Abstract
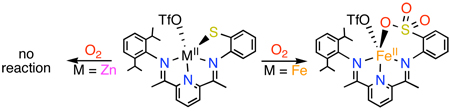
The synthesis of structural and functional models of the active site of the non-heme iron enzyme cysteine dioxygenase (CDO) is reported. A bis(imino)pyridine ligand scaffold was employed to synthesize a mononuclear ferrous complex, FeII(LN3S)(OTf) (1), which contains 3 neutral nitrogen and one anionic thiolato donor. Complex 1 is a good structural model of the Cys-bound active site of CDO. Reaction of 1 with O2 results in oxygenation of the thiolato sulfur, affording the sulfonato complex FeII(LN3SO3)(OTf) (2) under mild conditions. Isotope labeling studies show that O2 is the sole source of O atoms in the product, and that the reaction proceeds via a dioxygenase-type mechanism for two out of three O atoms added, analogous to the dioxygenase reaction of CDO. The zinc(II) analog, Zn(LN3S)(OTf) (4), was prepared and found to be completely unreactive toward O2, suggesting a critical role for FeII in the oxygenation chemistry observed for 1. To our knowledge, S-oxygenation mediated by an FeII-SR complex and O2 is unprecedented.
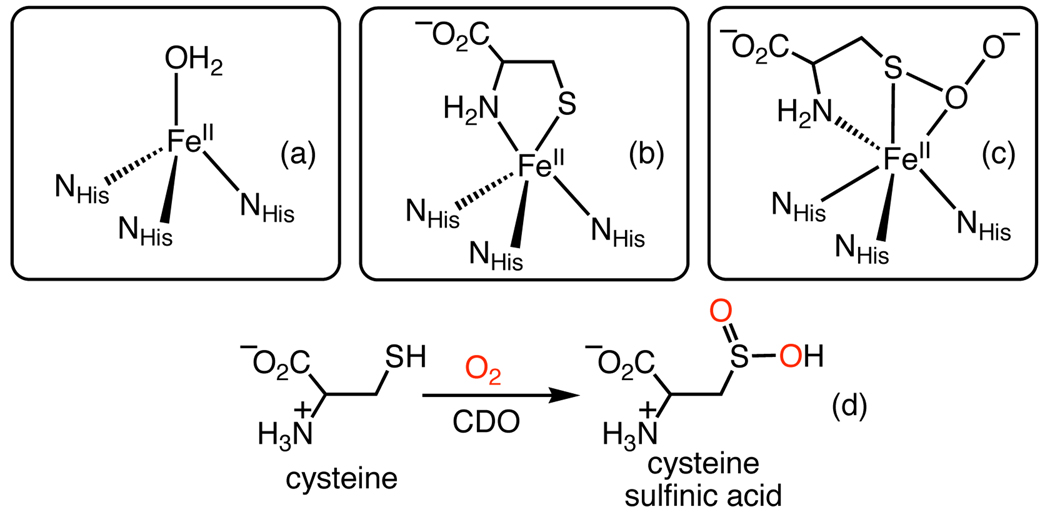
The utilization of O2 for the oxidation of organic substrates is a critical process carried out by metalloenzymes, and a highly desirable one for synthetic chemists to replicate. Cysteine dioxygenase (CDO) is a mononuclear non-heme iron enzyme that catalyzes the S-oxygenation of cysteine to cysteine sulfinic acid with O2 as oxidant (Figure 1).1 Loss of CDO function has been correlated with Alzheimer’s, Parkinson’s, and other neurological disorders. CDO contains a mononuclear FeII center bound by 3 His ligands, in contrast to the 2-His-1-carboxylate “facial triad” that is the canonical motif for non-heme Fe oxygenases. This unexpected structural variation suggests that the ligation of three neutral N donors may be important for CDO function.1h X-ray crystal structures of the native iron(II) CDO,1b a Cys-bound complex,1c and an intriguing Cys-persulfenate species1f have been determined (Figure 1). Little is known regarding the mechanism of CDO, although the persulfenate structure suggests an Fe-O2 intermediate may be important.
Figure 1.
Depiction of the active sites of CDO derived from X-ray crystallography for (a) the iron(II) resting state (b) the Cys-bound form and (c) a trapped persulfenate complex; (d) CDO reaction scheme.
Herein we describe the first structural and functional synthetic models of CDO. To obtain biologically relevant models, we targeted polydentate ligand platforms that would 1) provide 3 neutral N donors, 2) stabilize FeII, 3) allow for the facile incorporation of a thiolate donor and 4) include steric protection against the formation of O- or S-bridged Fe complexes. These criteria were met with the metal-templated synthesis of LN3S, a bis(imino)pyridine ligand in which a pendant thiolate donor has been incorporated.2 Herein it is shown that an FeII(LN3S) complex reacts with O2 via sulfur oxygenation. To our knowledge, S-oxygenation of a well-defined FeII-SR species with O2 is unprecedented.
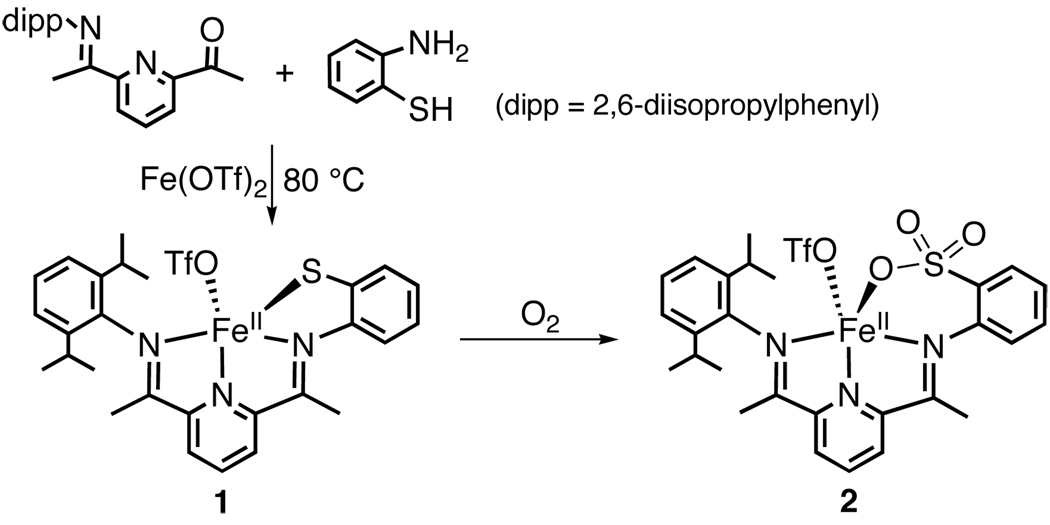
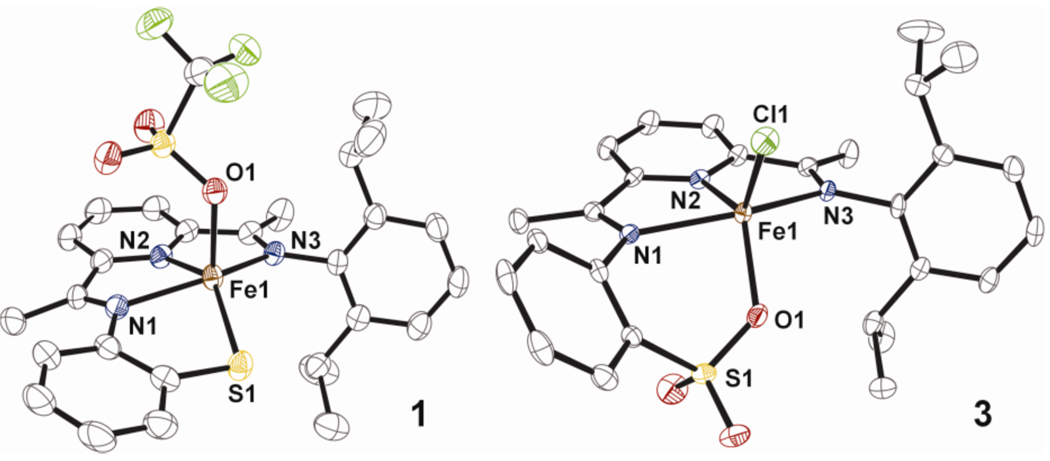
Reaction of the unsymmetrical ketone 2-(O=CMe)-6-(2,6-(iPr2-C6H3N=CMe)-C5H3N with 2-aminothiophenol in the presence of FeII(OTf)2 and Et3N at 80 °C in ethanol affords the desired dark brown FeII complex [FeII(LN3S)(OTf)] (1) in good yield (86%) (Scheme 1). The molecular structure of 1 is shown in Figure 2. The FeII ion is bound by the three neutral N donors and the thiolate S donor of the LN3S ligand in a distorted square pyramidal geometry (τ = 0.12), with the OTf− anion occupying the axial position. The diisopropyl substituents are projected orthogonal to the pseudo-equatorial N3S plane, providing significant steric protection of the metal center. The Fe-N/S/O distances are consistent with a high-spin FeII complex.3
Scheme 1.
Figure 2.
Displacement ellipsoid plots (50% probability level) of 1 and 3. The H atoms are omitted for clarity.
Addition of excess O2 to 1 in CH2Cl2 leads to an immediate color change from black to brown. Analysis of the reaction mixture by laser-desorption ionization mass spectrometry (LDIMS) shows the complete loss of starting material after 24 h and the appearance of a prominent ion at m/z 532.1, consistent with the triply-oxygenated cation [FeII(LN3SO3)]+ of 2 (Scheme 1). The reaction is solvent independent, giving the same product in CH3CN or THF. Reaction mixtures at earlier times (e.g. 5 – 180 min) contain starting material 1 ([FeII(LN3S)]+, m/z 484.2) and 2, together with a smaller peak at m/z 516.1, corresponding to a doubly-oxygenated product which disappears as the reaction proceeds. The peak at 516.1 is consistent with either a sulfinato (RSO2−) complex or a persulfenate species analogous to that seen for CDO. A sulfenato (RSO−) complex is not observed.
Attempts to crystallographically characterize 2 after O2 addition were unsuccessful. However, demetalation and acid hydrolysis (1 M HCl), followed by quantitative reverse-phase HPLC (H2O/CH3CN 95/5, 0.1% TFA) shows that the expected oxygenated organic fragment 2-H2N-C6H4SO3H is formed in good yield (60%). These data confirm that S-oxygenation occurs upon reaction of O2 with 1. EPR spectra at 15 K of mixtures of 1 + O2 reveal a signal for high-spin FeIII (g 4.3), but double-integration shows this signal accounts for less than 5 ± 2% of the total iron content. The lack of a significant EPR signal indicates a +2 oxidation state for 2. Quantitation with 1,10-phenanthroline yields a total FeII content of 91% after O2 addition (see Supporting Information).
Further support for the identity of 2 comes from the synthesis of a close analog. A template reaction with FeIICl2, unsymmetrical ketone 2-(O=CMe)-6-(2,6-(iPr2-C6H3N=CMe)-C5H3N, 2-H2N-C6H4SO3H and Et3N followed by re-crystallization from CH3CN/iPr2O affords [FeII(LN3SO3)(Cl)] (3) (Figure 2). The sulfonato group coordinates as expected to the FeII center, completing a distorted square pyramidal geometry (τ = 0.33) with the N and Cl donors. Thus complex 3 is a reasonable structural analog of the sulfonato product 2 proposed in Scheme 1.
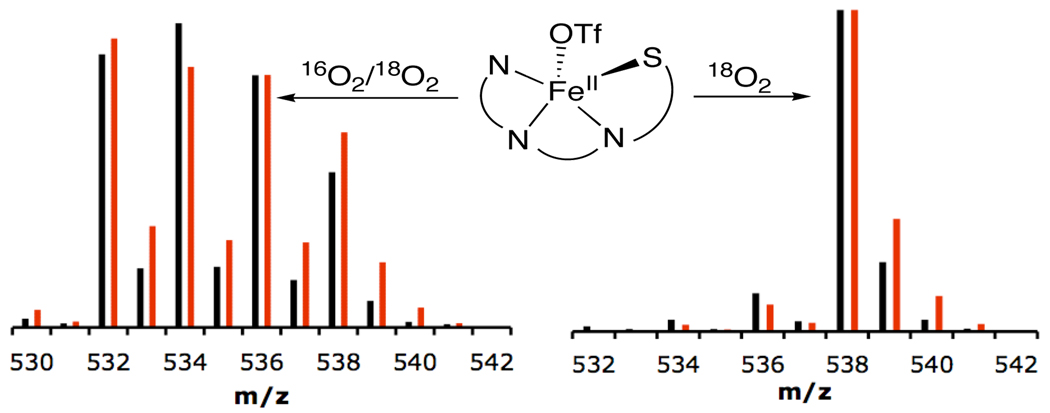
Isotopic labeling studies provide important mechanistic information regarding the oxygenation reaction. Addition of 18O2 (98%) to 1 results in fully labeled [FeII(LN3S18O3)]+ (Figure 3). In contrast, no 18O incorporation is observed when the reaction is run in the presence of excess H2 18O. Thus O2 is the source of S-oxygenation in 2, which parallels the results obtained from 18O-labeling studies with CDO.1f Two mechanistic possibilities for the formation of complex 2 are 1) incorporation of an intact molecule of O2 before or after the addition of a single O atom (2+1 case) or 2) single O atom addition for all three sulfonato oxygens (1+1+1 case).
Figure 3.
Oxygen isotope studies using LDIMS. 18O2/16O2 (~ 49/51) mixture (left) and 18O2 (98%) (right). Exptl (black), simulation (red).
Reaction of 1 with a mixture of 18O2/16O2 (~ 49:51), followed by LDIMS and statistical simulation of the isotopic distribution pattern in 2 provides a means for distinguishing these two possibilities.4 Simulations of the isotopic envelope show that the 2+1 mechanism is the dominant pathway (Figure 3 and Figure S1). This pathway indicates that a dioxygenase-type reaction is occurring, as seen for CDO. The failure to detect a singly-oxygenated sulfenato complex at earlier reaction times suggests that the third O atom is incorporated after dioxygenation, not before.
 |
(1) |
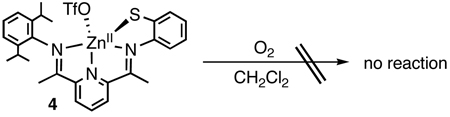
The role of the FeII ion in the S-oxygenation of 1 is not yet known, and mechanisms that involve both redox and non-redox pathways can be envisioned.1e However, synthesis of the redox-inert ZnII analog [Zn(LN3S)(OTf)] (4) provides some initial insights.5 Exposure of 4 to O2 for up to 7 d at 25 °C (eq 1) gives no reaction as determined by 1H NMR and LDIMS. Thus the requirement for iron(II), the native metal in CDO, appears to be critical for the S-oxygenation of 1.
There are only a few reports of O2-mediated S-oxygenation of FeIII-SR complexes.6,7 However, prior to the present study, the reaction of O2 with FeII-SR complexes has led only to the formation of FeIII-O-FeIII complexes, in lieu of S-oxygenates.8 Interestingly, Darensbourg observed that the site of O-capture (Fe vs S) in the reaction of FeII-SR + O2 resulted in the exclusive selection of Fe over S.8a Our findings establish that an FeII-SR complex, in the appropriate ligand environment, can selectively react with O2 to yield S-oxygenates. Further examination of 1 and related complexes should provide new, general insights regarding Fe/S/O2 reactivity.
Supplementary Material
Acknowledgment
The NIH (GM62309) is gratefully acknowledged for financial support. We thank Prof. S. Michel, S. J. Lee and J. Michalek for assistance with HPLC.
Footnotes
Supporting Information Available: Experimental details, spectra, and crystallographic data for complexes 1, 3, and 4. This material is available free of charge via the Internet at htpp://pubs.acs.org.
References
- 1.(a) McCoy JG, Bailey LJ, Bitto E, Bingman CA, Aceti DJ, Fox BG, Phillips GN., Jr Proc. Natl. Acad. Sci. USA. 2006;103:3084–3089. doi: 10.1073/pnas.0509262103. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Simmons CR, Liu Q, Huang QQ, Hao Q, Begley TP, Karplus PA, Stipanuk MH. J. Biol. Chem. 2006;281:18723–18733. doi: 10.1074/jbc.M601555200. [DOI] [PubMed] [Google Scholar]; (c) Ye S, Wu X, Wei L, Tang DM, Sun P, Bartlam M, Rao ZH. J. Biol. Chem. 2007;282:3391–3402. doi: 10.1074/jbc.M609337200. [DOI] [PubMed] [Google Scholar]; (d) Pierce BS, Gardner JD, Bailey LJ, Brunold TC, Fox BG. Biochemistry. 2007;46:8569–8578. doi: 10.1021/bi700662d. [DOI] [PubMed] [Google Scholar]; (e) Joseph CA, Maroney MJ. Chem. Commun. 2007:3338–3349. doi: 10.1039/b702158e. [DOI] [PubMed] [Google Scholar]; (f) Simmons CR, Krishnamoorthy K, Granett SL, Schuller DJ, Dominy JE, Jr, Begley TP, Stipanuk MH, Karplus PA. Biochemistry. 2008;47:11390–11392. doi: 10.1021/bi801546n. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Kleffmann T, Jongkees SAK, Fairweather G, Wilbanks SM, Jameson GNL. J. Biol. Inorg. Chem. 2009;14:913–921. doi: 10.1007/s00775-009-0504-x. [DOI] [PubMed] [Google Scholar]; (h) de Visser SP, Straganz GD. J. Phys. Chem. A. 2009;113:1835–1846. doi: 10.1021/jp809700f. [DOI] [PubMed] [Google Scholar]
- 2.Metal complexes of bis(imino)pyridine ligands are of interest for a wide range of applications. Scott J, Gambarotta S, Korobkov I, Budzelaar PHM. J. Am. Chem. Soc. 2005;127:13019–13029. doi: 10.1021/ja054152b. Gibson VC, Redshaw C, Solan GA. Chem. Rev. 2007;107:1745–1776. doi: 10.1021/cr068437y. Manuel TD, Rohde JU. J. Am. Chem. Soc. 2009;131:15582–15583. doi: 10.1021/ja9065943. Schöffel J, Rogachev AY, DeBeer George S, Burger P. Angew. Chem., Int. Ed. 2009;48:4734–4738. doi: 10.1002/anie.200901494. Russell SK, Darmon JM, Lobkovsky E, Chirik PJ. Inorg. Chem. 2010;49:2782–2792. doi: 10.1021/ic902162z.
- 3.Krishnamurthy D, Sarjeant AN, Goldberg DP, Caneschi A, Totti F, Zakharov LN, Rheingold AL. Chem. Eur. J. 2005;11:7328–7341. doi: 10.1002/chem.200500156. and references therein. [DOI] [PubMed] [Google Scholar]
- 4.(a) Farmer PJ, Solouki T, Mills DK, Soma T, Russell DH, Reibenspies JH, Darensbourg MY. J. Am. Chem. Soc. 1992;114:4601–4605. [Google Scholar]; (b) Farmer PJ, Solouki T, Soma T, Russell DH, Darensbourg MY. Inorg. Chem. 1993;32:4171–4172. [Google Scholar]; (c) Grapperhaus CA, Darensbourg MY, Sumner LW, Russell DH. J. Am. Chem. Soc. 1996;118:1791–1792. [Google Scholar]
- 5.Chohan BS, Shoner SC, Kovacs JA, Maroney MJ. Inorg. Chem. 2004;43:7726–7734. doi: 10.1021/ic049110n. [DOI] [PubMed] [Google Scholar]
- 6.(a) Heinrich L, Li Y, Vaissermann J, Chottard G, Chottard JC. Angew. Chem., Int. Ed. 1999;38:3526–3528. doi: 10.1002/(sici)1521-3773(19991203)38:23<3526::aid-anie3526>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]; (b) Noveron JC, Olmstead MM, Mascharak PK. J. Am. Chem. Soc. 2001;123:3247–3259. doi: 10.1021/ja001253v. [DOI] [PubMed] [Google Scholar]; (c) Galardon E, Giorgi M, Artaud I. Chem. Commun. 2004:286–287. doi: 10.1039/b312318a. [DOI] [PubMed] [Google Scholar]; (d) O'Toole MG, Kreso M, Kozlowski PM, Mashuta MS, Grapperhaus CA. J. Biol. Inorg. Chem. 2008;13:1219–1230. doi: 10.1007/s00775-008-0405-4. [DOI] [PubMed] [Google Scholar]
- 7.S-oxygenation by O2 has been observed for Fe(NO)x(SR)y complexes, but assigning formal oxidation states to the metal in these cases is not practical. Lee CM, Hsieh CH, Dutta A, Lee GH, Liaw WF. J. Am. Chem. Soc. 2003;125:11492–11493. doi: 10.1021/ja035292t. Chen HW, Lin CW, Chen CC, Yang LB, Chiang MH, Liaw WF. Inorg. Chem. 2005;44:3226–3232. doi: 10.1021/ic049105j.
- 8.(a) Musie G, Lai CH, Reibenspies JH, Sumner LW, Darensbourg MY. Inorg. Chem. 1998;37:4086–4093. doi: 10.1021/ic980475f. [DOI] [PubMed] [Google Scholar]; (b) Theisen RM, Shearer J, Kaminsky W, Kovacs JA. Inorg. Chem. 2004;43:7682–7690. doi: 10.1021/ic0491884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.