Abstract
Objective
Tissue factor (TF), a major initiator of blood coagulation, contributes to inflammation, atherosclerosis, angiogenesis, and vascular remodeling. Pharmacological agonists of soluble guanylate cyclase (sGC) attenuate systemic and pulmonary hypertension, vascular remodeling, and platelet aggregation. However, the influence of these novel pharmacophores on TF is unknown.
Methods and Results
We evaluated effects of BAY 41-2272 and BAY 58-2667 on expression and activity of TF in human monocytes and umbilical vein endothelial cells (HUVECs). Both compounds reduced expression of active TF protein in monocytes stimulated with lipopolysaccharide, as demonstrated by immunoblotting and a TF procoagulant activity assay. In-cell Western assay revealed that this effect was associated with a marked reduction of total and surface TF presentation. Furthermore, BAY 41-2272 and BAY 58-2667 decreased TF protein expression and the TF-dependent procoagulant activity in HUVECs stimulated with TNF-α. The sGC agonists also suppressed transcriptional activity of NF-κB. A siRNA-mediated knockdown of the α1-subunit of sGC in monocytes and HUVECs confirmed that the inhibitory effect of BAY 41-2272 and BAY 58-2667 on TF expression is mediated through the sGC-dependent mechanisms.
Conclusions
Inhibition of TF expression and activity by sGC agonists might provide therapeutic benefits in cardiovascular diseases associated with enhanced procoagulant and inflammatory response.
Keywords: tissue factor, procoagulant activity, soluble guanylate cyclase, BAY 41-2272, BAY 58-2667
Tissue factor (TF), a transmembrane glycoprotein, is a major initiator of the extrinsic blood coagulation pathway. Tissue factor binds to factor FVII/FVIIa, and this complex activates factors FIX and FX, leading to generation of thrombin and fibrin deposition.1 In addition to its prominent role in coagulation, TF-dependent signaling pathways contribute to a variety of pathological processes, including inflammation, atherosclerosis, angiogenesis, and vascular remodeling.2,3 Tissue factor is expressed in the vascular wall,4,5 whereas monocytes seem to be its major source in circulating human blood.6 An increased TF expression in monocytes is associated with disseminated intravascular coagulation, acute coronary syndrome, sepsis, and other prothrombotic conditions.7 Expression of TF is also markedly upregulated in the lung vasculature in pulmonary arterial hypertension,8 a disease associated with pulmonary vascular remodeling and localized thrombosis. Because TF is an important player in vascular remodeling,9 inhibition of TF expression could be beneficial to offset the progression of pulmonary arterial hypertension and other cardiovascular diseases.
Earlier studies have indicated an inhibitory effect of cyclic guanosine 3′–5′ monophosphate (cGMP) on expression of TF in monocytes.10 Cyclic GMP is produced from guanosine-5′-triphosphate through activation of soluble guanylate cyclase (sGC) by nitric oxide leading to vasodilation, as well as inhibition of platelet aggregation and vascular smooth cell proliferation.11 Nitric oxide–independent agonists of sGC have been recently developed and shown to attenuate systemic and pulmonary hypertension, vascular remodeling, and platelet aggregation in several experimental models.11–16 In addition, emerging clinical evidence suggests that these novel pharmacophores might offer a significant advantage over current therapies in pulmonary arterial hypertension and heart failure.17,18 It is, however, unknown whether sGC plays any regulatory role in TF biology. Therefore, the goal of the present investigation was to elucidate whether pharmacological agonists of sGC would alter the expression and functional activity of TF in human monocytes and vascular endothelial cells.
We found that the sGC stimulator BAY 41-2272 and the sGC activator BAY 58-2667 (cinaciguat) attenuated the expression and procoagulant activity of TF in monocytes stimulated with bacterial lipopolysaccharide (LPS). Similar inhibitory effect of both sGC agonists on TF was also observed in human umbilical vein endothelial cells (HUVECs) stimulated with tumor necrosis factor–α (TNF-α). Furthermore, BAY 41-2272 and BAY 58-2667 suppressed transcriptional activity of NF-κB. Using a small interfering RNA (siRNA)-mediated knockdown of the α1 subunit of sGC in monocytes and endothelial cells, we confirmed that the inhibitory effect of the sGC agonists on TF expression is mediated through the sGC-dependent mechanisms.
Methods
For detailed descriptions of the Methods, please see supplemental material available online at http://atvb.ahajournals.org.
Cell Preparation and Experimental Protocols
We used a whole-blood system to test the potential of BAY 41-2272 and BAY 58-2667 to attenuate TF activation in monocytes stimulated with LPS. Blood sampling was performed according to the study protocol approved by the Regional Committee for Medical Research Ethics. Informed consent was obtained from 6 healthy volunteers of both sexes (age 24 to 59 years). Human blood was exposed to BAY 41-2272 (1, 10, 100, or 200 µmol/L) or BAY 58-2667 (1, 10, 50, or 100 µmol/L) for 10 or 45 minutes before the LPS challenge. Thereafter, whole blood aliquots were stimulated by adding 5 ng/mL of LPS (strain 026:B6; Difco Laboratories) for 2 hours at 37°C. Mononuclear cells (MNCs) were obtained by density centrifugation using a Lymphoprep solution (Axis-Shield). We also studied the potential of both sGC agonists to inhibit TF activation in human vascular endothelium in a model of resting or TNF-α–stimulated HUVECs (ATCC, Manassas, VA). Forty-eight hours after plating, the cells were starved overnight and exposed to 0.1, 1, 10, 50, or 100 µmol/L of BAY 41-2272 or BAY 58-2667 for 2 hours. Thereafter, HUVECs were stimulated with 10 ng/mL of TNF-α (Sigma-Aldrich) for 4 hours.
RNA Interference and Nucleofection
We evaluated whether the effects of BAY 41-2272 and BAY 58-2667 on TF were dependent on the presence of functional sGC by performing experiments in human monocytes or HUVECs with knocked-down expression of the α1 subunit of sGC (sGCα1). For sGCα1-silencing, 3 million monocytes or HUVECs were nucleofected with 3 µg of siRNA using a Nucleofector II device (Lonza Cologne). For assessment of nucleofection efficiency in the sGCα1-silencing experiments, 1 µg of the green fluorescent protein (GFP)-encoding plasmid pGFP-C1 was used. The quality of the anti-TF antibody used in this study was controlled in a TF knockdown experiment, where HUVECs were nucleofected with TF-specific siRNA. In separate experiments, nucleofected monocytes were reintroduced to whole blood samples, which were previously depleted for MNCs,6 and the highest effective concentrations of BAY 41-2272 or BAY 58-2667 preceding the LPS stimulation were applied as described above.
NF-κB Reporter Gene Assay
MNCs and HUVECs were nucleofected with pNF-κB-conA-LUC and pCMV-bgal reporter plasmids (Clontech Laboratories) using the Nucleofector II device together with a human monocyte nucleofector kit or a HUVEC nucleofector kit (Lonza Gologne). After nucleofection, the cells were seeded into 24-well plates and kept in a 5% CO2/air incubator at 37°C. After a 6-hour stabilization period, the cells were treated with the highest effective concentrations of BAY 41-2772 or BAY 58-2667 for 45 minutes before stimulation with LPS or for 2 hours before stimulation with TNF-α. Luciferase and β-galactosidase activities were assessed by a dual light reporter gene assay kit (Tropix; Promega) on a Lumoskan RS microplate reader (Labsystems Oy).
Western Blotting
TF protein levels in the lysates of isolated monocytes or harvested HUVECs were detected by probing the membranes with a mouse antihuman TF monoclonal antibody (clone TF9 10H10; Calbiochem) and a horseradish peroxidase (HRP)-conjugated goat antimouse secondary antibody (BD Biosciences Pharmingen). Densitometrical readings of TF immunopositive bands were used for statistical comparisons. Protein loading was determined by probing the membranes with a rabbit antihuman β-actin polyclonal antibody (Calbiochem) and a HRP-conjugated goat antirabbit antibody (BD Biosciences Pharmingen). The efficacy of sGCα1 silencing in the RNA interference assay was ascertained by a monoclonal antisGCα1 antibody (Abcam), and the expression of TF protein was used as a readout in the sGCα1-knockdown experiments. Levels of IκBα in monocytes and HUVECs were measured by using a mouse antihuman IκBα antibody (Cell Signaling Technology).
In-Cell Western Assay
We used the in-cell Western (ICW) assay to characterize total and surface TF levels in resting and stimulated monocytes.19 Isolated human monocytes were immunostained with mouse antihuman TF and rabbit antihuman glyceraldehydephosphate dehydrogenase (GAPDH; Sigma-Aldrich) antibodies. The signals from primary antibodies were amplified with IRDye800CW-conjugated goat anti-mouse (Rockland Immunochemicals) and Alexa680-conjugated goat antirabbit (Invitrogen) secondary antibodies.
Flow Cytometry
After completion of treatments, HUVECs were harvested and immunostained with an Alexa488-conjugated (Alexa-488 Protein Labeling Kit; Invitrogen) mouse antihuman TF monoclonal antibody (Calbiochem). Flow cytometry was performed using a FACSCalibur flow cytometer calibrated with Calibrate beads (BD Biosciences).
TF Procoagulant Activity
TF was measured in the intact cells and in frozen/thawed preparations of monocytes using a two-stage clotting assay based on the ability of TF to accelerate the activation of factor X by factor VIIa as previously described.20
Determinations of cGMP and Cell Viability
Concentrations of cGMP in lysates of MNCs and HUVECs were quantified using a cGMP enzyme-immunoassay kit (Amersham Pharmacia Biotech). Viability of MNCs and HUVECs after various treatments was assessed by flow cytometry analysis of propidium iodide (PI) uptake by cells as previously described.21
Data Analysis
Each experiment was performed at least 4 times. Samples, where applicable, were assayed in triplicates. Data are expressed as mean±SEM. The treatment effects were tested by ANOVA followed by a Holm-Sidak post hoc test (SigmaStat 3.0; Systat Software). Probability values <0.05 were considered statistically significant.
Results
BAY 41-2272 and BAY 58-2667 Inhibit Expression and Activity of TF in Monocytes
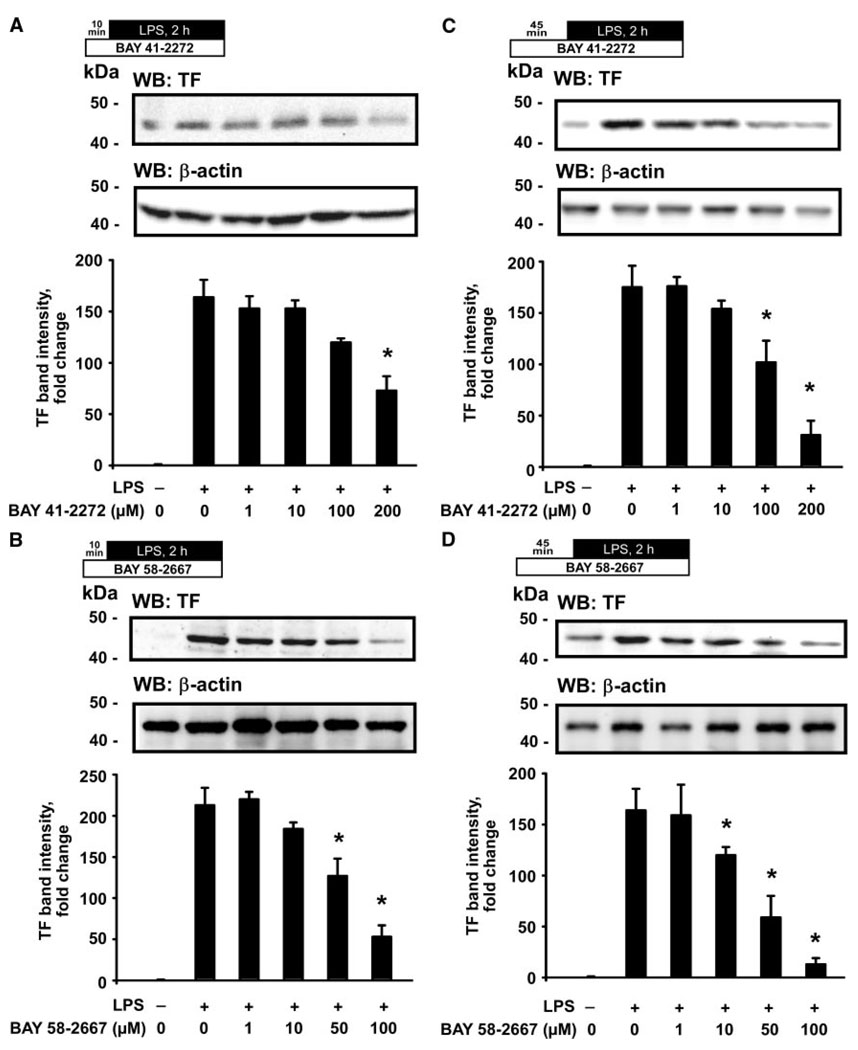
We first tested whether the sGC agonists would alter TF protein expression in monocytes, stimulated with LPS, in a whole blood system. A 2-hour LPS exposure resulted in a strong and reproducible elevation of the TF immunopositive band intensity on Western blots (Figure 1, second lanes). Pretreatment with BAY 41-2272 (200 µmol/L) for 10 minutes before stimulation with LPS produced a significant reduction in TF band intensity (Figure 1A). A 10-minute pretreatment with BAY 58-2667 resulted in a more potent reduction of TF band intensity, demonstrating this effect already at the 50-µmol/L concentration (Figure 1B). Increasing the duration of the pretreatment to 45 minutes produced a marked inhibition of TF protein levels starting at 100 µmol/L of BAY 41-2272 and at 10 µmol/L of BAY 58-2667 (Figure 1C and 1D).
Figure 1.
Agonists of sGC inhibit the LPS-induced expression of TF in human monocytes. Immunoblots illustrate a dose-dependent reduction of TF protein expression in the LPS-stimulated monocytes after pretreatment with BAY 41-2272 (A) or BAY 58-2667 (B) for 10 minutes and BAY 41-2272 (C) or BAY 58-2667 (D) for 45 minutes. Plots represent changes in the TF band density relative to resting control cells. WB indicates Western blotting. Data are mean±SEM. *P<0.05 vs the LPS-stimulated cells.
Because activation of TF encompasses externalization of TF protein onto the cell surface,22 we next studied effects of both sGC agonists on levels of surface and total TF in human monocytes using the ICW assay.19 Stimulation with LPS induced a reproducible increase of surface and total TF protein levels, which were respectively 7- and 10-fold higher than in resting cells (supplemental Figure I). Pretreatment of whole blood with BAY 41-2272 (200 µmol/L for 10 minutes and 100 µmol/L for 45 minutes before LPS) significantly reduced surface and total expression of TF in monocytes, as compared to the LPS-stimulated control cells. Pretreatment with BAY 58-2667 produced a more potent inhibition of surface and total TF expression, because this effect was already observed at the 50-µmol/L concentration (supplemental Figure II).
BAY 41-2272 and BAY 58-2667 attenuated TF protein levels in monocytes via regulation of TF mRNA expression. Specifically, a 10-minute pretreatment of monocytes with BAY 41-2272 (200 µmol/L) or BAY 58-2667 (50 µmol/L) produced a significant reduction in TF mRNA levels, when compared to the control cells stimulated with LPS alone (supplemental Figure IIIA and IIIB). This inhibitory effect was enhanced after a 45-minute pretreatment with both sCG agonists (supplemental Figure IIIC and IIID).
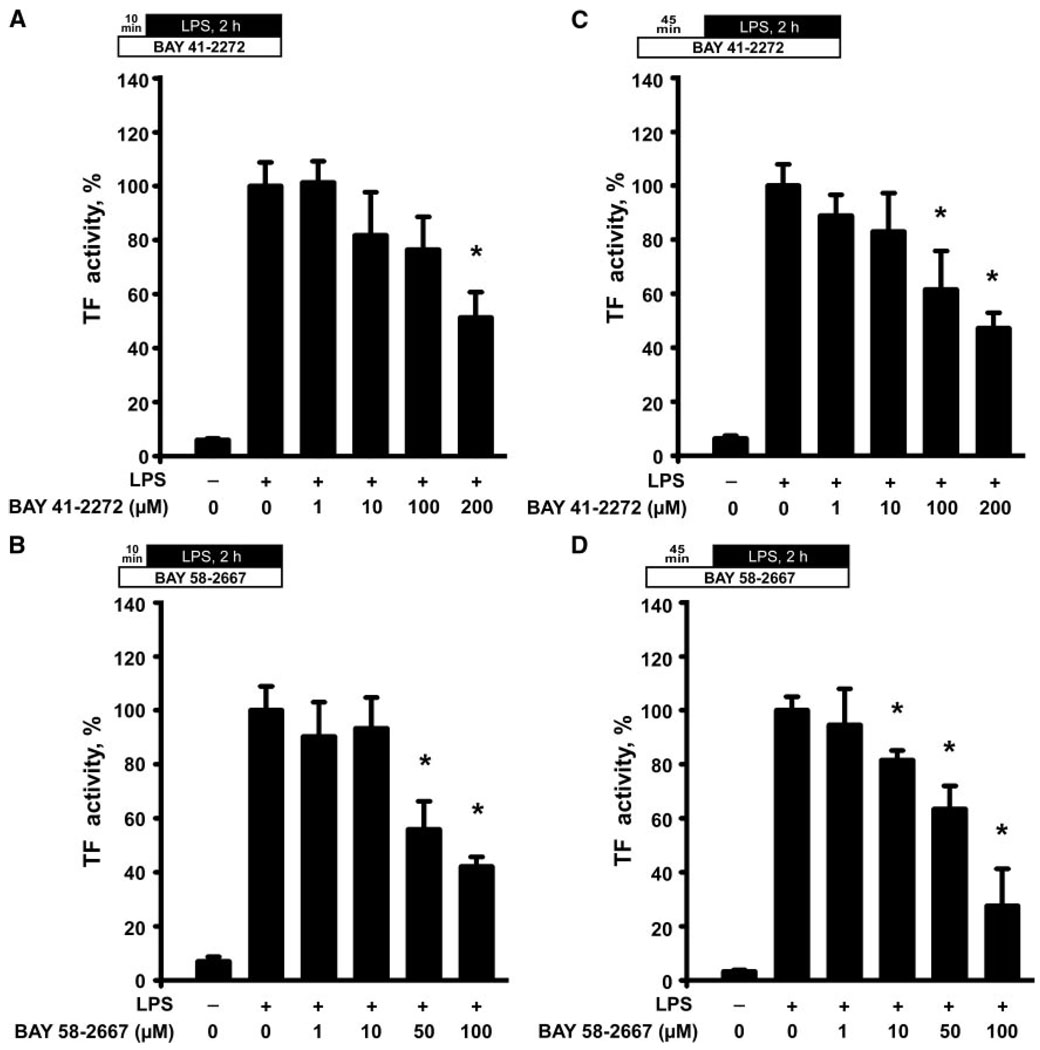
Because activity of TF is not solely regulated by the presentation of TF antigen on the surface of monocytes, but also via other mechanisms, generalized as the “TF decryption phenomenon,”23 we evaluated its functional activity, using a TF-dependent thrombin generation assay.20 After a 10-minute pretreatment, BAY 41-2272 (200 µmol/L) and BAY 58-2667 (50 µmol/L) inhibited the LPS-induced TF activity in monocytes (Figure 2A and 2B). A 45-minute pretreatment produced a more potent reduction of TF activity in the LPS-stimulated monocytes: BAY 41-2272 exhibited its inhibitory effect at 100 µmol/L, whereas BAY 58-2667 inhibited TF activity already at 10 µmol/L (Figure 2C and 2D).
Figure 2.
Agonists of sGC attenuate the LPS-induced increase in TF functional activity in human monocytes. Plots illustrate a dose-dependent reduction of TF procoagulant activity in the LPS-stimulated monocytes after pretreatment with BAY 41-2272 (A) or BAY 58-2667 (B) for 10 minutes and BAY 41-2272 (C) or BAY 58-2667 (D) for 45 minutes. Data are mean±SEM and represent percent changes from the LPS-stimulated cells. *P<0.05 vs the LPS-stimulated cells.
BAY 41-2272 and BAY 58-2667 Inhibit Expression and Activity of TF in Human Endothelial Cells
Because endothelial cells are a source of TF in blood vessels, we used this cell type as a model system to test effects of the sGC agonists on TF. Using flow cytometry of HUVECs immunostained against TF, we found that a 6-hour treatment with BAY 41-2272 (50 and 100 µmol/L) significantly reduced the TF-dependent mean fluorescence intensity (MFI) in resting HUVECs (supplemental Figure IVA and IVC). The population of cells presenting TF on their surface was also reduced (supplemental Figure IVB). In addition, lysates of resting HUVECs contained less TF protein and exhibited a significantly lower TF activity (supplemental Figure IVD and IVE).
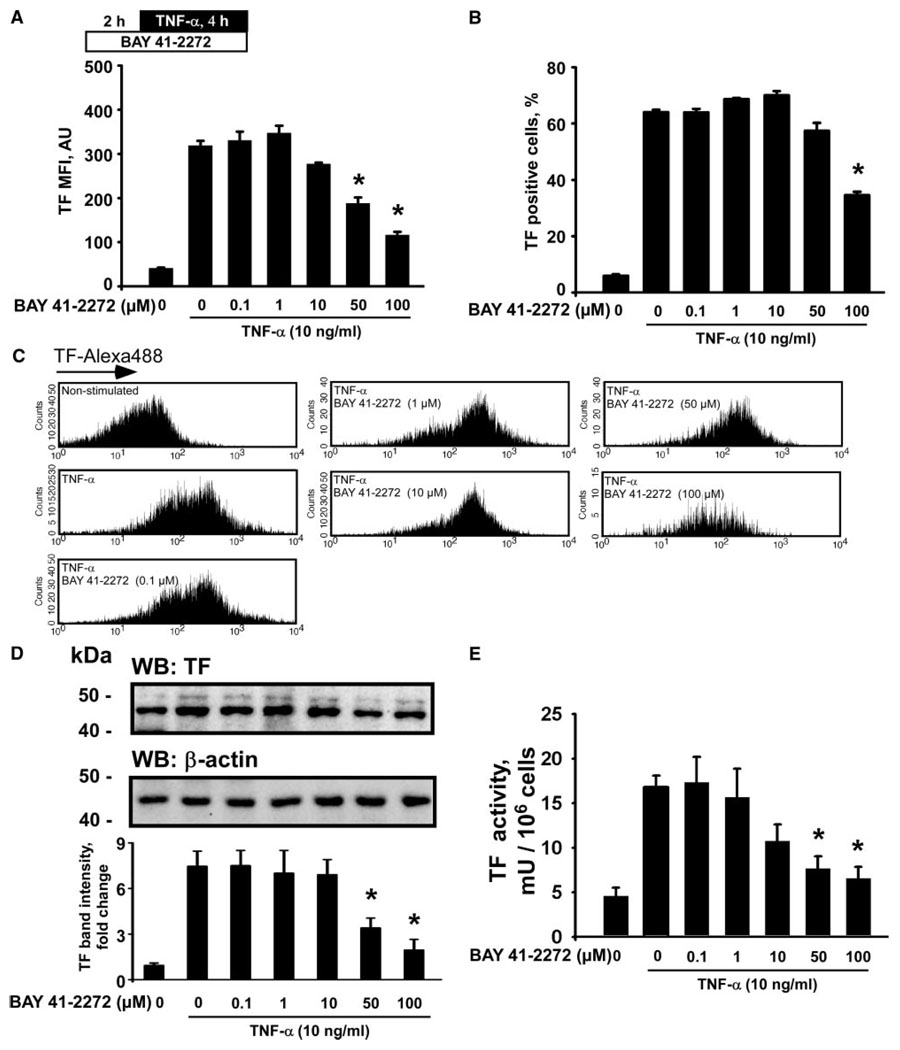
Furthermore, we evaluated effects of BAY 41-2272 in HUVECs challenged with TNF-α. TNF-α induced a marked elevation of TF protein expression, an expansion of a TF-positive cell population, and an increase of TF procoagulant activity in cell lysates (Figure 3). Pretreatment with BAY 41-2272 (50 and 100 µmol/L) for 2 hours before stimulation with TNF-α resulted in a significant reduction of TF MFI, as compared to the cells stimulated with TNF-α alone (Figure 3A and 3C). The population of TF-positive endothelial cells was also significantly reduced after 100 µmol/L of BAY 41-2272 (Figure 3B). In addition, BAY 41-2272 (50 and 100 µmol/L) reduced TF protein and activity levels in HUVEC lysates (Figure 3D and 3E).
Figure 3.
BAY 41-2272 inhibits TF expression and functional activity induced by TNF-α in human endothelial cells. TF-Alexa488 mean fluorescence intensities (MFI) (A), populations of TF-presenting cells (B and C), TF protein expression (D), and TF procoagulant activity (E) in HUVECs treated with BAY 41-2272 for 2 hours before stimulation with TNF-α. Plot in D represents changes in the TF band density relative to resting control cells. WB indicates Western blotting. Data are mean±SEM. *P<0.05 vs the TNF-α–stimulated cells.
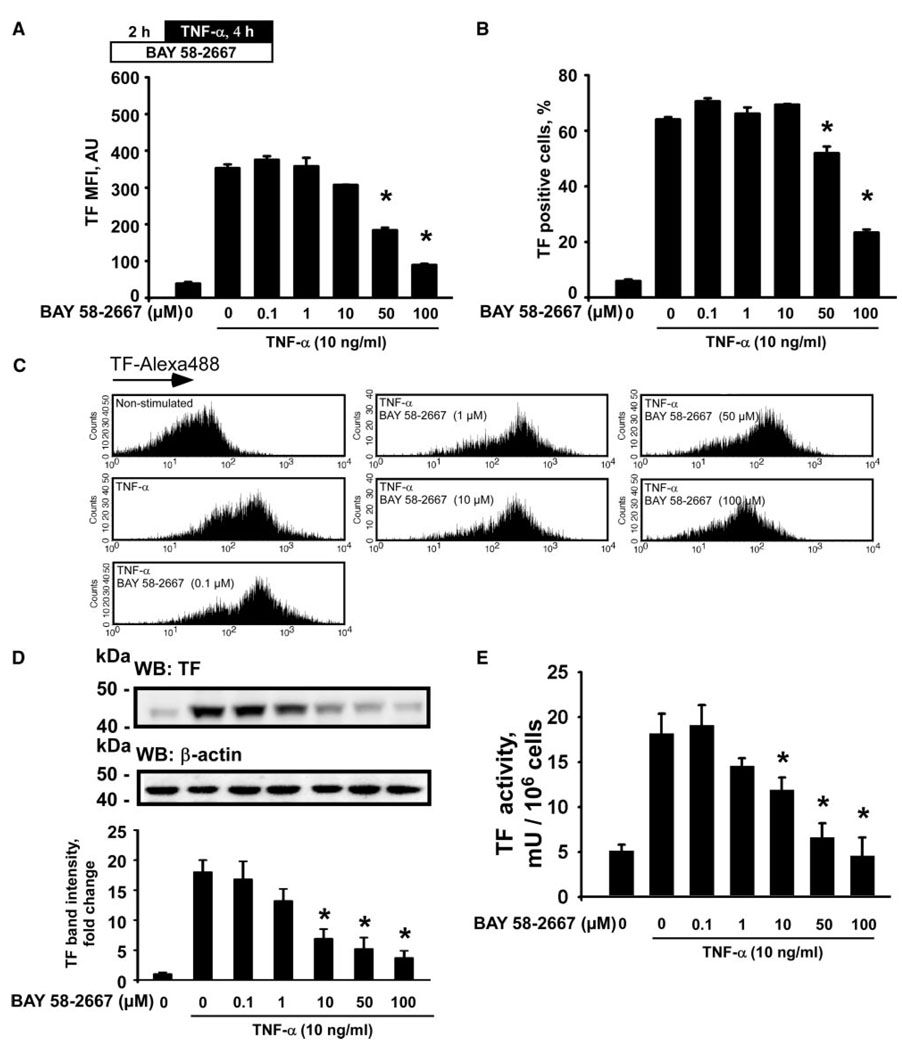
A 6-hour treatment of resting HUVECs with BAY 58-2667 significantly reduced TF MFI and a number of TF-positive cells when applied at concentrations of 10 µmol/L and higher (supplemental Figure VA through VC). We also observed a reduction of TF protein and activity levels in lysates of resting endothelial cells after 50 and 100 µmol/L of BAY 58-2667 (supplemental Figure VD and VE). Pretreatment of HUVECs with BAY 58-2667 (50 and 100 µmol/L) for 2 hours before TNF-α significantly reduced TF MFI and the population of the cells, expressing TF on their surface (Figure 4A through 4C). In addition, BAY 58-2667, at concentrations of 10 µmol/L and higher, attenuated TF protein expression and TF activity levels in lysates of HUVECs stimulated with TNF-α (Figure 4D and 4E).
Figure 4.
BAY 58-2667 inhibits TF expression and functional activity induced by TNF-α in human endothelial cells. TF-Alexa488 mean fluorescence intensities (MFI) (A), populations of TF-presenting cells (B and C), TF protein expression (D), and TF procoagulant activity (E) in HUVECs treated with BAY 58-2667 for 2 hours before stimulation with TNF-α. Plot in D represents changes in the TF band densities relative to resting control cells. WB indicates Western blotting. Data are mean±SEM. *P<0.05 vs the TNF-α–stimulated cells.
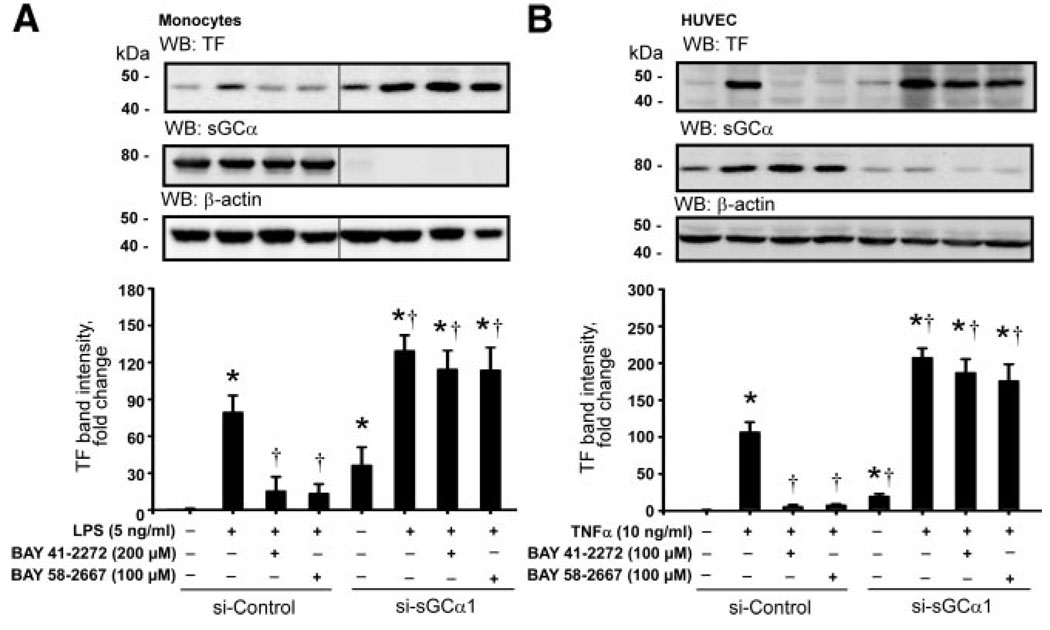
Inhibition of the TF System by BAY 41-2272 and BAY 58-2667 Is Dependent on the Expression of the α1 Subunit of sGC and Mediated via NF-κB
To evaluate the contribution of functional sGC in the response of the TF system to the sGC agonists, we used a siRNA-mediated knockdown of expression of the α1 subunit of sGC in human monocytes and endothelial cells. As shown in Figure 5, nucleofection of isolated monocytes and HUVECs with the sGCα1-specific siRNA resulted in a profound reduction of endogenous sGCα1 protein expression. Interestingly, TF band intensity was significantly stronger in resting monocytes and HUVECs nucleofected with siRNA against sGCα1 than in the cells nucleofected with control siRNA. Stimulation with LPS and TNF-α elicited an increase in TF protein levels in the cells expressing sGCα1, and this response was enhanced in the cells lacking sGCα1. However, after a knockdown of the sGCα1 expression in monocytes and HUVECs, BAY 41-2272 and BAY 58-2667 lost their potential to attenuate the LPS- or TNF-α–induced TF protein expression, even when applied at the maximal concentrations.
Figure 5.
Inhibition of the LPS- or TNF-α–induced expression of TF by sGC agonists is dependent on the expression of functional sGC. A siRNA-mediated knock-down of the α1 subunit of sGC resulted in a loss of the inhibitory potential of BAY 41-2272 and BAY 58-2667 on the LPS-induced TF expression in human monocytes (A) and on the TNF-α–induced TF expression in HUVECs (B). Plots represent changes in the TF band density relative to resting control cells. WB indicates Western blotting. Data are mean±SEM. *P<0.05 vs resting control cells; †P<0.05 vs the LPS- or TNF-α–stimulated cells.
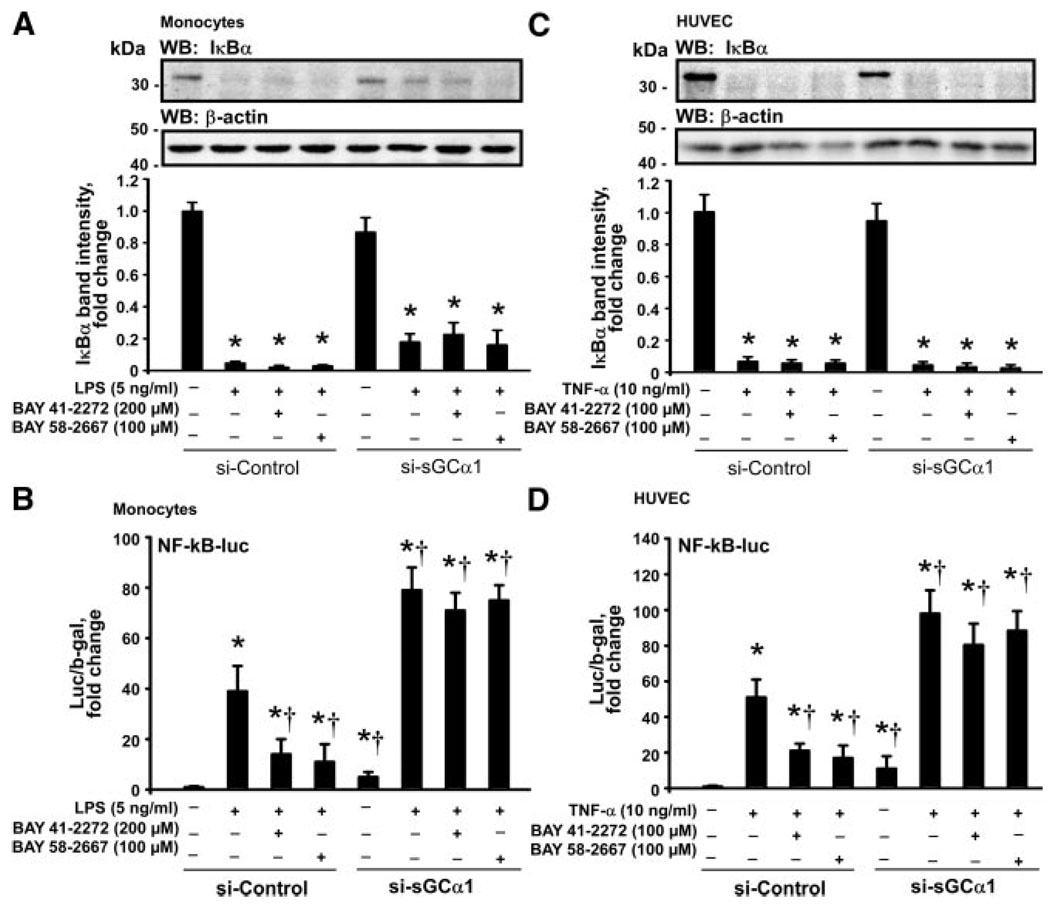
Figure 6 demonstrates that the loss of protein levels of IκBα in the LPS-stimulated monocytes and the TNF-α– stimulated HUVECs was independent of the sGCα1 expression and was not affected by BAY 41-2272 and BAY 58-2667. A knockdown of sGCα1 resulted in a significantly elevated NF-kB transcriptional activity in monocytes and HUVECs under resting conditions and particularly after stimulation with LPS or TNF-α, respectively. Both sGC agonists markedly attenuated the transcriptional activation of the NF-κB–sensitive reporter in the stimulated cells nucleofected with control siRNA but not in the stimulated cells nucleofected with siRNA against sGCα1.
Figure 6.
Agonists of sGC attenuate the transcriptional activity of NF-κB. BAY 41-2272 and BAY 58-2667 did not affect the loss of IκBα induced by LPS in monocytes (A) and by TNF-α in HUVECs (C), and this effect was independent of the sGCα1 expression. Both sGC agonists reduced the transcriptional activation of NF-κB–driven reporter gene induced by LPS in monocytes (B) and by TNF-α in HUVECs (D) in a sGCα1-sensitive manner. WB indicates Western blotting. Data are mean±SEM. *P<0.05 vs resting control cells; †P<0.05 vs the LPS- or TNF-α– stimulated cells.
Intracellular cGMP Concentrations
LPS and TNF-α significantly increased intracellular cGMP concentrations in monocytes and HUVECs, respectively, nucleofected with control siRNA (supplemental Figure VI). However, no such response occurred in the cells nucleofected with siRNA against sGCα1. BAY 41-2272 and BAY 58-2667 further increased intracellular cGMP concentrations in stimulated control monocytes and HUVECs but not in the stimulated sGCα1 knockdown cells.
Assessment of Cell Viability and Validation of the Anti-TF Antibody
None of the treatments applied in the present investigation had any significant effect on the fraction of apoptotic cells (supplemental Figure VII). Because most of the data on TF expression was obtained by using a single anti-TF antibody, we tested this antibody specificity by using a siRNA-mediated knockdown of TF expression. The Western blot shown on supplemental Figure VIII illustrates a nearly complete loss of TF immunopositive band in a sample of monocytes nucleofected with the TF-specific siRNA.
Discussion
Our investigation reveals that the sGC agonists BAY 41-2272 and BAY 58-2667 attenuate protein expression and function of TF in human monocytes stimulated with LPS, as demonstrated by Western blotting, real time RT-PCR, and the TF procoagulant activity assay. This response is associated with a marked reduction of total and surface TF presentation, as assessed by the ICW assay. Furthermore, BAY 41-2272 and BAY 58-2667 reduce TF protein expression in HUVECs, both under resting conditions and after stimulation with TNF-α. This effect is accompanied by a reduction of the TF-dependent procoagulant activity in the lysates of HUVECs under the same experimental conditions. Importantly, the inhibitory effects of both sGC agonists on TF expression are dependent on the presence of functional sGC, because the siRNA-mediated knockdown of the α1 subunit of sGC render stimulated monocytes and HUVECs insensitive to BAY 41-2271 and BAY 58-2667.
In the present study, BAY 41-2272 and BAY 58-2667 increased intracellular cGMP concentrations and decreased the expression of active TF protein, as well as the surface TF presentation in human monocytes and vascular endothelial cells stimulated, respectively, with LPS and TNF-α. These data are in agreement with an earlier investigation demonstrating an inhibitory role of cyclic nucleotides in regulation of TF expression in monocytes.10 Importantly, proinflammatory mediators such as LPS per se have been shown to increase intracellular levels of cGMP,24 as we also found in the present study. Apparently, further elevation of cGMP levels through pharmacological stimulation of sGC produces a self-limiting negative regulation of the inflammatory response. Of note, the observed changes in TF expression and functional activity in monocytes and HUVECs could not be attributed to the differences in the survival of cells after various treatments, as the fraction of apoptotic cells remained unchanged.
The concentrations of the sGC agonists used to achieve an acute pharmacological response in our in vitro investigation were higher than the concentrations previously tested to produce hemodynamic responses in vivo.12–14 However, an important finding was that the effects of BAY 41-2271 and BAY 58-2667 in monocytes were time-dependent, as both compounds elicited a stronger inhibitory activity on TF when the exposure time before the LPS stimulation was extended from 10 to 45 minutes. Thus, it is likely that with chronic administration the effective concentrations of these compounds required to elicit a significant inhibitory effect on TF in vivo would be much lower.
In humans, the promoter of F3 gene contains a binding site for NF-κB, which mediates responses to TNF-α and LPS.25,26 Although TNF-α and LPS can regulate TF expression by virtue of posttranscriptional stabilization of TF mRNA,27,28 upregulation of TF expression in response to inflammatory stimuli is largely dependent on the transcriptional activity of NF-κB. In the present study, we demonstrate that inhibition of TF by the sGC agonists in both monocytes and HUVECs is mediated at the level of the transcriptional regulation of NF-κB and not upstream of IκBα, because the loss of IκBα protein after stimulation with LPS and TNF-α was not affected by BAY 41-2272 and BAY 58-2667. Our findings are in agreement with Pan et al, who have reported that YC-1, a nonspecific nitric oxide–independent stimulator of sGC, reduced the LPS-induced expression of inflammatory cytokines in mononuclear cells via inhibition of NF-κB.29 Similarly, attenuation of the LPS-induced NF-κB activity by YC-1 has been recently shown in microglial cells.30
In circulating blood monocytes, regulation of TF expression and functional activity depends on interactions with other cell types, notably platelets. Platelets play a crucial role in facilitation of the LPS-induced TF expression in monocytes. 31 Furthermore, platelets promote thrombin generation by providing a catalytic surface for the TF/FVIIa/FXa complex and release FVa,32 and can also affect monocyte TF expression by releasing platelet-derived factors and inducing TNF-α.20,33,34 The cross-talk between monocytes and platelets results in synthesis and surface exposure of various adhesion molecules, such as P-selectin, lymphocyte function-associated antigen-1, membrane-activated complex-1,35 and CD40 with its CD40-ligand, which enhance platelet-monocyte interactions and TF expression by monocytes.36,37 Interestingly, BAY 41-2272 has been recently shown to inhibit platelet activity16 and down-regulate the expression of P-selectin,38 supporting our findings that these novel pharmacophores may exert antithrombotic effects by reducing TF expression in circulating blood monocytes and vascular endothelial cells.
The presence of functional sGC is crucial for BAY 41-2272 and BAY 58-2667 to elicit the inhibitory effect on TF expression, because the siRNA-mediated knockdown of the α1 subunit of sGC completely negated this effect. Interestingly, TF protein levels were significantly higher in resting monocytes and HUVECs in which sGCα1 expression was knocked-down as compared to the resting cells nucleofected with control siRNA. Likewise, the TF expression was also greatly enhanced in the sGCα1-deficient cells after stimulation with LPS and TNF-α. These findings indicate that in human monocytes and endothelial cells sGC plays an important regulatory role by controlling the TF system. Furthermore, the downregulation of TF expression and functional activity by BAY 41-2272 and BAY 58-2667 are mediated through the sGC-dependent mechanisms involving the suppression of transcriptional activity of NF-κB. Taken together, our data strongly suggest that inhibition of TF expression and activity by sGC agonists might provide therapeutic benefits in cardiovascular diseases associated with enhanced prothrombotic and inflammatory responses.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported in part by departmental funds, the Northern Norway Regional Health Authority (to M.A.S. and J.B.H.), and the Intramural Research Program of NIH/NIAAA (to P.P.).
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
Disclosures
J.P.S. is a full-time employee of Bayer Schering Pharma AG and a coinventor in several patent applications on sGC agonists.
References
- 1.Mackman N. Role of tissue factor in hemostasis and thrombosis. Blood Cells Mol Dis. 2006;36:104–107. doi: 10.1016/j.bcmd.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 2.Croce K, Libby P. Intertwining of thrombosis and inflammation in atherosclerosis. Curr Opin Hematol. 2007;14:55–61. doi: 10.1097/00062752-200701000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Ruf W, Mueller BM. Tissue factor in cancer angiogenesis and metastasis. Curr Opin Hematol. 1996;3:379–384. doi: 10.1097/00062752-199603050-00008. [DOI] [PubMed] [Google Scholar]
- 4.Stemerman MB, Pitlick FA, Dembitzer HM. Electron microscopic immunohistochemical identification of endothelial cells in the rabbit. Circ Res. 1976;38:146–156. doi: 10.1161/01.res.38.3.146. [DOI] [PubMed] [Google Scholar]
- 5.Colucci M, Balconi G, Lorenzet R, Pietra A, Locati D, Donati MB, Semeraro N. Cultured human endothelial cells generate tissue factor in response to endotoxin. J Clin Invest. 1983;71:1893–1896. doi: 10.1172/JCI110945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Egorina EM, Sovershaev MA, Olsen JO, Osterud B. Granulocytes do not express but acquire monocyte-derived tissue factor in whole blood: evidence for a direct transfer. Blood. 2008;111:1208–1216. doi: 10.1182/blood-2007-08-107698. [DOI] [PubMed] [Google Scholar]
- 7.Steffel J, Luscher TF, Tanner FC. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation. 2006;113:722–731. doi: 10.1161/CIRCULATIONAHA.105.567297. [DOI] [PubMed] [Google Scholar]
- 8.White RJ, Meoli DF, Swarthout RF, Kallop DY, Galaria II, Harvey JL, Miller CM, Blaxall BC, Hall CM, Pierce RA, Cool CD, Taubman MB. Plexiform-like lesions and increased tissue factor expression in a rat model of severe pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L583–L590. doi: 10.1152/ajplung.00321.2006. [DOI] [PubMed] [Google Scholar]
- 9.Riewald M, Ruf W. Orchestration of coagulation protease signaling by tissue factor. Trends Cardiovasc Med. 2002;12:149–154. doi: 10.1016/s1050-1738(02)00153-6. [DOI] [PubMed] [Google Scholar]
- 10.Lyberg T. Effect of cyclic AMP and cyclic GMP on thromboplastin (factor III) synthesis in human monocytes in vitro. Thromb Haemost. 1983;50:804–809. [PubMed] [Google Scholar]
- 11.Evgenov OV, Pacher P, Schmidt PM, Hasko G, Schmidt HH, Stasch JP. NO-independent stimulators and activators of soluble guanylate cyclase: discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evgenov OV, Ichinose F, Evgenov NV, Gnoth MJ, Falkowski GE, Chang Y, Bloch KD, Zapol WM. Soluble guanylate cyclase activator reverses acute pulmonary hypertension and augments the pulmonary vasodilator response to inhaled nitric oxide in awake lambs. Circulation. 2004;110:2253–2259. doi: 10.1161/01.CIR.0000144469.01521.8A. [DOI] [PubMed] [Google Scholar]
- 13.Dumitrascu R, Weissmann N, Ghofrani HA, Dony E, Beuerlein K, Schmidt H, Stasch JP, Gnoth MJ, Seeger W, Grimminger F, Schermuly RT. Activation of soluble guanylate cyclase reverses experimental pulmonary hypertension and vascular remodeling. Circulation. 2006;113:286–295. doi: 10.1161/CIRCULATIONAHA.105.581405. [DOI] [PubMed] [Google Scholar]
- 14.Evgenov OV, Kohane DS, Bloch KD, Stasch JP, Volpato GP, Bellas E, Evgenov NV, Buys ES, Gnoth MJ, Graveline AR, Liu R, Hess DR, Langer R, Zapol WM. Inhaled agonists of soluble guanylate cyclase induce selective pulmonary vasodilation. Am J Respir Crit Care Med. 2007;176:1138–1145. doi: 10.1164/rccm.200707-1121OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boerrigter G, Costello-Boerrigter LC, Cataliotti A, Tsuruda T, Harty GJ, Lapp H, Stasch JP, Burnett JC., Jr Cardiorenal and humoral properties of a novel direct soluble guanylate cyclase stimulator BAY 41–2272 in experimental congestive heart failure. Circulation. 2003;107:686–689. doi: 10.1161/01.cir.0000055737.15443.f8. [DOI] [PubMed] [Google Scholar]
- 16.Hobbs AJ, Moncada S. Antiplatelet properties of a novel, non-NO-based soluble guanylate cyclase activator, BAY 41–2272. Vascul Pharmacol. 2003;40:149–154. doi: 10.1016/s1537-1891(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 17.Grimminger F, Weimann G, Frey R, Voswinckel R, Thamm M, Bolkow D, Weissmann N, Muck W, Unger S, Wensing G, Schermuly RT, Ghofrani HA. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J. 2009;33:785–792. doi: 10.1183/09031936.00039808. [DOI] [PubMed] [Google Scholar]
- 18.Lapp H, Mitrovic V, Franz N, Heuer H, Buerke M, Wolfertz J, Mueck W, Unger S, Wensing G, Frey R. Cinaciguat (BAY 58–2667) improves cardiopulmonary hemodynamics in patients with acute decompensated heart failure. Circulation. 2009;119:2781–2788. doi: 10.1161/CIRCULATIONAHA.108.800292. [DOI] [PubMed] [Google Scholar]
- 19.Egorina EM, Sovershaev MA, Osterud B. In-cell Western assay: a new approach to visualize tissue factor in human monocytes. J Thromb Haemost. 2006;4:614–620. doi: 10.1111/j.1538-7836.2005.01781.x. [DOI] [PubMed] [Google Scholar]
- 20.Osterud B. Platelet activating factor enhancement of lipopolysaccharide-induced tissue factor activity in monocytes: requirement of platelets and granulocytes. J Leukoc Biol. 1992;51:462–465. [PubMed] [Google Scholar]
- 21.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–1461. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- 22.Egorina EM, Sovershaev MA, Bjorkoy G, Gruber FX, Olsen JO, Parhami-Seren B, Mann KG, Osterud B. Intracellular and surface distribution of monocyte tissue factor: application to intersubject variability. Arterioscler Thromb Vasc Biol. 2005;25:1493–1498. doi: 10.1161/01.ATV.0000168413.29874.d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bach RR. Tissue factor encryption. Arterioscler Thromb Vasc Biol. 2006;26:456–461. doi: 10.1161/01.ATV.0000202656.53964.04. [DOI] [PubMed] [Google Scholar]
- 24.Connelly L, Jacobs AT, Palacios-Callender M, Moncada S, Hobbs AJ. Macrophage endothelial nitric-oxide synthase autoregulates cellular activation and pro-inflammatory protein expression. J Biol Chem. 2003;278:26480–26487. doi: 10.1074/jbc.M302238200. [DOI] [PubMed] [Google Scholar]
- 25.Mackman N, Brand K, Edgington TS. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THP-1 monocytic cells requires both activator protein 1 and nuclear factor kappa B binding sites. J Exp Med. 1991;174:1517–1526. doi: 10.1084/jem.174.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin E, Baeuerle PA, Waldher R, Kisiel W, Ziegler R, Stern DM, Nawroth PP. Mechanism of the tumor necrosis factor alpha-mediated induction of endothelial tissue factor. J Biol Chem. 1995;270:26419–26432. doi: 10.1074/jbc.270.44.26419. [DOI] [PubMed] [Google Scholar]
- 27.Scarpati EM, Sadler JE. Regulation of endothelial cell coagulant properties. Modulation of tissue factor, plasminogen activator inhibitors, and thrombomodulin by phorbol 12-myristate 13-acetate and tumor necrosis factor. J Biol Chem. 1989;264:20705–20713. [PubMed] [Google Scholar]
- 28.Brand K, Fowler BJ, Edgington TS, Mackman N. Tissue factor mRNA in THP-1 monocytic cells is regulated at both transcriptional and posttranscriptional levels in response to lipopolysaccharide. Mol Cell Biol. 1991;11:4732–4738. doi: 10.1128/mcb.11.9.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pan SL, Guh JH, Peng CY, Chang YL, Cheng FC, Chang JH, Kuo SC, Lee FY, Teng CM. A potential role of YC-1 on the inhibition of cytokine release in peripheral blood mononuclear leukocytes and endotoxemic mouse models. Thromb Haemost. 2005;93:940–948. doi: 10.1160/TH04-03-0195. [DOI] [PubMed] [Google Scholar]
- 30.Lu DY, Tang CH, Liou HC, Teng CM, Jeng KC, Kuo SC, Lee FY, Fu WM. YC-1 attenuates LPS-induced proinflammatory responses and activation of nuclear factor-kappaB in microglia. Br J Pharmacol. 2007;151:396–405. doi: 10.1038/sj.bjp.0707187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pinder PB, Hunt JA, Zacharski LR. In vitro stimulation of monocyte tissue factor activity by autologous platelets. Am J Hematol. 1985;19:317–325. doi: 10.1002/ajh.2830190402. [DOI] [PubMed] [Google Scholar]
- 32.Osterud B, Rapaport SI, Lavine KK. Factor V activity of platelets: evidence for an activated factor V molecule and for a platelet activator. Blood. 1977;49:819–834. [PubMed] [Google Scholar]
- 33.Neumann FJ, Marx N, Gawaz M, Brand K, Ott I, Rokitta C, Sticherling C, Meinl C, May A, Schomig A. Induction of cytokine expression in leukocytes by binding of thrombin-stimulated platelets. Circulation. 1997;95:2387–2394. doi: 10.1161/01.cir.95.10.2387. [DOI] [PubMed] [Google Scholar]
- 34.Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH, Prescott SM, Zimmerman GA. Activated platelets signal chemokine synthesis by human monocytes. J Clin Invest. 1996;97:1525–1534. doi: 10.1172/JCI118575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neumann FJ, Zohlnhofer D, Fakhoury L, Ott I, Gawaz M, Schomig A. Effect of glycoprotein IIb/IIIa receptor blockade on platelet-leukocyte interaction and surface expression of the leukocyte integrin Mac-1 in acute myocardial infarction. J Am Coll Cardiol. 1999;34:1420–1426. doi: 10.1016/s0735-1097(99)00350-2. [DOI] [PubMed] [Google Scholar]
- 36.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 37.Lindmark E, Tenno T, Siegbahn A. Role of platelet P-selectin and CD40 ligand in the induction of monocytic tissue factor expression. Arterioscler Thromb Vasc Biol. 2000;20:2322–2328. doi: 10.1161/01.atv.20.10.2322. [DOI] [PubMed] [Google Scholar]
- 38.Ahluwalia A, Foster P, Scotland RS, McLean PG, Mathur A, Perretti M, Moncada S, Hobbs AJ. Antiinflammatory activity of soluble guanylate cyclase: cGMP-dependent down-regulation of P-selectin expression and leukocyte recruitment. Proc Natl Acad Sci U S A. 2004;101:1386–1391. doi: 10.1073/pnas.0304264101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.