Fig. S1) were grown on soil or Jiffy pellets (Jiffy Products International). For bacterial growth assays, plants were grown for 2 weeks in a controlled-environment chamber under short-day conditions (65% humidity and 23°C/22°C day/night temperatures); for ROS assays using leaf discs, plants were grown for a further 2 weeks under greenhouse conditions (21°C/19°C day/night temperatures).
Oxidative Burst Measurements
Oxidative burst was measured in intact seedlings (Khairullin and Akhmetova, 2001), and flg22 treatment was performed as outlined below (Supplemental Fig. S9). Arabidopsis seedlings were grown under sterile conditions on 96-well microtiter plates (467 μmol of light, 60% humidity, and 21°C/19°C day/night temperatures), each in 100 μL of Murashige and Skoog medium supplied with Nitch vitamins for 14 d under short-day conditions. In order to reduce variation between individual samples, growth medium was exchanged with water containing 10 nm flg22, incubated for 1 h, replaced with water, and further incubated for 1 h. ROS production was triggered with 100 nm flg22 applied together with 20 μm luminol and 1 μg per 100 μL of horseradish peroxidase. Luminescence was measured by a Centro LB 960 microplate luminometer (Berthold Technologies). Each plate was measured over a period of 40 min in 13 cycles. ROS detection using leaf discs from soil-grown adult and seedling plants was performed as described by Gomez-Gomez et al. (1999). All ROS measurements were repeated at least three times with similar results. The measurement values flanking the maximal level of ROS production, representing a time interval of 3.5 min, were determined, and their sum was calculated and referred to as Σ RLUmax (where RLU = relative light units). Statistical analysis was done using Student’s t test.
Seedling Growth Arrest
Flg22-triggered seedling growth arrest was essentially done as described by Gomez-Gomez et al. (1999). The mean of seedling growth without flg22 treatment was set at 100%, and percentage growth in the presence of 100 nm flg22 was calculated. Statistical analysis was done using Student’s t test.
Protein Detection and MAP Kinase Assays
Protein extraction and immunoblot analyses were essentially done as described previously (Häweker et al., 2010). In-gel MAP kinase assays were carried out as reported by Chinchilla et al. (2007). Briefly, 17-d-old in vitro-grown seedlings were treated with 100 nm flg22, and samples were harvested at intervals. Total protein was extracted and subjected to SDS-polyacrylamide gels supplied with 0.25 mg mL−1 myelin basic protein as kinase substrate. MAP kinase activation was determined by phosphoimaging (Typhoon 8600 PhosphorImager and Image Eraser; Molecular Dynamics).
Quantitative Real-Time PCR Analysis
Arabidopsis genotypes were grown in vitro as described for ROS measurements of seedlings. RNA was extracted using the RNeasy Plant Mini Kit (Qiagen) followed by TURBO DNase (Applied Biosystems) treatments to remove genomic DNA. Subsequent cDNA synthesis was done using the SuperScript II RNase H− Reverse Transcriptase (Invitrogen). Quantitative real-time PCR was performed in the presence of qPCR SYBR Green JumpStart Taq ReadyMix (Sigma-Aldrich). The following primer pairs were used for PCR amplification: FLS2 (5′-ACTCTCCTCCAGGGGCTAAGGAT-3′ and 5′-AGCTAACAGCTCTCCAGGGATGG-3′), BAK1 (5′-ACCGCCTCCTATCTCTCCTACACC-3′ and 5′-CTGGGTCCTCTTCAGCTGGTACA-3′), RbohD (5′-GCCGAGCCGTATCTCCATTC-3′ and 5′-TCCAATGCCGAGACCTACGA-3′), and Tubulin4 (5′-AGGGAAAGGAAGAGAGGAAG-3′ and 5′-GCTGGCTAATCCTACCCTTTGG-3′). PCR amplification was monitored in triplicate using the Chromo4 detection system (Bio-Rad). Amplification of Tubulin4 (At1g04820) served as an internal control for normalization, and gene transcript levels were calculated with reference to expression in the Col-0 wild type and averaged to 1. Statistical analysis was done by Student’s t test using R software (http://www.R-project.org).
Bacterial Infections
Bacterial infection assays were modified from Zipfel et al. (2004). Briefly, Pseudomonas syringae pv tomato DC3000 ΔAvrPto/ΔAvrPtoB (Rosebrock et al., 2007) was spray inoculated onto leaf surfaces of 2-week-old seedlings at 108 colony-forming units mL−1. All aerial parts were harvested 4, 24, and 72 h after pathogen treatment and surface sterilized. Two seedlings from each of 16 samples were pooled, and bacteria were extracted by grinding in 10 mm MgCl2. Samples were diluted and plated on medium containing appropriate antibiotics. For each Arabidopsis genotype, eight samples, each comprising two seedlings, were analyzed. The results of three independent experiments were combined, and statistical analysis (ANOVA and subsequent posthoc test by Tukey’s honestly significant difference) was done using R software (http://www.R-project.org).
Stomatal Aperture
Two-week-old seedlings were grown in vitro under standard conditions and then transferred to 100 mE m−2 s−1 light for at least 3 h in order to ensure that most stomata were open. The seedlings were vacuum infiltrated 10 min in a water solution without or with 3 μm flg22 or 108 colony-forming units mL−1 PtoDC3000 ΔAvrPto/ΔAvrPtoB (Rosebrock et al., 2007) and incubated for 2 h. The lower epidermis of six leaves of independent seedlings was mounted on glass slides and imaged using a Zeiss Axiophot microscope. Stomatal aperture of at least 140 stomata was determined as the ratio between width and length (Lemichez et al., 2001; Morillon and Chrispeels, 2001) using ImageJ software. Statistical analysis (Student’s t test) was done using R software.
Additional information about materials and methods used are provided in Supplemental Materials and Methods S1.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Overview of mutants with known roles in plant defense and stress signaling tested for flg22-induced ROS production.
Supplemental Figure S2. Oxidative burst in ethylene-insensitive mutants.
Supplemental Figure S3. Steady-state accumulation of ethylene.
Supplemental Figure S4. Oxidative burst in ethylene signaling mutants at adult stage.
Supplemental Figure S5. Flg22-induced ROS in wounded seedling material.
Supplemental Figure S6. Imaging of stomata.
Supplemental Figure S7. Flg22-stimulated responses in lines with altered ROS production.
Supplemental Figure S8. FLS2 accumulation in lines with altered ROS production.
Supplemental Figure S9. Flg22-stimulated oxidative burst.
Supplemental Table S1. Transcript abundance of genes involved in plant defense and stress signaling in different mutant backgrounds.
Supplemental Table S2. Transcript abundance of genes involved in plant defense and stress signaling upon various treatments.
Supplemental Table S3. Potential transcription factor binding sites within 1,000 bp of FLS2 promoter sequence.
Supplemental Materials and Methods S1. Oxidative burst measurements in intact adult plants; ethylene measurements; microscopic analysis; reverse transcription-PCR analysis.
Supplementary Material
Acknowledgments
We thank J.E. Parker and R. O’Connell (Max-Planck-Institute, Cologne, Germany) for their support and for critically reading the manuscript. D. Chinchilla and T. Boller (University of Basel, Basel), J. Kangasjärvi (University of Helsinki, Helsinki), J. Leung (Institut des Sciences du Végétal, Paris), and M. Torres (Madrid Technical University, Madrid) are thanked for kindly providing materials.
References
- Adams-Phillips L, Wan J, Tan X, Dunning FM, Meyers BC, Michelmore RW, Bent AF. (2008) Discovery of ADP-ribosylation and other plant defense pathway elements through expression profiling of four different Arabidopsis-Pseudomonas R-avr interactions. Mol Plant Microbe Interact 21: 646–657 [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Assmann SM, Snyder JA, Lee YRJ. (2001) ABA-deficient (aba1) and ABA-insensitive (abi1-1, abi2-1) mutants of Arabidopsis have a wild-type stomatal response to humidity. Plant Cell Environ 23: 387–395 [Google Scholar]
- Bent AF, Innes RW, Ecker JR, Staskawicz BJ. (1992) Disease development in ethylene-insensitive Arabidopsis thaliana infected with virulent and avirulent Pseudomonas and Xanthomonas pathogens. Mol Plant Microbe Interact 5: 372–379 [DOI] [PubMed] [Google Scholar]
- Bethke G, Unthan T, Uhrig JF, Pöschl Y, Gust AA, Scheel D, Lee J. (2009) Flg22 regulates the release of an ethylene response factor substrate from MAP kinase 6 in Arabidopsis thaliana via ethylene signaling. Proc Natl Acad Sci USA 106: 8067–8072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker AB, Kende H. (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16: 1–18 [DOI] [PubMed] [Google Scholar]
- Boller T, Felix G. (2009) A Renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Chagué V, Danit LV, Siewers V, Schulze-Gronover C, Tudzynski P, Tudzynski B, Sharon A. (2006) Ethylene sensing and gene activation in Botrytis cinerea: a missing link in ethylene regulation of fungus-plant interactions? Mol Plant Microbe Interact 19: 33–42 [DOI] [PubMed] [Google Scholar]
- Chao Q, Rothenberg M, Solano R, Roman G, Terzaghi W, Ecker JR. (1997) Activation of the ethylene gas response pathway in Arabidopsis by the nuclear protein ETHYLENE-INSENSITIVE3 and related proteins. Cell 89: 1133–1144 [DOI] [PubMed] [Google Scholar]
- Chen H, Xue L, Chintamanani S, Germain H, Lin H, Cui H, Cai R, Zuo J, Tang X, Li X, et al. (2009) Ethylene-insensitive3 and ethylene-insensitive-like1 repress salicylic acid induction deficient2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell 21: 2527–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. (2006) The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell 20: 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JDG, Felix G, Boller T. (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM. (2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323: 95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Fengler KA, Yu I, Lippok B, Smith RK, Jr, Bent AF. (2000) The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc Natl Acad Sci USA 97: 9323–9328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Autréaux B, Toledano MB. (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8: 813–824 [DOI] [PubMed] [Google Scholar]
- Desikan R, Hancock JT, Bright J, Harrison J, Weir I, Hooley R, Neill SJ. (2005) A role for ETR1 in hydrogen peroxide signaling in stomata guard cells. Plant Physiol 137: 832–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Last K, Harrett-Williams R, Tagliavia C, Harter K, Hooley R, Hancock JT, Neill SJ. (2006) Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. Plant J 47: 907–916 [DOI] [PubMed] [Google Scholar]
- Ecker JR. (1995) The ethylene signal transduction pathway in plants. Science 268: 667–675 [DOI] [PubMed] [Google Scholar]
- Felix G, Duran JD, Volko S, Boller T. (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18: 265–276 [DOI] [PubMed] [Google Scholar]
- Galletti R, Denoux C, Gambetta S, Dewdney J, Ausubel FM, De Lorenzo G, Ferrari S. (2008) The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea. Plant Physiol 148: 1695–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göhre V, Spallek T, Häweker H, Mersmann S, Mentzel T, Boller T, de Torres M, Mansfield JW, Robatzek S. (2008) Plant pattern-recognition receptor FLS2 is directed for degradation by the bacterial ubiquitin ligase AvrPtoB. Curr Biol 18: 1824–1832 [DOI] [PubMed] [Google Scholar]
- Gomez-Gomez L, Boller T. (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Gomez-Gomez L, Felix G, Boller T. (1999) A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J 18: 277–284 [DOI] [PubMed] [Google Scholar]
- Hao D, Ohme-Takagi M, Yamasaki K. (2003) A modified sensor chip for surface plasmon resonance enables a rapid determination of sequence specificity of DNA-binding proteins. FEBS Lett 536: 151–156 [DOI] [PubMed] [Google Scholar]
- Häweker H, Rips S, Koiwa H, Salomon S, Saijo Y, Chinchilla D, Robatzek S, von Schaewen A. (2010) Pattern recognition receptors require N-glycosylation to mediate plant immunity. J Biol Chem 285: 4629–4636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S, Sasaki K, Hibi T, Yoshida H, Uchida E, Kosugi S, Kato T, Mie T, Ito H, Katou S, et al. (2009) Involvement of two rice ETHYLENE INSENSITIVE3-LIKE genes in wound signaling. Mol Genet Genomics 282: 517–529 [DOI] [PubMed] [Google Scholar]
- Kangasjärvi J, Jaspers P, Kollist H. (2005) Signalling and cell death in ozone-exposed plants. Plant Cell Environ 28: 1021–1036 [Google Scholar]
- Kende H. (1993) Ethylene biosynthesis. Annu Rev Plant Physiol Plant Mol Biol 44: 283–307 [Google Scholar]
- Khairullin RM, Akhmetova IE. (2001) Luminol-dependent chemiluminescence analysis of chitooligosaccharide-induced rapid production of hydrogen peroxide by intact wheat seedlings. Biochemistry 66: 282–285 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenburg M, Roman G, Feldmann KA, Ecker JR. (1993) CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72: 427–441 [DOI] [PubMed] [Google Scholar]
- Knoester M, van Loon LC, van den Heuvel J, Hennig J, Bol JF, Linthorst HJ. (1998) Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc Natl Acad Sci USA 95: 1933–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosugi S, Ohashi Y. (2000) Cloning and DNA-binding properties of a tobacco Ethylene-Insensitive3 (EIN3) homolog. Nucleic Acids Res 28: 960–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak MJ, Mori CI, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI. (2003) NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemichez E, Wu Y, Sanchez JP, Mettouchi A, Mathur J, Chua NH. (2001) Inactivation of AtRac1 by abscisic acid is essential for stomatal closure. Genes Dev 15: 1808–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JH, Liu YQ, Lü P, Lin HF, Bai Y, Wang XC, Chen YL. (2009) A signaling pathway linking nitric oxide production to heterotrimeric G protein and hydrogen peroxide regulates extracellular calmodulin induction of stomatal closure in Arabidopsis. Plant Physiol 150: 114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Zhang S. (2004) Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16: 3386–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCourt P. (1999) Genetic analysis of hormone signalling. Annu Rev Plant Physiol Plant Mol Biol 50: 219–243 [DOI] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Koczan J, Nomura K, He SY. (2006) Plant stomata function in innate immunity against bacteria invasion. Cell 126: 969–980 [DOI] [PubMed] [Google Scholar]
- Miller G, Schlauch K, Tam R, Cortes D, Torres MA, Shulaev V, Dangl JL, Mittler R. (2009) The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci Signal 2: ra45. [DOI] [PubMed] [Google Scholar]
- Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD, Werck-Reichart D, Ausubel FM. (2010) Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell 22: 973–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischina TE, Zeier J. (2007) Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J 50: 500–513 [DOI] [PubMed] [Google Scholar]
- Morillon R, Chrispeels MJ. (2001) The role of ABA and the transpiration stream in the regulation of the osmotic water permeability of leaf cells. Proc Natl Acad Sci USA 98: 14138–14143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse TS, Bottrill AR, Jones AME, Peck SC. (2007) Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. Plant J 51: 931–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nühse TS, Peck SC, Hirt H, Boller T. (2000) Microbial elicitors induce activation and dual phosphorylation of the Arabidopsis thaliana MAPK 6. J Biol Chem 275: 7521–7526 [DOI] [PubMed] [Google Scholar]
- Overmyer K, Tuominen H, Kettunen R, Betz C, Langebartels C, Sandermann H, Jr, Kangasjärvi J. (2000) Ozone-sensitive Arabidopsis rcd1 mutant reveals opposite roles for ethylene and jasmonate signaling pathways in regulating superoxide-dependent cell death. Plant Cell 12: 1849–1862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieterse CM, van Wees SC, van Pelt JA, Knoester M, Laan R, Gerrits H, Weisbeek PJ, van Loon LC. (1998) A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell 10: 1571–1580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogány M, von Rad U, Grün S, Dongó A, Pintye A, Simoneau P, Bahnweg G, Kiss L, Barna B, Durner J. (2009) Dual roles of reactive oxygen species and NADPH oxidase RBOHD in an Arabidopsis-Alternaria pathosystem. Plant Physiol 151: 1459–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robatzek S, Chinchilla D, Boller T. (2006) Ligand-induced endocytosis of the pattern recognition receptor FLS2 in Arabidopsis. Genes Dev 20: 537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosebrock TR, Zeng LR, Brady JJ, Abramovitch RB, Xiao FM, Martin GB. (2007) A bacterial E3 ubiquitin ligase targets a host protein kinase to disrupt plant immunity. Nature 448: 370–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweighofer A, Kazanaviciute V, Scheikl E, Teige M, Doczi R, Hirt H, Schwanninger M, Kant M, Schuurink R, Mauch F, et al. (2007) The PP2C-type phosphatase AP2C1, which negatively regulates MPK4 and MPK6, modulates innate immunity, jasmonic acid, and ethylene levels in Arabidopsis. Plant Cell 19: 2213–2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, He P, Li J, Heese A, Peck SC, Nürnberger T, Martin GB, Sheen J. (2008) Bacterial effectors target the common signaling partner BAK1 to disrupt multiple MAMP receptor-signaling complexes and impede plant immunity. Cell Host Microbe 4: 17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirichandra C, Gu D, Hu HC, Davanture M, Lee S, Djaoui M, Valot B, Zivy M, Leung J, Merlot S, et al. (2009) Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Lett 583: 2982–2986 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL. (2005) Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr Opin Plant Biol 8: 397–403 [DOI] [PubMed] [Google Scholar]
- Torres MA, Dangl JL, Jones JD. (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99: 517–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JDG. (1998) Six Arabidopsis thaliana homologues of the human respiratory burst oxidase (gp91phox). Plant J 14: 365–370 [DOI] [PubMed] [Google Scholar]
- Trujillo M, Altschmied L, Schweizer P, Kogel KH, Hückelhoven R. (2006) Respiratory burst oxidase homologue A of barley contributes to penetration by the powdery mildew fungus Blumeria graminis f. sp. hordei. J Exp Bot 57: 3781–3791 [DOI] [PubMed] [Google Scholar]
- Tsuda K, Sato M, Stoddard T, Glazebrook J, Katagiri F. (2009) Network properties of robust immunity in plants. PLoS Genet 5: e1000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Loon LC, Geraats BP, Linthorst HJ. (2006) Ethylene as a modulator of disease resistance in plants. Trends Plant Sci 11: 184–191 [DOI] [PubMed] [Google Scholar]
- Wang KL, Li H, Ecker JR. (2002) Ethylene biosynthesis and signaling networks. Plant Cell 14: 131–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KL, Yoshida H, Lurin C, Ecker JR. (2004) Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature 428: 945–950 [DOI] [PubMed] [Google Scholar]
- Wang L, Tsuda K, Sato M, Cohen JD, Katagiri F, Glazebrook J. (2009) Arabidopsis CaM binding protein CBP60g contributes to MAMP-induced SA accumulation and is involved in disease resistance against Pseudomonas syringae. PLoS Pathog 5: e1000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka H, Numata N, Nakajima K, Katou S, Kawakita K, Rowland O, Jones JD, Doke N. (2003) Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to Phytophthora infestans. Plant Cell 15: 706–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, He SY. (2010) A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol 153: 1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Shao F, Li Y, Cui H, Chen L, Li H, Zou Y, Long C, Lan L, Chai J, et al. (2007) A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell Host Microbe 1: 175–185 [DOI] [PubMed] [Google Scholar]
- Zhang W, He SY, Assmann SM. (2008) The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J 56: 984–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel C, Robatzek S, Navarro L, Oakeley EJ, Jones JDG, Felix G, Boller T. (2004) Bacterial disease resistance in Arabidopsis through flagellin perception. Nature 428: 764–767 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.