Abstract
Despite intensive research, efforts to reduce the mortality of septic patients have failed. Adenosine is a potent extracellular signaling molecule, and its levels are elevated in sepsis. Adenosine signals through G-protein–coupled receptors and can regulate the host’s response to sepsis. In this study, we studied the role of A2B adenosine receptors in regulating the mortality and inflammatory response of mice following polymicrobial sepsis. Genetic deficiency of A2B receptors increased the mortality of mice suffering from cecal ligation and puncture-induced sepsis. The increased mortality of A2B knockout mice was associated with increased levels of inflammatory cytokines and chemokines and augmented NF-κB and p38 activation in the spleen, heart, and plasma in comparison with wild-type animals. In addition, A2B receptor knockout mice showed increased splenic apoptosis and phosphatase and tensin homolog activation and decreased Akt activation. Experiments using bone-marrow chimeras revealed that it is the lack of A2B receptors on nonhematopoietic cells that is primarily responsible for the increased inflammation of septic A2B receptor-deficient mice. These results indicate that A2B receptor activation may offer a new therapeutic approach for the management of sepsis.
Systemic illness triggered by microbial invasion of normally sterile parts of the body is referred to as sepsis. Sepsis is a major health care problem; current estimates suggest that the incidence of sepsis is ~750,000 annually, and sepsis is responsible for >200,000 deaths each year in the United States (1). The innate immune system recognizes the presence of invading pathogens and initiates an inflammatory response to control the invading pathogen. This systemic inflammatory response often leads to organ injury and dysregulated apoptosis (2), which are thought to be major contributors to septic mortality (3).
The purine nucleoside adenosine is an endogenous signaling molecule, and its extracellular levels rapidly increase in septic patients owing to the increased breakdown of adenine nucleotides (ATP and ADP) in ischemic, injured, and inflamed tissues (4). Extracellular adenosine elicits its cellular effects by binding to four subtypes of G-protein–coupled adenosine receptors, termed A1, A2A, A2B, and A3 (5). The different adenosine receptors activate divergent intracellular pathways in a cell- and tissue-specific manner (6–8). Targeting adenosine receptors for pharmacological intervention in various illnesses is appealing, but the multifaceted regulation of tissue function by adenosine receptors makes their role in regulating complex pathophysiological responses, such as those occurring during sepsis, difficult to predict (9). The role of A1, A2A, and A3 receptors in regulating the pathophysiological sequelae of sepsis has been addressed recently using the clinically relevant cecal ligation and puncture (CLP) mouse model of sepsis (10–12). Inactivation of both A1 and A3 adenosine receptors was found to result in increased mortality, inflammation, renal dysfunction, and hepatic injury in this model (10, 12). In contrast, Nemeth and coworkers (11) showed that A2A receptor inactivation protected from the lethal effect of sepsis, and this protection was associated with improved bacterial clearance, attenuated splenic apoptosis, and diminished inflammatory cytokine levels.
Because the role of A2B receptors in regulating mortality, bacterial growth, and inflammation in sepsis has not been investigated, the potential for targeting these receptors for pharmacological intervention remains unknown. Recent evidence obtained using A2B knockout (KO) mice and selective pharmacological agents has increased our understanding of the influence of A2B receptors in various pathophysiological conditions. The first report using A2B receptor KO mice demonstrated that they exhibit an inflamed phenotype with elevated cytokine levels and adhesion molecule expression in various organs when compared with their wild-type (WT) counterparts (13). Additionally, Yang and coworkers (14) found that genetic inactivation of A2B receptors resulted in increased vascular injury in a femoral artery injury model. Our previous in vivo studies have shown that vascular permeability (15) and pulmonary inflammation (16) are significantly increased in A2B KO mice subjected to hypoxia or mechanical ventilation. In contrast, A2B receptor inactivation has been shown to ameliorate the course of inflammatory colitis (17), emphasizing the complex manner in which A2B receptors regulate pathophysiological events during distinct disease processes.
In the current study, using genetic and pharmacological approaches, we examined the role of A2B receptors in regulating the host’s response to polymicrobial sepsis. We report that A2B receptors prevent sepsis-induced mortality and inflammation, which indicates that A2B receptors may be therapeutically targeted for the benefit of patients suffering from sepsis.
Materials and Methods
Experimental animals
A2B receptor KO mice were acquired from Deltagen (San Mateo, CA). Mice were backcrossed 10 generations onto a C57Blk/6J background. A2B KO mice were bred as described previously (18). At weaning, a 0.5-cm tail sample was removed for the purpose of DNA collection for genotyping. Genotyping was performed by RT-PCR using primers described previously (18). For pharmacological studies with the selective A2B receptor antagonist MRS 1754 8-[4-[((4-cyanophenyl)carbamoylmethyl)oxy]phenyl]-1,3-di(N-propyl)xanthine (Sigma-Aldrich, St. Louis, MO) and for WT controls, male C57Blk/6J mice were used that were purchased from The Jackson Laboratory (Bar Harbor, ME). All mice were maintained in accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals, and the experiments were approved by the New Jersey Medical School Animal Care Committee (Newark, NJ).
Generation of A2B receptor bone marrow-chimeric mice
A2B receptor bone marrow-chimeric mice were generated as previously described (15, 16). Bone marrow was ablated by radiation in WT mice followed by reconstitution with bone marrow derived from previously characterized mice gene-targeted for the A2B receptor and vice versa (A2B KO→A2B WT and A2B WT→A2B KO).
Cecal ligation and puncture
Polymicrobial sepsis was induced by subjecting 8–12-week-old A2B KO or WT mice to CLP as we have described previously (11). In brief, mice were anesthetized with pentobarbital (50 mg/kg), given i.p. Under aseptic conditions, a 2-cm midline laparotomy was performed to allow exposure of the cecum with adjoining intestine. Approximately two-thirds of the cecum was tightly ligated with a 3.0 silk suture, and the ligated part of the cecum perforated twice (through and through) with a 20-gauge needle. The cecum was then gently squeezed to extrude a small amount of feces (~4 mm diameter) from the perforation sites. The cecum was then returned to the peritoneal cavity, and the laparotomy was closed in two layers with 4.0 silk sutures. The mice were resuscitated with 1 ml physiological saline injected s.c. and returned to their cages with free access to food and water. The effect of pharmacological inactivation of A2B receptor in mice subjected to CLP was evaluated using C57BL/6J mice in a similar fashion to that described for the A2B receptor KO or WT mice. The 16-h point was used because in previous studies we have established that cytokine levels and bacterial counts subside sharply after this point (11). In this set of experiments, the mice were injected immediately before the operation with MRS1754 (0.5 mg/kg, s.c.) or its vehicle.
Collection of blood, peritoneal lavage fluid, and organs
Blood samples were obtained aseptically by cardiac puncture using heparinized syringes after opening the chest and were placed on ice into heparinized Eppendorf tubes until further processing for bacteriological analysis. After serial dilutions for bacteriological analysis were made (see below), the blood was centrifuged at 2000 × g for 10 min, and the recovered plasma was stored at −70°C until further use. For peritoneal lavage, the abdominal skin was cleansed with 70% ethanol, and the abdominal wall was exposed by opening the skin. Two milliliters of sterile physiological saline were then installed into the peritoneal cavity via an 18-gauge needle. The abdomen was massaged gently for 1 min while keeping the tip of the needle in the peritoneum, after which procedure peritoneal fluid was recovered through the needle. Recovered peritoneal lavage fluid was placed on ice until it was processed for bacteriological examination. After serially diluting the peritoneal lavage fluid to determine CFU numbers (see below), the peritoneal lavage fluid was centrifuged at 5000 × g for 10 min, and the supernatant was stored at −70°C until further analysis. Samples from spleen, thymus, lung, kidneys, heart, and liver were excised and immediately frozen in liquid nitrogen.
Quantification of bacterial CFUs from peritoneal lavage fluid and blood
A total of 100 μl blood or peritoneal lavage fluid was diluted serially in sterile physiological saline, and 50 μl each dilution was aseptically plated and cultured on trypticase blood agar plates (BD Biosciences, San Jose, CA) at 37°C. After 24 h, the number of bacterial colonies was counted. Quantitative cultures are expressed as CFUs per milliliter of blood or peritoneal lavage fluid.
Flow cytometric analysis of leukocyte subsets
The percent distribution of leukocyte subsets in blood and spleen cell suspensions was analyzed by specifically staining CD3+CD4+, CD3+CD8+ T cells, CD19+ B cells, and CD11b+ myeloid cells using Abs against CD markers conjugated with FITC, allophycocyanin, PerCP, or PE (BD Biosciences) in three- or four-color incubations. Aliquots of 0.1 ml whole blood or splenocyte suspension were incubated with the respective markers for 15 min followed by incubation with BD FACS lysing solution (BD Biosciences) for 7 min at 37°C. Cells were washed twice with BD FACS wash buffer and then fixed with 1% methanol-free formaldehyde. FACS acquisitions were performed in a centralized flow cytometry facility. At least 30,000 events were collected for each analysis.
Protein extraction and Western blot analysis
Frozen organs were homogenized in a Dounce homogenizer in modified radioimmunoprecipitation assay buffer (50 mM Tris HCl, 150 mM NaCl, 1 mM EDTA, 0.25% sodium deoxycholate, 1% Nonidet P-40, 1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 mM PMSF, and 1 mM Na3VO4). The lysates were centrifuged at 15,000 × g for 15 min, and the supernatant was recovered. Protein concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA). A total of 40 μg sample was separated on 8–12% Trisglycine gel (Invitrogen, Carlsbad, CA) and transferred to nitrocellulose membrane. The membranes were probed with polyclonal rabbit anti-cleaved caspase-3, polyclonal rabbit anti-cleaved poly (ADP-ribose) polymerase (PARP), polyclonal rabbit anti-IκBα, polyclonal rabbit anti–phospho-Akt, polyclonal rabbit anti–phospho-phosphatase and tensin homolog deleted on chromosome 10 (PTEN), polyclonal rabbit anti–phospho-p38, polyclonal rabbit anti–phospho-ERK1/2, and polyclonal rabbit anti–phospho-JNK Abs (all from Cell Signaling Technology, Beverly, MA). Thereafter, the membranes were incubated with a secondary HRP-conjugated anti-rabbit Ab (Santa Cruz Biotechnology, Santa Cruz, CA). HRP-conjugated polyclonal goat anti–β-actin Ab to assess equal loading was used from Santa Cruz Biotechnology. Bands were detected using ECL Western blotting Detection Reagent (Amersham Biosciences, Piscataway, NJ).
Isolation of nuclear protein extract from spleen and heart samples and detection of NF-κB activation
Cytosolic and nuclear protein fractions were isolated from spleen and heart samples using NE-PER Nuclear and Cytosolic Extraction reagent (Thermo Fisher Scientific, Waltham, MA), according to the manufacturer’s protocol. Quantification of NF-κB activation was made using Trans AM NF-κB p65 Chemilumi Transcription Factor Assay kit (Active Motif, Carlsbad, CA) using 2 μg splenic or heart nuclear cell extract according to the manufacturer’s suggestions.
Determination of cytokine and chemokine levels
Concentrations of TNF-α, the β form of pro-IL-1 (IL-1β), IL-6, IL-12 p40, IL-10, MIP-2, and MCP-1 were determined using commercially available ELISA kits (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions (19). The lower detection limit for all of these cytokines was 10 pg/ml.
Statistical analysis
Survival statistics were compared with a Kaplan-Meier curve and log-rank test. Two-tailed t testing was used to compare cytokine concentrations, CFUs, and other laboratory parameters. Statistical significance was assigned to p values <0.05.
Results
A2B receptor deficiency decreases survival without affecting bacterial load in CLP-induced sepsis
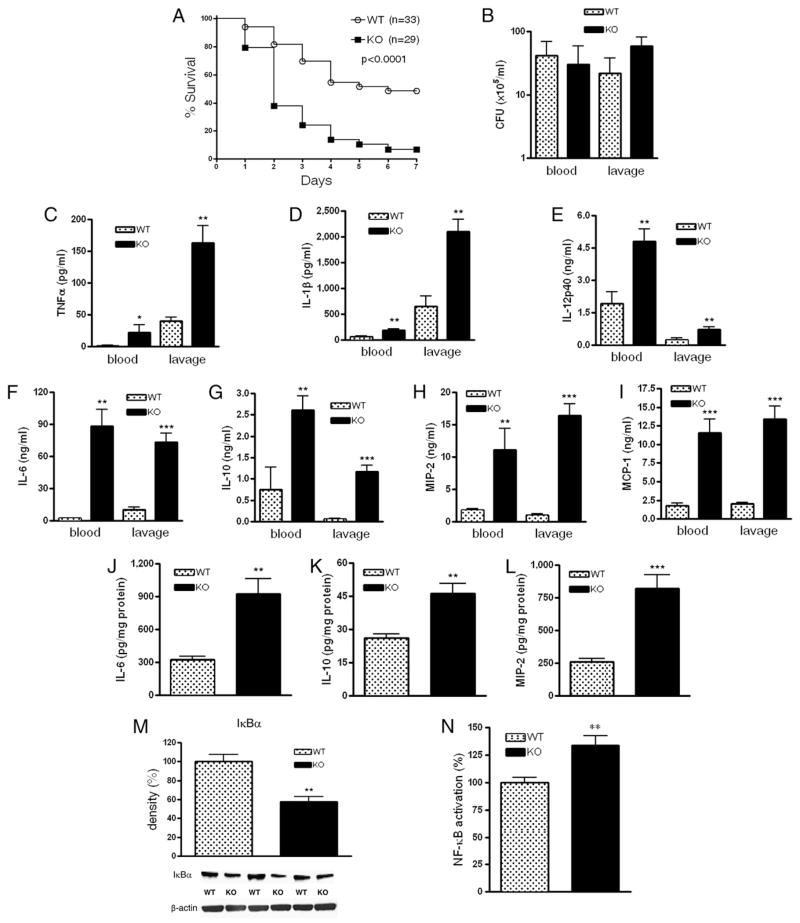
We first studied the effect of A2B deficiency on mortality in CLP-induced septic peritonitis by observing A2B WT and KO mice. As demonstrated in Fig. 1A, A2B receptor KO mice had significantly higher mortality rates compared with A2B WT mice, which became apparent on the second day of observation. On the seventh day following CLP, the mortality rate of A2B KO mice was markedly (by >40%) higher than that of A2B WT mice. No additional changes in mortality were detected when the mice were monitored for up to 10 d (data not shown). Bacterial translocation from the intestines into the peritoneal cavity and bloodstream invasion are important contributors to the mortality of mice in CLP-induced sepsis. As shown in Fig. 1B, we found no difference in the numbers of bacteria in the blood or peritoneal lavage fluid of A2BR KO mice when compared with WT animals at 16 h following the CLP procedure. Taken together, these studies document that despite the fact that A2B receptors do not affect bacterial invasion, A2B receptors prevent mortality in polymicrobial sepsis.
FIGURE 1.
A2B deficiency decreases survival and increases inflammation in polymicrobial sepsis. A, Surviving A2B KO and WT mice were counted every day for 7 d after inducing polymicrobial sepsis by way of CLP. WT, n = 33; KO, n = 29. p < 0.0001 versus WT. B, Blood and peritoneal lavage fluid obtained from A2B KO or WT mice 16 h post CLP were cultured on soytrypticase agar plates, and the number of bacterial colonies was counted (n = 12 mice per group). Serum and peritoneal lavage TNF-α (C), IL-1β (D), IL-12p40 (E), IL-6 (F), IL-10 (G), MIP-2 (H), and MCP-1 (I) levels were determined from samples collected 16 h post CLP using ELISA(n = 12/group). Splenic levels ofIL-6 (J), IL-10 (K), and MIP-2(L)weremeasured from protein extracts of spleens of A2B KOand WT mice (n = 12/group). M, IκBα levels were assessed using Western blotting of spleen protein extracts of A2B WTand KO mice (n = 6/group). Protein extracts were generated from spleen taken 16 h after sepsis induction. Bands were detected by ECL. All results (mean ± SEM) shown are representative of three experiments. N, p65 levels were assessed in nuclear fractions of spleens using NF-κB (p65) chemiluminescence assay kit. Results (mean ± SEM) shown are the summary of three independent experiments. WT, n = 29; KO, n = 31. *p < 0.05 versus WT; **p < 0.01 versus WT; ***p < 0.001 versus WT.
A2B receptor deficiency increases cytokine and chemokine production, and augments NF (NF)-κB activation in CLP-induced sepsis
Increased production of inflammatory cytokines and chemokines and activation of the transcription factor NF-κB are important components of a dysregulated immune response and mortality in septic mice. To investigate whether A2B receptor deficiency alters the release of inflammatory cytokines, we next determined concentrations of TNF-α, IL-1β, IL-12p40, IL-6, and IL-10 in both the plasma and peritoneal lavage fluid. We found markedly elevated cytokine levels in both the blood and peritoneal cavity of A2B KO animals (Fig. 1C–G). We then assessed the levels of MIP-2 and MCP-1, two crucial chemokines that mediate inflammatory responses in the plasma and peritoneal lavage fluid of A2B KO and WT mice subjected to CLP. Fig. 1H and 1I show that the release of both of these chemokines was higher in A2B KO animals than WT mice.
Because the spleen and liver are among the most important sources of inflammatory mediators in sepsis, in the next set of experiments, we studied the cytokine/chemokine response of these organs to CLP-induced sepsis. We found comparable concentrations of inflammatory mediators in the liver of A2B KO and WT mice (data not shown). However, A2B KO mice exhibited elevated splenic IL-6, IL-10, and MIP-2 levels when compared with their WT littermates (Fig. 1J–L). Cytokine levels in all sham-operated animals were below the detection limit (data not shown).
Microbial components and endogenous danger signals trigger the activation of signaling cascades, leading to induction of the NF-κB system during sepsis. Persistent activation of NF-κB may cause excessive inflammatory responses culminating in tissue injury, organ dysfunction, and death. Because we found elevated cytokine and chemokine levels in A2B KO mice undergoing sepsis, we studied the activation of NF-κB by measuring the levels of the inhibitory subunit of NF-κB (IκBα) in whole-cell extracts and the nuclear levels of the p65 NF-κB subunit in the spleen of septic animals. As Fig. 1M shows, the levels of IκBα were lower in the spleen of A2B KO mice than WT mice. In addition, we found that the nuclear level of p65 was significantly enhanced in A2B KO as compared with WT mice (Fig. 1N). These results together indicate that NF-κB activation is increased in A2B KO versus WT mice.
Taken together, these results suggest a prominent role for A2B receptors in harnessing inflammation during polymicrobial sepsis.
Pharmacological inactivation of A2B receptors enhances CLP-induced cytokine and chemokine levels
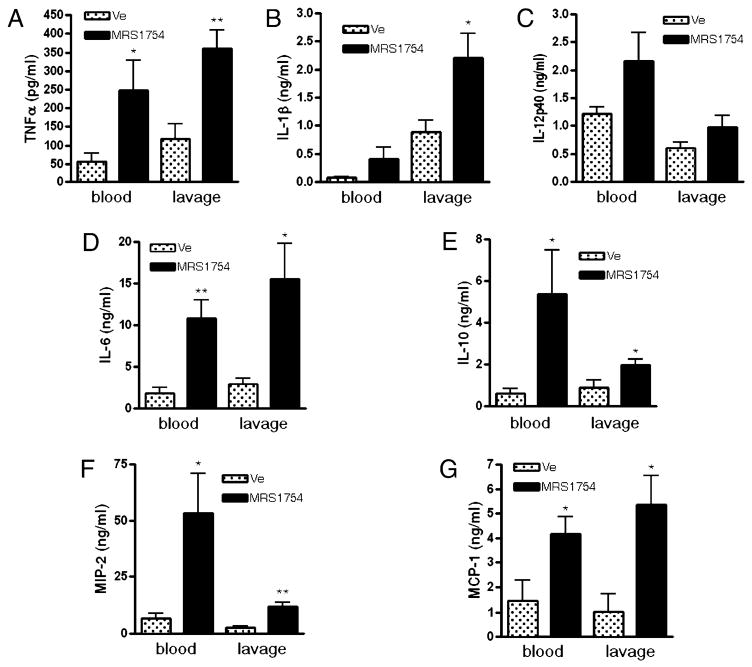
We then further examined the role of A2B receptors in modulating CLP-induced sepsis using a pharmacological approach. C57BL/6J mice were treated with the selective A2B receptor antagonist MRS1754 (0.5 mg/kg) or its vehicle, and cytokine and chemokine levels were measured in the blood and peritoneal lavage fluid of mice 16 h after the CLP procedure. Similar to genetic inactivation of A2B receptors, the concentration of TNF-α, IL-1β, IL-6, and IL-10 (Fig. 2A–E), as well as MIP-2 (Fig. 2F) and MCP-1 (Fig. 2G), was higher in both the blood and peritoneal lavage fluid of mice with pharmacological inactivation of A2B receptors. However, there was no difference in IL-12p40 levels between vehicle- and MRS1754-treated animals. These results confirm that inactivation of A2B receptors increases inflammation in polymicrobial sepsis.
FIGURE 2.
Pharmacological inactivation of A2B receptors enhances inflammatory mediator production in polymicrobial sepsis. Blood and peritoneal lavage fluid was obtained from mice injected with the A2B receptor-specific antagonist MRS1754 or vehicle (DMSO) 16 h after the CLP procedure, and TNF-α (A), IL-1β (B), IL-12p40 (C), IL-6 (D), IL-10 (E), MIP-2 (F), and MCP-1 (G) concentrations were determined using ELISA (n = 10/group). All results (mean ± SEM) shown are representative of three experiments. *p < 0.05 versus vehicle-injected mice; **p < 0.01 versus vehicle-injected mice.
Genetic ablation of A2B receptors enhances the levels of inflammatory mediators in the lungs of septic mice
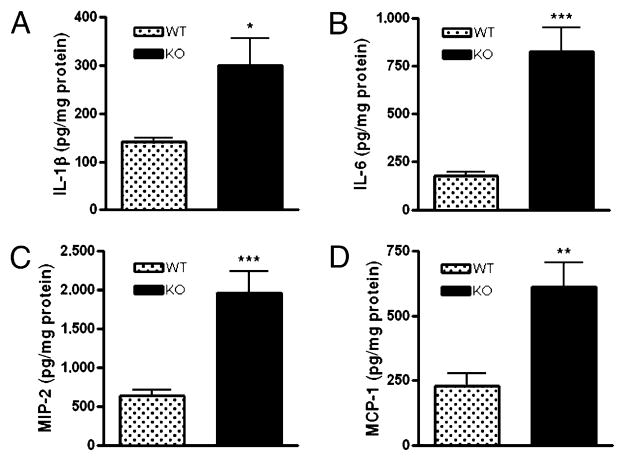
Lung inflammation is a complication of sepsis, often leading to acute respiratory distress syndrome and death. We thus investigated the effect of A2B receptor ablation on pulmonary inflammation in sepsis. We found augmented levels of IL-1β, IL-6, MIP-2, and MCP-1 in lung tissue of A2B KO versus WT mice when measured 16 h after the induction of sepsis (Fig. 3A–D). Cytokine levels in all sham-operated animals were below the detection limit (data not shown).
FIGURE 3.
Genetic ablation of A2B receptor enhances sepsis-induced pulmonary inflammatory cytokine and chemokine levels. Pulmonary concentrations of IL-1β (A), IL-6 (B), MIP-2 (C), and MCP-1 (D) were measured from pulmonary extracts of A2B KO and WT mice taken after 16 h of sepsis (n = 12/group). All results (mean ± SEM) shown are representative of three experiments. *p < 0.05 versus WT; **p < 0.01 versus WT; ***p < 0.001 versus WT.
These results suggest that A2B receptors prevent the increasing effect of sepsis on lung inflammatory cytokine levels.
A2B receptor deletion augments inflammatory mediator levels and NF-κB activation in the hearts of septic mice
A growing body of evidence indicates that sepsis induces cardiac injury and myocardial dysfunction in both experimental animals and humans. Moreover, the A2B receptor has an important protective role in preventing inflammation and tissue injury during cardiac ischemia (20). Therefore, we examined the levels of inflammatory mediators in heart tissue homogenates. Fig. 4A and 4B show that protein homogenates from A2B KO heart tissue contained higher levels of IL-6 and MCP-1 when compared with homogenates obtained from WT hearts. Cytokine levels in all sham-operated animals were below the detection limit (data not shown). Sepsis-induced cardiac inflammation is known to correlate with the phosphorylation of MAPK cascades, such as p38, ERK 1/2 (ERK1/2), and JNK (JNK1/2). Hence, we studied the phosphorylation of these kinases in hearts of A2B KO and WT animals after CLP. As Fig. 4C shows, p38 phosphorylation in hearts of A2B-deficient animals was enhanced when compared with WT animals. There was no difference in the phosphorylation of cardiac ERK1/2 and JNK1/2 between WT and KO mice following CLP (data not shown). Previous in vivo and in vitro studies have demonstrated that cardiac inflammation is generally associated with augmented activation of the NF-κB system (21). Consequently, we assessed the activation state of the NF-κB system by measuring IκBα protein levels and the nuclear level of p65 subunit of NF-κB in the heart. IκBα protein levels were decreased, and the nuclear level of p65 was elevated in the heart of animals lacking A2B receptors when compared with WT littermates (Fig. 4D, 4E). Thus, we conclude that A2B receptors diminish cardiac inflammatory mediator production and NF-κB activation during sepsis.
FIGURE 4.
A2B receptor deletion augments sepsis-induced inflammatory mediator levels and NF-κB activation in the heart. Cardiac levels of IL-6 (A) and MCP-1 (B) were assessed using ELISA. Protein extracts were prepared from hearts of A2B KO and WT mice isolated after 16 h of sepsis (n = 12/group). p38 phosphorylation (C) and IκBα (D) levels in heart protein extracts of A2B KO and WT mice were examined using Western blotting. Bands were detected by ECL. All results (mean ± SEM) shown are representative of three experiments (n = 6/group in each experiment). E, p65 levels were assessed in nuclear fraction of hearts using NF-κB (p65) chemiluminescence assay kit. Results (mean ± SEM) shown are the summary of three independent experiments. WT, n = 29; KO, n = 31. *p < 0.05 versus WT; **p < 0.01 versus WT.
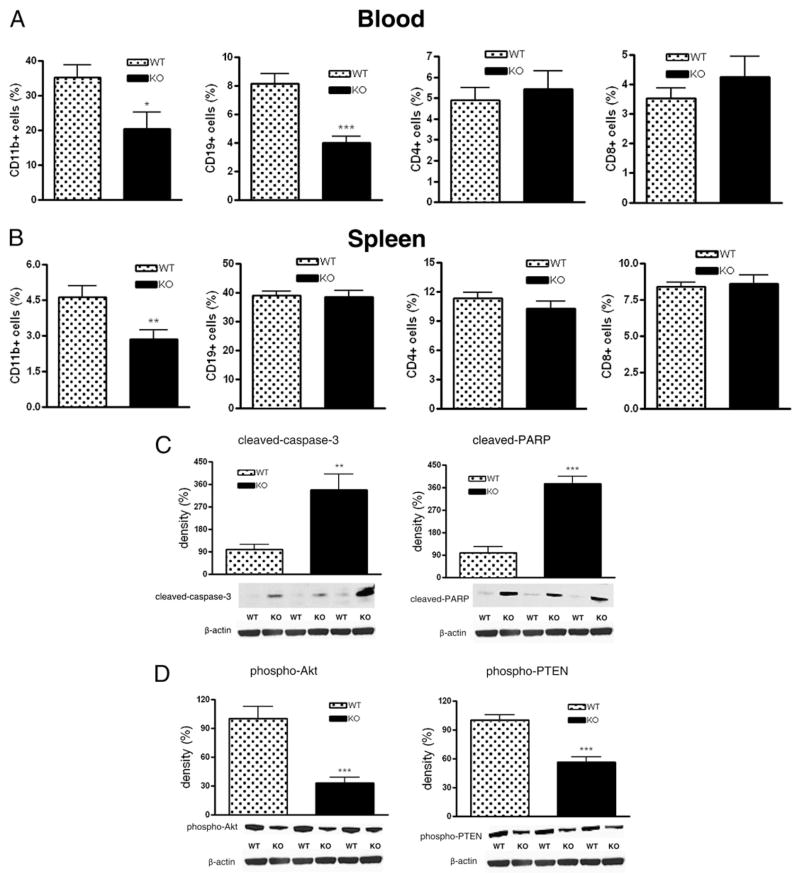
Lack of A2B adenosine receptors decreases the number of CD19+ and CD11b+ cells during CLP
CLP-induced sepsis decreased the number of total circulating leukocytes in both A2B receptor KO and WT mice to the same degree (data not shown). Analysis of WBC subsets indicated a decrease in the numbers of circulating CD11b+ cells and CD19+ B cells in KO versus WT animals, whereas the number of CD4+ and CD8+ T cells was comparable between WT and KO mice (Fig. 5A). Furthermore, the number of resident splenic CD11b+ cells but not other cell populations decreased in A2B KO mice as compared with their WT littermates (Fig. 5B).
FIGURE 5.
A2B receptor deficiency decreases the number of CD19+ and CD11b+ lymphocyte subsets and increases apoptosis. Numbers of various lymphocyte subsets were monitored in blood (A) and spleen (B) by flow cytometric analysis after 16 h of sepsis induced by CLP (n = 8/group). Proteolytic cleavage of caspase-3 and PARP (C) as well as phosporylation of Akt and PTEN (D) in spleen protein extracts from A2B WT and KO mice was examined using Western blotting. Bands were detected by ECL. All results (mean ± SEM) shown are representative of three experiments (n = 6/group in each experiment). *p < 0.05 versus WT; **p < 0.01 versus WT; ***p < 0.001 versus WT.
Genetic deletion of the A2B receptor increases apoptosis and downregulates PI3K/Akt signaling in the spleen
Sepsis provokes extensive immune cell apoptosis that contributes to immune dysregulation and mortality. Because proteolytic cleavage of caspase-3 and PARP is a good indicator of apoptosis, we tested whether A2B receptor deficiency would affect the cleavage of caspase-3 and PARP in the spleen and thymus of mice subjected to CLP. Fig. 5C shows that 16 h after the onset of sepsis, the cleavage of both caspase-3 and PARP in the spleen was markedly enhanced in A2B receptor KO mice. However, CLP-induced apoptosis in the thymus was comparable between A2B KO and WT mice (data not shown). We next surveyed some of the upstream intracellular signaling mechanisms regulating apoptosis in sepsis. The Ser/Thr kinase Akt is a key regulator of cell survival, and sepsis-induced reduction in its phosphorylation/activation is associated with enhanced apoptosis. Thus, we tested the hypothesis that the elevated apoptosis in A2B receptor KO mice that we observed may be due to impaired Akt phosphorylation/activation. We found decreased levels of phosphorylated/activated Akt in spleens from A2B KO mice subjected to CLP (Fig. 5D). PTEN is an endogenous inhibitor of Akt, and its phosphorylation results in its deactivation (22). We found diminished PTEN phosphorylation in spleens of A2B KO animals when compared with WT mice 16 h following the CLP insult (Fig. 5D), suggesting that A2B KO receptor deletion causes apoptosis via PTEN activation. Taken together, A2B receptors prevent splenic apoptosis in polymicrobial sepsis.
Loss of A2B receptors on nonhematopoietic cells is responsible for the increased cytokine response of A2B receptor-deficient mice
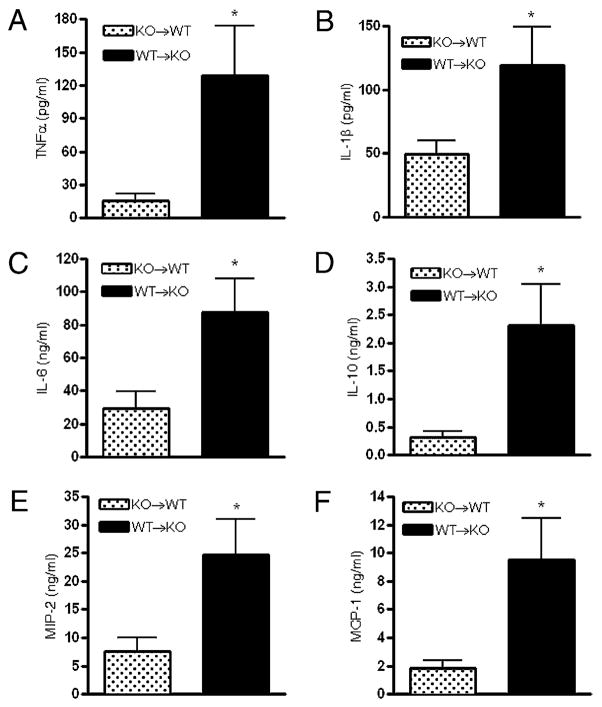
Previous studies employing bone marrow-chimeric mice have helped to clarify the role of the contribution of A2B receptors on hematopoietic versus nonhematopoietic cells to modulating inflammation in various injury models (13, 15, 16, 23). To determine whether the elevated cytokine levels that we detected in A2B KO mice subjected to CLP (Fig. 1) were due to loss of A2B receptors on hematopoietic or nonhematopoietic cells, we generated A2B receptor bone marrow-chimeric mice. A2B WT→A2B KO chimeras resulting in WT bone marrow and KO parenchyma exhibited higher CLP-induced cytokine (TNF-α, IL-1β, IL-6, and IL-10) (Fig. 6A–D), and chemokine (MIP-2 and MCP-1) (Fig. 6E, 6F) levels than A2B KO→A2B WT mice with WT parenchyma and KO bone marrow. This observation demonstrates that non–bone marrow-associated A2B receptors have a preeminent role in dampening TNF-α, IL-1β, IL-6, and IL-10 as well as chemokine production in sepsis.
FIGURE 6.
Lack of signaling through A2B receptors on nonhematopoietic cells is responsible for the increased cytokine response of A2B receptor-deficient mice. A–F, Blood was obtained from (A2B KO→A2B WT; A2B WT→A2B KO) A2B receptor bone marrow-chimeric mice 16 h post CLP. Plasma TNF-α (A), IL-1β (B), IL-6 (C), IL-10 (D), MIP-2 (E), and MCP-1 (F) concentrations were assessed using ELISA. Results are depicted as mean ± SEM of n = 10 A2B KO→A2B WTand n = 8 A2B WT→A2B KO mice. *p < 0.05 versus A2B WT→A2B KO bone marrow-chimeric mice.
Discussion
Studies employing both KO and pharmacological approaches have provided insights into the role of the various adenosine receptors in regulating the physiological response of the host to sepsis. These studies have revealed that deletion of either A1 or A3 adenosine receptors results in impaired survival in response to CLP-induced sepsis (10, 12). In contrast to observations with inhibition of A1 and A3 receptors, a previous study from our laboratory showed that A2A receptor inactivation protects animals from the lethal effect of sepsis (11). The most important novelty of the current study is that, similar to A1 and A3 receptors, blockade of A2B receptors decreased the survival rate of septic animals, pointing to a protective role of A2B receptors in sepsis.
Studies employing antibiotic therapy have demonstrated that systemic bacterial dissemination is a major factor contributing to the mortality of both experimental animals and humans during sepsis (24, 25). Our studies showed that bacterial dissemination was not different between A2B receptor KO and WT mice, suggesting that A2B receptors do not protect against mortality by controlling the systemic infection during sepsis. In addition to direct organ injury caused by multiplying bacteria, excessive inflammation is another factor that precipitates organ injury and eventually leads to death in sepsis. Our studies identify this inflammatory response as a major target of A2B receptors in preventing mortality in septic mice. We found that A2B receptors decreased inflammatory cytokine and chemokine levels both systematically and locally in organs, such as the lung, heart, and the peritoneal cavity. The role of A2B receptors in regulating inflammation is controversial. In some studies, A2B receptor KO mice exhibited elevated plasma levels of inflammatory mediators in the systemic circulation (13), lung (16), vasculature (14), and kidney (23). In contrast, in the dextran sodium sulfate-induced colitis model, A2B receptor KO mice had decreased levels of inflammatory mediators as well as an attenuated clinical course of colitis (17). In addition, A2B receptor inactivation suppressed pulmonary injury in adenosine deaminase-deficient mice (26). These observations underline the concept that the role of adenosine receptors in regulating inflammation is complex and unpredictable (8, 9, 27–29).
The mechanisms by which A2B receptor activation limits inflammation have not been defined in detail. Increased activation of NF-κB occurs during sepsis, and this activation is associated with higher rates of mortality and worse clinical outcome (30, 31). Our results demonstrating downregulation of the NF-κB system in the spleen by A2B receptors indicate that this decreased NF-κB activation may represent a central pathway by which A2B receptors decrease inflammatory cytokine production and injury in sepsis. Earlier studies on A1 and A3 receptor KO animals documented increased hepatic and kidney injury in response to CLP (10, 12). In the current study, we noted more pronounced cardiac inflammation in septic A2B KO animals, indicating heart protection as an additional potential mechanism of the protective effect of A2B receptors. Although inflammatory cytokine levels were elevated in the lungs of A2B KO animals, this did not translate into increased injury, as assessed using the myeloperoxidase assay and by measuring wet/dry ratio (edema) (data not shown). In contrast, the degree of liver and kidney injury as assessed by histological observation, IκBα degradation, and measuring inflammatory mediator levels was similar in A2B KO and WT mice subjected to CLP (data not shown).
Myocardial injury and cardiac dysfunction are major consequences of sepsis and contribute to the high mortality of this illness (32). It is well appreciated that an NF-κB– and p38-driven inflammatory response leading to overproduction of proinflammatory cytokines, such as IL-6 and TNF-α in the heart, is an important factor in mediating sepsis-induced cardiac injury (33–35). In this regard, our data showing increased cardiac inflammatory mediator production and NF-κB activation in A2B receptor KO mice suggest that A2B receptor may limit septic mortality by attenuating cardiac inflammation and maintaining cardiac contractility. This idea is supported by our recent observations demonstrating that A2B receptor activation is protective in cardiac ischemia (20).
Sepsis provokes extensive immune cell apoptosis, and mortality in this illness is thought to be, at least in part, a consequence of dysregulated immune cell death. The most affected immune cell populations are T and B lymphocytes, as well as dendritic cells (2, 36, 37). In our model, A2B receptors prevented apoptosis in the spleen. Because we found decreased numbers of CD11b+ but not CD4+ or CD8+ cells in the spleen of A2B KO mice, and dendritic cells express CD11b, we propose that A2B receptor activation prevents depletion of dendritic cells. Because polymorphonuclear neutrophils also express CD11b, an additional explanation for decreased CD11b expression in the spleen of A2B KO mice is that splenic polymorphonuclear neutrophil infiltration is decreased in KO animals. Because we observed diminished Akt signaling in the spleens of A2B KO animals, it is possible that A2B receptors protect against apoptosis by activating Akt. This proposition is supported by a recent study, which reported that inhibition of Akt signaling augmented apoptosis in the spleen and increased CLP-induced mortality of septic animals (38). In this regard, it is note-worthy that A2B receptors have been reported to activate the Akt system both in vitro (39) and in the ischemic heart (40). Finally, our results that A2B receptors limited activation of PTEN, a negative upstream regulator of Akt highlight a novel mechanism explaining the inhibitory effect of A2B receptor on apoptosis.
Results of a recent study utilizing A2B receptor bone marrow-chimeric mice indicated a primary role for A2B receptors on hematopoietic cells in decreasing the TNF-α and IL-6 response to peritoneal injection of LPS (13). In contrast, our experiments with A2B bone marrow chimeras revealed that regulation of plasma cytokine production in polymicrobial sepsis is associated with A2B signaling on nonhematopoietic cells. These results further emphasize the dissimilarity of our polymicrobial sepsis model and the endotoxemia model, in which LPS injection elicits a cytokine response. Our previous studies using different injury models also underscore the point that protection can be mediated by A2B receptors on nonhematopoietic-derived cells or on both hematopoietic and nonhematopoietic cells (15, 16). For example, we showed a critical role of vascular (nonhematopoietic) A2B receptors in limiting vascular leakage caused by hypoxia, and both nonhematopoietic and hematopoietic A2B receptors were responsible for suppressing neutrophil tissue accumulation following a hypoxic episode (15). In summary, the most intriguing result of this study is that inactivation of A2B receptors increases the mortality of mice subjected to polymicrobial sepsis. These results illustrate that A2B adenosine receptors have a beneficial effect on the outcome of sepsis and may represent a new therapeutic target for the management of patient with sepsis.
Acknowledgments
This work was supported by National Institutes of Health Grant R01GM66189 and the Intramural Research Program of the National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism, as well as the Hungarian Research Fund (CK 78275).
Abbreviations in this paper
- CLP
cecal ligation and puncture
- IL-1β
β form of pro-IL-1
- KO
knockout
- PARP
poly(ADP-ribose) polymerase
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 2.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci USA. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt H, Siems WG, Grune T, Grauel EL. Concentration of purine compounds in the cerebrospinal fluid of infants suffering from sepsis, convulsions and hydrocephalus. J Perinat Med. 1995;23:167–174. doi: 10.1515/jpme.1995.23.3.167. [DOI] [PubMed] [Google Scholar]
- 5.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53:527–552. [PMC free article] [PubMed] [Google Scholar]
- 6.Haskó G, Szabó C, Németh ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- 7.Haskó G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25:33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Sitkovsky MV, Lukashev D, Apasov S, Kojima H, Koshiba M, Caldwell C, Ohta A, Thiel M. Physiological control of immune response and inflammatory tissue damage by hypoxia-inducible factors and adenosine A2A receptors. Annu Rev Immunol. 2004;22:657–682. doi: 10.1146/annurev.immunol.22.012703.104731. [DOI] [PubMed] [Google Scholar]
- 9.Haskó G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallos G, Ruyle TD, Emala CW, Lee HT. A1 adenosine receptor knockout mice exhibit increased mortality, renal dysfunction, and hepatic injury in murine septic peritonitis. Am J Physiol Renal Physiol. 2005;289:F369–F376. doi: 10.1152/ajprenal.00470.2004. [DOI] [PubMed] [Google Scholar]
- 11.Németh ZH, Csóka B, Wilmanski J, Xu D, Lu Q, Ledent C, Deitch EA, Pacher P, Spolarics Z, Haskó G. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176:5616–5626. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HT, Kim M, Joo JD, Gallos G, Chen JF, Emala CW. A3 adenosine receptor activation decreases mortality and renal and hepatic injury in murine septic peritonitis. Am J Physiol Regul Integr Comp Physiol. 2006;291:R959–R969. doi: 10.1152/ajpregu.00034.2006. [DOI] [PubMed] [Google Scholar]
- 13.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116:1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang D, Koupenova M, McCrann DJ, Kopeikina KJ, Kagan HM, Schreiber BM, Ravid K. The A2b adenosine receptor protects against vascular injury. Proc Natl Acad Sci USA. 2008;105:792–796. doi: 10.1073/pnas.0705563105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111:2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118:3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolachala VL, Vijay-Kumar M, Dalmasso G, Yang D, Linden J, Wang L, Gewirtz A, Ravid K, Merlin D, Sitaraman SV. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology. 2008;135:861–870. doi: 10.1053/j.gastro.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Csóka B, Németh ZH, Virág L, Gergely P, Leibovich SJ, Pacher P, Sun CX, Blackburn MR, Vizi ES, Deitch EA, Haskó G. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood. 2007;110:2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Németh ZH, Bleich D, Csóka B, Pacher P, Mabley JG, Himer L, Vizi ES, Deitch EA, Szabó C, Cronstein BN, et al. Adenosine receptor activation ameliorates type 1 diabetes. FASEB J. 2007;21:2379–2388. doi: 10.1096/fj.07-8213com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckle T, Krahn T, Grenz A, Köhler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 21.Haudek SB, Spencer E, Bryant DD, White DJ, Maass D, Horton JW, Chen ZJ, Giroir BP. Overexpression of cardiac I-kappaBalpha prevents endotoxin-induced myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2001;280:H962–H968. doi: 10.1152/ajpheart.2001.280.3.H962. [DOI] [PubMed] [Google Scholar]
- 22.Edwin F, Singh R, Endersby R, Baker SJ, Patel TB. The tumor suppressor PTEN is necessary for human Sprouty 2-mediated inhibition of cell proliferation. J Biol Chem. 2006;281:4816–4822. doi: 10.1074/jbc.M508300200. [DOI] [PubMed] [Google Scholar]
- 23.Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enoh VT, Fairchild CD, Lin CY, Varma TK, Sherwood ER. Differential effect of imipenem treatment on wild-type and NK cell-deficient CD8 knockout mice during acute intra-abdominal injury. Am J Physiol Regul Integr Comp Physiol. 2006;290:R685–R693. doi: 10.1152/ajpregu.00678.2005. [DOI] [PubMed] [Google Scholar]
- 25.Leibovici L, Drucker M, Konigsberger H, Samra Z, Harrari S, Ashkenazi S, Pitlik SD. Septic shock in bacteremic patients: risk factors, features and prognosis. Scand J Infect Dis. 1997;29:71–75. doi: 10.3109/00365549709008668. [DOI] [PubMed] [Google Scholar]
- 26.Sun CX, Zhong H, Mohsenin A, Morschl E, Chunn JL, Molina JG, Belardinelli L, Zeng D, Blackburn MR. Role of A2B adenosine receptor signaling in adenosine-dependent pulmonary inflammation and injury. J Clin Invest. 2006;116:2173–2182. doi: 10.1172/JCI27303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohta A, Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- 28.Sitkovsky MV, Ohta A. The ‘danger’ sensors that STOP the immune response: the A2 adenosine receptors? Trends Immunol. 2005;26:299–304. doi: 10.1016/j.it.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Feoktistov I, Biaggioni I. Adenosine A2B receptors. Pharmacol Rev. 1997;49:381–402. [PubMed] [Google Scholar]
- 30.Böhrer H, Qiu F, Zimmermann T, Zhang Y, Jllmer T, Männel D, Böttiger BW, Stern DM, Waldherr R, Saeger HD, et al. Role of NFkappaB in the mortality of sepsis. J Clin Invest. 1997;100:972–985. doi: 10.1172/JCI119648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paterson RL, Galley HF, Dhillon JK, Webster NR. Increased nuclear factor kappa B activation in critically ill patients who die. Crit Care Med. 2000;28:1047–1051. doi: 10.1097/00003246-200004000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Krishnagopalan S, Kumar A, Parrillo JE, Kumar A. Myocardial dysfunction in the patient with sepsis. Curr Opin Crit Care. 2002;8:376–388. doi: 10.1097/00075198-200210000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Kapadia S, Lee J, Torre-Amione G, Birdsall HH, Ma TS, Mann DL. Tumor necrosis factor-alpha gene and protein expression in adult feline myocardium after endotoxin administration. J Clin Invest. 1995;96:1042–1052. doi: 10.1172/JCI118090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang H, Wang HY, Bassel-Duby R, Maass DL, Johnston WE, Horton JW, Tao W. Role of interleukin-6 in cardiac inflammation and dysfunction after burn complicated by sepsis. Am J Physiol Heart Circ Physiol. 2007;292:H2408–H2416. doi: 10.1152/ajpheart.01150.2006. [DOI] [PubMed] [Google Scholar]
- 35.Yang M, Wu J, Martin CM, Kvietys PR, Rui T. Important role of p38 MAP kinase/NF-kappaB signaling pathway in the sepsis-induced conversion of cardiac myocytes to a proinflammatory phenotype. Am J Physiol Heart Circ Physiol. 2008;294:H994–H1001. doi: 10.1152/ajpheart.01044.2007. [DOI] [PubMed] [Google Scholar]
- 36.Peck-Palmer OM, Unsinger J, Chang KC, McDonough JS, Perlman H, McDunn JE, Hotchkiss RS. Modulation of the Bcl-2 family blocks sepsis-induced depletion of dendritic cells and macrophages. Shock. 2009;31:359–366. doi: 10.1097/SHK.0b013e31818ba2a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peck-Palmer OM, Unsinger J, Chang KC, Davis CG, McDunn JE, Hotchkiss RS. Deletion of MyD88 markedly attenuates sepsis-induced Tand B lymphocyte apoptosis but worsens survival. J Leukoc Biol. 2008;83:1009–1018. doi: 10.1189/jlb.0807528. [DOI] [PubMed] [Google Scholar]
- 38.Williams DL, Li C, Ha T, Ozment-Skelton T, Kalbfleisch JH, Preiszner J, Brooks L, Breuel K, Schweitzer JB. Modulation of the phosphoinositide 3-kinase pathway alters innate resistance to polymicrobial sepsis. J Immunol. 2004;172:449–456. doi: 10.4049/jimmunol.172.1.449. [DOI] [PubMed] [Google Scholar]
- 39.Schulte G, Fredholm BB. The G(s)-coupled adenosine A(2B) receptor recruits divergent pathways to regulate ERK1/2 and p38. Exp Cell Res. 2003;290:168–176. doi: 10.1016/s0014-4827(03)00324-0. [DOI] [PubMed] [Google Scholar]
- 40.Solenkova NV, Solodushko V, Cohen MV, Downey JM. Endogenous adenosine protects preconditioned heart during early minutes of reperfusion by activating Akt. Am J Physiol Heart Circ Physiol. 2006;290:H441–H449. doi: 10.1152/ajpheart.00589.2005. [DOI] [PubMed] [Google Scholar]