Abstract
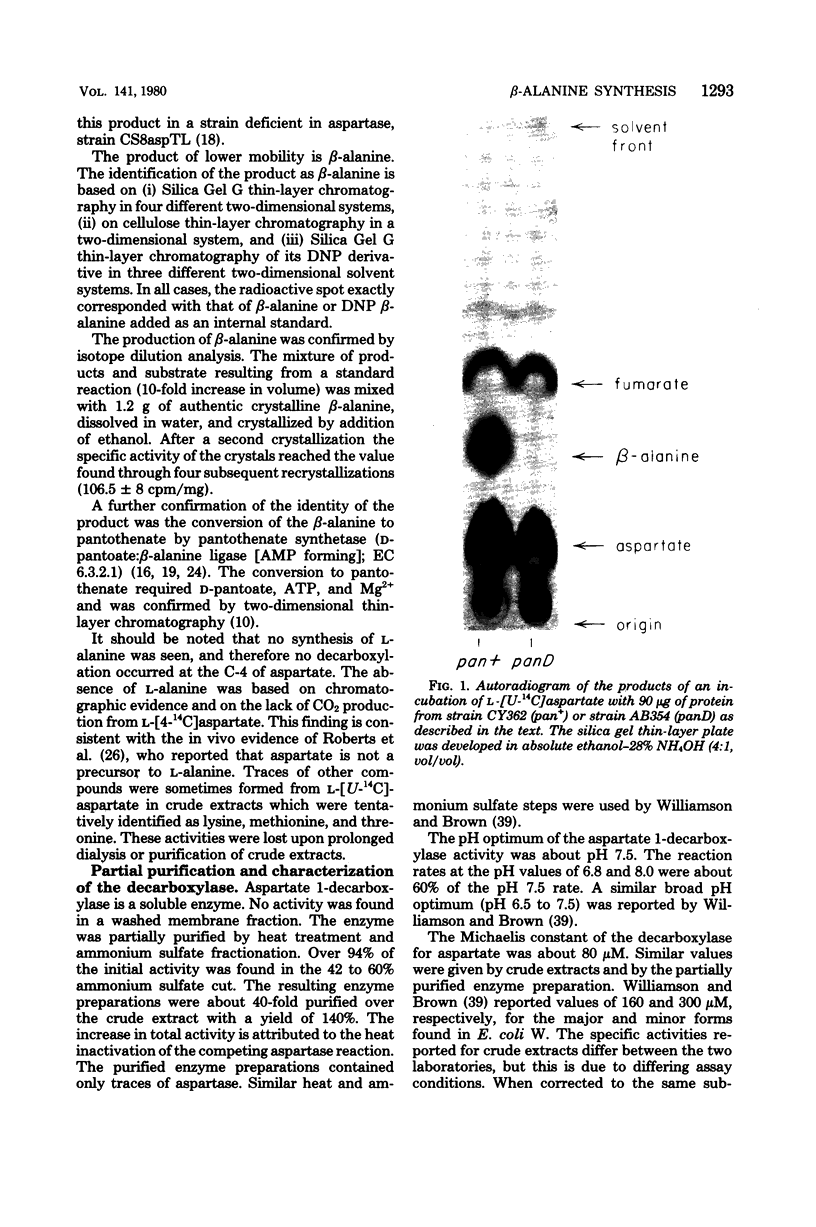
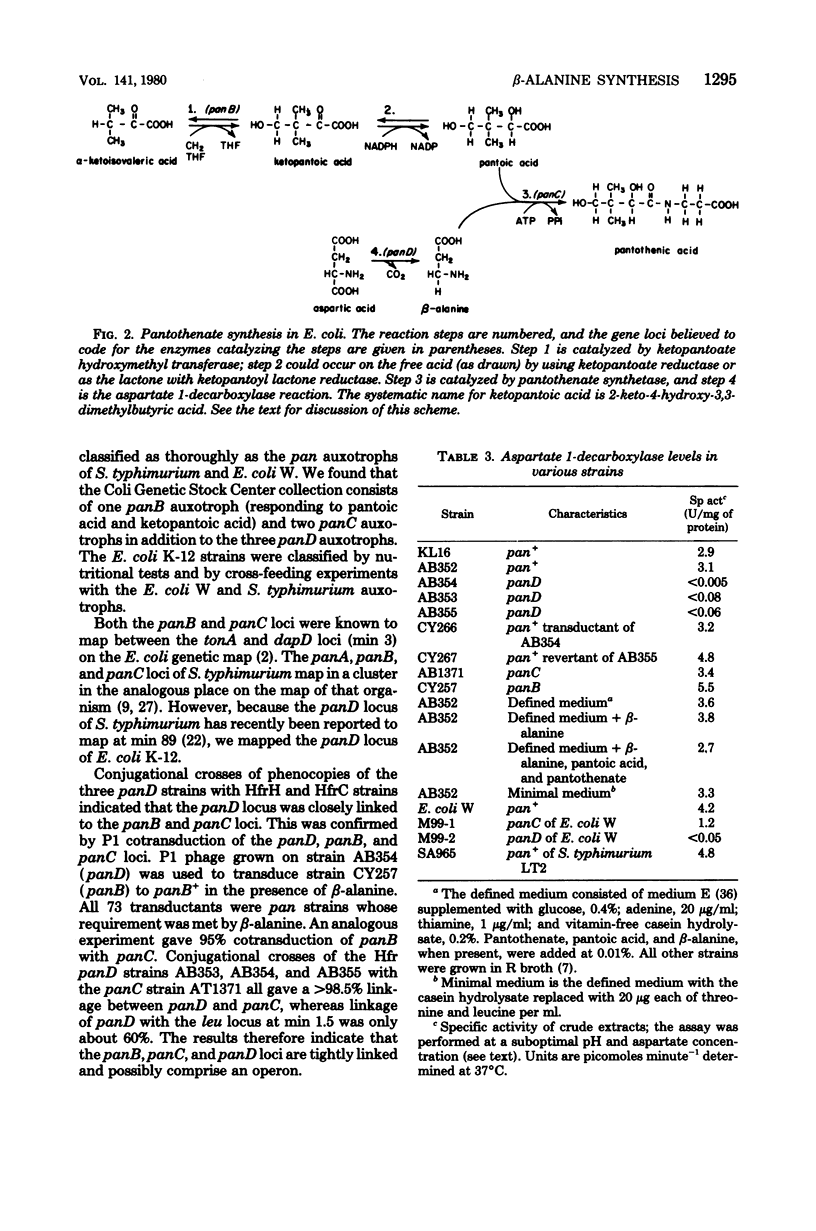
The enzyme, aspartate 1-decarboxylase (L-aspartate 1-carboxy-lyase; EC 4.1.1.15), that catalyzes the reaction aspartate leads to beta-alanine + CO2 was found in extracts of Escherichia coli. panD mutants of E. coli are defective in beta-alanine biosynthesis and lack aspartate 1-decarboxylase. Therefore, the enzyme functions in the biosynthesis of the beta-alanine moiety of pantothenate. The genetic lesion in these mutants is closely linked to the other pantothenate (pan) loci of E. coli K-12.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Vagelos P. R. Acyl carrier protein. 8. Studies of acyl carrier protein and coenzyme A in Escherichia coli pantothenate or betaalanine auxotrophs. J Biol Chem. 1966 Nov 25;241(22):5201–5204. [PubMed] [Google Scholar]
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROWN G. M. The metabolism of pantothenic acid. J Biol Chem. 1959 Feb;234(2):370–378. [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Silbert D. F., Wulff D. L. Mapping of the fabA locus for unsaturated fatty acid biosynthesis in Escherichia coli. J Bacteriol. 1972 Oct;112(1):206–211. doi: 10.1128/jb.112.1.206-211.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M. J., Trager P. G., Johnson J. B., Rubin S. H. Characterization of crystalline calcium pantothenate by physicochemical assay methods. J Pharm Sci. 1973 Jan;62(1):108–111. doi: 10.1002/jps.2600620121. [DOI] [PubMed] [Google Scholar]
- Klosterman H. J. Vitamin B6 antagonists of natural origin. Methods Enzymol. 1979;62:483–495. doi: 10.1016/0076-6879(79)62255-3. [DOI] [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- MAAS W. K., DAVIS B. D. Pantothenate studies. I. Interference by D-serine and L-aspartic acid with pantothenate synthesis in Escherichia coli. J Bacteriol. 1950 Dec;60(6):733–745. doi: 10.1128/jb.60.6.733-745.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAAS W. K. Pantothenate studies. III. Description of the extracted pantothenate-synthesizing enzyme of Escherichia coli. J Biol Chem. 1952 Sep;198(1):23–32. [PubMed] [Google Scholar]
- MUNKRES K. D., RICHARDS F. M. THE PURIFICATION AND PROPERTIES OF NEUROSPORA MALATE DEHYDROGENASE. Arch Biochem Biophys. 1965 Mar;109:466–479. doi: 10.1016/0003-9861(65)90391-7. [DOI] [PubMed] [Google Scholar]
- Marcus M., Halpern Y. S. Mapping of the aspartase gene in Escherichia coli K-12. Isr J Med Sci. 1969 May-Jun;5(3):413–415. [PubMed] [Google Scholar]
- Martin S. K., Miller L. H., Hicks C. U., David-West A., Ugbode C., Deane M. Frequency of blood group antigens in Nigerian children with falciparum malaria. Trans R Soc Trop Med Hyg. 1979;73(2):216–218. doi: 10.1016/0035-9203(79)90217-7. [DOI] [PubMed] [Google Scholar]
- Miyatake K., Nakano Y., Kitaoka S. Pantothenate synthetase from Escherichia coli [D-pantoate: beta-alanine ligase (AMP-forming), EC 6.3.2.1]. Methods Enzymol. 1979;62:215–219. doi: 10.1016/0076-6879(79)62221-8. [DOI] [PubMed] [Google Scholar]
- Ortega M. V., Cárdenas A., Ubiera D. panD, a new chromosomal locus of Salmonella typhimurium for the biosynthesis of beta-alanine. Mol Gen Genet. 1975 Sep 29;140(2):159–164. doi: 10.1007/BF00329783. [DOI] [PubMed] [Google Scholar]
- Parikh I., March S., Cuatercasas P. Topics in the methodology of substitution reactions with agarose. Methods Enzymol. 1974;34:77–102. doi: 10.1016/s0076-6879(74)34009-8. [DOI] [PubMed] [Google Scholar]
- Powers S. G., Snell E. E. Ketopantoate hydroxymethyltransferase. II. Physical, catalytic, and regulatory properties. J Biol Chem. 1976 Jun 25;251(12):3786–3793. [PubMed] [Google Scholar]
- SLOTNICK I. J. Dihydrouracil as a growth factor for a mutant strain of Escherichia coli. J Bacteriol. 1956 Aug;72(2):276–277. doi: 10.1128/jb.72.2.276-277.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLOTNICK I. J., WEINFELD H. Dihydrouracil as a growth factor for mutant strains of Escherichia coli. J Bacteriol. 1957 Aug;74(2):122–125. doi: 10.1128/jb.74.2.122-125.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E., Hartman P. E. Linkage map of Salmonella typhimurium, edition V. Microbiol Rev. 1978 Jun;42(2):471–519. doi: 10.1128/mr.42.2.471-519.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teller J. H., Powers S. G., Snell E. E. Ketopantoate hydroxymethyltransferase. I. Purification and role in pantothenate biosynthesis. J Biol Chem. 1976 Jun 25;251(12):3780–3785. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Van Den Bosch H., Williamson J. R., Vagelos P. R. Localization of acyl carrier protein in Escherichia coli. Nature. 1970 Oct 24;228(5269):338–341. doi: 10.1038/228338a0. [DOI] [PubMed] [Google Scholar]
- Wilken D. R., King H. L., Jr, Dyar R. E. Ketopantoic acid and ketopantoyl lactone reductases. Stereospecificity of transfer of hydrogen from reduced nicotinamide adenine dinucleotide phosphate. J Biol Chem. 1975 Mar 25;250(6):2311–2314. [PubMed] [Google Scholar]
- Wilken D. R., King H. L., Jr, Dyar R. E. Ketopantoyl lactone reductases. Methods Enzymol. 1979;62:209–215. doi: 10.1016/0076-6879(79)62220-6. [DOI] [PubMed] [Google Scholar]
- Williamson J. M., Brown G. M. Purification and properties of L-Aspartate-alpha-decarboxylase, an enzyme that catalyzes the formation of beta-alanine in Escherichia coli. J Biol Chem. 1979 Aug 25;254(16):8074–8082. [PubMed] [Google Scholar]



