Abstract
The pubertal transition has been identified as a time of risk for development of type 2 diabetes, particularly among vulnerable groups, such as African Americans (AAs). Documented ethnic differences in insulin secretory dynamics may predispose overweight AA adolescents to risk for type 2 diabetes. The objectives of this longitudinal study were to quantify insulin secretion and clearance in a cohort of 90 AA and European American (EA) children over the pubertal transition and to explore the association of genetic factors and adiposity with repeated measures of insulin secretion and clearance during this critical period. Insulin sensitivity was determined by intravenous glucose tolerance test (IVGTT) and minimal modeling; insulin secretion and clearance by C-peptide modeling; genetic ancestry by admixture analysis. Mixed-model longitudinal analysis indicated that African genetic admixture (AfADM) was independently and positively associated with first-phase insulin secretion within the entire group (P < 0.001), and among lean children (P < 0.01). When examined within pubertal stage, this relationship became significant at Tanner stage 3. Total body fat was a significant determinant of first-phase insulin secretion overall and among obese children (P < 0.001). Total body fat, but not AfADM, was associated with insulin clearance (P < 0.001). In conclusion, genetic factors, as reflected in AfADM, may explain greater first-phase insulin secretion among peripubertal AA vs. EA; however, the influence of genetic factors is superseded by adiposity. The pubertal transition may affect the development of the β-cell response to glucose in a manner that differs with ethnic/genetic background.
INTRODUCTION
The pubertal transition has been identified as a time of risk for development of type 2 diabetes, particularly among vulnerable groups, such as African Americans (AAs) who have been disproportionately burdened relative to European Americans (EAs) (1). The disparities may be related to racial/ethnic differences in the insulin secretory profile. It is well documented that healthy AA children and adults have higher postchallenge insulin concentration, relative to EA (2–4) and the greater insulin response among AA is apparent even after adjusting for insulin sensitivity (which is lower in AA) (3). The physiologic relevance and clinical implications of the difference are not known. An understanding of underlying mechanisms associated with insulin dynamics in healthy AA and EA adolescents, particularly during the pubertal transition, a critical period in growth and development, could serve as a potential avenue to understand the cause of ethnic disparities in disease prevalence.
Puberty is associated with a period of transient insulin resistance. We have shown that during the pubertal transition, changes in the acute insulin response to glucose (AIRg) do not fully compensate for the transient decrease in insulin sensitivity (5). However, this earlier study assessed only peripheral insulin concentrations during the first 10 min following glucose administration (AIRg). Because AIRg captures both secretion and clearance, and because compensatory events may occur after the acute response period, examination of β cell function throughout the test using C-peptide analysis is needed to accurately characterize the influence of ethnicity on insulin secretory dynamics during the pubertal transition.
Obesity is associated with lower insulin sensitivity, lower insulin clearance and altered insulin secretory dynamics (6–10) and may confound assessment of ethnic influences on these measures. Ethnic differences in insulin sensitivity and secretion that have been documented in nonobese populations have not consistently been observed when the subject population consisted of obese individuals (6,7,9,11). In analyses of early pubertal children, we have shown that both obesity and ethnicity are uniquely associated with insulin sensitivity index (SI) and AIRg (12). Furthermore, adolescence is a time during which children are at risk for gaining body fat. We have shown that AA girls, relative to EA girls, gain a greater amount of body fat following menarche (13). Thus, studies designed to examine ethnic influences on insulin secretion and action should take into account to potential confounding influence of adiposity.
Previous studies in children examining insulin secretion and clearance among ethnic groups have typically relied on cross-sectional analysis and used surrogate indices of insulin secretion (14,15) or insulin clearance (3,15). Longitudinal assessment quantifying insulin secretion and clearance in an adolescent population is very limited but may be especially useful in the identification of contributors to disparities in obesity and insulin dynamics.
The objectives of this longitudinal analysis were (i) to quantify insulin secretion and clearance in a cohort of AA and EA children and adolescents during the pubertal transition using mathematical modeling, and (ii) to examine potential independent associations of genetic admixture and body fat with insulin secretion and clearance. We tested the specific hypotheses that insulin secretion would be higher, and insulin clearance lower, among peripubertal children with greater African admixture, and that adiposity would override this association. This study extends existing knowledge on ethnic differences in insulin secretion and clearance in AA and EA children by providing a longitudinal perspective over the pubertal transition, identifying the contribution of obesity to changes in the insulin secretory profile, and through using robust measures of insulin dynamics and genetic admixture.
METHODS AND PROCEDURES
Subjects
Data were derived from a longitudinal observational study exploring the contribution of body fat distribution to disease risk in children. Data were collected for these analyses between 1999 and 2003. Recruitment approach and criteria have been detailed elsewhere (7). Data from 90 children and adolescents aged 8–16 years were available for present analyses; 44 subjects self-identified as EA, and 46 as AA. Not all subjects joined the study in the same year, and not all subjects completed all possible visits (239 tests with each subject having 1–4 tests). The study protocol was approved by the Institutional Review Board for Human Use at the University of Alabama at Birmingham.
Protocol
Annually (at approximately the same time each year), subjects were admitted into the General Clinical Research Center at the University of Alabama at Birmingham for an overnight stay. Anthropometric measurements were assessed by a registered dietitian, and sexual maturation was assessed by a pediatrician according to the criteria of Marshall and Tanner (16). Children were served a standard dinner meal and evening snack, with all food consumed before 2000 hours. After the overnight fast, a tolbutamide-modified IVGTT was performed. Body composition was determined by dual-energy X-ray absorptiometry. Female subjects who had started menstruating were tested in the follicular phase of their menstrual cycle.
Total and percent body fat
Body composition was determined by dual-energy X-ray absorptiometry (Lunar DPX-L, software version 1.5e; Lunar radiation, Madison, WI), as described previously (17).
Glucose, insulin, and C-peptide
Glucose was measured in 10-μl sera using an Ektachem DT II System (Johnson and Johnson, Rochester, NY). In our laboratory this analysis has a mean intra-assay coefficient of variation (CV) of 0.61% and a mean interassay CV of 1.45%. Insulin was assayed in duplicate 200-μl aliquots with a solid-phase radioimmunoassay (Diagnostic Products, Los Angeles, CA). In our laboratory this assay has a sensitivity of 1.9 μIU/ml, a mean intra-assay CV of 5%, and a mean interassay CV of 6%. C-peptide was measured in duplicate 25-μl aliquots with a double-antibody radioimmunoassay (Diagnostic Products). In our laboratory this assay has a sensitivity of 0.318 ng/ml, a mean intra-assay CV of 3.57%, and a mean interassay CV of 5.59%.
Reproductive hormones
Sera were analyzed for estradiol using a double-antibody radioimmunoassay (Diagnostic Products), and for total testosterone using a solid-phase immunoassay (Coat-A-Count Total Testosterone; Diagnostic Products). In our laboratory, assay sensitivity for estradiol is 15.42 pmol/l, mean intra-assay CV is 4.69%, and interassay CV is 6.0%. For testosterone, the sensitivity is 11.8 ng/dl and the intra- and interassay CV are 2.7% and 11.4%, respectively. Samples that had undetectable concentrations of hormone were assigned the minimum detectable value for that hormone.
IVGTT
On the morning after the overnight fast, a topical anesthetic was applied to the antecubital space of both arms, and flexible intravenous catheters were placed in both arms. Baseline samples were collected for hormone analysis. At time zero, glucose (25% dextrose; 11.4 g/m2) was administered intravenously. Blood samples (2 ml) were collected at the following times relative to glucose administration at 0 min: −15, −5, −1, 2, 3, 4, 5, 6, 8, 10, 14, 19, 22, 25, 30, 40, 50, 70, 100, 140, and 180 min. Tolbutamide (125 mg/m2) was injected intravenously at 20 min. Values for fasting insulin were obtained from the average of the baseline values. Glucose and insulin values were entered into the MINMOD computer program (Millenium 2003 version 5.16, Los Angeles, CA, copyright Richard N. Bergman) for derivation of the SI as described elsewhere (18).
Parameters of insulin secretion and clearance
Prehepatic insulin secretion rates and percent insulin extracted by the liver were calculated based on the Extended Combined model (19). This computer-based technique uses the insulin and C-peptide measures obtained at each time point during the IVGTT to generate estimates of prehepatic insulin secretion rate and percent hepatic extraction. First-phase insulin secretion was calculated as the area under the curve for insulin secretion rate for the first 10 min of the test; second-phase was calculated similarly from the remainder of the test; and total insulin secretion was the sum of the two phases of insulin secretion. Incremental first- and second-phase insulin secretion and incremental total insulin secretion were similarly calculated after subtraction of basal insulin secretion. The insulin secretion rates predicted by the model were corrected to mass per unit time by correcting for the C-peptide distribution space, assumed to be 6.02% body weight (20,21). All analyses were done using the MLAB software (Civilized Software, Bethesda, MD) with the weighted nonlinear least square approach, as published (20). Hepatic insulin clearance was expressed as the percent of total insulin secreted over the 180-min IVGTT. Absolute amount of hormone cleared over the 180-min IVGTT was calculated as the product of percent extraction and total insulin secretion.
Genetic admixture
Genetic material for all subjects was obtained. Individual estimates of genetic admixture were obtained by genotyping 22 ancestry informative markers, previously identified for parental European and African populations. Markers used for this study, their chromosomal and centimorgan location, and their allelic difference between European and African parental populations, have been published (22). Markers and techniques used for the identification of the ancestry informative markers have previously been described by Parra et al. (23) and are available through dbSNP (http://www.ncbi.nlm.nih.gov/SNP/) using the handle PSA-ANTH. Genotyping was performed at the Pennsylvania State University. Genotyping was done using molecular techniques such as the melting curve analysis of single-nucleotide polymorphisms (Mc-SNP) method described by Akey et al. (24) and agarose gel electrophoresis. All genotype information was translated into an individual value for genetic admixture using a maximum likelihood method, as described (22,25).
Socioeconomic status (SES)
Differences in health status, based on SES are well documented (26–28). SES was measured with the Hollingshead 4-factor index of social class (29), which combines the educational attainment and occupational prestige for the number of working parents in the child's family. Scores range from 8 to 66, with a higher score indicating higher theoretical social status.
Statistical analyses
As the study design included multiple (1–4) observations per subject, analyses were performed using a mixed linear model (PROC MIXED) to determine the relationship of the dependent variables with African genetic admixture (AfADM) and total body fat. Pubertal stage was modeled as a categorical variable, with stage 5 as the reference value. PROC MIXED was selected for analyses because of its ability to utilize multiple observations on one individual, account for missing data, and incorporate covariates. Statistical models incorporated age and pubertal stage as covariates, to adjust for changes in derived variables with age and maturation. Dependent variables were basal insulin secretion; incremental first-phase, second-phase, and total insulin secretion; percent insulin extraction; and absolute insulin cleared by the liver. Because some children remained at a given pubertal stage throughout several consecutive years of testing, an “age-nested-in-pubertal stage” component was included in the models. Although there was high colinearity between pubertal stage and age, use of the nested term was necessary to account for changes in the dependent variables that occurred with age, within a given pubertal stage (30). SES serves as a proxy for socioenvironmental contributions to differences in health status. Previous studies have documented that SES is related to differences in insulin dynamics (26–28). To account for the socioenvironmental contribution, SES was adjusted for in all models.
Exploratory analyses, including evaluation of the fit of the model by regression analyses, and testing normality of residuals, were used to identify the best-fit model for dependent variables. Variables that were not normally distributed were log transformed before analyses. This transformation yielded a normal distribution, and these transformed variables were used in all subsequent analyses. All statistical models were constructed to identify potential independent associations with AfADM and total body fat. All statistical models were adjusted by age, SES, pubertal stage, and age-nested-in-pubertal stage. The contribution of sex was little or none and therefore sex was not included in the final models. The final models for basal insulin secretion, incremental first- and second-phase insulin secretion, and incremental total insulin secretion included SI, and that for percent insulin extraction, included total insulin secretion. The inclusion of SI as a covariate eliminated potential confounding due to sex- (31), obesity-, or ethnicity-mediated effects on insulin sensitivity. To further explore trends over the pubertal transition, models were also analyzed according to pubertal stage.
Because body fat was independently related to several dependent variables, “obese” (n = 106 observations) and “lean” (n = 133 observations) subject subgroups were created by classifying each subject at each visit as either overweight/obese or lean according to their percent body fat. We used the cut-points of 30% fat in girls and 25% fat in boys for defining obesity in children established by Williams et al. (32) and recommended by others (33–35). In the mixed-model analysis every subject at each time point is classified according to lean or obese in the model and adjusted by pubertal stage. By doing so, this accounts for dynamic changes in body composition that may have occurred throughout the study. All statistical analyses were performed using SAS version 8.0 (SAS Institute, Cary, NC).
RESULTS
The descriptive characteristics and major outcome variables of the subjects are provided in Table 1; means are given by ethnicity and pubertal stage. AA matured at a faster rate, had lower SI, and higher AIRg.
Table 1.
Descriptive statistics and outcome measures by ethnicity and pubertal stage (mean ± s.d.)
| Pubertal stage |
|||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| European American | |||||
| n (observations)/(subjects) | 18/9 | 18/12 | 31/22 | 32/21 | 13/9 |
| Male subjects (n/%) | 5/56 | 5/42 | 4/18 | 6/29 | 5/56 |
| Lean subjects (n/%) | 6/67 | 8/75 | 16/73 | 10/48 | 4/44 |
| Age (years) | 11 ± 1 | 12 ± 1 | 14 ± 1** | 14 ± 1** | 15 ± 2 |
| Weight (kg) | 39 ± 13 | 45 ± 12 | 54 ± 12 | 66 ± 15 | 71 ± 11 |
| Total fat mass (kg) | 12 ± 8 | 16 ± 15 | 16 ± 10 | 23 ± 12 | 20 ± 13 |
| Percent fat (%) | 26 ± 12 | 27 ± 12 | 27 ± 10 | 33 ± 11 | 27 ± 14 |
| Estradiol (pg/ml) | |||||
| Males | 4 ± 2 | 4 ± 2 | 4 ± 2 | 5 ± 1 | 8 ± 7 |
| Females | 4 ± 0 | 4 ± 2 | 19 ± 27 | 33 ± 35 | 22 ± 12** |
| Testosterone (pg/ml) | |||||
| Males | 12 ± 0 | 13 ± 4 | 88 ± 131 | 230 ± 266 | 297 ± 204 |
| Females | 10 ± 0 | 12 ± 1 | 14 ± 4 | 24 ± 13 | 22 ± 12 |
| SI (×10–4/min/(uIU/ml)) | 9 ± 5* | 7 ± 4** | 6 ± 3** | 5 ± 3 | 6 ± 4* |
| Φ1 (nmol/min) | 10 ± 8 | 19 ± 21* | 14 ± 6** | 22 ± 17* | 16 ± 9*** |
| Φ2 (nmol/min) | 45 ± 68 | 80 ± 100 | 62 ± 33 | 104 ± 85 | 84 ± 79 |
| Total insulin secretion (nmol/min) | 55 ± 75 | 93 ± 107 | 75 ± 38* | 126 ± 100 | 100 ± 87* |
| Insulin extraction (%) | 49 ± 7 | 50 ± 7 | 50 ± 11 | 53 ± 7 | 54 ± 7 |
| Absolute insulin clearance (nmol/min) | 28 ± 43 | 47 ± 59 | 38 ± 23 | 67 ± 56 | 53 ± 40* |
| African American | |||||
| n (observations)/(subjects) | 14/9 | 20/17 | 24/19 | 33/26 | 35/16 |
| Male subjects (n/%) | 5/56 | 9/53 | 10/53 | 10/38 | 4/25 |
| Lean subjects (n/%) | 7/78 | 7/41 | 10/53 | 13/50 | 5/31 |
| Age (years) | 11 ± 1 | 11 ± 1 | 12 ± 1 | 13 ± 1 | 15 ± 1 |
| Weight (kg) | 38 ± 16 | 55 ± 19 | 63 ± 17 | 67 ± 16 | 72 ± 17 |
| Total fat mass (kg) | 10 ± 12 | 19 ± 14 | 20 ± 11 | 20 ± 12 | 24 ± 14 |
| Percent fat (%) | 21 ± 12 | 32 ± 12 | 30 ± 10 | 28 ± 11 | 32 ± 13 |
| Estradiol (pg/ml) | |||||
| Males | 4 ± 0 | 4 ± 0 | 4 ± 0 | 5 ± 2 | 7 ± 4 |
| Females | 4 ± 0 | 4 ± 1 | 17 ± 23 | 24 ± 31 | 42 ± 41 |
| Testosterone (pg/ml) | |||||
| Males | 12 ± 0 | 15 ± 3 | 93 ± 164 | 178 ± 217 | 280 ± 234 |
| Females | 12 ± 1 | 12 ± 0 | 17 ± 23 | 26 ± 18 | 31 ± 16 |
| SI (×10–4/min/(uIU/ml)) | 6 ± 2 | 3 ± 1 | 3 ± 2 | 3 ± 2 | 3 ± 2 |
| Φ1 (nmol/min) | 10 ± 8 | 40 ± 39 | 37 ± 31 | 46 ± 51 | 46 ± 29 |
| Φ2 (nmol/min) | 28 ± 25 | 120 ± 202 | 92 ± 103 | 122 ± 113 | 126 ± 81 |
| Total insulin secretion (nmol/min) | 38 ± 32 | 160 ± 238 | 129 ± 133 | 168 ± 157 | 173 ± 106 |
| Insulin extraction (%) | 44 ± 8 | 46 ± 10 | 46 ± 11 | 50 ± 10 | 51 ± 11 |
| Absolute insulin clearance (nmol/min) | 16 ± 13 | 78 ± 126 | 61 ± 60 | 84 ± 74 | 91 ± 62 |
EA vs. AA
P < 0.05
P < 0.01
P < 0.001.
Φ1, first-phase insulin secretion; Φ2, second-phase insulin secretion; SI, derived insulin sensitivity index.
Among children who reported their ethnicity as EA, AfADM ranged from 0 to 21%; among those who reported their ethnicity as AA, AfADM ranged from 57 to 100%. SES ranged from 14 to 66. On average, SES was higher among EA (51.88 ± 8.87, mean ± s.d.) relative to AA (32.13 ± 13.09; P < 0.001).
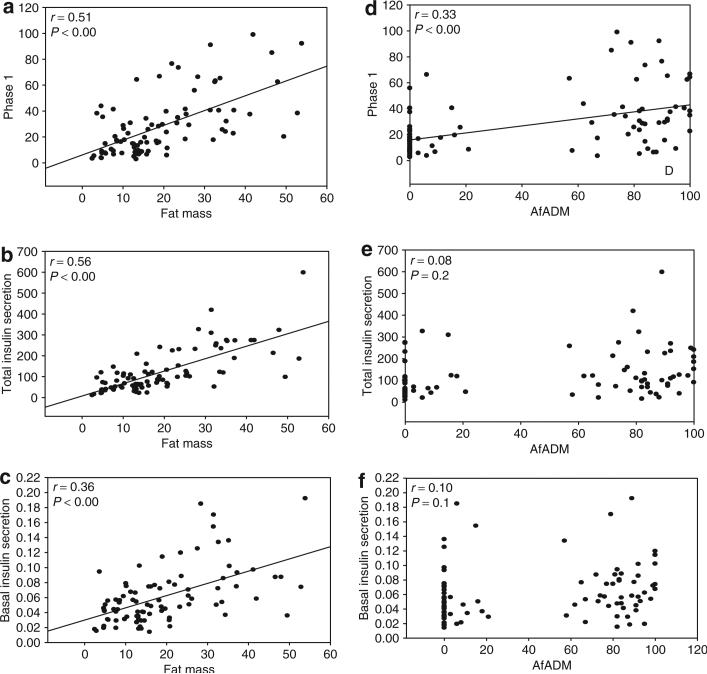
Results of mixed-model analyses for insulin secretion outcomes are shown in Table 2. In all insulin secretion models, SI was significantly and inversely related to insulin secretion. AfADM was positively related to first-phase insulin secretion (P < 0.001), but was not related to second-phase, total or basal insulin secretion. Total body fat was positively related to all measures of insulin secretion (P < 0.001) (Figure 1). When models were analyzed by Tanner stage, AfADM was a significant contributor to first-phase insulin secretion beginning at Tanner stage 3 (Table 3). When second-phase insulin secretion, basal insulin secretion, and total insulin secretion were analyzed by Tanner stage, AfADM was not significant (Table 3).
Table 2.
Mixed linear models for dependant variables reflecting insulin secretion
| Estimate | s.e. | P | |
|---|---|---|---|
| Basal insulin secretion | |||
| Intercept | –3.7638 | 1.0443 | <0.001 |
| S I | –0.2880 | 0.0731 | <0.001 |
| Total fat | 0.5751 | 0.0762 | <0.001 |
| AfADM | –0.0004 | 0.0015 | 0.806 |
| First-phase insulin secretion | |||
| Intercept | –0.3910 | 1.0118 | 0.700 |
| S I | –0.1800 | 0.0764 | 0.020 |
| Total fat | 0.5114 | 0.0855 | <0.001 |
| AfADM | 0.0064 | 0.0018 | <0.001 |
| Second-phase insulin secretion | |||
| Intercept | 0.6783 | 1.1909 | 0.570 |
| S I | –0.6696 | 0.0815 | <0.001 |
| Total fat | 0.4584 | 0.0835 | <0.001 |
| AfADM | –0.0024 | 0.0017 | 0.159 |
| Total insulin secretion | |||
| Intercept | 1.2628 | 1.0700 | 0.241 |
| S I | –0.5088 | 0.0729 | <0.001 |
| Total fat | 0.4676 | 0.0753 | <0.001 |
| AfADM | –0.0002 | 0.0015 | 0.878 |
All models were adjusted for age, pubertal stage, age-nested-in-pubertal stage, and SES.
AfADM, African genetic admixture; SES, socioeconomic status; SI, Insulin sensitivity index.
Figure 1.
Relationship (bivariate correlations) between (a–c) insulin secretion and total fat mass and (d–f) insulin secretion and African genetic admixture in the entire study population.
Table 3.
P values from mixed linear models reflecting the contribution of AfADM to insulin secretory profile by pubertal stage
| Pubertal stage | Φ1 | Φ2 | Basal | Total |
|---|---|---|---|---|
| I | 0.220 | 0.581 | 0.809 | 0.534 |
| II | 0.797 | 0.084 | 0.575 | 0.174 |
| III | 0.005 | 0.982 | 0.808 | 0.274 |
| IV | 0.018 | 0.502 | 0.169 | 0.142 |
| V | 0.031 | 0.732 | 0.443 | 0.424 |
Significant P values in boldface.
All models were adjusted for age, age-nested-in-pubertal stage, and SES.
AfADM, African genetic admixture; Φ1, first-phase insulin secretion; Φ2, second-phase insulin secretion; SES, socioeconomic status.
Results for mixed-model analyses for percent insulin extraction and absolute quantity of insulin cleared by the liver are shown in Table 4. Total insulin secretion was positively related to percent insulin extraction (P < 0.05). AfADM tended to be negatively related to percent insulin extraction (P = 0.055), but was not related to absolute insulin clearance. Total body fat was positively related to absolute insulin clearance (P < 0.001).
Table 4.
Mixed linear models for dependant variables reflecting insulin clearance
| Estimate | s.e. | P | |
|---|---|---|---|
| Percent insulin extraction | |||
| Intercept | 0.3465 | 0.1579 | 0.030 |
| Total insulin secretion | 0.0001 | 0.0000 | 0.018 |
| Total fat | –0.0009 | 0.0006 | 0.151 |
| AfADM | –0.0004 | 0.0002 | 0.055 |
| Absolute insulin clearance | |||
| Intercept | –0.0894 | 1.2095 | 0.941 |
| Total fat | 0.7125 | 0.0773 | <0.001 |
| AfADM | 0.0013 | 0.0017 | 0.413 |
All models were adjusted for age, pubertal stage, age-nested-in-pubertal stage, and SES.
AfADM, African genetic admixture; SES, socioeconomic status.
In “obese” children, SI (P = 0.01) and total fat (P < 0.001) were significant in the model for first-phase insulin secretion (Table 5). In the “lean” children, AfADM (P < 0.05) was the only significant variable in the model for first-phase insulin secretion (Table 5). Similarly, SI and total body fat were significant in the “obese” group in the models for other phases of secretion (data not shown). Total body fat remained a significant contributor to absolute insulin clearance in both “obese” and “lean” groups; AfADM was significantly and positively associated with absolute insulin clearance in the “lean” group (P < 0.01; data not shown). Otherwise, separate analyses of the models for second-phase, total, and basal insulin secretion, and for absolute insulin clearance in the “obese” and the “lean” subgroups, revealed no meaningful differences from the analogous models conducted in the entire cohort.
Table 5.
Mixed linear models for the dependant variable first-phase insulin secretion
| Estimate | s.e. | P | |
|---|---|---|---|
| First-phase insulin secretion (obese subjects only) | |||
| Intercept | –0.990 | 1.486 | 0.508 |
| S I | –0.281 | 0.105 | 0.010 |
| Total fat | 0.935 | 0.208 | <0.001 |
| AfADM | 0.005 | 0.002 | 0.062 |
| First-phase insulin secretion (lean subjects only) | |||
| Intercept | –1.378 | 1.624 | 0.419 |
| S I | 0.032 | 0.119 | 0.782 |
| Total fat | 0.344 | 0.141 | 0.145 |
| AfADM | 0.006 | 0.002 | 0.014 |
Models were conducted separately within the obese and lean subgroups. All models were adjusted for age, pubertal stage, age-nested-in-pubertal stage, and SES.
AfADM, African genetic admixture; SES, socioeconomic status; SI, insulin sensitivity index.
DISCUSSION
Results of this study expand our previous research on ethnic differences in insulin dynamics. Using mixed-model analysis, we were able to identify trends across puberty in the insulin secretory profile. We identified an association between first-phase insulin secretion and AfADM that began at Tanner stage 3, suggesting that the contribution of genetic make-up to β-cell function begins at reproductive maturity. Further, among peripubertal children, the relationship between AfADM and first-phase insulin secretion differed with adiposity. Among obese subjects, total body fat but not AfADM was a determinant of first-phase insulin secretion, whereas among lean subjects, AfADM was significant. AfADM tended to be associated with a lower percent, but not total, hepatic insulin clearance in the entire cohort. As such, genetic factors (ancestral genetic background) may play a role in determining postchallenge insulin responses in lean individuals, whereas the potential genetic effect influencing insulin dynamics is attenuated by a physiologic component among obese individuals. This is an important consideration to be taken into account in studies probing racial/ethnic and genetic contribution to diabetes risks.
The first phase of insulin secretion is essential for maintaining normoglycemia and achieving effective glucose homeostasis (36). There are few studies and to our knowledge no longitudinal studies in children that evaluate the ethnic differences in insulin secretory profile among obese and lean children over the pubertal transition. The physiological relevance of a greater first-phase insulin response in AA is not known, but alterations in insulin secretory dynamics may be an early event in the etiology of impaired glucose tolerance or “prediabetes” (36,37). It is possible that greater first-phase insulin secretion in response to a glucose challenge may lead to exhaustion of the pancreatic β-cells at a faster rate, and even to a loss of β-cell mass through increased susceptibility to apoptosis (38,39). Such stress on the pancreas may be particularly detrimental during puberty, when there is a transient decline in insulin sensitivity and an associated increase in β-cell demand. We have previously demonstrated in this population that hyper-insulinemia during the pubertal transition may lead to greater fat mass deposition in AA relative to EA (13). Whether the metabolic perturbations associated with puberty (i.e., insulin resistance, hyperinsulinemia) are more apparent among AA, and are related to increased disease risk in adulthood is not clear. Longitudinal data are needed to better understand the roles of insulin resistance and secretion in the development of chronic metabolic disease.
The determinants of greater first-phase insulin secretion among AA relative to EA have been unclear. Previous studies conducted by our group have suggested that AA children maintain a larger readily releasable pool of insulin in the pancreas, which could lead to increased first-phase β-cell sensitivity to glucose (3). We have previously demonstrated greater first-phase insulin secretion among AA is independent of lower SI among AA compared to EA (3). Further, in this study, inclusion of SI in all models controlled for any potential confounding due to differences in insulin sensitivity. Present data suggested that genetic factors may exert independent effects on insulin secretion during first-phase insulin secretion. Ethnic differences in factors regulating insulin secretion have been reported (40,41). Higher glucagon-like peptide-1 concentrations have been documented in obese AA adults, when compared to obese EA adults, both in the fasting state and during an oral glucose tolerance test (41). Glucagon-like peptide-1 is thought to increase β-cell proliferation (41,42) and may account for the increased β-cell responsiveness among AA.
In accordance with the findings of Weiss et al. (11) using an oral glucose tolerance test, our results indicated that total body fat had an independent effect on insulin secretion, such that greater total body fat was associated with greater insulin secretion. However, our study is unique in demonstrating that the effects of adiposity masked ethnic differences in insulin secretion. The mechanism through which adiposity affects insulin secretion is not known. Adipose tissue secretes free fatty acids and leptin, which have postulated effects on insulin secretion. Free fatty acids are believed to have an acute stimulatory effect on insulin secretion, but chronic exposure to elevated free fatty acids has been postulated to result in β-cell lipotoxicity, causing a decrease in insulin secretion (8). Leptin has been shown to upregulate glucagon-like peptide-1 secretion in some studies (43); glucagon-like peptide-1 in turn could stimulate insulin secretion. Leptin is also believed to directly affect pancreatic synthesis of insulin (44).
In this study, the independent association of AfADM with first-phase insulin secretion was not observed when the analyses were conducted in a subgroup of obese subjects. Similarly, no ethnic difference in first-phase insulin secretion, assessed with the clamp technique, was observed in a study involving obese AA and EA children (6). Furthermore, in this study, among obese but not lean children, total body fat was significantly and independently related to all phases of insulin secretion. These results suggested that adiposity may override the relative contributions of genetic factors in determining insulin secretion.
Higher AIRg previously documented among AA relative to EA also could be the result decreased hepatic insulin clearance. Lower percent hepatic insulin clearance, as determined by either the C-peptide to insulin molar ratio or mathematical modeling has been noted in both AA adults (15) and children (3,20), compared to EA. In addition, lower absolute insulin clearance, as assessed with the clamp technique, has been documented among AA relative to EA children (14). In this study, the inverse association between AfADM and percent insulin extraction over the 180-min test period approached significance (P = 0.055). Because the percent of insulin cleared by the liver is affected by the total amount of insulin secreted, we also calculated the absolute amount of insulin cleared by the liver. We postulated that AfADM would be associated with higher absolute insulin clearance, due to greater first-phase secretion observed among AA compared to EA. Although mean values for absolute insulin clearance were higher among AA than EA (Table 1), AfADM was not a significant determinant of absolute insulin clearance in the entire cohort. It is likely that the similar amount of insulin secreted over the 180-min test period between AA and EA resulted in a similar amount of absolute hormone cleared over this time.
Our results also indicated that total body fat was positively related to absolute insulin clearance. Previous studies suggested that obesity is associated with lower insulin extraction, as estimated by the C-peptide to insulin molar ratio (9,10). This discrepancy is likely explained by the strong positive association between obesity and insulin secretion, as was observed in this study. Higher insulin secretion by individuals in the obese group was likely attributable to greater absolute insulin clearance. However, it also is possible that the molar ratio, which involves only basal, fasting concentrations of C-peptide and insulin, does not accurately reflect insulin clearance under dynamic conditions.
Because this study was conducted in a healthy population of children, it avoided the potential confounding effects (beyond those that exist during reproductive maturation) of factors that may affect insulin secretion and action in adults, such as smoking, alcohol intake, and chronic obesity. Such research using healthy children is useful for detecting potential inherent physiological factors contributing to ethnic differences in insulin secretion and action. This study used refined measures of both insulin secretion and clearance to avoid the pitfalls associated with surrogate estimates (45,46). A limitation of our study was the absence of measures of physical activity and diet, which might have been be useful for identifying additional socioenvironmental contributors to the variance in insulin secretion.
In conclusion, greater AfADM was associated with greater first-phase insulin secretion among lean peripubertal children. This relationship became significant at pubertal stage 3, suggesting that the pubertal process affects the development of the β-cell response to glucose in a manner that differs with ethnic/genetic background. Among obese peripubertal children, adiposity appeared to mask the relation between genetic admixture and insulin secretion. Greater AfADM tended to be associated with lower percent, but not absolute, insulin clearance. An understanding of the differences in insulin dynamics based on body habitus and how these factors may differentially impact insulin dynamics are of particular significance to studies which probe a genetic contribution to diabetes risk. Longitudinal research is needed to determine the physiological ramifications, and potential association with disease risk, of relatively high first-phase insulin secretion among African Americans.
ACKNOWLEDGMENTS
We acknowledge the contributions of Tena Hilario-Hailey (project coordinator), Maryellen Williams and Kangmei Ren (laboratory analyses), Mark Shriver (admixture analysis), and the study participants and their families. This research was supported by NIH Grants R01DK58278, R01HD/HL33064, R01HG02154, R01DK53958, M01RR00032, and P30-DK56336. R.M.W. was supported by a grant from the American Diabetes Association. This research was funded by NICHD (MIG: R29 HD 32668 and R01 HD/HL 33064), Clinical Nutrition Research Center Grant P30-DK56336, and by General Clinical Research Center grant M01RR00032.
Footnotes
The first two authors contributed equally to this work.
DISCLOSURE: The authors declared no conflict of interest.
REFERENCES
- 1.Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khoury PR, Zeitler P. Increased Incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr. 1996;128(5 Pt 1):608–615. doi: 10.1016/s0022-3476(96)80124-7. [DOI] [PubMed] [Google Scholar]
- 2.Arslanian S, Suprasongsin C, Janosky JE. Insulin secretion and sensitivity in black versus white prepubertal healthy children. J Clin Endocrinol Metab. 1997;82:1923–1927. doi: 10.1210/jcem.82.6.4002. [DOI] [PubMed] [Google Scholar]
- 3.Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI. Contribution of insulin secretion and clearance to glucose-induced insulin concentration in African-American and Caucasian children. J Clin Endocrinol Metab. 2002;87:2218–2224. doi: 10.1210/jcem.87.5.8498. [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, D'Agostino R, Jr, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 5.Gower BA, Granger WM, Franklin F, Shewchuk RM, Goran MI. Contribution of insulin secretion and clearance to glucose-induced insulin concentration in African-American and Caucasian children. J Clin Endocrinol Metab. 2002;87:2218–2224. doi: 10.1210/jcem.87.5.8498. [DOI] [PubMed] [Google Scholar]
- 6.Bacha F, Saad R, Gungor N, Janosky JE, Arslanian S. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88:2534–2540. doi: 10.1210/jc.2002-021267. [DOI] [PubMed] [Google Scholar]
- 7.Gower BA, Nagy TR, Goran MI. Visceral fat, insulin sensitivity, and lipids in prepubertal children. Diabetes. 1999;48:1515–1521. doi: 10.2337/diabetes.48.8.1515. [DOI] [PubMed] [Google Scholar]
- 8.McGarry JD, Dobbins RL. Fatty acids, lipotoxicity and insulin secretion. Diabetologia. 1999;42:128–138. doi: 10.1007/s001250051130. [DOI] [PubMed] [Google Scholar]
- 9.Schuster DP, Kien CL, Osei K. Differential impact of obesity on glucose metabolism in black and white American adolescents. Am J Med Sci. 1998;316:361–367. doi: 10.1097/00000441-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Valera Mora ME, Scarfone A, Calvani M, Greco AV, Mingrone G. Insulin clearance in obesity. J Am Coll Nutr. 2003;22:487–493. doi: 10.1080/07315724.2003.10719326. [DOI] [PubMed] [Google Scholar]
- 11.Weiss R, Dziura JD, Burgert TS, et al. Ethnic differences in beta cell adaptation to insulin resistance in obese children and adolescents. Diabetologia. 2006;49:571–579. doi: 10.1007/s00125-005-0109-z. [DOI] [PubMed] [Google Scholar]
- 12.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50:2444–2450. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 13.Casazza K, Goran MI, Gower BA. Associations among insulin, estrogen, and fat mass gain over the pubertal transition in African American and European American girls. J Clin Endocrinol Metab. 2008;93:2610–2615. doi: 10.1210/jc.2007-2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arslanian S, Saad R, Lewy V, Danadian K, Janosky JE. Hyperinsulinemia in African-American children. Decreased insulin clearance and increased insulin secretion and its relationship to insulin sensitivity. Diabetes. 2002;51:3014–3019. doi: 10.2337/diabetes.51.10.3014. [DOI] [PubMed] [Google Scholar]
- 15.Osei K, Schuster DP. Ethnic differences in secretion, sensitivity, and hepatic extraction of insulin in black and white Americans. Diabetic Med. 1994;11:755–762. doi: 10.1111/j.1464-5491.1994.tb00349.x. [DOI] [PubMed] [Google Scholar]
- 16.Marshall WA, Tanner JM. Growth and physiological development during adolescence. Annu Rev Med. 1968;19:283–300. doi: 10.1146/annurev.me.19.020168.001435. [DOI] [PubMed] [Google Scholar]
- 17.Goran MI, Driscoll P, Johnson R, Nagy TR, Hunter GR. Cross-calibration of body composition techniques against dual-energy X-ray absorptiometry in young children. Am J Clin Nutr. 2006;63:299–305. doi: 10.1093/ajcn/63.3.299. [DOI] [PubMed] [Google Scholar]
- 18.Pacini G, Bergman RN. MINMOD: a computer program to calculate insulin sensitivity and pancreatic responsivity from the frequently sampled intravenous glucose tolerance test. Comput Methods Programs Biomed. 1986;23:113–122. doi: 10.1016/0169-2607(86)90106-9. [DOI] [PubMed] [Google Scholar]
- 19.Watanabe RM, Steil GM, Bergman RN. Critical evaluation of the combined model approach for estimation of prehepatic insulin secretion. Am J Physiol (Endocrinol Metab) 1998;274:E172–E183. doi: 10.1152/ajpendo.1998.274.1.E172. [DOI] [PubMed] [Google Scholar]
- 20.Goran MI, Bergman RN, Cruz ML, Watanabe R. Insulin resistance and associated compensatory responses in African-American and Hispanic children. Diabetes Care. 2002;25:2184–2190. doi: 10.2337/diacare.25.12.2184. [DOI] [PubMed] [Google Scholar]
- 21.Van Cauter E, Mestrez F, Sturis J, Polonsky KS. Estimation of insulin secretion rates from C-peptide levels. Diabetes. 1992;41:368–377. doi: 10.2337/diab.41.3.368. [DOI] [PubMed] [Google Scholar]
- 22.Gower BA, Fernandez JR, Beasley TM, Shriver MD, Goran MI. Using genetic admixture to explain racial differences in insulin-related phenotypes. Diabetes. 2003;52:1047–1051. doi: 10.2337/diabetes.52.4.1047. [DOI] [PubMed] [Google Scholar]
- 23.Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet. 1998;63:1839–1851. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akey JM, Sosnoski D, Parra E, et al. Melting curve analysis of SNPs (McSMP): a gel-free and inexpensive approach for SNP genotyping. Biotechniques. 2001;30:358–367. doi: 10.2144/01302tt05. [DOI] [PubMed] [Google Scholar]
- 25.Hanis CL, Chakraborty R, Ferrell RE, Schull WJ. Individual admixture estimates: disease associations and individual risk of diabetes and gallbladder disease among Mexican-Americans in Starr County, Texas. Am J Physiol Anthropol. 1986;70:433–441. doi: 10.1002/ajpa.1330700404. [DOI] [PubMed] [Google Scholar]
- 26.Delva J, O'Malley PM, Johnston LD. Racial/ethnic and socioeconomic status differences in overweight and health-related behaviors among American students: national trends 1986–2003. J Adolesc Health. 2006;39:536–545. doi: 10.1016/j.jadohealth.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Di FG, Mansueto P, Longo RA, Rini G, Carmina E. Influence of sociocultural factors on the ovulatory status of polycystic ovary syndrome. Fertil Steril. 2008 doi: 10.1016/j.fertnstert.2008.02.161. e-pub ahead of print 1 May 2008. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Liang H, Tussing L, et al. Obesity and related risk factors among low socio-economic status minority students in Chicago. Public Health Nutr. 2007;10:927–938. doi: 10.1017/S1368980007658005. [DOI] [PubMed] [Google Scholar]
- 29.Cirino PT, Chin CE, Sevcik RA, et al. Measuring socioeconomic status: reliability and preliminary validity for different approaches. Assessment. 2002;9:145–155. doi: 10.1177/10791102009002005. [DOI] [PubMed] [Google Scholar]
- 30.Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2002;50:2444–2450. doi: 10.2337/diabetes.50.11.2444. [DOI] [PubMed] [Google Scholar]
- 31.Moran A, Jacobs DR, Jr, Steinberger J, et al. Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation. 2008;117:2361–2368. doi: 10.1161/CIRCULATIONAHA.107.704569. [DOI] [PubMed] [Google Scholar]
- 32.Williams DP, Going SB, Lohman TG, et al. Body fatness and risk for elevated blood pressure, total cholesterol, and serum lipoprotein ratios in children and adolescents. Am J Public Health. 1992;82:358–363. doi: 10.2105/ajph.82.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dwyer T, Blizzard CL. Defining obesity in children by biological endpoint rather than population distribution. Int J Obes Relat Metab Disord. 1996;20:472–480. [PubMed] [Google Scholar]
- 34.Higgins PB, Gower BA, Hunter GR, Goran MI. Defining health-related obesity in prepubertal children. Obes Res. 2001;9:233–240. doi: 10.1038/oby.2001.27. [DOI] [PubMed] [Google Scholar]
- 35.Whincup PH, Gilg JA, Papacosta O, et al. Early evidence of ethnic differences in cardiovascular risk: cross sectional comparison of British South Asian and white children. BMJ. 2002;324:635. doi: 10.1136/bmj.324.7338.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Prato S, Tiengo A. The importance of first-phase insulin secretion: implications for the therapy of type 2 diabetes mellitus. Diabetes Metab Res Rev. 2001;17:164–74. doi: 10.1002/dmrr.198. [DOI] [PubMed] [Google Scholar]
- 37.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fridlyand LE, Philipson LH. Does the glucose-dependent insulin secretion mechanism itself cause oxidative stress in pancreatic beta cells? Diabetes. 2004;53:1942–1948. doi: 10.2337/diabetes.53.8.1942. [DOI] [PubMed] [Google Scholar]
- 39.Stefan N, Stumvoll M, Weyer C, Bogardus C, Tataranni PA, Pratley RE. Exaggerated insulin secretion in Pima Indians and African-Americans but higher insulin resistance in Pima Indians compared to African-Americans and Caucasians. Diabetic Med. 2004;21:1090–1095. doi: 10.1111/j.1464-5491.2004.01290.x. [DOI] [PubMed] [Google Scholar]
- 40.Arslanian S, Suprasongsin C. Differences in the in vivo insulin secretion and sensitivity of healthy black versus white adolescents. J Pediatr. 1996;129:440–443. doi: 10.1016/s0022-3476(96)70078-1. [DOI] [PubMed] [Google Scholar]
- 41.Velasquez-Mieyer PA, Cowan PA, Umpierrez GE, et al. Racial differences in glucagon-like peptide-1 (GLP-1) concentration and insulin dynamics during oral glucose tolerance test in obese subjects. Int J Obesity. 2003;27:1359–1364. doi: 10.1038/sj.ijo.0802415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meier JJ, Nauck MA. Glucagon-like peptide 1 (GLP-1) in biology and pathology. Diabetes Metab Res Rev. 2005;21:91–117. doi: 10.1002/dmrr.538. [DOI] [PubMed] [Google Scholar]
- 43.Anini Y, Brubaker PL. Role of leptin in the regulation of glucagon-like peptide-1 secretion. Diabetes. 2003;52:252–259. doi: 10.2337/diabetes.52.2.252. [DOI] [PubMed] [Google Scholar]
- 44.Seufert J. Leptin effects on pancreatic beta-cell gene expression and function. Diabetes. 2004;53(Suppl 1):S152–S158. doi: 10.2337/diabetes.53.2007.s152. [DOI] [PubMed] [Google Scholar]
- 45.Polonsky KS, Rubenstein AH. C-peptide as a measure of the secretion and hepatic extraction of insulin. Pitfalls and limitations. Diabetes. 1984;33:486–494. doi: 10.2337/diab.33.5.486. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe RM, Bergman RN. Accurate measurement of endogenous insulin secretion does not require separate assessment of C-peptide kinetics. Diabetes. 2000;49:373–382. doi: 10.2337/diabetes.49.3.373. [DOI] [PubMed] [Google Scholar]