Abstract
Vascular endothelial growth factor (VEGF) appears to be an important mediator of pathologic retinal angiogenesis. In understanding the mechanisms of pathologic retinal neovascularization, we found that VEGF activates PLD1 in human retinal microvascular endothelial cells, and this event is dependent on Src. In addition, VEGF activates protein kinase C-γ (PKCγ) via Src-dependent PLD1 stimulation. Inhibition of Src, PLD1, or PKCγ via pharmacologic, dominant negative mutant, or siRNA approaches significantly attenuated VEGF-induced human retinal microvascular endothelial cell migration, proliferation, and tube formation. Hypoxia also induced Src-PLD1-PKCγ signaling in retina, leading to retinal neovascularization. Furthermore, siRNA-mediated down-regulation of VEGF inhibited hypoxia-induced Src-PLD1-PKCγ activation and neovascularization. Blockade of Src-PLD1-PKCγ signaling via the siRNA approach also suppressed hypoxia-induced retinal neovascularization. Thus, these observations demonstrate, for the first time, that Src-dependent PLD1-PKCγ activation plays an important role in pathologic retinal angiogenesis.
Introduction
Angiogenesis is required for normal development and wound healing.1,2 Paradoxically, angiogenesis also plays a major role in the progression of various disorders, including tumor growth, retinopathy, and atherosclerosis.3–5 The pathologic ocular neovascularization that occurs in retinopathy of prematurity and proliferative diabetic retinopathy can lead to significant vision loss.6 Among the numerous factors identified thus far that stimulate pathologic angiogenesis, vascular endothelial growth factor (VEGF) appears to be the most potent by far.5,7 VEGF mediates its angiogenic effects via its tyrosine kinase receptors, VEGFR1 and VEGFR2.8–10 A large number of studies reveal that, although VEGFR2 mediates the angiogenic effects of VEGF during development and disease processes, VEGFR1 either antagonizes the actions of VEGFR2 or is only involved in the mediation of pathologic angiogenesis.5 Because circulating levels of VEGF are highly elevated in cancer and retinopathy patients, many current therapeutic strategies target VEGF function.11,12 VEGF is required for endothelial cell proliferation, migration, and permeability.13,14 One of the well-studied signaling molecules in VEGF-induced angiogenic events of endothelial cells (ECs) is Src, a nonreceptor tyrosine kinase.15 In addition, a role for phospholipase C-γ, phosphoinositide-3 kinase, and extracellular signal-regulated kinase in the mediation of VEGF signaling events in ECs has been demonstrated.16,17 Despite the high potency of VEGF in mediating angiogenesis, relatively less is known about the mechanisms by which VEGF influences the angiogenic signaling events. During the past decade, work from various laboratories has indicated that PLD1/2 plays a role in the regulation of cell migration and proliferation.18–20 PLD1/2 hydrolyzes membrane phospholipids and phosphatidylcholine and generates various lipid second messenger molecules, including diacylglycerol.21 The other category of signaling molecules involved in the regulation of cell migration and proliferation is protein kinase C (PKC), and these serine/threonine kinases are intertwined with signaling events of hormones, growth factors, and cytokines mediating a variety of biologic responses.22 In our ongoing studies of the molecular mechanisms of VEGF-induced angiogenic signaling, we have discovered that VEGF activates PLD1 downstream to Src. In addition, Src-dependent PLD1 activation is needed for PKCγ stimulation. Furthermore, activation of Src-PLD1-PKCγ signaling is required for VEGF-induced human retinal microvascular endothelial cell (HRMVEC) migration, proliferation, and tube formation. Interestingly enough, activation of Src-PLD1-PKCγ signaling is also needed for hypoxia-induced VEGF-mediated pathologic retinal neovascularization.
Methods
Reagents
Recombinant human VEGF165 was bought from R&D Systems (catalog no. 293-VE). Growth factor–reduced Matrigel (catalog no. 354250) was obtained from BD Biosciences. 1,6-Bis (cyclohexyloximinocarbonyl-amino) hexane (catalog no. ST-300) was purchased from BIOMOL Research Laboratories. PP1 (catalog no. 529579) and propranolol (catalog no. 537075) were purchased from EMD Chemicals (Calbiochem). High-molecular-weight (∼ 2 000 000) fluorescein-conjugated dextran (catalog no. FD2000S), 1-butanol (catalog no. B-7906), fluorescein isothiocyanate (FITC)–conjugated anti–rabbit IgG (catalog no. F-0382), and tetramethylrhodamine isothiocyanate (TRITC)–conjugated anti–rabbit IgG (catalog no. T6778) were purchased from Sigma-Aldrich. Anti-PKCα antibodies (SC-8393), anti-PKCγ antibodies (SC-211), anti-PKCδ antibodies (SC-213), anti-PLD1 antibodies (SC-025512), anti–Tie-2 antibodies (SC-324), and anti–β-tubulin antibodies (SC-9104) were purchased from Santa Cruz Biotechnology. Anti-PLD1 antibodies (catalog no. 3832), phospho-Src (Tyr416) antibodies (catalog no. 2101), phospho-PLD1 (Thr147) antibodies (catalog no. 3831), phospho-PKCα/βII (Thr638/641) antibodies (catalog no. 9375), phospho-PKCγ (Thr514) antibodies (catalog no. 9379), phospho-PKCδ (Tyr311) antibodies (catalog no. 2055), and phosphotyrosine antibodies (catalog no. 9411) were purchased from Cell Signaling Technology. Anti-Src antibodies (catalog no. 05-184) were purchased from Millipore. Anti-CD31 antibodies (catalog no. 550274) were purchased from BD Biosciences PharMingen. Phosphoserine/threonine/tyrosine antibodies (catalog no. ab15556) were purchased from Abcam. Amplex Red Phospholipase D Assay Kit (catalog no. A12219) was bought from Invitrogen. Human Scr siRNA (ON-TARGET plus nontargeting Pool D-001810-10), human PLD1 siRNA (ON-TARGET plus SMARTpool L-009413-00-0010), human PKCγ siRNA (ON-TARGET plus SMARTpool L-004654-00-0010), mouse Scr siRNA (ON-TARGET plus Nontargeting Pool D-001810-10-20), mouse Src siRNA (ON-TARGET plus SMARTpool L-040877-00-0010), mouse PLD1 siRNA (ON-TARGET plus SMARTpool L-040014-01-0020), mouse PKCγ siRNA (ON-TARGET plus SMARTpool L-050293-00-0010), and mouse VEGF siRNA (ON-TARGET plus SMARTpool L-040812-00-0020) were purchased from Thermo Electron. VECTOR M.O.M. Immunodetection Kit (catalog no. FMK-2201), Vectashield HardSet Mounting Medium for Fluorescence (catalog no. H-1400), and Vectashield HardSet Mounting Medium with DAPI (catalog no. H-1500) were purchased from Vector Laboratories. Alexa Fluor 350 goat anti–rat IgG (catalog no. A21093) was purchased from Invitrogen. The construction of Ad-GFP and Ad-dnSrc were described previously.23
Cell culture
HRMVECs (catalog no. ACBRI 181) were purchased from Applied Cell Biology Research Institute and grown in medium 131 containing microvascular growth supplements, 10 μg/mL gentamycin and 0.25 μg/mL amphotericin B. Cultures were maintained at 37°C in a humidified 95% air and 5% CO2 atmosphere. HRMVECs were growth-arrested by incubating in medium 131 for 24 hours and used to perform the experiments unless otherwise indicated.
Cell migration
Cell migration was performed as described previously.23
DNA synthesis
DNA synthesis was measured by [3H]-thymidine incorporation as described previously.24
PLD activity
PLD activity was measured using Amplex Red Phospholipase D Assay Kit following the manufacturer's instructions. Cell extracts containing an equal amount of protein from each sample were placed into a 96-well plate. Reactions were started by adding 100mM Amplex Red reagent, 2 U/mL horseradish peroxidase, 0.2 U/mL choline oxidase, and 0.5mM lecithin. After incubation at 37°C for 1 hour, fluorescence intensity of the samples was measured with a fluorescence microplate reader with excitation at 530 nm and fluorescence detection at 590 nm. The enzymatic activity was expressed as arbitrary fluorescence units.
Tube formation
Tube formation was measured as described previously.23 The images of tubelike structures were observed under a phase-contrast light microscope (Nikon Eclipse TS100; type, ADL; magnification 10×/0.25 NA) and the images were captured using a CCD camera (KP-D20AU, Hitachi) using Apple iMovie Version 7.1.4 software.
Western blotting
Cell or tissue extracts containing an equal amount of protein were resolved by electrophoresis on 0.1% sodium dodecyl sulfate and 10% polyacrylamide gels. The proteins were transferred electrophoretically to a nitrocellulose membrane. After blocking, the membrane was treated with appropriate primary antibodies followed by incubation with horseradish peroxidase-conjugated secondary antibodies. The antigen-antibody complexes were detected using a chemiluminescence reagent kit.
Transfections and transductions
HRMVECs were transfected with specific siRNA molecules at a final concentration of 100nM using Lipofectamine 2000 transfection reagent according to the manufacturer's instructions. When adenoviral vectors were used to down-regulate the function of a specific molecule, cells were transduced with adenovirus carrying green fluorescent protein (GFP) or target molecule at 40 multiplicity of infection per overnight in complete medium. After transfection or transductions, cells were quiesced in microvascular growth supplement–free medium 131 for 24 hours and used as required. In the case of in vivo transfections, siRNA molecules at 1 μg/0.5 μL/eye were injected intravitreally using a 33-gauge needle syringe.
Retinal angiogenesis
C57BL/6 mice pups with their nursing mothers were exposed to 75% oxygen regulated by an oxygen controller (BioSpherix Ltd) between P7 and P12. After exposure to hyperoxia, mice pups were administered vehicle, Scr siRNA, Src siRNA, PLD1 siRNA, PKCγ siRNA, or VEGF siRNA (1 μg/0.5 μL/eye) at P13, P14, and P15 by intravitreal injection using a 33-gauge needle.25 They were killed at P17 after intracardiac perfusion with high-molecular-weight FITC-dextran in phosphate-buffered saline. Eyes were enucleated and fixed in 4% paraformaldehyde for 24 hours at room temperature. Retinas were isolated, flat-mounted, and examined using a fluorescence microscope. Retinal vasculature was determined by calculating the ratio of fluorescence intensity to total retinal area. Retinal tufts were used to evaluate the retinal neovascularization. Neovascularization was quantified by dividing the area of tufts by total retinal area. All the experiments involving the use of animals were approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center (Memphis, TN).
Immunofluorescence staining
After blocking in normal goat serum, the cryosections (5 μm) were incubated with rabbit anti–mouse Tie-2 antibodies (1:100) and rat anti–mouse CD31 antibodies (1:100) in a humidified chamber for 1 hour at room temperature, followed by incubation with TRITC- or FITC-conjugated secondary antibodies. Fluorescence images were captured using a Zeiss inverted microscope (AxioVision AX10). To examine the phosphorylation of Src, PLD1, and PKCγ in retina, cryosections (5 μm) were blocked in M.O.M. Mouse Ig blocking reagent (Vector Laboratories), incubated with phosphotyrosine antibodies (1:100) or phosphoserine/threonine/tyrosine antibodies (1:100) in M.O.M. diluent solution, followed by incubation with biotinylated anti–mouse IgG and fluorescein-avidin DCS. After washing with phosphate-buffered saline and blocking in goat serum, the sections were incubated with rabbit anti–mouse-Src, PLD1, or PKCγ antibodies in combination with rat anti–mouse CD31 antibodies. The sections were probed with TRITC-conjugated goat anti–rabbit and Alexa Fluor-350–conjugated goat anti–rat antibodies. The sections were observed under a Zeiss inverted microscope (Zeiss AxioVision AX10; type, LD plan-Neofluar; magnification 40×/0.6 NA or type, plan-Apochromat; magnification 10×/0.45 NA) and fluorescence images were captured with a Zeiss AxioCam MRm camera using the microscope operating and image analysis software AxioVision Version 4.7.2 (Carl Zeiss Imaging Solutions GmbH).
Statistics
All experiments were repeated 3 times, and data are presented as mean plus or minus SD. The treatment effects were analyzed by Student t test, and P values less than .05 were considered to be statistically significant. In the case of Western blot analysis, immunofluorescence staining, and retinal angiogenesis, one set of data is shown.
Results
PLD1 mediates VEGF-induced HRMVEC DNA synthesis, migration, and tube formation
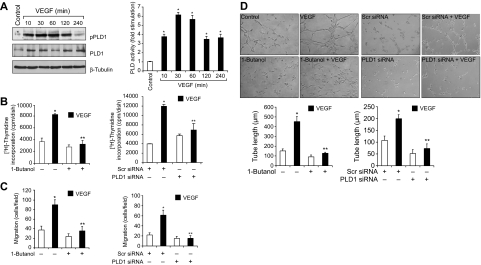
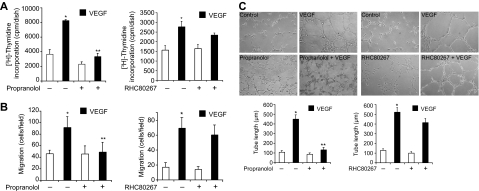
VEGF binds to its receptors VEGFR1/2 and stimulates a variety of signaling events leading to angiogenesis.7–10 To understand the mechanisms of VEGF-induced retinal neovascularization, we tested the role of PLD1. VEGF (40 ng/mL) induced PLD1 phosphorylation in a time-dependent manner in HRMVECs. Maximum increases in Thr147 phosphorylation of PLD1 occurred at 30 minutes, and these increases were sustained for at least 2 hours (Figure 1A). Consistent with its phosphorylation state, PLD activity was also increased in a time-dependent manner with maximum activity at 30 minutes in response to VEGF. In addition, either 1-butanol (0.25%), the pharmacologic inhibitor of PLD1,26 or PLD1 depletion by its siRNA attenuated VEGF-induced HRMVEC DNA synthesis, migration, and tube formation (Figure 1B-D). Because activation of PLD1 generates phosphatidic acid (PA), a potential mitogen,27 we asked the question whether PA or its conversion to diacylglycerol (DAG) is needed for VEGF-induced HRMVEC DNA synthesis, migration, and tube formation. To address this question, we tested the effect of propranolol, a specific inhibitor of PA phosphohydrolase (PAP).28 Propranolol (200μM) inhibited VEGF-induced HRMVEC DNA synthesis, migration, and tube formation, suggesting that conversion of PA to DAG is required for the observed responses (Figure 2). On the other hand, inhibition of DAG lipase activity using its pharmacologic inhibitor RHC8026729 had no significant effect on VEGF-induced HRMVEC DNA synthesis, migration, and tube formation (Figure 2). These findings indicate that PLD1-dependent DAG production is needed for VEGF-induced angiogenic responses of HRMVECs.
Figure 1.
VEGF-induced HRMVEC DNA synthesis, migration, and tube formation require PLD1 activation. (A) Quiescent HRMVECs were treated with and without 40 ng/mL VEGF for the indicated time periods, and cell extracts were prepared and analyzed PLD1 phosphorylation by Western blotting using its phosphospecific antibodies or measured its activity using a kit. The blot was reprobed sequentially with anti-PLD1 and anti–β-tubulin antibodies for normalization. (B) Quiescent HRMVECs in which PLD1 is inhibited by 1-butanol (0.25%) or down-regulated by its siRNA (100nM) were treated with and without VEGF (40 ng/mL) for 24 hours, and DNA synthesis was measured by [3H]-thymidine incorporation. (C-D) All the conditions were the same as in panel B except that cells were treated with and without VEGF (40 ng/mL) for 8 hours and either cell migration (C) or tube formation (D) was measured. (B-D) Bar graphs represent the quantitative analysis of 3 independent experiments. Values are mean ± SD. *P < .01 versus vehicle control or Scr siRNA control. **P < .01 versus VEGF or Scr siRNA plus VEGF.
Figure 2.
Propranolol, a PAP inhibitor, but not RHC 80267, a DAG lipase inhibitor, blocks VEGF-induced HRMVEC DNA synthesis, migration, and tube formation. (A-C) All the conditions were the same as in Figure 1B, C, and D, respectively, except that either propranolol (200μM) or RHC80267 (50μM) was added to cells 30 minutes before treatment with and without VEGF. Values are mean ± SD. *P < .01 versus vehicle control. **P < .01 versus VEGF.
PKCγ mediates VEGF-induced HRMVEC DNA synthesis, migration, and tube formation downstream to PLD1
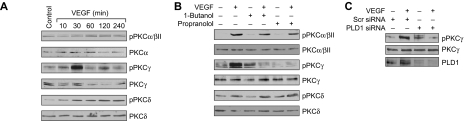
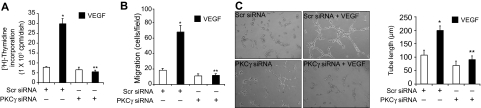
DAG is an essential lipid molecule for activation of conventional and novel PKC isozymes.22 To understand whether VEGF-induced PLD1 activation leads to stimulation of either conventional or novel PKC isozymes, we have studied the time course of VEGF activation of these serine/threonine kinases. Treatment with VEGF (40 ng/mL) resulted in a time-dependent stimulation of PKCα/βII, PKCγ, and PKCδ (Figure 3A). Next, we tested the role of PLD1 and PAP in VEGF-induced activation of these PKCs. 1-Butanol and propranolol, although having little or no effect on PKCα/βII or PKCδ phosphorylation, completely suppressed the phosphorylation of PKCγ (Figure 3B). In addition, siRNA-mediated depletion of PLD1 also attenuated VEGF-induced PKCγ phosphorylation (Figure 3C). To determine whether PKCγ plays a role in VEGF-induced HRMVEC angiogenic responses, we used an siRNA approach. Down-regulation of PKCγ by its siRNA attenuated VEGF-induced HRMVEC DNA synthesis, migration, and tube formation (Figure 4A-C).
Figure 3.
VEGF-induced PKCγ activation is mediated by PLD1 in HRMVECs. (A) Quiescent HRMVECs were treated with and without VEGF (40 ng/mL) for the indicated time periods, and cell extracts were prepared and analyzed by Western blotting for PKCα/βII, -γ, and -δ phosphorylation using their phosphospecific antibodies. (B) All the conditions were the same as in panel A, except that cells were treated with and without VEGF (40 ng/mL) in the presence and absence of 1-butanol (0.25%) or propranolol (200μM) for 30 minutes and cell extracts were prepared and analyzed by Western blotting for PKCα/βII, -γ, and -δ phosphorylation using their phosphospecific antibodies. (C) HRMVECs that were transfected with scrambled or PLD1 siRNA were quiesced, treated with and without VEGF (40 ng/mL) for 30 minutes, and cell extracts were prepared and analyzed by Western blotting for PKCγ phosphorylation using its phosphospecific antibodies. (A-C) The blots were reprobed with the antibodies of the indicated molecules for either normalization or to show the effect of siRNA on its target molecule levels.
Figure 4.
VEGF-induced HRMVEC DNA synthesis, migration, and tube formation require PKCγ activation. (A-C) All the conditions were the same as in Figure 1B, C, and D, respectively, except that cells were transfected with either scrambled or PKCγ siRNA and quiesced before subjecting them to VEGF-induced DNA synthesis, migration, or tube formation. (A-C) Bar graphs represent the quantitative analysis of 3 independent experiments. Values are mean ± SD. *P < .01 versus Scr siRNA. **P < .01 versus Scr siRNA plus VEGF.
Src mediates VEGF-induced PLD1-PKCγ activation
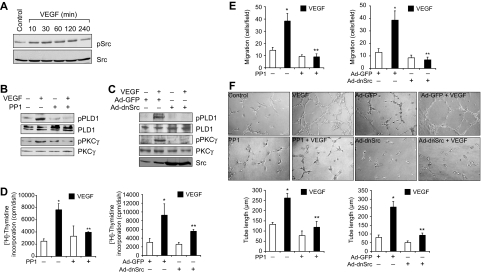
It was suggested that Src plays a role in VEGF-induced angiogenesis.15 To understand the upstream signaling events of PLD1-PKCγ activation by VEGF, we tested the role of Src. VEGF stimulated the tyrosine phosphorylation of Src in a time-dependent manner (Figure 5A). Interference with activation of Src by the use of its pharmacologic inhibitor PP130 or adenovirus-mediated expression of its dominant negative mutant significantly blocked VEGF-induced PLD1 and PKCγ activation (Figure 5B-C). Similarly, PP1 and dnSrc also blocked VEGF-induced HRMVEC DNA synthesis, migration, and tube formation (Figure 5D-F).
Figure 5.
Src mediates VEGF-induced PLD1-PKCγ signaling axis activation. (A) All the conditions were the same as in Figure 1A, except that the cell extracts were analyzed by Western blotting for Src phosphorylation using its phosphotyrosine antibodies. (B) Quiescent HRMVECs were treated with and without VEGF (40 ng/mL) for 30 minutes in the presence or absence of 10μM PP1, a potent Src inhibitor, and cell extracts were prepared and analyzed by Western blotting for PLD1 and PKCγ phosphorylation using their phosphospecific antibodies. (C) All the conditions were the same as in panel B, except that cells were transduced with Ad-GFP or Ad-dnSrc at 40 multiplicity of infection and quiesced before subjecting them to treatment with and without VEGF (40 ng/mL) for 30 minutes and analyzing the cell extracts for PLD1 and PKCγ phosphorylation. (A-C) The blots were reprobed with antibodies of the indicated molecules for normalization. The bottom blot in panel C shows overexpression of dominant negative Src. (D-F) Quiescent cells in which Src is inhibited by PP1 or its dominant negative mutant were treated with and without VEGF (40 ng/mL), and DNA synthesis, cell migration, and tube formation were measured as described in Figure 1B, C, and D, respectively. Values are mean ± SD. *P < .01 versus vehicle control or Ad-GFP control. **P < .01 versus VEGF or Ad-GFP plus VEGF.
Oxygen-induced retinal neovascularization requires Src-PLD1-PKCγ activation downstream to VEGF
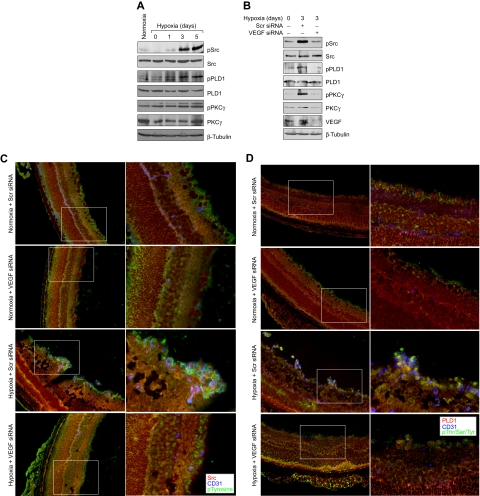
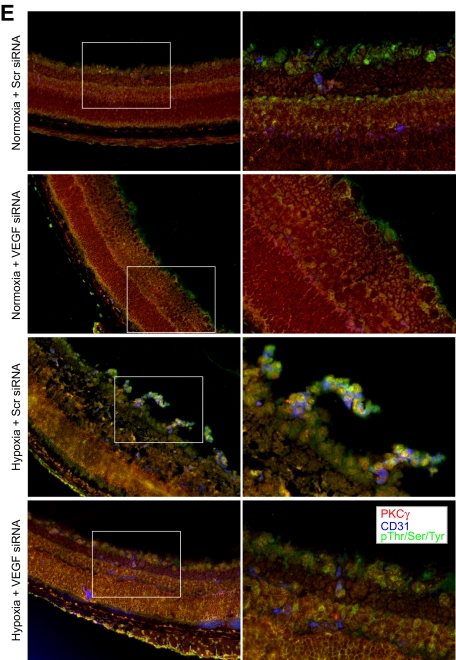
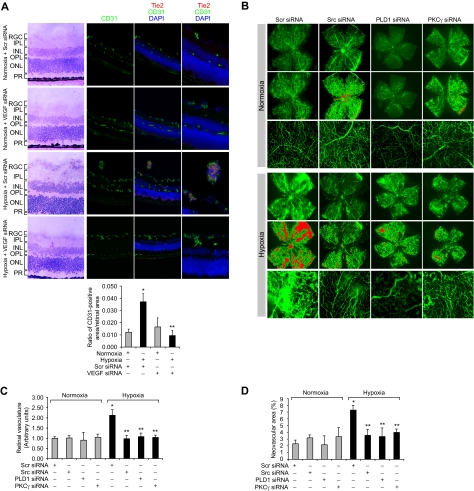
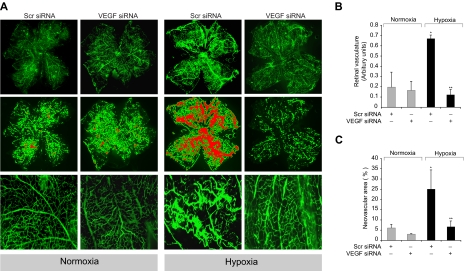
To understand the significance of Src-PLD1-PKCγ signaling in pathologic retinal angiogenesis, we used the oxygen-induced retinopathy model. We subjected mice pups to hyperoxia for 5 days (P7-P12) followed by a return to normoxia, thereby causing relative hypoxia. This treatment led to phosphorylation of Src, PLD1, and PKCγ in a time-dependent manner with maximum effect at 3 days and 5 days (Figure 6A). Previous studies have shown that hypoxia induces the expression of VEGF.12 To determine whether hypoxia-induced Src, PLD1, and PKCγ phosphorylation are dependent on VEGF production, we next tested the role of VEGF. Down-regulation of VEGF via its siRNA significantly attenuated hypoxia-induced Src, PLD1, and PKCγ phosphorylation (Figure 6B). To find the cell type in which hypoxia-induced Src, PLD1, and PKCγ phosphorylation occurs, we performed double or triple immunofluorescence studies. Immunofluorescence staining for Src, phosphotyrosine (PY20), and CD31 revealed that hypoxia induces Src phosphorylation both in the ganglion cell layer and in ECs of retina with heightened activation in the latter cell type (Figure 6C). Similarly, hypoxia induced the phosphorylation of PLD1 and PKCγ markedly in ECs (Figure 6D-E). Down-regulation of VEGF via intravitreal injection of its siRNA resulted in substantial inhibition of hypoxia-induced phosphorylation of Src, PLD1, and PKCγ in ECs (Figure 6C-E). Consistent with its effect on hypoxia-induced Src, PLD1, and PKCγ phosphorylation, depletion of VEGF levels by its siRNA suppressed retinal neovascularization as observed by the blockade of new capillary formation projecting into vitreous as well as a decrease in the number of CD31 and Tie2-positive cells, the former being a marker for ECs and latter being a marker for developing blood vessels (Figure 7A). Retinal flat mounts of FITC-dextran perfused mice showed that hypoxia, as expected, caused substantial increases in retinal neovascularization with the appearance of tufts and dilatation of blood vessels (Figure 7B-D). Furthermore, siRNA-mediated depletion of Src, PLD1, PKCγ, or VEGF dramatically reduced hypoxia-induced retinal neovascularization with reduction of tufts and absence of vasodilatation (Figures 7B-D, 8A-C).
Figure 6.
Hypoxia stimulates Src, PLD1, and PKCγ phosphorylation in a VEGF-dependent manner in retina. (A) C57BL/6 mice pups were exposed to 75% oxygen from P7 to P12, at which time they were returned to normoxia for the indicated time periods, enucleated, retinas were isolated, and analyzed by Western blotting for Src, PLD1, and PKCγ phosphorylation using their phosphospecific antibodies. (B) After exposure to hyperoxia, pups were returned to normoxia and administered 1 μg of Scr or VEGF siRNA at P12 and P13 by intravitreal injections. Retinas were isolated at P15, and the proteins were analyzed by Western blotting for Src, PLD1, and PKCγ phosphorylation using their phosphospecific antibodies. The blots were reprobed with antibodies of the indicated molecules for normalization. The blots in panels A and B that were probed with phosphospecific anti-PKCγ antibodies were also reprobed with either anti–β-tubulin or anti-VEGF antibodies for the purpose of lane loading control or to show the effect of VEGF siRNA on VEGF levels. (C) Retinas isolated at P15 were analyzed by double or triple immunofluorescence staining for phosphorylation of Src (C), PLD1 (D), and PKCγ (E) along with CD31 as described in “Methods.” The right column shows the higher magnification (original magnification ×40) of the selected areas of images shown in the left column.
Figure 7.
Down-regulation of VEGF, Src, PLD1, or PKCγ blocks hypoxia-induced retinal neovascularization. (A) After exposure to 75% oxygen, the mice pups were returned to normoxia, administered 1 μg of Scr or VEGF siRNA at P12 and P13 by intravitreal injections, and retinas were isolated at P15 and analyzed by double immunofluorescence staining for CD31 and Tie2 (top panel). Retinal vascularization was quantified by measuring the ratio of CD31-positive area to retinal area (bottom panel). The left column shows the histochemical staining of the respective retinal sections. The far right column shows the higher magnification (original magnification ×40) of retinal sections shown in the third column. (B-D) After exposure to 75% oxygen, the mice pups were returned to normoxia, administered 1 μg of Scr, Src, PLD1, or PKCγ siRNA at P13, P14, and P15 by intravitreal injections, and at P17 pups were anesthetized, perfused with FITC-dextran, sacrificed, enucleated, retinas were isolated, and flat mounts of whole retina were examined for retinal neovascularization (B). Neovascular tufts were highlighted with red (second and fifth rows). The third and sixth rows show the magnified section of a selected area of the images shown in the first and third rows, respectively. The images were captured using an Inverted Zeiss fluorescence microscope (AxioVision AX10), and fluorescence intensity in the total retinal area (C) and the area of neovascular tufts to total retinal area (D) was analyzed by Nikon NIS-Elements software Version AR3.1. Values are mean ± SD. *P < .01 versus normoxia plus scrambled siRNA control. **P < .01 versus hypoxia plus scrambled siRNA. RGC indicates retinal ganglion cell; IPL, inner plexiform layer; INL, inner nuclear layer; OPL, outer plexiform layer; ONL, outer nuclear layer; and PR, photoreceptors.
Figure 8.
Depletion of VEGF levels reduces hypoxia-induced retinal neovascularization. After exposure to 75% oxygen, the mice pups were returned to normoxia and administered 1 μg of Scr or VEGF siRNA at P13, P14, and P15 by intravitreal injections. Pups were anesthetized at P17, perfused with FITC-dextran, sacrificed, enucleated, retinas were isolated, and flat mounts of whole retina were examined for retinal neovascularization (A). Neovascular tufts were highlighted with red (second row). The third row shows the magnified (original magnification ×10) section of a selected area of the images shown in the first row (original magnification ×2). The images were captured using an Inverted Zeiss fluorescence microscope (AxioVision AX10), and fluorescence intensity in the total retinal area (B) and the area of neovascular tufts to total retinal area (C) was analyzed by Nikon NIS-Elements software Version AR3.1. Values are mean ± SD. *P < .01 versus normoxia plus scrambled siRNA control. **P < .01 versus hypoxia plus scrambled siRNA.
Discussion
VEGF has been found to be the major factor contributing to angiogenesis during development and disease.7,31 Circulating VEGF levels have been found to be elevated in patients with cancer, macular degeneration, and retinopathies.11,12 Indeed, many treatments that were approved by the Food and Drug Administration for cancer and retinal neovascularization are based on the inhibition of VEGF function.12 Among the 2 high-affinity VEGF receptors, VEGFR-2 was found to be the major mediator of both embryonic and pathologic angiogenesis.7,10 With regard to downstream signaling events of VEGFRs, phospholipase C-γ–dependent mitogen-activated protein kinase activation and phosphoinositide-3 kinase-Akt-mTOR–dependent S6K1 stimulation have been shown to be involved in VEGF-induced angiogenesis.16,17 In the present study, we observed that VEGF activates PLD1 via a mechanism involving Src in mediating HRMVEC proliferation, migration, and tube formation. Previous studies have demonstrated that PKCϵ mediates PLD1 activation in response to sphingosine 1-phosphate in lung endothelial cells.32 Similarly, it was observed that Src activation is required for PKCs in many cell types in response to several agonists.33 In light of these findings, it can be anticipated that Src via recruitment of PKC or some other serine/threonine kinases mediates VEGF-induced PLD1 activation in stimulating HRMVEC angiogenic responses. Because PLD1 generates PA, a potent mitogen,27 one could also expect that PA itself may be mediating the VEGF effects on HRMVEC proliferation, migration, and tube formation. However, this scenario appears unlikely as inhibition of PAP abrogated the VEGF-induced HRMVEC angiogenic responses. Instead, this observation along with the finding that inhibition of DAG lipase had no effect on VEGF-induced HRMVEC proliferation, migration, and tube formation suggest that DAG is the downstream effector lipid molecule of PLD1 in the observed angiogenic effects. Because DAG is required for activation of PKCs, particularly conventional and novel types, we can envision a role for one of these PKCs in mediating VEGF-induced HRMVEC angiogenic responses. The finding that down-regulation of PLD1 completely suppressed the VEGF-induced PKCγ activation indicates that VEGF activates this serine/threonine kinase via PLD1. The observation that down-regulation of PKCγ attenuates the VEGF effects on HRMVEC proliferation, migration, and tube formation supports a role for PKCγ downstream to PLD1 in VEGF-induced angiogenesis. The role of PKCγ in development, cell proliferation, and migration has also been demonstrated.34,35
The capacity of Src-PLD1-PKCγ signaling appears to be quite significant in mediating VEGF-induced angiogenic events in vivo in pathophysiologic models as well. The oxygen-induced retinopathy-induced regression in vessel density resulted in abnormal outgrowth of new vessels characterized by bulging, anastomose-like structures, and dilatation of vessels with leakiness after ischemia (hypoxia). Similar observations were noted by many studies previously.31,36 It is interesting that, after hyperoxia, Src, PLD1, and PKCγ are all phosphorylated and activation of these molecules requires VEGF. It is intriguing to note that phosphorylation of Src, PLD1, and PKCγ occurred specifically in ECs of the sprouting vessels. This demonstrates the precipitous capacity of VEGF in the activation of these signaling molecules in forming new blood vessels. The role of PLD1-PKCγ can also be much appreciated in hypoxia-induced angiogenesis because inhibition of either PLD1 or PKCγ negated the hypoxic effect on retinal neovascularization downstream to VEGF.
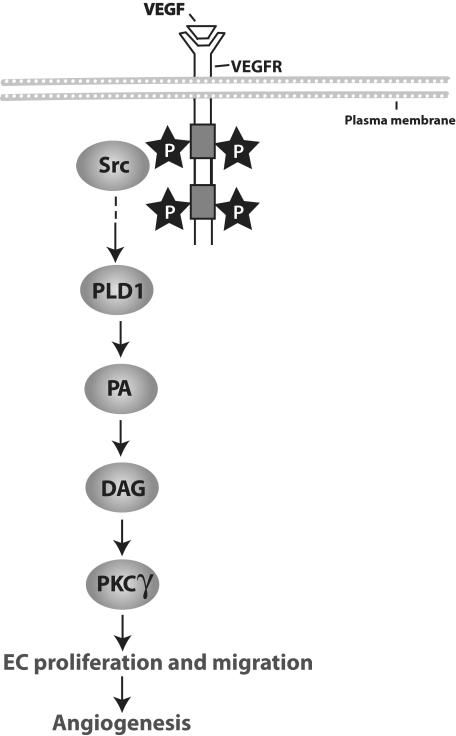
In conclusion, the present study provides the first evidence for the involvement of the Src-PLD1-PKCγ signaling axis as shown in Figure 9 in pathologic retinal neovascularization.
Figure 9.
A schematic flow chart depicting the Src-PLD1-PKCγ signaling axis in VEGF and hypoxia-induced pathologic retinal angiogenesis.
Acknowledgment
This work was supported by the National Eye Institute/National Institutes of Health (grant EY014856).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Q.Z. performed cell migration, DNA synthesis, tube formation, Western blot analysis, and retinal angiogenesis; D.W. performed immunofluorescence and retinal angiogenesis; V.K.-S. performed tube formation and Western blot analysis; L.G. performed retinal angiogenesis; D.A.J. provided scientific discussions and helped in writing the manuscript; G.J.T. helped in conducting retinal angiogenesis; and G.N.R. designed the experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gadiparthi N. Rao, Department of Physiology, University of Tennessee Health Science Center, 894 Union Ave, Memphis, TN 38163; e-mail: rgadipar@uthsc.edu.
References
- 1.Makanya AN, Hlushchuk R, Djonov VG. Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning, and remodeling. Angiogenesis. 2009;12(2):113–123. doi: 10.1007/s10456-009-9129-5. [DOI] [PubMed] [Google Scholar]
- 2.Kubota S, Takigawa M. CCN family proteins and angiogenesis: from embryo to adulthood. Angiogenesis. 2007;10(1):1–11. doi: 10.1007/s10456-006-9058-5. [DOI] [PubMed] [Google Scholar]
- 3.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alon T, Hemo I, Itin A, Pe'er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1(10):1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 5.Luttun A, Tjwa M, Moons L, et al. Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med. 2002;8(8):831–840. doi: 10.1038/nm731. [DOI] [PubMed] [Google Scholar]
- 6.Friedlander M. Fibrosis and diseases of the eye. J Clin Invest. 2007;117(3):576–586. doi: 10.1172/JCI31030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohela M, Bry M, Tammela T, Alitalo K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr Opin Cell Biol. 2009;21(2):154–165. doi: 10.1016/j.ceb.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 8.de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255(5047):989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 9.Terman BI, Dougher-Vermazen M, Carrion ME, et al. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun. 1992;187(3):1579–1586. doi: 10.1016/0006-291x(92)90483-2. [DOI] [PubMed] [Google Scholar]
- 10.Gille H, Kowalski J, Li B, et al. Analysis of biological effects and signaling properties of Flt-1 (VEGFR-1) and KDR (VEGFR-2): a reassessment using novel receptor-specific vascular endothelial growth factor mutants. J Biol Chem. 2001;276(5):3222–3230. doi: 10.1074/jbc.M002016200. [DOI] [PubMed] [Google Scholar]
- 11.Bradley J, Ju M, Robinson GS. Combination therapy for the treatment of ocular neovascularization. Angiogenesis. 2007;10(2):141–148. doi: 10.1007/s10456-007-9069-x. [DOI] [PubMed] [Google Scholar]
- 12.Aiello LP, Pierce EA, Foley ED, et al. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci U S A. 1995;92(23):10457–10461. doi: 10.1073/pnas.92.23.10457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Li X, Parra M, Verdin E, Bassel-Duby R, Olson EN. Control of endothelial cell proliferation and migration by VEGF signaling to histone deacetylase 7. Proc Natl Acad Sci U S A. 2008;105(22):7738–7743. doi: 10.1073/pnas.0802857105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8(11):1223–1234. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 15.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4(6):915–924. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 16.Takahashi T, Yamaguchi S, Chida K, Shibuya M. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 2001;20(11):2768–2778. doi: 10.1093/emboj/20.11.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitamura T, Asai N, Enomoto A, et al. Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat Cell Biol. 2008;10(3):329–337. doi: 10.1038/ncb1695. [DOI] [PubMed] [Google Scholar]
- 18.Ahn BH, Kim SY, Kim EH, et al. Transmodulation between phospholipase D and c-Src enhances cell proliferation. Mol Cell Biol. 2003;23(9):3103–3115. doi: 10.1128/MCB.23.9.3103-3115.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehman N, Di Fulvio M, McCray N, Campos I, Tabatabaian F, Gomez-Cambronero J. Phagocyte cell migration is mediated by phospholipases PLD1 and PLD2. Blood. 2006;108(10):3564–3572. doi: 10.1182/blood-2006-02-005959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang Y, Park IH, Wu AL, et al. PLD1 regulates mTOR signaling and mediates Cdc42 activation of S6K1. Curr Biol. 2003;13(23):2037–2044. doi: 10.1016/j.cub.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 21.Exton JH. Regulation of phospholipase D. FEBS Lett. 2002;531(1):58–61. doi: 10.1016/s0014-5793(02)03405-1. [DOI] [PubMed] [Google Scholar]
- 22.Dempsey EC, Newton AC, Mochly-Rosen D, et al. Protein kinase C isozymes and the regulation of diverse cell responses. Am J Physiol Lung Cell Mol Physiol. 2000;279(3):L429–L438. doi: 10.1152/ajplung.2000.279.3.L429. [DOI] [PubMed] [Google Scholar]
- 23.Cheranov SY, Karpurapu M, Wang D, Zhang B, Venema RC, Rao GN. An essential role for SRC-activated STAT-3 in 14,15-EET-induced VEGF expression and angiogenesis. Blood. 2008;111(12):5581–5591. doi: 10.1182/blood-2007-11-126680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karpurapu M, Wang D, Singh NK, Li Q, Rao GN. NFATc1 targets cyclin A in the regulation of vascular smooth muscle cell multiplication during restenosis. J Biol Chem. 2008;283(39):26577–26590. doi: 10.1074/jbc.M800423200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chikaraishi Y, Shimazawa M, Hara H. New quantitative analysis, using high-resolution images, of oxygen-induced retinal neovascularization in mice. Exp Eye Res. 2007;84(3):529–536. doi: 10.1016/j.exer.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Banno Y, Takuwa Y, Akao Y, et al. Involvement of phospholipase D in sphingosine 1-phosphate-induced activation of phosphatidylinositol 3-kinase and Akt in Chinese hamster ovary cells overexpressing EDG3. J Biol Chem. 2001;276(38):35622–35628. doi: 10.1074/jbc.M105673200. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Fanburg BL. Phospholipase D signaling in serotonin-induced mitogenesis of pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2008;295(3):L471–L478. doi: 10.1152/ajplung.00071.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asp L, Kartberg F, Fernandez-Rodriguez J, et al. Early stages of Golgi vesicle and tubule formation require diacylglycerol. Mol Biol Cell. 2009;20(3):780–790. doi: 10.1091/mbc.E08-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skinner JR, Shew TM, Schwartz DM, et al. Diacylglycerol enrichment of endoplasmic reticulum or lipid droplets recruits perilipin 3/TIP47 during lipid storage and mobilization. J Biol Chem. 2009;284(45):30941–30948. doi: 10.1074/jbc.M109.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh M, Gharavi NM, Choi J, et al. Oxidized phospholipids increase interleukin 8 (IL-8) synthesis by activation of the c-src/signal transducers and activators of transcription (STAT)3 pathway. J Biol Chem. 2004;279(29):30175–30181. doi: 10.1074/jbc.M312198200. [DOI] [PubMed] [Google Scholar]
- 31.Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- 32.Gorshkova I, He D, Berdyshev E, et al. Protein kinase C-epsilon regulates sphingosine 1-phosphate-mediated migration of human lung endothelial cells through activation of phospholipase D2, protein kinase C-zeta, and Rac1. J Biol Chem. 2008;283(17):11794–11806. doi: 10.1074/jbc.M800250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasirer-Friede A, Cozzi MR, Mazzucato M, De Marco L, Ruggeri ZM, Shattil SJ. Signaling through GP Ib-IX-V activates alpha IIb beta 3 independently of other receptors. Blood. 2004;103(9):3403–3411. doi: 10.1182/blood-2003-10-3664. [DOI] [PubMed] [Google Scholar]
- 34.Kramer KL, Barnette JE, Yost HJ. PKCgamma regulates syndecan-2 inside-out signaling during xenopus left-right development. Cell. 2002;111(7):981–990. doi: 10.1016/s0092-8674(02)01200-x. [DOI] [PubMed] [Google Scholar]
- 35.Wouters MM, Roeder JL, Tharayil VS, et al. Protein kinase Cγ mediates regulation of proliferation by the serotonin 5-hydroxytryptamine receptor 2B. J Biol Chem. 2009;284(32):21177–21184. doi: 10.1074/jbc.M109.015859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lofqvist C, Chen J, Connor KM, et al. IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc Natl Acad Sci U S A. 2007;104(25):10589–10594. doi: 10.1073/pnas.0702031104. [DOI] [PMC free article] [PubMed] [Google Scholar]